Abstract
Extracorporeal life support (ECLS) is used for the treatment of severe cardiogenic shock. However, pulmonary congestion can progress to a severe problem with ECLS therapy. We report our experience with the Impella system for severe pulmonary congestion with ECLS therapy. We used the Impella system for two patients, which led to successful unloading of the left ventricle. Impella implantation during ECLS support appears to be a promising concept. However, more evidence is required for further evaluation.
INTRODUCTION
Extracorporeal life support (ECLS) is commonly used for the treatment of severe cardiogenic shock (CS) [1, 2]. One severe problem involved with ECLS therapy is the development of pulmonary congestion [3, 4]. Absent or marginal remaining left ventricular ejection can lead to irreversible myocardial damage, fatal pulmonary edema (PE) and thrombosis in the left heart system. The Impella system is a temporary, minimally invasive, catheter-based left ventricular assist device designed to directly unload the left ventricle (LV). The Impella LP 2.5 (Abiomed, Danvers, MA) can be inserted percutaneously into the LV. The micro-axial pump provides non-pulsatile forward blood flow of up to 2.5 l/min from the LV into the aorta [5]. We have used the Impella system to unload the LV for two patients undergoing ECLS therapy with severe pulmonary congestion.
CASE REPORT
Patient 1
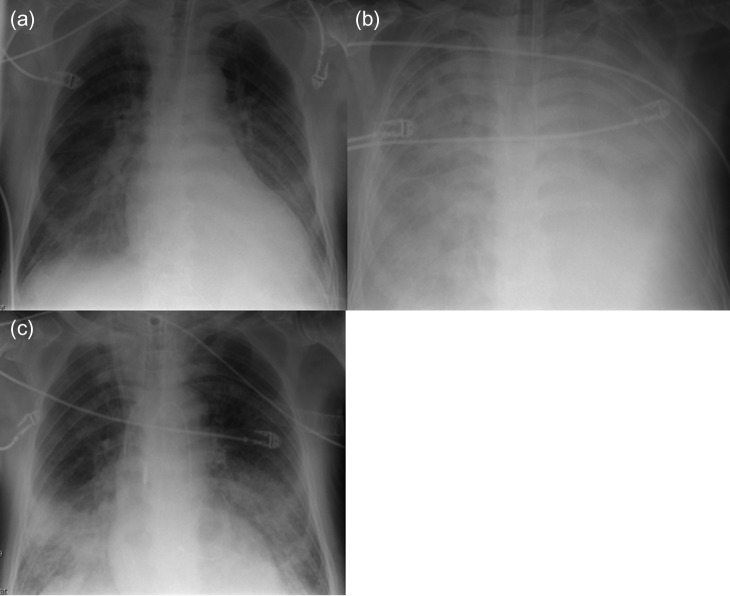
A 72-year-old man with a huge ventricular aneurysm due to a previous myocardial infarction in the anterior wall experienced an acute large myocardial infarction in the posterior wall due to total occlusion of the right coronary artery. Despite emergency coronary intervention at a regional hospital, his condition worsened and severe CS developed. After an emergency call, our ECLS team implanted ECLS percutaneously through the right femoral vessels, then the patient was transferred to our hospital. His condition rapidly stabilized. His left ventricular ejection fraction was ~20%, with a giant ventricular aneurysm on the anterior wall, akinesia of the posterior wall, and moderate mitral valve regurgitation (MR). On Day 7, chest radiography indicated that PE had worsened (Fig. 1a and b). Echocardiography revealed new onset of a small ventricular septum perforation that had not been detected by daily echocardiography. On Day 8, the Impella system was percutaneously implanted through the left femoral artery for direct unloading of the LV as therapy for PE. After Impella implantation, PE considerably improved (Fig. 1c). The flow from the Impella system was 2.0 l/min, whereas the flow from ECLS remained unchanged at 6 l/min; therefore, he had more output than before Impella implantation. However, his general condition had worsened due to sepsis and he died 2 days after Impella implantation.
Figure 1:
Radiography of Patient 1. (a) After ECLS implantation. (b) Before Impella implantation. (c) Two days after Impella implantation.
Patient 2
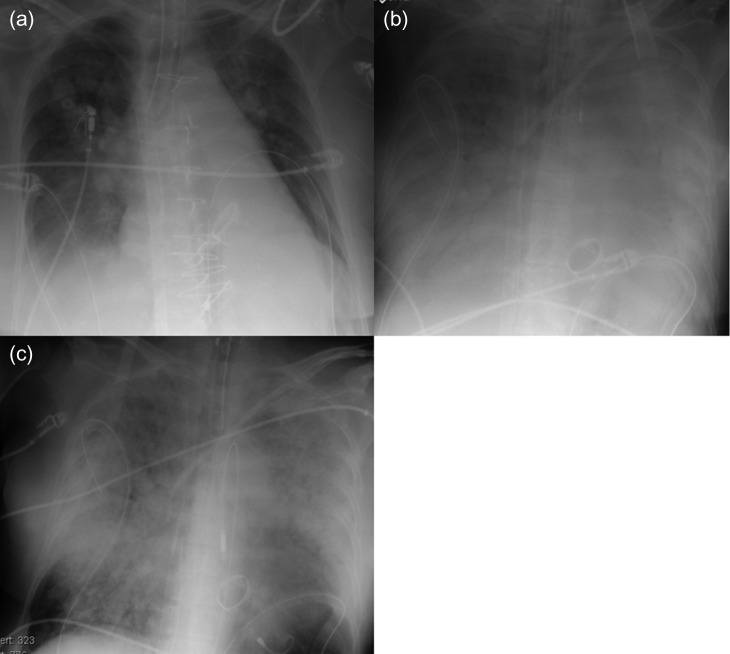
A 66-year-old woman experienced acute dyspnea and was admitted to a regional hospital. Echocardiography revealed severe MR with prolapse of the posterior leaflet. Acute coronary syndrome was ruled out by coronary catheterization. Her condition worsened on Day 2, when she needed a short period of cardiopulmonary resuscitation (CPR). After CPR, the patient was still in severe CS. Therefore, she was transferred to our hospital on the same day. ECLS was immediately implanted percutaneously through the right femoral vessels. Thereafter, her condition stabilized. Ten days after ECLS implantation, surgery including mitral valve replacement, annuloplasty of the tricuspid valve and closure of a giant atrial septum defect was performed. At the operation, an attempt to wean from the ECLS was done, but remained unsuccessful due to right heart failure. After surgery, multi-organ failure (MOF) with massive PE slowly developed (Fig. 2a and b). The Impella system was implanted percutaneously through the left femoral artery on postoperative Day 8 for direct unloading of the LV to treat PE; after implantation, PE showed clear regression on chest radiography (Fig. 2c). Flow from the Impella system was 2.0 l/min, whereas flow from ECLS remained unchanged at 5.5 l/min. Despite her higher systemic output, her general condition had worsened. She died 2 days after Impella implantation due to MOF.
Figure 2:
Radiography of Patient 2. (a) After open heart surgery. (b) Before Impella implantation. (c) Two days after Impella implantation.
Ventilation parameters and blood gas analyses
Ventilation parameters and results of blood gas analyses pre and post Impella-implantation are listed in Table 1. In both patients, the tidal and minute volumes of the lung were improved in the long run. Their hearts were able to sustain some degree of ejection and therefore blood oxygenation was not completely dependent on the ECLS system. Shortly after Impella-implantation, blood oxygenation initially worsened in the both patients, maybe because the less oxygenized blood from the massively edematous lung was increasingly mobilized into the systemic circulation. However, blood oxygenation recovered and sustainably increased after 24 h in both patients.
Table 1.
Ventilation parameters and blood gas analyses
| Parameter | 2 h after ECLS implantation | At the time of Impella implantation | 6 h after Impella implantation | 12 h after Impella implantation | 24 h after Impella implantation |
|---|---|---|---|---|---|
| Patient 1 | |||||
| pH | 7.34 | 7.34 | 7.31 | 7.42 | 7.4 |
| pO2 (mmHg) | 476 | 107 | 84 | 83.1 | 141 |
| PCO2 (mmHg) | 29.9 | 41.9 | 51 | 37.7 | 35 |
| FiO2 (%) | 100 | 45 | 75 | 50 | 35 |
| Frequency (/min) | 16 | 25 | 25 | 30 | 24 |
| Inspiratory pressure (mmHg) | 25 | 27 | 26 | 28 | 26 |
| Positive end-expiratory pressure (mmHg) | 10 | 10 | 10 | 12 | 12 |
| Tidal volume (ml) | 610 | 125 | 130 | 220 | 420 |
| Minute volume (l) | 9.6 | 5.6 | 3.0 | 6.8 | 12.8 |
| Lactate (mmol/l) | 4.1 | 3.5 | 1.7 | 1.8 | 2.5 |
| Patient 2 | |||||
| pH | 7.33 | 7.44 | 7.47 | 7.48 | 7.43 |
| pO2 (mmHg) | 99.5 | 149 | 70.9 | 155 | 148 |
| PCO2 (mmHg) | 38.9 | 37.4 | 44.3 | 40 | 36.4 |
| FiO2 (%) | 30 | 60 | 100 | 50 | 40 |
| Frequency (/min) | 12 | 16 | 11 | 11 | 11 |
| Inspiratory pressure (mmHg) | 24 | 16 | 32 | 32 | 30 |
| Positive end-expiratory pressure (mmHg) | 12 | 10 | 20 | 20 | 16 |
| Tidal volume (ml) | 301 | 460 | 74 | 140 | 193 |
| Minute volume (l) | 3.1 | 12.6 | 1.3 | 2.0 | 3.1 |
| Lactate (mmol/l) | 16 | 2.0 | 6.1 | 4.4 | 3.9 |
DISCUSSION
ECLS is the first choice of mechanical circulatory support for severe acute cardiopulmonary failure [1, 2]. One of the pitfalls of ECLS therapy is increased afterload of the LV, which leads to PE [3, 4]. Although the whole heart should be unloaded with the ECLS system, a small fraction of the blood volume still reaches the left atrium and ventricle while the ECLS system generates high LV afterload. Therefore, when left ventricular function is critically impaired, the LV cannot evacuate the blood against the increased afterload, resulting in left ventricular distention. This can lead to further deterioration of the LV and/or to severe irreversible PE [3, 4]. Some treatment options to treat PE associated with ECLS have been reported [6]. Percutaneous insertion of intra-aortic balloon pump (IABP) is popular option, but its effect on the unloading of the LV is limited. Direct unloading of the LA or LV with surgical insertion of cannula is effective but have relatively high risk of bleeding. Percutaneous atrial balloon septostomy or TandemHeart (Cardiac Assist, Pittsburgh, PA) trans-septal cannula implantation has been described as effective options, but these options are technically demanding to puncture appropriate size of defect at the exact location of the atrial septum [7, 8]. Impella implantation is more effective than IABP and easier to use than the LA unloading via atrial septum [6]. In our series, the LV could be successfully unloaded using the Impella system in all patients undergoing ECLS therapy. In usual cases, Impella 2.5LP are implanted by percutaneous cannulation of 13 Fr outer sheath, but ‘chimney’ graft technique or axillary artery access should be considered patients with small diameter of the femoral artery or peripheral arterial disease [9].
Until now, the optimal flow rate of ECLS for CS has not been well studied. However, patients with ongoing sepsis and/or MOF may need more cardiac output. For both patients in this series, the Impella system could generate blood flow 2.0 l/min without impairing the ECLS flow. Therefore, additional cardiac output could be an advantage of Impella implantation with ECLS therapy, especially for certain patients with a large body surface area or patients needing higher cardiac output due to increased end-organ oxygen consumption. Although the primary objective of the Impella system could be achieved for both patients, both of them died. For these patients, the timing of Impella implantation was probably too late; both patients already had ongoing MOF. Therefore, early intervention should be considered for effective use of the Impella system.
In conclusion, using the Impella system, the LV could be unloaded successfully in all patients in this series. It appears to be a promising concept, but more research is necessary.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None declared.
REFERENCES
- 1. Sayer GT, Baker JN, Parks KA. Heart rescue: the role of mechanical circulatory support in the management of severe refractory cardiogenic shock. Curr Opin Crit Care 2012;18:409–16. [DOI] [PubMed] [Google Scholar]
- 2. Kar B, Basra SS, Shah NR, Loyalka P. Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation 2012;125:1809–17. [DOI] [PubMed] [Google Scholar]
- 3. Avalli L, Maggioni E, Sangalli F, Favini G, Formica F, Fumagalli R. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J 2011;57:38–40. [DOI] [PubMed] [Google Scholar]
- 4. Kotani Y, Chetan D, Rodrigues W, Sivarajan VB, Gruenwald C, Guerguerian AM, et al. Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: current strategy and clinical outcomes. Artif Organs 2013;37:29–36. [DOI] [PubMed] [Google Scholar]
- 5. Lauten A, Engström AE, Jung C, Empen K, Erne P, Cook S, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-Registry. Circ Heart Fail 2013;6:23–30. [DOI] [PubMed] [Google Scholar]
- 6. Donker DW, Brodie D, Henriques JPS, Broomé M. Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion 2018;267659118794112 10.1177/0267659118794112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin YN, Chen YH, Wang HJ, Hung JS, Chang KC, Lo PH. Atrial septostomy for left atrial decompression during extracorporeal membrane oxygenation by inoue balloon catheter. Circ J 2017;81:1419–23. [DOI] [PubMed] [Google Scholar]
- 8. Bernhardt AM, Hillebrand M, Yildirim Y, Hakmi S, Wagner FM, Blankenberg S, et al. Percutaneous left atrial unloading to prevent pulmonary oedema and to facilitate ventricular recovery under extracorporeal membrane oxygenation therapy. Interact Cadiovasc Thorac Surg 2018;26:4–7. [DOI] [PubMed] [Google Scholar]
- 9. Murthy R, Brenes J, Dimas VV, Guleserian KJ. Ringed polytetrafluoroethylene (Gore-Tex) tunneled ‘chimney’ graft for pediatric use of Impella 2.5 axial flow pump. J Thorac Cardiovasc Surg 2014;147:1421–2. [DOI] [PubMed] [Google Scholar]