Abstract
AIM
To explore the more suitable concentration of glutamate or N-methyl-D-aspartic acid (NMDA) for intravitreal injection to establish a rat model of retinal neurodegeneration.
METHODS
We injected different doses of glutamate (20 or 50 nmol) or NMDA (40 nmol) into the vitreous chambers of rats, then measured the concentration of glutamate and retinal thickness, quantified apoptotic cells and determined the degree of tau hyperphosphorylation at different time points. T-test was used for comparison of two groups. One-way ANOVA and Turkey's multiple comparisons test were used for comparisons of different groups, and P values below 0.05 were considered statistically significant.
RESULTS
The glutamate level in the rats treated with 50 nmol of glutamate was twice that of the control group and persisted two weeks. Seven days after intravitreal injection of 50 nmol of glutamate, three parameters [inner retinal thickness (IRT), retinal thickness (RT) and ganglion cell layer (GCL) cell number] were reduced significantly. Furthermore, numerous TUNEL-positive cells were observed in the GCL one day after intravitreal injection of 50 nmol of glutamate, the expression of the apoptosis-related factor cleaved casepase-3 was markedly increased compared with the expression levels in the other treatment groups, and the expression levels of tau s396 and tau s404 were significantly increased compared with those in the control group.
CONCLUSION
This study demonstrates that the intravitreal injection of 50 nmol of glutamate can establish the more effective retinal neurodegeneration animal model relative to other treatment groups.
Keywords: retinal neurodegeneration, glutamate, excitotoxicity, animal model, glaucoma
INTRODUCTION
Primary glaucoma has become the second leading cause of blindness worldwide. Elevated intraocular pressure (IOP) is the only factor that can be controlled in clinical practice, and many basic studies of glaucoma have been based on an animal model of elevated IOP. However, vision and the visual field gradually deteriorate even in the presence of stable IOP in some patients[1], indicating that damage to the optic nerve is progressive. Currently, the exact pathogenesis of glaucoma is in-completely understood. A pathophysiological hallmark of glaucoma is gradual retinal neurodegeneration, particularly the degeneration and death of retinal ganglion cells (RGCs)[2], which are central nervous system (CNS) neurons that have their soma in the inner retina and their axons gathered in the optic nerve. RGCs convey visual information from the retina to the brain. Similar to other CNS neurons, RGCs are difficult to regenerate if they die. To some extent, glaucoma can be viewed as a neurodegenerative disease, similar to other degenerative CNS diseases such as Alzheimer's and Parkinson's disease, and is ultimately caused by deficits in neuronal function[3]–[6]. The degeneration of retinal neurons, including RGCs degeneration in glaucoma, occurs as a result of apoptotic mechanisms triggered by multiple factors. In addition to elevated IOP, other factors play important roles in glaucoma pathogenesis, including elevated glutamate concentrations, ischemia, oxidative stress, excessive production of nitric oxide, and reductions in neurotrophic factors[2].
Excitatory amino acids (EAAs) are considered to have an important role in the pathophysiology of CNS degeneration[7]–[8]. Glutamate is the most common EAA in the CNS, and N-methyl-D-aspartic acid (NMDA) is an analogue of glutamate. Many studies have shown that intravitreal injection of glutamate or NMDA can lead to rapid RGC degeneration and apoptosis[9]–[11]. Multiple studies have established glutamate-induced RGC-degeneration animal models of glaucoma[10]. However, the dose and duration of treatment varies greatly, and no comparisons of NMDA and glutamate are available; most studies have not tested the actual concentration of glutamate in vitreous fluid. Researchers have shown that glutamate concentrations in excess of 25 µmol/L cause excitotoxic damage to neurons[12]. According to the literatures [9],[12]–[15], the effective glutamate concentration, the actual vitreous volume of rat and time for test were taken into consideration, we selected 50 nmol and 20 nmol glutamate as well as 40 nmol NMDA for injection into the vitreous chambers of rats to induce retinal excitotoxic damage. Then, we detected the concentration of glutamate, measured retinal thickness (RT), counted apoptotic neurons of retina and assessed the degree of tau hyperphosphorylation at different time points[16]. The purpose of this study was to explore the more suitable concentration of glutamate or NMDA for injection into the vitreous chamber to establish an excitotoxic damage related rat retinal neurodegeneration model.
MATERIALS AND METHODS
Animals and Anesthesia
Adult male Sprague-Dawley rats weighing 180-200 g were purchased from the Laboratory Animal Center of Daping Hospital of Army Medical University. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals. The rats were housed in cages with free access to food and water and were maintained on a 12h light/dark cycle in a temperature-controlled room (22°C). Light intensity within the cages ranged from 9 to 24 lx. The animal experiments also followed our institutional guidelines. All surgical procedures were carried out under general anesthesia induced by intraperitoneal (i.p.) injection of 10% chloral hydrate (350 mg/kg). The animals were sacrificed with an i.p. injection of an overdose of 10% chloral hydrate at various time points after intravitreal injection.
Methods
Intravitreal injection
The rats were anesthetized by i.p. injection of 10% chloral hydrate (350 mg/kg). Before injection, pupillary dilatation was maintained by administering tropicamide phenylephrine eye drops 3 times for a total of 90 µL (Santen Pharmaceutical Co., Ltd., Japan), and topical anesthesia was achieved by administering 0.5% oxybuprocaine hydrochloride eye drops 3 times for a total of 90 µL (Santen Pharmaceutical Co., Ltd., Japan). Levofloxacin eye drops (Santen Pharmaceutical Co., Ltd., Japan) were administered 3 times for a total of 90 µL to clean conjunctival sacs.
Three microlitres of different drugs [40 nmol NMDA: 20 mmol/L (Sigma-Aldrich); 50 nmol glutamate (Solarbio): 25 mmol/L; 20 nmol glutamate: 25 mmol/L; when injecting less than 3 µL, 0.1 mol/L sterile phosphate-buffered saline (PBS; HyClone) was added to bring the volume to 3 µL] was injected into the right eye with a 30-gauge (G) needle (Hamilton). The needle punctured the center of the vitreous chamber approximately 1.5 mm from the limbus in the superior temporal quadrant. The tip of the needle was pointed in the direction of the optic nerve to avoid injuring the lens. The tip was visible through the dilated pupil, and the drug was slowly injected. The needle was left in the eye for an additional 20s to allow the eye to adjust to the increase in volume and then pulled out. The control group received the same volume of 0.1 mol/L of sterile PBS (HyClone) as this was used as the solvent for NMDA and glutamate.
The left eyes remained untreated. After injection, the eyes were treated 3 times with a total of 90 µL of levofloxacin eye drops (Santen Pharmaceutical Co., Ltd., Japan). While recovering from anesthesia, the animals were placed in their cages.
At least six animals were used for each experimental condition, and eyes were enucleated and processed for further analysis.
Determination of the glutamate concentration in vitreous humor samples by high-performance liquid chromatography
Vitreous humor samples were obtained from each eye of all animals immediately after euthanasia. A 30-G micro syringe was used to obtain each sample from the central region of the vitreous chamber. Care was taken to avoid contaminating the vitreous humor sample with blood or any other intraocular tissue. Samples were centrifuged at 13 000 rpm for 10min at 4°C. The supernatant was then transferred to new centrifuge tubes. Subsequently, 2 µL of HClO4 and 1 µL of K2CO3 (ratio: 10:2:1) were added to the vitreous humor samples (10 µL), followed by centrifugation twice at 13 000 rpm for 10min at 4°C. The glutamate concentrations in the vitreous humor samples were measured via high-performance liquid chromatography (HPLC; Waters e2695 Separations Module, WATERS Co., USA). The samples were run through a C18 column and analyzed by a 157 spectrofluorometric detector with the excitation wavelength set at 330 nm and the emission wavelength fixed at 450 nm[17].
Histology and Analysis
Paraffin sectioning
Eyes were immediately enucleated after euthanasia via anesthetic overdose followed by cervical dislocation. The cornea, lens and vitreous humor were removed after incubation with Davidson's fluid (2% of a 37%-40% solution of formaldehyde, 35% ethanol, 10% glacial acetic acid and 53% distilled water) for approximately 3h. The remainder of each eye cup was incubated in Davidson's fluid overnight. The tissue samples were dehydrated and embedded in paraffin wax. Microtomy was performed in the sagittal plane (parallel to the visual axis) from the temporal to nasal sides of the eye. Sections through the optic nerve, at a thickness of 5 µm, were mounted on slides for hematoxylin and eosin (H&E), TUNEL and immunofluorescence assays.
H&E: cell counting and morphometry
Sections were gradient dewaxed, washed with PBS 3 times and stained with H&E. To count the number of neurons and for morphometric analysis of RT, thirty-six images of six sections, with an interval of 30 µm per retina, were examined under 200× magnification, and six images were taken of each section from one ora serrata through the optic nerve to another ora serrata (two peripheral, two equatorial and two posterior images) using a microscope (Leica DM 1000). Images were processed with Image-Pro Plus 6.0 (Media Cybernetics, USA).
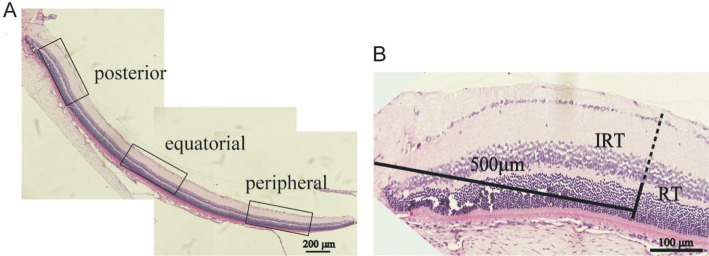
For cell counts, only cells with a generally round shape and a discernable nucleus and cytoplasm were selected. No attempt was made to distinguish the types of RGCs or displaced amacrine cells. Morphologically distinguishable glial cells and vascular endothelial cells were excluded from the cell count. The cell counting unit was defined as the total cell number of one posterior; one equatorial and one peripheral picture of a section (Figure 1A). The sum of twelve units from six sections was averaged to obtain the value of one eye. Six eyes were used for each time point in all groups.
Figure 1. Method of measurement.
A: The cell counting unit was defined as the total cell number of one posterior, one equatorial and one peripheral picture of a section; B: Approximately 500 µm from the center of optic disc of posterior images were used for analysis of IRT and RT.
Inner retinal thickness (IRT) was defined from the inner boundary of the ganglion cell layer (GCL) to the outer boundary of the inner nuclear layer (INL). Overall RT was defined from the inner boundary of the GCL to the outer boundary of the outer nuclear layer (ONL). For standardization, a site approximately 500 µm from the center of the optic disc in the posterior images was used for the analysis of RT (Figure 1B). Measurements of six posterior pictures from three sections were averaged to obtain the value of one eye. Six eyes were used for each time point in all groups.
Immunofluorescence
Sections were then prepared for immunofluorescence according to standard techniques. Briefly, specimens were sequentially dewaxed, washed with PBS 3 times and subjected to antigen retrieval in heated sodium citrate. Specimens were then treated with 0.1% TritonX-100 (Solarbio) and blocked in PBS containing 10% goat serum to reduce nonspecific binding. Then, sectioned specimens were treated with primary antibodies diluted with antibody dilution solution (Solarbio; rabbit anti-tau phospho s396, ab109390, Abcam; dilution 1:300 and rabbit anti-tau phospho s404, ab131338, Abcam; dilution: 1:300) overnight at 4°C. Sections were washed with PBS 3 times and incubated with a secondary antibody (ab150077, Abcam; Alexa Fluor 488-conjugated goat anti-rabbit; dilution: 1:300) for two hours at room temperature. The sections were then washed in PBS, and Hoechst 33342 (C0030, Solarbio; dilution: 1:2000) was used for nuclear staining for three minutes. After staining, the sections were washed with PBS and mounted on slides with florescent mounting medium. Negative controls for all antibody stains were performed using secondary antibodies only. Images were obtained with identical parameters with an SP-8 confocal microscope (Leica, Germany) at 200× magnification and were processed with Leica LAS AF Lite software (Leica, Germany) and Image-Pro Plus 6.0 (Media Cybernetics, USA). For further evaluations, six images per retina (two peripheral, two equatorial, and two posterior images) and six eyes for each time point were examined.
TUNEL assay
Apoptotic cell death was detected in retinal sections following the protocol provided with the TUNEL kit (Roche, Switzerland). Briefly, the deparaffinized sections were washed three times in double distilled water and PBS for 5min each. Specimens were incubated with proteinase K (20 mg/L) diluted with 10 mmol/L Tris-HCl for 15min on ice for permeabilization. For double immunofluorescent staining of Brn-3a and TUNEL, samples were treated with mouse anti-Brn-3a antibody (MAB1585, Millipore; dilution: 1:50) and goat anti-mouse IgG H&L (Cy3; ab97035, Abcam; dilution: 1:300). Subsequently, the TUNEL detection solution was added to the sections, and the samples were incubated for 40min at 37°C under dark and humid conditions. The sections were washed with PBS and incubated with Hoechst 33342 (C0030, Solarbio; dilution: 1:2000) for nuclear staining. After washing in PBS, the sections were mounted, and images were taken using an SP-8 confocal microscope (Leica, Germany) at 200× magnification and were processed with Leica LAS AF Lite software (Leica, Germany). Image-Pro Plus 6.0 (Media Cybernetics, USA) was used to quantify the total number of Brn-3a positive cells, TUNEL-positive cells and the total number of nuclei in the GCL. The apoptotic index was calculated as the number of TUNEL-positive cells/total number of cells in the GCL within one image (×100%). Three sections, with an interval of 30 µm per retina, were examined under 200× magnification, and six images were taken of each section from one ora serrata through the optic nerve to another ora serrata (two peripheral, two equatorial and two posterior images) with a microscope (Leica DM 1000). Six eyes were used for each time point of all groups.
Western blotting
For Western blotting, samples were boiled at 100°C for 5min in loading buffer. The proteins were electrophoresed in 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the separated proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore Ltd.). The membranes were then blocked with 5% bovine serum albumin (BSA; Solarbio, Beijing, China) for 1h and probed with primary antibodies diluted with antibody dilution solution (Beyotime, Shanghai, China) rabbit anti-tau phospho s396 (ab109390, Abcam; dilution 1:10 000), rabbit anti-tau phospho s404 (ab131338, Abcam; dilution: 1:1000), rabbit anti-tau (ab32057, Abcam; dilution: 1:5000), rabbit anti-caspase-3 (#9662, Cell Signaling; dilution: 1:1000), and mouse anti-beta actin (ab6276, Abcam; dilution: 1:5000) at 4°C overnight. Secondary antibodies diluted with antibody dilution solution (Beyotime, Shanghai, China) goat anti-mouse IgG H&L (HRP; ab6789, Abcam; dilution: 1:5000) or goat anti-rabbit IgG H&L (HRP; ab6721, Abcam; dilution: 1:5000) were applied for 1h. Images were taken with a gel imaging system (Aplegen, Gel Company, Inc, CA, USA). The protein bands were recorded and analyzed with LabWorks (version 4.6.0.65, UVP Company). The protein signal intensity was normalized to β-actin. We performed three repeats per analysis.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6.01 (GraphPad Software, USA). The data are presented as the mean±standard deviation (SD) with a minimum of n=3. The t-test was used for comparison of two groups. One-way ANOVA and Turkey's multiple comparisons test were used for comparisons of different groups, and P values below 0.05 were considered statistically significant.
RESULTS
Five eyes that suffered hypotension because of drug leakage, cataract and infection were excluded.
Glutamate Concentrations of Vitreous Humor
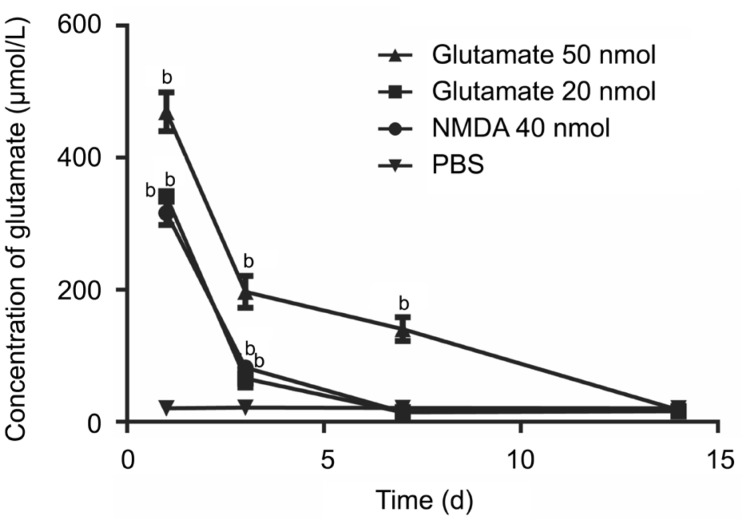
Injection of 50 nmol or 20 nmol of glutamate or 40 nmol of NMDA all caused significantly increased glutamate concentrations in the vitreous humor samples (P<0.05) compared with those in the PBS group. Injection of 50 nmol of glutamate resulted in a twofold greater level of glutamate than the normal level, which lasted for approximately 2wk (approximately 1wk following the other two treatments). The concentrations then gradually decreased to normal levels (Figure 2).
Figure 2. A rat model of glutamate analyses.
The glutamate concentration reached the peak the day after injection, then decreased gradually. There was good agreement between the injected values and the values measured by HPLC. aP<0.05; bP<0.01.
Changes in Retinal Morphology in the Glutamate-mediated Retinal Excitotoxic Damage Model
Decrease in cell number, disorganized cell arrangements and the appearance of vacuoles in the GCL were observed in the treated eyes (Figure 3A).
Figure 3. Time-dependent loss of inner retinal elements postinjection.
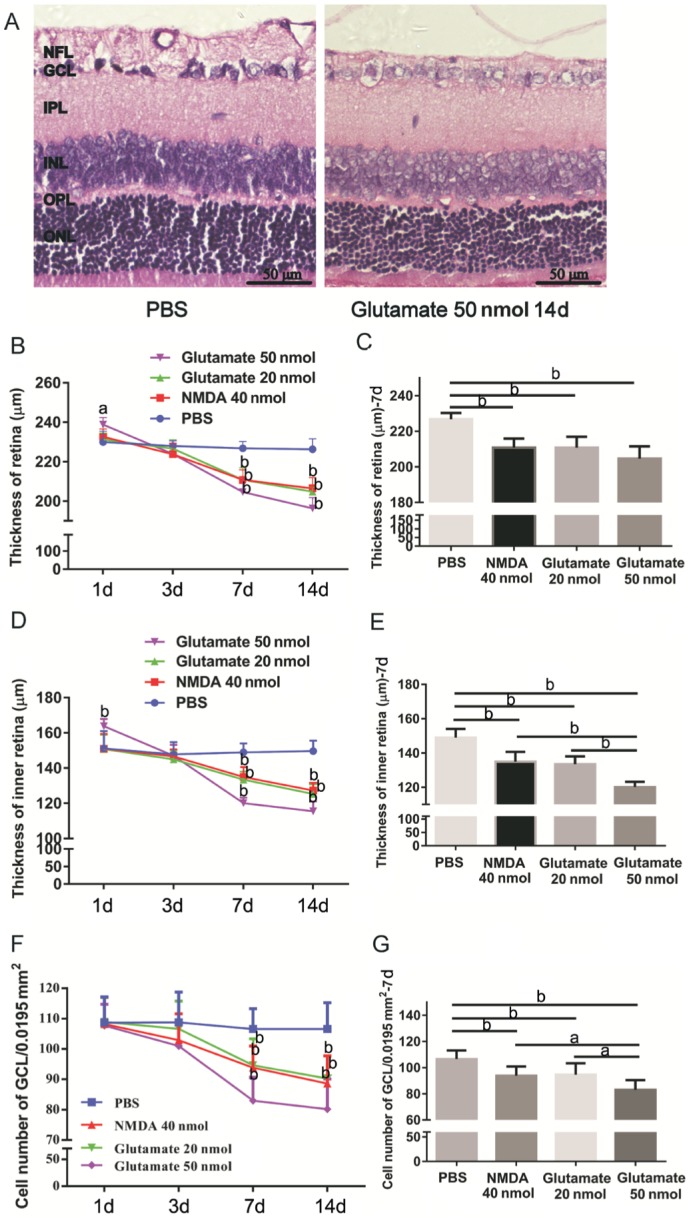
A: H&E stain of sagittal semithin section through the retina of rat eye 2wk after intravitreal injection of PBS and glutamate 50 nmol; B, D, F: Change of RT, IRT and cell number of GCL; C, E, G: RT, IRT and cell number of GCL 7d after intravitreal injection. aP<0.05; bP<0.01.
Except for the PBS group, the RT, IRT and GCL cell number all decreased 7d after intravitreal injection of 50 nmol or 20 nmol of glutamate or 40 nmol of NMDA, and these changes were time-dependent (Figure 3B, 3D and 3F).
Significant reductions in RT (µm) were noted 7d after intravitreal injections of 40 nmol of NMDA (210.833±5.037, P<0.01), 20 nmol of glutamate (210.833±6.080, P<0.01) and 50 nmol of glutamate (204.667±6.861, P<0.01) compared with that in the PBS group (226.833±3.430; Figure 3C). A decrease in IRT (µm) was apparent 7d after intravitreal injections of 40 nmol of NMDA (135±5.657, P<0.01), 20 nmol of glutamate (133.5±4.506, P<0.01), and 50 nmol of glutamate (120.167±5.648, P<0.01) compared with those in the PBS group (149±5.060; Figure 3E). There were statistically significant reductions in IRT (Figure 3E) and GCL cell number (Figure 3G) in the eyes treated with 50 nmol of glutamate compared with those in the other two treated groups (P<0.01). However, no statistically significant differences in RT were observed among the three treated groups (Figure 3C), although the RT with injection of 50 nmol of glutamate decreased more than those in the other two treated groups. Moreover, no statistically significant differences between the effects of 40 nmol of NMDA and 20 nmol of glutamate were observed in these three parameters (P>0.05).
Apoptosis in the Retina of the Glutamate-mediated Retinal Excitotoxic Damage Model
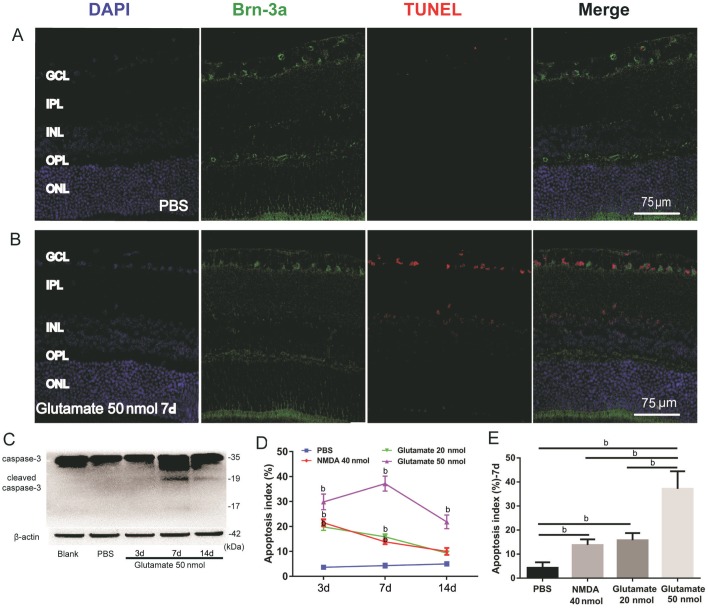
Only a few scattered TUNEL-positive cells were detected in the sections of eyes that received intravitreal injections of PBS (Figure 4A). Numerous TUNEL-positive cells observed in the GCL were Brn-3a positive, with only a few TUNEL-positive cells in the inner plexiform layer (IPL) and INL 7d after intravitreal injections of 50 nmol of glutamate (Figure 4B). The expression of the apoptosis-related factor cleaved caspase-3 was detected 7d after intravitreal injection of 50 nmol of glutamate (Figure 4C). In addition, these changes were time-dependent, and the apoptosis index peaked 7d after intravitreal injection of 50 nmol of glutamate and 3d for 20 nmol of glutamate or 40 nmol of NMDA, then decreased gradually (Figure 4D). There were no statistically significant differences between the effects of 40 nmol of NMDA and 20 nmol of glutamate at any time point (P>0.05; Figure 4D). Moreover, when compared with 20 nmol of glutamate, there was a significant increase in the apoptosis index of 50 nmol of glutamate 7d after intravitreal injection (P<0.01; Figure 4E). Very few TUNEL-positive cells were detected in the ONL, which may suggest that the excitotoxicity of glutamate has little effect on the outer retina in this glutamate-mediated retinal excitotoxic damage animal model.
Figure 4. TUNEL of retinae at various time points after intravitreal injection.
A, B: TUNEL of reinae from PBS group (B) and 50 nmol glutamate group (C); C: Relative protein level of cleaved caspase-3 (n=3); D-E: The apoptosis index of each group (n=6). Apoptosis index= (TUNEL positive cells/total cells of GCL) ×100%. aP<0.05; bP<0.01.
Tau Hyperphosphorylation in the Glutamate-mediated Retinal Excitotoxic Damage Model
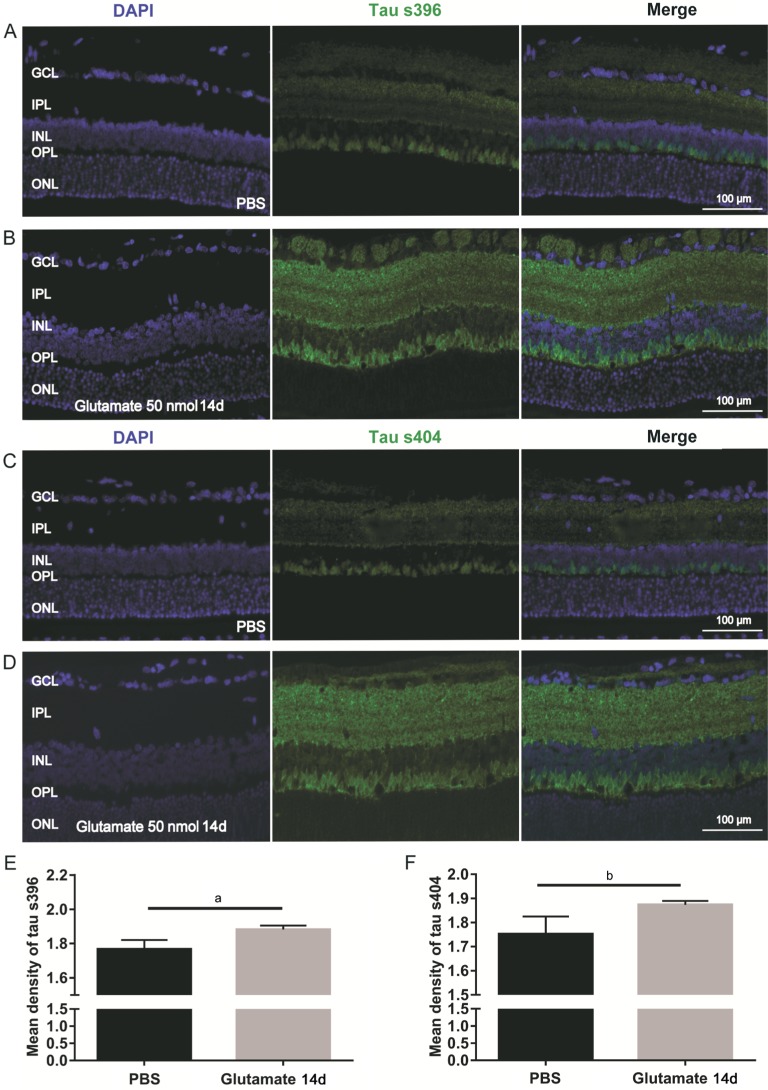
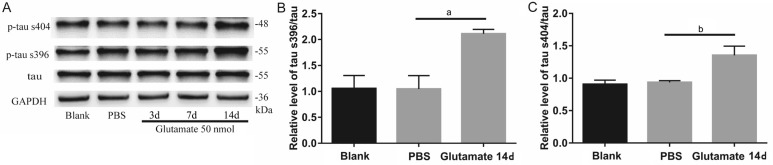
Normally, few hyperphosphorylated tau proteins are expressed in retinas. However, 14d after intravitreal injection of 50 nmol of glutamate, the expression of p-tau s396 and s404 increased significantly compared with that in the PBS group (Figure 5A-5D). The immunofluorescence assays revealed significant differences between the glutamate injection group and the PBS injection group in terms of the mean retinal expression densities of p-tau s396 (1.883±0.007712 vs 1.767±0.01809, P<0.01; Figure 5E) and p-tau s404 (1.874±0.005286 vs 1.752±0.02443, P<0.01; Figure 5F). The Western blot data were consistent with the results of the immunofluorescence assay, demonstrating significant differences between the glutamate group and the PBS group in the retinal expression of p-tau s396 (2.112±0.086 vs 1.048±0.257, P<0.05; Figure 6B) and p-tau s404 (1.354±0.141 vs 0.953±0.027, P<0.05; Figure 6C).
Figure 5. Upregulation of phosphorylated tau s396 and s404 14d after intravitreal injection of glutamate 50 nmol (Immunofluorescence).
A, C: Phosphorylated tau s396 (A) and s404 (C) of PBS groups; B, D: Phosphorylated tau s396 (B) and s404 (D) 14d after intravitreal injection of 50 nmol glutamate; E-F: Mean density of phosphorylated tau s396 and tau s404. aP<0.05; bP<0.01.
Figure 6. Upregulation of phosphorylated tau s396 and s404 14d after intravitreal injection of glutamate 50 nmol (Western blotting).
Relative protein level of phosphorylated tau s396 (n=3; B) and phosphorylated tau s404 (n=3; C). aP<0.05; bP<0.01.
DISCUSSION
Although most studies have demonstrated that intravitreal EAA concentrations increase in many ocular diseases, such as diabetic retinopathy, glaucoma, retinal detachment, retinal artery occlusion and central retinal vein occlusion[18]–[21], a few studies have shown that there were no significant changes in vitreous glutamate concentration between glaucoma eyes and control eyes[20],[22]–[23]. However, these studies were limited by a small number of study subjects[22], the use of some of the subjects included in several other protocols[23], and abnormalities in the control group[22]. It is possible that the epiretinal membrane or macular hole by itself could have elevated vitreous glutamate levels, thus lessening the difference between the study and control groups. Moreover, inappropriate handling of the samples before analysis and vitreous sample storage may have interfered with the results. Even if not statistically significant, the elevation of glutamate levels in vitreous glutamate may be physiologically sufficient to damage the retinal neurons, resulting in retinal neurodegeneration. Therefore, the possibility of glutamate-related excitotoxic effects on RGCs as a pathogenic factor of glaucoma should not be dismissed.
Glutamate levels are elevated not only in patients with visual field loss but also in those with glaucomatous optic nerve damage without field loss[24], suggesting that this aminoacidopathy may be present in the earlier stages of glaucoma and the elevated IOP may not be a necessary factor for glaucoma. Therefore, this glutamate-mediated retinal excitotoxic damage model as a normotensive glaucoma model may better represent the early stages of glaucoma. However, the dose and duration of glutamate or NMDA injected into the vitreous chamber varies greatly[9],[13]–[14],[25]. To explore the more suitable concentration of glutamate or NMDA to establish a rat retinal excitotoxic damage model, we injected different doses of those compounds into the vitreous chambers of rats and observed several indices in this study. According to the literature (Table 1)[9],[12]–[15],[25], the effective glutamate concentration, the true vitreous volume of the rat and time of test should be taken into consideration, and we selected 50 nmol and 20 nmol of glutamate as well as 40 nmol of NMDA to explore the more suitable concentration of glutamate or NMDA for establishing a rat retinal excitotoxic damage model.
Table 1. Literatures on excitatory toxicity of glutamate.
| Literature | Subject | Methods | Time of observation | Results |
| Kuehn et al, 2017[9] | rat | Intravitreal injection 20, 40 and 80 nmol NMDA or PBS | days 3 and 14 | NMDA 20 nmol/L had no effect on the percentage of Brn-3a+ cells. |
| Aihara et al, 2014[25] | rat RGCs | 5 or 25 µmol/L of glutamate; hyperbaric pressure | 72h | The survival rate of RGCs in the presence of 25 µmol/L of glutamate was significantly reduced; Hyperbaric pressure increased RGC susceptibility to glutamate toxicity. |
| Otori et al, 1998[12] | rat RGCs | 5-500 µmol/L of glutamate | 3d | Low doses of glutamate can activate AMPA-KA receptors in RGCs, which causes decreases in cell survival. |
| Vorwerk et al, 1996[14] | rat | Every 5d intravitreal injection of 2.5 nmol (1 µL) glutamate | 1h, 1, 3, 5, 10d, 1, 2, 3mo | No retinal pathology was observed 10d after intravitreal injection; About 42% of the RGCs were killed 3mo after intravitreal injection. |
| Siliprandi et al, 1992[13] | rat | 5 µL different concentrations of NMDA (2-200 nmol per injection) | 8d | Administration of NMDA resulted in a dose dependent loss of cells in the GCL and a reduction in thickness of the IPL. |
| Dureau et al, 2001[15] | rat | Intravitreal injections of 1, 3, 5, or 10 µL China ink | immediately | A 3 or 5 µL volume appears to have the best reproducibility with minimum loss of solution. |
NMDA: N-methyl-D-aspartic acid; AMPA-KA: Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-kainic acid; IPL: Inner plexform layer.
A recent study by Kuehn et al[9], that 20 nmol of NMDA had no effect on the percentage of RGCs and that injection of more than 40 nmol of NMDA reduced the percentage of RGCs in the retina. Some investigators have reported that a single injection of as little as 20 nmol of NMDA can be highly toxic to the RGC layer in the eyes of rats[13]. However, most of these studies did not measure the final concentrations of glutamate in vitreous samples. We observed that injections of 20 or 50 nmol of glutamate or 40 nmol of NMDA caused significantly increased glutamate concentrations in vitreous humor samples (P<0.05) compared with levels in the untreated group. However, 40 nmol of NMDA has effects that were approximately equivalent to those of 20 nmol of glutamate; almost all tested indices were similar, including the concentration of glutamate in vitreous samples, GCL cell number and IRT after intravitreal injection at each time point. More obvious changes in these parameters occurred in the group that received intravitreal injections of 50 nmol of glutamate, which resulted in twofold greater levels of glutamate than normal levels for approximately 2wk, while the elevated levels in the other two groups only lasted approximately 1wk. The levels then gradually decreased to normal. The predominant form of excitotoxicity of retinal neuron is mediated by overstimulation of the NMDA subtype of glutamate receptor, which, in turn, leads to excessive levels of intracellular calcium[26]. Non-NMDA receptors also may play a role in glutamate excitotoxicity[12],[21]. Intravitreal injection of glutamate may be better than NMDA with respect to the induction of excitotoxicity.
Many of the changes in retinal morphology after glutamate injection were similar to the pathological changes associated with glaucoma: a dramatic decrease in the thickness of the nerve fiber layer (NFL), a decrease in the GCL cell number, and a large number of vacuole-containing cells in the GCL. In addition, the number of TUNEL-positive cells in the GCL increased after injection, and 7d later, the number gradually decreased. Furthermore, the expression of the apoptosis-related factor cleaved caspase-3 was detected 7d after intravitreal injection of 50 nmol of glutamate. These data are consistent with the concept that three weeks after intravitreal injections, apoptotic processes in the retina are largely complete, and cellular deposits have been removed[11]. Seitz and Tamm[11] reported that a few hours after injections of 30 nmol of NMDA into the vitreous chambers of mice, the first apoptotic cells could be observed in the retina; 24h later, at least half of the cells in the GCL were TUNEL-positive. Given that the volume of the rat vitreous chamber is much larger than that of mice, the differences in the final concentrations of glutamate may cause different results.
Tau proteins are essential for microtubule assembly and microtubule stabilization. We suggest that tau proteins are closely related to retinal neurons degeneration, which is supported by the increased levels of tau proteins in the vitreous bodies of patients with glaucoma and diabetic retinopathy, the susceptibility of retinal neurons to tau-mediated neurodegeneration[27], and the fact that glaucoma exhibits signature pathological features of tauopathies, including tau accumulation, altered tau phosphorylation and tau protein missorting[28]. Phosphorylation is a critical posttranslational modification of tau proteins during development and in pathological conditions. Phosphorylation on residues s396 and s404 has been described in the brains of patients with tauopathies, and both residues are known to impair microtubule binding and facilitate tau aggregation[28]. Normally, few hyperphosphorylated tau proteins are expressed in the retina. In our experiment, after intravitreal injection of 50 nmol of glutamate, the expression of p-tau s396 and s404 in the retina increased significantly compared with that in the control group, which was accompanied by a sustained high concentration of glutamate in the vitreous humor for at least 2wk. These data suggest that the glutamate-induced retinal excitotoxic damage animal model is a good tool for investigating the pathogenesis of glaucoma.
In summary, our study demonstrates that intravitreal injections of glutamate or NMDA can cause glaucomatous retinal neurodegeneration. Moreover, treatment with 50 nmol of glutamate via rat intravitreal injection establishes a more effective retinal excitotoxic damage animal model.
Acknowledgments
Foundation: Supported by National Natural Science Foundation of China (No.81400398).
Conflicts of Interest: Gao L, None; Zheng QJ, None; Ai LQY, None; Chen KJ, None; Zhou YG, None; Ye J, None; Liu W, None.
REFERENCES
- 1.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for progression and glaucoma treatment the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 2.Almasieh M, Wilson AM, Morquette B, Vargas JLC, Polo AD. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease: the story so far. Nat Rev Neurol. 2015;12(1):15–27. doi: 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- 4.Thomson KL, Yeo JM, Waddell Briony, Cameron JR, Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst) 2015;1(2):136–143. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan TDL, Goldacre R, Goldacre MJ. Associations between primary open angle glaucoma, Alzheimer's disease and vascular dementia: record linkage study. Br J Ophthalmol. 2015;99(4):524. doi: 10.1136/bjophthalmol-2014-305863. [DOI] [PubMed] [Google Scholar]
- 6.McKinnon SJ. The cell and molecular biology of glaucoma: common neurodegenerative pathways and relevance to glaucoma. Invest Ophthalmol Vis Sci. 2012;53(5):2485–2487. doi: 10.1167/iovs.12-9483j. [DOI] [PubMed] [Google Scholar]
- 7.SA L, PA R. Excitory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Li P, Feng J, Wu M. Dysfunction of NMDA receptors in Alzheimer's disease. Neurol Sci. 2016;37(7):1039–1047. doi: 10.1007/s10072-016-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuehn S, Rodust C, Stute G, Grotegut P, Meißner W, Dick SRHB, Joachim SC. Concentration-dependent inner retina layer damage and optic nerve degeneration in a NMDA model. J Mol Neurosci. 2017;63(3-4):283–299. doi: 10.1007/s12031-017-0978-x. [DOI] [PubMed] [Google Scholar]
- 10.Lam TT, Abler AS, Kwong JMK, Tso MOM. N-methyl-D-aspartate (NMDA)-induced apoptosis in rat retina. Invest Ophthalmol Vis Sci. 1999;40(10):2391–2397. [PubMed] [Google Scholar]
- 11.Seitz R, Tamm ER. N-methyl-D-aspartate (NMDA)-mediated excitotoxic damage: a mouse model of acute retinal ganglion cell damage. Methods Mol Biol. 2013;935:99–109. doi: 10.1007/978-1-62703-080-9_7. [DOI] [PubMed] [Google Scholar]
- 12.Otori Y, Wei JY, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 1998;39(6):972–981. [PubMed] [Google Scholar]
- 13.Siliprandi R, Canella R, Carmignoto G, Schiavo N, Zanellato A, Zanoni R. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992;8(6):567–573. doi: 10.1017/s0952523800005666. [DOI] [PubMed] [Google Scholar]
- 14.Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA, Dreyer EB. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996;37(8):1618–1624. [PubMed] [Google Scholar]
- 15.Dureau P, Bonnel S, Menasche M, Dufier JL, Abitbol M. Quantitative analysis of intravitreal injections in the rat. Curr Eye Res. 2001;22(1):74–77. doi: 10.1076/ceyr.22.1.74.6974. [DOI] [PubMed] [Google Scholar]
- 16.Cuenca N, Pinilla I, Fernández-Sánchez L, Salinas-Navarro M, Alarcón-Martínez L, Avilés-Trigueros M, Pdl Villa, Imperial JMd, Villegas-Pérez MP, Vidal-Sanz M. Changes in the inner and outer retinal layers after acute increase of the intraocular pressure in adult albino Swiss mice. Exp Eye Res. 2010;91(2):273–285. doi: 10.1016/j.exer.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Dai S, An J, Li P, Chen X, Xiong R, Liu P, Wang H, Zhao Y, Zhu M, Liu X, Zhu P, Chen JF, Zhou Y. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151(4):1198–1207. doi: 10.1016/j.neuroscience.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during Ischemia and its relation to Neuronal Death. Arch Med Res. 2006;37(1):11–18. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Honkanen RA, Baruah S, Zimmerman MB, Khanna CL, Weaver YK, Narkiewicz J, Waziri R, Gehrs KM. Vitreous amino acid concentrations in patients with glaucoma undergoing vitrectomy. Arch Ophthalmol. 2003;121(2):183–188. doi: 10.1001/archopht.121.2.183. [DOI] [PubMed] [Google Scholar]
- 20.Carter-Dawson L, Crawford MLJ, Harwerth RS, III ELS, Feldman R, Shen FF, Mitchell CK, Whitetree A. Vitreal glutamate concentration in monkeys with experimental glaucoma. Invest Ophthalmol Vis Sci. 2002;43(8):2633–2677. [PubMed] [Google Scholar]
- 21.Mosinger JL, Price MT, Bai HY, Xiao H, Wozniak DF, Olney JW. Blockade of both NMDA and non-NMDA receptors is required for optimal protection against ischemic neuronal degeneration in the in vivo adult mammalian retina. Exp Neurol. 1991;113(1):10–17. doi: 10.1016/0014-4886(91)90140-8. [DOI] [PubMed] [Google Scholar]
- 22.Honkanen RA, Baruah S, Zimmerman MB, Khanna CL, Weaver YK, Narkiewicz J, Waziri R, Gehrs KM, Weingeist TA, Boldt HC, Folk JC, Russell SR, Kwon YH. Vitreous amino acid concentrations in patients with glaucoma undergoing vitrectomy. Arch Ophthalmol. 2003;121(2):183–188. doi: 10.1001/archopht.121.2.183. [DOI] [PubMed] [Google Scholar]
- 23.Wamsley S, Gabelt BAT, Dahl DB, Case GL, Sherwood RW, May CA, Hernandez MR, Kaufman PL. Vitreous glutamate concentration and axon loss in monkeys with experimental glaucoma. Arch Ophthalmol. 2005;123(1):64–70. doi: 10.1001/archopht.123.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114(3):299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 25.Aihara M, Chen YN, Uchida S, Nakayama M, Araie M. Hyperbaric pressure and increased susceptibility to glutamate toxicity in retinal ganglion cells in vitro. Mol Vis. 2014;20(20):606–615. [PMC free article] [PubMed] [Google Scholar]
- 26.Sucher NJ, Lei SZ, Lipton SA. Calcium channel antagonists attenuate NMDA receptor-mediated neurotoxicity of retinal ganglion cells in culture. Brain Research. 1991;551(1-2):297–302. doi: 10.1016/0006-8993(91)90944-q. [DOI] [PubMed] [Google Scholar]
- 27.Gasparini L, Crowther RA, Martin KR, Berg N, Coleman M, Goedert M, Spillantini MG. Tau inclusions in retinal ganglion cells of human P301S tau transgenic mice: effects on axonal viability. Neurobiol Aging. 2011;32(3):419–433. doi: 10.1016/j.neurobiolaging.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Chiasseu M, Cueva Vargas JL, Destroismaisons L, Vande Velde C, Leclerc N, Di Polo A. Tau accumulation, altered phosphorylation, and missorting promote neurodegeneration in glaucoma. J Neurosci. 2016;36(21):5785–5798. doi: 10.1523/JNEUROSCI.3986-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]