Abstract
Objective
To validate the necessity of increasing the examined lymph node (ELN) count for enhancing the accuracy of prognostic evaluation of gastric cancer (GC) patients after curative gastrectomy in multiple medical centers of China.
Methods
The clinicopathological data of 7,620 patients who underwent the curative resection for GC between 2001 and 2011 were included to demonstrate whether the ELN count is indispensable for enhancing the accuracy of prognostic evaluation of GC patients after surgery. After a meticulous stratification by using the cut-point survival analysis, all included 7,620 patients were allocated into three groups as: less than 16 (<16), between 16 and 30 (16−30), and more than 30 (>30) ELNs. Survival differences among various subgroups of GC patients were analyzed to assess the impact of the ELN count on the stage migration in accordance with the overall survival (OS) of GC patients.
Results
Survival analyses revealed that the ELN count was positively correlated with the OS (P=0.001) and was an independent prognostic predictor (P<0.01) of 7,620 GC patients. Stratum analysis showed that the accuracy of prognostic evaluation could be enhanced when the ELN count was no less than 16 (≥16) for node-negative patients and >30 for node-positive patients. Stage migrations were mainly detected in the various subgroups of patients with specific pN stages as follows: pN0 with 16−30 ELNs (pN016−30) and pN0 with >30 ELNs (pN0 >30), pN0 with <16 ELNs (pN0 <16) and pN1>30, pN1<16 and pN216−30, pN116−30 and pN2>30, pN3a<16 and pN3b16−30, and pN3a<16 and pN3b>30. These findings indicate that increasing the ELN count is a prerequisite to guarantee precisely prognostic evaluation of GC patients.
Conclusions
The ELN count should be proposed to be >30 for acquiring the accurate prognostic evaluation for GC patients, especially for node-positive patients.
Keywords: Stomach, neoplasm, lymph node, metastasis, prognosis
Introduction
Intraoperatively dissected lymph node (DLN) count is an essential parameter to ensure the curative quality of gastrectomy for gastric cancer (GC) (1). This parameter corresponds to the number of lymph nodes dissected by surgeons intraoperatively within a complete tissue specimen. Actually, DLNs should be individually separated from a complete tissue sample for detailed pathological examination after surgery because this procedure requires patience and endurance. Consequently, the examined lymph node (ELN) count after curative surgery for GC is frequently lower than the DLN count, and this condition may directly impede the accurate evaluation of the curative degree of surgery for GC (2). In theory, three essential elements can affect the ELN count, which may induce the migration of pN stage after surgery: 1) extent of intraoperative lymphadenectomy or the degree of lymph node dissection; 2) number of lymph nodes postoperatively separated from a complete tissue specimen, and 3) pathological confirmation of ELNs. A consensus for the extent of lympadenectomy in standard GC curative surgery should be recommended for D2 lymphadenectomy but not for all early diseases (3). However, a consensus on the sufficient ELN count based on the DLN count for accurate evaluation of the pN stage has not been researched.
Several studies have revealed the significantly positive association of ELN count with the number of metastatic lymph nodes in GC, but the quantitative assessment of the effects of ELN count on GC has remained controversial (4,5). The minimum ELN count required for proper staging is not mandatory according to the 8th edition TNM classification for GC (6), although an ELN count of ≥16 has been proposed by the American Joint Commission for Cancer (AJCC) to guarantee the accurate prognosis of pN stage since 2009 (7). Recently, Sano et al. (8) reported an international gastric cancer association staging project including 25,411 patients mainly from Japan (41.85%) and Korea (42.98%) to propose that the optimal ELN count had better achieve to be 30 or more. We also noticed that the mean values of ELN count of GC patients in Japan (39.4) and Korea (33.0) were identified to be higher than those in other Asia countries (24.8) and West countries (29.5) in that manuscript (8). It must be sure that no pN classification system can supersede the performance of an adequate lymph node dissection of ≥16 nodes as endorsed by any oncology practice guidelines [such as National Comprehensive Cancer Network (NCCN) guidelines] (9).
Considering the important effects of the number of metastatic lymph nodes on the accurate evaluation of pTNM classification and prognosis of GC patients after surgery, we should define the cut-off values of the ELN count to prevent the migration of pN stage in clinical settings. The present study aimed to elucidate whether the sub-classifications of ELN count should be designed to enhance the accuracy of both postoperative staging and accurate prognostic evaluation for GC patients. Survival differences among various subgroups of GC patients were analyzed on the basis of various cut-off values of the ELN count from three high-volume medical centers in North and South China.
Materials and methods
Patients
Between January 2001 and December 2011, 2,864 GC patients underwent surgical resection in the Department of Gastric Cancer in the Tianjin Medical University Cancer Hospital (TJMUCH), 3,043 GC patients underwent surgical resection in the Department of Surgical Oncology in the First Affiliated Hospital of China Medical University (CMUFAH) and 2,977 GC patients underwent surgical resection in the Department of Gastric and Pancreatic Surgery in the Sun Yat-sen University Cancer Center (SYSUCC), respectively. After approval from the institutional review boards of the TJMUCH, the CMUFAH and the SYSUCC, data from the cancer registries of three hospitals was obtained. Informed consent was obtained from all patients for being included in the study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Eligibility criteria included: 1) histologically proven primary adenocarcinoma of the stomach; 2) no history of gastrectomy or other malignancy; 3) absence of non-curative surgical factors including distant metastasis, positive peritoneal cytology, or peritoneal dissemination; 4) no Siewert-I or II esophagogastric junction (EGJ) tumor; 5) pathologically negative resection margins (R0 resection); 6) remaining alive during the initial hospital stay and during the first postoperative month; and 7) no administration with neoadjuvant radiochemotherapy. After applying these criteria, we found that 71 patients had the history of gastrectomy, 46 patients had suffered from other malignant diseases, 117 patients presented with distant metastasis in the operation, 106 patients presented with the peritoneal dissemination, 503 patients were diagnosed as the Siewert-I EGJ tumor after surgery, 105 patients were identified as the R1 resection cases, 38 patients were identified as the R2 resection cases, 74 patients died during the first postoperative month, and 204 patients were administered with neoadjuvant treatments. Ultimately, 7,620 GC patients were included in the study (Supplementary Figure S1 ).
S1.
Scheme of included patients after curative gastrectomy.
Surgical management
Primary tumors were resected en bloc by means of lymphadenectomy according to the guidelines of the Japanese Gastric Cancer Association (10). The surgical procedures were based mainly on the Japanese Gastric Cancer Treatment Guidelines (11). Patients with clinical T1 and N0 tumors underwent D1 or D1+ lymphadenectomy, and patients with clinical T2 or more advanced tumors and/or those with N1 or more advanced tumors underwent D2 or D2+ lymphadenectomy. Each medical center has a surgical expert who can perform the standard gastrectomy (including D1, D1+, D2, or D2+ lymph node dissection) in accordance with Japanese Gastric Cancer Treatment Guidelines.
Follow-up
After undergoing curative surgery, all 7,620 patients were followed up every 3 or 6 months for 2 years, then every 6 months for next 3 years, and annually thereafter until death. The median follow-up time for the entire cohort was 87 (range, 2−186) months. Follow-up of all patients included in this study was completed in October 2015.
Stage migration
Stage migrations were mainly defined as: 1) no statistical significance of the survival differences to be detected in several subgroups of patients with different ELNs in the specific pN stages, or 2) the significant survival differences to be detected in several subgroups of patients with the same ELNs in the different pN stages.
Statistical analysis
The cut-point survival analysis was adopted to determine the most appropriate cut-off values for the ELN count (12). Clinicopathological characteristics significantly related to patients’ survival were evaluated by the Kaplan-Meier method and Cox proportional hazards analysis. Chi-square test, likelihood ratio test and multinomial logistic regression were used for the correlation analysis of ELN count and the various clinicopathological characteristics. The Bayesian information criterion (BIC) value within a multinomial logistic regression model was calculated for each category to measure its discriminatory ability. A smaller BIC value indicated a better model for predicting outcome (13). Stratum analysis was adopted to evaluate the influence of the clinicopathological characteristics on the efficiency of prognostic prediction of the ELN count for patients and to demonstrate the migration of pN stage of patients in accordance with the varieties of the ELN count. Significance was defined as a P value <0.05. All statistical analyses were performed using IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA).
Results
General results
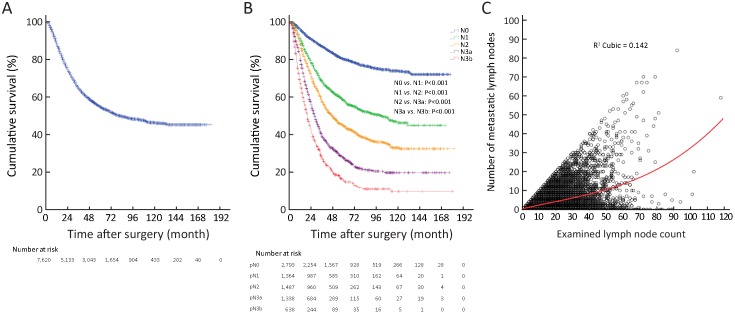
The 5-year survival rate (YSR) of 7,620 GC patients was 54.6%, with a median overall survival (OS) of 81.0 months (Supplementary Figure S2A ). Of the 7,620 GC patients, 2,793 (36.7%) were pN0 stage (node-negative) with 80.1% of 5-YSR, 1,364 (17.9%) were pN1 stage with 58.1% of 5-YSR, 1,487 (19.5%) were pN2 stage with 44.6% of 5-YSR, 1,337 (17.5%) were pN3a with 27.5% of 5-YSR, and 639 (8.4%) were pN3b stage with 9.9% of 5-YSR (Supplementary Figure S2B ). The data from 7,620 GC patients were analyzed, and the clinicopathological characteristics of patients are shown in Table 1 . Of the 7,620 GC patients, 2,318 (including 96.3% advanced cases) with 42.6% of 5-YSR underwent curative surgery in the TJMUCH, 2,868 (including 81.4% advanced cases) with 57.6% of 5-YSR were subjected to curative surgery in the CMUFAH, and 2,434 (including 86.6% advanced cases) with 62.9% of 5-YSR had their curative surgery in the SYSUCC. A total of 3,244 (42.6%) patients died at the end of the follow-up. The curve correlation between the number of metastatic lymph nodes and the ELN count is illustrated in Supplementary Figure S2C . In addition, the correlation between pN stage and ELN count was identified to be statistical significance in all 7,620 GC patients (P<0.001,Supplementary Table S1 ). According to the 7th edition TNM classification for GC, the minimal prerequisite for the prognostic evaluation of patients should be 16. However, the most appropriate cut-off value of the ELN count was 31 (>30), which were calculated in accordance with the method reported by Okajima (14) (Supplementary Table S2 and Figure 1A, B ).
S2.
Survival curve and correlation between the number of metastatic lymph nodes and the ELN count. (A) Survival curve of patients included in this study; (B) Survival curve of patients according to the N stage; (C) Curve correlation between the number of metastatic lymph nodes and examined lymph node (ELN) count.
1.
Clinicopathological characteristics and survival analyses of GC patients (N=7,620)
| Characteristics | n | 5-YSR (%) | χ2 | Univariate P value | HR (95% CI) | Multivariate P value |
| Gender | 2.443 | 0.118 | 2.227 (2.081−2.376) | 0.136 | ||
| Male | 5,378 | 54.0 | ||||
| Female | 2,242 | 56.0 | ||||
| Age at surgery (year) | 82.616 | <0.001 | 1.369 (1.276−1.469) | <0.001 | ||
| <60 | 3,986 | 59.4 | ||||
| ≥60 | 3,634 | 49.3 | ||||
| Tumor location | 320.657 | <0.001 | 0.950 (0.914−0.988) | 0.011 | ||
| Upper third | 2,023 | 49.1 | Reference | Reference | ||
| Middle third | 1,474 | 52.2 | 0.761 (0.636−0.911) | 0.003 | ||
| Lower third | 3,487 | 62.9 | 0.830 (0.730−0.944) | 0.004 | ||
| >2/3 stomach | 636 | 32.4 | 0.857 (0.744−0.988) | 0.034 | ||
| Tumor size (cm)* | 513.623 | <0.001 | 1.345 (1.245−1.452) | <0.001 | ||
| ≤4.0 | 3,626 | 68.4 | ||||
| >4.0 | 3,994 | 42.2 | ||||
| Lauren classification | 90.692 | <0.001 | 1.143 (1.061−1.230) | <0.001 | ||
| Intestinal | 3,215 | 61.2 | ||||
| Diffuse | 4,405 | 49.8 | ||||
| Type of gastrectomy | 439.340 | <0.001 | 0.790 (0.746−0.836) | <0.001 | ||
| TG | 1,741 | 36.4 | Reference | Reference | ||
| DG | 4,230 | 63.3 | 1.288 (1.093−1.518) | 0.003 | ||
| PG | 1,649 | 50.7 | 0.813 (0.665−0.993) | 0.042 | ||
| pT stage | 938.880 | <0.001 | 1.355 (1.303−1.410) | <0.001 | ||
| T1 | 945 | 95.7 | Reference | Reference | ||
| T2 | 1,409 | 67.8 | 0.122 (0.089−0.167) | <0.001 | ||
| T3 | 1,248 | 53.6 | 0.527 (0.454−0.612) | <0.001 | ||
| T4a | 3,528 | 42.4 | 0.603 (0.524−0.695) | <0.001 | ||
| T4b | 490 | 26.9 | 0.726 (0.644−0.818) | <0.001 | ||
| pN stage | 1,829.794 | <0.001 | 1.313 (1.227−1.405) | <0.001 | ||
| N0 | 2,793 | 80.1 | Reference | Reference | ||
| N1 | 1,364 | 58.1 | 0.177 (0.059−0.532) | 0.002 | ||
| N2 | 1,487 | 44.6 | 0.479 (0.388−0.591) | <0.001 | ||
| N3a | 1,338 | 27.5 | 0.524 (0.446−0.615) | <0.001 | ||
| N3b | 638 | 15.4 | 0.747 (0.658−0.847) | <0.001 | ||
| Ratio between metastatic
lymph nodes and ELNs (%) |
2,036.660 | <0.001 | 1.302 (1.206−1.406) | <0.001 | ||
| 0 | 2,797 | 80.0 | Reference | Reference | ||
| 0.1−10.0 | 1,101 | 66.0 | 0.915 (0.306−2.732) | 0.874 | ||
| 10.1−40.0 | 2,037 | 43.3 | 0.531 (0.438−0.675) | <0.001 | ||
| >40.0 | 1,685 | 19.5 | 0.751 (0.674−0.837) | <0.001 | ||
| ELN count* | 13.604 | 0.001 | 0.772 (0.726−0.822) | <0.001 | ||
| <16 | 2,292 | 51.7 | Reference | Reference | ||
| 16−30 | 3,482 | 55.3 | 1.630 (1.436−1.851) | <0.001 | ||
| Table 1 (continued) | ||||||
S1.
Correlation between clinicopathological characteristics and pN stage of GC patients (N=7,620)
| Characteristics | pN stage (n) | χ2 | P | ||||
| N0 | N1 | N2 | N3a | N3b | |||
| GC, gastric cancer; TG, total gastrectomy; DG, distal gastrectomy; PG, proximal gastrectomy; ELN, examined lymph node. The eighth edition of TNM classification for GC was adopted for postoperatively pathological stages of all included patients. | |||||||
| Gender | 15.823 | 0.003 | |||||
| Male | 1,985 | 986 | 1,065 | 932 | 410 | ||
| Female | 808 | 378 | 422 | 406 | 228 | ||
| Age at surgery (year) | 26.076 | <0.001 | |||||
| <60 | 1,522 | 656 | 732 | 715 | 361 | ||
| ≥60 | 1,271 | 708 | 755 | 623 | 277 | ||
| Tumor location | 210.795 | <0.001 | |||||
| Upper third | 741 | 410 | 444 | 311 | 117 | ||
| Middle third | 507 | 224 | 260 | 314 | 169 | ||
| Lower third | 1,389 | 642 | 636 | 584 | 236 | ||
| >2/3 stomach | 156 | 88 | 147 | 129 | 116 | ||
| Tumor size (cm) | 636.873 | <0.001 | |||||
| ≤4.0 | 1,812 | 636 | 592 | 431 | 155 | ||
| >4.0 | 981 | 728 | 895 | 907 | 483 | ||
| Lauren classification | 152.489 | <0.001 | |||||
| Intestinal | 1,363 | 620 | 610 | 438 | 184 | ||
| Diffuse | 1,430 | 744 | 877 | 900 | 454 | ||
| Type of gastrectomy | 363.556 | <0.001 | |||||
| TG | 435 | 250 | 355 | 407 | 294 | ||
| DG | 1,739 | 767 | 768 | 692 | 264 | ||
| PG | 619 | 347 | 364 | 239 | 80 | ||
| pT stage | 1,334.542 | <0.001 | |||||
| T1 | 792 | 84 | 51 | 18 | 0 | ||
| T2 | 637 | 267 | 259 | 177 | 69 | ||
| T3 | 365 | 256 | 286 | 225 | 116 | ||
| T4a | 915 | 674 | 785 | 780 | 374 | ||
| T4b | 84 | 83 | 106 | 138 | 79 | ||
| ELN count | 745.581 | <0.001 | |||||
| <16 | 1,031 | 495 | 522 | 244 | 0 | ||
| 16−30 | 1,203 | 613 | 646 | 762 | 258 | ||
| >30 | 559 | 256 | 319 | 332 | 380 | ||
S2.
Prognostic effects in 7,620 GC patients depending on different cut-off ELN counts
| Cut-off
ELN count |
Number of patients
with ≤ cut-off ELN count |
χ2 | P |
| P values were calculated by the log-rank test for survival curves that were generated by the Kaplan-Meier method. *, the most appropriate cut-off value of the ELN count was 31 (P<0.05). | |||
| 5 | 337 | 0.284 | 0.594 |
| 6 | 499 | 2.742 | 0.098 |
| 7 | 632 | 4.364 | 0.037 |
| 8 | 799 | 6.166 | 0.013 |
| 9 | 990 | 7.439 | 0.006 |
| 10 | 1,166 | 7.202 | 0.007 |
| 11 | 1,361 | 9.352 | 0.002 |
| 12 | 1,575 | 11.621 | 0.001 |
| 13 | 1,797 | 12.401 | <0.001 |
| 14 | 1,991 | 8.568 | 0.001 |
| 15 | 2,292 | 11.879 | 0.001 |
| 16 | 2,624 | 12.522 | <0.001 |
| 17 | 2,892 | 7.803 | 0.005 |
| 18 | 3,176 | 6.436 | 0.011 |
| 19 | 3,421 | 12.681 | <0.001 |
| 20 | 3,681 | 16.524 | <0.001 |
| 21 | 3,978 | 19.309 | <0.001 |
| 22 | 4,205 | 18.388 | <0.001 |
| 23 | 4,420 | 17.481 | <0.001 |
| 24 | 4,645 | 13.527 | <0.001 |
| 25 | 4,852 | 9.702 | 0.002 |
| 26 | 5,041 | 11.965 | 0.001 |
| 27 | 5,255 | 11.050 | 0.001 |
| 28 | 5,456 | 11.112 | 0.001 |
| 29 | 5,623 | 7.944 | 0.005 |
| 30 | 5,774 | 5.949 | 0.015 |
| 31* | 5,903 | 5.191 | 0.023 |
| 32 | 6,055 | 3.681 | 0.055 |
| 33 | 6,182 | 3.353 | 0.067 |
| 34 | 6,303 | 1.716 | 0.190 |
| 35 | 6,424 | 1.998 | 0.157 |
| 36 | 6,526 | 2.601 | 0.107 |
| 37 | 6,616 | 1.018 | 0.313 |
| 38 | 6,699 | 1.446 | 0.229 |
| 39 | 6,780 | 1.026 | 0.311 |
| 40 | 6,852 | 0.798 | 0.372 |
| 41 | …… | …… | …… |
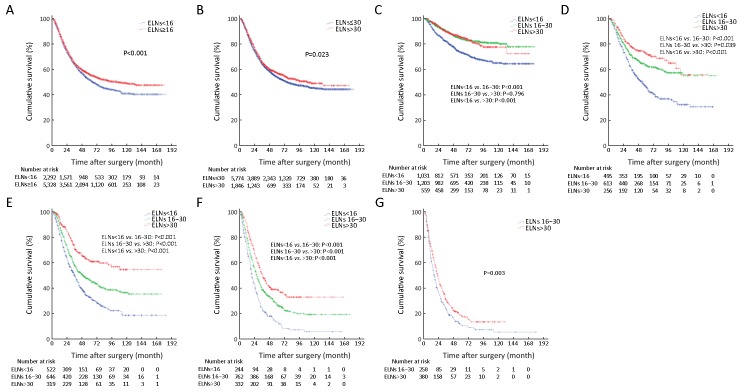
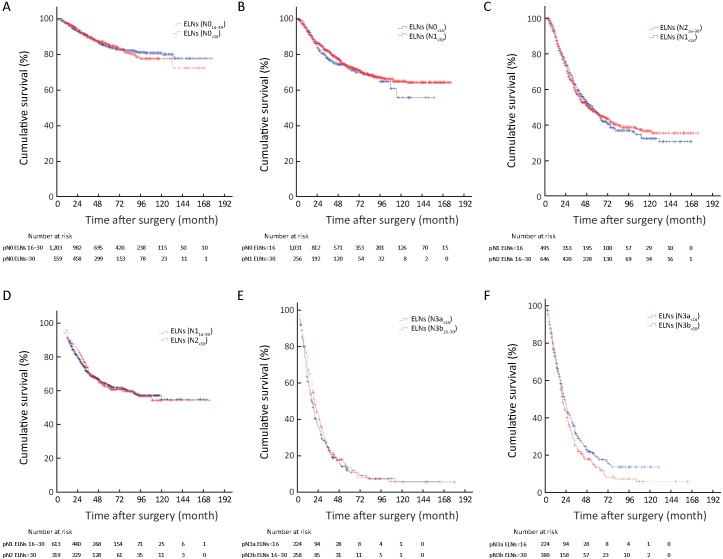
1.
Survival curve of patients according to cut-off value and pN stage. (A) Survival curve of patients according to the cut-off value of 16 examined lymph nodes (ELNs) (P<0.001); (B) Survival curve of patients according to the cut-off value of 31 ELNs (P=0.023); (C) Survival curve of patients with pN0 stage according to the ELN count; (D) Survival curve of patients with pN1 stage according to the ELN count; (E) Survival curve of patients with pN2 stage according to the ELN count; (F) Survival curve of patients with pN3a stage according to the ELN count; (G) Survival curve of patients with pN3b stage according to the ELN count (P=0.003).
Survival analysis
Univariate analysis revealed that the following nine clinicopathological characteristics were significantly associated with OS after the 7,620 GC patients undergoing curative surgery: age at surgery, tumor location, tumor size, Lauren classification, type of gastrectomy, depth of tumor invasion (pT stage), pN stage, ratio between metastatic lymph nodes and ELNs, and ELN count (Table 1 ). These characteristics were then included in a multivariate Cox proportional hazard model (forward stepwise procedure) to adjust for the effects of covariates. Multivariate analysis indicated that the ELN count [hazard ratio (HR), 0.772, P<0.001], age at surgery, tumor location, tumor size, Lauren classification, type of gastrectomy, pT stage, pN stage, and ratio between metastatic lymph nodes and ELNs (Table 1 ) were independent predictors of the OS of all GC patients postoperatively.
Relationship between clinicopathological characteristics and ELN count of GC patients
With the Chi-square test analysis, we demonstrated that gender, age at surgery, tumor location, tumor size, Lauren classification, type of gastrectomy, pT stage and pN stage were significantly related to the ELNs of all 7,620 included GC patients. Furthermore, the multinominal logistic regression model showed that both gender and tumor size presented the smallest BIC values indicating the most intensive relationship to the ELNs of GC patients (Table 2 ).
2.
Relationship between clinicopathological characteristics and ELN count of GC patients (N=7,620)
| Characteristics | Cases for ELN count [n (%)] | χ2 | P | Likelihood ratio
test P value |
BIC value | ||
| <16 | 16−30 | >30 | |||||
| ELN, examined lymph node; GC, gastric cancer; TG, total gastrectomy; DG, distal gastrectomy; PG, proximal gastrectomy; BIC, Bayesian information criterion. The eighth edition of TNM classification for GC was adopted for postoperatively pathological stages of all included patients. | |||||||
| Gender | 22.949 | <0.001 | 0.041 | 6,180 | |||
| Male | 1,699 (22.3) | 2,432 (31.9) | 1,247 (16.4) | ||||
| Female | 593 (7.8) | 1,050 (13.8) | 599 (7.9) | ||||
| Age at surgery (year) | 90.274 | <0.001 | <0.001 | 6,223 | |||
| <60 | 1,020 (13.4) | 1,883 (24.7) | 1,083 (14.2) | ||||
| ≥60 | 1,272 (16.7) | 1,599 (21.0) | 763 (10.0) | ||||
| Tumor location | 398.163 | <0.001 | <0.001 | 6,223 | |||
| Upper third | 899 (11.8) | 857 (11.2) | 267 (3.5) | ||||
| Middle third | 283 (3.7) | 688 (9.0) | 503 (6.6) | ||||
| Lower third | 900 (11.8) | 1,656 (21.7) | 931 (12.2) | ||||
| >2/3 stomach | 210 (2.8) | 281 (3.7) | 145 (1.9) | ||||
| Tumor size (cm) | 19.963 | <0.001 | 0.029 | 6,181 | |||
| ≤4.0 | 1,126 (14.8) | 1,705 (22.4) | 795 (10.4) | ||||
| >4.0 | 1,166 (15.3) | 1,777 (23.3) | 1,051 (13.8) | ||||
| Lauren classification | 16.818 | <0.001 | 0.392 | ||||
| Intestinal | 1,034 (13.6) | 1,465 (19.2) | 716 (9.4) | ||||
| Diffuse | 1,258 (16.5) | 2,017 (26.5) | 1,130 (14.8) | ||||
| Type of gastrectomy | 500.060 | <0.001 | <0.001 | 6,291 | |||
| TG | 360 (4.7) | 774 (10.2) | 607 (8.0) | ||||
| DG | 1,129 (14.8) | 2,018 (26.5) | 1,083 (14.2) | ||||
| PG | 803 (10.5) | 690 (9.1) | 156 (2.0) | ||||
| pT stage | 66.037 | <0.001 | <0.001 | 6,195 | |||
| T1 | 276 (3.6) | 455 (6.0) | 214 (2.8) | ||||
| T2 | 383 (5.0) | 654 (8.6) | 372 (4.9) | ||||
| T3 | 313 (4.1) | 560 (7.3) | 375 (4.9) | ||||
| T4a | 1,189 (15.6) | 1,576 (20.7) | 763 (10.0) | ||||
| T4b | 131 (1.7) | 237 (3.1) | 122 (1.6) | ||||
| pN stage | 745.581 | <0.001 | <0.001 | 6,902 | |||
| N0 | 1,031 (13.5) | 1,203 (15.8) | 559 (7.3) | ||||
| N1 | 495 (6.5) | 613 (8.0) | 256 (3.4) | ||||
| N2 | 522 (6.9) | 646 (8.5) | 319 (4.2) | ||||
| N3a | 244 (3.2) | 762 (10.0) | 332 (4.4) | ||||
| N3b | 0 (0) | 258 (3.4) | 380 (5.0) | ||||
Effects of other clinicopathological characteristics on ELN count for predicting prognosis of GC patients
Stratum analysis through Kaplan-Meier analysis was conducted to investigate the potential effects of other clinicopathological characteristics on the ELN count prognostic prediction for all GC patients. In Table 3 , ELN≥16 was considered as the minimal and optimal prerequisite for the accurate prognostic evaluation of the following subgroups of patients: pN0 stage, age at surgery ≥60 years, tumor sizes of ≤4.0 cm, diffuse Lauren classification, and 0% ratio between metastatic lymph nodes and ELNs.
3.
Effects of clinicopathological characteristics on ELN count for predicting prognosis of GC patients
| Characteristics | ELN count | 5-YSR (%) | ELN<16 | ELN 16−30 | |||
| χ2 | P | χ2 | P | ||||
| Age at surgery (year) | |||||||
| <60 | <16 | 58.5 | − | − | − | − | |
| 16−30 | 60.0 | 0.611 | 0.434 | − | − | ||
| >30 | 59.1 | 0.045 | 0.832 | 0.175 | 0.676 | ||
| ≥60 | <16 | 46.2 | − | − | − | − | |
| 16−30 | 49.7 | 4.178 | 0.041 | − | − | ||
| >30 | 54.5 | 10.214 | 0.001 | 3.007 | 0.083 | ||
| ≥16 | 51.2 | 8.504 | 0.004 | − | − | ||
| Tumor location | |||||||
| Upper third | <16 | 45.7 | − | − | − | − | |
| 16−30 | 51.8 | 6.765 | 0.009 | − | − | ||
| >30 | 51.5 | 2.869 | 0.090 | 0.044 | 0.834 | ||
| Middle third | <16 | 53.7 | − | − | − | − | |
| 16−30 | 49.7 | 0.504 | 0.478 | − | − | ||
| >30 | 54.8 | 0.152 | 0.679 | 1.575 | 0.209 | ||
| Lower third | <16 | 61.9 | − | − | − | − | |
| 16−30 | 63.0 | 0.225 | 0.635 | − | − | ||
| >30 | 63.8 | 0.527 | 0.468 | 0.184 | 0.668 | ||
| >2/3 stomach | <16 | 31.5 | − | − | − | − | |
| 16−30 | 33.8 | 1.264 | 0.261 | − | − | ||
| >30 | 30.9 | 0.594 | 0.441 | 0.044 | 0.835 | ||
| Tumor size (cm) | |||||||
| ≤4.0 | <16 | 65.0 | − | − | − | − | |
| 16−30 | 69.6 | 4.734 | 0.030 | − | − | ||
| >30 | 70.9 | 6.090 | 0.014 | 0.747 | 0.388 | ||
| ≥16 | 70.0 | 7.063 | 0.008 | − | − | ||
| >4.0 | <16 | 39.0 | − | − | − | − | |
| 16−30 | 41.7 | 4.526 | 0.033 | − | − | ||
| >30 | 46.9 | 13.728 | <0.001 | 4.515 | 0.034 | ||
| Lauren classification | |||||||
| Intestinal | <16 | 59.2 | − | − | − | − | |
| 16−30 | 62.4 | 1.117 | 0.291 | − | − | ||
| >30 | 62.2 | 0.955 | 0.328 | 0.052 | 0.820 | ||
| Diffuse | <16 | 45.6 | − | − | − | − | |
| 16−30 | 50.3 | 10.764 | 0.001 | − | − | ||
| >30 | 54.0 | 17.336 | <0.001 | 2.370 | 0.124 | ||
| ≥16 | 51.6 | 17.444 | <0.001 | − | − | ||
| Type of gastrectomy | |||||||
| TG | <16 | 32.4 | − | − | − | − | |
| 16−30 | 35.2 | 0.818 | 0.366 | − | − | ||
| >30 | 40.5 | 7.883 | 0.005 | 5.413 | 0.020 | ||
| Table 3 (continued) | |||||||
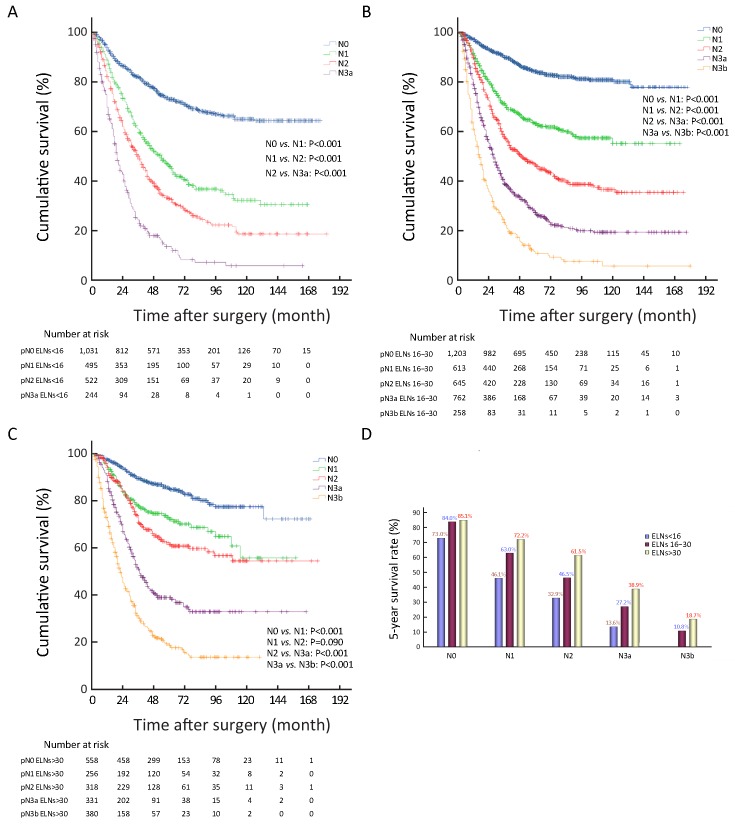
We also found that the 5-YSR of node-negative patients with ELNs≥16 was significantly higher than those with ELNs<16 (P<0.001), while the 5-YSR of node-negative patients with ELNs 16−30 was not significantly different from that of the node-negative patients with ELNs>30 (P=0.796;Figure 1C ). On the other hand, the 5-YSR of node-positive patients (pN1−3a) with ELNs 16−30 was significantly higher than that of the patients with ELNs<16 (P<0.001), and the 5-YSR of node-positive patients (pN1−3a) with ELNs>30 was also significantly higher than that of the patients with ELNs 16−30 (P<0.05;Figures 1D−F ). In addition, the 5-YSR of pN3b patients with ELNs 16−30 was significantly different from that of pN3b patients with ELNs>30 (10.8%vs. 18.7%, P=0.003; Figure 1G ).
In Table 3 , ELNs>30 was also identified as a potentially optimal prerequisite for the prognostic evaluation of patients with tumor sizes of >4.0 cm (Figure 2A ), and as the minimal and optimal prerequisite for the prognostic evaluation of patients who underwent total gastrectomy, and pT4b stage patients (Figure 2B, C ).
2.
Superiorities of the examined lymph nodes (ELNs) >30 for prognostic evaluation in patients. (A) Superiorities of the ELNs>30 for prognostic evaluation in patients with tumor size of >4.0 cm; (B) Superiorities of the ELNs>30 for prognostic evaluation in patients underwent total gastrectomy; (C) Superiorities of the ELNs>30 for prognostic evaluation in pT4b stage patients; (D) Superiorities of the ELNs>30 for prognostic evaluation in all included 7,620 patients.
In order to make the above results more convincing, we have adopted the univariate COX proportional hazards analysis to evaluate the impact of the ELN count on GC patients’ prognosis (Table 4 ). Ultimately, we found that the ELNs>30 had the significant impact on discriminating the prognosis of subgroups of patients in clinicopathological characteristics, such as tumor size (P=0.031), type of gastrectomy (P=0.015), and pN stage (P<0.001).
4.
ELN count predicting prognosis of GC patients (univariate Cox regression)
| Characteristics | ELN<16 | ELN 16−30 | ELN>30 | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| ELN, examined lymph node; GC, gastric cancer; 5-YSR, 5-year survival rate; HR, hazard ratio; 95% CI, 95% confidence interval; The eighth edition of TNM classification for GC was adopted for postoperatively pathological stages of all included patients. | ||||||||
| Age at surgery | Reference | Reference | 1.130 (1.028−1.242) | 0.012 | 1.037 (0.948−1.135) | 0.432 | ||
| Tumor location | Reference | Reference | 1.118 (1.015−1.232) | 0.024 | 1.033 (0.944−1.131) | 0.480 | ||
| Tumor size | Reference | Reference | 1.243 (1.131−1.367) | <0.001 | 1.104 (1.009−1.267) | 0.031 | ||
| Lauren classification | Reference | Reference | 1.210 (1.101−1.330) | <0.001 | 1.064 (0.973−1.164) | 0.173 | ||
| Type of gastrectomy | Reference | Reference | 1.268 (1.150−1.398) | 0.001 | 1.118 (1.022−1.225) | 0.015 | ||
| pT stage | Reference | Reference | 1.139 (1.036−1.252) | 0.007 | 1.052 (0.962−1.152) | 0.269 | ||
| pN stage | Reference | Reference | 2.176 (1.960−2.415) | <0.001 | 1.354 (1.234−1.486) | <0.001 | ||
| Ratio between metastatic and ELNs | Reference | Reference | 1.151 (1.046−1.266) | 0.004 | 1.031 (0.942−1.228) | 0.510 | ||
Stage migration analysis of ELN count
Univariate survival analysis revealed that the survival of patients with ELNs 16−30 was not significantly different from that of patients with ELNs>30 in the all 7,620 included patient cohort (P=0.262) (Figure 2D ), although ELN count was identified as an independent predictor of patients’ prognostic evaluation in this study. In Tables 1 and 2 , several stage migrations were observed in the pN stage of all GC patients. Stage migrations were mainly detected in several subgroups of patients with specific pN stages: 1) pN0 with ELNs 16−30 (pN016−30) and pN0 with ELNs>30 (pN0>30) (P=0.796); 2) pN0 with ELNs<16 (pN0<16) and pN1>30 (P=0.444); 3) pN1<16 and pN216−30 (P=0.857); 4) pN116−30 and pN2>30 (P=0.815); 5) pN3a<16 and pN3b16−30 (P=0.302); and 6) pN3a<16 and pN3b>30 (P=0.060) (Figures 3A−F ).
3.
Stage migrations impact on the patients’ survival in the various subgroups of patients between different pN stages. (A) Stage migrations impact on the patients’ survival in the various subgroups of patients between pN016−30 and pN0>30 (P=0.796); (B) Stage migrations impact on the patients’ survival in the various subgroups of patients between pN0<16 and pN1>30 (P=0.444); (C) Stage migrations impact on the patients’ survival in the various subgroups of patients between pN1<16 and pN216−30 (P=0.857); (D) Stage migrations impact on the patients’ survival in the various subgroups of patients between pN116−30 and pN2>30 (P=0.815); (E) Stage migrations impact on the patients’ survival in the various subgroups of patients between pN3a<16 and pN3b16−30 (P=0.302); (F) Stage migrations impact on the patients’ survival in the various subgroups of patients between pN3a<16 and pN3b>30 (P=0.060).
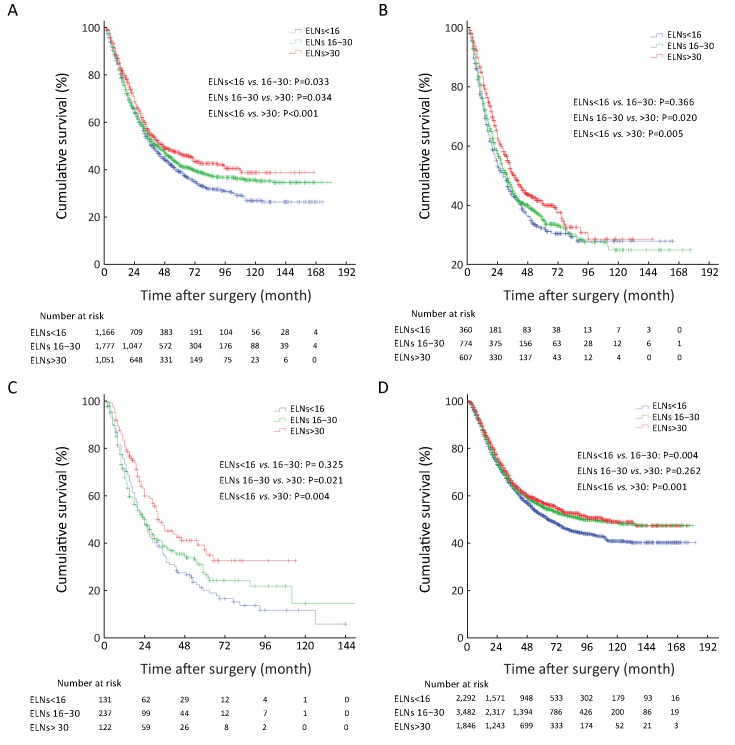
In addition, stage migration of pN stage was also analyzed in different ELN count in all 7,620 GC patients by using the univariate survival analysis. We demonstrated that the stage migration of pN stage was only found in subgroup of patients with ELNs>30 as between pN1 and pN2 stage patients (Figures 4A−C , Supplementary Table S3 ).
4.
Stage migrations in different subgroups of patients with various examined lymph node (ELN) count survival curve of patients with different ELNs according to pN stage. (A) Stage migrations in different subgroups of patients with various ELN count survival curve of patients with ELNs<16 according to pN stage; (B) Stage migrations in different subgroups of patients with various ELN count survival curve of patients with ELNs 16−30 according to pN stage; (C) Stage migrations in different subgroups of patients with various ELN count survival curve of patients with ELNs>30 according to pN stage; (D) Stage migrations in the different subgroups of patients with various ELN count histograms of patients’ 5-year survival rate according to pN stage in various ELN counts.
S3.
Stage migration of pN stage in different ELN count in all 7,620 GC patients by using univariate survival analysis
| ELN count | n | pN0 | pN1 | pN2 | pN3a | pN3b | |||||||||
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | χ2 | P | ||||||
| ELN, examined lymph nodes; GC, gastric cancer. The eighth edition of TNM classification for GC was adopted for postoperatively pathological stages of all included patients. | |||||||||||||||
| <16 | |||||||||||||||
| pN0 | 1,031 | ||||||||||||||
| pN1 | 495 | 121.562 | <0.001 | ||||||||||||
| pN2 | 522 | 273.329 | <0.001 | 20.801 | <0.001 | ||||||||||
| pN3a | 244 | 473.813 | <0.001 | 117.991 | <0.001 | 49.616 | <0.001 | − | |||||||
| pN3b | − | − | − | − | − | − | − | ||||||||
| 16−30 | |||||||||||||||
| pN0 | 1,203 | ||||||||||||||
| pN1 | 613 | 104.777 | <0.001 | ||||||||||||
| pN2 | 646 | 314.039 | <0.001 | 32.409 | <0.001 | ||||||||||
| pN3a | 762 | 720.338 | <0.001 | 166.551 | <0.001 | 58.686 | <0.001 | ||||||||
| pN3b | 258 | 930.922 | <0.001 | 283.744 | <0.001 | 163.020 | <0.001 | 45.069 | <0.001 | − | − | ||||
| >30 | |||||||||||||||
| pN0 | 559 | ||||||||||||||
| pN1 | 256 | 19.348 | <0.001 | ||||||||||||
| pN2 | 319 | 46.828 | <0.001 | 2.879 | 0.090 | ||||||||||
| pN3a | 332 | 201.424 | <0.001 | 51.863 | <0.001 | 36.004 | <0.001 | ||||||||
| pN3b | 380 | 462.036 | <0.001 | 159.319 | <0.001 | 147.778 | <0.001 | 42.406 | <0.001 | − | − | ||||
Discussion
Nodal involvement, which is one of the strongest predictors of GC prognosis, is mainly the potential root of disease relapse among patients after surgery (15-19). Our previous study showed that patients with ELNs≥16 had a significantly median OS than those with ELNs<16 after curative surgery because of the underestimation of the N stage of patients with ELNs<16 (20). We also demonstrated that the insufficient ELN count may be a potential risk factor of the postoperative recurrence of GC patients even in node-negative patients (17). Consistent with the 6th edition of Union for International Cancer Control (UICC)/AJCC TNM classification for GC, the median survival of patients with perigastric lymph node metastasis, serosal involvement, and ratio of positive lymph nodes less than 25% or patients without adjuvant chemotherapy in the ELN>15 group was comparatively longer than that of patients with homologous clinicopathologic variables in the ELN<15 group (1). We further demonstrated that ELNs<16 are more significantly associated with high rates of local regional recurrence and peritoneal dissemination in node-negative GC patients than ELNs≥16 (21).
At present, the clinicopathological data of 7,620 GC patients from three medical centers in South and North China represented the basic disease information and general therapeutic level of GC, especially advanced disease, in China. Similar to other researchers, we found that the 5-YSR of GC patients with ELNs≥16 was significantly higher than that of patients with ELNs<16 (P<0.001) (Figure 1A ). There is still no consensus to mandate a minimal requirement of ELN count for the accurate staging and prognostic evaluation of GC, although ELNs≥16 has been proposed in the latest edition GC pTNM classification.
In this study, patients were divided into two groups based on cut-off ELN numbers between 5 and 40. For each cut-off value of ELNs, the survival rates of patients between these two groups were compared. When the cut-off values of ELNs was between 7 and 31, the prognosis of patients who had less than the cut-off values of ELNs was significantly worse than that of patients who had the cut-off values or more ELNs (Supplementary Table S2 ), suggesting that a retrieval of more than 30 ELNs might be recommended as a sufficient ELN count for lymph node staging. Note that this is different from the Japanese researchers’ report that a majority of GC patients in China were advanced stage cases at the time of initial diagnosis (14), which may incur stage migration of lymph node metastasis in Chinese GC patients with insufficient ELN count in theory. Table 2 shows that pN stage had the significant relationship with ELN count of GC patients, indicating that increasing the ELN count could enhance the detection ratio of positive nodes partly. In Supplementary Figure S2C , the general trend of the correlation between the metastatic lymph nodes and the ELN count implied that the number of positive lymph nodes was partly based on the ELN count. In Table 3 , pN stage was remarkably influenced by the ELN count during the evaluation of the survival of GC patients.
ELNs>30 could be considered to enhance the discrimination of the survival difference among some subgroups of node-positive patients (pN1−3), although the survival of node-negative patients (pN0) with ELNs 16−30 was not significantly different from that of patients with ELNs>30 (P=0.796,Table 3 and Figure 1C ). Therefore, we analyzed that the curve of ELNs>30 should be considered as the essential threshold to guarantee the accuracy of pN+ stage for inhibiting the stage migration of positive nodal involvement, which might be contributed to precisely prognostic evaluation of GC patients. Table 3 and Figures 1D−G also show that ELNs>30 significantly contributed to the improvement of the survival discrimination in all node-positive GC patients.Figure 4 shows the survival rate differences in accordance with pN stage among the various subgroups of patients with different ELN count. It seems to be deduced that the 5-YSR of all GC patients with ELNs<16 can be increased by 12.1% for pN0 cases, 26.1% for pN1 cases, 28.6% for pN2 cases, and 25.3% for pN3a cases when ELN count increases to more than 30 for all patients with ELNs<16. Other GC patient subgroups, including those with tumor size >4.0 cm, those who underwent total gastrectomy, and those in pT4b stage also contributed to enhancing the survival discrimination by ELNs>30 (Figure 2 ).
Stage migrations in lymph node metastasis were also identified among several subgroups of GC patients in this study. Figure 3B indicates that node-negative patients with ELNs<16 were potentially high-risk false node-negative cases in this patient cohort because of the smaller ELN count with a lower accuracy of pN staging. Similarly, pN1<16 stage should be defined as ELNs>30 for the comparatively accurate prognostic discrimination of patients (Figure 3C, D ). Patients with pN116−35 or pN3a<16 demonstrated pN staging migration as the ELN count increased (Figure 3E, F ). Therefore, an increase in ELN count could enhance the accuracy of prognostic evaluation by pN stage through the inhibition of stage migration for node-positive GC patients.
Of course, we have to admit that there are some limitations to this study. All patients are come from Chinese population in this study, which perhaps result in little bias of detection results comparing to the other race. In viewing of about 42% of worldwide GC patients occurring in China, the large scale patient-based cases are capable of possessing certain representative significance.
Conclusions
Our analysis suggests that ELN count should be determined to accurately assess the status of lymph node metastasis among GC patients. For node-positive GC patients, ELN>30 should be recommended to avoid stage migration after surgery.
Acknowledgements
This study was supported in part by grants from the Programs of National Natural Science Foundation of China (No. 81572372, No. 81172080, No. 81201773, No. 81572372), National Key Research and Development Program (MOST-2016YFC1303202), National Precision Medicine Research Program (2017YFC0908300), the Application Foundation and Advanced Technology Program of Tianjin Municipal Science and Technology Commission (15JCYBJC24800) and the National Key Clinical Specialist Construction Programs of China (2013-544).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Jingyu Deng, Email: dengery@126.com.
Zhiwei Zhou, Email: zhouzhw@sysucc.org.cn.
Huimian Xu, Email: xuhuimian@126.com.
Han Liang, Email: tjlianghan@126.com.
References
- 1.Deng JY, Liang H, Sun D, et al. Outcome in relation to numbers of nodes harvested in lymph node-positive gastric cancer. Eur J Surg Oncol. 2009;35:814–9. doi: 10.1016/j.ejso.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Deng J, Yamashita H, Seto Y, et al. Increasing the number of examined lymph nodes is prerequisite for improvement accurate evaluation the overall survival of node-negative gastric cancer patients. Ann Surg Oncol. 2017;24:745–53. doi: 10.1245/s10434-016-5513-8. [DOI] [PubMed] [Google Scholar]
- 3.Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 4.de Manzoni G, Verlato G, Roviello F, et al. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer. 2002;87:171–4. doi: 10.1038/sj.bjc.6600432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvier AM, Haas O, Piard F, et al. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94:2862–6. doi: 10.1002/cncr.10550. [DOI] [PubMed] [Google Scholar]
- 6.Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. New York: Wiley, 2017.
- 7.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours, seventh edition. New York: Wiley, 2009.
- 8.Sano T, Coit DG, Kim HH, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217–25. doi: 10.1007/s10120-016-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.In H, Solsky I, Palis B, et al. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann Surg Oncol. 2017;24:3683–91. doi: 10.1245/s10434-017-6078-x. [DOI] [PubMed] [Google Scholar]
- 10.Jaehne J, Meyer HJ, Maschek H, et al. Lymphadenectomy in gastric adenocarcinoma. A prospective and prognostic study. Arch Surg. 1992;127:290–4. doi: 10.1001/archsurg.1992.01420030052010. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 12.Smith DD, Schwarz RR, Schwartz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–24. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 13.Deng J, Liang H, Ying G, et al. Clinical significance of the methylated cytosine-phosphate-guanine sites of protocadherin-10 promoter for evaluating the prognosis of gastric cancer. J Am Coll Surg. 2014;219:904–13. doi: 10.1016/j.jamcollsurg.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Okajima W, Komatsu S, Ichikawa D, et al. Prognostic impact of the number of retrieved lymph nodes in patients with gastric cancer. J Gastroenterol Hepatol. 2016;31:1566–71. doi: 10.1111/jgh.13306. [DOI] [PubMed] [Google Scholar]
- 15.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Deng J. Evaluation of rational extent lymphadenectomy for local advanced gastric cancer. Chin J Cancer Res. 2016;28:397–403. doi: 10.21147/j.issn.1000-9604.2016.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng J, Liang H, Sun D, et al. Prognosis of gastric cancer patients with negative node metastasis following curative resection. Outcomes of the survival and recurrence. Can J Gastroenterol. 2008;22:835–9. doi: 10.1155/2008/761821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tóth D, Bíró A, Varga Z, et al. Comparison of different lymph node staging systems in prognosis of gastric cancer: a bi-institutional study from Hungary. Chin J Cancer Res. 2017;29:323–32. doi: 10.21147/j.issn.1000-9604.2017.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong JY, Kim MG, Ha TK, et al. Prognostic factors on overall survival in lymph node negative gastric cancer patients who underwent curative resection. J Gastric Cancer. 2012;12:210–6. doi: 10.5230/jgc.2012.12.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng J, Zhang R, Pan Y, et al. Comparison of the staging of regional lymph nodes using the sixth and seventh editions of the tumor-node-metastasis (TNM) classification system for the evaluation of overall survival in gastric cancer patients: findings of a case-control analysis involving a single institution in China. Surgery. 2014;156:64–74. doi: 10.1016/j.surg.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Jiao XG, Deng JY, Zhang RP, et al. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer. World J Gastroenterol. 2014;20:3640–8. doi: 10.3748/wjg.v20.i13.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]