Abstract
Objective
Examine the impact of the 2011 shortage of the drug cytarabine on patient receipt and timeliness of induction treatment for Acute Myeloid Leukemia (AML).
Study Design
A retrospective cohort was utilized to examine odds of receipt of inpatient induction chemotherapy and time to first dose across major (N = 105) and moderate (N = 316) shortage time periods as compared to a nonshortage baseline (N = 1,147).
Data Collection/Extraction Methods
De‐identified patient data from 2008 to 2011 Surveillance, Epidemiology, and End Results (SEER) were linked to 2007–2013 Medicare claims and 2007–2013 Hospital Characteristics.
Principal Findings
Compared to prior nonshortage time period, patients diagnosed during a major drug shortage were 47 percent less likely (p < .05) to receive inpatient chemotherapy within 14 days of diagnosis. Patients who were younger, had a lower Charlson Comorbidity score, and for whom AML was a first primary cancer were prioritized across all periods.
Conclusions
Period of major shortage of a generic oncolytic, without an equivalent therapeutic substitute, reduced timely receipt of induction chemotherapy treatment. More favorable economic and regulatory policies for generic drug suppliers might result in greater availability of essential, older generic drug products that face prolonged or chronic shortage.
Keywords: Cytarabine, drug shortage, receipt of treatment, timeliness of treatment, prioritization, acute myeloid leukemia
The Food and Drug Administration (FDA) considers a drug entity to be in a state of shortage when the total supply of all interchangeable versions of a drug across manufacturers is inadequate to meet current patient demand (U.S. Food and Drug Administration 2014b). The number of drugs meeting the FDA's shortage definition continuously rose from 2007 to 2011 (Fox 2014), with total number of drugs listed almost tripling from 61 in 2005 to 178 in 2010 (Stencel 2014). There are numerous anecdotal reports throughout this period described physicians being forced to choose between patients when allocating scarce quantities of the drug, as well as reports of physicians placing patients in a waiting queue for receipt of treatment (Gogineni, Shuman, and Emanuel 2013; Helwick 2013; McBride et al. 2013).
Sterile injectable formulations have consistently dominated drug shortage lists, accounting for more than 70 percent of drugs in shortage from 2011 to 2014, and more than 5 percent from 2014 to 2016 (U.S. Food and Drug Administration 2017). Drug families with the highest number of shortages included central nervous system (CNS) agents, antibiotics, and chemotherapy drugs (Fox, Sweet, and Jensen 2014).
An investigation of the first commercialization approval dates (i.e., approved new drug application, NDA) (U.S. Food and Drug Administration 2015) for oncolytic injectables incurring a supply interruption found time on market often to be in excess of a 7–9‐year effective patent term of market availability, subjecting these drug entities to price erosion. Of the 49 injectable oncolytics recorded by the University of Utah Drug Information Services incurring at least one shortage episode between 2007 and 2013 (Fox 2015), 43 (88 percent) were found to have an NDA date prior to 2000, and 25 (51 percent) prior to 1990 (U.S. Food and Drug Administration 2015) (Appendix SA2).
Acute Myeloid Leukemia (AML) is regarded as an oncologic emergency due to the rapid rate of cell division and a patient's primary dependence upon chemotherapeutic agents for eradication (Sekeres et al. 2009; Roboz 2012; Stein and Tallman 2012; National Cancer Institute 2017a). Prompt initiation of chemotherapy is an important aspect of treatment, with rates of complete remission and overall survival days reported to be reduced in persons less than age 60 with a delay in treatment as little as five days (Sekeres et al. 2009).
Long‐term survival depends on complete remission of the leukemic burden, followed by receipt of either postremission consolidation chemotherapy or hematopoietic stem cell transplant (Stein and Tallman 2012). Treatment should be sufficiently aggressive to achieve complete remission, because partial remission offers no substantial survival benefit (Jourdan et al. 2001; Walter et al. 2010; National Cancer Institute 2017b). Survival time among persons age 60 and above foregoing aggressive induction treatment is estimated to be about four months (Döhner et al. 2010; Burnett, Wetzler, and Lowenberg 2011).
Worldwide, treatment of adults with AML consists of induction chemotherapy with a cytarabine/anthracycline combination, followed by one to four cycles of consolidation chemotherapy or a stem cell transplant. The induction phase is operationalized as seven days of intravenous (IV) cytarabine plus three days of either daunorubicin or idarubicin, based on randomized controlled trial results from trials conducted in the 1980s (Rai et al. 1981; Yates et al. 1982; Preisler et al. 1987; Stein and Tallman 2012). While the anthracycline component of a regimen can vary, cytarabine remains the core. Successive consolidation therapy with high‐dose cytarabine should follow as soon as a patient has sufficiently recovered from myelosuppression.
DNA testing is now commonly performed to identify patients with histology markers more favorable to a treatment response (Khwaja et al. 2016). Nongenetic negative prognosticators include advanced age, a white blood cell count greater than 100,000 cells per cubic millimeter at the time of diagnosis, history of myelodysplastic syndrome, history of prior receipt of chemotherapy, infection at the time of diagnosis, and penetration of leukemia cells into the central nervous system (Jiang et al. 2009; Tefferi and Vardiman 2009). Prolonged time to induce remission is also believed to be a negative prognostic factor (American Cancer Society 2015).
Acute promyelocytic leukemia (APL) (I‐O‐C type 9866) diagnosis is the one exception to use of cytarabine. Rather, this variant of AML is typically treated with a cocktail comprised of tretinoin, idarubicin, and arsenic trioxide (O'Donnell et al. 2013; National Comprehensive Cancer Network 2014). The absence of indication for use of cytarabine across this subgroup allows these patients to be used to perform a “falsification” robustness check.
Unlike prior studies attempting to understand drug substitution patterns during a period of compromised supply (Goodman et al. 2012; Havrilesky et al. 2012; Jain et al. 2012; Metzger, Billett, and Link 2012; Patel et al. 2013; Poi et al. 2013; Trifilio et al. 2013; Berger et al. 2014; Nickel et al. 2014), our primary objective was to understand the impact of a chemotherapy drug shortage on cancer patients with a time‐sensitive diagnosis in the absence of a therapeutic substitute. A cohort of patients diagnosed with incident AML during calendar year 2011 when cytarabine market availability was constrained provided the data for our study of accessibility and timeliness to treatment during a recognized shortage period. Additionally, we examined the extent to which there were differences in receipt of cytarabine treatment by the patient age and primary versus secondary malignancy status, as older patients and patients for whom AML is not a first primary cancer may have been less likely to receive treatment.
Methods
Data
The two primary data sources for this analysis were the National Cancer Institute (NCI) SEER‐18 linked Patient Entitlement and Diagnosis Summary File (PEDSF) and Medicare Parts A & B claims (linked claims). The PEDSF file included patients from18 registry regions crossing 12 states, approximating 28 percent of the U.S. population (Warren et al. 2002; NCI Epidemiology and End Results Program 2017). In addition, a de‐identified linked hospital characteristics file was sourced for identification of academic affiliation, hospital bed size, and NCI affiliation. The associated research protocol was approved by the Institutional Review Board at University of Illinois at Chicago.
The starting SEER PEDSF file contained records for 27,565 patients with an incident diagnosis for leukemia of any type or etiology during calendar years 2008 through 2011. Minimum requirement for inclusion was a SEER‐recorded diagnosis of AML between 1/1/2008 and 12/31/2011 as manifest by an ICD‐0‐3 site group of 1.2.
Patients were excluded due to non‐AML leukemia subtype, AML diagnosis not conferred prior to death/autopsy, multiple diagnosis dates, entitlement reason of end stage renal disease (ESRD), noncontinuous Medicare Part A or B coverage, leukemia site of origin other than blood or bone marrow, missing diagnosis month/year, age at diagnosis <65 or >74, insufficient claims on file, or inpatient IV chemotherapy having taken place 1–30 days prior to diagnosis date (Appendix SA3).
Key outcome measures included receipt of inpatient chemotherapy and time to first dose inpatient chemotherapy, conditional upon receipt.
The “Aday and Andersen framework” guided the selection of independent control variables (Aday and Andersen 1974; Aday et al. 2004). Within this framework, health policy serves as a vehicle able to affect characteristics of the patient population at risk and environment and health care delivery system, each of which may lead to changes in the utilization of health care services. Accordingly, independent control variables included basic patient demographics (gender, marital status, race, age), health history (Charlson Comorbidity Score, whether AML was first primary cancer, histology favorability), and patient residential and socioeconomic measures (region of the United States, urban versus rural, and census tract poverty level indicator above 20 percent). Health system control variables included hospital bed size, teaching status, and presence of any NCI affiliation.
Histology favorability definitions utilized were consistent with the WHO I‐O‐C3 classification system (World Health Organization 2008). Charlson score was calculated utilizing both hospital and physician office diagnosis codes from claims for the prior (12‐month lead in) and index (month of diagnosis) periods, following the methodology developed by Klabunde, Harlan, and Warren (2006), Klabunde et al (2000), and for which SAS macros exclusive of cancer diagnosis are provided by NCI (National Cancer Institute 2017ab). Health facets of interest not controlled for were infection status at diagnosis and leukemia cell penetration into the CNS, given difficulty of articulating these from Medicare claims data.
Claims for diagnostic procedures associated with a bone marrow biopsy (Döhner et al. 2010; Seiter 2016) and cytogenetic and immuno‐phenotyping tests were utilized to arrive at an estimated calendar date of diagnosis at day/month/year level of specificity as opposed to month/year supplied in the PEDSF file (Appendix SA4). Medicare Part A hospital claims beginning 30 days prior to the assigned date of diagnosis were searched for presence of an ICD‐9‐CM procedure code value of 99.25 without a concomitant ICD‐9‐CM procedure code of “0392,” “9649,” “5497,” and “3491” (nonintravenous routes) to identify instances of administration of intravenous chemotherapy. As explicit drug name is not included in Medicare Part A claims, additional criteria were applied to minimize likelihood of identification of a drug protocol other than a [7 + 3] cytarabine plus anthracycline combination. Criteria enforced included a requirement for a 99.25 chemotherapy administration procedure code to be greater than or equal to the date of AML diagnosis, minimum 7‐day length of stay (LOS) during first treatment, and absence of outpatient administration of azacitidine or decitabine (hypo‐methylating agents) within the first 60 days following diagnosis.
Cytrarabine sales volume in grams as reported in The IMS National Sales Perspective Database (IMSHealth.com) was traced from January 1, 2008 to December 21, 2014 to estimate level of compromise across the FDA‐reported cytarabine shortage period of December 12, 2010–October 20, 2011 (U.S. Food and Drug Administration 2014a). Designations of “major shortage” and “moderate shortage” were applied to the calendar periods between January 1 and March 31, 2011, and between April 1, 2011 and December 31, 2011, respectively, with approximate reductions of 60 percent and 33 percent in the supply of cytarabine, respectively. Calendar years 2008–2010 provided a nonshortage control period.
Statistical Analysis
Proportion of patients receiving one or more doses of qualifying inpatient chemotherapy within 7, 14, 28, and 60 days post estimated date of diagnosis by shortage period was compared using a Pearson chi‐square statistic.
The following logistic regression model was used to estimate the receipt of inpatient chemotherapy within 7, 14, 28, and 60 days of diagnosis:
| (1) |
where π i is a binary indicator for receipt of treatment within 7, 14, 28, or 60 days, η is a constant (intercept), β 1 and β 2 are the coefficients for exposure to major and moderate shortage conditions as compared to nonshortage at time of AML diagnosis, respectively, and β 3–β 16 represent the coefficients for a full set of covariates previously described. Standard errors were clustered at the hospital level.
Median number of days to first qualifying dose of inpatient chemotherapy were estimated for the subset of the sample diagnosed during each respective shortage time period, restricting the sample to those patients who did not receive a hypo‐methylating agent as outpatient within the first 60 days following diagnosis. An upper bound of 60 days was imposed upon this measurement, so as not to skew the comparison with extreme positive outliers for reasons other than limited drug supply. As the data were overdispersed, negative binomial models were utilized to estimate the association of shortage status with time to first treatment, conditional on receipt of treatment.
A two‐part hurdle model was estimated to allow for evaluation of both receipt and timeliness of treatment within a single model. The first part was a logistic model of whether a patient received any treatment and the second part was a zero‐truncated negative binomial model to generate an incident rate ratio for having received treatment within a designated time window, conditional on receipt of treatment. The following equation is applied (Hilbe 2007; Cameron and Trivedi 2013):
| (2) |
where Y indicates whether patient i has received any treatment at time t, Z is comprised of a three‐category shortage exposure variable as previously described and X controls for a full list of covariates.
Given the absence of any equivalent drug substitute, bivariate logistic regression models were constructed to test for potential substitution of a nontherapeutically equivalent drug within the first 30 and 60 days following leukemia diagnosis, which might prolong survival though not induce a full remission (Huls 2015; Khwaja et al. 2016).
A series of robustness checks were performed to evaluate impact of a slight shift in predicted diagnosis date as well as modifications to the attribution algorithm used to determine a patient's first date of inpatient chemotherapy. The first two tests varied the number of attribute points required for inclusion of either a diagnostic date determinate or induction proxy assignment. A third robustness check relaxed the hypo‐methylating agent exclusionary period from 60 to 30 days.
A final robustness check consisted of a falsification test performed on the previously removed subset of patients with a diagnosis of acute promyelocytic leukemia, an AML subtype for which cytarabine is not utilized, to understand if a potential unmeasured period effect may have been responsible for shortage exposure findings.
Results
Patient demographic characteristics and relevant health history by shortage period are summarized in Table 1. Diagnosis records spanned 1568 patients and 875 hospitals. There were no significant differences in patient demographic characteristics across respective major, moderate, and nonshortage (control) time periods. Significant differences in health attributes across shortage and nonshortage groups were noted for AML is first primary cancer, history of myelodysplastic syndrome, and prior receipt of a hypo‐methylating agent.
Table 1.
Patient Demographics, Health History, and Historical Receipt of Inpatient Chemotherapy
| Variables | Total, N (%) | Major Shortageo, N (%) | Moderate Shortageo, N (%) | Non Shortageo, N (%) | p‐value |
|---|---|---|---|---|---|
| Sample size (N) | 1,568 | 105 | 316 | 1,147 | n/a |
| Demographics | |||||
| Age at AML diagnosisa | 0.198 | ||||
| Mean (SD) | 70.13 (2.57) | 69.79 (2.58) | 70.19 (2.65) | 70.15 (2/55) | 0.970 |
| Median (min/max) | 70 (65,74) | 70 (65,74) | 70 (65,74) | 70 (65,74) | 1.000 |
| Age 65–69 | 654 (41.7) | 52 (49.5) | 135 (42.7) | 467 (40.7) | |
| Age 70–74 | 914 (58.3) | 53 (50.5) | 181 (57.3) | 680 (59.3) | |
| Sexa | |||||
| Male | 899 (57.3) | 66 (62.9) | 185 (58.5) | 648 (56.5) | 0.401 |
| Female | 669 (42.7) | 39 (37.1) | 131 (41.5) | 499 (43.5) | |
| Marital statusa | |||||
| Single/never married | 139 (8.9) | (IS) | (IS) | 101 (8.8) | 0.420 |
| Married/domestic partner | 987 (62.9) | 65 (61.9) | 194 (61.4) | 728 (63.5) | |
| Sep/widowed/divorced | 360 (23) | 25 (23.8) | 68 (21.5) | 267 (23.3) | |
| Unknown | 82 (5.2) | (IS) | (IS) | 51 (4.4) | |
| Racea | |||||
| Non‐Hispanic white | 1348 (86.1) | 94 (89.5) | 263 (84) | 991 (86.4) | 0.657 |
| Black | 109 (7) | (IS) | (IS) | 79 (6.9) | |
| Other | 108 (6.9) | (IS) | (IS) | 77 (6.7) | |
| U.S. Census Regionb , c | |||||
| Region 1 (Northeast) | 264 (16.8) | (IS) | (IS) | 197 (17.2) | 0.816 |
| Region 2 (Midwest) | 207 (13.2) | (IS) | (IS) | 157 (13.7) | |
| Region 3 (South) | 435 (27.7) | 32 (30.5) | 85 (26.9) | 318 (27.7) | |
| Region 4 (West) | 662 (42.2) | 48 (45.7) | 139 (44) | 475 (41.4) | |
| Urban or Rural countyc | |||||
| <2,500 urban population | 44 (2.8) | (IS) | (IS) | (IS) | 0.616 |
| Sociodemographic | |||||
| Medicare entitlement “old age”d | 1,299 (82.8) | 86 (81.9) | 269 (85.1) | 944 (82.3) | 0.482 |
| Medicaid dual eligiblee | 217 (13.8) | 15 (14.3) | 38 (12) | 164 (14.3) | 0.579 |
| Health history | |||||
| AML is first primary cancere | 964 (61.5) | 56 (53.3) | 160 (50.6) | 748 (65.2) | 0.000 |
| Charlson Scoref | |||||
| Mean (SD) | 1.157 (1.482) | 1.200 (1.534) | 1.272 (1.570) | 1.122 (1.451) | 0.116 |
| Median (min, max) | 1 (0, 9) | 1 (0, 7) | 1 (0, 8) | 1 (0, 9) | 1.000 |
| HX Myelodysplastic Syndrome (238)g | 392 (25) | 34 (32.4) | 98 (31) | 260 (22.7) | 0.002 |
| HX of multiple myeloma (203)h | 28 (1.8) | (IS) | (IS) | (IS) | 0.799 |
| HX of ALL (204)i | 26 (1.7) | (IS) | (IS) | (IS) | 0.805 |
| HX other leukemias (206–209)j | 50 (3.2) | (IS) | (IS) | 35 (3.1) | 0.471 |
| HX receipt of a hypo‐methylating agentk | 84 (5.4) | (IS) | (IS) | 44 (3.8) | 0.000 |
| History of prior therapeutic radiation | |||||
| Any dose/any type per registry | 35 (2.3) | (IS) | (IS) | (IS) | 0.416 |
| History of radiation as noted in claimsl | 34 (2.17) | (IS) | (IS) | 22 (1.92) | 0.393 |
| ICD‐O‐3 histologic typem , n | |||||
| Less favorable | 1,534 (98.2) | 101 (97.1) | 313 (99.1) | 1,120 (98.1) | 0.350 |
As recorded within PEDSF Registry files.
Northeast = Connecticut, New Jersey; Midwest = Iowa, Michigan; South = Georgia, Kentucky, Louisiana; West = California, New Mexico, Utah, Washington.
Geographic program data points reported by SEER are from the record of the first diagnosis of cancer at age 65 or older. Zip codes reported by SEER as a component of the PEDSF file are sourced from Medicare enrollment file in the year of first diagnosis at age 65 or older or the last diagnosis if never 65 (Adamo, Dickie, and Ruhl 2016).
Subjects with initial entitlement reason of end stage renal failure excluded from sample. Patients aged 65 at time of diagnosis may have originally qualified for Medicare coverage secondary to a documented disability.
Medicare‐Medicaid dual eligibility determination based upon Medicaid eligibility indicator sourced from Medicare Part D enrollment file.
AML represent first malignant patient cancer. This measure takes into account all reportable malignant, in situ, benign, and borderline primary tumors over the lifetime of a patient, regardless of geographical location at time diagnosed.
Charlson Comorbidity Index calculated using formula published by the National Cancer Institute (NCI) which excludes a cancer morbidity component score. The syntax utilized includes the Deyo adaptation to the Charlson comorbidity index) inclusive of several procedure codes reflecting the Romano adaptation. The calculated score is a summation of prior plus index values calculated based upon a 12‐month lead in period continuing through month of diagnosis (13 months in total) using method amended by Klabunde et al. which factors in reported diagnosis from carrier claims (http://https://healthcaredelivery.cancer.gov/SEERmedicare/program/comorbidity.html [accessed October 7, 2016]).
ICD‐9‐CM (Center for Disease Control and Prevention and National Center for Health Statistics) diagnosis code of 238.x, where “x” represents any digit.
ICD‐9‐CM (Center for Disease Control and Prevention & National Center for Health Statistics) diagnosis code of 203.x, where “x” represents any digit.
ICD‐9‐CM (Center for Disease Control and Prevention & National Center for Health Statistics) diagnosis code of 204,x, where “x” represents any digit.
ICD‐9‐CM (Center for Disease Control and Prevention & National Center for Health Statistics) diagnosis code of 206.x 209.x, where “x” represents any digit.
As detected by presence of HCPCS (Centers for Medicare and Medicaid Services [CMS] 2018) codes J9025 (azacitidine) or J0894 (decitabine) in Medicare Part B outpatient claims.
As detected by ICD‐9‐CM (Center for Disease Control and Prevention and National Center for Health Statistics) procedure code 92.2x or HCPCS (Centers for Medicare and Medicaid Services [CMS] 2018) codes 61793, 77261, 77262, 77263, 77280, 77285, 77290,77295, 77299, 77300, 77331, 77332, 77333, 77334, 7399, 77401, 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77413, 77414, 77416, 77418, 77419, 77420, 77425, 77427, 77430, 77431, 77432, 77470, 77499, 77761, 77762, 77763, 77776, 77777, 77778, 77789, 0190T, 0520F, A4650, D5983, G0231, G0232, G0233.
ICD‐0‐3 histological grouping category. All patients included within sample have an AYAWHO classification of 02 which equates to acute myeloid leukemia, as based upon ICD‐0‐3 (World Health Organization 2008) hematopoietic codes and WHO classification of tumors of haematopoietic and lymphoid tissues. Persons with an ICD‐0‐3 subtype consistent with acute promyelocytic leukemia have been excluded as this disease group is typically NOT treated with cytarabine (standard protocol is 5‐azacytidine + arsenic).
More favorable subtypes include 9896 Acute myeloid leukemia t(8;21)(q22;q22) RUNX1‐RUNX1T1 and 9871 AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22)) CBFB‐MYH11. Less favorable subtypes include 9840 Acute erythroid leukemia (M6 type); 9861 Acute myeloid leukemia; 9865 AML w/t(6;9)(p23;q34) DEK‐NUP2145; 9867 Acute myelomonocytic leukemia; 9869 Acute myeloid leuk. inv(3)(q21;q26.2) or t(3;3)(q21;q26.2); RPN1‐EVI1; 9871 Ac. myelomonocytic leuk. w abn. mar. eosinophils; 9872 AMLwith minimal differentiation; 9873 AML without maturation; 9874 AML with maturation; 9891 Acute monoblastic and monocytic leukemia; 9895 AML with myelodysplasia‐related changes (multilineage dysplasia); 9896 Acute myeloid leukemia, t(8;21)(q22;q22); 9897 Acute myeloid leukemia with t(9;11)(p22;q23);MLLT3‐MLL; 9910 Acute megakaryoblastic leukemia; 9920 Therapy‐related (acute) myeloid neoplasm (Khwaja et al. 2016; Ruhl et al.2018).
Shortage periods: Major Shortage = first quarter 2011; Moderate Shortage = quarters 2–4, 2011; N‐Nonshortage = calendar years 2008–2010.
Descriptive statistics were compared using two‐sided Student‐T tests or Anova for continuous variables, Pearson Chi‐Square (Pearson 1900) or Fishers’ Exact (Fisher 1922) for categorical variables and the Wilcoxon Rank‐Sum test (Wilcoxon 1945) for comparison of medians.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; AYA, adolescents and young adults; CCI, Charlson Comorbidity Index; DGN, diagnosis; HX, history; NOS, not otherwise described; SD, standard deviation; SEER, surveillance, epidemiology and end results; WHO, World Health Organization.
(IS) Insufficient Sample Size: counts suppressed to protect patient confidentiality. [Correction added on 24 September 2018, after first online publication: in Table 1, some data in columns “Major Shortage”, “Moderate Shortage” and “Non Shortage” have been changed to “(IS)” and a corresponding footnote has been added.]
A three‐way comparison (F‐test) showed statistically significant differences in proportion of patients receiving treatment within 14 (p < .05) and 28 days (p < .1) post diagnosis across shortage periods. By the time 60 days had elapsed, a statistically significant difference was no longer found. A two‐way comparison restricted to the proportion of patients treated during periods of major versus nonshortage conditions revealed statistically significant differences in proportion treated (p < .05) at 7, 14, and 28 days post diagnosis (Appendix SA5).
A nonparametric Wilcoxon Rank Sum test (Wilcoxon 1945) approached statistical significant differences in median days to first qualifying dose for persons diagnosed during a period of major versus nonshortage for the sample in full (p = .053), for the subgroup of patients for whom AML was not a first primary cancer (p = .049), and for the subgroup of patients aged 70–74 years (p = .083). Figure 1 provides a graphical progression of days to first dose by patient age and primary cancer status.
Figure 1.
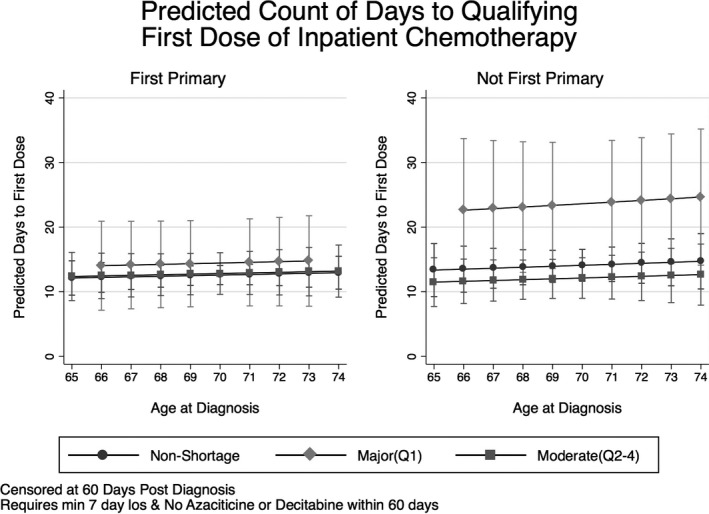
Predicted Days to First Dose by Chronological Age and AML Primary Status with Robust Standard Errors Clustered at the Hospital Level
Multivariate logistic regression models failed to reveal a statistically significant difference in odds of receipt of inpatient chemotherapy at an elapsed time period of 7 days for patients diagnosed during a period of major shortage; however, odds of receipt of inpatient chemotherapy was found to be 47 percent less (OR = 0.531, p < .05) for the sample at large and 62 percent less for the subgroup of patients in which AML was not a first primary cancer (OR = 0.382, p < .1) by 14 days post diagnosis. At 28 days post diagnosis, comparative odds for receipt of chemotherapy during a period of major shortage was 31 percent less than baseline but no longer statistically significant (Figure 2).
Figure 2.
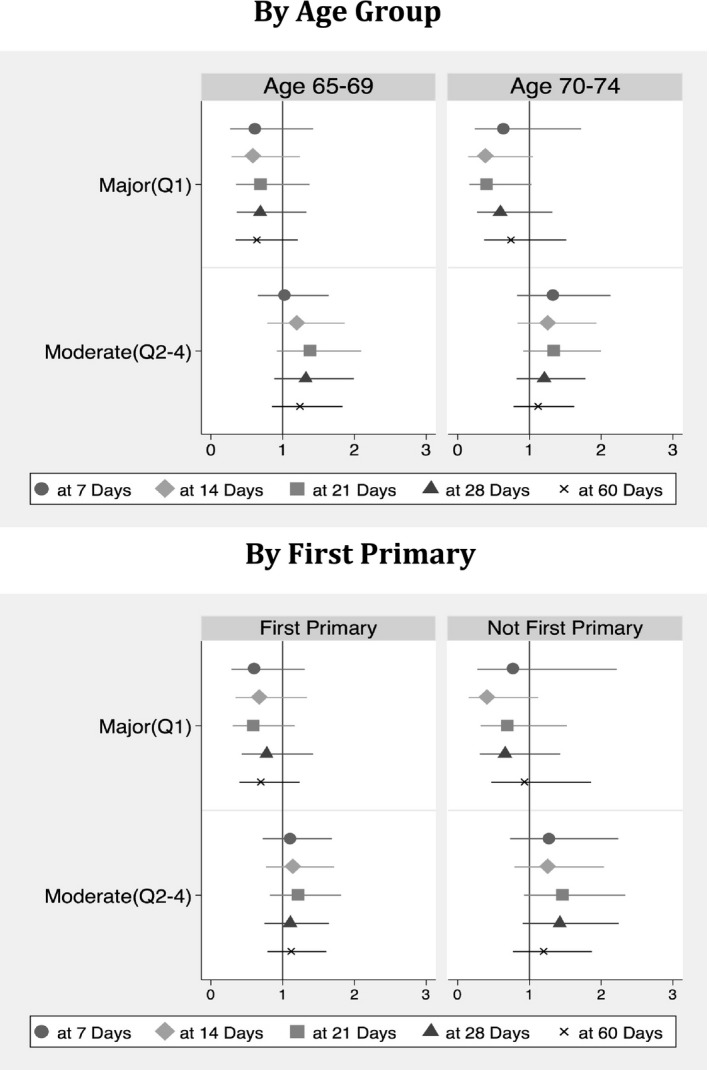
Multivariate Odds Ratios for Receipt of Chemotherapy by Shortage Period When Controlling for Gender, Marital Status, Age Group, Race, Original Reason for Medicare Eligibility, Charlson Score, First Primary Cancer, Histology Favorability, Census Region at Diagnosis, Rural Geography, Bed Size, NCI Affiliation, Teaching Status, and Census Tract Poverty Level with Robust Standard Errors Clustered at the Hospital Level
There was no significant difference in odds of receipt of IV chemotherapy for the sample at large diagnosed during a nine‐month period of moderate supply compromise; however, persons aged 65–69 for whom AML was not a first primary cancer were found to be more likely to have received treatment (OR = 2.558, p < .01) at 28 days post diagnosis than counterparts diagnosed during the baseline nonshortage period (not shown).
As shown in Table 2, two‐part hurdle model findings suggest access to inpatient chemotherapy treatment for persons diagnosed during period of major shortage to be just over half of that seen during periods of nonshortage (OR = 0.531, p < .05). For patients treated within 14 days of diagnosis, there was no relative difference in timeliness of treatment (IRR = 0.983, NS), holding all other factors constant. Timeliness of treatment waned with days elapsed, with model estimates suggesting it to have taken on average 30 percent longer (IRR = 1.301, p < .1) holding all other variables constant, when the time window following diagnosis is widened to 60 days.
Table 2.
Full Sample Multivariate Hurdle Model at 14, 28, and 60 Days of Observation
| Variables | (1) | (2) | (3) | (4) | (5) | (6) |
|---|---|---|---|---|---|---|
| 14 days | 14 days | 28 days | 28 days | 60 days | 60 days | |
| Logit OR (95% CI) | Neg‐Binomial IRR (95% CI) | Logit OR (95% CI) | Neg‐Binomial IRR (95% CI) | Logit OR (95% CI) | Neg‐Binomial IRR (95% CI) | |
| Major (Q1) | 0.531** | 0.983 | 0.667 | 1.211 | 0.716 | 1.301* |
| (0.300–0.939) | (0.774–1.249) | (0.408–1.090) | (0.933–1.573) | (0.453–1.132) | (0.958–1.766) | |
| Moderate (Q2‐4) | 1.175 | 1.033 | 1.197 | 1.022 | 1.134 | 0.974 |
| (0.864–1.599) | (0.914–1.168) | (0.890–1.609) | (0.877–1.190) | (0.840–1.533) | (0.796–1.192) | |
| Male gender | 0.830 | 1.125** | 0.816* | 1.147** | 0.790** | 1.131 |
| (0.657–1.049) | (1.002–1.265) | (0.648–1.028) | (1.001–1.315) | (0.630–0.992) | (0.955–1.340) | |
| Never married | 0.917 | 1.002 | 0.889 | 0.963 | 0.968 | 1.080 |
| (0.594–1.414) | (0.850–1.181) | (0.583–1.356) | (0.769–1.207) | (0.618–1.515) | (0.822–1.420) | |
| Sep/Divorce/Widow | 0.815 | 0.992 | 0.801 | 0.990 | 0.850 | 1.168 |
| (0.612–1.086) | (0.853–1.154) | (0.603–1.063) | (0.839–1.169) | (0.642–1.126) | (0.942–1.449) | |
| Marital unknown | 0.677 | 1.172* | 0.662 | 1.179 | 0.727 | 1.216 |
| (0.384–1.193) | (0.989–1.388) | (0.387–1.132) | (0.895–1.552) | (0.435–1.214) | (0.895–1.651) | |
| Age 65–69 | 1.597*** | 1.013 | 1.577*** | 0.916 | 1.602*** | 0.870* |
| (1.277–1.998) | (0.909–1.129) | (1.264–1.967) | (0.812–1.034) | (1.281–2.003) | (0.756–1.000) | |
| Black | 0.974 | 1.172 | 0.988 | 1.104 | 0.820 | 0.944 |
| (0.610–1.554) | (0.936–1.467) | (0.624–1.562) | (0.874–1.395) | (0.521–1.292) | (0.688–1.296) | |
| Other Race | 1.496* | 0.987 | 1.388 | 0.872 | 1.654** | 1.022 |
| (0.999–2.241) | (0.832–1.170) | (0.936–2.057) | (0.683–1.112) | (1.061–2.579) | (0.772–1.353) | |
| Medicare old age | 1.103 | 1.011 | 1.107 | 0.992 | 1.031 | 0.919 |
| (0.774–1.571) | (0.873–1.172) | (0.789–1.552) | (0.840–1.172) | (0.730–1.457) | (0.739–1.143) | |
| Charlson Score | 0.884*** | 1.041** | 0.906** | 1.054** | 0.929* | 1.061** |
| (0.808–0.966) | (1.003–1.080) | (0.833–0.985) | (1.007–1.102) | (0.855–1.008) | (1.007–1.118) | |
| First primary | 1.701*** | 0.870** | 1.676*** | 0.854** | 1.779*** | 0.876* |
| (1.325–2.183) | (0.776–0.975) | (1.321–2.126) | (0.745–0.978) | (1.398–2.263) | (0.749–1.025) | |
| Histology favorable | 2.424** | 0.741* | 2.114* | 0.607** | 1.823 | 0.527* |
| (1.104–5.319) | (0.549–1.001) | (0.960–4.656) | (0.395–0.933) | (0.815–4.078) | (0.273–1.018) | |
| Northeast | 1.146 | 1.109 | 1.176 | 0.994 | 1.385* | 1.139 |
| (0.823–1.596) | (0.945–1.302) | (0.858–1.613) | (0.827–1.193) | (0.991–1.938) | (0.923–1.405) | |
| Midwest | 1.018 | 0.910 | 0.981 | 0.842 | 1.150 | 1.044 |
| (0.700–1.482) | (0.768–1.079) | (0.674–1.427) | (0.673–1.055) | (0.748–1.768) | (0.829–1.314) | |
| South | 0.944 | 0.989 | 1.018 | 0.985 | 1.045 | 0.972 |
| (0.693–1.288) | (0.851–1.149) | (0.757–1.369) | (0.833–1.166) | (0.774–1.409) | (0.797–1.186) | |
| Rural address | 0.643 | 1.178 | 0.919 | 1.250 | 0.829 | 1.130 |
| (0.300–1.377) | (0.850–1.633) | (0.454–1.863) | (0.916–1.706) | (0.395–1.737) | (0.759–1.683) | |
| CT poverty > 20% | 0.993 | 1.029 | 1.033 | 1.024 | 1.094 | 1.006 |
| (0.706–1.395) | (0.874–1.210) | (0.755–1.413) | (0.860–1.220) | (0.794–1.507) | (0.813–1.244) | |
| Less than 200 beds | 0.714* | 1.194*** | 0.761 | 1.237** | 0.748* | 1.200 |
| (0.496–1.028) | (1.046–1.363) | (0.546–1.059) | (1.033–1.482) | (0.535–1.047) | (0.965–1.493) | |
| NCI affiliation | 0.807 | 0.963 | 0.926 | 1.087 | 1.017 | 1.183 |
| (0.542–1.202) | (0.788–1.176) | (0.625–1.372) | (0.891–1.326) | (0.664–1.557) | (0.952–1.470) | |
| Teaching hospital | 1.262* | 1.013 | 1.226 | 0.996 | 1.142 | 0.855* |
| (0.976–1.631) | (0.895–1.146) | (0.948–1.586) | (0.867–1.144) | (0.872–1.496) | (0.725–1.009) | |
| Constant | 0.019*** | 0.412*** | 0.014*** | 0.330*** | 0.010*** | 0.286*** |
| (0.011–0.033) | (0.318–0.534) | (0.008–0.023) | (0.244–0.446) | (0.006–0.016) | (0.202–0.406) | |
| Observations | 1,499 | 1,499 | 1,499 | 1,499 | 1,499 | 1,499 |
The following criteria must have been met in entirety for a 99.25 procedure code date to be attributed as an eligible induction proxy: (1) presence of a 99.25 ICD‐9‐CM (Center for Disease Control and Prevention and National Center for Health Statistics) code without a concurrent ICD‐9‐CM procedure codes of “0392,” “9649,” “5497,” and “3491”; (2) date of first 99.25 ICD‐9‐CM code must be equal to or greater than estimated date of diagnosis; (3) LOS must be a minimum of 7 days, or if less than 7 days patient must have been expired; (4) Patient must not have any claims for either azacitidine or decitiabine in the first 60 days following estimated date of diagnosis.
Major Shortage‐first quarter 2011 as compared to 2008, quarter 1 through 2010 quarter 4.
Moderate Shortage‐ quarters 2–4, 2011 as compared to 2008, quarter 1 through 2010 quarter 4.
Medicare Old Age‐ initial qualifying reason for Medicare eligibility was “old age.”
Favorable Histology includes WHO subtypes 9896 Acute myeloid leukemia) t(8;21)(q22;q22) RUNX1‐RUNX1T1 and 9871 AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22)) CBFB‐MYH11 (World Health Organization 2008; Khwaja et al. 2016; Ruhl et al. 2018).
Geographical regions: Northeast = Connecticut, New Jersey; Midwest = Iowa, Michigan; South = Georgia, Kentucky, Louisiana; West = California, New Mexico, Utah, Washington.
Hospital has any level of affiliation with National Cancer Institute in calendar year 2010.
Cluster robust 95% confidence intervals displayed in parentheses.
***p < .01, **p < .05, *p < .1.
CI, confidence interval; CT, census tract; IRR, incident rate ratio; NCI, National Cancer Institute; OR, odds ratio; Sep/widow/divorced, separated, widowed or divorced.
Additional subgroup analysis (not shown) suggested that patients for whom AML was a first primary cancer were more likely to have received treatment at each successive time point, as compared to persons for whom AML was a second or later cancer. The most notable finding was the 60‐day IRR estimate comparing timeliness of treatment (IRR = 1.488, p < .05), suggesting it to have taken on average close to 50 percent longer for persons in which AML was a secondary or later cancer to have begun induction treatment during a period of major shortage as compared to baseline.
Factors that were positively associated with the receipt of treatment having controlled for the type of shortage period included female gender (14 days), AML as first primary cancer, younger age, lower Charlson score, urban address, hospital teaching status (14 days), and hospital bed size greater than 200. Charlson comorbidity score was a significant correlate of treatment for persons for whom AML was a first primary cancer, but not for persons for whom AML was a secondary or later cancer. Significant overlap was found between attributes influencing odds of treatment with those demonstrating a relationship with timeliness of treatment.
As shown in Table 3, findings from logistic regression models testing for outpatient use of a nontherapeutically equivalent drug within 30 days of diagnosis were consistent with substitution. Patients diagnosed during a period of major shortage were found to be 75 percent more likely (OR = 1.75, p < .05) and moderate shortage 54 percent more likely (OR = 1.544, p < .05) than persons diagnosed during a period of nonshortage to have received azacitidine or decitabine as an outpatient within the first 30 days following diagnosis. A final set of substitution models restricted to the subset of patients without evidence of receipt of a hypo‐methylating agent during the year prior to diagnosis found even greater differences in substitution of either azacitidine or decitabine at both 30 and 60 days post diagnosis.
Table 3.
Bivariate Odds of Substitution of Azacitidine or Decitabine in Total and by Age and Primary Status
| Section 1: Use of Azacitabine or Decitabine within 30 Days following Diagnosis as Outpatient | |||||||
|---|---|---|---|---|---|---|---|
| Variables | (1) | (2) | (3) | (4) | (5) | (6) | (7) |
| Full Sample OR (95% CI) | First Primaryc OR (95% CI) | Not Firstd OR (95% CI) | First Primaryc Age 65–69 OR (95% CI) | First Primaryc Age 70–74 OR (95% CI) | Not Firstd Age 65–69 OR (95% CI) | Not Firstd Age 70–74 OR (95% CI) | |
| Major (Q1)a | 1.751** | 1.574 | 1.604 | 1.200 | 2.169* | 2.202 | 1.105 |
| (1.006–3.047) | (0.645–3.840) | (0.777–3.312) | (0.394–3.651) | (0.879–5.351) | (0.648–7.484) | (0.436–2.801) | |
| Moderate (Q2‐4)b | 1.544** | 1.559 | 1.265 | 1.023 | 2.033** | 1.835 | 1.569* |
| (1.069–2.230) | (0.877–2.772) | (0.777–2.060) | (0.469–2.230) | (1.158–3.569) | (0.779–4.322) | (0.920–2.677) | |
| Observations | 1,568 | 964 | 604 | 410 | 551 | 218 | 329 |
| Section 2: Use of Azacitabine or Decitabine within 60 Days following Diagnosis as Outpatiente | |||||||
|---|---|---|---|---|---|---|---|
| Major (Q1)a | 1.531* | 1.621 | 1.276 | 1.204 | 2.140* | 1.655 | 1.190 |
| (0.946–2.478) | (0.811–3.242) | (0.648–2.513) | (0.396–3.664) | (0.868–5.275) | (0.558–4.907) | (0.493–2.872) | |
| Moderate (Q2–4)b | 1.636*** | 1.531* | 1.478* | 1.027 | 2.006** | 1.489 | 1.468 |
| (1.210–2.213) | (0.976–2.402) | (0.973–2.245) | (0.471–2.238) | (1.144–3.518) | (0.687–3.226) | (0.884–2.437) | |
| Observations | 1,568 | 964 | 604 | 411 | 553 | 243 | 361 |
| Section 3: Use of Azacitabine or Decitabine within 30 to 60 Days as Outpatient in Patients Not Receiving Either Drug Prior to Diagnosis | ||
|---|---|---|
| Variables | (1) | (2) |
| Full Sample within 30‐day post, not pre OR (95% CI) | Full Sample within 60‐day post, not pre OR (95% CI) | |
| Major (Q1)a | 1.857** | 1.620* |
| (1.032–3.344) | (0.980–2.680) | |
| Moderate (Q2–4)b | 1.543** | 1.643*** |
| (1.036–2.299) | (1.193–2.262) | |
| Observations | 1,484 | 1,484 |
Major Shortage‐first quarter 2011 as compared to 2008, quarter 1 through 2010 quarter 4.
Moderate Shortage‐ quarters 2–4, 2011 as compared to 2008, quarter 1 through 2010 quarter 4.
First Primary describes subgroup of patients in which AML was first primary cancer.
Not First describes subgroup of patients in which AML was Not a patient's first primary cancer.
HCPCS (Centers for Medicare and Medicaid Services [CMS] 2018) codes used to identify Azacitidine is J9025 and Decitibine J0894 respectively.
Robust 95% confidence intervals show in exponential form in parentheses.
***p < .01, **p < .05, *p < .1.
Findings from robustness checks 1, 2, and 3 (not shown), which introduced variation into the applied diagnostic and induction date algorithms were directionally in line with modeled results. In addition, the falsification test performed on the subset of patients with APL for which cytarabine is not utilized (check 4—not shown) failed to uncover any unmeasured period effect coinciding with cytarabine shortage dates.
Discussion
This research represents the first quantitative evaluation of a disruption in the supply of a generic oncolytic drug agent with no close or distant therapeutic substitute for the diagnosis on receipt of and time to treatment. With the exception of Metzger et al.'s study of the consequences of a shortage of the drug mechlorethamine among a pediatric ALL population (Metzger, Billett, and Link 2012), this is the only multisite study to have investigated potential compromises in patient access to treatment absent of a clear substitute. Further, this research investigated which subgroups of patients were more versus less likely to receive treatment and differences in length of delays encountered across subgroups. Hence, this research serves as a foundation for future researchers looking to evaluate implications of a drug shortage, absent a therapeutic substitute.
We found the odds of receipt of inpatient chemotherapy for patients diagnosed during a period of major shortage to be 53 percent of those for patients diagnosed during the prior nonshortage period. Conversely, there was not a significant difference in odds of receipt of IV chemotherapy within 14 days of diagnosis for patients diagnosed during the period of moderate supply disruption compared to the nonshortage period.
Age appears to have been a factor considered by clinicians when faced with rationing of a scarce chemotherapeutic, with the subgroup of persons aged 70–74 having experienced a larger negative directional shift in odds of receipt than persons aged 60–65. Given the small sample size for the major shortage period, confidence intervals were wide and estimates were not significant.
Median time (days) to first qualifying dose of inpatient IV chemotherapy was found to be 11 days during a period of major shortage versus 7 days during a period of nonshortage for the sample at large, with no significant difference identified for patients for whom AML was a first primary cancer (8 vs. 7 days). Conversely, a delta of 8.5 days was found for the patient subgroup for whom AML was not a first primary cancer (17.5 vs. 9 days).
While not considered to be a therapeutic substitute, increased utilization of hypo‐methylating agents within the first 30 days following an AML diagnosis was identified for persons diagnosed during major (OR = 1.75) and moderate (OR = 1.544) shortage periods. Substitution appears to have been most prominent among persons aged 70–74 for whom AML was a first primary cancer. When the same substitution outcome was examined at an elapsed time period of 60 days post diagnosis, odds ratios for receipt of a substitute therapeutic were found to be 1.531 and 1.636 during periods of major and moderate shortages, respectively, as compared to nonshortage.
Four different robustness checks were performed, the first three designed to assess plausibility of assigned definitions for date of diagnosis and identification of first treatment receipt and the fourth to assess for the presence of an otherwise unobserved period effect as a potential source of shortage exposure findings. Nondifferential findings from this fourth robustness check performed using the sample of patients with APL (N = 77) whose diagnosis is the one exception to use of cytarabine, provides the strongest evidence suggestive of a causal basis for our findings. Should the shift in odds ratios visualized in the main analysis for the subset of patients diagnosed during a period of major shortage be a manifestation of omitted variable bias as opposed to being a consequence of exposure to drug shortage conditions, one would have expected to observe the same directional shift in odds ratios with the APL subgroup. However, greater rather than lower odds ratios were identified at elapsed time periods of 7 and 14 days for persons diagnosed during a period of major shortage as compared to nonshortage, which migrate down to a value of 1.0 (bivariate) or 1.12 (multivariate) by 21 days of observation. While we expected the odds ratio to be equal to 1.0 at all measurement time points, it is possible that the early elevation observed in the falsification patient subset was tied to a general prevailing urgency to get patients into treatment as quickly as possible, given the large number of drugs undergoing a shortage across calendar year 2011.
The literature is mixed on the role and value of aggressive induction therapy in older adults, which is one explanation why the level of treatment compromise estimated, might be less dire than some might have anticipated. While Lowenburg et al. previously reported a median difference in survival time of 10 weeks (21 vs. 11 weeks), not all clinicians are proponents of aggressive induction treatment in older patients.
Burnett et al. caution that while 50 percent of patients aged 60–74 with no comorbidities and scores of 0 or 1 based on the World Health Organization/Eastern Cooperative Oncology Group Performance Status Scale (WHO/ECOG PS) are expected to achieve complete remission after 1–2 cycles, unfavorable cytogenetics reduce this proportion to approximately 30 percent (Burnett et al. 2010; Burnett, Wetzler, and Lowenberg 2011). Appelbaum et al. in turn speak to high mortality rates following induction, pointing out how 31 percent of patients aged 66–74 with a performance score of two, and 47 percent of patients with a performance score of three, are likely to die within 30 days of induction (Appelbaum et al. 2006). Juliusson et al. in turn counter that the majority of AML patients up to 80 years of age should be considered fit for attempt of intensive induction treatment, citing Swedish registry data findings suggestive that standard intensive induction therapy decreases, rather than increases, 8‐week death rates in most patients up to 80 years of age (Juliusson et al. 2009); Juliusson 2009).
The findings in this study are subject to a number of limitations. First, the use of Medicare fee‐for‐service claims data did not permit for identification of care delivered elsewhere, such as through the Veterans Health Care System, state or county health care facilities, or via a clinical trial. To address this limitation, patients were required to have Medicare Parts A and B enrollment starting 12 months prior to month of diagnosis and exclusive reliance on Medicare claims data for identification of treatment received.
Second, SEER registry data lacks calendar date level of specificity for time of diagnosis and fails to track functional performance scores. Hence, we developed and implemented a diagnostic date algorithm based upon sequential calendar dates of diagnostic procedures likely to comprise an initial leukemia work up to improve upon diagnostic date precision, and calculated a Charlson Comorbidity score adjustment factor to control for comorbid health states.
Third, documentation of initial receipt of chemotherapy within SEER registry files is regarded as incomplete and not recommended for analytic use (Du et al. 2006; Noone et al. 2016; National Cancer Institute 2017a). Further, Medicare Inpatient claims files fail to specify the exact identity of chemotherapeutic entity administered while inpatient. To address this data gap, an algorithm was developed requiring presence of an ICD‐9 procedure code corresponding with administration of IV chemotherapy, a minimum length of stay was imposed, and receipt of a hypo‐methylating agent within 60 days following diagnosis as outpatient was disallowed, accompanied by sensitivity testing. Despite the level of rigor implemented to identify and test our definition for a cytarabine induction proxy, it is possible that a portion of patients counted as having received inpatient [7 + 3] cytarabine‐containing induction chemotherapy regimen may have received either azacitidine or decitabine, given the upward shift in usage identified both prior to and post diagnosis in Part B claims. Unfortunately, due to data limitations, we cannot address this possibility.
It is important to acknowledge the absence of sufficiently tight confidence intervals to achieve a high level of statistical significant given the modest number of patients (N = 105) diagnosed during the three month period categorized as major shortage and the controversy over placing an older patient on aggressive chemotherapy, even during periods of nonshortage.
While a series of negative directional shifts in odds ratio point values was observed across consecutive time periods, the majority were not found to be statistically significant at an alpha value of 0.05. Consequently, additional research with larger sample sizes is recommended to further our understanding of access to treatment ramifications of a shortage of a chemotherapeutic absent a therapeutically equivalent substitute.
As the price of a generic molecule erodes to a point close to the marginal cost of manufacturing, the level of incentive for a pharmaceutical manufacturer to remain in a market wanes. Wiske, Ogbechie, and Schulman (2015) speaks to how observed drug shortages and price spikes are negative outcomes associated with a malfunctioning marketplace (Wiske, Ogbechie, and Schulman 2015). They further call out how the generic pharmaceutical market is challenged by both financial and time‐related entry barriers, unlike more efficient commodities markets. To address these challenges, those authors proposed provocative strategies of restricted generic market entry and tax‐incentives for long‐term contracting between generic drug manufacturers and large distributors or other methods to create a futures market for generic drugs.
In January 2018, Intermountain Healthcare announced a collaboration consisting of members from 450 hospitals has been formed to combat rising drug costs and recurrent drug shortages (Intermountain Healthcare 2018). This press release suggests one impetus for the formation of this new nonprofit drug company is to stabilize the available supply of essential generic medications administered in hospitals and health systems. The effectiveness of this new initiative to reduce drug shortages will need to be assessed over time.
Findings from this study can be used to inform legislative bodies of the consequences of a supply disruption on patient access to a time sensitive treatment. While the Orphan Drug Act of 1983 has provided a stimulus to attract pharmaceutical manufacturers to have interest in newer therapies targeted for use in relatively small populations, these same financial incentives do not apply to the sale of older generic drugs with expired patent exclusivity prior to passage of this act. Further, not only are these older pharmaceuticals subject to relatively small marginal returns, a manufacturer's ability to adjust price may be subject to the terms of multiyear contractual agreements between pharmaceutical manufacturers and group purchasing organizations.
This study was conducted to understand ramifications of a market's failure to supply sufficient quantities of a generic oncolytic absent a therapeutically equivalent substitute on patient access and timeliness of treatment. Patients diagnosed during a major cytarabine shortage were approximately half as likely to receive inpatient chemotherapy within two weeks of diagnosis. Persons who were younger, had a lower versus higher Charlson Comorbidity score, and for whom AML was a first primary cancer appear to have been prioritized. Substitution of a nonequivalent drug was also identified.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2: First Commercialization Date.
Appendix SA3: Consort Diagram.
Appendix SA4: Diagnostic Procedure Codes.
Appendix SA5: Chemotherapy Receipt.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: Described research was conducted while Nancy Hedlund was a PhD candidate within the School of Public Health at the University of Illinois, Chicago (UIC). Financial support for licensing of Seer‐Medicare data was received from the School of Public Health at UIC.
Disclosure: None.
Disclaimer: None.
References
- Aday, L. , and Andersen R.. 1974. “A Framework for the Study of Access to Medical Care.” Health Services Research 9 (3): 208–20. [PMC free article] [PubMed] [Google Scholar]
- Adamo, M. , Dickie L., and Ruhl J.. 2016. SEER Program Coding and Staging Manual 2016. Bethesda, MD: National Cancer Institute, 20850‐9765. U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute. Available at https://seer.cancer.gov/manuals/2016/SPCSM_2016_maindoc.pdf [Google Scholar]
- Aday, L. , Begley C., Lairson D., and Balkrishnan R.. 2004. Evaluating the Healthcare System: Effectiveness, Efficiency, and Equity, 3d Edition Chicago, IL: Health Administration Press. [Google Scholar]
- American Cancer Society . 2015. “Leukemia–Acute Myeloid (Myelogenous)” [accessed on June 21, 2015]. Available at http://www.cancer.org/acs/groups/cid/documents/webcontent/003110-pdf
- Appelbaum, F. R. , Gundacker H., Head D. R., Slovak M. L., Willman C. L., Godwin J. E., Anderson J. E., and Petersdorf S. H.. 2006. “Age and Acute Myeloid Leukemia.” Blood 107 (9): 3481–5. 10.1182/blood-2005-09-3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, J. L. , Smith A., Zorn K. K., Sukumvanich P., Olawaiye A. B., Kelley J., and Krivak T. C.. 2014. “Outcomes Analysis of an Alternative Formulation of PEGylated Liposomal Doxorubicin in Recurrent Epithelial Ovarian Carcinoma During the Drug Shortage Era.” OncoTargets and Therapy 7: 1409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett, A. , Wetzler M., and Lowenberg B.. 2011. “Therapeutic Advances in Acute Myeloid Leukemia.” Journal of Clinical Oncology 29 (5): 487–94. [DOI] [PubMed] [Google Scholar]
- Burnett, A. K. , Hills R. K., Milligan D. W., Goldstone A. H., Prentice A. G., McMullin M.‐F., Duncombe A., Gibson B., and Wheatley K.. 2010. “Attempts to Optimize Induction and Consolidation Treatment in Acute Myeloid Leukemia: Results of the MRC AML12 Trial.” Journal of Clinical Oncology 28 (4): 586–95. 10.1200/jco.2009.22.9088 [DOI] [PubMed] [Google Scholar]
- Cameron, C. , and Trivedi P.. 2013. Regression Analysis of Count Data, 2d Edition. New York: Cambridge University Press. [Google Scholar]
- Center for Disease Control and Prevention, and National Center for Health Statistics . 2018. “International Classification of Diseases, Ninth Revision (ICD‐9)” [accessed on July 26, 2018]. Available at https://www.cdc.gov/nchs/icd/icd9.html
- Centers for Medicare and Medicaid Services [CMS] . 2018, May, 7. “HCPT (CPT in HCPCS)–Synopsis. Unified Medical Language System (UMLS)”. [accessed on May 7, 2018]. Available at https://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/HCPT/index.html
- Döhner, H. , Estey E., Amadori S., et al. 2010. “Diagnosis and Management of Acute Myeloid Leukemia in Adults: Recommendations from an International Expert Panel, on Behalf of the European LeukemiaNet.” Blood 115: 453–74. [DOI] [PubMed] [Google Scholar]
- Du, X. L. , Key C. R., Dickie L., Darling R., Delclos G. L., Waller K., and Zhang D.. 2006. “Information on Chemotherapy and Hormone Therapy From Tumor Registry had Moderate Agreement With Chart Reviews.” Journal of Clinical Epidemiology 59 (1): 53–60. https://10.1016/j.jclinepi.2005.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. A. 1922. “On the Interpretation of χ2 From Contingency Tables, and the Calculation of P.” Journal of the Royal Statistical Society 85 (1): 87–94. [Google Scholar]
- Fox, E . 2014. “National Drug Shortages.” American Society of Health System Pharmacists. Available at http://www.ashp.org/DocLibrary/Policy/DrugShortages/Drug-Shortages-Statistics.pdf
- Fox, E. R . 2015. Drug Shortage Database. University of Utah Drug Information Service, Version current through 12/31/14.
- Fox, E. , Sweet B., and Jensen V.. 2014. “Drug Shortages: A Complex Health Care Crisis.” Mayo Clinic Proceedings 89 (3): 361–73. [DOI] [PubMed] [Google Scholar]
- Gogineni, K. , Shuman K. L., and Emanuel E. J.. 2013. “Survey of Oncologists about Shortages of Cancer Drugs.” New England Journal of Medicine 369 (25): 2463–4. [DOI] [PubMed] [Google Scholar]
- Goodman, A. , Sullivan L., Lafleur K., Penson R., Schorge J., Del Carmen M., Boruta II D., Growdon W., Krasner C., and Birrer M.. 2012. “Impact of Drug Shortages on Cancer Care: The Story of Pegylated Liposomal Doxorubicin in Ovarian Cancer.” Gynecologic Oncology 125: S66–7. 10.1016/j.ygyno.2011.12.159 [DOI] [Google Scholar]
- Havrilesky, L. J. , Garfield C. F., Barnett J. C., and Cohn D. E.. 2012. “Economic Impact of Paclitaxel Shortage in Patients with Newly Diagnosed Ovarian Cancer.” Gynecologic Oncology 125 (3): 631–4. [DOI] [PubMed] [Google Scholar]
- Helwick, C . 2013. “Two Surveys Confirm Drug Shortages are a Persistent Problem, Increasing Costs.” American Health and Drug Benefits 6 (6):1–5. [Google Scholar]
- Hilbe, J. 2007. Negative Binomial Regression, 2d Edition New York City, NY: Cambridge University Press. [Google Scholar]
- Huls, G. 2015. “Azacitidine in AML: A Treatment Option?” Blood 126 (3): 283–5. [DOI] [PubMed] [Google Scholar]
- Intermountain Healthcare . 2018. “Leading U.S. Health Systems Announce Plans to Develop a Not‐for‐Profit Generic Drug Company” [accessed on April 16, 2016]. Available at https://intermountainhealthcare.org/news/2018/01/leading-us-health-systems-announce-plans-to-develop-a-not-for-profit-generic-drug-company/
- Jain, P. , Gupta S., Yim B., and Mullane M. R.. 2012. “5‐Flourouracil (5FU) Shortage: Clinical Implications and Costs in a Single Month.” Journal of Clinical Oncology 30 (4 Suppl. 1): 663. [Google Scholar]
- Jiang, Y. , Dunbar A., Gondek L. P., Mohan S., Rataul M., O'Keefe C., Sekeres M., Saunthararajah Y., and Maciejewski J. P.. 2009. “Aberrant DNA Methylation is a Dominant Mechanism in MDS Progression to AML.” Blood 113 (6): 1315–25. 10.1182/blood-2008-06-163246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, E. , Reiffers J., Stoppa A. M., Sotto J. J., Attal M., Bouabdallaha R., Marit G., Fégueux N., Boulat O., Dastugue N., Boiron J. M., Fabères C., Gastaut J. A., Maraninchi D., and Blaise D.. 2001. “Outcome of Adult Patients with Acute Myeloid Leukemia Who Failed to Achieve Complete Remission after One Course of Induction Chemotherapy: A Report from the BGMT Study Group: for the BGMT.” Leukemia & Lymphoma 42 (1–2): 57–65. [DOI] [PubMed] [Google Scholar]
- Juliusson, G. 2011. “Older Patients With Acute Myeloid Leukemia Benefit From Intensive Chemotherapy: An Update From the Swedish Acute Leukemia Registry.” Clinical Lymphoma, Myeloma & Leukemia 11 (Suppl 1): S54–S59. 10.1016/j.clml.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Juliusson, G. , Antunovic P., Derolf Å., Lehmann S., Möllgård L., Stockelberg D., Tidefelt U., Wahlin A., and Höglund M.. 2009. “Age and Acute Myeloid Leukemia: Real World Data on Decision to Treat and Outcomes From the Swedish Acute Leukemia Registry.” Blood 113 (18): 4179–4187. 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- Khwaja, A. , Bjorkholm M., Gale R. E., Levine R. L., Jordan C. T., Ehninger G., Bloomfield C. D., Estey E., Burnett A., Cornelissen J. J., Scheinberg D. A., Bouscary D., and Linch D. C.. 2016. “Acute Myeloid Leukaemia.” Nature Reviews Disease Primers 2: 16010. [DOI] [PubMed] [Google Scholar]
- Klabunde, C. N. , Potosky A. L., Legler J. M., and Warren J. L.. 2000. “Development of a Comorbidity Index Using Physician Claims Data.” Journal of Clinical Epidemiology 53 (12): 10. [DOI] [PubMed] [Google Scholar]
- Klabunde, C. N. , Harlan L. C., and Warren J. L.. 2006. “Data Sources for Measuring Comorbidity: A Comparison of Hospital Records and Medicare Claims for Cancer Patients.” Medical Care 44 (10): 921–8. [DOI] [PubMed] [Google Scholar]
- McBride, A. , Holle L. M., Westendorf C., Sidebottom M., Griffith N., Muller R. J., and Hoffman J. M.. 2013. “National Survey on the Effect of Oncology Drug Shortages on Cancer Care.” American Journal of Health‐System Pharmacy 70 (7): 609–17. [DOI] [PubMed] [Google Scholar]
- Metzger, M. L. , Billett A., and Link M. P.. 2012. “The Impact of Drug Shortages on Children with Cancer–The Example of Mechlorethamine.” New England Journal of Medicine 367 (26): 2461–3. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute . 2017a, February 24. “Seer Medicare: Defining the Date of Diagnosis and Treatment” [accessed on July 31, 2018]. Available at http://healthcaredelivery.cancer.gov/seermedicare/considerations/date.html
- National Cancer Institute . 2017b, March 15. “Comorbidity SAS Macro (2014 Version).” Healthcare Delivery Research [accessed on July 30, 2018]. Available at https://healthcaredelivery.cancer.gov/seermedicare/considerations/macro-2014.html
- National Cancer Institute . 2018, February 7. “Adult Acute Myeloid Leukemia Treatment” [accessed on July 30, 2018]. Available at http://www.cancer.gov/cancertopics/pdq/treatment/adultAML/healthprofessional
- National Comprehensive Cancer Network . 2014, April 1. “UPDATES: NCCN Guidelines® and NCCN Compendium” [accessed on November 30, 2016]. Available at https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=307
- NCI Epidemiology and End Results Program . 2017. “About the SEER Registries.” Overview of SEER Program [accessed on January 4, 2017]. Available at https://seer.cancer.gov/registries/
- Nickel, R. , Keller F., Bergsagel J., Cooper T., Daves M., Sabnis H., and Lew G.. 2014. “Mitoxantrone as a Substitute for Daunorubicin During Induction in Newly Diagnosed Lymphoblastic Leukemia and Lymphoma.” Pediatric Blood and Cancer 61 (5): 810–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone, A. M. , Lund J. L., Mariotto A., Cronin K., McNeel T., Deapen D., and Warren J. L.. 2016. “Comparison of SEER Treatment Data With Medicare Claims.” Medical Care 54 (9): e55–e64. 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, M. R. , Tallman M. S., Abboud C. N., Altman J. K., Appelbaum F. R., Arber D. A., Attar E., Borate U., Coutre S. E., Damon L. E., Lancet J., Maness L. J., Marcucci G., Martin M. G., Millenson M. M., Moore J. O., Ravandi F., Shami P. J., Smith B. D., Stone R. M., Strickland S. A., Wang E. S., Gregory K. M., and Naganuma M.. 2013. “Acute Myeloid Leukemia, Version 2.2013.” Journal of the National Comprehensive Cancer Network 11 (9): 1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S. , Leblebjian H., Harris C., Scullion‐Fowler B., and Bartel S.. 2013. “Assessment of Toxicities of Two Brands of Pegylated Liposomal Doxoubicin at a Large Academic Institution.” Journal of Oncology Pharmacy Practice 19: 12. [Google Scholar]
- Pearson, K . 1900. “On the Criterion That a Given System of Deviations From the Probable in the Case of a Correlated System of Variables Is Such That It Can Be Reasonably Supposed to Have Arisen From Random Sampling.” Philosophical Magazine (Series 5) 50 (302): 157–175. [Google Scholar]
- Poi, M. J. , Berger M., Lustberg M., Layman R., Shapiro C. L., Ramaswamy B., Mrozek E., Olson E., and Wesolowski R.. 2013. “Docetaxel‐Induced Skin Toxicities in Breast Cancer Patients Subsequent to Paclitaxel Shortage: A Case Series and Literature Review.” Supportive Care in Cancer 21 (10): 2679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler, H. , Davis R. B., Kirshner J., Dupre E., Richards F. 3rd, Hoagland H. C., Kopel S., Levy R. N., Carey R., and Schulman P.. 1987. “Comparison of Three Remission Induction Regimens and Two Postinduction Strategies for the Treatment of Acute Nonlymphocytic Leukemia: A Cancer and Leukemia Group B Study.” Blood 69 (5): 1441–9. [PubMed] [Google Scholar]
- Rai, K. R. , Holland J. F., Glidewell O. J., et al. 1981. “Treatment of Acute Myelocytic Leukemia: A Study by Cancer and Leukemia Group B.” Blood 58 (6): 1203–12. [PubMed] [Google Scholar]
- Roboz, G. J. 2012. “Current Treatment of Acute Myeloid Leukemia.” Current Opinion in Oncology 24 (6): 711–9. [DOI] [PubMed] [Google Scholar]
- Ruhl, J. , Adamo M., Dickie L., and Negoita S.. 2018. “Hematopoietic and Lymphoid Neoplasm Coding Manual” [accessed on July 26, 2018]. Available at https://seer.cancer.gov/tools/heme/Hematopoietic_Instructions_and_Rules.pdf
- Seiter, K. , Talavera F., Sacher R., Besa E., and Adoo C.. 2016. “Acute Myelogenous Leukemia Workup.” MedScape Reference, Drug Diseases and Procedures [accessed on July 29, 2018]. Available at http://emedicine.medscape.com/article/197802-workup#showall [Google Scholar]
- Sekeres, M. A. , Elson P., Kalaycio M. E., Advani A. S., Copelan E. A., Faderl S., Kantarjian H. M., and Estey E.. 2009. “Time From Diagnosis to Treatment Initiation Predicts Survival in Younger, but not Older, Acute Myeloid Leukemia Patients.” Blood 113 (1): 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, E. M. , and Tallman M. S.. 2012. “Remission Induction in Acute Myeloid Leukemia.” International Journal of Hematology 96 (2): 164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stencel, E. 2014. “Health Policy Brief: Drug Shortages” Health Affairs [accessed on July 29, 2018]. Available at https://www.rwjf.org/en/library/research/2014/09/drug-shortages-.html [Google Scholar]
- Tefferi, A. , and Vardiman J. W.. 2009. “Myelodysplastic Syndromes.” New England Journal of Medicine 361 (19): 1872–85. [DOI] [PubMed] [Google Scholar]
- Trifilio, S. , Zhou Z., Mehta J., Czerniak C., Pi J., Greenberg D., Koslosky M., Pantiru M., and Altman J.. 2013. “Idarubicin Appears Equivalent to Dose‐Intense Daunorubicin for Remission Induction in Patients with Acute Myeloid Leukemia.” Leukemia Research 37 (8): 868–71. [DOI] [PubMed] [Google Scholar]
- U.S. Food Drug Administration . 2014a. “Current and Resolved Drug Shortages and Discontinuations Reported to FDA.” FDA Drug Shortages [accessed on July 26, 2018]. Available at http://www.accessdata.fda.gov/scripts/drugshortages/default.cfm [Google Scholar]
- U.S. Food and Drug Administration . 2014b. “Manual of Policies and Procedures, Center for Drug Evaluation and Research, Office of the Center Director, Drug Shortage Management“. MAPP 4190.1 Rev 2. Available at https://www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/ManualofPoliciesProcedures/ucm079936.pdf
- U.S. Food and Drug Administration . 2015. “Drugs@FDA: FDA Approved Drug Products” [accessed on July 26, 2018]. Available at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm
- U.S. Food and Drug Administration . 2017. “Drug Shortages Infographic” [accessed on July 26, 2018]. Available at https://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM441583.pdf
- Walter, R. B. , Kantarjian H. M., Huang X., Pierce S. A., Sun Z., Gundacker H. M., Ravandi F., Faderl S. H., Tallman M. S., Appelbaum F. R., and Estey E.. 2010. “Effect of Complete Remission and Responses Less Than Complete Remission on Survival in Acute Myeloid Leukemia: A Combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study.” Journal of Clinical Oncology 28 (10): 1766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, J. , Klabunde C., Schrag D., Bach P., and Riley G.. 2002. “Overview of the SEER‐Medicare Data: Content, Research Applications, and Generalizability to the United States Elderly Population.” Medical Care 40 Supplement 8(0025‐7079 (Print)):IV‐3‐18. [DOI] [PubMed] [Google Scholar]
- Wilcoxon, F. 1945. “Individual Comparisons by Ranking Methods.” Biometrics Bulletin 1 (6): 80–83. [Google Scholar]
- Wiske, C. P. , Ogbechie O. A., and Schulman K. A.. 2015. “Options to Promote Competitive Generics Markets in the United States.” Journal of the American Medical Association 314 (20): 2129–30. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2008. “WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues” In The International Agency for Research on Cancer (IARC), Vol. 2, Revised 4th Edition, edited by Swerdlow S. H., Campo E., Harris N. L., Jaffe E. S., Pileri S. A., Stein H., and Thiele J., pp. 129–165 [accessed on July 29, 2018]. Available at http://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Who-Classification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017 [Google Scholar]
- Yates, J. , Glidewell O., Wiernik P., Cooper M. R., Steinberg D., Dosik H., Levy R., Hoagland C., Henry P., Gottlieb A., Cornell C., Berenberg J., Hutchison J. L., Raich P., Nissen N., Ellison R. R., Frelick R., James G. W., Falkson G., Silver R. T., Haurani F., Green M., Henderson E., Leone L., and Holland J. F.. 1982. “Cytosine Arabinoside with Daunorubicin or Adriamycin for Therapy of Acute Myelocytic Leukemia: A CALGB Study.” Blood 60 (2): 454–62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: First Commercialization Date.
Appendix SA3: Consort Diagram.
Appendix SA4: Diagnostic Procedure Codes.
Appendix SA5: Chemotherapy Receipt.


