Abstract
Objective
To identify the optimal timing of in‐person physician visit after hospital discharge to yield the largest reduction in readmission among elderly or chronically ill patients.
Data Sources/Study Setting/Extraction Methods
We extracted insurance billing data on 620,656 admissions for any cause from 2002 to 2009 in Quebec, Canada.
Study Design
We used flexible survival models to estimate inverse probability weights for the precise timing (days) of in‐person physician visit after discharge and weighted competing risk outcome models.
Principal Findings
Readmission reduction associated with in‐person physician visits (compared to none) was seen early after discharge, with 67.8 fewer readmissions per 1,000 discharges if physician visit occurred within 7 days (95 percent CI: 66.7–69.0), and 110.0 fewer readmissions within 21 days (95 percent CI: 108.2–111.7). The period of largest contribution to readmission reduction was seen in the first 10 days, while physician visits occurring later than 21 days after discharge did not further contribute to reducing hospital readmissions. Larger risk reductions were observed among patients in the highest morbidity level and for in‐person follow‐up with a primary care physician rather than a medical specialist.
Conclusions
When provided promptly, postdischarge in‐person physician visit can prevent many readmissions. The benefits appear optimal when such visit occurs within the first 10 days, or at least within the first 21 days of discharge.
Keywords: Cohort analysis, administrative data uses, ambulatory/outpatient care
Hospital readmissions have been a target of health care policy in the United States and in other countries, either as a quality measure of hospital care or as a marker of poor integration of the health care delivery system. That a fair portion of hospital readmissions may be preventable indicates an opportunity for containing cost and for improving the quality of patient care (PriceWaterhouse Coopers’ Health Research Institute 2008; Burton 2012). Timely outpatient follow‐up after discharge has been a key component of contemporary efforts to promote better care coordination and address readmissions (Kim and Flanders 2013; Kripalani et al. 2014). In‐person physician visits represent an opportunity to manage care after hospitalization. Patients who see a physician shortly after discharge may ask questions about their hospitalization, and physicians may monitor and address problems related to the patient's transition from hospital to community (Sommers and Cunningham 2011; Brooke et al. 2014; Canadian Institute of Health Information 2015). Incentive billing codes effective January 2013 for postdischarge care coordination (e.g., face‐to‐face visit within 14 or 7 days after discharge) (Centers for Medicare and Medicaid Services 2013) and readmission penalties under the Affordable Care Act have been implemented in the United States.
Measuring the preventive effect of timely outpatient follow‐up using observational data presents several challenges. First, the probability of receiving physician follow‐up may change over the postdischarge period, and failing to account for changing temporal patterns may introduce bias. Second, patient health status during an admission may also exert a time‐dependent effect on the conditional probability of receiving early follow‐up. These challenges are further compounded by the consideration that those who died or were readmitted early after discharge are likely different in their propensity to have received follow‐up. No studies to date have addressed all of these methodological challenges.
Previous research has generated mixed results on the association between postdischarge follow‐up and readmission. A number of observational studies reported that various patient populations receiving outpatient follow‐up have a lower risk of 30‐day readmission, including surgical patients (Brooke et al. 2014; Saunders et al. 2014), medical patients (Sin et al. 2002; Hernandez et al. 2010; Muus et al. 2010; Sharma et al. 2010; Leschke et al. 2012; Erickson et al. 2014; Baker et al. 2015), and chronically ill patients (Misky, Wald, and Coleman 2010; Lin, Barnato, and Degenholtz 2011; Hubbard et al. 2014; Jackson et al. 2015). A study by Jackson et al. (2015) reported large inverse associations between early follow‐up and readmission; however, the study did not account for geographic‐, physician‐, and hospital‐level factors, as well as time‐dependent effects of covariates and competing risk by death. Similarly, another large study found that hospitals with higher rates of outpatient physician follow‐up within 7 days of discharge were associated with approximately 10 percent lower rates of readmission (relative effect) among patients with heart failure; this association was not significant when examining rates of follow‐up within 14 days (Hernandez et al. 2010). The authors addressed time‐dependent confounding by illness severity by design (i.e., comparing hospitals instead of patients) and accounted for competing risk by death. However, there were limitations to the hospital‐level comparisons because they did not account for the key components of an ideal transition in care (e.g., discharge planning or timeliness of information transfer). In contrast, a number of studies found no association (Fidahussein et al. 2014; Field et al. 2015; Thygesen et al. 2015). The heterogeneity in the literature may be attributed to variations in the clinical characteristics of the study population, in the definition of timely follow‐up (i.e., within 7, 14, or 30 days of discharge) and of comparison groups, and in the analytical approach (Saunders et al. 2014).
From a policy perspective, the optimal timing of physician follow‐up following discharge and whether the type of follow‐up plays a role in reducing hospital readmissions remain unclear. Our primary goal was to identify the critical time window at which a postdischarge in‐person physician visit yields the greatest reduction in hospital readmissions among elderly or chronically ill patients (compared to no follow‐up visit). We also estimated effects by type of physician visit (by a primary care physician, by a medical specialist, or both) and by patient morbidity level.
Setting, Study Design, and Cohort
We constructed a cohort of elderly or chronically ill patients from population‐based claims database that includes continuously insured patients under the universal public health insurance plan in the province of Québec. Québec is Canada's largest province by area and second largest by population with 8.2 million inhabitants. Approximately 96 percent of residents have public health insurance which covers all services provided in‐hospital and by a general practitioner or by a medical specialist regardless of where the service is provided (e.g., outpatient clinics or hospitals) (Régie de l'assurance maladie du Québec 2013). The Régie de l'Assurance Maladie du Québec (RAMQ) administers the plan and pays doctors directly for the services that they provide. For this study, we linked data from RAMQ databases using a unique lifetime identifier encrypted from the personal health insurance number. RAMQ databases contain information about patient demographics, physician claims, and hospital admissions. We also linked information about the specialty of the billing physician for all physician services, and characteristics of the primary care physician who enrolled a patient into their practice, including practice type and characteristics, number of patients and services provided, and income source. Primary and specialist medical care in Quebec is predominantly funded via fee‐for‐service payments, with only a small portion of primary care physicians paid in part by salary for services provided within community health centers (<5 percent of physicians receive more than 20 percent of their income from salary, our data). In this study, primary care physician refers to a physician that specialized in family medicine, while medical specialist refers to a physician that specialized in any other medical fields (e.g., internal medicine or surgery).
Patients were selected into the cohort if they visited a primary care physician between November 2002 and January 2005, and if during that visit they met the criteria for “vulnerable patient,” that is, if a patient is either 70 years old or above or has one or more specified chronic health conditions: chronic obstructive pulmonary disease (COPD), moderate‐to‐severe asthma, pneumonia, cardiovascular disease, cancer associated with chemotherapy or radiotherapy treatments, cancer in a terminal phase, diabetes, alcohol or hard drug withdrawal, drug addiction treated with methadone, HIV/AIDS, or a degenerative disease of the nervous system (Régie de l'assurance maladie du Québec 2006). Since 2002 in Quebec, primary care physicians enroll patients into their practice as “vulnerable” by billing an incentive fee code. For each included patient, 5 years of health insurance billing data were extracted beginning on the date of their enrollment as vulnerable.
For this analysis, we created a dataset consisting of all hospital admissions that occurred during the 5‐year follow‐up corresponding to an overall study period between November 2002 and December 2009. The unit of analysis was the index admission, which we defined as a hospital admission for any cause. We excluded index admissions to long‐term care facilities and those that resulted in a discharge or a transfer to another facility. We further excluded index admissions for mental health and pregnancy/child birth using principal diagnosis codes (International Classification of Disease [ICD], 9th and 10th revisions), same‐day readmissions, admissions with in‐hospital death, pediatric admissions, and admissions with a hospital stay lasting 30 days or more (exclusions also apply to readmissions). These admissions were excluded because they represent patient subgroups that likely differ with regard to the patterns of use and need of health care services. We excluded admissions from Northern Quebec due to the extreme remoteness of these regions. We extracted billing data on the index admission and on any medical service (outpatient or inpatient) provided in the 60 days following the hospital discharge, including the date and type (primary care or specialty care) of all outpatient services. Data for patients with multiple index admissions meeting our criteria were also included.
Methods
Outcome Variable
For each index admission, we counted the number of calendar days that elapsed since discharge to the day a patient was readmitted; the time to a readmission for any cause consisted of our primary outcome variable. All observations were censored at 60 days after hospital discharge. There is no consensus in the scientific literature on the analytical time frame for postdischarge outcomes (American Hospital Association 2011; Shams, Ajorlou, and Yang 2012; Fischer et al. 2014). We chose a reasonably short time frame (i.e., 60 days after discharge) to avoid introducing bias from unmeasured factors unrelated to the index admission and because previous works by van Walraven et al. (2011) found that the proportion of readmissions deemed avoidable was highest early after hospital discharge and decreased significantly with increasing time following discharge.
Exposure Variable
We examined visits up to 30 days of hospital discharge because this is the time frame commonly suggested by clinical practice guidelines (e.g., follow‐up within 7 days, 14 days, or 30 days after discharge). We first identified outpatient physician visits defined as any physician service billed in establishments other than the emergency department, including hospital outpatient clinics and office‐based practices. We recorded the time to the first in‐person physician visit by counting the number of calendar days that elapsed since the patient was discharged to the day that the first outpatient service of any type was billed.
Control Variables
We controlled for several patient‐, physician‐, and hospital‐level factors that are associated with the receipt of postdischarge follow‐up and are plausible risk factors for readmission. At the patient level, we included age, sex, neighborhood socioeconomic status, and residential geographic location category. We used a material deprivation index based on small geographic units (population of 400–700 persons) as a measure of neighborhood socioeconomic status (Pampalon 2003; Gauthier et al. 2009; Pampalon, Gamache, and Hamel 2010). We used a categorical variable representing the patient's residential geographic location as a function of the proximity to an urban center and to a tertiary or secondary referral hospital to serve as a proxy for geographic access to care. Patient health and health care service utilization were represented by the following variables measured at index admission: length of index hospital stay, cumulative number of previous admissions since study entry (i.e., hospital admissions recorded previous to an index admission are counted since patient registration as vulnerable), the time since previous use of inpatient care, relative intensity of hospital resource use (Éco‐Santé Québec 2012), and major diagnostic category. We used the Johns Hopkins Adjusted Clinical Group (ACG) Case‐Mix System (“The Johns Hopkins ACG Case Mix System” 2003) to rank patients into Resource Utilization Bands (RUBs), which comprised five categories (healthy, low, moderate, high morbidity, or very high morbidity); patients in our cohort were in the “moderate,” “high,” or “very high” morbidity subgroups only. Patients were assigned to a RUB category based on diagnostic codes and ACGs for inpatient and outpatient use in the calendar year preceding the index admissions. Patients expected to use similar quantity and intensity of health care resources are in the same RUB category (Mittmann et al. 2014; Manitoba Centre for Health Policy 2015). Finally, we included characteristics of the enrolling primary care physician (age, sex, years in practice, the total number of patients, and income source, e.g., short‐term care establishment, salary, emergency services) and dummy variables for each hospital and calendar year of index admission. To account for missing data, we used a dummy variable (0/1) for categorical data (i.e., material deprivation index) and single imputation for discrete variables (i.e., physician characteristics—total number of patients and income of enrolling physician).
Stratifying Variables
We stratified our analyses by patient morbidity level (moderate, high morbidity, or very high morbidity) and by type of physician follow‐up provided. For the latter, we considered an in‐person visit by a primary care physician only, by a medical specialist only, or by both a primary care physician and a medical specialist (i.e., joint effect), irrespective of the order in which they occurred.
Statistical Analysis
Inverse Probability Weights (IPW)
We used stabilized IPW to make exposure groups (i.e., had in‐person physician visit at time t vs. did not) comparable on measured covariates (we refer readers to the paper by Austin & Stuart for best practice guidelines on IPW, which we followed) (Garrido et al. 2014; Austin and Stuart 2015). Our approach to constructing IPW was similar to that described by Naimi et al. (2014) on constructing IPW for continuous exposures, except that our exposure (i.e., in‐person physician visits) followed a time‐to‐failure distribution. We did this to best reflect the underlying propensity of the timing of physician office visits given covariates. In other words, whether a patient sees a physician following discharge may depend on several factors, and those factors may also play an important role in the timing of such a visit. For example, sicker patients may be more likely to visit their physician earlier after hospital discharge. Previous studies have shown that time‐specific approaches performed better than the conventional approach (i.e., probability of treatment averaged over time) for confounding adjustment (Dusetzina, Mack, and Sturmer 2013; Mack et al. 2013; Dilokthornsakul et al. 2014).
We modeled the time‐to‐failure distribution of IPW using flexible parametric survival regression (Royston and Lambert 2011). This technique models the effect of covariates on the probability of treatment received on each day after discharge and allows for flexibility in modeling baseline hazard and in incorporating complex time‐dependent effects through the use of restricted cubic splines (Royston and Parmar 2002; Lambert and Royston 2009). To obtain stabilized IPW, we derived from an unadjusted model the predicted probability of exposure actually received. We then divided this probability by the conditional predicted probability of treatment received derived from fully adjusted models. To examine the effects by subgroup of patient morbidity, the probability in the numerator of the stabilized weights was conditional on the patient morbidity level. To examine independent and joint effects by type of physician follow‐up, we multiplied the stabilized weight for follow‐up by primary care physician and the stabilized weight for follow‐up by a medical specialist. We assessed the validity of analytical weights according to published balance diagnostics in IPW analysis; standardized differences between exposure groups >10 percent on any covariate (and for each day after discharge) after weighting indicate risk of bias (Appendix SA3) (Austin and Stuart 2015). We considered evidence of model fit for exposure models based on the Akaike information criterion and the Bayesian information criterion. We also estimated models to investigate effect heterogeneity by type of physician visit (primary care physician, medical specialist, or both). We accounted for clustering at the hospital level by using fixed effects for hospitals in our models. Details of models are presented in Appendix SA2.
Marginal Structural Models
We estimated and modeled the subdistribution cumulative incidence functions of readmission using flexible parametric survival models adapted to account for competing risk of death (Hinchliffe and Lambert 2013). All models were weighted by IPW to estimate marginal differences in cumulative incidence functions (from this point onward referred to as risk, i.e., risk of readmission had everyone had an in‐person physician visit minus the risk of readmission had everyone not had an in‐person physician visit). We used the clustered bootstrap to obtain 95 percent confidence intervals (CIs) on the differences in cumulative incidence between exposure groups. We used Stata MP 14 for all analyses.
Sensitivity Analyses
In sensitivity analyses, we compared IPW diagnostics and effect estimates over time by whether the weights were derived using a time‐specific approach or using a conventional approach via logistic regression.
Results
After exclusions, we included a total of 312,377 patients representing 620,656 index admissions (Figure 1). The number of deaths occurring during the 60 days after discharge was higher among patients who did not have an in‐person physician visit than among patients who did (Figure 1).
Figure 1.
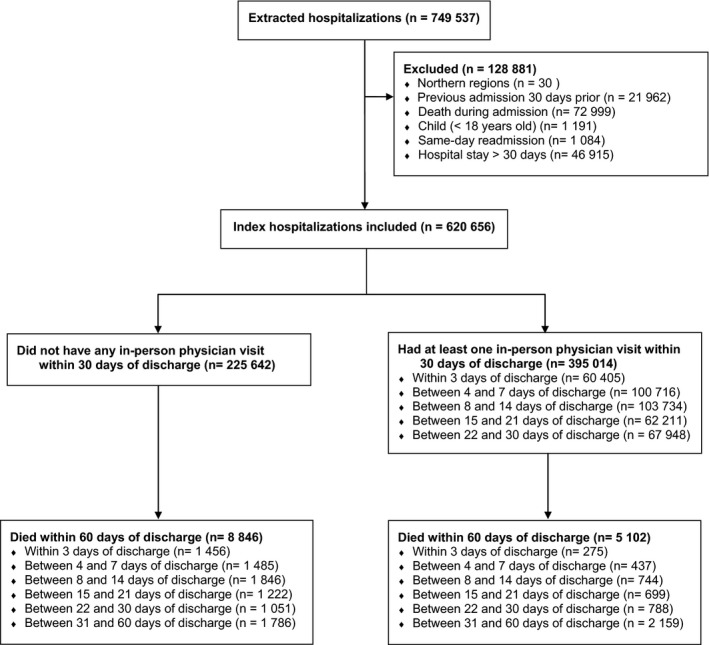
Selection of Hospital Admissions for the Study
As expected, the distribution of baseline characteristics measured at index admission differed across groups (Table 1). After weighting by IPW, we found no standardized differences >10 percent (Appendix SA3).
Table 1.
Patient Characteristics at Index Admission, Quebec (Canada) 2002–2009
| Characteristics and Measures | No. (%) | |
|---|---|---|
| Had Physician Visit within 30 Days of Discharge (N = 395,014) | Did Not Have Physician Visit within 30 Days of Discharge (N = 225,642) | |
| Sex, female | 202,268 (51.2) | 124,972 (55.4) |
| Age, years | ||
| 18–34 | 2,284 (0.6) | 1,480 (0.7) |
| 35–49 | 13,992 (3.5) | 6,558 (2.9) |
| 50–64 | 63,551 (16.1) | 28,294 (12.5) |
| 65–79 | 195,295 (49.4) | 100,664 (44.6) |
| ≥80 | 119,892 (30.4) | 88,646 (39.3) |
| Length of hospital stay, days | ||
| 0–2 | 98,779 (25.0) | 48,230 (21.4) |
| 3–6 | 130,755 (33.1) | 70,786 (31.4) |
| 7–13 | 107,812 (27.3) | 63,751 (28.25) |
| 14–20 | 37,386 (9.5) | 25,575 (11.3) |
| 21–30 | 20,282 (5.1) | 17,210 (7.6) |
| No. of previous admissions | ||
| 0 | 129,625 (32.8) | 69,000 (31.0) |
| 1 | 95,901 (24.3) | 53,038 (23.5) |
| 2 | 60,548 (15.3) | 34,002 (15.1) |
| ≥3 | 108,940 (27.6) | 68,702 (30.5) |
| Time since previous admission | ||
| 30 days to 3 months | 85,202 (21.5) | 47,568 (21.1) |
| 3–6 months | 35,525 (9.0) | 21,384 (9.5) |
| 6 months to 1 year | 43,775 (11.1) | 26,336 (11.7) |
| ≥1 year | 100,887 (25.5) | 60,454 (26.8) |
| Cost of hospitalizations,a median (IQR) | $4,351 ($2,852–$6,948) | $4,581 ($2,950–$7,560) |
| Morbidity, % | ||
| Moderate | 64,264 (16.3) | 41,684 (18.5) |
| High | 111,242 (28.2) | 62,720 (27.8) |
| Very high | 219,508 (55.6) | 121,238 (53.7) |
| Time since enrollment with primary care physician | ||
| 3 months or less | 29,786 (7.5) | 13,802 (6.1) |
| 3–6 months | 26,461 (6.7) | 12,887 (5.7) |
| 6 months to 1 year | 48,372 (12.3) | 27,899 (12.4) |
| 1–2 years | 88,168 (22.3) | 48,821 (21.6) |
| ≥2 years | 202,227 (51.2) | 122,233 (54.2) |
| Material deprivation quintile | ||
| Q1 (low) | 55,175 (14.0) | 27,782 (12.3) |
| Q2 | 65,434 (16.6) | 34,948 (15.5) |
| Q3 | 78,484 (19.9) | 43,085 (19.1) |
| Q4 | 84,729 (21.5) | 48,168 (21.4) |
| Q5 (high) | 85,773 (21.7) | 54,073 (24.0) |
| Geographic region | ||
| Urban/university | 135,206 (34.2) | 74,226 (32.9) |
| Suburban | 159,604 (40.4) | 83,530 (37.0) |
| Intermediate | 80,112 (20.3) | 50,782 (22.5) |
| Rural | 19,227 (4.9) | 16,064 (7.1) |
Material deprivation quintile: 25.419 (6.4%) and 17,586 (7.8%) in patients who received follow‐up and those who did not, respectively; geographic region: 865 (0.2%) and 1,040 (0.5%) in patients who had in‐person physician visit and those who did not, respectively.
Costs in current Canadian dollars are based on resource intensity weights for an admission multiplied by its unit cost per fiscal year.
IQR, interquartile range.
Table 2 presents the results obtained from marginal structural survival models weighted by IPW, which correspond to the cumulative difference in the risk of readmission; 95 percent CIs show a statistically significant reduction associated with the intervention if the lower limit is >0. In‐person physician visit that occurred within 7 days of discharge resulted in approximately 68 fewer hospital readmissions per 1,000 discharges (Table 2; any physician; full sample). The reduction in hospital readmissions increased over time and was cumulatively largest by the 21st postdischarge day, with approximately 110 fewer readmissions per 1,000 discharges (Table 2). Physician visits occurring later than 21 days after discharge did not further contribute to reducing hospital readmissions, as seen by the risk reduction estimates at 30 and 60 days after discharge (105 and 88 fewer hospital readmissions per 1,000 discharges, respectively).
Table 2.
Adjusted Difference in Riska of Readmission between Patients Who Had a Postdischarge In‐Person Physician Visit and Those Who Did Not
| Adjusted Risk Difference per 1,000 Discharges (95% CIb) | ||||
|---|---|---|---|---|
| Days Since Hospital Discharge | Full Sample N = 620,656 | Morbidity Level | ||
| Moderate N = 105,948 | High N = 173,962 | Very High N = 340,746 | ||
| Follow‐up with any physician | ||||
| 7 | 67.8 (66.7–69.0) | 20.5 (19.0–21.9) | 40.7 (34.2–47.2) | 101.2 (86.6–115.9) |
| 14 | 102.5 (100.9–104.1) | 32.0 (29.9–34.1) | 63.4 (56.1–70.8) | 151.7 (135.8—167.6) |
| 21 | 110.0 (108.2–111.7) | 36.1 (33.6–38.5) | 71.5 (64.1–78.8) | 164.6 (149.2–180.0) |
| 30 | 105.2 (103.2–107.2) | 36.5 (33.8–39.3) | 72.0 (64.5–79.6) | 159.1 (143.8–174.4) |
| 60 | 87.8 (85.5–90.1) | 34.0 (30.5–37.5) | 65.0 (57.1–72.9) | 129.1 (114.4–143.8) |
| Follow‐up with a primary care physician (independent effect)c | ||||
| 7 | 69.6 (68.3–71.0) | 20.3 (17.8–22.8) | 30.3 (10.2–50.5) | 80.3 (29.0–131.7) |
| 14 | 104.4 (102.5–106.2) | 32.4 (29.7–35.1) | 61.0 (45.1–76.9) | 150.6 (114.8–186.6) |
| 21 | 113.0 (110.8–115.2) | 37.4 (34.3–40.6) | 73.8 (59.1–88.5) | 172.9 (141.5–204.2) |
| 30 | 110.3 (107.8–112.9) | 38.8 (35.1–42.5) | 76.8 (62.5–91.0) | 171.5 (142.5–200.4) |
| 60 | 97.0 (93.7–100.3) | 37.3 (32.6–41.9) | 70.1 (57.3–82.8) | 140.8 (117.1–164.4) |
| Follow‐up with a medical specialist (independent effect)d | ||||
| 7 | 55.3 (54.0–56.6) | 17.3 (15.7–19.0) | 38.0 (29.8–46.2) | 92.2 (74.0–110.4) |
| 14 | 78.5 (76.6–80.4) | 25.1 (22.6–27.6) | 50.3 (41.2–59.5) | 118.9 (99.0–138.7) |
| 21 | 79.9 (77.6–82.1) | 27.2 (24.2–30.3) | 54.0 (44.6–63.4) | 122.8 (102.8–142.9) |
| 30 | 72.5 (69.9–75.0) | 27.0 (23.5–30.5) | 53.6 (43.7–63.4) | 116.6 (96.4–136.7) |
| 60 | 55.2 (52.2–58.2) | 25.4 (20.9–29.9) | 50.3 (39.5–61.1) | 96.8 (77.0–116.6) |
| Follow‐up with a primary care physician and medical specialist (joint effect)e | ||||
| 7 | 77.6 (76.4–78.7) | 23.4 (22.0–24.8) | 67.5 (22.3–112.8) | 118.2 (51.6–184.7) |
| 14 | 125.8 (124.1–127.5) | 39.5 (37.4–41.7) | 109.6 (69.3–149.9) | 224.7 (156.6–292.7) |
| 21 | 141.0 (138.9–143.1) | 46.7 (43.0–49.4) | 116.7 (86.9–146.4) | 247.8 (194.3–301.2) |
| 30 | 136.9 (134.4–139.4) | 47.8 (44.3–51.4) | 108.5 (85.5–131.4) | 230.6 (188.0–273.3) |
| 60 | 115.1 (111.6–118.5) | 43.8 (38.4–49.2) | 87.0 (68.8–105.2) | 173.0 (140.1–205.9) |
Accounts for competing risk of death.
Clustered bootstrap 95% CIs.
Independent effect corresponding to effect of in‐person primary care physician visit had everyone not had a visit with a medical specialist within 30 days of discharge.
Independent effect corresponding to effect of in‐person physician visit with a medical specialist had everyone not had a visit with primary care physician within 30 days of discharge.
Joint effect corresponding to the reduction in the risk of readmission had everyone had a physician visit with both a medical specialist and with a primary care physician within 30 days of discharge.
We observed differences in the magnitude of risk reductions across morbidity levels. The largest was observed among patients with very high morbidity, with approximately 101 and 165 fewer hospital readmissions per 1,000 discharges if a physician visit occurred within 7 and 21 days after discharge, respectively. The risk reduction was largest at 30 days after discharge among patients in the moderate (37 fewer readmissions per 1,000 discharges) and high (72 fewer hospital readmissions per 1,000 discharges) morbidity subgroups.
The independent and joint effects by type of physician visit are also shown in Table 2. At 21 days after discharge, 80 fewer hospital readmission per 1,000 discharges were attributable to the independent effect of an in‐person physician visit with a medical specialist, and 113 fewer hospital readmissions were attributable to the independent effect of a physician visit with a primary care physician. Having a postdischarge physician visit with both led to 141 fewer readmissions by 21 days after discharge (Table 2).
Figure 2 graphs the readmission risk reduction attributable to in‐person physician as it cumulates over days after discharge, by patient morbidity level. A steeper slope means a greater contribution toward reducing hospital readmissions, while a downward slope means a negative contribution to the cumulative risk reduction. There were similar trends across three morbidity levels: (1) a statistically significant readmission risk reduction effect beginning a few days after discharge (as seen by the 95 percent confidence bands that do not overlap the 0—horizontal line); (2) a steeper positive slope occurred in the early days after discharge (approximately up to day 10), representing a larger contribution toward reducing readmissions during this early period; and (3) horizontal or downward slopes occurred later (approximately beyond day 20), representing no additional contribution. The slopes were most pronounced among very high morbidity patients, indicating even greater reduction effect of early timing (e.g., before day 10), in contrast to greater loss if in‐person physician visit occurs late (e.g., after day 20; Figure 2).
Figure 2.
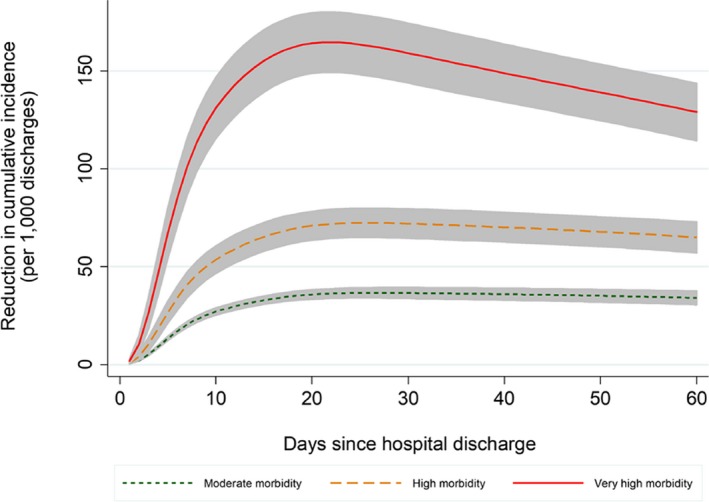
- Notes. *Accounts for competing risk of death.
Sensitivity Analyses
Diagnostics based on mean stabilized IPW, standard errors, range, and standardized differences were comparable across exposure model specifications (time‐to‐failure vs. logistic regression); both had a mean close to 1, small standard errors, and a reasonable range (Appendix SA3) (Cole and Hernan 2008; Austin and Stuart 2015). When we compared IPW estimated using time‐to‐failure models versus those estimated using logistic regression, the former showed changes in the probability of receiving postdischarge physician visit over time, whereas the latter did not (Appendix SA4). We also graphed the mean stabilized IPW by day since hospital discharge derived by logistic regression and by time‐to‐failure models. The mean IPW estimated by logistic regression: (1) were further away from 1 in the early postdischarge period (see Appendix SA4), (2) slightly overestimated the effect of a physician visit in the early postdischarge period, and (3) slightly underestimated the effect in the later postdischarge period (Appendix SA5).
Discussion
Our results show that to yield the greatest reduction in the numbers of hospital readmissions, a physician visit should occur optimally within approximately 10 days and at least within 21 days of discharge. Our findings also suggest that postdischarge care with a primary care physician rather than by a medical specialist is associated with a larger reduction in the risk of readmission.
Consistent with previous research, the “timeliness” effect of in‐person physician visit on reducing hospital readmissions was seen most strikingly in the most medically complex patients (Jackson et al. 2015). We provide more conservative estimates; Jackson et al. (2015) reported more than 20 percent‐point reduction in the risk of readmission among patients with multiple chronic conditions and more than 30 percent‐point among those with high clinical complexity. The discrepancy may be explained by different categorization of patient clinical complexity and by differences in our methodological approach, which included better adjustment for more covariates acting as important confounders, flexible modeling of time‐dependent effects, and accounting for competing risk by death.
Our findings also suggest that an in‐person visit with a primary care physician contributed more toward reducing the risk of readmission than follow‐up by a medical specialist. This adds to the small body of evidence on the association between the type of postdischarge physician follow‐up and readmission rates. Findings from one cohort study showed that follow‐up by a physician who has a longitudinal relationship to the patient was associated with lower rates of readmissions than follow‐up by a physician without such relationship (McAlister et al. 2013), while another one found no association (Hernandez et al. 2010). Another study showed that postdischarge follow‐up care by a primary care physician only and by a psychiatrist only was similarly associated with lower rates of 180‐day readmissions among patients with a mental health diagnosis; these authors, however, restricted the analysis to patients who survived or were not readmitted within 30 days of discharge (Kurdyak et al. 2014).
The primary focus of this study is on the optimal timing of in‐person physician visits; it does not provide evidence of superiority over other interventions. There is a vast evidence base in support of other interventions to reduce hospital readmissions, including nurse‐led interventions (Naylor et al. 2004; Latour et al. 2006; Chiu and Newcomer 2007; Chow et al. 2008; Bobay, Yakusheva, and Weiss 2011; Li et al. 2014), telehealth (Kirk 2014; Li et al. 2014; Soong et al. 2014; Tang, Fujimoto, and Karliner 2014; Olsen, Courtemanche, and Hodach 2015), and home health‐based interventions (Naylor et al. 1999; Robertson and Kayhko 2001). Unfortunately, our data did not allow us to account for nonphysician interventions and support for discharged patients. Further, claims data do not fully capture severity of illness and functional status, which could have biased our findings in either direction. For example, patients at very high risk of readmission due to the severity of their condition or due to functional limitations may (1) have not been able to receive follow‐up within 30 days or (2) have received home care or a phone follow‐up after discharge from hospital (which do not appear in our data); either of these scenarios would have likely biased our results away from the null. Other unmeasured factors such as mental health and peer or community support after discharge could have had a similar impact on our results. In contrast, our lack of data on nurse follow‐up after discharge could have biased our results toward the null if patients receiving follow‐up by a nurse may be less likely to see a physician and also less likely to be readmitted to the hospital. Understanding the full scope of how outpatient care affects readmission, including the role of nurses, is particularly important given the context of primary health care reforms focused on team‐based care (e.g., the patient‐centered medical home) that are being promoted as potential solutions to care fragmentation and system inefficiencies. Finally, this research primarily focused on the timing of a postdischarge visit with a physician on readmission, and we did not consider the volume or the comprehensiveness of postdischarge care, nor did we examine other undesirable postdischarge outcomes (i.e., return to the emergency department).
This study has methodological strengths. We used patient‐level data and we accounted for time‐invariant hospital differences using hospital‐level fixed effects. We further accounted for time‐dependent effects of patient's health and health utilization on the propensity to have a postdischarge physician visit using a novel approach (IPW). Our sensitivity analyses contributed evidence that this novel approach performed better than logistic regression in reducing bias in the early days after discharge (i.e., within 14 days). Further, the flexible modeling approach that we used to characterize the timeliness of follow‐up care and its time‐dependent effect on the risk of readmission allowed us to draw inference on the critical time window that provided the most benefit to patients, particularly for those with a very high level of morbidity. To date, no other study had incorporated the time dependency of outpatient follow‐up, with respect to both the exposure and the outcome. We also accounted for competing risk by death, which allows for a fully transparent interpretation of the results.
Implications for Policy and Practice
The evidence we provide supports initiatives to ensure the timeliness of in‐person follow‐up visits after discharge. Meeting the 10‐day postdischarge window appears to be optimal to help in reducing hospital readmissions, while meeting the 21‐day window appears to be critical. For patients with very high morbidity, timing is especially key and should be the target of policy and practice initiatives at the system, hospital, and community level. Such opportunities may include (but are not limited to) payment incentives for care coordination activities, supporting standardized information technologies, developing hospital/clinical guidelines for postdischarge care, developing performance indicators based on follow‐up interventions, and interprofessional collaboration. Our results also support the important role of primary care in the postdischarge period; more research is needed to better understand the role that primary care practitioners play in the care transition.
Conclusion
The timing of physician follow‐up after hospital discharge is highlighted as a key intervention point in medical care to prevent a very large number of hospital readmissions among the elderly or chronically ill. Physician follow‐up should occur as early as necessary, or ideally within 10 days, and at least within 21 days after hospital discharge. Further, primary care physician follow‐up may contribute more to reducing the risk of readmission than follow‐up with a medical specialist; future investigations to address this hypothesis are needed.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2: Models.
Appendix SA3: Description of Inverse Probability Weights*.
Appendix SA4: Sensitivity Analysis on IPW Model Specification.
Appendix SA5: Comparison of Results Obtained by Time‐Specific Approach or Conventional Approach.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: Bruno D. Riverin was funded by the Fonds de recherche du Québec – Santé – Unité SUPPORT du Québec and by a Partnership for Health Systems Improvement grant from the Canadian Institutes of Health Research. Patricia Li was funded by a Chercheur‐boursier clinicien Junior 1 from the Fonds de la Recherche du Québec–Santé and the Ministère de la Santé et des Services sociaux du Québec and a New Investigator Salary Award from the Canadian Institutes of Health Research. Erin Strumpf was funded by a Chercheur‐boursier Junior 1 from the Fonds de la Recherche du Québec–Santé and the Ministère de la Santé et des Services sociaux du Québec. Bruno D. Riverin, Patricia Li, Ashley I. Naimi, and Erin Strumpf have no financial relationships relevant to this article to disclose.
Disclosure: None.
Disclaimer: None.
References
- American Hospital Association . 2011. Examining the Drivers of Readmissions and Reducing Unnecessary Readmissions for Better Patient Care. Washington, DC: American Hospital Association. [Google Scholar]
- Austin, P. C. , and Stuart E. A.. 2015. “Moving Towards Best Practice When Using Inverse Probability of Treatment Weighting (IPTW) Using the Propensity Score to Estimate Causal Treatment Effects in Observational Studies.” Statistics in Medicine 34 (28): 3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, H. , Oliver‐McNeil S., Deng L., and Hummel S. L.. 2015. “Regional Hospital Collaboration and Outcomes in Medicare Heart Failure Patients: See You in 7.” JACC Heart Failure 3 (10): 765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobay, K. L. , Yakusheva O., and Weiss M. E.. 2011. “Outcomes and Cost Analysis of the Impact of Unit‐Level Nurse Staffing on Post‐Discharge Utilization.”Nursing Economics 29 (2): 69–78, 87. [PubMed] [Google Scholar]
- Brooke, B. S. , Stone D. H., Cronenwett J. L., Nolan B., DeMartino R. R., MacKenzie T. A., Goodman D. C., and Goodney P. P.. 2014. “Early Primary Care Provider Follow‐Up and Readmission after High‐Risk Surgery.” JAMA Surgery 149 (8): 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, R. 2012. “Health Policy Brief: Care Transitions.” Health Affairs.
- Canadian Institute of Health Information . 2015. “Physician Follow‐Up after Hospital Discharge: Progress in Meeting Best Practices” [accessed on April 21, 2018]. Available at https://secure.cihi.ca/free_products/Physician-Follow-Up-Study-mar2015_EN.pdf
- Centers for Medicare and Medicaid Services . 2013. “2013 Medicare Physician Fee Schedule.” [PubMed]
- Chiu, W. K. , and Newcomer R.. 2007. “A Systematic Review of Nurse‐Assisted Case Management to Improve Hospital Discharge Transition Outcomes for the Elderly.”Professional Case Management 12 (6): 330–6; quiz 337–338. [DOI] [PubMed] [Google Scholar]
- Chow, S. K. , Wong F. K., Chan T. M., Chung L. Y., Chang K. K., and Lee R. P.. 2008. “Community Nursing Services for Postdischarge Chronically Ill Patients.” Journal of Clinical Nursing 17 (7B): 260–71. [DOI] [PubMed] [Google Scholar]
- Cole, S. R. , and Hernan M. A.. 2008. “Constructing Inverse Probability Weights for Marginal Structural Models.” American Journal of Epidemiology 168 (6): 656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilokthornsakul, P. , Chaiyakunapruk N., Schumock G. T., and Lee T. A.. 2014. “Calendar Time‐Specific Propensity Score Analysis for Observational Data: A Case Study Estimating the Effectiveness of Inhaled Long‐Acting Beta‐Agonist on Asthma Exacerbations.” Pharmacoepidemiology and Drug Safety 23 (2): 152–64. [DOI] [PubMed] [Google Scholar]
- Dusetzina, S. B. , Mack C. D., and Sturmer T.. 2013. “Propensity Score Estimation to Address Calendar Time‐Specific Channeling in Comparative Effectiveness Research of Second Generation Antipsychotics.” PLoS ONE 8 (5): e63973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Éco‐Santé Québec . 2012. “ANNEXE 13: Les APR‐DRG V24 et le NIRRU.” [accessed on March 5, 2016]. Available at http://www.ecosante.fr/QUEBFRA/11300.html
- Erickson, K. F. , Winkelmayer W. C., Chertow G. M., and Bhattacharya J.. 2014. “Physician Visits and 30‐Day Hospital Readmissions in Patients Receiving Hemodialysis.” Journal of the American Society of Nephrology 25 (9): 2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidahussein, S. S. , Croghan I. T., Cha S. S., and Klocke D. L.. 2014. “Posthospital Follow‐Up Visits and 30‐Day Readmission Rates in Chronic Obstructive Pulmonary Disease.” Risk Management and Healthcare Policy 7: 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, T. S. , Ogarek J., Garber L., Reed G., and Gurwitz J. H.. 2015. “Association of Early Post‐Discharge Follow‐Up by a Primary Care Physician and 30‐Day Rehospitalization among Older Adults.” Journal of General Internal Medicine 30 (5): 565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, C. , Lingsma H. F., Marang‐van de Mheen P. J., Kringos D. S., Klazinga N. S., and Steyerberg E. W.. 2014. “Is the Readmission Rate a Valid Quality Indicator? A Review of the Evidence.” PLoS ONE 9 (11): e112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, M. M. , Kelley A. S., Paris J., Roza K., Meier D. E., Morrison R. S., and Aldridge M. D.. 2014. “Methods for Constructing and Assessing Propensity Scores.” Health Services Research 49 (5): 1701–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, J. , Haggerty J., Lamarche P., Levesque J. F., Morin D., Pineault R., and Sylvain H.. 2009. “Entre adaptabilité et fragilité: les conditions d'accès aux services de santé des communautés rurales et éloignées” [accessed on April 21, 2018]. Available at http://www.inspq.qc.ca/pdf/publications/1014_ConditionsAccesServSanteCommunRurales.pdf
- Hernandez, A. F. , Greiner M. A., Fonarow G. C., Hammill B. G., Heidenreich P. A., Yancy C. W., Peterson E. D., and Curtis L. H.. 2010. “Relationship between Early Physician Follow‐Up and 30‐Day Readmission among Medicare Beneficiaries Hospitalized for Heart Failure.” Journal of the American Medical Association 303 (17): 1716–22. [DOI] [PubMed] [Google Scholar]
- Hinchliffe, S. R. , and Lambert P. C.. 2013. “Flexible Parametric Modelling of Cause‐Specific Hazards to Estimate Cumulative Incidence Functions.” BMC Medical Research Methodology 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, M. , Frost S., Siu K., Quon N., and Esposito D.. 2014. “Association between Outpatient Visits Following Hospital Discharge and Readmissions among Medicare Beneficiaries with Atrial Fibrillation and Other Chronic Conditions.” American Journal of Medical Quality 29 (3): 206–12. [DOI] [PubMed] [Google Scholar]
- Jackson, C. , Shahsahebi M., Wedlake T., and DuBard C. A.. 2015. “Timeliness of Outpatient Follow‐Up: An Evidence‐Based Approach for Planning after Hospital Discharge.” Annals of Family Medicine 13 (2): 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. S. , and Flanders S. A.. 2013. “In the Clinic. Transitions of Care. “Annals of Internal Medicine 158 (5 Pt 1): ITC3‐1. [DOI] [PubMed] [Google Scholar]
- Kirk, C. 2014. “Telephone Follow‐Up of Older People after Hospital Admissions.” Current Aging Science 7 (2): 144–53. [DOI] [PubMed] [Google Scholar]
- Kripalani, S. , Theobald C. N., Anctil B., and Vasilevskis E. E.. 2014. “Reducing Hospital Readmission Rates: Current Strategies and Future Directions.” Annual Review of Medicine 65: 471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdyak, P. , Vigod S., Newman A., and Stukel T.. 2014. “Shared Care and Outcomes Following Mental Health Discharge.” Paper presented at the Canadian Association for Health Services and Policy Research, Toronto, ON.
- Lambert, P. C. , and Royston P.. 2009. “Further Development of Flexible Parametric Models for Survival Analysis.” Stata Journal 9: 265–90. [Google Scholar]
- Latour, C. H. , de Vos R., Huyse F. J., de Jonge P., van Gemert L. A., and Stalman W. A.. 2006. “Effectiveness of Post‐Discharge Case Management in General‐Medical Outpatients: A Randomized. Controlled Trial.” Psychosomatics 47 (5): 421–9. [DOI] [PubMed] [Google Scholar]
- Leschke, J. , Panepinto J. A., Nimmer M., Hoffmann R. G., Yan K., and Brousseau D. C.. 2012. “Outpatient Follow‐Up and Rehospitalizations for Sickle Cell Disease Patients.” Pediatric Blood & Cancer 58 (3): 406–9. [DOI] [PubMed] [Google Scholar]
- Li, J. , Wang H., Xie H., Mei G., Cai W., Ye J., Zhang J., Ye G., and Zhai H.. 2014. “Effects of Post‐Discharge Nurse‐Led Telephone Supportive Care for Patients with Chronic Kidney Disease Undergoing Peritoneal Dialysis in China: A Randomized Controlled Trial.” Peritoneal Dialysis International 34 (3): 278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. Y. , Barnato A. E., and Degenholtz H. B.. 2011. “Physician Follow‐up Visits after Acute Care Hospitalization for Elderly Medicare Beneficiaries Discharged to Noninstitutional Settings.” Journal of the American Geriatrics Society 59 (10): 1947–54. [DOI] [PubMed] [Google Scholar]
- Mack, C. D. , Glynn R. J., Brookhart M. A., Carpenter W. R., Meyer A. M., Sandler R. S., and Sturmer T.. 2013. “Calendar Time‐Specific Propensity Scores and Comparative Effectiveness Research for Stage III Colon Cancer Chemotherapy.” Pharmacoepidemiology and Drug Safety 22 (8): 810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitoba Centre for Health Policy . 2015. “Term: Resource Utilization Bands (RUBs)” [accessed on April 21, 2018]. Available at http://mchp-appserv.cpe.umanitoba.ca/viewDefinition.php?definitionID=104613
- McAlister, F. A. , Youngson E., Bakal J. A., Kaul P., Ezekowitz J., and van Walraven C.. 2013. “Impact of Physician Continuity on Death or Urgent Readmission after Discharge among Patients with Heart Failure.” CMAJ Canadian Medical Association Journal 185 (14): E681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misky, G. J. , Wald H. L., and Coleman E. A.. 2010. “Post‐Hospitalization Transitions: Examining the Effects of Timing of Primary Care Provider Follow‐Up.” Journal of Hospital Medicine (Online) 5 (7): 392–7. [DOI] [PubMed] [Google Scholar]
- Mittmann, N. , Liu N., Porter J., Seung S. J., Hon Isogai P. K., Saskin R., Cheung M. C., Leighl N. B., Hoch J. S., Trudeau M., Evans W. K., Dainty K. N., and Earle C. C.. 2014. “Utilization and Costs of Home Care for Patients with Colorectal Cancer: A Population‐Based Study.”CMAJ Open 2 (1): E11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muus, K. J. , Knudson A., Klug M. G., Gokun J., Sarrazin M., and Kaboli P.. 2010. “Effect of Post‐Discharge Follow‐Up Care on Re‐Admissions among US Veterans with Congestive Heart Failure: A Rural‐Urban Comparison.” Rural and Remote Health 10 (2): 1447. [PubMed] [Google Scholar]
- Naimi, A. I. , Moodie E. E., Auger N., and Kaufman J. S.. 2014. “Constructing Inverse Probability Weights for Continuous Exposures: A Comparison of Methods.” Epidemiology 25 (2): 292–9. [DOI] [PubMed] [Google Scholar]
- Naylor, M. D. , Brooten D., Campbell R., Jacobsen B. S., Mezey M. D., Pauly M. V., and Schwartz J. S.. 1999. “Comprehensive Discharge Planning and Home Follow‐Up of Hospitalized Elders: A Randomized Clinical Trial.” Journal of the American Medical Association 281 (7): 613–20. [DOI] [PubMed] [Google Scholar]
- Naylor, M. D. , Brooten D. A., Campbell R. L., Maislin G., McCauley K. M., and Schwartz J. S.. 2004. “Transitional Care of Older Adults Hospitalized with Heart Failure: A Randomized. Controlled Trial.” Journal of the American Geriatrics Society 52 (5): 675–84. [DOI] [PubMed] [Google Scholar]
- Olsen, R. , Courtemanche T., and Hodach R.. 2015. “Automated Phone Assessments and Hospital Readmissions.” Population Health Management 19: 120–4. [DOI] [PubMed] [Google Scholar]
- Pampalon, R. 2003. “Indice de défavorisation matérielle et sociale: son application au secteur de la santé et du bien‐être. SANTÉ, SOCIÉTÉ ET SOLIDARITÉ, 1.”
- Pampalon, R. , Gamache P., and Hamel D.. 2010. “Indice de défavorisation matérielle et sociale du Québec: Suivi méthodologique de 1991 à 2006 “[accessed on April 21, 2018]. Available at Québec: http://www.inspq.qc.ca/pdf/publications/1176_IndiceDefavorisation1991A2006.pdf
- PriceWaterhouse Coopers’ Health Research Institute . 2008. “The Price of Excess: Identifying Waste in Healthcare, 2008 “[accessed on March 5, 2016]. Available at http://www.pwc.com/us/en/healthcare/publications/the-price-of-excess.jhtml
- Régie de l'assurance maladie du Québec . 2006. “4. Ententes particulières. Brochure 1 ‐ Omnipraticiens. No. Seq. 33.”
- Régie de l'assurance maladie du Québec . 2013. Rapport d’études et statistics St@tRAMQ [accessed on April 21, 2018]. Available at https://www4.prod.ramq.gouv.qc.ca/IST/CD/CDF_DifsnInfoStats/CDF1_CnsulInfoStatsCNC_iut/DifsnInfoStats.aspx?ETAPE_COUR=2&LANGUE=fr-CA
- Robertson, K. A. , and Kayhko K.. 2001. “Cost Analysis of an Intensive Home Follow‐Up Program for First‐Time Post‐Myocardial Infarction Patients and Their Families.” Dynamics 12 (4): 25–31. [PubMed] [Google Scholar]
- Royston, P. , and Lambert P. C.. 2011. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, TX: Stata Press. [Google Scholar]
- Royston, P. , and Parmar M. K.. 2002. “Flexible Parametric Proportional‐Hazards and Proportional‐Odds Models for Censored Survival Data, with Application to Prognostic Modelling and Estimation of Treatment Effects.” Statistics in Medicine 21: 2175–97. [DOI] [PubMed] [Google Scholar]
- Saunders, R. S. , Fernandes‐Taylor S., Rathouz P. J., Saha S., Wiseman J. T., Havlena J., Matsumura J., and Kent K. C.. 2014. “Outpatient Follow‐Up versus 30‐Day Readmission among General and Vascular Surgery Patients: A Case for Redesigning Transitional Care.” Surgery 156 (4): 949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams, I. , Ajorlou S., and Yang K.. 2012. “A Predictive Analytics Approach to Reducing Avoidable Hospital Readmission (Vol. 1402.5991): arXiv.”
- Sharma, G. , Kuo Y. F., Freeman J. L., Zhang D. D., and Goodwin J. S.. 2010. “Outpatient Follow‐Up Visit and 30‐Day Emergency Department Visit and Readmission in Patients Hospitalized for Chronic Obstructive Pulmonary Disease.” Archives of Internal Medicine 170 (18): 1664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin, D. D. , Bell N. R., Svenson L. W., and Man S. F.. 2002. “The Impact of Follow‐Up Physician Visits on Emergency Readmissions for Patients with Asthma and Chronic Obstructive Pulmonary Disease: A Population‐Based Study.” American Journal of Medicine 112 (2): 120–5. [DOI] [PubMed] [Google Scholar]
- Sommers, A. , and Cunningham P. J.. 2011. “Physician Visits after Hospital Discharge: Implications for Reducing Readmissions” [accessed on March 5, 2016]. Available at http://www.nihcr.org/Reducing_Readmissions.html
- Soong, C. , Kurabi B., Wells D., Caines L., Morgan M. W., Ramsden R., and Bell C. M.. 2014. “Do Post Discharge Phone Calls Improve Care Transitions? A Cluster‐Randomized Trial.” PLoS ONE 9 (11): e112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Fujimoto J., and Karliner L.. 2014. “Evaluation of a Primary Care‐Based Post‐Discharge Phone Call Program: Keeping the Primary Care Practice at the Center of Post‐Hospitalization Care Transition.” Journal of General Internal Medicine 29 (11): 1513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Johns Hopkins ACG Case Mix System . (2003). Version 6.0 Release Notes (Vol. PC (DOS/WIN/NT) and Unix Version 6.0 – April, 2003). Baltimore: Johns Hopkins Bloomberg School of Public Health. [Google Scholar]
- Thygesen, L. C. , Fokdal S., Gjorup T., Taylor R. S., Zwisler A. D., and Prevention of Early Readmission Research G . 2015. “Can Municipality‐Based Post‐Discharge Follow‐Up Visits Including a General Practitioner Reduce Early Readmission among the Fragile Elderly (65 + Years Old)? A Randomized Controlled Trial.” Scandinavian Journal of Primary Health Care 33 (2): 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Walraven, C. , Jennings A., Taljaard M., Dhalla I., English S., Mulpuru S., Blecker S., and Forster A. J.. 2011. “Incidence of Potentially Avoidable Urgent Readmissions and Their Relation to All‐Cause Urgent Readmissions.” CMAJ 183 (14): E1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: Models.
Appendix SA3: Description of Inverse Probability Weights*.
Appendix SA4: Sensitivity Analysis on IPW Model Specification.
Appendix SA5: Comparison of Results Obtained by Time‐Specific Approach or Conventional Approach.


