Abstract
Objective
To examine the relationship between antihypertensive drug deintensification and recurrent falls in long‐term care.
Data Sources/Settings
Department of Veterans Affairs (VA) inpatient, outpatient, and purchased care data, Minimum Data Set assessments from VA nursing homes (NHs), and Medicare claims from fiscal years 2010 – 2015.
Study Design
We identified NH residents with evidence of overaggressive antihypertensive treatment, defined as systolic blood pressure (SBP) 80–120 and an index fall. Recurrent fall, hospitalization, and mortality within 30 days were compared between veterans whose antihypertensive medications were deintensified versus those whose antihypertensive medications were not using propensity score methods (PSM).
Principal Findings
Among 2,212 NH residents with possibly overaggressive antihypertensive treatment, 11 percent experienced antihypertensive drug deintensification. Lower blood pressure, >1 antihypertensive drug, no congestive heart failure, fracture from index fall, and older age were associated with higher likelihood of deintensification. Antihypertensive deintensification was associated with statistically significant (p‐value < .01) lower risk of recurrent fall among residents with SBP 80–100 (marginal effect = −11.4 percent; PSM = −13.6 percent) and higher risk of death among residents with SBP 101–120 (marginal effect = 2.1 percent, p‐value = .07; with PSM = 4.3 percent, p‐value = .04).
Conclusions
Results provide some needed evidence and guidelines for deintensifying antihypertensive medication among frail older residents; since hypertension is prevalent among 54 percent of NH residents, the potential impact of new evidence is great.
Keywords: Hypertension, drug deintensification, fall prevention, long‐term care
Although some trials suggest better outcomes with hypertension treatment in older adults(Beckett et al. 2008; Williamson et al. 2016), it is unclear whether frail older adults in nursing homes (NHs) experience a net benefit or net harm from aggressive hypertension management. On the one hand, NH residents may benefit from excellent control of hypertension through a decrease in adverse cardiovascular events (Davis et al. 2017; Manning and Wolfson 2017). On the other hand, NH residents may be harmed by orthostatic hypotension, syncope, presyncope, and falls (Finucane et al. 2017; Sexton et al. 2017; Williamson et al. 2016), with such harms leading to higher long‐term mortality risk (Benetos et al. 2015).
As a result, medication review and deintensification (“deprescribing”) are a component of many NH (and geriatrics) safety improvement strategies (Wouters et al. 2017). Deprescribing interventions commonly promote deintensification of medications for chronic conditions (e.g., diabetes, hypertension) when there are signs that treatment is overaggressive (Bain et al. 2008; Scott et al. 2013). Although there is growing evidence to support less aggressive use of antidiabetic medication in older adults in NHs (Hsu et al. 2017; Lipska et al. 2015), evidence regarding less aggressive use of antihypertensive medication is less developed, and parameters for when hypertension treatment should be considered overaggressive are not established.
The objectives of this study were, in an observational cohort of frail older NH residents, to 1) describe the frequency of antihypertensive deintensification during scenarios suggesting overaggressive treatment, 2) identify characteristics of residents associated with antihypertensive deintensification, and 3) examine the association between antihypertensive deintensification and subsequent falls.
Our conceptual framework depicted in Figure 1 identifies three general factor types that would motivate deintensification. Situational or precipitating factors are those acute conditions that are triggers to action. Those include occurrence of low blood pressure, adverse drug effects such as falls and acute change in health such as a new or exacerbated illness, behavioral symptoms, or decline in cognitive or physical function. Situational or precipitating factors are mediated by resident underlying condition including the stage of life‐limiting disease, concurrent chronic conditions, and general frailty along with ongoing treatments such as polypharmacy. Beyond those resident characteristics, NH culture and behavior and physician or advanced practice practitioner practices are likely to provide a context in which deintensification decisions are made. We hypothesized that medication deintensification would be associated with resident characteristics that reflect less potential benefit and greater potential harm from treatment, namely older age and greater disease or disability burden. We further hypothesized that after adjusting for differences, antihypertensive deintensification would be associated with fewer subsequent falls, since falls are a possible adverse drug effect of antihypertensive medication use in our conceptual framework (Figure 1).
Figure 1.
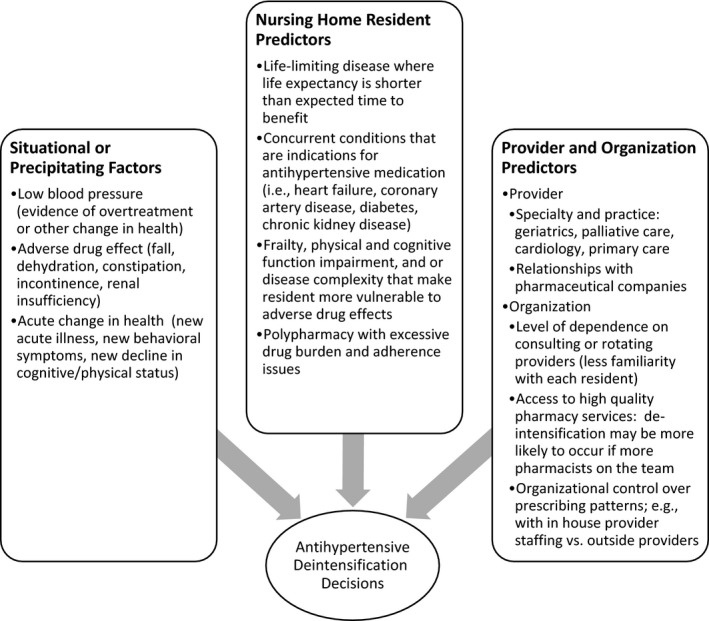
Factors That Could Influence Antihypertensive Deintensification Decisions
Methods
Data
We used the Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) and other sources to obtain fiscal years 2010–15 data on vital signs (including blood pressure values, height, and weight); vital status and enrollment data; VA inpatient and NH (also called Community Living Center) stays; outpatient encounters; and ICD‐9‐CM (the international classification of diseases, ninth revision, clinical modification) diagnoses noted in those records. We also used Medicare inpatient, outpatient, hospice, and carrier file claims to identify Medicare‐paid utilization and to extract diagnoses. Medication doses ordered and given were captured with high completeness by VA's CDW bar‐coded medication administration data. We obtained Minimum Data Set (MDS) NH resident assessments (MDS version 2.0 until June 2012 and MDS version 3.0 from July 2013) from the VA Office of Geriatrics and Extended Care. MDS assessments were conducted at VA NH admission, quarterly, and at the time of a change in a resident's condition. MDS assessment items included cognitive function, physical function, continence, psychosocial well‐being, diagnoses, health conditions, communication/hearing problems, nutritional status, and medication exposure. Trained clinical staff complete the MDS based on direct observations of residents for whom they are responsible (Morris et al. 1992). Studies have established the reliability, internal consistency, and validity of commonly used MDS items (Abt Associates, Inc. 2001; Mor et al. 2011); interrater reliability is adequate or better (kappa ≥ 0.40) for 88 percent of the items (Sgadari et al. 1997).
Inclusion Criteria
We identified long‐stay residents treated for hypertension residing in all 132 VA NHs. To be included, residents had to be ≥65 years old, have a stay ≥91 days between 10/01/09 and 09/30/15, and have at least 1 year of prior VA enrollment. For individuals who had more than 1 NH stay separated by time in the community, we included only the longest stay. To be included, residents had to have a diagnosis of hypertension, as defined by an ICD‐9‐CM code 401.xx‐405.xx or item I.1.h checked in MDS 2.0 or item I0700 in MDS 3.0 in the 1 year before baseline(Simonson, Han, and Davidson 2011), and be treated with at least one first‐line antihypertensive medication (thiazide diuretic, calcium channel blocker, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, or beta‐blocker) according to a major U.S. guideline (James et al. 2014).
Included individuals also fulfilled criteria for an index clinical scenario in which antihypertensive deintensification might have been indicated. We characterized this scenario as a low blood pressure and a fall within 3 days of each other, suggesting clinically significant low blood pressure (Figure 2). We identified the lowest systolic blood pressure (SBP) measurement of each day and defined low blood pressure as a SBP value between 80 and 120 (Sussman et al. 2015), with SBP 80–100 as definitely low blood pressure and SBP 101–120 as possibly low blood pressure. We defined a fall as an ICD‐9‐CM code E880‐E888 during VA NH stay or in emergency department encounter. We excluded falls associated with hospital admission as index events because we wanted to examine scenarios in which antihypertensive prescribing decisions were determined by chronic blood pressure management considerations rather than acute blood pressure management or other acute cardiovascular disease management considerations.
Figure 2.
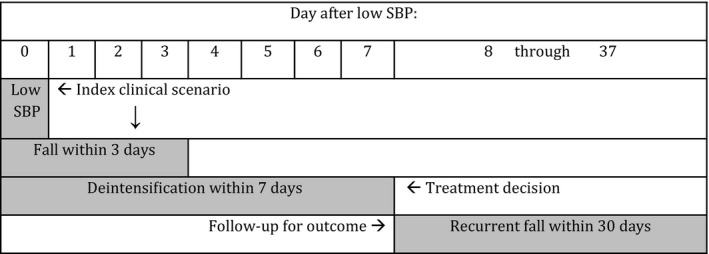
Study Design. The Index Date was the Date of Low Blood Pressure, Followed by a Fall within 3 Days Note. A Treatment Decision Period of 7 Days in Which Deintensification Could Have Occurred Began on the Index Date. An Outcome Period of 30 Days Began at the End of the Treatment Decision Period.
Key Measures
The index date was the date of measurement of low blood pressure. We defined antihypertensive deintensification as discontinuation of 1 or more first‐line hypertension medications without substitution within 7 days of the index date (Figure 2). The primary health outcome was recurrent fall, defined as an ICD‐9‐CM code of E880‐E888 in any encounter within 30 days of the end of the treatment decision period (Figure 2). Secondary outcomes were hospital admission and mortality within 30 days of the end of the treatment decision period. In a sensitivity analysis, we examined as an outcome hospitalizations due to cardiovascular disease as a possible adverse consequence of antihypertensive deintensification, using ICD‐9‐CM codes for malignant hypertension, coronary artery disease, myocardial infarction, heart failure, peripheral vascular disease, and cerebrovascular disease (see Appendix SA2: Table S5 for description of ICD‐9‐CM codes).
Covariates
We included in our models variables representing factors that could influence deintensification decisions (Figure 1) and factors that could influence the occurrence of primary and secondary outcomes. Covariates included demographics (age, gender, race), aggregate Elixhauser comorbidity score (Elixhauser et al. 1998), individual conditions that might affect prescribing decisions for antihypertensive medications (congestive heart failure, kidney failure, diabetes, ischemic heart disease, cardiac arrhythmias, and chronic pulmonary disease), and limited life expectancy (hospice classification or prognosis of <6 months (MDS 2.0 item P.1.a.o./MDS 3.0 items O0100 and J1400)). We identified dementia if item I.1.q. or I.1.u. in MDS 2.0 or item I4200 or I4800 in MDS 3.0 was checked or if we found a diagnosis from a list of ICD‐9‐CM codes (290.xx, 291.2, 292.82, 294.1x, 294.8, 331.0–331.2, and 332.83) in the MDS or in VA or Medicare data in the 1 year before the index date. For cognitive function, the MDS Cognitive Performance Scale (CPS; Morris et al. 1994) uses five MDS items assessing consciousness, short‐term memory, decision making skills, communication ability, and ability to feed oneself, to create a hierarchical cognitive scale from 0 (no impairment) to 6 (severely impaired). The CPS has excellent reliability with estimates in the range of 0.66–0.88, and high sensitivity (>90 percent) and specificity (>85 percent) using the Mini‐mental state examination as the gold standard (Hartmaier et al. 1994; Hartmaier et al. 1995). The Cognitive Function Scale (CFS; Thomas et al. 2017) combines the CPS with results from the Brief Interview of Mental Status (Chodosh et al. 2008). For physical function, the MDS Activities of Daily Living (ADL)‐Long Form scale(Morris, Fries, and Morris 1999) employs seven MDS items assessing ability to perform the tasks of self‐hygiene, dressing, toileting, transfer, locomotion, bed mobility, and eating. Each item is scored from independent to totally dependent (0–4) and the scores are summed for a total scale range of 0–28. This scale was shown to predict average daily minutes of care provided by nursing assistants(Morris, Fries, and Morris 1999). The transfer, locomotion, and bed mobility items identify residents who were completely nonambulatory and/or totally dependent on staff assistance for transfer and mobility, and thus potentially less likely to fall. We also considered as covariates medication regimen (number of antihypertensive drugs, number of other drugs, and drug class indicators) on the index date and other drug classes associated with falls (antipsychotics, antianxiety, antidepressants, neuromotor, and nonselective antihistamines) received during 7 days of index date. Finally, we also considered as covariates characteristics of the index clinical scenario, including SBP 80–100 versus 101–120, number of days between low SBP and fall, fracture associated with the index fall (using same‐day ICD‐9‐CM codes), and a significant worsening of health status measured by the use of antibiotics, intravenous/intramuscular (IV/IM) medication, and/or presence of a “significant change in status” MDS assessment. Covariates representing cognitive and physical function, medication utilization, and characteristics of the index clinical scenario were ascertained up to and including day 7 following the index date in order to capture up‐to‐date resident health and treatment status at the beginning of the follow‐up period.
Analysis
To describe antihypertensive deintensification, we compared the distribution of covariates among veterans who did or did not experience antihypertensive deintensification within 7 days of the index date.
Multivariable logistic regression was employed to examine the association between antihypertensive deintensification and resident index date SBP (80–100 vs. 101–120), age, comorbidities, limited life expectancy, physical and cognitive impairments, clinical conditions, number of drugs, drug class indicators, fracture associated with the index fall, markers of significant worsening of health status, and number of days between low SBP and index fall. Odds ratio and 95 percent confidence interval were calculated for each predictor variable.
We estimated separate multivariable logistic regressions for each of three outcomes: recurrent fall, hospital admission, and mortality. The independent variable was antihypertensive deintensification. A priori we hypothesized that the impact of deintensification would be different among residents with different degrees of low SBP, so we also included an interaction term between the two groups of low SBP (80–100 vs. 101–120) and the treatment (identifier of antihypertensive deintensification). Models controlled for other resident characteristics. In a sensitivity analysis, we applied logistic regression models only to residents who were not lost to follow‐up during the 30‐day follow‐up period, in order to address the concern that a difference in falls was due to incomplete follow‐up when a veteran died or was discharged from the NH or end of study period.
To further optimize matching in this observational study, we conducted propensity score analyses with scaled Kernel weighting to balance the observed individual characteristics between treatment group (deintensification) and comparison group (no deintensification) (D'Agostino 1998; Garrido et al. 2014; Rubin 1997). The average effect of deintensification on individuals who were deintensified (average effect on the treated) was estimated using Kernel weighted logistic regression. Confidence intervals were obtained by bootstrapping (Garrido et al. 2014).
Results
The study identified 2,212 veterans who were long‐stay residents of VA NHs who fulfilled inclusion criteria and experienced an index clinical scenario. Study veterans’ characteristics are presented in Table 1. Veterans were 80.5 years old on average, 97.7 percent male and 85.4 percent white. They had moderate physical function impairment (average ADL score 12.9 of 28 maximum—indicating assistance needed in most ADLs), 57.7 percent had a diagnosis of dementia, and 11.4 percent were on hospice or had a <6‐month life expectancy. Forty‐six percent took two or more antihypertensive medications at baseline. In the 7 days after the index date, 239 (10.8 percent) had an antihypertensive medication discontinued and were classified as having experienced antihypertensive deintensification.
Table 1.
Description of Resident Outcomes, Antihypertensive Drug Utilization, and Factors Potentially Impact Deintensification Decisions (See Figure 1)
| All Residents | Among Deintensified Residents | Among Non‐Deintensified Residents | p‐Value | |
|---|---|---|---|---|
| (N = 2212) | (N = 239, 10.8%) | (N = 1973, 89.2%) | ||
| Outcomes in the 30‐day follow‐up | ||||
| Fall | 18.8 | 17.2 | 19.0 | .50 |
| Death | 3.0 | 5.4 | 2.1 | <0.01 |
| Hospitalization | 13.4 | 17.6 | 13.1 | .06 |
| Antihypertensive drug utilization | ||||
| Number of antihypertensive medications prescribeda (%) | ||||
| One | 53.8 | 37.7 | 55.8 | <.0001 |
| Two or more | 46.2 | 62.3 | 44.3 | |
| Antihypertensive drug classes prescribeda (%) | ||||
| Angiotensin converting enzyme inhibitor | 43.3 | 55.2 | 41.8 | <.0001 |
| Angiotensin receptor blocker | 7.1 | 8.4 | 7.0 | .44 |
| Beta‐blocker | 67.7 | 62.3 | 68.3 | .06 |
| Calcium channel blocker | 31.2 | 39.8 | 30.1 | .00 |
| Thiazide diuretic | 8.3 | 15.1 | 7.5 | <.0001 |
| Situational or precipitating factors | ||||
| Number of Nonhypertensive drugsa | 11.5 | 11.3 | 11.6 | .42 |
| Other drug classes prescribedb (%) | ||||
| Antipsychotics | 37.3 | 41.4 | 36.9 | .17 |
| Antianxiety | 33.7 | 43.5 | 32.5 | .00 |
| Antidepressants | 68.2 | 63.6 | 68.7 | .11 |
| Neuromotor | 40.6 | 43.1 | 40.3 | .40 |
| Antihistamines (nonselective) | 11.6 | 17.6 | 10.9 | .00 |
| Cardiovascular | 50.5 | 57.3 | 49.7 | .03 |
| Intravenous or intramuscular drugs | 1.8 | 2.9 | 1.7 | .17 |
| Antibiotics | 14.7 | 13.4 | 14.9 | .55 |
| Low SBP 80–100 (%) | 20.5 | 26.4 | 19.8 | .02 |
| Days from low SBP to index fall (%) | ||||
| 0 | 21.7 | 18.8 | 22 | .14 |
| 1 | 14.6 | 12.6 | 14.8 | |
| 2 | 19.0 | 16.7 | 19.3 | |
| 3 | 44.8 | 51.9 | 43.9 | |
| Significant change in statusc | 1.3 | 2.5 | 1.1 | .07 |
| Fractured | 25.7 | 39.8 | 24 | <.0001 |
| Resident predictors | ||||
| Age (years) | 80.5 | 81.1 | 80.4 | .22 |
| Male gender (%) | 97.7 | 96.2 | 97.8 | .13 |
| Race (%) | ||||
| White | 85.4 | 86.2 | 85.3 | .49 |
| Black or African American | 11.4 | 9.6 | 11.6 | |
| Other | 3.3 | 4.2 | 3.1 | |
| Comorbidity score (Elixhauser) | 6.3 | 6.2 | 6.3 | .79 |
| Chronic conditions (%) | ||||
| Congestive heart failure | 39.5 | 32.2 | 40.3 | .02 |
| Kidney failure | 35.0 | 37.7 | 34.7 | .36 |
| Diabetes | 50.8 | 48.5 | 51.1 | .46 |
| Ischemic heart disease | 53.2 | 49.8 | 53.6 | .26 |
| Cardiac tachy‐arrhythmias | 41.0 | 41.8 | 40.9 | .78 |
| Cardiac brady‐arrhythmias | 25.5 | 29.7 | 25 | .12 |
| Chronic pulmonary disease | 39.9 | 36.4 | 40.3 | .25 |
| Dementia | 57.7 | 53.1 | 58.3 | .13 |
| Physical function (ADL score) | 12.9 | 13.2 | 12.9 | .58 |
| Cognitive performancee | ||||
| CPS 0–2; CFS 1–2 | 58.5 | 58.7 | 58.5 | .31 |
| CPS 3–4; CFS 3 | 29.7 | 26.5 | 30 | |
| CPS 5–6; CFS 4 | 11.8 | 14.8 | 11.5 | |
| Hospice or <6 months life expectancy (%) | 11.4 | 11.7 | 11.3 | .87 |
Drugs prescribed on the day of the index low SBP event.
Drugs prescribed within 7 days after the index low SBP event.
Nursing home resident assessment for significant change in health status after index fall and within 7 days after the index low SBP event.
Fracture identified using ICD‐9‐CM diagnosis on the day of index fall.
CPS‐Cognitive Performance Scale from nursing home resident assessment MDS2.0; CFS‐Cognitive Function Scale from nursing home resident assessment MDS3.0.
ADL, Activities of Daily Living.
In a multivariable logistic regression model with antihypertensive drug deintensification as the dependent variable (Table 2), older age, SBP 80–100 (as compared to 101–120), no diagnosis of congestive heart failure, diagnosis of cardiac brady‐arrhythmias, occurrence of fracture associated with the index fall, and receipt of >1 antihypertensive drug were associated with a higher likelihood of experiencing antihypertensive drug deintensification, and in particular angiotensin converting enzyme inhibitor and thiazide diuretics. There were no significant associations between antihypertensive drug deintensification and dementia status, physical function, aggregate comorbidity, or hospice/limited life expectancy. In addition, residents in the deintensification group were more likely to receive 2 classes of medication associated with falling: antianxiety medications and nonselective antihistamines.
Table 2.
Logistic Regression Model Results of the Association between Resident Characteristics and Antihypertensive Drug Deintensification (N = 2212)
| Characteristics | Odds Ratio | SE | 95% CI | p‐Value | |
|---|---|---|---|---|---|
| Antihypertensive drug utilizationa | |||||
| Number of antihypertensive medications prescribed | |||||
| One | Reference | ||||
| Two or more | 1.92 | 0.32 | 1.02 | 3.63 | .04 |
| Antihypertensive drug classes prescribeda | |||||
| Angiotensin converting enzyme inhibitor | 1.67 | 0.28 | 0.98 | 2.87 | .06 |
| Angiotensin receptor blocker | 1.18 | 0.37 | 0.57 | 2.43 | .66 |
| Beta‐blocker | 0.71 | 0.28 | 0.41 | 1.23 | .22 |
| Calcium channel blocker | 1.15 | 0.25 | 0.70 | 1.88 | .58 |
| Thiazide diuretic | 1.96 | 0.28 | 1.13 | 3.42 | .02 |
| Situational or precipitating factors | |||||
| Number of Nonhypertensive drugsa | 0.97 | 0.02 | 0.92 | 1.01 | .11 |
| Other drugs prescribedb | |||||
| Antipsychotics | 1.14 | 0.18 | 0.80 | 1.64 | .47 |
| Antianxiety | 1.76 | 0.18 | 1.25 | 2.49 | .00 |
| Antidepressants | 0.79 | 0.18 | 0.56 | 1.13 | .20 |
| Neuromotor | 1.31 | 0.18 | 0.93 | 1.85 | .12 |
| Antihistamines (nonselective) | 1.64 | 0.23 | 1.05 | 2.57 | .03 |
| Cardiovascular | 1.30 | 0.17 | 0.92 | 1.82 | .13 |
| IV or IM drugs | 1.44 | 0.48 | 0.57 | 3.68 | .44 |
| Antibiotics | 0.75 | 0.24 | 0.47 | 1.20 | .22 |
| Low SBP 80–100 | 1.66 | 0.19 | 1.14 | 2.41 | .01 |
| Days from low SBP to index fall | |||||
| 0 | Reference | ||||
| 1 | 1.09 | 0.27 | 0.63 | 1.86 | .77 |
| 2 | 1.24 | 0.26 | 0.74 | 2.07 | .41 |
| 3 | 1.62 | 0.22 | 1.06 | 2.48 | .03 |
| Significant change in statusc | 1.91 | 0.53 | 0.67 | 5.39 | .22 |
| Fractured | 2.53 | 0.18 | 1.77 | 3.62 | <.0001 |
| Resident predictors | |||||
| Age (years) | 1.03 | 0.01 | 1.01 | 1.05 | .01 |
| Female gender | 1.52 | 0.46 | 0.61 | 3.77 | .37 |
| Race | |||||
| White | Reference | ||||
| Black or African American | 0.76 | 0.28 | 0.44 | 1.31 | .32 |
| Other | 1.55 | 0.42 | 0.69 | 3.50 | .29 |
| Comorbidity score (Elixhauser) | 1.06 | 0.04 | 0.97 | 1.16 | .17 |
| Chronic conditions | |||||
| Congestive heart failure | 0.54 | 0.21 | 0.36 | .82 | .00 |
| Kidney failure | 1.24 | 0.19 | 0.86 | 1.78 | .26 |
| Diabetes | 0.86 | 0.18 | 0.61 | 1.22 | .40 |
| Ischemic heart disease | 1.04 | 0.18 | 0.72 | 1.49 | .84 |
| Cardiac tachy‐arrhythmias | 1.17 | 0.19 | 0.81 | 1.69 | .40 |
| Cardiac brady‐arrhythmias | 1.47 | 0.19 | 1.01 | 2.14 | .04 |
| Chronic pulmonary disease | 0.89 | 0.18 | 0.62 | 1.27 | .52 |
| Dementia | 0.76 | 0.20 | 0.51 | 1.11 | .15 |
| Physical function (ADL score) | 1.00 | 0.01 | 0.98 | 1.03 | .83 |
| Cognitive performance[Link] | |||||
| CPS 0–2; CFS 1–2 | Reference | ||||
| CPS 3–4; CFS 3 | 1.02 | 0.22 | 0.67 | 1.55 | .93 |
| CPS 5–6; CFS 4 | 1.42 | 0.29 | 0.81 | 2.49 | .22 |
| Hospice or <6 months life expectancy | 1.18 | 0.24 | 0.73 | 1.90 | .50 |
CPS‐Cognitive Performance Scale from nursing home resident assessment MDS2.0; CFS‐Cognitive Function Scale from nursing home resident assessment MDS3.0.
Drugs prescribed on the day of the index low SBP event.
Drugs prescribed within 7 days after the index low SBP event.
Nursing home resident assessment for significant change in health status after index fall and within 7 days after the index low SBP event.
Fracture identified using ICD‐9‐CM diagnosis on the day of index fall.
ADL, Activities of Daily Living; IM, intramuscular; IV, intravenous.
In multivariable logistic regression models (not propensity weighted), the associations between antihypertensive deintensification and recurrent fall (the primary outcome) varied by SBP levels (coefficient of interaction term between level of SBP (80–100 vs. 101–120) and antihypertensive deintensification = −1.20; p‐value = .04; see Appendix SA2: Table S1). Thus, we present results stratified by SBP level. Among veterans with SBP 80–100, antihypertensive deintensification was associated with a lower risk of falling (adjusted marginal effect (AME) = −11.4 percent; p‐value < .01) but not statistically significantly associated with risk of death (AME = 2.6 percent; p‐value = .23) or hospitalization (AME = 1.4 percent; p‐value = .76) (Table 3). Among residents with SBP 101–120, antihypertensive deintensification was not statistically significantly associated with risk of falling (AME = −0.7 percent; p‐value = .82) or hospitalization (AME = 1.5 percent; p‐value = .54), but was marginally associated with increased risk of death (AME = 2.1 percent; p‐value = .07) (Table 3). The sensitivity analyses with residents not lost to follow‐up produced results consistent with these results (see Appendix SA2: Table S2 for full model estimates). Antihypertensive deintensification was not associated with hospitalization due only to cardiovascular disease (see Appendix SA2: Table S4 for full model estimates).
Table 3.
Separate Logistic Regression Model Results of the Association between Deintensification and Fall, Death, and Hospitalization in Veterans with SBP 80–100 and SBP 100–120, Controlling for Covariates. See Appendix SA2: Tables S1 and S3 for Full Model Estimates
| Subgroup | Outcome (%) | Deintensification | Multivariable Adjusteda | Propensity Weighteda | |||||
|---|---|---|---|---|---|---|---|---|---|
| % Among “Yes” | % Among “No” | Marginal Effect % | SE % | p‐Value | Marginal Effect % | SE % | p‐Value | ||
| SBP 80–100 (N = 453) | Fall | 6.4 | 17.7 | −11.4 | 0.03 | <.01 | −13.6 | 0.04 | <.01 |
| Death | 7.9 | 3.1 | 2.6 | 0.02 | .23 | 2.3 | 0.03 | .47 | |
| Hospitalization | 17.5 | 14.6 | 1.4 | 0.04 | .76 | 0.7 | 0.06 | .90 | |
| SBP 101–120 (N = 1759) | Fall | 21.0 | 19.3 | −0.7 | 0.03 | .82 | 0.9 | 0.03 | .79 |
| Death | 6.8 | 2.3 | 2.1 | 0.01 | .07 | 4.3 | 0.02 | .04 | |
| Hospitalization | 17.6 | 12.7 | 1.5 | 0.02 | .54 | 2.4 | 0.03 | .41 | |
Controlling for demographics, cognitive and physical function, hospice, comorbidity, drug class, use of antibiotics and medication, significant change in health status and fracture associated with index fall (see Appendix SA2: Tables S1 and S3 for full model estimates).
In a propensity score analysis, resident characteristics were balanced between the treatment (deintensification) and comparison groups after Kernel weighting (bandwidth 0.02). The mean standardized percentage bias remaining between the two groups after weighting was <5 percent for all resident characteristics (see Appendix SA2: Figure S1). Among residents with SBP 80–100, antihypertensive deintensification was associated with a lower probability of falling (weighted marginal effect (WME) = −13.6 percent; p‐value < .01) but not associated with the probability of death (WME = 2.3 percent; p‐value = .47) or hospitalization (WME = 0.7 percent; p‐value = .90) (Table 3). Among residents with SBP 101–120, antihypertensive deintensification was associated with a higher probability of death (WME = 4.3 percent; p‐value = .04) but not associated with the probability of falling (WME = 0.9 percent; p‐value = .79) or hospitalization (WME = 2.4 percent; p‐value = .41) (Table 3).
Discussion
We found that among NH residents who were diagnosed with hypertension and received antihypertensive medication, 11 percent underwent antihypertensive deintensification during a clinical scenario (low SBP and a fall) suggesting overaggressive treatment. Older residents, those with lower SBP, more antihypertensive prescriptions, fracture associated with the index fall and absence of congestive heart failure were more likely to experience antihypertensive deintensification. However, most resident predictors including end‐of‐life status, physical function impairment, and dementia diagnosis were not associated with the likelihood of deintensification. Among residents with definitely low SBP (80–100), antihypertensive deintensification was associated with lower risk of falling but not associated with risk of hospitalization or death. Among residents with possibly low SBP (101–120), antihypertensive deintensification was associated with higher risk of death but not associated with risk of falling or hospitalization, suggesting an SBP of 100 as a possible threshold for distinguishing benefit (at SBP ≤ 100) from harm (at SBP > 100) from antihypertensive deintensification.
There are several possible explanations that clinicians did not commonly respond to evidence of overaggressive treatment of hypertension in this study's population. First, a provider may have been unaware of the low SBP and/or fall because of incomplete nurse‐provider communication. Second, residents may have had other compelling indications for treatment with the studied medication classes (e.g., congestive heart failure). Third, providers face a lack of evidence and guidelines for deintensifying antihypertensive medication among frail older residents. Our study found that deintensification decisions were tailored to some prognostic and health factors, such as lower SBP, older age, and absence of congestive heart failure, but surprisingly not to function and end‐of‐life status. This is consistent with a study that showed that diabetes treatment intensity was not tailored to functional status in older adults, despite the harms of intensive treatment likely exceeding the benefits in those with significant disability (Lipska et al. 2015). In addition, prescribing practice in NHs is influenced not only by resident characteristics but also by organization and practice patterns not measured in this study that may outweigh resident factors (Carter, Montpetite, and Jump 2017).
Our finding of an association between antihypertensive deintensification and fewer falls among frail NH residents is congruent with two previous studies of deintensification to prevent falls: a pharmacist‐led intervention targeted to residents on psychoactive medications that reduced fall rates by 66 percent, and a prescribing modification program for primary care physicians that reduced falls by 39 percent (Campbell et al. 1999; van der Velde et al. 2007). The latter study found a greater fall prevention benefit for cardiovascular drug withdrawal than for psychoactive drug withdrawal. Although blood pressure treatment with a SBP goal of 120 was shown to prevent cardiovascular events in community‐dwelling adults older than 75 years without increasing fall risk (Williamson et al. 2016), most NH residents and geriatrics patients have greater disease and function burden, higher fall rates, and worse survival than that study's subjects (Sexton et al. 2017). Since hypertension is the most common chronic condition among NH residents in the United States, with 54 percent of all residents affected (Simonson, Han, and Davidson 2011), the potential impact of new evidence is great.
Our finding of an association between antihypertensive deintensification and greater 30‐day mortality has several possible explanations. On the one hand, deintensification of antihypertensive medication could cause an increase in mortality from an increase in cardiovascular events. However, in a sensitivity analysis we found no association between antihypertensive deintensification and hospitalizations for a vascular event (e.g., stroke, myocardial infarction). In addition, the separation in mortality occurred primarily during the first 10 days of follow‐up and then plateaued; if the association were causal, we would expect a steadily increasing divergent survival during the follow‐up period. Instead, we believe the association between antihypertensive deintensification and mortality could be a result of deintensification being a marker of residents with more severe illness who were more likely to die for other reasons. Evidence to support this is the attenuation of the association when measures of severe illness were added, such as receipt of antibiotics and IV/IM therapy.
Our study has several limitations. First, this was an observational study where the intervention of antihypertensive deintensification was not randomly assigned but possibly related to unmeasured resident and other parameters. We conducted a propensity score analysis and balanced a set of observed individual characteristics between intervention (deintensification) and comparison groups; however, propensity score analysis is also limited to observed variables and cannot account for unmeasured differences in resident health status. Although our analysis was able to control for a wide range of clinical and nonclinical resident characteristics, we lacked some measures of terminal condition such as do‐not‐resuscitate status that would help disentangle the reasons for the association between deintensification and short‐term mortality. Future studies may benefit from a careful review of resident medical history/records. Second, the clinical scenario (low SBP followed by a fall within three days) created in this study to mark overaggressive treatment was tailored to available data. The actual scenario faced by clinicians may be far more complex. Nevertheless, our results are likely applicable to older adults who have function limitations and who have signs of overaggressive antihypertensive treatment, no matter where they reside. Results are also likely applicable to residents who have low blood pressure readings but do not have a fall and to residents who fall but whose blood pressure around the time of the fall is unknown (e.g., who fall in the community) or whose blood pressure is labile and reactive to measurement. Third, this study used VA data only. The findings may not be generalizable to older adults outside of VA NHs. Fourth, our analysis does not account for facility quality of care or fall prevention interventions at the NH level. Nevertheless, in support of a true association between antihypertensive deintensification and fewer falls are the observations that the association with falls exceeds the magnitude of the association with mortality and it persists even when excluding those who died or who are lost to follow‐up. Moreover, these results are motivated by a physiological mechanism. In this study, we examined medication use and correlates among veterans who resided in U.S. Department of VA NHs. The VA is an integrated health care system with a highly developed electronic health record system which is assembled system‐wide by national data centers. The 132 VA NHs use electronically surveilled bar‐coded medication administration, and vital signs are recorded and tracked in the electronic health record and assembled into the national databases. Thus, it is an ideal system in which to conduct an observational study on medication use, blood pressure, and related outcomes.
We found that antihypertensive deintensification was uncommon among veterans residing in VA NHs who experienced possibly overaggressive treatment of hypertension. Not deintensifying medications can be seen as an inertia which may be a result of clinical uncertainty and a relative lack of evidence and guidelines. Decisional uncertainty rises as patients transition from robust to frail as the risk of harm from treatment increases (Budnitz et al. 2011) and drug administration becomes more burdensome to patients and caregivers (Mitchell et al. 2009). In addition, deintensification strategies and guidelines usually promote discontinuation of medications that have high potential to harm with little possible future benefit (AGS 2015; Bain et al. 2008; Garfinkel and Mangin 2010; Iyer et al. 2008; Tjia et al. 2015), and focus on medications classified as “inappropriate” for older adults based on expert pharmacologic review (AGS 2015). Our finding that antihypertensive deintensification was associated with fewer falls among older NH residents is a signal that deintensification of antihypertensive under these circumstances may also be beneficial, and a prospective randomized trial may be warranted. At least, in frail older adults, clinicians should repeatedly re‐evaluate intensity of blood pressure management, taking into account the resident's prognosis, goals of care, and an individualized estimate of the benefits and harms associated with the intensity of antihypertensive medication.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2: Table S1. Separate Logistic Regression Models of Recurrent Fall, Death and Hospitalization on Antihypertensive Drug Deintensification, Controlling for Resident Characteristics.
Table S2. Separate Logistic Regression Models of Recurrent Fall, Death and Hospitalization on Antihypertensive Drug Deintensification Among Residents Who Were Not Lost to Follow‐Up, Controlling for Resident Characteristics.
Table S3. Separate Logistic Regression Models of Recurrent Fall, Death and Hospitalization on Antihypertensive Drug Deintensification with Propensity Score Weighted Sample (Kernel Weighting, Bandwidth 0.02)§, Controlling for Resident Characteristics.
Table S4. Logistic Regression Model of Hospitalization Due Only to Cardiovascular Disease on Antihypertensive Drug Deintensification, Controlling for Resident Characteristics.
Table S5. Description of ICD‐9‐CM Codes for Cardiovascular Diseases.
Figure S1. Balance of Resident Characteristics between Deintensification and no Deintensification Groups before and after Propensity Score Weighting (Kernel Weighting Bandwidth 0.02).
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This project was funded by Department of Veteran Affairs Office of Geriatrics and Extended Care Data & Analysis Center, National Institute on Aging (P30 AG028741) and the Donaghue Foundation.
Disclaimer: None.
Disclosures: None.
References
- Abt Associates, Inc . 2001. Identification and Evaluation of Existing Quality Indicators that are Appropriate for Use in Long Term Care Settings. Baltimore, MD: Centers for Medicare and Medicaid Services. [Google Scholar]
- AGS . 2015. “American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.” Journal of the American Geriatrics Society 63: 2227‐46. [DOI] [PubMed] [Google Scholar]
- Bain, K. T. , Holmes H. M., Beers M. H., Maio V., Handler S. M., and Pauker S. G.. 2008. “Discontinuing Medications: A Novel Approach for Revising the Prescribing Stage of the Medication‐Use Process.” Journal of the American Geriatrics Society 56: 1946‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett, N. S. , Peters R., Fletcher A. E., Staessen J. A., Liu L., Dumitrascu D., Stoyanovsky V., Antikainen R. L., Nikitin Y., Anderson C., Belhani A., Forette F., Rajkumar C., Thijs L., Banya W., Bulpitt C. J., and Study Group Hyvet.. 2008. “Treatment of Hypertension in Patients 80 Years of Age or Older.”New England Journal of Medicine 358: 1887‐98. [DOI] [PubMed] [Google Scholar]
- Benetos, A. , Labat C., Rossignol P., Fay R., Rolland Y., Valbusa F., Salvi P., Zamboni M., Manckoundia P., Hanon O., and Gautier S.. 2015. “‘Treatment with Multiple Blood Pressure Medications, Achieved Blood Pressure, and Mortality in Older Nursing Home Residents: The PARTAGE Study.” JAMA Internal Medicine 175: 989‐95. [DOI] [PubMed] [Google Scholar]
- Budnitz, D. S. , Lovegrove M. C., Shehab N., and Richards C. L.. 2011. “Emergency Hospitalizations for Adverse Drug Events in Older Americans.”New England Journal of Medicine 365: 2002‐12. [DOI] [PubMed] [Google Scholar]
- Campbell, A. J. , Robertson M. C., Gardner M. M., Norton R. N., and Buchner D. M.. 1999. “Psychotropic Medication Withdrawal and a Home‐Based Exercise Program to Prevent Falls: A Randomized, Controlled Trial.” Journal of the American Geriatrics Society 47: 850‐3. [DOI] [PubMed] [Google Scholar]
- Carter, R. R. , Montpetite M. M., and Jump R. L. P.. 2017. “Mixed‐Methods Pilot Study to Assess Perceptions of Antimicrobial Stewardship in Nursing Homes.” Journal of the American Geriatrics Society 65: 1073‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh, J. , Edelen M. O., Buchanan J. L., Yosef J. A., Ouslander J. G., Berlowitz D. R., Streim J. E., and Saliba D.. 2008. “Nursing Home Assessment of Cognitive Impairment: Development and Testing of a Brief Instrument of Mental Status.” Journal of the American Geriatrics Society 56: 2069‐75. [DOI] [PubMed] [Google Scholar]
- D'Agostino Jr, R. B. 1998. “Propensity Score Methods for Bias Reduction in the Comparison of a Treatment to a Non‐Randomized Control Group.” Statistics in Medicine 17: 2265‐81. [DOI] [PubMed] [Google Scholar]
- Davis, J. C. , Best J. R., Khan K. M., Dian L., Lord S., Delbaere K., Hsu C. L., Cheung W., Chan W., and Liu‐Ambrose T.. 2017. “Slow Processing Speed Predicts Falls in Older Adults with a Falls History: 1‐Year Prospective Cohort Study.” Journal of the American Geriatrics Society 65: 916‐23. [DOI] [PubMed] [Google Scholar]
- Elixhauser, A. , Steiner C., Harris D. R., and Coffey R. M.. 1998. “Comorbidity Measures for Use with Administrative Data.” Medical Care 36: 8‐27. [DOI] [PubMed] [Google Scholar]
- Finucane, C. , O'Connell M. D., Donoghue O., Richardson K., Savva G. M., and Kenny R. A.. 2017. “Impaired Orthostatic Blood Pressure Recovery is Associated with Unexplained and Injurious Falls.” Journal of the American Geriatrics Society 65: 474‐82. [DOI] [PubMed] [Google Scholar]
- Garfinkel, D. , and Mangin D.. 2010. “Feasibility Study of a Systematic Approach for Discontinuation of Multiple Medications in Older Adults: Addressing Polypharmacy.” Archives of Internal Medicine 170: 1648‐54. [DOI] [PubMed] [Google Scholar]
- Garrido, M. M. , Kelley A. S., Paris J., Roza K., Meier D. E., Morrison R. S., and Aldridge M. D.. 2014. “Methods for Constructing and Assessing Propensity Scores.” Health Services Research 49: 1701‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmaier, S. L. , Sloane P. D., Guess H. A., and Koch G. G.. 1994. “The MDS Cognition Scale: A Valid Instrument for Identifying and Staging Nursing Home Residents with Dementia Using the Minimum Data Set.” Journal of the American Geriatrics Society 42: 1173‐9. [DOI] [PubMed] [Google Scholar]
- Hartmaier, S. L. , Sloane P. D., Guess H. A., Koch G. G., Mitchell C. M., and Phillips C. D.. 1995. “Validation of the Minimum Data Set Cognitive Performance Scale: Agreement with the Mini‐Mental State Examination.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 50: M128‐33. [DOI] [PubMed] [Google Scholar]
- Hsu, A. , Gan S., Cenzer‐Stijacic I., and Lee S. J.. 2017. “Glycemic Control and Functional Decline in Nursing Home Residents with Diabetes.” JAMA Internal Medicine 177: 130‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, S. , Naganathan V., McLachlan A. J., and Le Couteur D. G.. 2008. “Medication Withdrawal Trials in People Aged 65 Years and Older: A Systematic Review.” Drugs and Aging 25: 1021‐31. [DOI] [PubMed] [Google Scholar]
- James, P. A. , Oparil S., Carter B. L., Cushman W. C., Dennison‐Himmelfarb C., Handler J., Lackland D. T., LeFevre M. L., MacKenzie T. D., Ogedegbe O., Smith S. C. Jr, Svetkey L. P., Taler S. J., Townsend R. R., Wright J. T. Jr, Narva A. S., and Ortiz E.. 2014. “2014 Evidence‐Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8).” Journal of the American Medical Association 311: 507‐20. [DOI] [PubMed] [Google Scholar]
- Lipska, K. J. , Ross J. S., Miao Y., Shah N. D., Lee S. J., and Steinman M. A.. 2015. “Potential Overtreatment of Diabetes Mellitus in Older Adults with Tight Glycemic Control.” JAMA Internal Medicine 175: 356‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, K. J. , and Wolfson L. I.. 2017. “Decreasing Fall Risk: Intensive Cognitive Training and Blood Pressure Control.” Journal of the American Geriatrics Society 65: 906‐8. [DOI] [PubMed] [Google Scholar]
- Mitchell, S. L. , Teno J. M., Kiely D. K., Shaffer M. L., Jones R. N., Prigerson H. G., Volicer L., Givens J. L., and Hamel M. B.. 2009. “The Clinical Course of Advanced Dementia.” New England Journal of Medicine 361: 1529‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor, V. , Intrator O., Unruh M. A., and Cai S.. 2011. “Temporal and Geographic Variation in the Validity and Internal Consistency of the Nursing Home Resident Assessment Minimum Data Set 2.0.” BMC Health Services Research 11: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. N. , Fries B. E., Mehr D. R., Hawes C., Phillips C., Mor V., and Lipsitz L. A.. 1994. “MDS Cognitive Performance Scale.” J Gerontol 49: M174‐82. [DOI] [PubMed] [Google Scholar]
- Morris, J. N. , Fries B. E., and Morris S. A.. 1999. “Scaling ADLs within the MDS.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 54: M546‐53. [DOI] [PubMed] [Google Scholar]
- Morris, J. N. , Hawes C., Murphy K., et al. 1992. Multistate Nursing Home Case Mix and Quality Demonstration Training Manual. Natick, MA: Eliott Press. [Google Scholar]
- Rubin, D. B. 1997. “Estimating Causal Effects from Large Data Sets Using Propensity Scores.” Annals of Internal Medicine 127: 757‐63. [DOI] [PubMed] [Google Scholar]
- Scott, I. A. , Gray L. C., Martin J. H., Pillans P. I., and Mitchell C. A.. 2013. “Deciding When to Stop: Towards Evidence‐Based Deprescribing of Drugs in Older Populations.” Evidence‐Based Medicine 18: 121‐4. [DOI] [PubMed] [Google Scholar]
- Sexton, D. J. , Canney M., O'Connell M. D. L., Moore P., Little M. A., O'Seaghdha C. M., and Kenny R. A.. 2017. “Injurious Falls and Syncope in Older Community‐Dwelling Adults Meeting Inclusion Criteria for SPRINT.” JAMA Internal Medicine 177: 1385‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgadari, A. , Morris J. N., Fries B. E., Ljunggren G., Jonsson P. V., DuPaquier J. N., and Schroll M.. 1997. “Efforts to Establish the Reliability of the Resident Assessment Instrument.” Age and Ageing 26 (Suppl 2): 27‐30. [DOI] [PubMed] [Google Scholar]
- Simonson, W. , Han L. F., and Davidson H. E.. 2011. “Hypertension Treatment and Outcomes in US Nursing Homes: Results from the US National Nursing Home Survey.” Journal of the American Medical Directors Association 12: 44‐9. [DOI] [PubMed] [Google Scholar]
- Sussman, J. B. , Kerr E. A., Saini S. D., Holleman R. G., Klamerus M. L., Min L. C., Vijan S., and Hofer T. P.. 2015. “Rates of Deintensification of Blood Pressure and Glycemic Medication Treatment Based on Levels of Control and Life Expectancy in Older Patients with Diabetes Mellitus.” JAMA Internal Medicine 175: 1942‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K. S. , Dosa D., Wysocki A., and Mor V.. 2017. “The Minimum Data Set 3.0 Cognitive Function Scale.” Medical Care 55: e68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjia, J. , Field T., Mazor K., Lemay C. A., Kanaan A. O., Donovan J. L., Briesacher B. A., Peterson D., Pandolfi M., Spenard A., and Gurwitz J. H.. 2015. “Dissemination of Evidence‐Based Antipsychotic Prescribing Guidelines to Nursing Homes: A Cluster Randomized Trial.” Journal of the American Geriatrics Society 63: 1289‐98. [DOI] [PubMed] [Google Scholar]
- van der Velde, N. , Stricker B. H., Pols H. A., and van der Cammen T. J.. 2007. “Risk of Falls after Withdrawal of Fall‐Risk‐Increasing Drugs: A Prospective Cohort Study.” British Journal of Clinical Pharmacology 63: 232‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, J. D. , Supiano M. A., Applegate W. B., Berlowitz D. R., Campbell R. C., Chertow G. M., Fine L. J., Haley W. E., Hawfield A. T., Ix J. H., Kitzman D. W., Kostis J. B., Krousel‐Wood M. A., Launer L. J., Oparil S., Rodriguez C. J., Roumie C. L., Shorr R. I., Sink K. M., Wadley V. G., Whelton P. K., Whittle J., Woolard N. F., Wright J. T. Jr, and Pajewski N. M.. 2016. “Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial.” Journal of the American Medical Association 315: 2673‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters, H. , Scheper J., Koning H., et al. 2017. “Discontinuing Inappropriate Medication Use in Nursing Home Residents: A Cluster Randomized Controlled Trial.” Annals of Internal Medicine 167: 609‐17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: Table S1. Separate Logistic Regression Models of Recurrent Fall, Death and Hospitalization on Antihypertensive Drug Deintensification, Controlling for Resident Characteristics.
Table S2. Separate Logistic Regression Models of Recurrent Fall, Death and Hospitalization on Antihypertensive Drug Deintensification Among Residents Who Were Not Lost to Follow‐Up, Controlling for Resident Characteristics.
Table S3. Separate Logistic Regression Models of Recurrent Fall, Death and Hospitalization on Antihypertensive Drug Deintensification with Propensity Score Weighted Sample (Kernel Weighting, Bandwidth 0.02)§, Controlling for Resident Characteristics.
Table S4. Logistic Regression Model of Hospitalization Due Only to Cardiovascular Disease on Antihypertensive Drug Deintensification, Controlling for Resident Characteristics.
Table S5. Description of ICD‐9‐CM Codes for Cardiovascular Diseases.
Figure S1. Balance of Resident Characteristics between Deintensification and no Deintensification Groups before and after Propensity Score Weighting (Kernel Weighting Bandwidth 0.02).


