With an estimated 390 million infections per year, the four serotypes of dengue virus (DENV) cause the most important mosquito-borne viral disease in humans. The dengue vaccine Dengvaxia was licensed; however, its low efficacy among dengue-naive individuals and increased risk of causing severe dengue in children highlight the need for a better understanding of the role of human antibodies in immunity against DENV. DENV suspensions contain mature, immature, and partially immature particles. We investigated the binding of 22 human monoclonal antibodies (MAbs) to the DENV envelope protein on particles with different maturation states. Potently neutralizing MAbs had higher relative maximum binding and avidity to mature particles than weakly neutralizing MAbs. This was supported by analysis of MAb repertoires and polyclonal sera from patients with primary DENV infection. Together, these findings suggest that mature particles may be the optimal form of presentation of the envelope protein to induce more potent neutralizing antibodies against DENV.
KEYWORDS: dengue virus, envelope, mature particles, monoclonal antibody, neutralization
ABSTRACT
The four serotypes of dengue virus (DENV) cause the most important mosquito-borne viral disease in humans. The envelope (E) protein is the major target of neutralizing antibodies and contains 3 domains (domain I [DI], DII, and DIII). Recent studies reported that human monoclonal antibodies (MAbs) recognizing DIII, the D1/DII hinge, the E-dimer epitope, or a quaternary epitope involving DI/DII/DIII are more potently neutralizing than those recognizing the fusion loop (FL) of DII. Due to inefficient cleavage of the premembrane protein, DENV suspensions consist of a mixture of mature, immature, and partially immature particles. We investigated the neutralization and binding of 22 human MAbs to DENV serotype 1 (DENV1) virions with differential maturation status. Compared with FL MAbs, DIII, DI/DII hinge, and E-dimer epitope MAbs showed higher maximum binding and avidity to mature particles relative to immature particles; this feature may contribute to the strong neutralizing potency of such MAbs. FL-specific MAbs required 57 to 87% occupancy on mature particles to achieve half-maximal neutralization (NT50), whereas the potently neutralizing MAbs achieved NT50 states at 20 to 38% occupancy. Analysis of the MAb repertoire and polyclonal sera from patients with primary DENV1 infection supports the immunodominance of cross-reactive anti-E antibodies over type-specific antibodies. After depletion with viral particles from a heterologous DENV serotype, the type-specific neutralizing antibodies remained and showed binding features shared by potent neutralizing MAbs. Taken together, these findings suggest that the use of homogeneous mature DENV particles as an immunogen may induce more potent neutralizing antibodies against DENV than the use of immature or mixed particles.
IMPORTANCE With an estimated 390 million infections per year, the four serotypes of dengue virus (DENV) cause the most important mosquito-borne viral disease in humans. The dengue vaccine Dengvaxia was licensed; however, its low efficacy among dengue-naive individuals and increased risk of causing severe dengue in children highlight the need for a better understanding of the role of human antibodies in immunity against DENV. DENV suspensions contain mature, immature, and partially immature particles. We investigated the binding of 22 human monoclonal antibodies (MAbs) to the DENV envelope protein on particles with different maturation states. Potently neutralizing MAbs had higher relative maximum binding and avidity to mature particles than weakly neutralizing MAbs. This was supported by analysis of MAb repertoires and polyclonal sera from patients with primary DENV infection. Together, these findings suggest that mature particles may be the optimal form of presentation of the envelope protein to induce more potent neutralizing antibodies against DENV.
INTRODUCTION
Dengue virus (DENV) belongs to the Flavivirus genus of the Flaviviridae family. There are four serotypes (DENV serotype 1 [DENV1], DENV2, DENV3, and DENV4) that cause the most common and significant arboviral disease in humans (1). Approximately 390 million DENV infections occur annually, with 25% of these being apparent infections, including dengue fever and the severe forms of disease, dengue hemorrhagic fever and dengue shock syndrome (1–4). Although the live-attenuated chimeric yellow fever-dengue vaccine Dengvaxia has been licensed in several countries, it is recommended only for persons who have experienced previous DENV infection. The moderate efficacy (∼60%) of Dengvaxia in the presence of neutralizing antibodies during phase 2b and 3 trials, its lower efficacy among dengue-naive individuals than among dengue-experienced individuals (∼40 versus ∼80%), and the increased risk of hospitalization and severe dengue among young vaccinated children highlight the need for a better understanding of humoral responses following natural DENV infection (5–9).
DENV contains a positive-sense single-stranded RNA genome encoding one polyprotein, which is cleaved into three structural proteins, the capsid, premembrane (prM), and envelope (E) proteins, and seven nonstructural proteins (10). E protein, present on the surface of the virion, mediates virus entry and is the major target of neutralizing antibodies (4, 10). The ectodomain of E protein has three domains. Domain I (DI) is located in the center; domain II (DII), an elongated domain containing the fusion loop (FL) at its tip, is involved in dimerization and membrane fusion; and domain III (DIII), an immunoglobulin-like domain, is involved in receptor binding and stabilization of trimers during fusion (10–13).
In the Flavivirus genus, there are several serocomplexes, including the DENV serocomplex, the Japanese encephalitis virus serocomplex, the tick-borne encephalitis virus serocomplex, and yellow fever virus as a single member. Anti-E antibodies that recognize members of two or more serocomplexes, members within the same serocomplex, or a single member are categorized as group-reactive (GR), complex-reactive (CR), or type-specific (TS) antibodies, respectively (14). Previous studies of mouse anti-E monoclonal antibodies (MAbs) revealed that different categories of MAbs recognize different epitopes and have different neutralizing potency; murine GR MAbs mainly recognize the highly conserved residues in the FL of DII, whereas CR and TS murine MAbs recognize different but overlapping residues in DIII (15–19). TS MAbs were generally more potent at neutralizing the virus than CR or GR MAbs (17, 19). Studies of human MAbs have shown that GR MAbs recognize either FL or both FL and bc loop residues in DII (20–22); TS MAbs recognize DIII residues, the quaternary epitope, or the DI/DII hinge (DI/IIh) region (23–30); and CR MAbs recognize DIII, E-dimer epitope 2 (EDE2), or E-dimer epitope 1 (EDE1), which involve FL and other residues including the N-linked glycan at residue 153 (23, 24, 31, 32). Several of these TS MAbs, such as 2D22, 14c10, 5J7, and 1F4, and CR MAbs, such as C8 and C10, neutralize potently.
DENV enters the cell through receptor-mediated endocytosis. The low-pH environment in the endosome causes a conformational change of E protein and fusion between viral and endosomal membranes (10, 11, 33, 34). DENV particles assemble in the membranes derived from the rough endoplasmic reticulum (ER), where the immature virions bud into the lumen of the ER and transport through the secretory pathway (10, 34). Within the low-pH environment of the trans-Golgi network, the furin or furin-like protease cleaves the prM protein on immature virions into M protein and pr peptide, which is then released under neutral pH in the culture medium to form mature particles (34–36). However, prM cleavage is inefficient, leading to a mixture of mature, immature, and partially immature DENV particles in virus suspensions produced in tissue culture (37, 38). Based on cryo-electron microscopy (cryo-EM) images, the proportions of mature, immature, and partially immature DENV2 particles derived from C6/36 insect cells were reported to be 55, 3, and 42%, respectively (38). Using anti-prM and anti-E MAbs in a virion-capture enzyme-linked immunosorbent assay (ELISA), the prM-containing DENV2 particles in culture supernatants derived from dendritic cells, C6/36 insect cells, Vero monkey kidney cells, and 293T human embryonic kidney cells were 13, 54, 52, and 63%, respectively (31). The infectivity of immature particles was 10,000-fold lower than that of wild-type (WT) (mixed-maturity-state) particles (39, 40).
Previous studies of human antibodies to DENV primarily focused on DENV particles derived from culture supernatants, which are a mixture of mature, immature, and partially immature particles. Little is known about how antibodies recognize DENV particles with different maturation states in relationship to neutralization. In this study, we investigated the neutralization and binding of 22 human anti-E MAbs on DENV1 virions with different maturation states. The findings suggest that a higher relative maximum binding (Bmax) and a higher avidity of binding to mature particles may contribute to the stronger neutralizing activities of potently neutralizing MAbs. Analysis of twelve polyclonal serum samples from subjects with a history of primary DENV1 infection revealed that TS neutralizing antibodies remained after depletion of cross-reactive antibodies and showed binding characteristics shared by potently neutralizing MAbs. These findings have implications for the rational design and testing of new dengue vaccine candidates.
RESULTS
Binding of different categories of MAbs to mature, immature, or mixed phenotype particles.
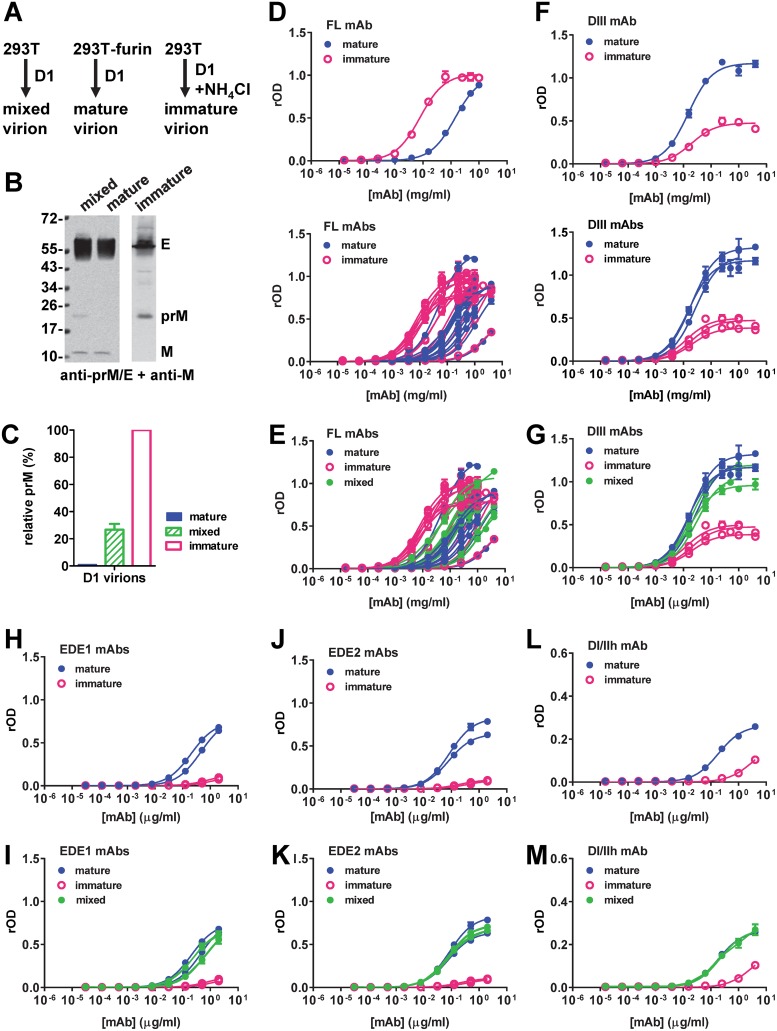
Table 1 summarizes the source, binding specificity, domain, epitope, and neutralizing potency of 22 human MAbs in this study. Mature, mixed, or immature DENV1 particles were generated from 293T-furin cells, 293T cells, or 293T cells in the presence of ammonium chloride, respectively, and verified by Western blot (WB) analysis (Fig. 1A and B). The prM protein content relative to that in immature particles was determined to be 0.9 or 26.7% for mature or mixed particles, respectively (Fig. 1C) (31). We first examined the curves for the binding of different categories of MAbs to mature or immature particles. While FL-specific MAbs bound mature and immature particles with similar Bmax values, they reached the Bmax for binding to immature particles at lower concentrations, suggesting a higher binding avidity to immature particles (Fig. 1D). The binding curves for mixed particles were between those for mature and immature particles (Fig. 1E). In contrast, DIII MAbs showed a higher Bmax to mature particles than to immature particles, suggesting higher epitope accessibility on mature particles (Fig. 1F and G). Similarly, DI/IIh, EDE1, and EDE2 MAbs had a higher Bmax to mature particles than to immature particles (Fig. 1H, J, and L). Although the binding curves for mixed particles were between those for mature and immature particles, they were closer to those for mature particles than to those for immature particles, probably due to the low prM content of the mixed particles (26.7%) prepared in this study (Fig. 1I, K, and M).
TABLE 1.
Source, binding specificity, epitope, and neutralizing potency of human monoclonal antibodies in this studye
| MAb | Patient identifier | Infection and serotypea | Category | Binding specificityb | Domain(s)c | Epitope(s)d | NT50 (μg/ml) for D1 | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| DVG1.3 | DVG | Primary D1 | FL | GR | I/II | L107 | 0.52 | 21 |
| DVG6.3 | DVG | Primary D1 | FL | GR | I/II | W101, F108 | >2 | 21 |
| DVG7.5 | DVG | Primary D1 | FL | GR | I/II | W101, F108 | >2 | 21 |
| DVG12.2 | DVG | Primary D1 | FL | GR | I/II | L107, F108, T76 | >2 | 21 |
| DVG17.12 | DVG | Primary D1 | III | TS D1 | III | G383, E384, K385 | 0.005 | 21 |
| DV470.12 | Donor 12 | Secondary D1 | III | TS D1 | III | G383, E384, K385, 79E | 0.01 | 23 |
| DV87.1 | Donor 12 | Secondary D1 | III | CR D1–D3 | III | K307, E311, L389, W391 | 0.007 | 23 |
| DV28.1 | Donor 12 | Secondary D1 | FL | GR | I/II | W101, F108 | 0.37 | 23 |
| DV143.6 | Donor 12 | Secondary D1 | FL | GR | I/II | L107, D290, 67 | 0.50 | 23 |
| DV291.3 | Donor 12 | Secondary D1 | FL | GR | I/II | W101, F108 | 0.55 | 23 |
| DV291.7 | Donor 12 | Secondary D1 | FL | GR | I/II | 101W, D290 | 0.92 | 23 |
| DV378.12 | Donor 12 | Secondary D1 | FL | GR | I/II | W101, F108, D290 | 0.87 | 23 |
| 749B6 | 749 | Secondary D1 | FL | GR | I/II | W101, F108 | >2 | 21, 31 |
| 751B6 | 751 | Secondary D1 | FL | GR | I/II | W101, F108, G78 | 0.53 | 21, 31 |
| 751B11 | 751 | Secondary D1 | FL | GR | I/II | W101, F108, G106 | 0.65 | 21, 31 |
| 753C1 | 753 | Secondary D1 | FL | GR | I/II | W101, F108, L107 | 0.14 | 21, 31 |
| 4L5 | Donor 1 | Secondary | FL | GR | I/II | W101, 79E, 86S | 0.18 | 27 |
| 752B10 | 752 | Primary | EDE1 | CR | I/II | E49, Q77, W101, N134, I161, A162, P169, T200, Q323, W391, F392 | 0.061 | 31 |
| 752-2C8 | 752 | Primary | EDE1 | CR | I/II | E49, Q77, W101, I161, A162, T200, Q323, W391, F392 | 0.131 | 31 |
| 747B8 | 747 | Secondary D2 | EDE2 | CR | I/II | E49, Q77, W101, N134, N153, T155, I161, A162, P169, T200, E203, Q323, W391, F392 | 0.030 | 31 |
| 747D8 | 747 | Secondary D2 | EDE2 | CR | I/II | E49, Q77, W101, N134, N153, T155, I161, A162, P169, T200, K202, E203, L308, K310, Q323, W391, F392 | 0.021 | 31 |
| 1F4 | Donor 1 | Secondary | I/II hinge | TS | I/II | K47, N52, K136, E157, T160, T161, T163, G274 | 0.11 | 27 |
Primary or secondary DENV infection and the serotype infecting the patients were determined as reported previously (41).
GR, group reactive; CR, complex reactive; TS, type specific.
The domain was based on the location of the epitope residues or the binding to recombinant DI/II or DIII reported previously (21, 23, 27, 31).
Epitope residues were reported previously or identified in this study (DV470.12, 87.1, 28.1, 143.6, 291.3, 291.7, 378.12).
D1, DENV1; D2, DENV2; D3, DENV3.
FIG 1.
Binding of different categories of human anti-E MAbs to mature, immature, or mixed particles. (A) Outline of preparation of mature, mixed, and immature DENV1 (D1) virions from 293T-furin cells, 293T cells, and 293T cells in the presence of ammonium chloride, respectively. (B) WB analysis of mature, mixed, or immature DENV1 virions purified by sucrose gradient ultracentrifugation and probed with DENV-immune human serum (anti-prM/E) and rabbit serum against M-peptide (anti-M). The values on the left are molecular masses (in kilodaltons). (C) The relative prM contents of the different particles were determined by a virion ELISA, which used a murine MAb (FL0251) for capture and human MAbs (anti-E MAb DVG17.12, anti-prM MAb DVB 59.3) for detection. (D to M) Binding curves of different categories of human MAbs to mature, immature, or mixed particles based on virion-capture ELISAs, including FL-specific MAbs (D, E), DIII MAbs (F, G), EDE1 MAbs (H, I), EDE2 MAbs (J, K), and DI/IIh MAb (L, M). The relative OD (rOD) compared to the OD of DENV-immune human serum is presented. Data are the means and standard deviations for duplicates from one representative experiment of two.
Relationship between neutralizing potency and binding characteristics of mature and immature particles.
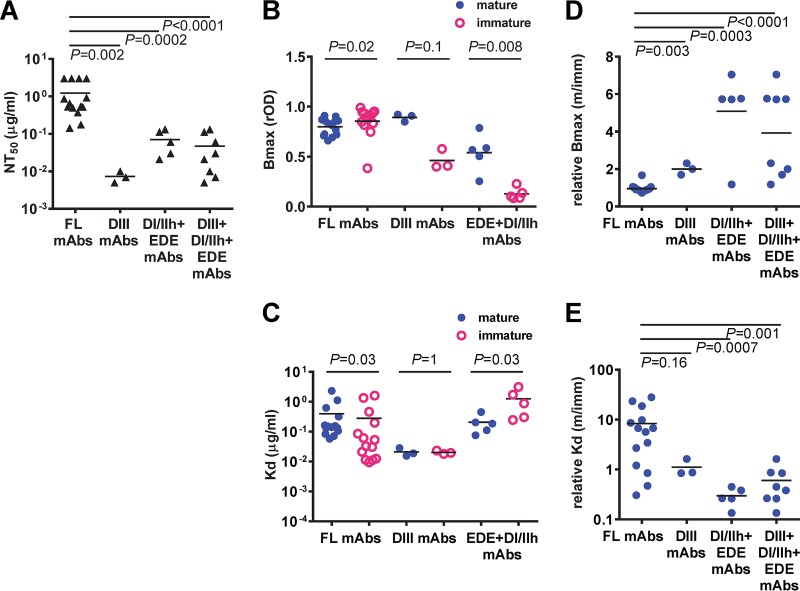
The half-maximal neutralization (NT50) values of FL-specific MAbs were significantly higher than those of DIII, DI/IIh, and EDE MAbs together or DIII and non-DIII (DI/IIh plus EDE) MAbs alone (P < 0.0001, 0.002, and 0.0002, respectively, two-tailed Mann-Whitney test) (Fig. 2A). DI/IIh and EDE MAbs had a higher Bmax to mature particles than to immature particles (P = 0. 008, two-tailed Mann-Whitney test) (Fig. 2B), suggesting that higher epitope accessibility to mature particles than to immature particles may account for their strong neutralizing potency. In addition, DI/IIh and EDE MAbs had lower dissociation constant (Kd) values for mature particles than for immature particles (P = 0.03, two-tailed Mann-Whitney test) (Fig. 2C), suggesting that the higher binding avidity to mature particles also contributes to the potent neutralization. In contrast, the higher binding avidity of FL-specific MAbs to immature particles than to mature particles may account for their lower neutralizing potency.
FIG 2.
Comparison of neutralizing potency, maximum binding, and dissociation constants of different categories of human anti-E MAbs to mature, immature, or mixed particles. (A) DIII, EDE, and DI/IIh MAbs were more potent neutralizers than FL-specific MAbs. (B, C) Comparison of the Bmax (B) and Kd (C) for mature or immature DENV1 particles within each category of MAbs, including FL, DIII, and EDE plus DI/IIh MAbs. (D, E) Comparison of the relative Bmax (D) and relative Kd (E) for mature versus immature DENV1 particles, Bmax (m/imm) and Kd (m/imm), respectively, between FL, DIII, and EDE plus DI/IIh MAbs. Bmax and Kd were determined by nonlinear regression analysis (GraphPad Prism software, version 6.0). Data are the means for duplicates from two experiments. P values were based on the two-tailed Mann-Whitney test.
Since the DENV produced in cultured cells consists of a mixture of mature, immature, and partially immature particles, we calculated the relative Bmax (the ratio of the Bmax of mature particles to that of immature particles) as another parameter. We found the potent neutralizing MAbs (DIII, DI/IIh, and EDE MAbs together) had higher relative Bmax values than FL-specific MAbs (P < 0.0001, two-tailed Mann-Whitney test) (Fig. 2D). In addition, these potent neutralizing MAbs had lower relative Kd values (the ratio of the Kd of mature particles to the Kd of immature particles) than FL-specific MAbs (P = 0.001, two-tailed Mann-Whitney test) (Fig. 2E). Taken together, these findings suggest that the higher relative epitope accessibility and binding avidity of potent neutralizing MAbs to mature particles, which are infectious particles, than those of MAbs to immature particles, which are 10,000-fold less infectious, may contribute to their potent neutralizing activity (39, 40).
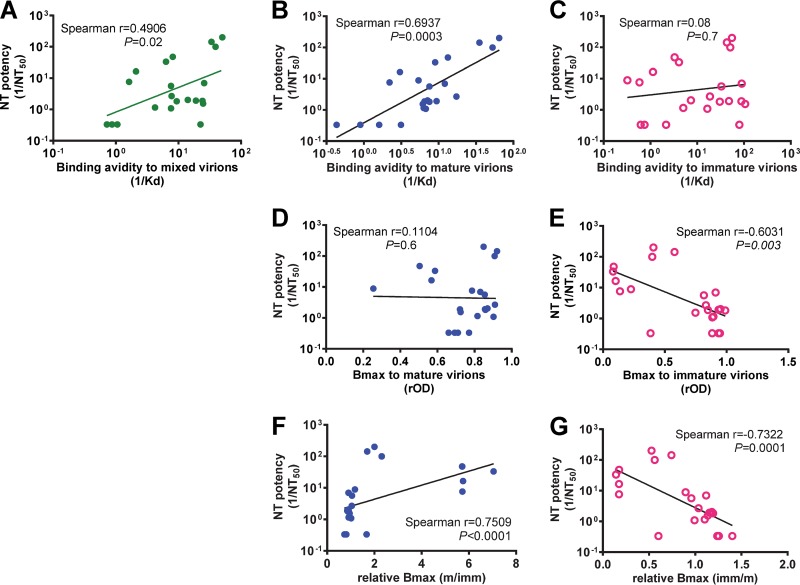
We further examined the relationship between neutralizing potency, binding avidity, and Bmax of all 22 MAbs (as a group) to different particles. As shown in Fig. 3A, a positive correlation between neutralizing potency and binding avidity to mixed particles was found (Spearman correlation coefficient [r] = 0.4906, P = 0.02). A stronger correlation between neutralizing potency and binding avidity to mature particles (r = 0.6937, P = 0.0003) than to immature particles was observed (Fig. 3B and C). While the neutralizing potency correlated inversely with Bmax to immature particles, it did not correlate with Bmax to mature particles (Fig. 3D and E), probably due to the low Bmax to mature particles for some potent neutralizing MAbs (such as DI/IIh and EDE MAbs) compared with that for FL-specific MAbs (Fig. 2B). Using the relative Bmax in the analysis, the neutralizing potency correlated with the relative Bmax to mature particles and inversely with the relative Bmax to immature particles (r = 0.7509 and P < 0.0001 and r = −0.7322 and P = 0.0001, respectively; Fig. 3F and G).
FIG 3.
Relationship between neutralizing (NT) potency and binding parameters to mature, mixed, or immature particles. (A) Relationship between neutralizing potency and binding avidity to mixed DENV1 particles. (B, D, F) Relationship between neutralizing potency and binding avidity (B), Bmax to mature DENV1 particles (D), and relative Bmax (mature/immature [m/imm]) (F). (C, E, G) Relationship between neutralizing potency and binding avidity (C), Bmax to immature DENV1 particles (E), and relative Bmax (immature/mature [imm/m]) (G). Neutralizing potency and binding avidity were defined by 1/NT50 and 1/Kd, respectively. Bmax and Kd were determined by nonlinear regression analysis (GraphPad Prism software, version 6.0). Data are means for duplicates from two experiments. The Spearman correlation test was performed.
Neutralization and percent occupancy of mature and mixed particles by different MAbs.
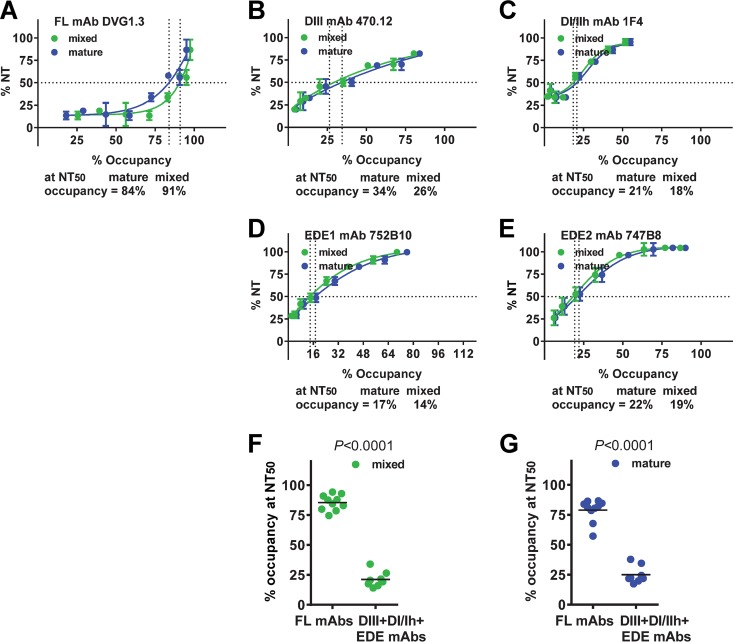
As shown in Fig. 4A, the percent occupancy of an FL-specific MAb (DVG1.3) to mature and mixed particles increased as the percent neutralization increased. At the concentration of NT50, the percent occupancy for mature or mixed particles reached 84% or 91%, respectively. A similar trend was observed for 10 FL-specific MAbs, in that the percent occupancy to mature or mixed particles was 57 to 87% or 75 to 94%, respectively (4 were not tested due to NT50 values of >2 μg/ml). For a DIII MAb (470.12), the percent occupancy to mature or mixed particles at the NT50 concentration was much lower, 34% or 26%, respectively (Fig. 4B). Similarly, other categories of potent neutralizing MAbs, including a DI/IIh MAb (1F4), EDE1 MAb (752B10), or EDE2 MAb (747B8), had the percent occupancy for mature/mixed particles at NT50 of 21/18%, 17/14%, and 22/19%, respectively (Fig. 4C to E). Taken together, the percent occupancy of three categories of potent neutralizing MAbs (DIII, DI/IIh, and EDE MAbs) to mature or mixed particles at the NT50 concentration was significantly lower than that of FL-specific MAbs (P < 0.0001, two-tailed Mann-Whitney test) (Fig. 4F and G).
FIG 4.
Relationship between neutralization and percent occupancy of mature and mixed particles for different categories of anti-E MAbs. (A to E) Representative example of FL (A), DIII (B), DI/IIh (C), EDE1 (D), and EDE1 (E) MAbs and percent occupancy of these MAbs at the NT50 concentration. (F, G) Comparison of percent occupancy of mixed (F) or mature (G) particles at the NT50 concentration between FL MAbs and potent neutralizing MAbs (including DIII, DI/IIh, and EDE MAbs). The curves were determined by nonlinear regression analysis (GraphPad Prism software, version 6.0). Data are the means and/or standard deviations (for A to E) for duplicates from two experiments. P values were based on the two-tailed Mann-Whitney test.
Analysis of the repertoire of anti-E MAbs derived from the same individual.
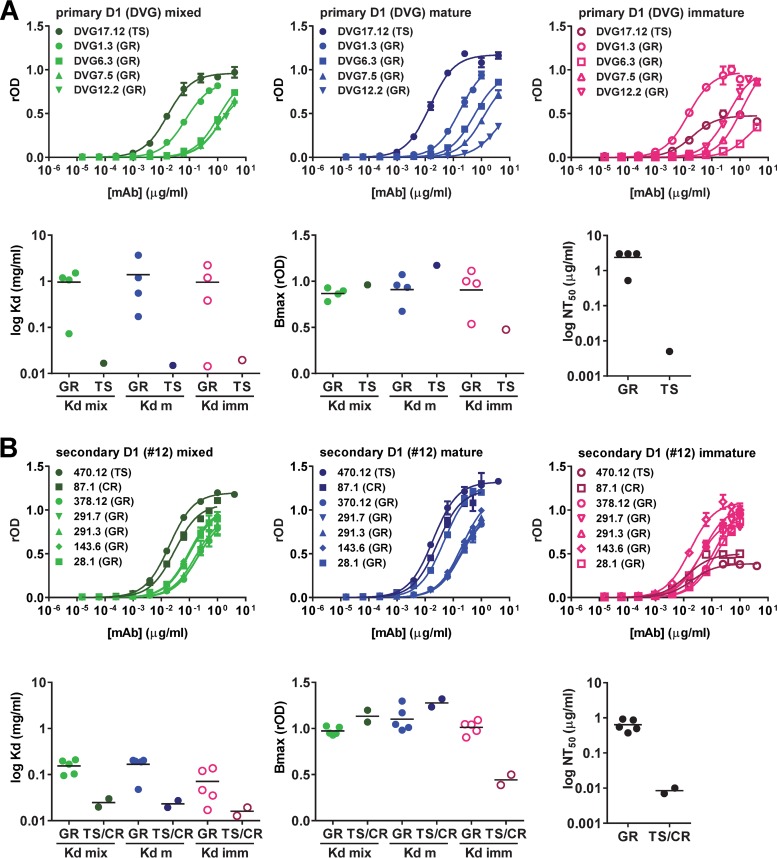
Previous studies of polyclonal human sera after DENV infection revealed that a significant proportion of anti-E antibodies was cross-reactive (either GR or CR), whereas a minor proportion was TS (15, 19–21, 41–43). To better understand the binding of the anti-E antibody repertoire to immature, mature, or mixed particles, we examined different anti-E MAbs derived from two individuals, one with primary DENV1 infections (DVG) and another with secondary DENV1 infections (donor 12) (Table 1). The five MAbs derived from DVG included four GR MAbs and one DENV1-TS MAb. As shown in Fig. 5A, the TS MAb had a higher binding avidity to mixed or mature particles than the four GR MAbs, whereas GR MAbs had a higher Bmax to immature particles. Consistent with this finding, the TS MAb had a stronger neutralization potency than the four GR MAbs. The seven MAbs derived from donor 12 included five GR MAbs, one CR MAb, and one DENV1 TS MAb (Fig. 5B). The TS and CR MAbs showed a higher binding avidity to mixed or mature particles than the GR MAbs, whereas the GR MAbs showed a higher Bmax to immature particles. Notably, the TS and CR MAbs had a stronger neutralization potency than the five GR MAbs.
FIG 5.
Analysis of repertoire of anti-E MAbs derived from the same individual. (A) The five MAbs derived from a case with primary DENV1 (D1) infection (DVG) include four GR MAbs and one DENV1 TS MAb. (B) The seven MAbs derived from a case with secondary DENV1 infection (donor 12) include five GR MAbs, one CR MAb, and one DENV1-TS MAb. The binding curves, Kd, and Bmax for mature (m), mixed (mix), or immature (imm) particles, as well as the NT50 are presented. GR, group reactive; CR, complex reactive; TS, type specific. Data are the means and/or standard deviations for duplicates from one representative experiment of two.
Analysis of polyclonal dengue-immune human sera.
We next investigated 12 serum samples from subjects following primary DENV1 infection (Table 2). We used a previously described depletion protocol with inactivated DENV3, a heterologous serotype, to remove cross-reactive antibodies in the serum from case ID26 (Fig. 6A) (41). In agreement with previous reports (25, 41), the DENV1 TS neutralizing activity remained in the DENV3-depleted serum (Fig. 6B). We then examined the binding of mock- or DENV3-depleted serum to mature or immature DENV1 particles. Compared with mock-depleted serum, which bound immature particles slightly better than mature particles (endpoint titer, 114,408 versus 68,831), the DENV3-depleted serum showed greatly reduced binding to both particles (endpoint titers, 15,315 and 14,180) (Fig. 6C to E). The relative endpoint titer (ratio of the endpoint titer of mature to immature particles) increased from 0.60 to 0.93 (Fig. 6C and D). Comparing the 12 mock-depleted and DENV3-depleted serum samples, an increase in the relative endpoint titer was observed after removing cross-reactive antibodies (P = 0.027, Wilcoxon signed-rank test) (Fig. 6E), suggesting that the TS neutralizing antibodies in polyclonal serum bind to mature particles better than immature particles.
TABLE 2.
Basic information of DENV-immune subjects in this studya
| Patient identifier | Time of sampling (mo)b | Yr, location |
|---|---|---|
| ID4 | 15 | 2006, Kaohsiung, Taiwan |
| ID7 | 15 | 2006, Kaohsiung, Taiwan |
| ID26 | 3 | 2006, Kaohsiung, Taiwan |
| ID33 | 3 | 2006, Kaohsiung, Taiwan |
| H1001 | 3 | 2015, Hawaii, USA |
| H1003 | 4.5 | 2015, Hawaii, USA |
| H1004 | 4.5 | 2015, Hawaii, USA |
| K1002 | 5 | 2015, Hawaii, USA |
| K1014 | 8.5 | 2015, Hawaii, USA |
| K1015 | 8.5 | 2015, Hawaii, USA |
| K1017 | 6.5 | 2015, Hawaii, USA |
| K1018 | 6.5 | 2015, Hawaii, USA |
All patients had a primary infection with DENV1. The primary or secondary DENV infection and the serotype infecting the patients were determined as reported previously (41).
Sampling time post-symptom onset.
FIG 6.
Analysis of polyclonal human sera. (A) Serum from a case with a prior primary DENV1 infection (patient ID26) was mock or DENV3 (D3) depleted and tested with a virion ELISA. (B) Neutralization curve for DENV1 of mock-depleted or DENV3-depleted serum from patient ID26. (C, D) Binding curves of mature or immature DENV1 particles for mock-depleted (C) or DENV3-depleted (D) serum. The endpoint titers for binding to mature or immature particles and the ratio (mature/immature) were determined based on four-parameter nonlinear regression analysis (GraphPad Prism software, version 6.0). (E) Endpoint titers for mature or immature particles and relative endpoint titers (mature/immature) of mock-depleted or DENV3-depleted sera from 12 patients following primary DENV1 infection. Data are the means and/or standard deviations for duplicates from two experiments. The two-tailed Wilcoxon signed-rank test was used.
DISCUSSION
We carried out an in-depth analysis of 22 human MAbs of different categories focusing on the neutralizing potency and binding characteristics of DENV1 virions with different maturation states. Our study presents three major findings. First, the potent neutralizing MAbs recognizing DIII, the DI/DII hinge, and EDE have a higher relative Bmax and avidity for binding to mature particles, the infectious virions, than FL-specific MAbs. Second, potent neutralizing MAbs require a lower occupancy on mature particles to achieve 50% neutralization than FL-specific MAbs. Third, the analysis of the MAb repertoires and polyclonal sera from subjects with a history of primary DENV1 infection supports the immunodominance of cross-reactive antibodies over TS neutralizing antibodies, which remained after depletion experiments and shared the features of potent neutralizing MAbs. Altogether, our findings suggest that mature DENV particles may represent an ideal immunogen to induce potent neutralizing antibodies against DENV.
Previous studies of murine MAbs against DENV2 reported that DIII TS potently neutralizing MAbs require a lower occupancy on DENV2 particles to achieve 50% neutralization than CR weakly neutralizing MAbs (44). Similarly, studies of murine MAbs against West Nile virus (WNV) have shown that DIII potent neutralizing MAbs can achieve 50% neutralization at 25% occupancy on WNV particles (45, 46). The binding to and percent occupancy of particles with different maturation states were not examined in those studies. We found that different categories of potently neutralizing human MAbs (DIII, DI/II hinge, EDE1, and EDE2) shared a common feature, namely, a requirement for low occupancy (20 to 38%) on mature particles, the infectious particles, to achieve 50% neutralization compared with that for FL-specific MAbs (57 to 87%). A similar trend was also observed for the mixed particles (Fig. 4F). It is worth noting that since the FL epitope is exposed more times on the mixed (or immature) virions, it would be expected to observe that a lower percent occupancy of FL-specific MAbs would be needed to reach 50% neutralization on mixed (or immature) virions, whereas a higher percent occupancy would be needed to reach 50% neutralization on mature virions. However, we used only mixed virions rather than mature (or immature) virions in our neutralization assays. Therefore, a higher percent occupancy for mixed virions than for mature virions was observed (Fig. 4F and G), probably due to the better binding of FL-specific MAbs to mixed virions than to mature virions.
Since 12 MAbs from our panel were derived from two donors (DVG and donor 12), we were able to investigate the anti-E MAb repertoire from the same individual. The majority of anti-E Mabs from the two donors were GR, and only two were TS, and the TS MAbs had a stronger neutralizing potency than the GR MAbs (Fig. 5). Analysis of 12 polyclonal serum samples from patients following primary DENV1 infection revealed that they bind immature particles comparably to mature particles (Fig. 6E). After removal of cross-reactive antibodies via depletion experiments, the proportion of TS antibodies calculated by a previously reported method (41) ranged from 12 to 43%, and that of cross-reactive (GR and CR) antibodies ranged from 57 to 88% (Fig. 7), supporting the predominance of cross-reactive antibodies following DENV infection (15, 19–21, 41–43).
FIG 7.
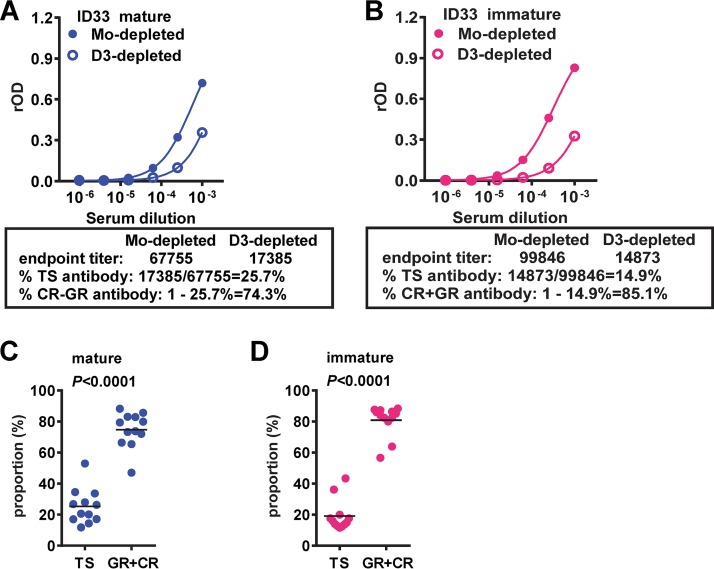
Determination of the proportion of TS antibodies in polyclonal sera. (A, B) Serum from a case with prior primary DENV1 infection (ID33) was mock or DENV3 (D3) depleted and titrated on mature (A) or immature (B) DENV1 particles. The endpoint titers of mock-depleted or DENV3-depleted sera were determined based on four-parameter nonlinear regression analysis (GraphPad Prism software, version 6.0). The proportion (in percent) of TS antibodies is equal to (endpoint titer of DENV3-depleted serum/endpoint titer of mock-depleted serum) × 100. The percentage of GR plus CR antibodies equals 100 − percentage of TS antibodies. (C, D) Comparison of the percentage of TS antibodies and the percentage of GR plus CR antibodies in sera from 8 patients following primary DENV1 infection based on mature (C) and immature (D) particles. Data are the means for duplicates from two experiments. The two-tailed Mann-Whitney test was used.
Since the discovery of potent neutralizing human MAbs targeting DI/IIh, DIII residues, quaternary epitopes on DI, DII, and DIII, and EDE, several studies have attempted to identify the epitopes or domains on E protein recognized by TS neutralizing antibodies in polyclonal sera following primary DENV infection. These TS neutralizing antibodies may contribute to long-lived protection against the infecting serotype, and if so, their epitopes would represent ideal targets for each component of a tetravalent dengue vaccine. Experiments using depletion protocols, mutant viruses, or chimeric viruses containing transplanted epitopes to examine the gain or loss of neutralization have shown that, despite some individual variations, TS antibodies recognizing residues proximal to the DI/II hinge, DIII complex epitope residues, and DI/II hinge residues contribute greatly to neutralizing activity following primary DENV1 immunization, primary DENV2 infection/immunization, or primary DENV4 infection/immunization, respectively (47–50; reviewed in reference 51).
The observations that these potent neutralizing human MAbs do not bind recombinant E protein or DIII alone and that their epitopes involve multiple residues of different domains from adjacent E monomers (such as the epitopes for MAbs 14c10, 5J7, and 2D22) suggest the importance of the proper conformation and arrangement of E protein on the surface of virions (or virus-like particles [VLP]) to induce such potent neutralizing antibodies (25–32). Recent studies have shown that stabilized E dimers can be recognized by some of these potent neutralizing MAbs (including MAbs 2D22, C8, and C10) (52, 53) and could represent components of new subunit vaccine candidates. The findings presented here showing that these potent neutralizing MAbs recognize mature particles better than immature particles suggest that mature particles may represent a promising immunogen to induce potent neutralizing antibodies against DENV. It is worth noting that mature particles contain little pr protein compared with mixed particles and are less likely to induce anti-prM antibodies, which are cross-reactive and weakly neutralizing and contribute to antibody-dependent enhancement in vitro (31, 40, 54, 55).
There are several limitations of this study. First, we focused on DENV1 particles with different maturation states and their interactions with different anti-E MAbs that were derived from subjects with a history of primary or secondary DENV1 infections as well as polyclonal sera from subjects following primary DENV1 infection. Whether the information is applicable to other DENV serotypes remains to be investigated in the future. Second, all the DENV particles with different maturation states were derived from human 293T cultured cells. Future studies are needed to determine if DENV particles produced from other cell lines (including those from mosquito origin), from primary cells, or in vivo have similar properties. Third, future cryo-EM studies of other categories of human anti-E and anti-prM MAbs bound to DENV particles with different maturation states might provide new insights to further our understanding of the interactions between human antibodies and DENV. These studies will facilitate a better understanding of the mechanisms of protection and enhancement and point the way toward new strategies for DENV vaccination.
MATERIALS AND METHODS
Dengue-immune sera and ethics statement.
Serum samples from twelve reverse transcription-PCR-confirmed DENV1 cases collected at 3 to 15 months postinfection were included in this study (Table 2). The subjects were adult dengue cases with primary DENV1 infection based on a monotypic neutralization pattern (41); four were from the Kaohsiung Medical University Hospital in Kaohsiung, Taiwan, in 2006, and eight were from the Hilo Medical Center in Big Island, Hawaii, during the 2015-2016 outbreak. With the approval of the Institutional Review Board of the Kaohsiung Medical University and the University of Hawaii at Manoa (CHS number 23786 and CHS number 17568), written informed consent was obtained. All serum samples involved in this study were coded for anonymity.
WB analysis.
WB analysis was performed as described previously (41, 42). Mature, mixed, or immature DENV1 virions purified from sucrose gradient ultracentrifugation were prepared in nonreducing sample buffer and subjected to 12% polyacrylamide gel electrophoresis, followed by transfer to a nitrocellulose membrane (Hybond-C Extra; GE Healthcare) and hybridization with dengue-immune serum plus rabbit serum against M peptide (resides 6 to 27; Pacific Immunology) (56) and secondary antibody (IRDye 800CW-conjugated goat anti-human IgG at 1:10,000). The signal was detected by use of LiCor Odyssey classic imaging system (LiCor Biosciences) and analyzed by Image Studio software (41).
Preparation of mature, immature, and mixed DENV1 virions.
To generate immature, mixed, or mature particles, DENV1 was inoculated onto 293T cells in the presence or absence of 20 mM ammonium chloride and onto 293T-furin cells (a stable clone expressing furin), respectively, at a multiplicity of infection of 0.25 to 1. Culture supernatants collected at day 7 were concentrated by use of an Amicon column (Ultra-15 centrifugal filter device; Amicon) and purified by 15 to 60% sucrose gradient ultracentrifugation (SW41 rotor at 17,600 rpm; Beckman) at 4°C for 18 h, followed by buffer exchange with 1× phosphate-buffered saline (PBS).
Virion-capture ELISA.
Flat-bottom 96-well plates were coated with a murine DIII MAb (FL0251), which binds mature, immature, or mixed particles similarly well, at 4°C overnight, followed by blocking with 1% bovine serum albumin in 1× PBS for 1 h and addition of purified and UV-inactivated mature, immature, or mixed DENV1 particles, anti-E MAb (at different concentrations) or a positive-control serum, and anti-mouse IgG conjugated with horseradish peroxidase, each at 37°C for 1 h, and then tetramethylbenzidine (TMB) substrate and stop solution at the final step (21). The optical density (OD) at a wavelength of 450 nm with a reference wavelength of 650 nm was read, and the relative OD (rOD) was normalized by the OD of the positive-control serum sample (sample 17) in the same ELISA, which bound mature, immature, or mixed particles similarly well. The comparative amounts of the different particles were determined on the basis of the OD of serum sample 17 (about 1.0) during the titration prior to ELISA. Binding curves, Bmax, percent Bmax, and Kd were determined by nonlinear regression analysis (GraphPad Prism software, version 6.0) (21). The relative Bmax and relative Kd, which were the ratio of the Bmax and Kd of mature particles to those of immature particles, respectively, were calculated. To determine the prM content in particles, a virion-capture ELISA was performed for mature, immature, or mixed DENV1 particles and probed with a human anti-prM MAb (DVB59.3) or anti-E MAb (DVG17.12). The relative prM content of mature and mixed DENV1 particles was determined by the ratio of the OD detected by the anti-prM MAb to that detected by anti-E MAb normalized by the OD ratio of immature DENV1 particles (31).
Virion ELISA.
DENV virions (DENV1 to DENV4) or WNV VLP derived by ultracentrifugation of culture supernatants of virus-infected/mock-infected Vero cells or WNV prM/E plasmid-transfected 293T cells were UV inactivated (for virions), diluted in coating buffer, and coated on flat-bottom 96-well plates at 4°C overnight, followed by blocking and incubation with primary antibodies (serum at a 1:500 dilution) and secondary antibodies (41). After a final wash and incubation with TMB substrate and stop solution, the OD at 450 nm was read with a reference wavelength of 650 nm (41–43).
Depletion of cross-reactive antibodies.
To deplete cross-reactive (GR or CR) antibodies, sera (1:20 dilution in 1× PBS) were incubated with UV-inactivated DENV3 (from Vero cells) or pellets derived from the culture supernatants of mock-infected Vero cells in 1× PBS at 37°C for 1 h and ultracentrifuged at 150,000 × g and 4°C for 1 h to remove bound cross-reactive antibodies (41). Mock- or DENV3-depleted sera were tested for neutralization and by virion ELISAs to verify the depletion. Fourfold serial dilutions (starting from 1:1,000) of mock- or DENV3-depleted sera were also tested with a DENV1 virion ELISA (mature or immature particles) to determine the endpoint titer, which was the reciprocal of the highest dilutions of postdepletion serum that reached an OD value greater than the cutoff (the mean OD value plus 3 standard deviations of the mean for dengue-naive sera at a 1:1,000 dilution) using four-parameter nonlinear regression analysis (GraphPad Prism software, version 6.0). The proportion (in percent) of TS antibodies was equal to (endpoint titer of DENV3-depleted serum/endpoint titer of mock-depleted serum × 100. The percentage of cross-reactive (GR and CR) antibodies was equal to 100 − the percentage of TS antibodies.
Epitope mapping.
Epitopes of human MAbs were determined by a dot blot assay using lysates derived from 293T cells transfected with the wild-type DENV1 prM/E construct or each of the 67 surface-exposed E alanine mutants, followed by verification with capture ELISA using WT or mutant VLP as described previously (19, 21).
Microneutralization test.
Flat-bottom 96-well plates were seeded with Vero cells (3 × 104 cells per well) 24 h prior to infection. Twofold serial dilutions of serum were mixed with 50 focus-forming units of DENV1 (Hawaii strain), DENV2 (NGC strain), DENV3 (CH53489), or DENV4 (H241 strain) at 37°C for 1 h. The mixtures were added to each well, followed by incubation for 48 to 70 h, removal of medium, and fixation as described previously (21, 41). After adding murine MAb 4G2 and a secondary antibody mixture (IRDye 800CW-conjugated goat anti-mouse IgG at 1:10,000 and the DRAQ5 fluorescent probe at 1:10,000), the signal (800 nm/700 nm fluorescence) was detected by the LiCor Odyssey classic imaging system (LiCor Biosciences) and analyzed by Image Studio software to determine percent neutralization at different concentrations and NT50 (21, 57).
Percent occupancy of mature and mixed particles.
Based on the binding curve of each MAb, the Bmax was determined by nonlinear regression analysis, and percent Bmax, which represents the percent occupancy on mature or mixed particles at different concentrations, was determined. Based on the neutralization curve of each MAb, the percent neutralization was plotted against the percent occupancy on mature or mixed particles, and the percent occupancy at the NT50 concentration was calculated.
Statistical analysis.
Neutralizing potency and binding avidity were assessed by 1/NT50 and 1/Kd, respectively. The two-tailed Mann-Whitney test was used to determine the difference in Kd, Bmax, or NT50 between two groups, and the two-tailed Spearman correlation test was used to determine the relationship between neutralizing potency and avidity or Bmax by GraphPad Prism software (version 6.0). The two-tailed Wilcoxon signed-rank test was used to determine the difference in endpoint titer and relative endpoint titer between two groups (mock and DENV3 depletion) (GraphPad Prism software, version 6.0).
ACKNOWLEDGMENTS
We thank F. Sallusto at the Institute for Research in Biomedicine, Università Della Svizzera Italiana, Switzerland, for kindly providing 7 human MAbs.
This work was supported by awards R01AI110769-01 (to W.-K.W.) from the National Institute of Allergy and Infectious Diseases and P30GM114737 from the National Institute of General Medical Sciences, NIH; grants MOHW107-TDU-B-212-123006 (to J.-J.T.) from the Ministry of Health and Welfare and NHRI-MR-107-PP-38 (to J.-J.T.) from the National Health Research Institute, Taiwan; grants 095541/Z/11/Z and 203224/Z/16/Z (to G.S.) from The Wellcome Trust; and MR/N012658/1 (to G.S.) from the Newton-Medical Research Council and the National Institute for Health Research Oxford Biomedical Research Centre, UK.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Guzman MG, Harris E. 2015. Dengue. Lancet 385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2009. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control, 3rd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Murphy BR, Whitehead SS. 2011. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 5.Guy B, Briand O, Lang J, Saville M, Jackson N. 2015. Development of the Sanofi Pasteur tetravalent dengue vaccine: one more step forward. Vaccine 33:7100–7111. doi: 10.1016/j.vaccine.2015.09.108. [DOI] [PubMed] [Google Scholar]
- 6.Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortés M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M, CYD-TDV Dengue Vaccine Working Group 2015. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 7.Wilder-Smith A, Vannice KS, Hombach J, Farrar J, Nolan T. 2016. Population perspectives and World Health Organization recommendations for CYD-TDV dengue vaccine. J Infect Dis 214:1796–1799. doi: 10.1093/infdis/jiw341. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB. 2016. Critique of World Health Organization recommendation of a dengue vaccine. J Infect Dis 214:1793–1795. doi: 10.1093/infdis/jiw340. [DOI] [PubMed] [Google Scholar]
- 9.Sanofi. 2017. Sanofi updates information on dengue vaccine. Press release, 30 November Sanofi, Paris, France. [Google Scholar]
- 10.Pierson TC, Diamond MS. 2013. Flaviviruses, p 747–794. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 6th ed Lippincott William & Wilkins, Philadelphia, PA. [Google Scholar]
- 11.Modis Y, Ogata S, Clements D, Harrison SC. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 12.Crill WD, Roehrig JT. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol 75:7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao M, Sánchez-San Martín C, Zheng A, Kielian M. 2010. In vitro reconstitution reveals key intermediate states of trimer formation by the dengue virus membrane fusion protein. J Virol 84:5730–5740. doi: 10.1128/JVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 15.Crill WD, Hughes HR, Delorey MJ, Chang GJ. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gromowski GD, Barrett AD. 2007. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Sukupolvi-Petty S, Austin K, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. 2007. Type and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol 81:12816–12826. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrestha B, Brien JD, Sukupolvi-Petty S, Austin K, Edeling MA, Kim T, O'Brien KM, Nelson CA, Johnson S, Fremont DH, Diamond MS. 2010. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog 6:e1000823. doi: 10.1371/journal.ppat.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, Wu YC, Lai CY, Lu CH, Huang JH, Chang GJ, Wu HC, Wang WK. 2012. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high-throughput assay. PLoS Negl Trop Dis 6:e1447. doi: 10.1371/journal.pntd.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, Bazzone LE, Hogancamp G, Figueroa Sierra M, Fong RH, Yang ST, Lin L, Robinson JE, Doranz BJ, Chernomordik LV, Michael SF, Schieffelin JS, Isern S. 2013. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J Virol 87:52–66. doi: 10.1128/JVI.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai WY, Lai CY, Wu YC, Lin HE, Edwards E, Jumnainsong A, Kliks S, Halstead S, Mongkolsapaya J, Screaton GR, Wang WK. 2013. High avidity and potent neutralizing cross-reactive human monoclonal antibodies derived from secondary dengue virus infection. J Virol 87:12562–12575. doi: 10.1128/JVI.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, Austin SK, Sukupolvi-Petty S, Diamond MS, Crowe JE Jr. 2013. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 4:e00873-13. doi: 10.1128/mBio.00873-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey RA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brian J, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. 2011. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr, de Silva AM. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teoh EP, Kukkaro PP, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 4:139ra83. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 27.Smith SA, de Alwis AR, Kose N, Jadi RS, de Silva AM, Crowe JE Jr. 2014. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J Virol 88:12233–12241. doi: 10.1128/JVI.00247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fibriansah G, Tan JL, Smith SA, de Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Wang J, Harris E, de Silva A, Crowe JE Jr, Lok SM. 2014. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol Med 6:358–371. doi: 10.1002/emmm.201303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fibriansah G, Ibarra KD, Ng TS, Smith SA, Tan JL, Lim XN, Ooi JS, Kostyuchenko VA, Wang J, de Silva AM, Harris E, Crowe JE Jr, Lok SM. 2015. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 349:88–91. doi: 10.1126/science.aaa8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fibriansah G, Tan JL, Smith SA, de Alwis R, Ng TS, Kostyuchenko VA, Jadi RS, Kukkaro P, de Silva AM, Crowe JE, Lok SM. 2015. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 6:6341. doi: 10.1038/ncomms7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinsky A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, Grimes JM, Tsai WY, Lai CY, Wang WK, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR. 2015. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, Girard-Blanc C, Petres S, Shepard WE, Desprès P, Arenzana-Seisdedos F, Dussart P, Mongkolsapaya J, Screaton GR, Rey FA. 2015. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 33.Kielian M, Rey FA. 2006. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol 4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol 3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 35.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. 2008. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 36.Perera R, Kuhn RJ. 2008. Structural proteomics of dengue virus. Curr Opin Microbiol 11:369–377. doi: 10.1016/j.mib.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG, Fremont DH. 2009. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J 28:3269–3276. doi: 10.1038/emboj.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, Kasinrerk W, Malasit P, Kuhn RJ, Sittisombut N. 2010. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J Virol 84:8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zybert IA, van der Ende-Metselaar H, Wilschut J, Smit JM. 2008. Functional importance of dengue virus maturation: infectious properties of immature virions. J Gen Virol 89:3047–3051. doi: 10.1099/vir.0.2008/002535-0. [DOI] [PubMed] [Google Scholar]
- 40.Rodenhuis-Zybert IA, van der Schaar HM, da Silva Voorham JM, van der Ende-Metselaar H, Lei HY, Wilschut J, Smit JM. 2010. Immature dengue virus: a veiled pathogen? PLoS Pathog 6:e1000718. doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai WY, Durbin A, Tsai JJ, Whitehead S, Wang WK. 2015. Complexity of neutralization antibodies against multiple dengue viral serotypes after heterotypic immunization and secondary infection revealed by in-depth analysis of cross-reactive antibodies. J Virol 89:7348–7362. doi: 10.1128/JVI.00273-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai CY, Tsai WY, Lin SR, Kao CL, Hu SP, King CC, Wu HC, Wang WK. 2008. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol 82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai CY, Williams KL, Wu YC, Knight S, Balmaseda A, Harris E, Wang WK. 2013. Analysis of cross-reactive antibodies recognizing the fusion loop of envelope protein and correlation with neutralizing antibody titers in Nicaraguan dengue cases. PLoS Negl Trop Dis 7:e2451. doi: 10.1371/journal.pntd.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gromowski GD, Barrett ND, Barrett AD. 2008. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J Virol 82:8828–8837. doi: 10.1128/JVI.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont DH, Diamond MS. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. 2008. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4:229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, Pierson TC. 2013. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS Pathog 9:e1003761. doi: 10.1371/journal.ppat.1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallichotte EN, Widman DG, Yount BL, Wahala WM, Durbin A, Whitehead S, Sariol CA, Crowe JE Jr, de Silva AM, Baric RS. 2015. A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 6:e01461-15. doi: 10.1128/mBio.01461-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nivarthi UK, Kose N, Sapparapu G, Widman D, Gallichotte E, Pfaff JM, Doranz BJ, Weiskopf D, Sette A, Durbin AP, Whitehead SS, Baric R, Crowe JE Jr, de Silva AM. 2017. Mapping the human memory B cell and serum neutralizing antibody responses to dengue virus serotype 4 infection and vaccination. J Virol 91:e02041-16. doi: 10.1128/JVI.02041-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrade DV, Katzelnick LC, Widman DG, Balmaseda A, de Silva AM, Baric RS, Harris E. 2017. Analysis of individuals from a dengue-endemic region helps define the footprint and repertoire of antibodies targeting dengue virus 3 type-specific epitopes. mBio 8:e01205-17. doi: 10.1128/mBio.01205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai WY, Lin HE, Wang WK. 2017. Complexity of human antibody response to dengue virus: implication for vaccine development. Front Microbiol 8:1372. doi: 10.3389/fmicb.2017.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metz SW, Gallichotte EN, Brackbill A, Premkumar L, Miley MJ, Baric R, de Silva AM. 2017. In vitro assembly and stabilization of dengue and Zika virus envelope protein homo-dimers. Sci Rep 7:4524. doi: 10.1038/s41598-017-04767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouvinski A, Dejnirattisai W, Guardado-Calvo P, Vaney MC, Sharma A, Duquerroy S, Supasa P, Wongwiwat W, Haouz A, Barba-Spaeth G, Mongkolsapaya J, Rey FA, Screaton GR. 2017. Covalently linked dengue virus envelope glycoprotein dimers reduce exposure of the immunodominant fusion loop epitope. Nat Commun 8:15411. doi: 10.1038/ncomms15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith SA, Nivarthi UK, de Alwis R, Kose N, Sapparapu G, Bombardi R, Kahle KM, Pfaff JM, Lieberman S, Doranz BJ, de Silva AM, Crowe JE Jr. 2015. Dengue virus prM-specific human monoclonal antibodies with virus replication enhancing properties recognize a single immunodominant antigenic site. J Virol 90:780–789. doi: 10.1128/JVI.01805-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Alwis R, Williams KL, Schmid MA, Lai CY, Patel B, Smith SA, Crowe JE, Wang WK, Harris E, de Silva AM. 2014. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog 10:e1004386. doi: 10.1371/journal.ppat.1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. 2014. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J Virol 88:11726–11737. doi: 10.1128/JVI.01140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Govindarajan D, Meschino S, Guan L, Clements DE, ter Meulen JH, Casimiro DR, Coller BA, Bett AJ. 2015. Preclinical development of a dengue tetravalent recombinant subunit vaccine: immunogenicity and protective efficacy in nonhuman primates. Vaccine 33:4105–4116. doi: 10.1016/j.vaccine.2015.06.067. [DOI] [PubMed] [Google Scholar]