HBV research has been greatly hampered by the lack of robust cell culture and small animal models of HBV infection and propagation. The discovery of NTCP as an HBV receptor has greatly impacted the field of HBV research. Although HBV infection of NTCP-expressing human and murine hepatocyte cell lines has been demonstrated, its replication in cell culture appeared inefficient. To further improve cell culture systems of HBV infection and replication, we constructed NTCP-expressing HepG2 and AML12 cell lines that are highly permissive to HBV infection. More significantly, we found that DMSO and hydrocortisone markedly enhanced HBV transcription and replication in human and murine hepatocytes when added to the cell culture medium. These new cell culture models of HBV infection and replication will facilitate HBV research and antiviral drug discovery towards the ultimate elimination of chronic hepatitis B virus infection.
KEYWORDS: AML12, DMSO, HepG2, NTCP, PHH, hepatitis B virus, HBV, hydrocortisone, infection, morphogenesis, replication
ABSTRACT
Hepatitis B virus (HBV) is a major cause of chronic liver diseases, including hepatitis, cirrhosis, and hepatocellular carcinoma. HBV research has been hampered by the lack of robust cell culture and small animal models of HBV infection. The discovery of sodium taurocholate cotransporting polypeptide (NTCP) as an HBV receptor has been a landmark advance in HBV research in recent years. Ectopic expression of NTCP in nonpermissive HepG2, Huh7, and AML12 cell lines confers HBV susceptibility. However, HBV replication in these human and murine hepatocyte cell lines appeared suboptimal. In the present study, we constructed stable NTCP-expressing HepG2 and AML12 cell lines and found that HBV permissiveness is correlated with NTCP expression. More significantly, we developed robust HBV cell culture models by treating the HBV-infected cells with dimethyl sulfoxide (DMSO) and hydrocortisone, which significantly promoted HBV replication and production. Mechanistic studies suggested that hydrocortisone significantly enhanced the transcription and expression of PGC1α and HNF4α, which are known to promote HBV transcription and replication. These new human and murine hepatocyte culture systems of HBV infection and replication will accelerate the determination of molecular aspects underlying HBV infection, replication, and morphogenesis in human and murine hepatocytes. We anticipate that our HBV cell culture models will also facilitate the discovery and development of antiviral drugs towards the ultimate eradication of chronic hepatitis B virus infection.
IMPORTANCE HBV research has been greatly hampered by the lack of robust cell culture and small animal models of HBV infection and propagation. The discovery of NTCP as an HBV receptor has greatly impacted the field of HBV research. Although HBV infection of NTCP-expressing human and murine hepatocyte cell lines has been demonstrated, its replication in cell culture appeared inefficient. To further improve cell culture systems of HBV infection and replication, we constructed NTCP-expressing HepG2 and AML12 cell lines that are highly permissive to HBV infection. More significantly, we found that DMSO and hydrocortisone markedly enhanced HBV transcription and replication in human and murine hepatocytes when added to the cell culture medium. These new cell culture models of HBV infection and replication will facilitate HBV research and antiviral drug discovery towards the ultimate elimination of chronic hepatitis B virus infection.
INTRODUCTION
Hepatitis B virus (HBV) chronically infects approximately 240 million people worldwide, with severe consequences, including chronic hepatitis, cirrhosis, liver failure, and hepatocellular carcinoma (HCC) (1, 2). Human HBV is the prototype member of the Hepadnaviridae family, consisting of small DNA viruses with genomes comprised of partially double-stranded DNA (3, 4). HBV enters hepatocytes via receptor-mediated endocytosis. Upon HBV cell entry and uncoating, the partially double-stranded DNA genome, in a relaxed circular conformation (rcDNA), is transported into the nucleus and converted to a covalently closed circular DNA (cccDNA) (5–8). The rcDNA-to-cccDNA conversion is believed to be carried out by cellular enzymes, including DNA polymerase kappa and ligase (9, 10). The cccDNA serves as a template for RNA transcription by the cellular polymerase II (Pol II) RNA polymerase to produce viral mRNAs and a terminally redundant pregenomic RNA (pgRNA). The viral mRNAs and pgRNA encode 7 proteins (HBs), including three different forms (L, M, and S) of envelope proteins, precore (HBe precursor), core (HBc), X (HBx), and pol (reverse transcriptase). The pgRNA, together with the attached pol, is encapsidated by the core protein to form a nucleocapsid in which reverse transcription of the pgRNA takes place, resulting in the virion’s rcDNA genome. The syntheses of viral RNA and DNA are modulated by many different cellular proteins (3). Most of our current knowledge about HBV DNA replication is derived from numerous studies with recombinant systems and DNA transfection methods. However, relatively little is known about the molecular mechanisms underlying each step of the HBV infectious cycle, largely due to the lack of a robust cell culture model of HBV infection and propagation.
The discovery of sodium taurocholate cotransporting polypeptide (NTCP) as the HBV receptor (also the hepatitis delta virus [HDV] receptor) has been a landmark advance in HBV research in recent years (11). Human hepatocellular carcinoma cell lines HepG2 and Huh-7 and an immortalized mouse hepatocyte cell line, AML12, expressing NTCP have been shown to be susceptible to HBV infection and replication, albeit inefficiently (11–20). AML12 appears to be the only murine hepatocyte cell line known to support HBV infection and replication when expressing NTCP (13). The expression of NTCP in transgenic mice conferred HDV but not HBV susceptibility (21), suggesting the existence of murine restriction factor(s) of HBV replication or the lack of cellular factor(s) essential for HBV infection and/or replication (22). It has long been known that HBV cccDNA could not be detected in transgenic mouse lineages (23). Therefore, there is an urgent need to develop more robust human and murine hepatocyte culture models of HBV infection and propagation for antiviral drug discovery and the determination of the molecular aspects governing each step of the HBV life cycle. In the present study, we have developed stable NTCP-expressing HepG2 and AML12 cell lines that are highly permissive to HBV infection. HBV replication in the NTCP-expressing HepaG2 and AML12 cell lines was greatly enhanced by treatment with dimethyl sulfoxide (DMSO) and hydrocortisone. These robust cell culture models of HBV infection and propagation will be valuable for the determination of the importance of cellular factors modulating HBV infection, replication, and morphogenesis. Additionally, these new HBV cell culture systems will facilitate the discovery of effective new classes of antiviral drugs toward the eradication of chronic hepatitis B virus infection.
RESULTS
Correlation of HBV infection and NTCP expression.
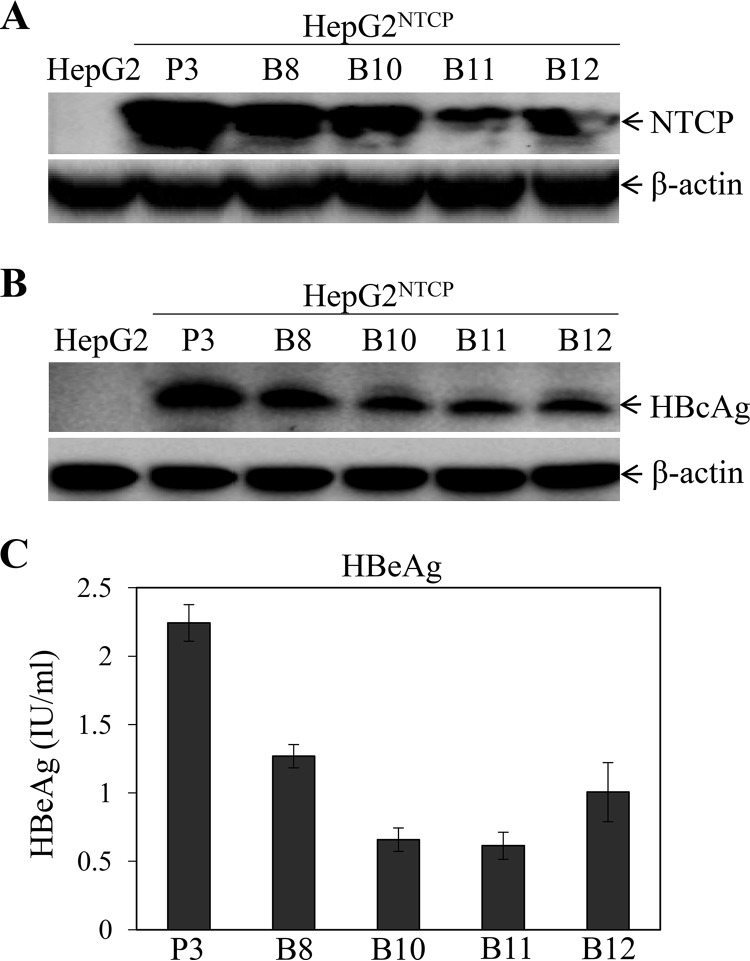
HBV research has been hampered by the lack of a robust cell culture system for HBV infection until the discovery of NTCP as the HBV receptor (11). A number of groups have developed stable NTCP-expressing HepG2, Huh7, and AML12 cell lines and demonstrated that NTCP expression in nonpermissive hepatocytes confers HBV susceptibility (11–20). However, HBV replication in the NTCP-reconstituted human and murine hepatocytes appeared suboptimal. To develop robust cell culture models of HBV infection and propagation, we sought to overexpress the HBV receptor NTCP under the control of a human apoE promoter and enhancer in a liver-specific expression vector, pLiv7 (24). Upon DNA transfection and selection with blasticidin or puromycin, stable NTCP-expressing HepG2 and AML12 cell clones were screened for NTCP expression by Western blotting. The expression of NTCP among different cell clones was further validated by immunofluorescence assay (IFA) (data not shown). Several HepG2 cell lines expressing various levels of NTCP (HepG2NTCP) were chosen for the determination of their susceptibility to HBV infection. The puromycin-resistant HepG2NTCP cell clone 3 (HepG2NTCP-P3) expressed the highest level of NTCP compared to blasticidin-resistant HepG2NTCP cell clones (B8, B10, B11, and B12) (Fig. 1A). As a result, HepG2NTCP-P3 was the most susceptible to HBV infection, as determined by the levels of HBc antigen (HBcAg) in the HBV-infected cells (Fig. 1B), as well as the levels of HBeAg in the cell culture supernatants (Fig. 1C). There is a close correlation between HBV infection and NTCP expression among different NTCP-expressing HepG2 cell lines, suggesting that the level of NTCP expression is important for HBV infection.
FIG 1.
Correlation of NTCP expression and HBV susceptibility. (A) HepG2 cells were transfected with pLiv7/Blast/NTCP and pLiv7/Puro/NTCP, respectively. Upon selection with blasticidin (B8, B10, B11, and B12) or puromycin (P3), cell clones were picked up and expanded. The expression of NTCP was detected by Western blotting using an NTCP-specific monoclonal antibody. (B) HepG2NTCP cell lines were infected with HBV in the presence of 4% PEG 8000 for 12 h. Upon removal of unbound HBV and PEG by washing with PBS, the HBV-infected cells were incubated with fresh DME-F12 medium containing 3% FBS. After 4 days postinfection (p.i.), cells were lysed in a RIPA buffer. HBcAg in cell lysates was detected by Western blotting using an HBc-specific monoclonal antibody. (A, B) The housekeeping gene β-actin was used as an internal control. (C) The levels of HBeAg in the supernatants were quantified by chemiluminescence immunoassay. Average values (±SD) derived from three experiments are plotted.
Promotion of HBV replication by DMSO and hydrocortisone.
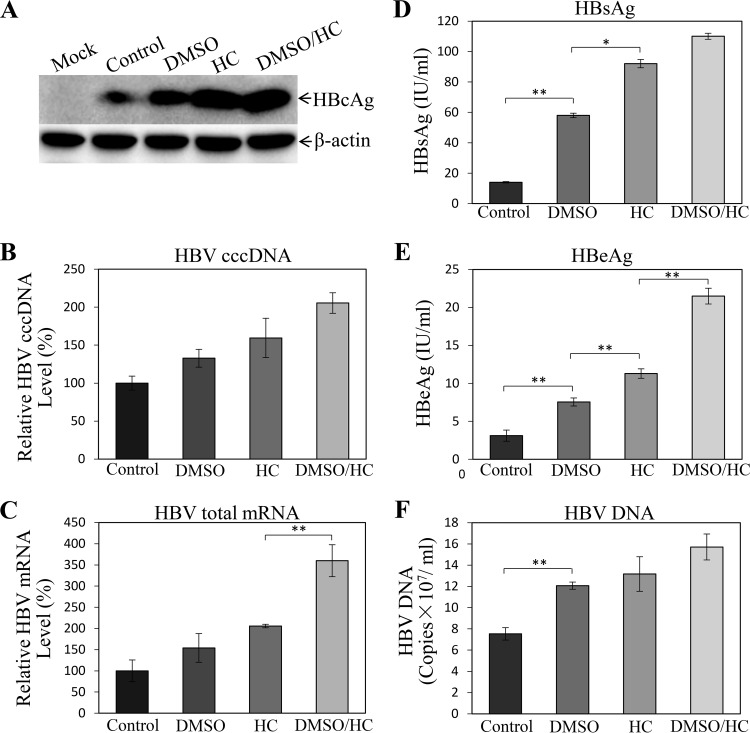
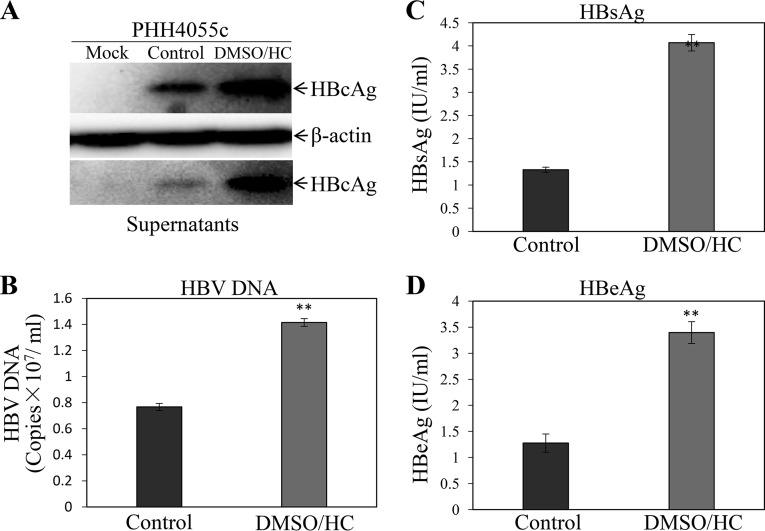
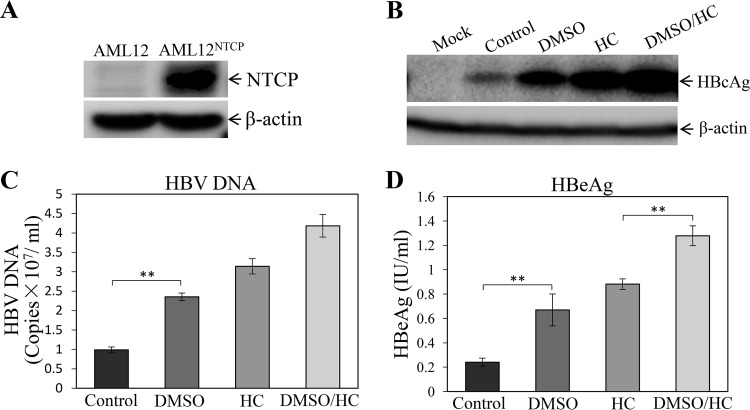
Although the NTCP-expressing HepG2 cell lines were susceptible to HBV infection, they did not appear to support robust HBV replication, based on lower levels of HBcAg in the cells and HBeAg in the cell culture supernatants (Fig. 1). Both DMSO and hydrocortisone were previously used as differentiation inducers of normal human hepatocytes and the human hepatoma cell line HepaRG, which were shown to be permissive to HBV infection (25–27). Hydrocortisone was also reported to enhance the replication of many other different viruses (28–33). To test whether DMSO and hydrocortisone would promote HBV replication, the HBV-infected HepG2NTCP-P3 cells were treated with 1% DMSO, 5 μg/ml hydrocortisone, or both for 4 days. The levels of HBcAg, HBV cccDNA, and total HBV mRNAs in the HBV-infected HepG2NTCP-P3 cells were determined by Western blotting, quantitative PCR (qPCR), and qRT-PCR, respectively. The cell culture supernatants were collected for quantification of HB surface protein antigen (HBsAg), HBeAg, and HBV DNA. Interestingly, DMSO modestly increased the levels of HBcAg (Fig. 2A), HBV cccDNA (Fig. 2B), and mRNAs (Fig. 2C) in the cells, by 1.8, 1.4, and 1.5 times, respectively. Likewise, the levels of HBsAg (Fig. 2D), HBeAg (Fig. 2E), and HBV DNA (Fig. 2F) in the supernatants of DMSO-treated cells were 4-, 2.5-, and 1.6-fold higher. Hydrocortisone resulted in even higher levels of HBV replication than DMSO, as shown by 3-, 1.6-, and 2-fold-higher levels of HBcAg, cccDNA, and mRNAs in the infected cells and 7-, 3.7-, and 1.8-fold increases of HBsAg, HBeAg, and HBV DNA in the supernatants (Fig. 2). The combination of DMSO and hydrocortisone further enhanced the levels of HBcAg, HBV cccDNA, and mRNAs in the cells and HBsAg, HBeAg, and HBV DNA in the supernatants, which were the highest among the different treatment groups (Fig. 2). The question arose whether DMSO and hydrocortisone would also promote HBV transcription and replication in primary human hepatocytes (PHHs) and AML12NTCP cells. HBV-infected PHHs and AML12NTCP cells were cultured in the presence of 1% DMSO and 5 μg/ml of hydrocortisone for 4 days. Similarly, DMSO and hydrocortisone significantly enhanced HBV replication in PHHs, as shown by the 3-fold increase of HBcAg in the cells and 1.8-, 3-, and 2.7-fold-higher levels of HBV DNA, HBsAg, and HBeAg in the supernatants, respectively (Fig. 3). We also constructed stable AML12NTCP cells (Fig. 4A). HBV transcription and replication in AML12NTCP cells were also greatly induced by treatment with DMSO and hydrocortisone. DMSO resulted in a 3-fold increase of HBcAg in the cells and 2.4- and 3-fold-higher levels of HBV DNA and HBeAg in the supernatants (Fig. 4). Hydrocortisone enhanced HBV transcription and replication in the AML12NTCP cells to even higher magnitudes, as shown by a 4-fold increase of HBcAg in the cells and about 3- and 4-fold-higher levels of HBV DNA and HBeAg, respectively, in the supernatants (Fig. 4). The combination of DMSO and hydrocortisone markedly promoted HBV transcription and replication in the AML12NTCP cells, as shown by a 5-fold increase of HBcAg in the cells and 4.2- and 5.5-fold-higher levels of HBV DNA and HBeAg in the supernatants (Fig. 4). Taken together, these results demonstrate that DMSO and hydrocortisone could promote HBV transcription and replication in HepG2NTCP cells, PHHs, and AML12NTCP cells.
FIG 2.
Enhancement of HBV replication by DMSO and hydrocortisone. HepG2NTCP-P3 cells were infected with HBV at an MOI of 100 genome copies (GC) in the presence of 4% PEG 8000 for 12 h. Upon removal of unbound HBV and PEG 8000, HBV-infected cells were incubated with DME-F12 medium containing 1% DMSO, 5 μg/ml hydrocortisone (HC), or both (DMSO/HC). (A) After culturing for 4 days, cell lysates were collected for detection of HBcAg by Western blotting. (B) HBV cccDNA in the cells was quantified by qPCR, using mitochondrial DNA as an internal control for normalization. (C) Total RNA was extracted from HBV-infected cells with TRIzol reagent and was reverse transcribed with superscript III. Resulting cDNAs were quantified by qPCR. (D to F) The levels of HBsAg (D) and HBeAg (E) in the supernatants were quantified by chemiluminescence immunoassay. HBV DNA in the supernatants was quantified by qPCR (F). (B to F) Average values (±SD) derived from three experiments are plotted. *, P < 0.05; **, P < 0.01.
FIG 3.
Enhancement of HBV transcription and replication in PHHs by DMSO and hydrocortisone. PHHs were infected with HBV at an MOI of 100 GC in the presence of 4% PEG 8000 for 12 h. The HBV-infected PHHs were cultured in the PHH maintenance medium without (Control) or with 1% DMSO and 5 μg/ml HC (DMSO/HC) for 4 days by changing medium every day. At 4 days p.i., the HBV-infected PHHs were lysed in a RIPA buffer and the cell culture supernatants were collected. (A) The levels of HBcAg in cell lysates were determined by Western blotting. (B to D) The levels of HBV DNA (B), HBsAg (C), and HBeAg (D) in the supernatants were quantified by qPCR or chemiluminescence immunoassay. Average values (±SD) derived from three experiments are plotted. **, P < 0.01.
FIG 4.
Promotion of HBV transcription and replication in the AML12NTCP cells by DMSO and hydrocortisone. AML12NTCP cells were infected with HBV at an MOI of 100 GC in the presence of 4% PEG 8000 for 12 h. Upon removal of unbound HBV and PEG 8000, the HBV-infected AML12NTCP cells were cultured in DME-F12 medium containing 3% FBS and 1% DMSO, 5 μg/ml HC, or both (DMSO/HC). (A, B) After 4 days p.i., the HBV-infected AML12NTCP cells were lysed in a RIPA buffer and the levels of NTCP (A) and HBcAg (B) in cell lysates were measured by Western blotting. (C, D) The levels of HBV DNA (C) and HBeAg (D) in the cell culture supernatants were quantified by qPCR and chemiluminescence immunoassay, respectively. Average values (±SD) derived from three experiments are plotted. **, P < 0.01.
Optimization of conditions for HBV infection and replication.
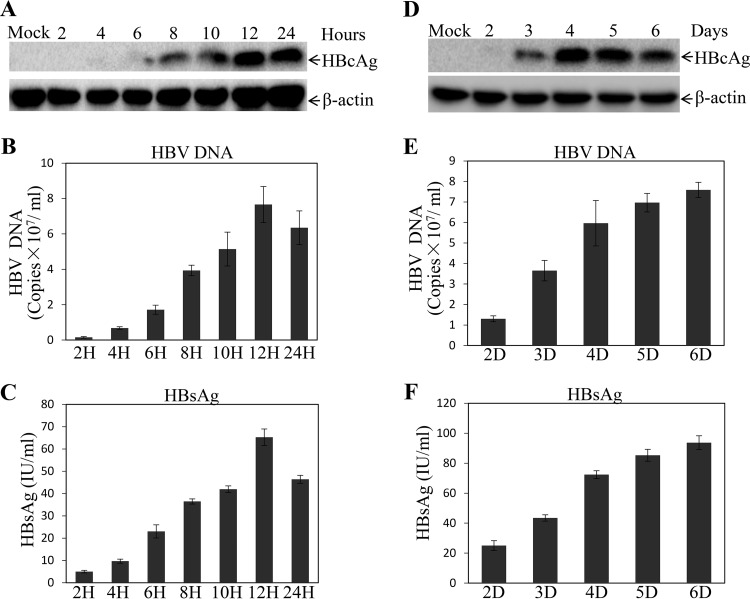
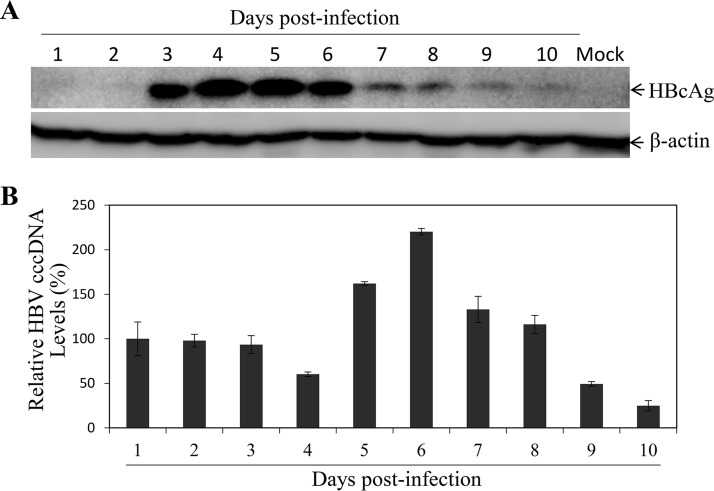
To optimize the conditions for HBV infection and replication in the NTCP-expressing human and murine hepatocytes, we determined the optimal time for HBV infection and replication and compared different media and fetal bovine serum (FBS) concentrations. It was found that 12 h of HBV infection reached the highest levels of HBV transcription and replication, based on the levels of HBcAg in the cells (Fig. 5A) and HBV DNA (Fig. 5B) and HBsAg (Fig. 5C) in the supernatants. Longer HBV infection time did not further increase the levels of HBcAg, HBV DNA, or HBsAg. A 4-day incubation after HBV infection was sufficient for the detection of HBcAg in the cells (Fig. 5D), although the levels of HBV DNA (Fig. 5E) and HBsAg (Fig. 5F) in the supernatants appeared to continue to rise after day 4. These results were further confirmed by a longer kinetic study of HBV replication upon treatment with DMSO and hydrocortisone (Fig. 6). HBcAg reached the highest levels at day 4 and day 5 postinfection and then gradually decreased after day 6 (Fig. 6A). Similarly, the levels of HBV cccDNA initially decreased at day 4 and then rose to the highest level at day 6, followed by a gradual reduction after day 7 (Fig. 6B). It is not clear why HBV replication fluctuated at different time points. Additionally, we determined the effects of different media on HBV transcription and replication and found that Dulbecco’s modified Eagle’s medium (DMEM) and DMEM-Ham’s nutrient mixture F-12 (DME-F12 medium) but not William E medium could support efficient HBV replication (data not shown). We also titrated FBS, DMSO, and hydrocortisone concentrations using the optimal infection (12 h) and incubation (4 days) times determined as described above. Similar to the results of a recent study (16), 3% FBS was optimal for HBV transcription and replication, as higher FBS concentrations actually reduced the levels of HBcAg in the cells and of HBsAg, HBeAg, and HBV DNA in the supernatants (data not shown). Likewise, 1% DMSO and 5 μg/ml hydrocortisone resulted in the highest levels of HBV transcription and replication. Higher concentrations of DMSO and hydrocortisone actually reduced HBV transcription and replication due to cytotoxicity (data not shown). Thus, the optimal conditions for HBV infection and replication include a 12-h infection time, 96-h culturing period, and DME-F12 medium containing 3% FBS, 1% DMSO, and 5 μg/ml hydrocortisone.
FIG 5.
Optimization of conditions for HBV infection and replication. (A to C) HepG2NTCP-P3 cells were infected with HBV at an MOI of 100 GC in the presence of 4% PEG 8000 for various times (2, 4, 6, 8, 10, 12, and 24 h). HBV-infected cells were cultured in DME-F12 medium containing 3% FBS, 1% DMSO, and 5 μg/ml hydrocortisone. (A) After 4 days p.i., the HBV-infected cells were lysed and the levels of HBcAg were determined by Western blotting. (B) HBV DNA in the supernatants was quantified by qPCR. (C) The levels of HBsAg in the supernatants were quantified by chemiluminescence immunoassay. (D to F) Optimal time of HBV replication was determined by HBV infection with an MOI of 100 GC in the presence of 4% PEG 8000 for 12 h. The HBV-infected cells were lysed in a RIPA buffer at different time points (2, 3, 4, 5, and 6 days) after HBV infection. The levels of HBcAg in the infected cells (D) and of HBV DNA (E) and HBsAg (F) in the supernatants were determined by Western blotting, qPCR, and chemiluminescence immunoassay, respectively. (B, C, E, and F) Average values (±SD) derived from three experiments are plotted.
FIG 6.
Kinetics of HBcAg and HBV cccDNA synthesis upon induction with DMSO and hydrocortisone. HepG2NTCP-P3 cells were infected with HBV at an MOI of 100 GC in the presence of 4% PEG 8000 for 12 h. HBV-infected cells were cultured in DME-F12 medium containing 3% FBS, 1% DMSO, and 5 μg/ml hydrocortisone. (A) At every day postinfection, the HBV-infected cells were lysed and the levels of HBcAg were determined by Western blotting. (B) HBV cccDNA in the cells was subjected to Hirt extraction, followed by digestion with T5 exonuclease and quantification by qPCR using cccDNA-specific primers and probe. Average values (±SD) derived from three experiments are plotted.
Validation of the optimized HBV cell culture conditions.
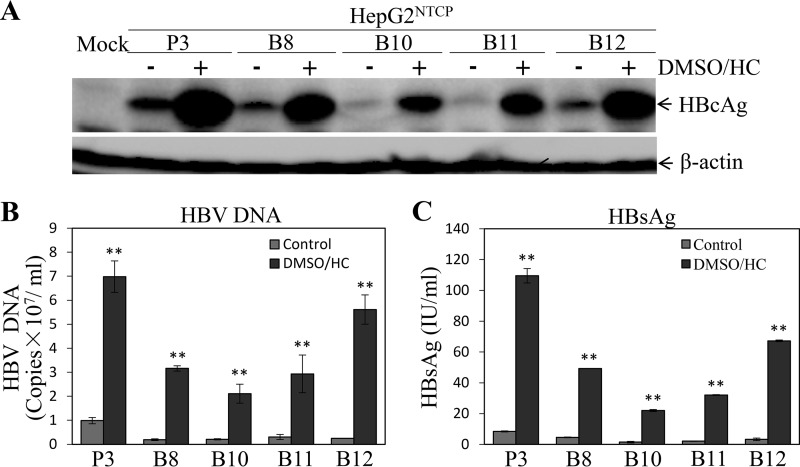
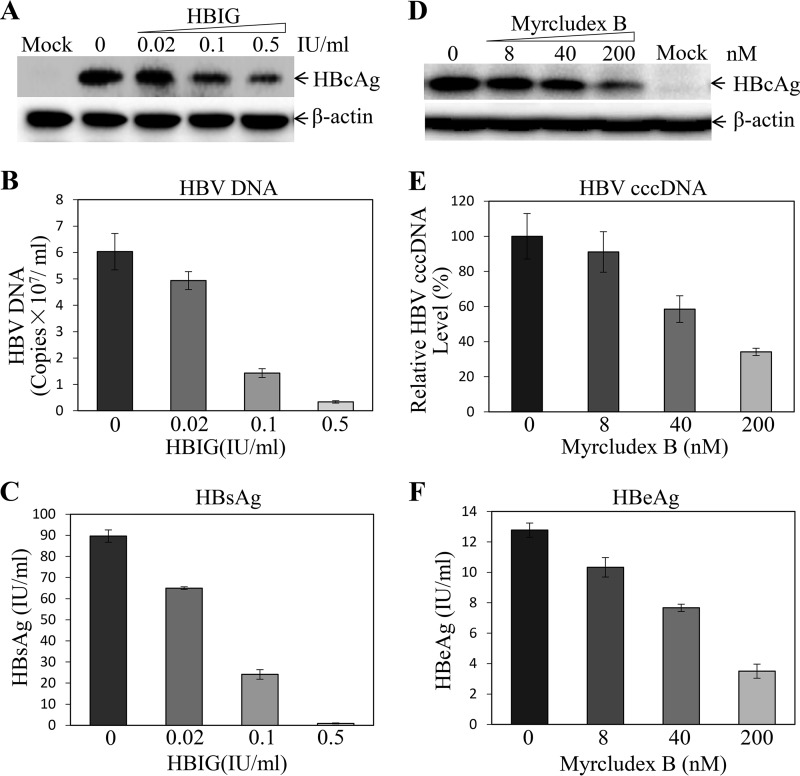
To validate the conditions for HBV infection and replication determined as described above, we compared different HepG2NTCP cell lines cultured in the presence and absence of DMSO and hydrocortisone. The HBV-infected HepG2NTCP cell lines were incubated with DME-F12 medium containing 1% DMSO, 5 μg/ml hydrocortisone, and 3% FBS for 4 days. Strikingly, the combination of DMSO and hydrocortisone promoted HBV replication in all NTCP-expressing HepG2 cell lines by 7 to 20 times (depending on cell lines) compared to its replication in the control medium without DMSO and hydrocortisone (Fig. 7). We also validated HBV infection using hepatitis B immune globulin (HBIG) and the HBV entry inhibitor myrcludex B. As expected, HBIG effectively neutralized HBV infectivity in a dose-dependent manner (Fig. 8). HBIG at a concentration of 0.5 IU/ml resulted in a 74% reduction of HBcAg (Fig. 8A) in the cells and an 82% decreased level of HBV DNA (Fig. 8B) and 99% lower HBsAg (Fig. 8C) in the cell culture supernatants. Similarly, myrcludex B inhibited HBV infection, as demonstrated by 70% reduction of HBcAg (Fig. 8D) and 66% lower HBV cccDNA (Fig. 8E) in the cells, as well as a 3.7-fold decrease of HBeAg (Fig. 8F) in the supernatants at 200 nM myrcludex B. These data suggest that our robust HBV cell culture system can be used to screen HBV inhibitors.
FIG 7.
Comparison of HBV transcription and replication efficiencies with and without DMSO and hydrocortisone (DMSO/HC) treatment among different HepG2NTCP cell lines. Different HepG2NTCP cell lines were infected with HBV at an MOI of 100 GC in the presence of 4% PEG 8000 for 12 h and then cultured in DME-F12 medium without (−) or with 1% DMSO and 5 μg/ml HC (+). After 4 days p.i., the HBV-infected cells were lysed in a RIPA buffer and cell culture supernatants were collected. (A) HBcAg in the cell lysates was detected by Western blotting. (B, C) The levels of HBV DNA (B) and HBsAg (C) in the supernatants were quantified by qPCR and chemiluminescence immunoassay, respectively. Average values (±SD) derived from three experiments are plotted. **, P < 0.01.
FIG 8.
Inhibition of HBV infection by HBIG and myrcludex B. HepG2NTCP-P3 cells were infected with HBV at an MOI of 100 GC in the presence of 4% PEG 8000 and increasing concentrations of HBIG (0, 0.02, 0.1, and 0.5 IU) or myrcludex B (0, 80, 40, and 200 nM) for 12 h. The HBV-infected cells were cultured with DME-F12 medium containing 3% FBS, 1% DMSO, and 5 μg/ml HC. After 4 days p.i., the HBV-infected cells were lysed in a RIPA buffer. (A, D, and E) The levels of HBcAg (A, D) and HBV cccDNA (E) in the HBV-infected cells were determined by Western blotting and qPCR, respectively. (B, C, and F) The levels of HBV DNA (B), HBsAg (C), and HBeAg (F) in the cell culture supernatants were quantified by qPCR or chemiluminescence immunoassay. Average values (±SD) derived from three experiments are plotted.
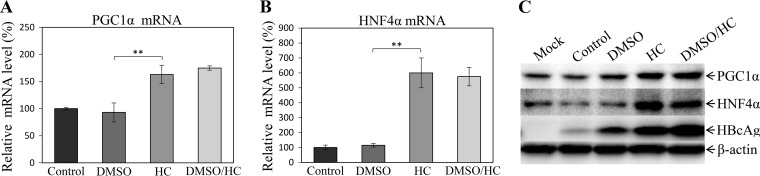
Induction of HBV transcription factor by hydrocortisone.
It was previously reported that glucocorticoid could induce the expression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) (34, 35), which was found to modulate HBV gene transcription through a nuclear transcription factor, hepatocyte nuclear factor 4α (HNF4α) (36, 37). To investigate the mechanism of action of hydrocortisone for the promotion of HBV transcription and replication, we determined the levels of PGC1α (Fig. 9A) and HNF4α (Fig. 9B) gene transcription and expression (Fig. 9C) in the HBV-infected HepG2NTCP-P3 cells that were treated with DMSO, hydrocortisone, or both. The PGC1α and HNF4α mRNAs and proteins were quantified by qRT-PCR and Western blotting, respectively. The levels of PGC1α mRNA (Fig. 9A) and HNF4α mRNA (Fig. 9B) were increased by 1.6- and 6-fold, respectively, in the HBV-infected cells when treated with hydrocortisone or DMSO-hydrocortisone. Their corresponding protein levels were also enhanced by 1.8 to 2.0 times in the hydrocortisone-treated HepG2NTCP-P3 cells compared to the levels in parental or DMSO-treated HepG2NTCP-P3 cells (Fig. 9). These findings suggest that hydrocortisone but not DMSO promoted HBV transcription, probably through the induction of PGC1α and HNF4α gene transcription and expression. This is consistent with the previous findings that DMSO induced the transcription of a number of cellular genes, except for PGC1α and HNF4α (17). It is possible that other gluconeogenic genes and nuclear factors involved in the regulation of HBV transcription and replication might also be induced by hydrocortisone treatment (36, 37). Future investigations are warranted to determine other cellular genes important for HBV transcription and replication.
FIG 9.
Induction of PGC1α and HNF4α gene transcription and expression by hydrocortisone. HepG2NTCP-P3 cells were infected with HBV at an MOI of 100 GC in the presence of 4% PEG 8000 for 12 h. The HBV-infected cells were cultured in DME-F12 medium containing 1% DMSO, 5 μg/ml HC, or both (DMSO/HC). (A, B) After 4 days p.i., the levels of PGC1α mRNA (A) and HNF4α mRNA (B) in the HBV-infected cells were quantified by a real-time RT-PCR method. Average values (±SD) derived from three experiments are plotted. **, P < 0.01. (C) The levels of PGC1α and HNF4α expression were determined by Western blotting using PGC1α- and HNF4α-specific monoclonal antibodies. β-Actin was used as an internal control.
DISCUSSION
We have developed robust human and murine hepatocyte culture systems with significantly enhanced efficiency of HBV infection and replication by using several strategies. First, we used a liver-specific vector to overexpress the HBV receptor NTCP without tagging. We screened a number of stable HepG2 cell clones and identified a puromycin-resistant HepG2NTCP cell clone (HepG2NTCP-P3) with the highest level of NTCP expression and the greatest susceptibility to HBV infection and/or replication. It appears that there is a close correlation between NTCP expression and HBV infection among different NTCP-expressing HepG2 cell clones (Fig. 1), suggesting the importance of the level of NTCP in HBV susceptibility. More significantly, we found that DMSO and hydrocortisone in combination could greatly enhance HBV transcription and replication when added to the infected cells (Fig. 2). We had previously tried to detect HBV infection and replication using the methods reported by others but failed to detect significant levels of HBV replication in the NTCP-expressing HepG2 cells by Western blotting, even after 10 days of HBV infection (data not shown). Now we could detect high levels of HBcAg in the HBV-infected cells as early as 3 days after HBV infection when 1% DMSO and 5 μg/ml hydrocortisone were added into the cell culture medium (Fig. 5 and 6). Using this induction medium, we have determined the optimal time required for HBV infection and replication. A 12-h HBV infection along with a 96-h incubation after HBV infection appeared to be sufficient for robust detection of HBcAg in the cells and of HBsAg, HBeAg, and HBV DNA in the cell culture supernatants. A longer time of HBV infection and further incubation after 5 days postinfection actually caused a reduction of HBcAg in the cells (Fig. 5 and 6). The exact reason(s) for the reduction of HBV infection and/or replication by longer infection and replication times are not clear. It might be due to cytotoxicity caused by the 4% polyethylene glycol 8000 (PEG 8000) that was used to facilitate HBV infection. The reduction of HBV transcription and replication after 5 days of incubation could be due to cell growth arrest, depletion of nutrients, and/or resistance to hydrocortisone induction. It was previously found that high cell density (overconfluence) resulted in an inhibition of hepatitis C virus (HCV) replication due to depletion of the intracellular nucleoside pool (38). However, we could not reverse the decrease of HBV transcription and/or replication by changing the medium every other day (Fig. 6).
DMSO and hydrocortisone promoted HBV replication not only in the HepG2NTCP cells but also in PHHs and the NTCP-expressing murine hepatocytes (AML12 cell line) (Fig. 3 and 4). It was also reported that hydrocortisone could enhance the replication of other viruses, including herpes simplex virus (31), human cytomegalovirus (39), Epstein-Barr virus (40), respiratory syncytial virus (29), and polyomavirus (30). The mechanism of action of DMSO for the enhancement of HBV replication is not clear, although it was found to induce the transcription of a number of cellular genes (17). It did not affect the expression of the HBV transcription factor HNF4α, unlike hydrocortisone (Fig. 8) (17). Its promotion of HBV replication is probably through the induction of hepatocyte differentiation, as previously demonstrated by others (25–27). Apart from DMSO, hydrocortisone induced the transcription and expression of PGC1α and HNF4α (Fig. 9), which was previously shown to promote HBV transcription and replication (41–43). Consistent with this interpretation, the levels of HBV RNAs and HBcAg in the cells and of HBeAg in the supernatants were markedly increased when hydrocortisone was added to culture medium (Fig. 7 and data not shown). Therefore, the enhancement of HBV transcription and gene expression by DMSO and hydrocortisone made possible the robust detection of HBV proteins by biochemical assays like Western blotting.
In summary, we have developed human and murine hepatocyte culture models of robust HBV infection and replication. These novel HBV propagation systems will be valuable for the determination of virus-host interaction and the underlying molecular mechanisms of cellular factors in HBV infection, replication, and morphogenesis. Their application to antiviral drug screening and evaluation will accelerate the identification and development of new classes of drugs toward the ultimate eradication of chronic hepatitis B virus infection.
MATERIALS AND METHODS
Cells and virus.
HepG2 and murine hepatocyte AML12 (ATCC) cell lines, as well as the HepAD38 cell line, were maintained in DME-F12 medium (HyClone) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 0.1 mM nonessential amino acids, 1× sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma) at 37°C in a 5% CO2 incubator. Primary human hepatocytes (PHHs) were from Lonza (lot number HUM4055C) and were cultured according to the manufacturer’s instructions. Cell culture flasks and plates were coated with 50 μg/ml rat tail collagen type I (Corning). HBV derived from HepAD38 cells was concentrated by precipitation with 10% polyethylene glycol 8000 (PEG 8000) (Hampton Research). The genome copy numbers of HBV DNA were quantified by a real-time PCR method.
Antibodies, peptide, and chemicals.
The HBV core-specific mouse monoclonal antibody was from Tokyo Future Style. Goat anti-HNF4α and anti-PGC1α polyclonal antibodies, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and rabbit anti-goat IgG secondary antibodies, and normal mouse IgG were all purchased from Santa Cruz Biotechnology. A human β-actin monoclonal antibody (AC15) was purchased from Sigma-Aldrich. HRP-conjugated goat anti-rabbit antibody was from Cell Signaling. Hepatitis B immune globulin (HBIG) was from Nanyue Biopharmaceuticals, Inc., China. NTCP-specific mouse monoclonal antibody was developed in the laboratory. Myrcludex B was kindly provided by Stephan Urban (44, 45).
Plasmid DNA construction.
The DNA fragment containing an EM-7 promoter and a blasticidin resistance gene was amplified by PCR using pcDNA6/TR (Invitrogen) as a template and synthetic oligonucleotides EM7P/Sac II (5ʹ-TCGCCGCGGAGGCCTAGGCTTT TG-3ʹ) and SV40pA/Sal I (5ʹ-GCGCGGTCGACGGTATACAGACATGATAA GATACATTG-3ʹ) as primers. The PCR DNA was digested with restriction enzymes Sac II and Sal I and inserted into the vector pLiv7 between the Sac II and Sal I sites, resulting in a vector designated pLiv7/Blast. The puromycin resistance gene was amplified by PCR using primers Puro/Nhe I (5ʹ-CTAGCTAGCGCCACCATGACCGAGTACAAGC-3ʹ) and Puro/Not I (5ʹ-CATTATATGCGGCCGCTCAGGCACCGGGCTTG-3ʹ) and pLentilox-IRES-Puro (Addgene) as the template. The Puro PCR product was digested with Nhe I and Not I and cloned into the vector peGFP/N1 (Clontech) between the Nhe I and Not I sites (replacing the enhanced green fluorescent protein [eGFP] gene). The DNA fragment containing the CMV promoter, puromycin resistance gene, and simian virus 40 (SV40) poly(A) signal was amplified by PCR using pCMV/Sac I (5ʹ-GCGCGAGCTCTTAATTAATAGTAATCAATTACGG-3ʹ) and SV40pA/Xho I (5ʹ-GCGCTCGAGCTTAAGATACATTGATGAG-3ʹ) as primers. The resulting PCR DNA fragment was cloned into the pLiv7 vector, which was designated pLiv7/Puro. The HBV receptor NTCP was amplified by PCR using primers NTCP/Kpn I (5ʹ-CTAGCTAGCGGTACCATGGAGGCCCACAACGC-3ʹ) and NTCP/Xho I (5ʹ-GCGCTCGAGCTAGGCTGTGCAAGGG-3ʹ) and pLVX-IRES-ZsGreen/NTCP as the template (18). The NTCP PCR DNA was digested with Kpn I and Xho I and inserted into the above-described pLiv7/BLAST and pLiv7/Puro vectors between the Kpn I and Xho I sites, resulting in plasmid vectors designated pLiv7/Blast/NTCP and pLiv7/Puro/NTCP, which were confirmed by DNA sequence analysis.
Selection of stable NTCP-expressing HepG2 and AML12 cell lines.
HepG2 and AML12 cells were transfected with pLiv7/Blast/NTCP and pLiv7/Puro/NTCP, respectively, using lipofectamine 3000 reagent (ThermoFisher Scientific). Upon DNA transfection, HepG2 and AML12 cell clones were selected with 5 μg/ml blasticidin or 2 μg/ml puromycin. After 2 to 3 weeks of selection with blasticidin or puromycin, individual cell clones were picked up and expanded. The expression of NTCP in the blasticidin- or puromycin-resistant cells was detected by Western blotting using an NTCP-specific monoclonal antibody. The permissiveness of NTCP-expressing HepG2 (HepG2NTCP) and AML12 (AML12NTCP) cells to HBV infection was determined by an HBV infection assay.
HBV infection.
HepG2NTCP and AML12NTCP cells were infected with HBV at a multiplicity of infection (MOI) of about 100 copies of genome equivalent in the presence of 4% PEG 8000 for 12 h except where otherwise indicated. Upon washing with 1× PBS three times, HBV-infected cells were cultured in DME-F12 medium containing 3% FBS with or without the addition of 1% DMSO, 5 μg/ml hydrocortisone, or both for 4 days or as indicated.
DNA and RNA extraction.
HBV DNA was extracted from the cells or from the medium using DNA isolation kits from Qiagen according to the manufacturer’s protocol. Total RNA was extracted with the RNeasy minikit (Qiagen) according to the manufacturer’s protocol. HBV cccDNA in the cells was extracted with the Hirt method (46) and was further treated with T5 exonuclease (New England BioLabs), which degrades DNAs with open 5ʹ and 3ʹ ends, such as HBV rcDNA, similarly to exonucleases Exo I and III (8).
Real-time PCR and RT-PCR.
HBV genomic DNA was quantified by a real-time PCR method using two HBV-specific primers, 5′-GAGTGTGGATTCGCACTCC-3′ (forward) and 5′-GAGGCGAGGGAGTTCTTCT-3′ (backward). HBV cccDNA was quantified by a real-time qPCR method reported by others (9). The two cccDNA-specific primers and probe were HBVcccDNA/F (5′-TCATCTGCCGGACCGTGTGC-3′), HBVcccDNA/R (5′-TCCCGATACAGAGCTGAGGCGG-3′), and HBVcccDNA/P (5ʹ-FAM-TTCAAGCCTCCAAGCTGTGCCTTGGGTGGC-TAMRA-3ʹ). The cellular mitochondrial DNA (COX3 gene) used as an internal control was quantified using primers mitoF (5ʹ-CCCTCTCGGCCCTCCTAATAACCT-3′) and mitoR (5ʹ-GCCTTCTCGTATAACATCGCGTCA-3ʹ), as described previously (9). The qPCR was carried out with TaqMan SYBR green master mix (Applied Biosystems) or iTaq Universal Probes supermix (Bio-Rad) by running a cycle at 95°C for 10 min and 40 cycles at 95°C for 15 s and 60°C for 60 s. For quantification of mRNAs of HBV and specific cellular genes, cDNAs were synthesized from total RNA using SuperScript III (Invitrogen) according to the manufacturer’s protocol, followed by PCR with TaqMan SYBR green master mix using gene-specific primers. The primer pairs for HBV mRNAs were HBVtF (5ʹ-TCACCAGCACCATGCAAC-3ʹ) and HBVtR (5ʹ-AAGCCACCCAAGGCACAG-3ʹ), based on the protocol of a previous report (11). The primer pairs for quantification of PGC1α mRNA were 5′-TCCTCACAGAGACACTAGACAG-3′ (forward) and 5′-CTGGTGCCAGTAAGAGCTTCT-3′ (backward). The levels of HNF4α mRNA were determined using the primer set 5ʹ-CTGCAGGCTCAAGAAATGCTT-3ʹ (forward) and 5ʹ-TCATTCTGGACGGCTTCCTT-3ʹ (backward).
HBsAg and HBeAg chemiluminescence immunoassay.
HBV surface antigen (HBsAg) and e antigen (HBeAg) in the cell culture supernatants were determined using HBsAg and HBeAg chemiluminescence immunoassay kits from Autobio Diagnostics Co. (Zhengzhou, China) according to the manufacturer’s instructions, as described previously (16). The levels of HBsAg and HBeAg were determined as international units per milliliter (IU/ml).
Western blotting.
The protein concentrations of cell lysates were determined using a protein assay reagent (Bio-Rad). Thirty-five micrograms of total protein for each sample were separated using 10% or 18% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane using a semidry blotter (Bio-Rad). After blocking with 5% nonfat milk, the membrane was incubated with anti-HBc and anti-β-actin monoclonal antibodies, followed by HRP-conjugated goat anti-mouse IgG. Protein images were visualized using an enhanced chemiluminescence (ECL) staining kit and a Chemi-Doc MP imaging system (Bio-Rad).
Statistical analysis.
Graphical representation and statistical analyses were performed by using Prism 5 software (GraphPad Software). Results are shown as mean values ± standard deviations (SD) of the data obtained from at least three independent experiments. Comparisons between samples were done using the paired two-tailed t test. A P value of <0.05 was considered to show a statistically significant difference.
ACKNOWLEDGMENTS
This work was supported by a pilot grant from the UAB Comprehensive Cancer Center (grant number P30 CA013148) and Center for AIDS Research (CFAR), an NIH-funded program (grant number P30 AI027767).
We thank Christopher Seeger (Fox Chase Cancer Center) for providing us with valuable reagents, John M. Taylor (Gladstone Institute of Cardiovascular Diseases, UCSF) for the pLiv7 vector, and Stephan Urban for myrcludex B. We are grateful to Jianming Hu (Penn State University College of Medicine) and Haitao Guo (Indiana University) for sharing protocols and unpublished information and suggestions.
REFERENCES
- 1.Ganem D, Prince AM. 2004. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med 350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ. 2009. Chronic hepatitis B: update 2009. Hepatology 50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 3.Seeger C, Mason WS. 2015. Molecular biology of hepatitis B virus infection. Virology 479-480:672–686. doi: 10.1016/j.virol.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, Michel ML, Autran B, Cosset FL, Strick-Marchand H, Trepo C, Kao JH, Carrat F, Lacombe K, Schinazi RF, Barre-Sinoussi F, Delfraissy JF, Zoulim F. 2015. Towards an HBV cure: state-of-the-art and unresolved questions—report of the ANRS workshop on HBV cure. Gut 64:1314–1326. doi: 10.1136/gutjnl-2014-308943. [DOI] [PubMed] [Google Scholar]
- 5.Gao W, Hu J. 2007. Formation of hepatitis B virus covalently closed circular DNA: removal of genome-linked protein. J Virol 81:6164–6174. doi: 10.1128/JVI.02721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo H, Jiang D, Zhou T, Cuconati A, Block TM, Guo JT. 2007. Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J Virol 81:12472–12484. doi: 10.1128/JVI.01123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo H, Mao R, Block TM, Guo JT. 2010. Production and function of the cytoplasmic deproteinized relaxed circular DNA of hepadnaviruses. J Virol 84:387–396. doi: 10.1128/JVI.01921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J, Cui X, Gao L, Hu J. 2017. Identification of intermediate in hepatitis B virus CCC DNA formation and sensitive and selective CCC DNA detection. J Virol 91:e00539-17. doi: 10.1128/JVI.00539-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q, Yan R, Hu J, Cai D, Mitra B, Kim ES, Marchetti A, Zhang H, Wang S, Liu Y, Huang A, Guo H. 2017. The role of host DNA ligases in hepadnavirus covalently closed circular DNA formation. PLoS Pathog 13:e1006784. doi: 10.1371/journal.ppat.1006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi Y, Gao Z, Xu G, Peng B, Liu C, Yan H, Yao Q, Sun G, Liu Y, Tang D, Song Z, He W, Sun Y, Guo JT, Li W. 2016. DNA polymerase kappa is a key cellular factor for the formation of covalently closed circular DNA of hepatitis B virus. PLoS Pathog 12:e1005893. doi: 10.1371/journal.ppat.1005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto M, Watashi K, Tsukuda S, Aly HH, Fukasawa M, Fujimoto A, Suzuki R, Aizaki H, Ito T, Koiwai O, Kusuhara H, Wakita T. 2014. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem Biophys Res Commun 443:808–813. doi: 10.1016/j.bbrc.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 13.Lempp FA, Qu B, Wang YX, Urban S. 2016. Hepatitis B virus infection of a mouse hepatic cell line reconstituted with human sodium taurocholate cotransporting polypeptide. J Virol 90:4827–4831. doi: 10.1128/JVI.02832-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Zong L, Sureau C, Barker L, Wands JR, Tong S. 2016. Unusual features of sodium taurocholate cotransporting polypeptide as a hepatitis B virus receptor. J Virol 90:8302–8313. doi: 10.1128/JVI.01153-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W. 2015. The hepatitis B virus receptor. Annu Rev Cell Dev Biol 31:125–147. doi: 10.1146/annurev-cellbio-100814-125241. [DOI] [PubMed] [Google Scholar]
- 16.Michailidis E, Pabon J, Xiang K, Park P, Ramanan V, Hoffmann HH, Schneider WM, Bhatia SN, de Jong YP, Shlomai A, Rice CM. 2017. A robust cell culture system supporting the complete life cycle of hepatitis B virus. Sci Rep 7:16616. doi: 10.1038/s41598-017-16882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, Stindt J, Koniger C, Nassal M, Kubitz R, Sultmann H, Urban S. 2014. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Seeger C, Sohn JA. 2014. Targeting hepatitis B virus with CRISPR/Cas9. Mol Ther Nucleic Acids 3:e216. doi: 10.1038/mtna.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan R, Zhang Y, Cai D, Liu Y, Cuconati A, Guo H. 2015. Spinoculation enhances HBV infection in NTCP-reconstituted hepatocytes. PLoS One 10:e0129889. doi: 10.1371/journal.pone.0129889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Zhao K, Yao Y, Yuan Y, Pei R, Wang Y, Chen J, Hu X, Zhou Y, Chen X, Wu C. 2017. Productive HBV infection of well-differentiated, hNTCP-expressing human hepatoma-derived (Huh7) cells. Virol Sin 32:465–475. doi: 10.1007/s12250-017-3983-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, Ren B, Mao F, Jing Z, Li Y, Liu Y, Peng B, Yan H, Qi Y, Sun Y, Guo JT, Sui J, Wang F, Li W. 2015. Hepatitis D virus infection of mice expressing human sodium taurocholate co-transporting polypeptide. PLoS Pathog 11:e1004840. doi: 10.1371/journal.ppat.1004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lempp FA, Mutz P, Lipps C, Wirth D, Bartenschlager R, Urban S. 2016. Evidence that hepatitis B virus replication in mouse cells is limited by the lack of a host cell dependency factor. J Hepatol 64:556–564. doi: 10.1016/j.jhep.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Chisari FV. 1996. Hepatitis B virus transgenic mice: models of viral immunobiology and pathogenesis. Curr Top Microbiol Immunol 206:149–173. [DOI] [PubMed] [Google Scholar]
- 24.Simonet WS, Bucay N, Lauer SJ, Taylor JM. 1993. A far-downstream hepatocyte-specific control region directs expression of the linked human apolipoprotein E and C-I genes in transgenic mice. J Biol Chem 268:8221–8229. [PubMed] [Google Scholar]
- 25.Gripon P, Diot C, Theze N, Fourel I, Loreal O, Brechot C, Guguen-Guillouzo C. 1988. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J Virol 62:4136–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. 2002. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A 99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isom I, Georgoff I, Salditt-Georgieff M, Darnell JE Jr.. 1987. Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J Cell Physiol 105:2877–2885. doi: 10.1083/jcb.105.6.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa J, Yee C, Troost T, Rabson AS. 1974. Effect of dexamethasone on herpes simplex virus type 2 infection in vitro. Nature 252:745–746. doi: 10.1038/252745a0. [DOI] [PubMed] [Google Scholar]
- 29.Domachowske JB, Bonville CA, Ali-Ahmad D, Dyer KD, Easton AJ, Rosenberg HF. 2001. Glucocorticoid administration accelerates mortality of pneumovirus-infected mice. J Infect Dis 184:1518–1523. doi: 10.1086/324664. [DOI] [PubMed] [Google Scholar]
- 30.Morhenn V, Rabinowitz Z, Tomkins GM. 1973. Effects of adrenal glucocorticoids on polyoma virus replication. Proc Natl Acad Sci U S A 70:1088–1089. doi: 10.1073/pnas.70.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiyama Y, Rapp F. 1979. Regulation of persistent infection with herpes simplex virus in vitro by hydrocortisone. J Virol 31:841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh JO. 1970. Enhancement of virus multiplication and interferon production by cortisone in ocular herpesvirus infection. J Immunol 104:1359–1363. [PubMed] [Google Scholar]
- 33.Refaeli Y, Levy DN, Weiner DB. 1995. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc Natl Acad Sci U S A 92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rog-Zielinska EA, Craig MA, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes MC, Hardie DG, Smith GL, Chapman KE. 2015. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1alpha. Cell Death Differ 22:1106–1116. doi: 10.1038/cdd.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JW, Park SY, You YH, Ham DS, Park HS, Lee SH, Yang HK, Yoon KH. 2014. Targeting PGC-1alpha to overcome the harmful effects of glucocorticoids in porcine neonatal pancreas cell clusters. Transplantation 97:273–279. doi: 10.1097/01.TP.0000438627.68225.25. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Oropeza CE, Kaestner KH, McLachlan A. 2009. Limited effects of fasting on hepatitis B virus (HBV) biosynthesis in HBV transgenic mice. J Virol 83:1682–1688. doi: 10.1128/JVI.02208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shlomai A, Paran N, Shaul Y. 2006. PGC-1alpha controls hepatitis B virus through nutritional signals. Proc Natl Acad Sci U S A 103:16003–16008. doi: 10.1073/pnas.0607837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson HB, Tang H. 2006. Effect of cell growth on hepatitis C virus (HCV) replication and a mechanism of cell confluence-based inhibition of HCV RNA and protein expression. J Virol 80:1181–1190. doi: 10.1128/JVI.80.3.1181-1190.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lathey JL, Spector SA. 1991. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J Virol 65:6371–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magrath IT, Pizzo PA, Novikovs L, Levine AS. 1979. Enhancement of Epstein-Barr virus replication in producer cell lines by a combination of low temperature and corticosteroids. Virology 97:477–481. doi: 10.1016/0042-6822(79)90360-X. [DOI] [PubMed] [Google Scholar]
- 41.Raney AK, Johnson JL, Palmer CN, McLachlan A. 1997. Members of the nuclear receptor superfamily regulate transcription from the hepatitis B virus nucleocapsid promoter. J Virol 71:1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raney AK, Kline EF, Tang H, McLachlan A. 2001. Transcription and replication of a natural hepatitis B virus nucleocapsid promoter variant is regulated in vivo by peroxisome proliferators. Virology 289:239–251. doi: 10.1006/viro.2001.1169. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y, Li J, Ou JH. 2004. Regulation of hepatitis B virus core promoter by transcription factors HNF1 and HNF4 and the viral X protein. J Virol 78:6908–6914. doi: 10.1128/JVI.78.13.6908-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gripon P, Cannie I, Urban S. 2005. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol 79:1613–1622. doi: 10.1128/JVI.79.3.1613-1622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulze A, Schieck A, Ni Y, Mier W, Urban S. 2010. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J Virol 84:1989–2000. doi: 10.1128/JVI.01902-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirt B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol 26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]