Only one HIV-1 candidate vaccine strategy has shown protection, albeit marginally (31%), against HIV-1 acquisition, and correlates of protection suggested that a multifunctional CD4 T cell immune response may be important for this protective effect. Therefore, the functional phenotypes of HIV-specific CD4 T cell responses induced by different phase I and phase II clinical trials were assessed to better show how different vaccine strategies influence the phenotype and function of HIV-specific CD4 T cell immune responses. The significance of this research lies in our comprehensive comparison of the compositions of the T cell immune responses to different HIV vaccine modalities. Specifically, our work allows for the evaluation of vaccination strategies in terms of their success at inducing Tfh cell populations.
KEYWORDS: CD4 T cells, HIV vaccine, RV144, Tfh cells, human immunodeficiency virus
ABSTRACT
To date, six vaccine strategies have been evaluated in clinical trials for their efficacy at inducing protective immune responses against HIV infection. However, only the ALVAC-HIV/AIDSVAX B/E vaccine (RV144 trial) has demonstrated protection, albeit modestly (31%; P = 0.03). One potential correlate of protection was a low-frequency HIV-specific CD4 T cell population with diverse functionality. Although CD4 T cells, particularly T follicular helper (Tfh) cells, are critical for effective antibody responses, most studies involving HIV vaccines have focused on humoral immunity or CD8 T cell effector responses, and little is known about the functionality and frequency of vaccine-induced CD4 T cells. We therefore assessed responses from several phase I/II clinical trials and compared them to responses to natural HIV-1 infection. We found that all vaccines induced a lower magnitude of HIV-specific CD4 T cell responses than that observed for chronic infection. Responses differed in functionality, with a CD40 ligand (CD40L)-dominated response and more Tfh cells after vaccination, whereas chronic HIV infection provoked tumor necrosis factor alpha (TNF-α)-dominated responses. The vaccine delivery route further impacted CD4 T cells, showing a stronger Th1 polarization after dendritic cell delivery than after intramuscular vaccination. In prime/boost regimens, the choice of prime and boost influenced the functional profile of CD4 T cells to induce more or less polyfunctionality. In summary, vaccine-induced CD4 T cell responses differ remarkably between vaccination strategies, modes of delivery, and boosts and do not resemble those induced by chronic HIV infection. Understanding the functional profiles of CD4 T cells that best facilitate protective antibody responses will be critical if CD4 T cell responses are to be considered a clinical trial go/no-go criterion.
IMPORTANCE Only one HIV-1 candidate vaccine strategy has shown protection, albeit marginally (31%), against HIV-1 acquisition, and correlates of protection suggested that a multifunctional CD4 T cell immune response may be important for this protective effect. Therefore, the functional phenotypes of HIV-specific CD4 T cell responses induced by different phase I and phase II clinical trials were assessed to better show how different vaccine strategies influence the phenotype and function of HIV-specific CD4 T cell immune responses. The significance of this research lies in our comprehensive comparison of the compositions of the T cell immune responses to different HIV vaccine modalities. Specifically, our work allows for the evaluation of vaccination strategies in terms of their success at inducing Tfh cell populations.
INTRODUCTION
A cornerstone of vaccine-mediated protective immunity is the ability to safely induce an immune response that recognizes the agent as foreign, destroys it, and “remembers” it so that the same type of microorganism is more quickly cleared upon subsequent encounters. Most successful vaccines confer protection through antibody (Ab) production (1), which often relies on help from the cellular arm of the immune system. In particular, T follicular helper (Tfh) cells play a crucial role during the germinal center reaction to induce B cell proliferation, antibody affinity maturation, and B cell differentiation to promote effective antibody responses. Developing an HIV vaccine that induces a comprehensive and well-orchestrated immune response composed of both an optimal and well-guided T cell response and optimal antibody-inducing B cell responses has proven difficult (2). Various vaccine strategies have been explored in clinical trials to examine optimal antigen immunogenicity while maintaining safety. Given the correlates of protection of licensed vaccines, almost all studies have focused on inducing antibody responses or CD8 T cell responses; however, understanding the quantity and quality of CD4 T cell responses induced by vaccination may be critical for improving the immunogenicity of vaccines (3). Interestingly, the RV144 vaccine trial showed a modestly but significantly reduced risk of HIV acquisition (31.2%; P = 0.04; 95% confidence interval, 1 to 51%) (4). The RV144 trial employed a prime-boost regimen consisting of a prime with a recombinant canarypox virus vector, ALVAC-HIV (vCP1521), and a bivalent AIDSVAX gp120 B/E boost. The ALVAC-HIV vaccine was administered at 0, 4, 12, and 24 weeks, and boosting with gp120 occurred at weeks 12 and 24. Nonneutralizing IgG antibody against the gp70V1V2 scaffold envelope correlated with a decreased risk of HIV acquisition, and ex vivo, this antibody has been demonstrated to mediate functional responses, such as antibody-dependent cellular cytotoxicity (ADCC) (5). Follow-up correlate analysis of HIV-specific CD4 T cell responses revealed production of a particular combination of cytokines (6), suggesting that Tfh cell help may also be important. Thus, understanding the properties of Tfh cells necessary to enable development of broadly neutralizing Abs against HIV may be the key to understanding how to elicit protective immune responses. Advantageously, several reports have described the presence of antigen-specific Tfh cell responses in the periphery, allowing the analysis of qualitative differences that are induced by different vaccines (7–10).
A variety of strategies have been applied to HIV vaccine design. The most common strategy utilizes replication-deficient viral vectors (11, 12), which are often combined with a protein in a prime-boost strategy (13). It is well established that the antigen and mode of delivery used for vaccines affect the strength and specificity of the induced immune response in ways that are not yet fully understood (14, 15). Indeed, adjuvanted protein antigens and killed viruses are efficient at inducing antibody responses, while live attenuated viruses induce a more well-rounded immune response which more closely resembles natural immunity (16). Some vaccine vectors direct immune responses more toward CD8 T cells, while others stimulate CD4 T cells. Similarly, different vaccine vectors induce T cell responses with different functional profiles (17–20).
The consequences of these differences between immunization modalities translate to differences in functionality, neutralization efficiency, and longevity of the antibody responses and thus impact vaccine efficacy. However, how vaccine-induced CD4 T cell responses are influenced by different vaccination approaches has not been well characterized. We therefore investigated the quantity and quality of the CD4 T cell responses elicited by several different immunization platforms from phase I/II clinical trials, especially focusing on cytokine profiles associated with Tfh cells in the periphery.
RESULTS
To determine potential differences in HIV vaccination strategies in regard to maturation, differentiation, and functionality of CD4 T cell responses, we used cryopreserved peripheral blood mononuclear cells (PBMCs) collected at peak immunogenicity from participants of several phase I/II trials utilizing different vaccination strategies (summarized in Table 1). Vaccination regimens in these trials varied widely in the nature of the primary immunization (DNA or protein), the vector used for immunogen delivery (e.g., canarypox virus vector based or modified vaccinia virus Ankara [MVA] based), the nature of the boosts (e.g., DNA or oligomeric Env protein), and the respective adjuvants (e.g., MF59 or alum).
TABLE 1.
Summary of phase I HIV vaccine trials used for comparison
| Trial no. | Source (reference) | Strategy | Vector or boost | Immunogen(s) | Adjuvant | Immunization times (wk) |
|---|---|---|---|---|---|---|
| RV138 | Eller et al. (21) | Canarypox virus vector | ALVAC-HIV (vCP205) | Env/Gag/Pro—autologous DC, i.d., i.m. | 0, 4, 12, 24 | |
| RV172 | Kibuuka et al. (35) | DNA prime, Ad5 boost | Multiclade DNA | Gag/Pol/Nef/Env | 0, 4, 8 | |
| rAd5 | Matching proteins (except Nef) | 24 | ||||
| RV114 | Pitisuttithum et al. (36) | Protein | Protein | rgp120 (SF2) | MF59 | 0, 4, 16 |
| RV132 | Thongcharoen et al. (24) | Canarypox virus vector prime, canarypox virus vector/protein boost | ALVAC-HIV (vCP1521) | 92TH023 gp120-LAI gp41 + LAI Gag/protein | 0, 4, 12, 24 | |
| Protein (oligomeric gp160) | 92TH023 gp120-LAI gp41 + LAI Gag/protein | PCPP | 12, 24 | |||
| Protein (bivalent gp120) | CM235 (100 μg) + SF2 (50 μg) | MF59 | 12, 24 | |||
| RV135 | Nitayaphan et al. (25) | Canarypox virus vector prime, canarypox virus vector/protein boost | ALVAC-HIV (vCP1521) | 92TH023 gp120-LAI gp41 + LAI Gag/protein | 0, 4, 12, 24 | |
| AIDSVAX (B/E) | MN (B) and A244 (E) gp120 | Alum | 12, 24 | |||
| RV158 | Currier et al. (22) | Cowpox virus vector | MVA-CDMR | gp160 (CM235, E), Gag/Pol (CM240, A) | 0, 4, 12 | |
| RV144 | Rerks-Ngarm et al. (4) | Canarypox virus vector prime, canarypox virus vector/protein boost | ALVAC-HIV (vCP1521) | 92TH023 gp120-LAI gp41 + LAI Gag/protein | 0, 4, 12, 24 | |
| Bivalent protein (AIDSVAX [B/E]) | MN (B) and A244 (E) gp120 | Alum | 12, 24 |
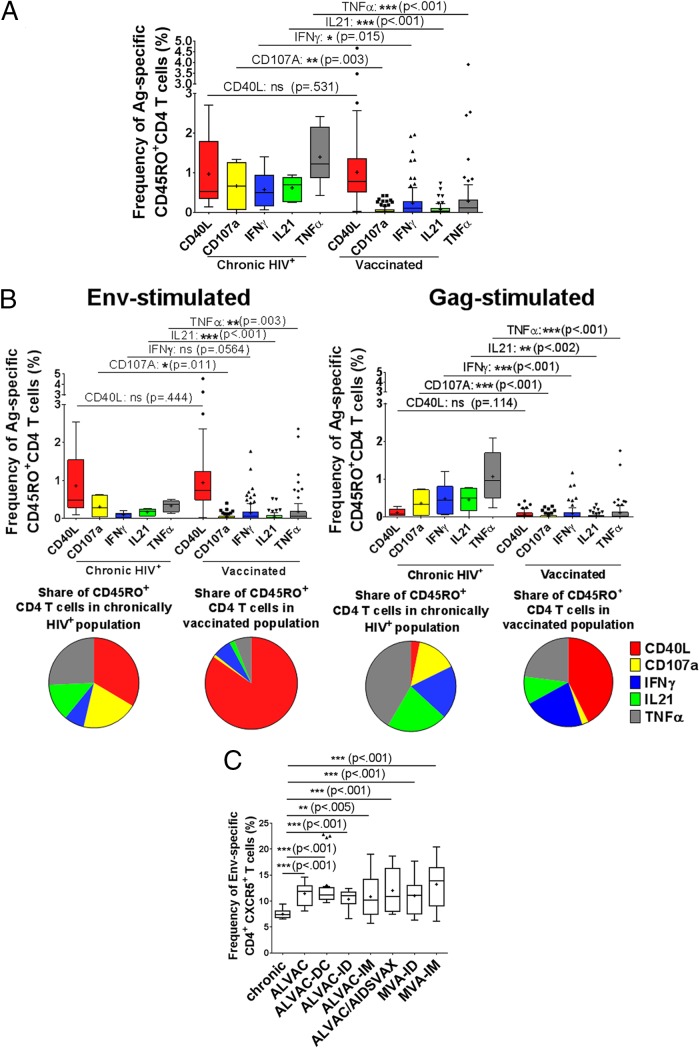
To address the functional quality of the HIV-specific CD4 T cell responses induced by vaccination, we began by comparing the HIV-specific CD4 T cell responses induced by treatment-naive chronic HIV infection with those induced by vaccines, without preselecting for responders. We performed multicolor flow cytometry on PBMCs to identify antigen-specific CD4 memory cells (CD45RO+ CD4+ cells) after stimulation with HIV antigens (Env/Gag) and then determined the functional profiles for gamma interferon (IFN-γ), interleukin-21 (IL-21), CD40 ligand (CD40L), tumor necrosis factor alpha (TNF-α), and CD107a. For chronically HIV-positive, treatment-naive individuals, a significant proportion of CD4 memory T cells (1% ± 0.9% of CD45RO+ cells) were HIV specific (Fig. 1A). In particular, we found that the majority of the HIV-specific CD4 T cells expressed TNF-α (1.4% of memory cells), followed by CD40L (∼1%), CD107a (∼0.7%), and IFN-γ/IL-21 (∼0.6% [each]). We next assessed quantitative and qualitative differences in HIV-specific CD4 T cell responses elicited at peak immunogenicity during vaccine trials and those seen in chronic HIV infection. In total, the immune response induced by vaccines was significantly lower (accumulatively, 1.7% of total CD45RO+ T cells were either CD40L, CD107A, IFN-γ, IL-21, or TNF-α positive after restimulation) than that induced by chronic HIV infection (4.2% positivity) (P < 0.001) (Fig. 1A). Interestingly, while the CD40L expression levels on CD4 T cells were comparable between chronic HIV infection and vaccination, CD107a-, IFN-γ-, IL-21-, and TNF-α-expressing cell levels were significantly lower in vaccinated individuals (P = 0.015 to P < 0.001). To determine whether age or gender may have accounted for the observed differences, we assessed differences in functional profiles for participants separated by age group or gender, respectively. However, we observed no significant differences in functional profiles between male and female participants or by age group (data not shown).
FIG 1.
Total frequency of the immunological response to HIV potential T cell epitope peptide stimulation in memory T cells. (A) Blood was drawn from chronically HIV-infected patients. In parallel, blood was collected 24 weeks after vaccination with ALVAC-HIV encoding Gag and Env. PBMCs isolated from the blood were stimulated with HIV peptide pools and analyzed via flow cytometry for the frequency of Env/Gag-specific memory T cells (CD45RO+) expressing either CD40L, CD107a, IFN-γ, IL-21, or TNF-α. (B) PBMCs from chronically HIV-infected (chronic; n = 6) or ALVAC-HIV-vaccinated (n = 97) patients were stimulated with either HIV Env or Gag potential T cell peptide pools and the response measured as described above. (C) PBMCs from uninfected (n = 6), chronically HIV-positive (n = 18), and vaccine trial (ALVAC [n = 21], ALVAC-DC [n = 18], ALVAC-ID [n = 21], ALVAC-IM [n = 21], ALVAC/AIDSVAX [n = 18], MVA-ID [n = 39], and MVA-IM [n = 30]) patients were compared by use of the frequencies of CXCR5-expressing CD4+ T cells after Env stimulation (results for Gag were similar [data not shown]). For statistical analysis, the Mann-Whitney U test was performed, and the representation of P values by asterisks is shown according to the style of the New England Journal of Medicine.
Next, differences in the functional profiles of CD4 T cell responses against Gag or Env were determined after vaccination and for chronic HIV infection. Overall, the immune responses induced by Gag were significantly different from those induced by Env. Env-specific CD4 T cell responses in chronically HIV-positive, treatment-naive individuals were dominated by CD40L (0.9%) (Fig. 1B, left panel), followed by CD107a+ or TNF-α+ (0.3%), IL-21+ (0.2%), and IFN-γ+ (0.1%) cells. A similar pattern of Env-specific CD4 T cell responses was also observed in vaccinees, but at lower levels (0.9% CD40L+, 0.2% TNF-α+, and 0.1% IFN-γ+, CD107a+, or IL-21+ cells). In contrast, Gag-specific CD4 T cell responses were dominated by TNF-α (1.1%) expression, followed by IFN-γ+ (0.5%) or IL-21+ (0.5%) and CD107a+ (0.4%) cells, whereas CD40L was expressed at low levels (0.1%) (Fig. 1B, right panel). After vaccination, there were fewer Gag-specific CD4 T cell responses (0.3% of CD4+ CD45RO+ T cells) than those induced by chronic infection (2.5%), indicating protein-specific differences in the induction of immune responses.
We next analyzed the frequency of pTfh markers, including programmed cell death protein 1 (PD-1), C-X-C chemokine receptor 5 (CXCR5), and the transcription factor and proto-oncogene musculoaponeurotic fibrosarcoma oncogene homolog (cMAF). Interestingly, we observed that there was a significantly larger proportion of Env-specific CXCR5+ CD4 T cells present after vaccination than that during chronic HIV infection, suggesting a specific induction of Tfh cells that is detectable after vaccination in the periphery (Fig. 1C). There was no significant difference in the expression of PD-1 or cMAF between vaccinees and those with natural HIV infection (data not shown).
Taken together, these observations show that HIV-specific CD4 T cell responses elicited after vaccination differ significantly from those observed in chronic HIV infection. In particular, the functionalities of the immune responses induced by the HIV proteins Gag and Env are markedly different between natural infection and vaccination. In addition, more Tfh cells were induced after vaccination than during natural HIV infection.
We next determined whether the route of vaccine delivery may affect the elicited HIV-specific CD4 T cell response. Both the RV138 (ALVAC; canarypox virus vector based) and RV158 (MVA vector based) trials used intradermal (i.d.) or intramuscular (i.m.) injection for vaccine delivery (21, 22). In addition, the RV138 trial also used autologous peptide-loaded dendritic cells (ALVAC DC). Overall, the qualities of the immune responses upon i.d. versus i.m. or DC application of the ALVAC and MVA vaccines did not differ strongly in terms of eliciting populations of CD4 memory T cells (Fig. 2A); however, the frequency of CD40L+ cells was significantly lower and the IL-21+ CD4 T cell responses higher in the ALVAC i.d. group than in the MVA i.d. group (P < 0.05). In contrast, response profiles did not differ significantly between the ALVAC DC, ALVAC i.m., and MVA i.m. groups. Yet on pooling the data and comparing responses for both i.d. versus both i.m. modalities, there were no significant differences in response profiles (Fig. 2B), suggesting that the route of vaccination alone does not significantly alter the elicited immune response. Instead, the choice of primary strategy combined with the vaccination route is key to guiding the immune response in different directions, e.g., ALVAC administered i.d. for fewer CD40L+ and more IL-21+ CD4 T cells and MVA administered i.d. for more CD40L+ and fewer IL-21+ CD4 T cells. Taken together, our data demonstrate that both the i.d. and i.m. routes of administration of different vaccines promote CD4 T cell responses similarly dominated by CD40L expression, with smaller contributions by IFN-γ, IL-21, and TNF-α, and most importantly with significant differences between ALVAC and MVA i.d. delivery but no differences between vaccines administered i.m. in terms of the overall expression profiles. The prime-boost strategy has recently gained popularity and is a commonly used approach. In this setting, the immune system is primed by one vaccine candidate and boosted with either the same (homologous) or a different (heterologous) vaccine candidate (23). To understand the impacts of different boosts on the same prime, we made use of the RV132 and RV135 vaccine trials (the latter was a precursor study to the RV144 trial), in which the same prime, ALVAC, was boosted with either bivalent gp120 (bi-gp120), oligomeric gp160 (o-gp160), or AIDSVAX (A244) (24, 25).
FIG 2.
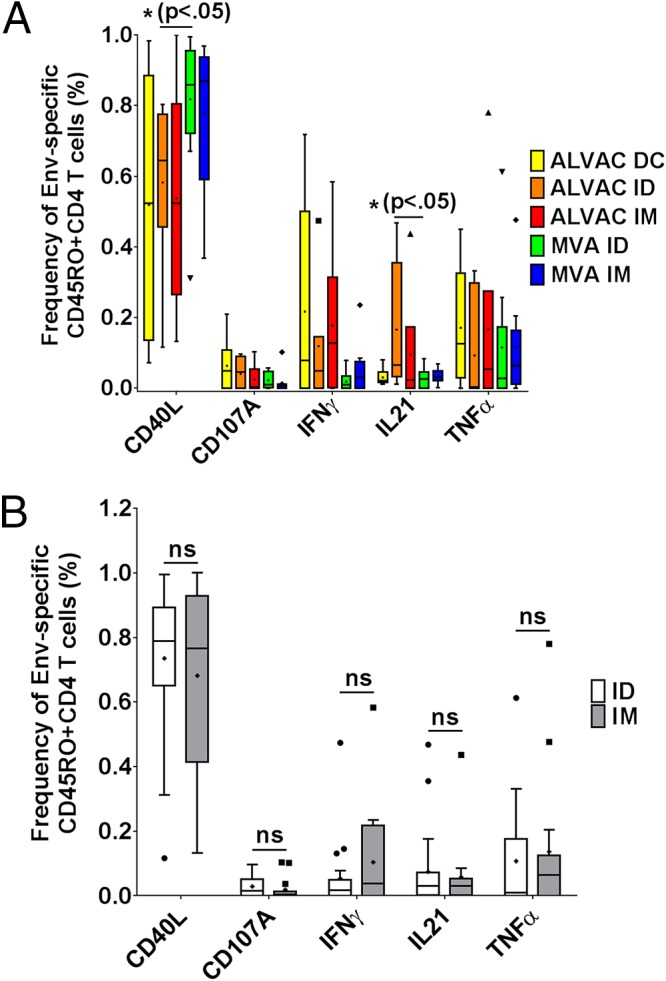
Effect of vaccine administration route on overall frequency of responses to stimulation with HIV Env peptides and on the contribution of each cytokine to the total response. (A) Twenty-four weeks after ALVAC (canarypox virus vector based) or MVA (modified vaccinia virus Ankara vector based) vaccination was given either via different routes (intradermally [n = 13 for MVA and n = 7 for ALVAC] or intramuscularly [n = 9 for MVA and n = 6 for ALVAC]) or, in the case of ALVAC, with autologous DC (n = 6), PBMCs from peripheral blood were analyzed for the frequency of Env-specific memory T cells by flow cytometry. After statistical analysis of all data via the Mann-Whitney U test, significant differences were labeled with the significance level and the corresponding P value. (B) Env-specific responses to the modified vaccinia virus Ankara vector-based vaccine delivered intradermally (n = 20) or intramuscularly (n = 15) were analyzed to determine the contributions of the molecules CD40L, CD107a, IFN-γ, IL-21, and TNF-α. Each experiment was repeated with blood from at least 6 donors, and the data were analyzed for statistically significant differences by the Mann-Whitney U test. ns, not significant; ID, intradermal; IM, intramuscular.
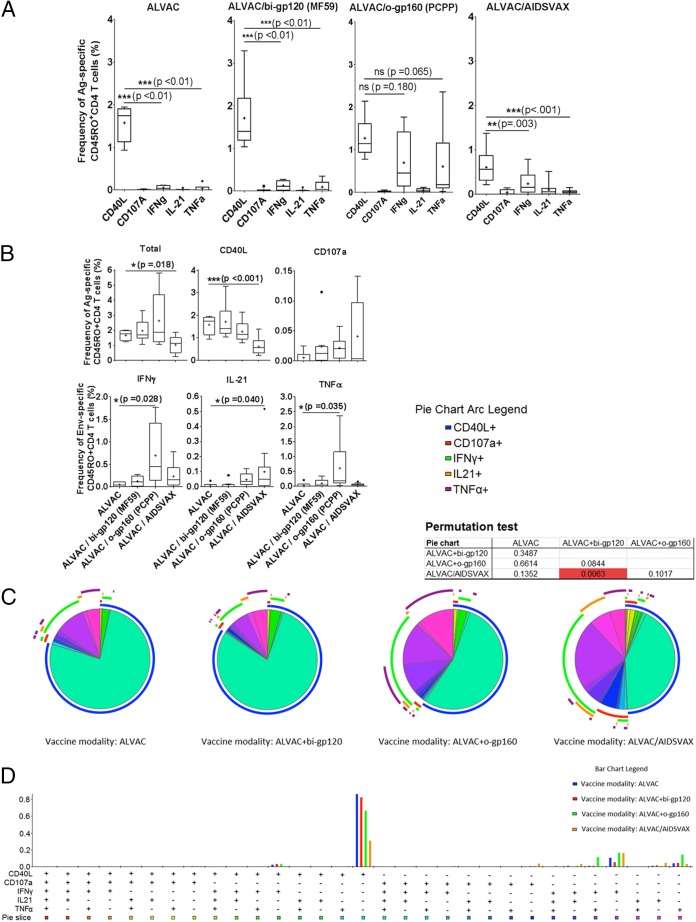
ALVAC alone induced Env-specific CD4 T cell responses, with CD40L expression (1.57% of CD4 T cells) being the most dominant immune response, while IFN-γ and IL-21 responses were comparatively low (0.05 and 0.04%, respectively) (Fig. 3A). The addition of a protein boost with bivalent gp120 did not significantly increase any of the responses. In contrast, when ALVAC was boosted with the oligomeric gp160 protein, the functional profile of the CD4 T cell responses shifted significantly, toward an IFN-γ- and TNF-α-dominated Th1-type immune response (0.7% IFN-γ+ cells and 0.6% TNF-α+ cells), but this boost did not significantly alter the CD40L expression in comparison to that with the bivalent gp120 boost. Interestingly, boosting ALVAC with the gp120 protein A244 (ALVAC/AIDSVAX) shifted the immune response away from a purely Th1-dominated response and toward a more balanced profile composed of cytolytic and IL-21+ CD4 T cells, the latter of which we previously identified as peripheral T follicular helper cells. Phenotypically, vaccination boosts did not lead to any significant increase in the Tfh cell-associated marker CXCR5, cMAF, or PD-1 (data not shown), indicating that phenotypic assessment of vaccine-induced Tfh cell responses is not sufficient for describing differences in the antigen-specific induced CD4 T cell populations (26). Among the responses induced by the four different prime and boost vaccination strategies, both ALVAC and ALVAC/bi-gp120 responses were dominated by CD40L (Fig. 3B). In contrast, the ALVAC/o-gp160 strategy led to a more complex response, inducing IFN-γ- and TNF-α-expressing cells at frequencies similar to those of cells expressing CD40L, whereas ALVAC/AIDSVAX induced only IFN-γ-expressing cells (besides CD40L-expressing cells). Overall, ALVAC/AIDSVAX induced functional responses at lower frequencies than those for the other vaccine modalities (Fig. 3A).
FIG 3.
Comparison of responses to Env induced by ALVAC versus ALVAC prime/boost vaccines (studies RV132 and RV135). (A) PBMCs from peripheral blood collected 24 weeks after vaccine administration were analyzed for dominance of each subpopulation of Env-specific CD4 memory cells, and the data are shown as a comparison of the different prime/boost strategies. (B) Frequencies of Env-specific memory T cells expressing either CD40L, IFN-γ, IL-21, TNF-α, or CD107a or the total frequency of CD45RO+ cells. Sample sizes were as follows: for ALVAC, n = 6; for ALVAC/bi-gp120, n = 8; for ALVAC/o-gp160, n = 6; and for ALVAC/AIDSVAX, n = 12. (C) SPICE analysis of the composition of the immune response after antigen stimulation, shown as the % CD45RO+ CD4+ cells after different prime/boost strategies (each pie chart represents one strategy). Pie chart arcs illustrate the contribution of each expressed protein (CD40L, CD107a, IFN-γ, IL-21, or TNF-α) to every single-, double-, triple-, or quadruple-positive population. (D) Contributions of CD40L, CD107a, IFN-γ, IL-21, and TNF-α expression to the CD45RO+ CD4+ immune response after different prime/boost strategies.
Interestingly, cells from the ALVAC and ALVAC/bi-gp120 groups largely expressed only CD40L (76% and 72% of cells, respectively), and both expression profiles were comparable, whereas the ALVAC/o-gp160 and ALVAC/AIDSVAX vaccines produced more differentiated multifunctional CD4 T cell response profiles (Fig. 3C and D). After the o-gp160 boost, only 54% of the memory cells were exclusively CD40L positive. Of the remaining CD4 memory T cells, most were either singly positive for IFN-γ (14%) or TNF-α (12%) or doubly positive for IFN-γ and TNF-α (9%) (Fig. 3C). ALVAC/AIDSVAX vaccination induced a different profile among memory T cells: 42% were CD40L+, and the remaining cells were predominantly IFN-γ positive (23%) or, at lesser frequencies, singly positive for IL-21 (7%), TNF-α (5%), or CD107a (5%). The differences between ALVAC and ALVAC/AIDSVAX were significant for the total frequency of antigen-specific cells and the frequencies of CD40L- and IL-21-positive cells (Fig. 3B). Differences between ALVAC and ALVAC/o-gp160 were significant for IFN-γ and TNF-α. Similarly, we observed significant differences between the ALVAC/bi-gp120 and ALVAC/AIDSVAX modalities by comparing pie charts using a permutation test (SPICE analysis).
In summary, among the 4 prime-boost strategies, ALVAC/o-gp160 vaccination induced the highest levels of Env-specific CD40L+, IFN-γ+, and TNF-α+ CD4 T cells, but ALVAC/AIDSVAX induced the most diverse CD4 T cell profile, with the strongest HIV-specific IL-21+ CD4 T cell responses. Yet, ALVAC/AIDSVAX overall produced the smallest amount of antigen-specific CD4 memory T cells.
DISCUSSION
Vaccine-induced CD4 T cell responses, particularly Tfh responses, are thought to be critical for the generation of high-affinity, long-lasting antibody responses. Over 400 clinical studies have been performed using diverse combinations of vaccine regimens, vectors, routes of vaccine administration, adjuvants, immunogens, and study populations (27). While almost all trials have evaluated the breadth, magnitude, function, and specificity of vaccine-induced antibody responses, only a few have evaluated the function or lack of HIV-specific CD4 T cell responses, despite their importance for antibody induction.
In the present study, we assessed how different vaccination strategies influence the qualitative profiles of CD4 T cell responses and how these responses compare to CD4 T cell responses naturally induced by HIV infection. While the selection of phase I vaccine trials was limited mainly to variation of similar vaccine components by use of constructs including MVA, ALVAC, DNA, and prime/boost, we found some patterns that were consistent in all phase I vaccine trials analyzed. Overall, we observed that the vaccine-induced CD4 T cell responses were quantitatively lower after vaccination than during natural HIV infection. This was expected given the chronic nature of immune stimulation observed in ongoing viral infections. However, we did observe similar frequencies of CD40L+ CD4 T cell responses after vaccination overall, but when we compared the functional profiles of Env- versus Gag-specific memory CD4 T cells, we observed almost opposite characteristics, in that the Env response was dominated by CD40L, whereas the Gag-specific CD4 T cell responses were skewed toward a Th1, TNF-α-dominant functional profile.
The effects of routes of vaccination have been investigated mostly with a focus on differences in the induced humoral immune responses (28) or cellular CD8 T cell responses (29–31). However, only a few studies have focused on CD4 T cells, and they demonstrated increased levels of Tfh cells after intradermal vaccination (32). In this context, we observed that intradermal ALVAC vaccination indeed induced the largest number of Tfh cell-associated responses as measured by the number of HIV-specific IL-21+ CD4 T cells (7), while DC-delivered ALVAC vaccination skewed them toward a Th1 (IFN-γ/TNF-α) phenotype.
Besides different routes of administration, we demonstrated that the functional profile of CD4 T cells can still be influenced during the prime and boost phases. Generally, an important reason for adding boosts to a vaccination strategy is to enhance antibody affinity and production (33) by inducing additional rounds of affinity maturation (34). However, the impact on the quality of CD4 T cell responses induced by prime/boost vaccination strategies has not previously been appreciated. Here we made use of the fact that several different vaccination strategies utilized the same priming agent (ALVAC) but used different boosts, including bivalent gp120 (ALVAC/bi-gp120), oligomeric gp160 (ALVAC/o-gp160), and gp120 (ALVAC/AIDSVAX). When responses were resolved by cytokine expression, the four strategies were differentially advantageous for the stimulation of different cell populations, with ALVAC and ALVAC/bi-gp120 producing the highest frequencies of CD40L-expressing cells. Surprisingly, there was a difference in outcome between ALVAC boosted with bi-gp120 and that boosted with o-gp160 in that the response to the bi-gp120 boost was not significantly different from that to the ALVAC vaccination alone. In contrast, the o-gp160 boost skewed the ALVAC-induced immune response to generate more IFN-γ+ and TNF-α+ Env-specific CD4 T cells than those observed with ALVAC alone.
ALVAC/AIDSVAX, on the other hand, was the most advantageous for the induction of CD107a+ and IL-21+ populations, and although the frequencies were extremely low, this might point to the induction of a small population of cytotoxic CD4 T cells and Tfh cells. We observed that boosting with AIDSVAX A244 induced the most diverse HIV-specific CD4 T cell profile. The significance of this is substantiated by Lin et al.'s computational analysis of the T cell functional response profile in the RV144 trial. Their reanalysis of the RV144 CD4 T cell subset composition via the COMPASS algorithm found two correlates of HIV protection after vaccination (6): the polyfunctional CD4 T cell subset (CD40L+ IL-2+ IL-4+ IFN-γ+ TNF-α+) showed the strongest significance, but the presence of triple-positive (CD40L+ IL-2+ IL-4+) cells also correlated with the vaccine's efficiency at protection from HIV infection. Additionally, our data show that the RV144 vaccine produced the highest frequency of Tfh cells among any of the vaccination strategies compared. This boost in the amount of Tfh cells may be responsible for the characteristic antibody profile observed in the RV144 trial. While more in-depth studies are required to evaluate the relative contributions of the expression of each of these functional markers and cytokines to protection, our study suggests that what made the choice of prime/boost used in the RV144 trial more successful than similar combinations might be its ability to induce polyfunctional T cell subsets. While the selection of vaccine trials tested here is limited in comparison to the vast number of vaccine strategies that have been tested so far, we nevertheless observed a striking difference in the functional profile. Thus, assessing qualities of vaccine-induced CD4 T cell responses in further trials may help to selectively improve future generations of HIV vaccines.
In summary, our work highlights the qualitative and quantitative differences in CD4+ T cell responses elicited by different HIV vaccination strategies and by chronic infection. In addition, the functional profile of Env-specific CD4 memory T cells was significantly different from that of Gag-specific cells. By comparing vaccination routes, we found that intradermal administration of an ALVAC prime resulted in the largest population of cells expressing Tfh cell-associated markers. The highest level of protection against HIV infection observed in any HIV vaccination trial was achieved with the ALVAC/AIDSVAX modality (RV144 trial). Thus, the diverse cellular responses achieved with this vaccine, which include cytotoxic CD4 T cells as well as Tfh marker-positive cells, point to a promising path for eliciting the most effective CD4 T cells. Our in-depth analysis of the cellular role in a semiprotective immune response against HIV represents an important tool for making go/no-go decisions in future clinical trials.
MATERIALS AND METHODS
Specimens collected from clinical trials.
Peripheral blood mononuclear cells (PBMCs) collected from the clinical trials summarized in Table 1 were used in this study. Trial designs and outcomes are described in detail elsewhere (4, 21, 22, 24, 25, 35, 36). Briefly, participants in the RV114, RV132, RV135, and RV144 trials were from Thailand, whereas subjects in the RV138 trial were U.S. residents, those in the RV158 trial were from either the United States or Thailand, and the RV172 trial was executed in Uganda, Kenya, and Tanzania. All subjects were healthy, HIV-seronegative adults (18 to 55 years old) with low-risk behavior. Participants in the RV172 study were administered plasmid DNA encoding clade A Gag, Pol, and Nef and clade A, B, and C Envs at weeks 0, 4, and 8, followed by a recombinant adenovirus type 5 (Ad5) boost (1 × 1010 or 1 × 1011 PFU) with homologous proteins at month 24 (35). Participants of the RV114 study received three bivalent gp120 (clade B/E) protein immunizations in MF59 adjuvant, at weeks 0, 4, and 16 (36). Participants of the RV132 trial received a canarypox virus vector (ALVAC) prime encoding HIVenv, HIVgag, and HIVpol at weeks 0, 4, 12, and 24, followed by an Env protein boost with either oligomeric gp160 in PCPP adjuvant or bivalent gp120 in MF59 adjuvant at weeks 12 and 24 (24). RV144 participants received the same priming modality, but the protein boosts consisted of bivalent gp120 protein in alum adjuvant (AIDSVAX) (4). Finally, RV158 participants were administered three immunizations with a vaccinia virus (MVA-CMDR) encoding HIV-1 gp160, Gag, and Pol, delivered either intramuscularly or intradermally, at 0, 4, and 12 weeks (22). Uninfected specimens and specimens from chronically HIV-infected individuals were obtained from the RV229 and RV149 trials, respectively. Cryopreserved PBMCs were obtained from peak immunogenicity times, roughly 2 weeks after the final immunization for each group.
All individuals participating in this study provided written informed consent. Ethical approval was obtained from institutional review boards in each country as well as from the Human Subjects Protection Branch at the Walter Reed Army Institute of Research.
Stimulation.
Cryopreserved PBMCs were thawed and allowed to rest overnight at 37°C and 5% CO2 at a concentration of about 1 × 106 to 2 × 106 cells/ml in R10 medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin [100 U/ml], and streptomycin [100 μg/ml]). The next day, PBMCs were resuspended to a concentration of 1 × 106 to 2 × 106 cells/ml in R10 medium containing anti-CD28/anti-CD49d costimulatory antibodies (1 μg/ml) (clones L293 and L25; BD Biosciences) in 5-ml fluorescence-activated cell sorter (FACS) tubes. PBMCs were stimulated for 6 h with 15-mer overlapping peptide pools comprising HIV Gag, Pol, Nef, or Env potential T cell epitopes (NIH AIDS Reagent Program) at a concentration of 1 μg/ml. Matched PBMCs stimulated with staphylococcus enterotoxin B (SEB) and unstimulated cells served as positive and negative controls, respectively.
Multiparameter flow cytometry.
During stimulation, pretitrated amounts of LEAF anti-CD40 (clone HB14; BioLegend), phycoerythrin (PE)-Cy5-conjugated anti-CD107a (BD Pharmingen), and allophycocyanin (APC)-Cy7-conjugated anti-CD40L (clone 24-31; BioLegend) antibodies were added to the medium. Thirty minutes into the stimulation, brefeldin A (Sigma) and monensin (BD Biosciences) were added to the medium to facilitate the detection of intracellular cytokines. After stimulation, PBMCs were washed with phosphate-buffered saline (PBS) and stained with an amine-reactive dye (LIVE/DEAD Aqua Blue; Invitrogen) at room temperature for 30 min. Cells were subsequently washed in cold staining buffer (PBS with 0.5% bovine serum albumin) and surface stained for 30 min at 4°C with surface CXCR5-Alexa Fluor 488 (RF8B2; BD Pharmingen), CD8-PE-CD594 (RDA-T8; BD Horizon), PD-1–eFluor 650 (eBioJ105; eBioscience), and CD45RO-BV711 (UCHL1; BioLegend) antibodies. After washing, cells were fixed and permeabilized using 2% paraformaldehyde and 1× CytoPerm wash buffer (BD Biosciences). Subsequently, cells were resuspended in 100 μl 1× CytoPerm containing IL-21–PE (4BG1; BioLegend), IFN-γ–PE–Cy7 (4S-B3; BD Pharmingen), cMAF-eFluor 660 (symOF1; eBioscience), TNF-α–Alexa Fluor 700 (MAb 11; BD Pharmingen), CD4-BV421 (RPA-T4; BioLegend), and CD3-Qdot605 (UCHT1; Life Technologies) antibodies.
Flow cytometric data were collected with an LSR II flow cytometer and FACSDiva software (BD Biosciences). Compensation was performed with single-stained capture beads (CompBeads; BD Biosciences) and an amine-reactive dye (ArC; Invitrogen). Cytometer settings were standardized using multifluorescence calibration beads (Rainbow fluorescent particles; Spherotech). The total number of cells in each sample was determined for analysis, and data were analyzed with FlowJo, version 9.4.1 (TreeStar). Initial gating used side scatter area versus forward scatter area to delineate the lymphocytes in the population, followed by doublet removal using a plot of side scatter height versus side scatter area. CD3+ LIVE/DEAD Aqua gating identified viable T cells. Following this gating, events were sequentially gated on CD4+ CD8− and CD4+ CD45RO+ for memory cells or CD4+ CXCR5+ for pTfh cells. Following identification of these subsets, each respective function was analyzed by creating a single gate. All functional responses shown were subjected to background subtraction on the basis of the unstimulated control for each patient, as described previously (37); all response data equal to or less than the 75th percentile for the background-subtracted values were removed. Coexpression analysis of IFN-γ, TNF-α, IL-21, CD40L, and CD107a was performed with a Boolean gating strategy and the PESTLE and SPICE software suite (NIH/M. Roederer [37]).
Statistical analysis.
Statistical analysis was performed with Prism V6.07 (GraphPad). Differences in magnitudes of responses between immunization groups were compared by Kruskal-Wallis analysis followed by Dunn's multiple-comparison test. For comparisons of Boolean populations, Wilcoxon signed-rank tests were performed using SPICE 5.1 software. P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
We thank Jintanat Ananworanich for critical reviews of the manuscript and for input on the project.
This work was funded by the National Institutes of Health (NIH; R01 AI091450-01 and R01 AI094602-01) and a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD). This work was further supported by DFG STR1069/2-1.
The following were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 PTE Gag and Env peptides.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained here are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 2.Gamble LJ, Matthews QL. 2010. Current progress in the development of a prophylactic vaccine for HIV-1. Drug Des Devel Ther 5:9–26. doi: 10.2147/DDDT.S6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havenar-Daughton C, Carnathan DG, Torrents de la Pena A, Pauthner M, Briney B, Reiss SM, Wood JS, Kaushik K, van Gils MJ, Rosales SL, van der Woude P, Locci M, Le KM, de Taeye SW, Sok D, Mohammed AU, Huang J, Gumber S, Garcia A, Kasturi SP, Pulendran B, Moore JP, Ahmed R, Seumois G, Burton DR, Sanders RW, Silvestri G, Crotty S. 2016. Direct probing of germinal center responses reveals immunological features and bottlenecks for neutralizing antibody responses to HIV Env trimer. Cell Rep 17:2195–2209. doi: 10.1016/j.celrep.2016.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 5.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin L, Finak G, Ushey K, Seshadri C, Hawn TR, Frahm N, Scriba TJ, Mahomed H, Hanekom W, Bart PA, Pantaleo G, Tomaras GD, Rerks-Ngarm S, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Michael NL, Kim JH, Robb ML, O'Connell RJ, Karasavvas N, Gilbert P, De Rosa SC, McElrath MJ, Gottardo R. 2015. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol 33:610–616. doi: 10.1038/nbt.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz BT, Teigler JE, Pissani F, Oster AF, Kranias G, Alter G, Marovich M, Eller MA, Dittmer U, Robb ML, Kim JH, Michael NL, Bolton D, Streeck H. 2016. Circulating HIV-specific interleukin-21(+)CD4(+) T cells represent peripheral Tfh cells with antigen-dependent helper functions. Immunity 44:167–178. doi: 10.1016/j.immuni.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Pissani F, Streeck H. 2014. Emerging concepts on T follicular helper cell dynamics in HIV infection. Trends Immunol 35:278–286. doi: 10.1016/j.it.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dan JM, Lindestam Arlehamn CS, Weiskopf D, da Silva Antunes R, Havenar-Daughton C, Reiss SM, Brigger M, Bothwell M, Sette A, Crotty S. 2016. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol 197:983–993. doi: 10.4049/jimmunol.1600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. 2013. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mothe B, Climent N, Plana M, Rosas M, Jimenez JL, Munoz-Fernandez MA, Puertas MC, Carrillo J, Gonzalez N, Leon A, Pich J, Arnaiz JA, Gatell JM, Clotet B, Blanco J, Alcami J, Martinez-Picado J, Alvarez-Fernandez C, Sanchez-Palomino S, Guardo AC, Pena J, Benito JM, Rallon N, Gomez CE, Perdiguero B, Garcia-Arriaza J, Esteban M, Lopez Bernaldo de Quiros JC, Brander C, Garcia F, RISVAC-03 Study Group. 2015. Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVA-B) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1. J Antimicrob Chemother 70:1833–1842. doi: 10.1093/jac/dkv046. [DOI] [PubMed] [Google Scholar]
- 12.Franchini G, Gurunathan S, Baglyos L, Plotkin S, Tartaglia J. 2004. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Expert Rev Vaccines 3:S75–S88. doi: 10.1586/14760584.3.4.S75. [DOI] [PubMed] [Google Scholar]
- 13.Russell ND, Graham BS, Keefer MC, McElrath MJ, Self SG, Weinhold KJ, Montefiori DC, Ferrari G, Horton H, Tomaras GD, Gurunathan S, Baglyos L, Frey SE, Mulligan MJ, Harro CD, Buchbinder SP, Baden LR, Blattner WA, Koblin BA, Corey L, National Institute of Allergy and Infectious Diseases HIV Vaccine Trials Network. 2007. Phase 2 study of an HIV-1 canarypox vaccine (vCP1452) alone and in combination with rgp120: negative results fail to trigger a phase 3 correlates trial. J Acquir Immune Defic Syndr 44:203–212. doi: 10.1097/01.qai.0000248356.48501.ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol 77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asmuth DM, Brown EL, DiNubile MJ, Sun X, del Rio C, Harro C, Keefer MC, Kublin JG, Dubey SA, Kierstead LS, Casimiro DR, Shiver JW, Robertson MN, Quirk EK, Mehrotra DV. 2010. Comparative cell-mediated immunogenicity of DNA/DNA, DNA/adenovirus type 5 (Ad5), or Ad5/Ad5 HIV-1 clade B gag vaccine prime-boost regimens. J Infect Dis 201:132–141. doi: 10.1086/648591. [DOI] [PubMed] [Google Scholar]
- 16.Cox RJ, Brokstad KA, Ogra P. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 59:1–15. [DOI] [PubMed] [Google Scholar]
- 17.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, Bailer R, Graham BS, Roederer M, Koup RA. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med 204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacGregor RR, Ginsberg R, Ugen KE, Baine Y, Kang CU, Tu XM, Higgins T, Weiner DB, Boyer JD. 2002. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. AIDS 16:2137–2143. doi: 10.1097/00002030-200211080-00005. [DOI] [PubMed] [Google Scholar]
- 19.Edupuganti S, Weber D, Poole C. 2004. Cytotoxic T-lymphocyte responses to canarypox vector-based HIV vaccines in HIV-seronegative individuals: a meta-analysis of published studies. HIV Clin Trials 5:259–268. doi: 10.1310/W9J3-0PTB-FX1V-Y3FX. [DOI] [PubMed] [Google Scholar]
- 20.Baden LR, Walsh SR, Seaman MS, Cohen YZ, Johnson JA, Licona JH, Filter RD, Kleinjan JA, Gothing JA, Jennings J, Peter L, Nkolola J, Abbink P, Borducchi EN, Kirilova M, Stephenson KE, Pegu P, Eller MA, Trinh HV, Rao M, Ake JA, Sarnecki M, Nijs S, Callewaert K, Schuitemaker H, Hendriks J, Pau MG, Tomaka F, Korber BT, Alter G, Dolin R, Earl PL, Moss B, Michael NL, Robb ML, Barouch DH, IPCAVD006/RV380/HIV-V-A002 Study Group. 2018. First-in-human randomized, controlled trial of mosaic HIV-1 immunogens delivered via a modified vaccinia Ankara vector. J Infect Dis 218:633–644. doi: 10.1093/infdis/jiy212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eller MA, Slike BM, Cox JH, Lesho E, Wang Z, Currier JR, Darden JM, Polonis VR, Vahey MT, Peel S, Robb ML, Michael NL, Marovich MA. 2011. A double-blind randomized phase I clinical trial targeting ALVAC-HIV vaccine to human dendritic cells. PLoS One 6:e24254. doi: 10.1371/journal.pone.0024254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currier JR, Ngauy V, de Souza MS, Ratto-Kim S, Cox JH, Polonis VR, Earl P, Moss B, Peel S, Slike B, Sriplienchan S, Thongcharoen P, Paris RM, Robb ML, Kim J, Michael NL, Marovich MA. 2010. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 5:e13983. doi: 10.1371/journal.pone.0013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musich T, Robert-Guroff M. 2016. New developments in an old strategy: heterologous vector primes and envelope protein boosts in HIV vaccine design. Expert Rev Vaccines 15:1015–1027. doi: 10.1586/14760584.2016.1158108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thongcharoen P, Suriyanon V, Paris RM, Khamboonruang C, de Souza MS, Ratto-Kim S, Karnasuta C, Polonis VR, Baglyos L, Habib RE, Gurunathan S, Barnett S, Brown AE, Birx DL, McNeil JG, Kim JH, Thai AIDS Vaccine Evaluation Group. 2007. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J Acquir Immune Defic Syndr 46:48–55. [DOI] [PubMed] [Google Scholar]
- 25.Nitayaphan S, Pitisuttithum P, Karnasuta C, Eamsila C, de Souza M, Morgan P, Polonis V, Benenson M, VanCott T, Ratto-Kim S, Kim J, Thapinta D, Garner R, Bussaratid V, Singharaj P, el-Habib R, Gurunathan S, Heyward W, Birx D, McNeil J, Brown AE, Thai AIDS Vaccine Evaluation Group. 2004. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis 190:702–706. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- 26.Buranapraditkun S, Pissani F, Teigler JE, Schultz BT, Alter G, Marovich M, Robb ML, Eller MA, Martin J, Deeks S, Michael NL, Streeck H. 2017. Preservation of peripheral T follicular helper cell function in HIV controllers. J Virol 91:e00497-. doi: 10.1128/JVI.00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu DC, O'Connell RJ. 2017. Progress in HIV vaccine development. Hum Vaccin Immunother 13:1018–1030. doi: 10.1080/21645515.2016.1276138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viegas EO, Tembe N, Nilsson C, Meggi B, Maueia C, Augusto O, Stout R, Scarlatti G, Ferrari G, Earl PL, Wahren B, Andersson S, Robb ML, Osman N, Biberfeld G, Jani I, Sandstrom E. 27 November 2017. Intradermal HIV-1 DNA immunization using needle-free Zetajet injection followed by HIV-modified vaccinia virus Ankara vaccination is safe and immunogenic in Mozambican young adults: a phase I randomized controlled trial. AIDS Res Hum Retroviruses doi: 10.1089/AID.2017.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liard C, Munier S, Arias M, Joulin-Giet A, Bonduelle O, Duffy D, Shattock RJ, Verrier B, Combadiere B. 2011. Targeting of HIV-p24 particle-based vaccine into differential skin layers induces distinct arms of the immune responses. Vaccine 29:6379–6391. doi: 10.1016/j.vaccine.2011.04.080. [DOI] [PubMed] [Google Scholar]
- 30.Liard C, Munier S, Joulin-Giet A, Bonduelle O, Hadam S, Duffy D, Vogt A, Verrier B, Combadiere B. 2012. Intradermal immunization triggers epidermal Langerhans cell mobilization required for CD8 T-cell immune responses. J Invest Dermatol 132:615–625. doi: 10.1038/jid.2011.346. [DOI] [PubMed] [Google Scholar]
- 31.Mann JF, McKay PF, Fiserova A, Klein K, Cope A, Rogers P, Swales J, Seaman MS, Combadiere B, Shattock RJ. 2014. Enhanced immunogenicity of an HIV-1 DNA vaccine delivered with electroporation via combined intramuscular and intradermal routes. J Virol 88:6959–6969. doi: 10.1128/JVI.00183-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin C, Bonduelle O, Nuttens C, Primard C, Verrier B, Boissonnas A, Combadiere B. 2017. Critical role for skin-derived migratory DCs and Langerhans cells in TFH and GC responses after intradermal immunization. J Invest Dermatol 137:1905–1913. doi: 10.1016/j.jid.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Rerks-Ngarm S, Pitisuttithum P, Excler JL, Nitayaphan S, Kaewkungwal J, Premsri N, Kunasol P, Karasavvas N, Schuetz A, Ngauy V, Sinangil F, Dawson P, deCamp AC, Phogat S, Garunathan S, Tartaglia J, DiazGranados C, Ratto-Kim S, Pegu P, Eller M, Karnasuta C, Montefiori DC, Sawant S, Vandergrift N, Wills S, Tomaras GD, Robb ML, Michael NL, Kim JH, Vasan S, O'Connell RJ, RV305 Study Team. 2017. Randomized, double-blind evaluation of late boost strategies for HIV-uninfected vaccine recipients in the RV144 HIV vaccine efficacy trial. J Infect Dis 215:1255–1263. doi: 10.1093/infdis/jix099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khurana S, Coyle EM, Dimitrova M, Castellino F, Nicholson K, Del Giudice G, Golding H. 2014. Heterologous prime-boost vaccination with MF59-adjuvanted H5 vaccines promotes antibody affinity maturation towards the hemagglutinin HA1 domain and broad H5N1 cross-clade neutralization. PLoS One 9:e95496. doi: 10.1371/journal.pone.0095496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, Shaffer D, Eller LA, Kibaya R, Eller MA, Schindler KB, Schuetz A, Millard M, Kroll J, Dally L, Hoelscher M, Bailer R, Cox JH, Marovich M, Birx DL, Graham BS, Michael NL, de Souza MS, Robb ML. 2010. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-uninfected East Africans (RV 172). J Infect Dis 201:600–607. doi: 10.1086/650299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitisuttithum P, Nitayaphan S, Thongcharoen P, Khamboonruang C, Kim J, de Souza M, Chuenchitra T, Garner RP, Thapinta D, Polonis V, Ratto-Kim S, Chanbancherd P, Chiu J, Birx DL, Duliege AM, McNeil JG, Brown AE, Thai AIDS Vaccine Evaluation Group. 2003. Safety and immunogenicity of combinations of recombinant subtype E and B human immunodeficiency virus type 1 envelope glycoprotein 120 vaccines in healthy Thai adults. J Infect Dis 188:219–227. doi: 10.1086/376506. [DOI] [PubMed] [Google Scholar]
- 37.Roederer M. 2011. Multiple stained samples are not appropriate compensation controls. Cytometry A 79:591–593. doi: 10.1002/cyto.a.21093. [DOI] [PubMed] [Google Scholar]