Abstract
Viral hepatitis is one of the major public health concerns around the world but until recently it has drawn little attention or funding from global health policymakers. Every year 1.4 million people die from viral hepatitis-related cirrhosis and liver cancer. However, the majority of the infected population are unaware of their condition. This population have significant obstacles to overcome such as lack of awareness, vulnerability, increased migration, disease stigma, discrimination, as well as poor health resources, conflict in policy development and program implementation. Despite implementing infection control measures over the last few decades eradication or significant disease reduction remains elusive. This study aims to present the current global prevalence status and examines potential elimination strategies. The information for this research were obtained through a systematic review, published scientific literatures, the official websites of various government organisations, international public health organisations and internationally recognised regulatory bodies over a period of 40 years between 1978 and 2018.
Keywords: Cirrhosis, Global epidemiology, Outreach clinic, Liver cancer, Vaccination, Viral hepatitis
Core tip: Viral hepatitis is a serious disease, which results in a high number of fatalities that increases each year, with the majority of infected people being unaware of their condition. Although many infection control measures have been employed with the expectation of reducing the spread of the virus, eradication or significant disease reduction remains a long way off. The global burden of the disease remains significant. This mini-review presents the current global prevalence status and examines potential elimination strategies.
INTRODUCTION
Viral hepatitis results from inflammation of the liver, caused by a viral infection[1]. Although “epidemic jaundice” has existed since ancient civilisation, it is only in the last few decades that viral aetiologies of hepatitis have been identified. Almost all such infections are caused by five viruses, namely hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV). Viral hepatitis is a major public health concern, infecting millions of people annually; some infections subsequently lead to hepatocellular carcinoma (HCC), liver cirrhosis and fatalities among significant proportion of patients. The World Health Organization (WHO) estimated that 1 in 3 people in the world have been infected by either HBV or HCV[1,2] and 1.3 million people have died as a result of this disease in 2015. It has been reported that 2 billion people have been infected with HBV, approximately 185 million of those people are infected with HCV and 20 million people are infected with HEV[3,4]. In high endemic regions more than 90% children get infected by HAV by the age of 10 although few develop complications[5]. About 2.3 billion people of the world are infected with one or more of the hepatitis viruses.
Viral hepatitis results in around 1.4 million deaths each year, HBV and HCV are responsible for about 90% of these fatalities, whilst the remaining 10% of fatalities are caused by other hepatitis viruses[6,7]. Although viral hepatitis is a major public health problem across the globe it has not been prioritised until now. Lately, the“2030 Agenda for Sustainable Development Goals” of WHO has identified specific actions to prevent viral hepatitis[8,9].
Prevention and control strategies for viral hepatitis such as raising awareness through public education, vaccination, blood transfusion safety strategies, early diagnosis and effective medical support can be implemented, and novel interventions are available. In this review, global epidemiology of viral hepatitis and effective control methods are summarised. Data from key recent observational studies, clinical trials, case reports or case series were identified and their data synthesised to summarise the disease burden, geographical distribution and effective control measures of viral hepatitis.
SEARCH STRATEGY
Key databases searched were Medline, EMBASE, CINAHL; websites of international medical associations/public health organisations such as European Association for the study of Liver, American Association for the study of Liver Disease, the Asian Pacific Association for the Study of Liver and Gastroenterology Society of Australia. Databases including Science Citation Index were expanded by using database-specific controlled vocabulary (where available) and general free text terms. Other relevant websites were also explored, including those of the Global Health Library, Centres for Disease Control and Prevention, WHO, United States Food and Drug Administration, European Medicines Agency, and Australian Government Department of Health. In addition other search engines such as Google Scholar, Research Gate and Science Direct were explored using the key search terms. The current status of viral hepatitis, action plans and options for controlling outbreaks have been discussed.
EPIDEMIOLOGY OF VIRAL HEPATITIS
Hepatitis A
Millions of people became infected with HAV by ingesting contaminated food and drinking water. The infection rate is strongly related to access to safe drinking water and socio-economic indicator. Generally, all high-income global regions have very low levels of HAV endemicity (< 50% of population), while low-income regions have high levels of endemicity (> 90% of population). Middle-income regions of society both have intermediate and low levels of endemicities[9,10]. Globally, only 1.5 million clinical cases of HAV are reported annually while the rate of infection is much higher[11]. In highly endemic countries nearly all children get infected at an early age, with mostly asymptomatic exposure, but acquire lifelong immunity. Paradoxically, in low endemic countries most children and adults remain susceptible to symptomatic infection and disease burden is high[11]. Global distribution is depicted in Figure 1[12].
Figure 1.
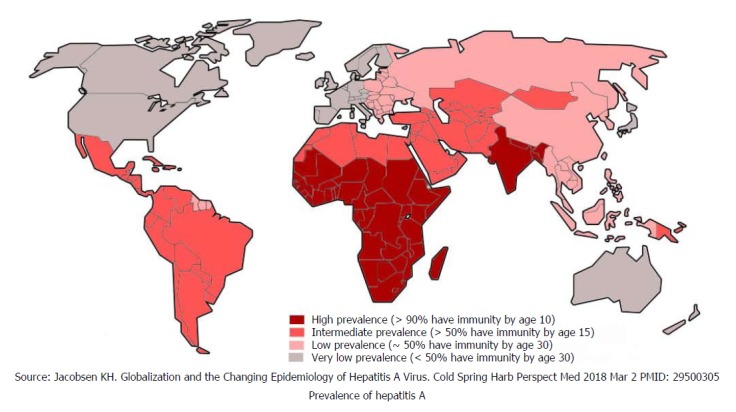
Global distribution of hepatitis A.
Hepatitis B
Hepatitis B is globally one of the most common and severe infectious diseases that leads to significant morbidity and mortality[13]. Approximately one-third of the World’s population have been infected with HBV. Around 5% of this population are chronic carriers and a quarter of these carriers develop serious liver diseases such as chronic hepatitis, cirrhosis and hepatic carcinoma[14]. Every year, 780000 HBV-related deaths are documented around the globe[14]. Figure 2 shows global distribution of HBV[15]. The risk of developing chronic infection and subsequent complications is inversely related to the age of infection. There is about 90% risk of developing chronic infection and subsequent complications if the infection occurs prenatally. In sub-Saharan Africa and East Asian countries HBV usually is predominantly transmitted via perinatal or horizontal route. However, in developed countries most infections occur to young adults through injecting drug use or high-risk sexual behavior[15].
Figure 2.
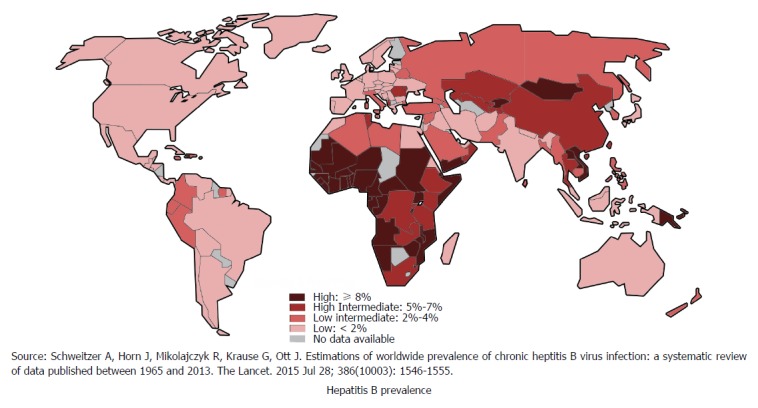
Global distribution of hepatitis B.
Hepatitis C
It is estimated that 71 million people globally have chronic hepatitis C infection[16], who are at risk of developing liver cirrhosis and liver cancer[17]. This accounts for 399000 deaths every year. Among various genotypes of HCV, genotype 1 is the most prevalent which accounts for 46% of all HCV infections, followed by genotype 3, which is 22% prevalent. Genotype 2 and 4 each has 13% prevalence[18]. Figure 3 illustrates global prevalence and genotype distribution[19]. As death occurs decades after being infected, people dying of liver conditions are often not linked to underlying viruses. The death certificates of people with HCV infections are increasing in the United States. Despite this, one study estimated, that the number of patients documented in the United States to have HCV infection in 2010 only represented one-fifth of the patients dying from HCV related illness that year[20].
Figure 3.
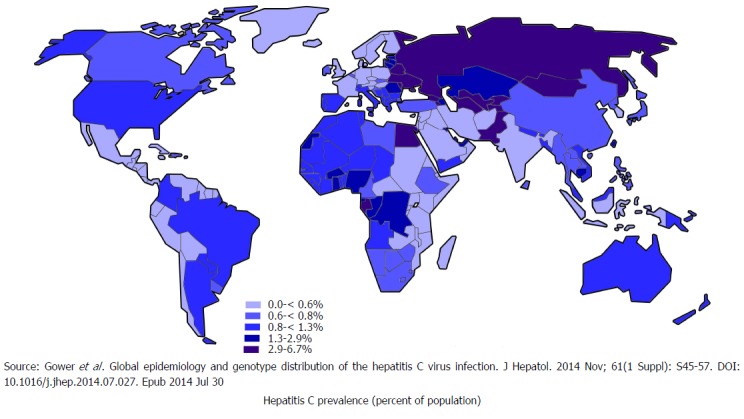
Global distribution of hepatitis C.
Hepatitis D
HDV is commonly seen in the people who are exposed to infected blood products or infected needles of previously infected HBV[21]. Globally, 5% of hepatitis B surface antigen positive people are co-infected with HDV[22]. Approximately 18 million people are infected with HDV globally[23]. HDV infection is likely to be declining worldwide as result of successful HBV immunisation, and improvement in socioeconomic status but data to show this from most parts of the globe is lacking as demonstrated in Figure 4[22].
Figure 4.
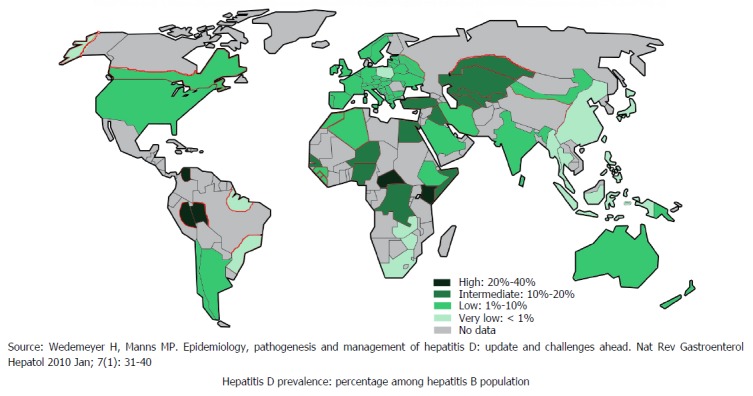
Global distribution of hepatitis D.
Hepatitis E
HEV causes food and waterborne diseases with outbreaks seen worldwide. These outbreaks are mostly seen in countries with limited access to clean water, sanitation and poor hygiene[24]. It is estimated that approximately 20 million people are infected with HEV annually worldwide[25] and 44000 of these infections result in deaths. Figure 5 shows worldwide HEV distribution[25]. Although HEV has similar route of transmission to HAV and does not lead to chronicity acute infection with HEV carries 3.3% mortality risk, which is significant[24].
Figure 5.
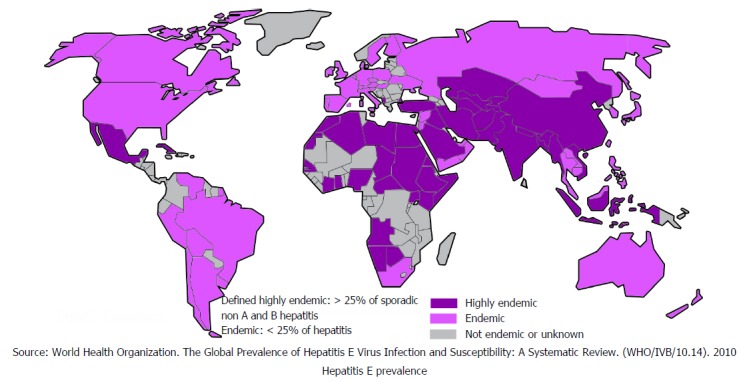
Global distribution of hepatitis E.
GEOGRAPHIC DISTRIBUTION OF VIRAL HEPATITIS
African continent
In highly endemic areas that include parts of Africa and Asia, a large proportion of individuals in the population are immune to HAV, and epidemics of hepatitis A are uncommon[26]. Some of the highest prevalence of HAV is observed in sub-Saharan countries where nearly all of the population develops HAV immunity. Disease burden for HBV is also high among the sub-Saharan population. About 6.1% of the African population have HBV infection and around 18 million people have chronic HCV infection[27,28]. One of the biggest concern associated with the high prevalence of HAV is that people in the infected regions have little awareness of the disease and are not adequately educated about treatment and preventative measures[28].
Americas
The prevalence of hepatitis A is high in the Americas, with exception of high-income North American countries. However, there has been slight reduction in Central American regions in recent years. In intermediate endemic regions such as, Central and South America, childhood transmission is not very frequent. Adolescents and adults are susceptible to symptomatic infection, and outbreaks are common[26]. Almost 50% of people infected with HAV have developed immunity against this virus by the age 15[29]. About 7-9 million people of Caribbean countries and Latin America are anti-HCV positive, and an estimated 0.7% of the population are infected with hepatitis B. The Americas have the lowest prevalence rate of HBV with the exception of Haiti[29]. Due to people immigrating into the United States a large number of new cases of HBV have been imported into the country in recent decades[30,31].
Although data on HDV are lacking in most parts of the world it has been well reported in North America. Few data are available for the epidemiology of HEV. Studies from Brazil have shown that 3% of the population are infected with HEV and another study from Bolivia has found that 1.7% to 16.2% of their population are HEV positive[32,33].
Eastern Mediterranean region
The incidence of HAV has decreased in North African and Middle Eastern countries in the last decades and now have intermediate prevalence (Figure 1)[12]. It has been reported that around 3.3% of people from this region are infected with HBV and about 800000 people are HCV positive. Interestingly, among the Egyptian population up to 14.5 million are HCV positive and 7.8 million have developed chronic HCV[34,35]. Eastern Mediterranean regions have low-intermediate endemicity with the exception of Somalia, Djibouti and Sudan which demonstrate a higher prevalence. The percentage of the population infected with HBV in the following countries are: Pakistan 4.5%[36], Yemen 12.7%-18.5%, Sudan 5%-8.2%[37,38], and Somalia 5.6%-21.3%[35]; HEV has been seen commonly reported in Sudan, South Sudan, Pakistan and Somalia[39] (Figure 5[25]).
European continent
The prevalence of HAV increases from west to east; childhood transmission is less frequent in Eastern Europe while adult transmission is more common[40]. Western Europe continues to enjoy very low prevalence of HAV but community wide outbreaks are frequently reported[26]. In 1996, the recorded prevalence of HAV was 15.1 per 100000 people, with this number decreasing to 3.9 in 2006[40]. Despite a declining incidence of HAV in Central and Eastern Europe it was considered a serious public health issue due to outbreaks occurring in Czech Republic, Slovakia and Latvia in 2008[41]. Although prevalence of HBV is low in the European continent, it rises eastward[42]. In Europe, approximately 1.4 million people have chronic HBV, while 9 million people have chronic HCV [40]. The number of deaths due to HBV and HCV infections are 36000 and 86000 per year respectively[43,44].
South-East Asia
In most parts of South East Asia HAV seroprevalence continues to be very high, but recent reports suggest that in some parts such as India infection rates are declining. In eastern regions HAV now has low endemicity. Five million people die from chronic viral hepatitis each year in the South-East region[44,45]. About 100 million people have a chronic HBV infection. The prevalence of chronic viral hepatitis in this region is 30 times more than that of human immunodeficiency virus (HIV)[46]. Unfortunately, about 65% of HBV infected patients and 75% of HCV infected people are unaware of the infection[47,48]. About 10 million people in this region carry hepatitis C[49]. It is estimated that 12 million cases of HEV infections are seen every year in this region[50-52]. This number accounts for more than half of the global cases. In this region, every year an estimated 6.5 million symptomatic cases of hepatitis E have been reported as well as 400000 cases of hepatitis A[53-56]. The true incidence is likely to be much higher.
Western Pacific region
This region of the world represents 28% of world’s population. In this part of the world more people die because of viral hepatitis than HIV, malaria and tuberculosis together[43]. Prevalence of HAV is low in high-income regions such as Australia and the Asia-pacific. The East Asian population is also seeing a decline in the incidence of HAV[57] as socio-economic condition in this region continues to improve. Overall, 350 million people live with HBV infection in this region, which is the half of world’s HBV cases[58,59]. This is also the region, which represents 60% of liver cancer worldwide[60]. The prevalence of HCV in East Asia is 1%-2% while some countries of this region such as Taiwan and Vietnam have a prevalence of 4.4% and 2.9%, respectively[61,62]. Of the 150 million people chronically infected with hepatitis C globally, more than 60 million live in Western Pacific region[62]. An estimated 3% of the deaths in this region are attributed to acute hepatitis B and E related complications[62].
ACTION PLANS FOR CONTROLLING VIRAL HEPATITIS
An effective approach to the prevention of viral hepatitis requires multiple strategies as outlined below.
Education
Education programs directed towards disease awareness lowers disease transmission[54,62]. Large portion of chronic hepatitis sufferers in developing countries are not aware of their condition. Awareness campaigns to educate entire community and implementation of local health measures are important[50]. These include training local communities on how to perform safe blood transfusion, and establishing efficient screening protocols for transfusion of donated bloods. Health education should include, administration of safe injections both in healthcare settings as well amongst as intravenous drug users and implementation of safer sex practices. Furthermore, there should be occupational safety trainings for health workers[63-65]. It is also important to effectively communicate and emphasise the importance of testing for the virus follow up visits and monitoring treatment to help eliminate viral hepatitis[66,67].
Improvement of socio-economic condition
Improvement in socioeconomic status has shown to reduce the prevalence of all types of viral hepatitis. Government bodies should ensure universal access to clean water and encourage hygienic food handling and safety practices, and should implement improved sanitation systems[65,68]. The practice of safe disposal of medical waste should be ensured in health sectors.
Screening and early detection
Screening, early detection and initiation of treatment will prevent further transmission of the virus and reduce morbidities and mortalities among infected individuals[68,69]. The first step should be to give the correct medical advice and initiate antiviral medication, if available. Unfortunately, implementing these steps is often challenging in developing countries where access to health care is limited and treatment is often unaffordable[70,71].
Vaccination
Vaccination campaigns for HAV and HBV infections are central to WHO’s drive to eradicate hepatitis globally[71-73]. To ensure the maximum implementation of its vision, WHO has provided technical guidance and support to reduce disease transmission such as ensuring safer blood transfusion, disposable needles, etc. Hepatitis A vaccine should be available to susceptible individuals in low and intermediate endemic areas[71].
Although hepatitis B vaccine is effective in the prevention of disease only 27% of newborns worldwide receive a birth dose of hepatitis B vaccine[71]. Birth dose vaccination of HBV is critical to prevent mother to child transmission as late vaccination is not fully effective in breaking mother-to-child transmission chain. Coordination between vaccination and maternal health services should be established effectively[60]. Currently, a number of treatment options are available for HBV that will improve long-term survival. However, treatment is not accessible in many countries due to high cost[72].
More than 350000 people die from HCV infection every year[73,74]. Currently, there is no vaccine to protect individuals from HCV due to the peculiarity of the virus. HCV is an RNA virus and mutates rapidly making vaccine development difficult[75]. However, traditional curative treatment is available based on genotyping of the virus, and safe blood transfusion strategies similar to that of HBV can be undertaken to prevent spread. The traditional genotype-based therapies with interferon, has been shown to be moderately successful in the sustained elimination of the viral genome[76].
However, the introduction of pan-genotypic treatments for all genotypes of hepatitis C has been a major breakthrough in scientific research. Recent clinical trials of the once-daily combination therapy of sofosbuvir and velpatasvir have shown sustained virologic response rates of about 95%, irrespective of prior treatment or presence of liver cirrhosis across all genotypes[68]. The pan-genotypic success achieved with this combination of sofosbuvir/velpatasvir represents a safe and curative therapy for HCV, but high cost is the major limitation of this therapy. Once the therapy is widely accessible to all infected patients, this will eventually decrease the burden of disease, economic burden on health care, with a subsequent decline in morbidity and mortality[69].
HDV requires HBV for replication and can only infect simultaneously with HBV or as superinfection with HBV[21]. Currently, no specific recommended treatment for HDV is recommended[23]. Management and preventative strategies for HDV are instead directed towards HBV. There is one vaccine under development against HEV in China, which is yet to implemented routinely[76].
Implementing WHO global model
The viral hepatitis outbreaks can be controlled with comprehensive global action plans and collaborations. A number of models have been used for viral hepatitis management. WHO has a vision and its goal is to eliminate viral hepatitis worldwide as a major public health problem[69-74]. In this global strategy, five core interventions have been proposed and the targeted areas are vaccination plans for hepatitis B, A and E, prevention of vertical transmission of hepatitis B, injection and blood products safety, harm reduction and treatment[77-79].
Multi-sectoral coordination
Elimination of viral hepatitis requires strong national and international commitments. Comprehensive action plans for prevention, screening, diagnosis and treatment of viral hepatitis should be implemented through collaborations between government, health service providers and society[78,79].
Nurse-led approach
This model for hepatitis C management was established in prison and provided advice on harm minimisation, diagnosis and treatment. HCV infected persons often face obstacles to access treatment such as not being aware of availability of modern therapies, high cost, fear and distrust of healthcare professionals. Some studies suggested that nurse-led models provided a good opportunity for instituting intervention against transmission and spread of HCV but it was minimally successful in reducing HCV transmission among prisoners[79-81]. However, it has also been shown that professional care and specialist-managed treatment models for chronic HCV do result in improved treatment uptake and low disease burden[49,82].
Peer navigation model
People who recover successfully from the infection can work closely with multidisciplinary clinical care team to offer extensive viral hepatitis support, care and access to treatment specifically for those who have barriers to clinical care[70]. Major obstacles that hinder care among HCV infected intravenous drug users should be overcome by strategies such as on-site treatment, addiction management plan, multidisciplinary teams work, intensive model of care, directly peer observed treatment and group treatment[79-81]. It has been shown that combination of clinical and behavioral interventions can result in reduction of HCV among substance users[80-82].
The prevalence of HCV infection among intravenous recreational drug users remains high worldwide. Despite the availability of well-tolerated successful treatment, morbidity and mortality due to liver disease among people with HCV infection is still increasing. The Kirby Institute, The University of New South Wales Sydney and the International Network on Hepatitis in Substance Users have organised an expert roundtable panel to evaluate current issues and implemented future research priorities for the prevention and management of HCV among people who inject drugs. International experts in drug and alcohol, infectious diseases, and hepatology have come together on one platform to identify the current scientific evidence, issues in research, and develop research priorities[79].
Outreach treatment
Providing outreach services is important in viral hepatitis management. One example includes mobile health clinics which are an innovative and flexible way to provide healthcare for chronic viral hepatitis patients. They have been proven effective in giving the health screenings, initiating chronic disease management and providing immediate intervention when required. The mobile van clinic has been a novel approach for controlling viral hepatitis[75].
HCV is prevalent among the injecting drug users especially those in prison, the aboriginal population and people coming from culturally and linguistically diverse background. These are the people who are disconnected from traditional health providers and have poor retention in care systems. To engage these people in the health care system and to provide the appropriate treatment requires innovative action plans[75,49].
The above-mentioned mobile outreach vans will bring the treatment services to these people and will also make it easier for them to access the health services particularly for those who have comorbid psychiatric and substance use disorders. It will create a link between clinical and community-based settings and will remove geographic, socioeconomic and structural obstacles. Successful treatment of HCV infections will decrease the risk of chronicity of the disease and liver cancer, improve the quality of life and will also increase the survival rates[77,78]. The mobile outreach system will also help in reducing the transmission of disease by providing the early treatment, improved viral clearance and reduced risk behaviors[75].
Post-exposure prophylaxis
Many cases of viral hepatitis occur among health workers due to accidental needle injuries. Preventing work-related accidents in health organisations should be urgently reviewed. Prompt IgG treatment option should be in place as soon as exposure to virus is confirmed. This treatment may stop the infection from developing. Patients exposed to viruses should undergo similar treatment. This prompt strategy could serve as an efficient therapeutic modality and prevent development of infection and minimise outbreaks.
CONCLUSION
Although there has been some success with preventative strategies globally, still many hurdles need to be overcome if we are to reduce viral transmission significantly. WHO has published its technical report manual for the development and assessment of national viral hepatitis plans in 2017[49]. This guidance could help to control viral hepatitis outbreaks. These actions need to be strengthened and reinforced in order to stop the outbreaks and provide a viral hepatitis-free future for the next generation.
One of the important actions to be adopted to control outbreak is prompt immune serum treatment. WHO can include post-exposure prophylaxis in their global strategy which at first can be implemented in resource-rich settings and gradually adopted in developing and underdeveloped countries. For global success in controlling viral hepatitis, international organisations can establish round tables to exchange ideas for action plans. There is no one single measure strong enough to curb viral hepatitis epidemics but having a global vision and implementing multiple strategies will go some way towards reducing global disease burden.
Footnotes
Conflict-of-interest statement: Nothing to declare.
Manuscript source: Invited manuscript
Peer-review started: May 29, 2018
First decision: July 9, 2018
Article in press: October 16, 2018
Specialty type: Medicine, research and experimental
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kao JT, Nozic D, Preda C S- Editor: Ji FF L- Editor: A E- Editor: Song H
Contributor Information
Meryem Jefferies, Drug Health, Western Sydney Local Health District, North Parramatta NSW 2151, Australia. meryemjefferies@gmail.com.
Bisma Rauff, Westmead Institute for Medical Research, Westmead Hospital, Sydney Medical School University of Sydney, Westmead NSW 2145, Australia.
Harunor Rashid, National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases, the Children’s Hospital at Westmead, and the Discipline of Child and Adolescent Health, Sydney Medical School, Westmead, NSW 2145, Australia.
Thao Lam, Drug Health, Western Sydney Local Health District, North Parramatta NSW 2151, Australia.
Shafquat Rafiq, Croydon University Hospital NHS Trust, Croydon SE23 2SP, United Kingdom.
References
- 1.World Health Organization. Prevention and Control of Viral Hepatitis Infection: Frame Work for Global Action. 2012. Available from: http://www.who.int/hepatitis/publications/Framework/en/
- 2.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 3.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 4.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653–6657. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Sixty Third World Health Assembly - Viral Hepatitis (WHA63.18) Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA63-REC1/WHA63_REC1-en.pdf.
- 7.Wiktor SZ, Hutin YJ. The global burden of viral hepatitis: better estimates to guide hepatitis elimination efforts. Lancet. 2016;388:1030–1031. doi: 10.1016/S0140-6736(16)31018-2. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Eliminate Hepatitis: WHO. Available from: http://www.who.int/news-room/detail/27-07-2017-eliminate-hepatitis-who.
- 9.Hutin Y, Low-Beer D, Bergeri I, Hess S, Garcia-Calleja JM, Hayashi C, Mozalevskis A, Rinder Stengaard A, Sabin K, Harmanci H, et al. Viral Hepatitis Strategic Information to Achieve Elimination by 2030: Key Elements for HIV Program Managers. JMIR Public Health Surveill. 2017;3:e91. doi: 10.2196/publichealth.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. The global prevalence of hepatitis A virus infection and susceptibility: a systematic review. Geneva: World Health Organization; 2010. [Google Scholar]
- 11.Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:68–73. doi: 10.4254/wjh.v4.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsen KH. Globalization and the Changing Epidemiology of Hepatitis A Virus. Cold Spring Harb Perspect Med. 2018;pii:a031716. doi: 10.1101/cshperspect.a031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:74–80. doi: 10.4254/wjh.v4.i3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma SK, Saini N, Chwla Y. Hepatitis B virus: inactive carriers. Virol J. 2005;2:82. doi: 10.1186/1743-422X-2-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 16.Tun W, Vu L, Adebajo SB, Abiodun L, Sheehy M, Karlyn A, Njab J, Ahonsi B, Issa BK, Idogho O. Population-based prevalence of hepatitis B and C virus, HIV, syphilis, gonorrhoea and chlamydia in male injection drug users in Lagos, Nigeria. Int J STD AIDS. 2013;24:619–625. doi: 10.1177/0956462413477553. [DOI] [PubMed] [Google Scholar]
- 17.El Khoury AC, Wallace C, Klimack WK, Razavi H. Economic burden of hepatitis C-associated diseases: Europe, Asia Pacific, and the Americas. J Med Econ. 2012;15:887–896. doi: 10.3111/13696998.2012.681332. [DOI] [PubMed] [Google Scholar]
- 18.Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 19.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Mahajan R, Xing J, Liu SJ, Ly KN, Moorman AC, Rupp L, Xu F, Holmberg SD; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006-2010. Clin Infect Dis. 2014;58:1055–1061. doi: 10.1093/cid/ciu077. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Hepatitis D. WHO Fact sheets. Available from: http://www.who.int/news-room/fact-sheets/detail/hepatitis-d.
- 22.Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31–40. doi: 10.1038/nrgastro.2009.205. [DOI] [PubMed] [Google Scholar]
- 23.Farci P. Delta hepatitis: an update. J Hepatol. 2003;39 Suppl 1:S212–S219. doi: 10.1016/s0168-8278(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 24.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. The global prevalence of hepatitis E virus infection and susceptibility: a systematic review. Geneva: World Health Organization; 2010. [Google Scholar]
- 26.Nelson N. Chapter 3, Infectious diseases related to travel. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/hepatitis-a.
- 27.World Health Organization. Hepatitis B. Available from: http://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- 28.World Health Organization. Hepatitis C. Weekly Epidemiological Record. 2009;84:405–420. [Google Scholar]
- 29.Díez-Padrisa N, Castellanos LG; PAHO Viral Hepatitis Working Group. Viral hepatitis in Latin America and the Caribbean: a public health challenge. Rev Panam Salud Publica. 2013;34:275–281. [PubMed] [Google Scholar]
- 30.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B--United States, 1974-2008. PLoS One. 2011;6:e27717. doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Combating Hepatitis B and C to reach elimination by 2030. Available from: http://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.pdf.
- 32.Bricks G, Senise JF, Pott Junior H, Grandi G, Passarini A, Caldeira DB, Carnaúba Junior D, Moraes HAB, Granato CFH, Castelo A. Seroprevalence of hepatitis E virus in chronic hepatitis C in Brazil. Braz J Infect Dis. 2018;22:85–91. doi: 10.1016/j.bjid.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dell’Amico MC, Cavallo A, Gonzales JL, Bonelli SI, Valda Y, Pieri A, Segund H, Ibañez R, Mantella A, Bartalesi F, et al. Hepatitis E virus genotype 3 in humans and Swine, Bolivia. Emerg Infect Dis. 2011;17:1488–1490. doi: 10.3201/eid1708.100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elgharably A, Gomaa AI, Crossey MM, Norsworthy PJ, Waked I, Taylor-Robinson SD. Hepatitis C in Egypt - past, present, and future. Int J Gen Med. 2016;10:1–6. doi: 10.2147/IJGM.S119301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esmat G. Hepatitis C in the Eastern Mediterranean Region. East Mediterr Health J. 2013;19:587–588. [PubMed] [Google Scholar]
- 36.Ali M, Idrees M, Ali L, Hussain A, Ur Rehman I, Saleem S, Afzal S, Butt S. Hepatitis B virus in Pakistan: a systematic review of prevalence, risk factors, awareness status and genotypes. Virol J. 2011;8:102. doi: 10.1186/1743-422X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gacche RN, Kaid AM. Epidemiology of viral hepatitis B and C infections in ibb city, yemen. Hepat Mon. 2012;12:460–462. doi: 10.5812/hepatmon.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badawi MM, Mohammed AA, Mohammed MS, Saeed MM, Ali EY, Khalil A. A Diagnostic Laboratory-Based Study on Frequency and Distribution of Viral Hepatitis B and C Among Sudanese. Open Virol J. 2017;11:98–107. doi: 10.2174/1874357901711010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Hepatitis E. Available from: http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-e.
- 40.Lemon SM. Hepatitis A virus. In: Webster RG and Granoff A, eds, editors. Encyclopedia of Virology. London: Academic Press Ltd; 1994. pp. 546–554. [Google Scholar]
- 41.Buchancová J, Svihrová V, Legáth L, Osina O, Urban P, Fenclová Z, Zibolenová J, Rosková D, Murajda L, Hudecková H. Occupational viral hepatitis in the Slovak and the Czech Republic. Cent Eur J Public Health. 2013;21:92–97. doi: 10.21101/cejph.a3790. [DOI] [PubMed] [Google Scholar]
- 42.Ishizaki A, Bouscaillou J, Luhmann N, Liu S, Chua R, Walsh N, Hess S, Ivanova E, Roberts T, Easterbrook P. Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low- and middle-income countries. BMC Infect Dis. 2017;17:696. doi: 10.1186/s12879-017-2767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol. 2013;8:371–380. doi: 10.2217/fvl.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David AM; Steering Committee for Prevention and Control of Infectious Diseases. Hepatitis A outbreaks--methods of intervention in South-East Asian countries. Int J Infect Dis. 2004;8:201–209. doi: 10.1016/j.ijid.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Giles-Vernick T, Hejoaka F, Sanou A, Shimakawa Y, Bamba I, Traoré A. Barriers to Linkage to Care for Hepatitis B Virus Infection: A Qualitative Analysis in Burkina Faso, West Africa. Am J Trop Med Hyg. 2016;95:1368–1375. doi: 10.4269/ajtmh.16-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohd Hanafiah K, Jacobsen KH, Wiersma ST. Challenges to mapping the health risk of hepatitis A virus infection. Int J Health Geogr. 2011;10:57. doi: 10.1186/1476-072X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. doi: 10.1101/cshperspect.a021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Global Hepatitis Report, 2017. Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 50.Scotto G, Bulla F, Campanale F, Tartaglia A, Fazio V. [Hepatitis E] Infez Med. 2013;21:175–188. [PubMed] [Google Scholar]
- 51.Teshale EH, Hu DJ. Hepatitis E: Epidemiology and prevention. World J Hepatol. 2011;3:285–291. doi: 10.4254/wjh.v3.i12.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. Hepatitis B. Available from: http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-B.
- 53.Lemon SM. Type A viral hepatitis: epidemiology, diagnosis, and prevention. Clin Chem. 1997;43:1494–1499. [PubMed] [Google Scholar]
- 54.Singh PK. Towards ending viral hepatitis as a public health threat: translating new momentum into concrete results in South-East Asia. Gut Pathog. 2018;10:9. doi: 10.1186/s13099-018-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sa-nguanmoo P, Posuwan N, Vichaiwattana P, Wutthiratkowit N, Owatanapanich S, Wasitthankasem R, Thongmee T, Poovorawan K, Theamboonlers A, Vongpunsawad S, et al. Swine is a possible source of hepatitis E virus infection by comparative study of hepatitis A and E seroprevalence in Thailand. PLoS One. 2015;10:e0126184. doi: 10.1371/journal.pone.0126184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon JG, Choi MJ, Yoon JW, Noh JY, Song JY, Cheong HJ, Kim WJ. Seroprevalence and disease burden of acute hepatitis A in adult population in South Korea. PLoS One. 2017;12:e0186257. doi: 10.1371/journal.pone.0186257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. Viral hepatitis in the Western Pacific. Available from: http://www.wpro.who.int/hepatitis/hepatitis_hepatitiscp_viral_hepatitiswpr/en/
- 58.Wiesen E, Diorditsa S, Li X. Progress towards hepatitis B prevention through vaccination in the Western Pacific, 1990-2014. Vaccine. 2016;34:2855–2862. doi: 10.1016/j.vaccine.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 59.Lim SG, Aghemo A, Chen PJ, Dan YY, Gane E, Gani R, Gish RG, Guan R, Jia JD, Lim K, et al. Management of hepatitis C virus infection in the Asia-Pacific region: an update. Lancet Gastroenterol Hepatol. 2017;2:52–62. doi: 10.1016/S2468-1253(16)30080-2. [DOI] [PubMed] [Google Scholar]
- 60.Varghese C, Carlos MC, Shin HR. Cancer burden and control in the Western Pacific region: challenges and opportunities. Ann Glob Health. 2014;80:358–369. doi: 10.1016/j.aogh.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ [PubMed]
- 62.Nur YA, Groen J, Elmi AM, Ott A, Osterhaus AD. Prevalence of serum antibodies against bloodborne and sexually transmitted agents in selected groups in Somalia. Epidemiol Infect. 2000;124:137–141. doi: 10.1017/s0950268899003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruneau J, Zang G, Abrahamowicz M, Jutras-Aswad D, Daniel M, Roy E. Sustained drug use changes after hepatitis C screening and counseling among recently infected persons who inject drugs: a longitudinal study. Clin Infect Dis. 2014;58:755–761. doi: 10.1093/cid/cit938. [DOI] [PubMed] [Google Scholar]
- 64.Heffernan A, Barber E, Cook NA, Gomaa AI, Harley YX, Jones CR, Lim AG, Mohamed Z, Nayagam S, Ndow G, et al. Aiming at the Global Elimination of Viral Hepatitis: Challenges Along the Care Continuum. Open Forum Infect Dis. 2017;5:ofx252. doi: 10.1093/ofid/ofx252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutin YJ, Bulterys M, Hirnschall GO. How far are we from viral hepatitis elimination service coverage targets? J Int AIDS Soc. 2018;21 Suppl 2:e25050. doi: 10.1002/jia2.25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez SA, Fierer DS, Talal AH. Medical and Behavioral Approaches to Engage People Who Inject Drugs Into Care for Hepatitis C Virus Infection. Addict Disord Their Treat. 2017;16:S1–S23. doi: 10.1097/ADT.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grebely J, Bruneau J, Lazarus JV, Dalgard O, Bruggmann P, Treloar C, Hickman M, Hellard M, Roberts T, Crooks L, et al. Research priorities to achieve universal access to hepatitis C prevention, management and direct-acting antiviral treatment among people who inject drugs. Int J Drug Policy. 2017;47:51–60. doi: 10.1016/j.drugpo.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mir F, Kahveci AS, Ibdah JA, Tahan V. Sofosbuvir/velpatasvir regimen promises an effective pan-genotypic hepatitis C virus cure. Drug Des Devel Ther. 2017;11:497–502. doi: 10.2147/DDDT.S130945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C--the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther. 2015;41:497–520. doi: 10.1111/apt.13090. [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization. The growing threats of Hepatitis B and Hepatitis C in the Eastern Mediterranean Region: A call for action. Available from: http://applications.emro.who.int/docs/EM_RC56_3_en.pdf.
- 71.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 72.Morano JP, Zelenev A, Lombard A, Marcus R, Gibson BA, Altice FL. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. J Community Health. 2014;39:922–934. doi: 10.1007/s10900-014-9932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lloyd AR, Clegg J, Lange J, Stevenson A, Post JJ, Lloyd D, Rudge G, Boonwaat L, Forrest G, Douglas J, et al. Safety and effectiveness of a nurse-led outreach program for assessment and treatment of chronic hepatitis C in the custodial setting. Clin Infect Dis. 2013;56:1078–1084. doi: 10.1093/cid/cis1202. [DOI] [PubMed] [Google Scholar]
- 74.Grebely J, Dore GJ, Morin S, Rockstroh JK, Klein MB. Elimination of HCV as a public health concern among people who inject drugs by 2030 - What will it take to get there? J Int AIDS Soc. 2017;20:22146. doi: 10.7448/IAS.20.1.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 76.Aggarwal R. Hepatitis e: epidemiology and natural history. J Clin Exp Hepatol. 2013;3:125–133. doi: 10.1016/j.jceh.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skipper C, Guy JM, Parkes J, Roderick P, Rosenberg WM. Evaluation of a prison outreach clinic for the diagnosis and prevention of hepatitis C: implications for the national strategy. Gut. 2003;52:1500–1504. doi: 10.1136/gut.52.10.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leonard NR, Banfield A, Riedel M, Ritchie AS, Mildvan D, Arredondo G, Cleland CM, Gwadz MV. Description of an efficacious behavioral peer-driven intervention to reduce racial/ethnic disparities in AIDS clinical trials. Health Educ Res. 2013;28:574–590. doi: 10.1093/her/cyt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evon DM, Golin CE, Ruffin R, Ayres S, Fried MW. Novel patient-reported outcomes (PROs) used in a pilot and feasibility study of a Cognitive Behavioral Coping Skills (CBCS) group intervention for patients with chronic hepatitis C. Pilot Feasibility Stud. 2018;4:92. doi: 10.1186/s40814-018-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cunningham EB, Hajarizadeh B, Amin J, Bretana N, Dore GJ, Degenhardt L, Larney S, Luciani F, Lloyd AR, Grebely J; HITS-p Investigators. Longitudinal injecting risk behaviours among people with a history of injecting drug use in an Australian prison setting: The HITS-p study. Int J Drug Policy. 2018;54:18–25. doi: 10.1016/j.drugpo.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 82.Taherkhani R, Farshadpour F. Global elimination of hepatitis C virus infection: Progresses and the remaining challenges. World J Hepatol. 2017;9:1239–1252. doi: 10.4254/wjh.v9.i33.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]


