Introduction
Immune checkpoint inhibitor therapies are used in the treatment of various cancers to stimulate an antitumor immune response. Checkpoint inhibitors, such as ipilimumab, pembrolizumab, and nivolumab, act by targeting inhibitory pathways and blocking the immune-evading effects of certain malignancies.1 The theory of so-called adaptive immune resistance suggests that tumors express numerous ligands that serve to downregulate the immune response. One such mechanism is through the interaction of programmed cell death-1 protein (PD-1) on T cells and programmed cell death-1 ligand (PD-L1) expressed by tumor cells. Nivolumab, an anti–PD-1 monoclonal antibody used for melanoma, non–small cell lung cancer (NSCLC), renal cell carcinoma, and more, prevents this interaction to allow immune-mediated destruction of the tumor.1 The immune activation triggered by nivolumab can also induce a nonspecific decrease in self-tolerance with subsequent development of autoimmune reactions. These immune-related adverse events can occur in multiple organ systems, including cutaneous, gastrointestinal (colitis, hepatitis), endocrine (hypophysitis, thyroiditis), respiratory (pneumonitis), and other toxicities.2, 3
The occurrence of vitiligolike depigmentation is a relatively common immune-related adverse event associated with nivolumab treatment of melanoma, with reported incidence ranging from 3.4% to 25.7%.4, 5, 6 This autoimmune reaction is thought to occur as a result of an immune response against healthy melanocyte antigens. Recently, however, more attention has been drawn to the role of vitiligo as a prognostic indicator of treatment success in melanoma immunotherapy.5, 6
Here we present a case of nivolumab-associated vitiligo in the treatment of a patient with NSCLC. Interestingly, nivolumab-associated vitiligo is rarely reported in nonmelanoma malignancies.7, 8
Case
The patient is a 75-year-old woman with stage IV NSCLC diagnosed in December 2015. Six months after initiating chemotherapy with carboplatin and pemetrexed, her disease progressed. Next-generation sequencing found no actionable mutations. Tumor expression of PD-L1 was done through a commercial vendor (Pathline Progressive Pathology, Ramsey, NJ) and found to be low-positive (PD-L1 expression on 1% to 24% of cytoplasmic and membranous locations on tumor with 1+ intensity). She started second-line nivolumab at 3 mg/kg every 2 weeks and had a partial response to therapy. Dosing was later changed to a flat dose, 240 mg every 2 weeks, according to changes in the drug label. Her treatment course was complicated by grade 1 pneumonitis, arthralgias, and thyroiditis followed by hypothyroidism requiring thyroid hormone replacement. Given persistent, asymptomatic pneumonitis, decision was made to take a 6-week treatment break. Subsequent imaging found radiographic improvement in pneumonitis, and treatment with nivolumab was resumed at 240 mg every 3 weeks per clinical discretion. Notably, she had a history of hypothyroidism secondary to subtotal thyroidectomy for hyperthyroidism at age 35.
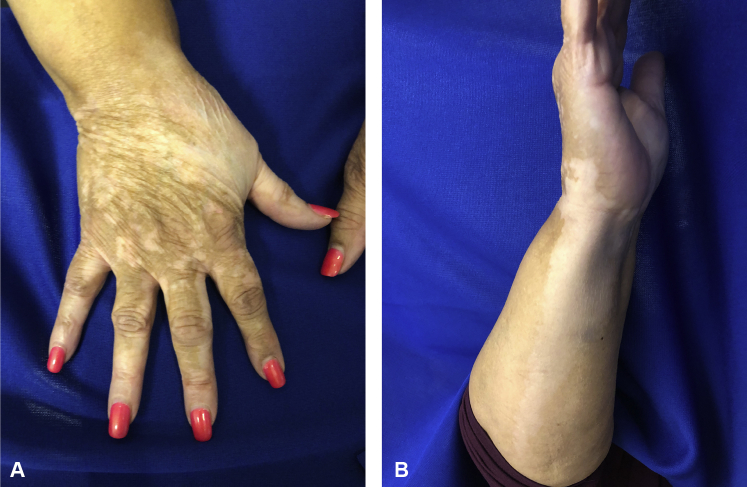
Approximately 20 months after first initiating nivolumab, the patient noticed a single patch of depigmentation on her dorsal right hand with subsequent spread to her forearm. At her presentation to the dermatology department 22 months into treatment with nivolumab, she had multiple depigmented macules on her right dorsal hand with a large confluent, well-demarcated patch extending down her ventral forearm (Fig 1). She also had depigmented patches with irregular, well-demarcated borders periorally, on her chin, bilateral cheeks, and anterior neck (Fig 2). There was no associated scale or erythema. Her oral mucosa was clear. Wood's lamp examination confirmed depigmentation of the patches.
Fig 1.
A, Multiple well-demarcated, depigmented macules with some coalescence and a large depigmented patch on the dorsal hand. B, Large, depigmented patch on ventral forearm.
Fig 2.
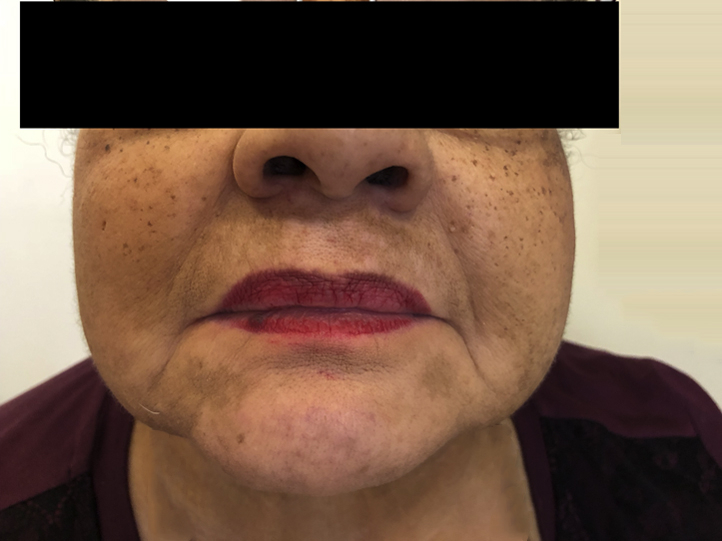
Multiple perioral, depigmented macules, some coalescing into patches.
Two 5-mm punch biopsies were taken, one from a depigmented patch on her right ventral forearm and one from normal pigmented skin on her right extensor forearm. SOX-10 stain showed complete absence of junctional melanocytes in depigmented skin, consistent with vitiligo (Fig 3). There was no marked lymphocytic infiltrate. The biopsy from normal skin found no abnormalities and SOX-10 stain found a normal number of junctional melanocytes. Notably, she has no history of autoimmune disease or dermatologic conditions, and her concurrent medication list was noncontributory. She started taking triamcinolone 0.1% cream, but stopped this after 2 months due to no effect, and tacrolimus 0.1% ointment daily for the facial patches. Throughout this time, imaging showed stable lung disease without progression.
Fig 3.

SOX-10 staining of depigmented skin shows absence of melanocytes, consistent with vitiligo. There is no marked lymphocytic infiltrate.
Discussion
Vitiligo is a complex process caused by the destruction of melanocytes and is associated with numerous genetic and autoimmune conditions.4 Even before the advent of immunotherapy, vitiligo development was described as an independent favorable prognostic factor in melanoma, regardless of treatment, suggesting immune activation against melanocyte antigens.9 More recently, attention has turned to the prognostic value of vitiligo during the treatment of melanoma as well. Patients undergoing immunotherapy treatment for melanoma who get vitiligo have shown a significantly improved progression-free survival and overall survival.6
Notably, vitiligo has been almost exclusively reported as an adverse event associated with the treatment of melanoma, with only 2 published cases of vitiligo occurring during immunotherapy for a nonmelanoma malignancy. One was associated with the treatment of NSCLC, similar to our patient, with the onset of vitiligo 6 days after initiating treatment.7 In the other case, a patient with a history of acute myeloid leukemia in remission and NSCLC was started on nivolumab to prevent recurrence of acute myeloid leukemia; vitiligo developed 20 months after treatment initiation.8 The exact mechanism remains unknown. Previous studies found absence of melanocytes with a marked T-cell infiltration in areas of depigmentation, similar to that of classic vitiligo.4
The continued reporting of vitiligolike depigmentation in the setting of checkpoint inhibitor therapy for nonmelanoma malignancies is important and suggests this phenomenon does arise independent of melanoma but as a direct result of anti–PD-1 therapy. It is possible that NSCLC shares a common antigen with melanoma and healthy melanocytes, which the activated T cells target. Whether treatment-associated vitiligo also provides independent prognostic value in the treatment of nonmelanoma malignancy remains unknown. There are published data, however, to suggest that other nivolumab-associated immune-related adverse events during NSCLC treatment, such as interstitial pneumonia and thyroid dysfunction, predict more favorable outcomes.10
Larger studies are needed to examine the role of vitiligo as a prognostic factor in the use of immunotherapy for nonmelanoma malignancies. This phenomenon may parallel the positive prognostic value observed in melanoma treatment.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Nisha Mohindra has served on the advisory board of AztraZeneca, Genentech, and Abbvie. Cory Kosche and Dr Choi have no conflicts of interest to disclose.
References
- 1.Shrimali R.K., Janik J.E., Abu-Eid R., Mkrtichyan M., Khleif S.N. Programmed death-1 & its ligands: promising targets for cancer immunotherapy. Immunotherapy. 2015;7(7):777–792. doi: 10.2217/imt.15.49. [DOI] [PubMed] [Google Scholar]
- 2.Collins L.K., Chapman M.S., Carter J.B., Samie F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–128. doi: 10.1016/j.currproblcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Yoest J. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. ImmunoTargets Ther. 2017;6:73–82. doi: 10.2147/ITT.S126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boniface K., Dutriaux C., Prey S., Taieb A., Seneschal J. Vitiligo-like lesions in patients receiving anti–programmed cell death-1 therapies are distinct from spontaneously occurring active vitiligo. J Am Acad Dermatol. 2018;78(1) doi: 10.1016/j.jaad.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y., Tanaka R., Asami Y. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2016;44(2):117–122. doi: 10.1111/1346-8138.13520. [DOI] [PubMed] [Google Scholar]
- 6.Teulings H.-E., Limpens J., Jansen S.N. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 7.Uenami T., Hosono Y., Ishijima M. Vitiligo in a patient with lung adenocarcinoma treated with nivolumab: a case report. Lung Cancer. 2017;109:42–44. doi: 10.1016/j.lungcan.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Yin E., Totonchy M., Leventhal J. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: a novel finding. JAAD Case Rep. 2017;76(6) doi: 10.1016/j.jdcr.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bystryn J.C. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123(8):1053–1055. [PubMed] [Google Scholar]
- 10.Sato K., Akamatsu H., Murakami E. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]