Introduction
Checkpoint inhibitors used for cancer immunotherapy may also result in autoimmunity.1, 2 We present a case of Wong-type dermatomyositis complicating treatment with nivolumab, an inhibitor of the programmed cell death 1 (PD-1) receptor. Wong-type dermatomyositis is a rare variant of classic dermatomyositis with concurrent clinical and histopathologic findings mimicking pityriasis rubra pilaris.3 Our report of nivolumab-related Wong-type dermatomyositis expands the clinical spectrum of autoimmune diseases associated with checkpoint inhibitors.
Report of a patient
A 67-year-old man with metastatic renal cell carcinoma enrolled in a clinical trial of cabiralizumab and nivolumab combination treatment was admitted for an exfoliative rash. The rash started following sun exposure and worsened immediately after his last infusion of nivolumab and cabiralizumab. He received treatment for 6 months and received a total of 13 infusions of nivolumab, 3 mg/kg, and cabiralizumab, 4 mg/kg. He had not taken any other new drugs. He had no muscle weakness or other symptoms.
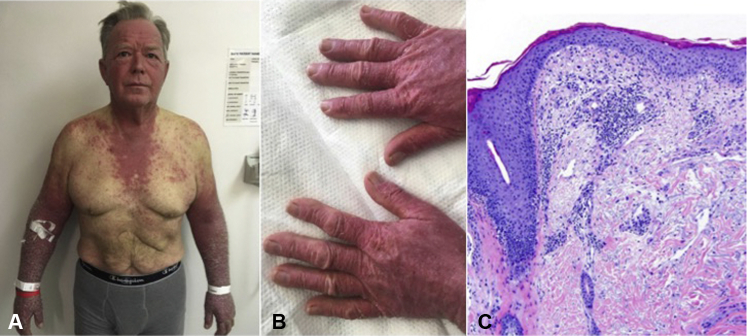
On physical examination, there was a photodistributed eruption of violaceous papules with shellac-like scale coalescing into confluent plaques over the central face including eyelids and nasolabial folds, V-neck chest, nape, dorsal hands, and forearms. There was marked facial edema, most prominent in a periocular distribution, and discrete papules on the dorsal fingers coalescing into plaques (Fig 1, A and B). The rash did not spare some photoprotected sites such as the submental skin, nasolabial folds, or upper eyelids as would be expected in a phototoxic eruption. Skin biopsy found superficial perivascular lymphocytic infiltrate with epidermal atrophy, vacuolar change, and dermal mucin; direct immunofluorescence testing was negative (Fig 1, C). No significant laboratory abnormalities were noted including autoantibodies to double-stranded DNA, Smith, RNP, Ro, La, Jo1, Mi2, and SRP antigens. Creatine kinase and aldolase were normal. Immunotherapy was held, and topical corticosteroids were started with improvement of the rash.
Fig 1.
A, The patient first presented with a photodistributed violaceous, exfoliative eruption composed of papules with shellac-like scale coalescing into plaques. B, The dorsal hands show erythematous papules coalescing into plaques. C, Skin biopsy shows epidermal atrophy, vacuolar change, dermal mucin deposition, and a superficial lymphocytic infiltrate.
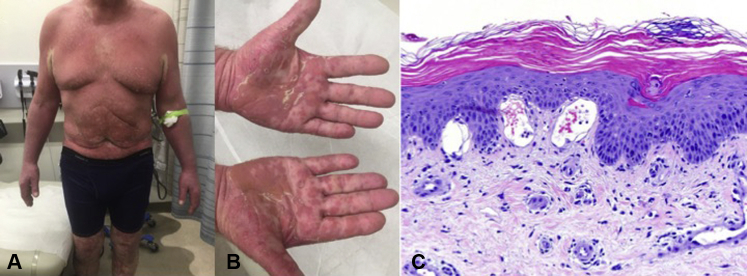
Two weeks later, the patient presented with erythroderma and hyperkeratotic follicular papules, with islands of sparing accentuated on the chest, axillae, and neck (Fig 2, A and B). His palms and soles had orange, waxy keratoderma. A biopsy found psoriasiform epidermal hyperplasia, sparse lymphocytic infiltrate, and accentuated cornification with parakeratotic foci and follicular hyperkeratosis (Fig 2, C). Basal vacuolar alteration and dermal mucin were concurrently present. Muscle enzymes remained normal. Wong-type dermatomyositis (sine myositis) was diagnosed based on initial clinical and histopathologic findings consistent with cutaneous dermatomyositis with later features mimicking classic pityriasis rubra pilaris.
Fig 2.
A and B, On follow-up, the patient had erythroderma with islands of sparing and an orange waxy keratoderma on his hands and feet. C, A second biopsy found psoriasiform epidermal hyperplasia, sparse lymphocytic infiltrate, and accentuated cornification with parakeratotic foci and follicular hyperkeratosis. Note that the dermal mucin and vacuolar interface change are still present.
The patient was treated with prednisone (1 mg/kg/d) tapered over 2 months with transition to methotrexate, 10 mg weekly. His renal cell carcinoma did not improve with nivolumab and cabiralizumab treatment, so he was withdrawn from the clinical trial and transitioned to alternative chemotherapy. His rash resolved within 1 month. He remains in remission at 9-month follow-up and was tapered off prednisone and methotrexate.
Discussion
Immune-related adverse events associated with PD-1 inhibitors commonly involve the skin, and reports of vitiligo, psoriasis, lichenoid dermatitis, eczematous dermatitis, and lupus-like reactions have entered the literature.1, 2 There is 1 report of an inflammatory myopathy complicating nivolumab therapy that was described as dermatomyositis sine dermatitis and 1 case of dermatomyositis with classic cutaneous features induced by nivolumab.4, 5 However, there are no reports of Wong-type dermatomyositis during anti–PD-1 therapy. Wong-type dermatomyositis is a rare variant of dermatomyositis with fewer than 30 cases reported.3 This case adds to the literature regarding immune-related adverse events associated with PD-1 inhibitors, expanding the spectrum of PD-1 inhibitor– related cutaneous inflammatory reaction patterns.
The mechanism leading to immune-related adverse events is not fully understood. PD-1 inhibitors may stimulate immune activity against tumor-associated antigens that cross-react with normal tissues. In this case, renal cell carcinoma overexpresses carbonic anhydrase, which is present in skeletal muscle and the epidermal basement membrane and thus represents a plausible target for drug-induced autoimmunity similar to dermatomyositis.6 Autoantibodies to carbonic anhydrase have also been observed in patients with closely related diseases such as systemic lupus erythematosus and Sjögren syndrome.7 Classic autoantigens may be absent in PD-1–related autoimmunity, as therapy may induce reactions to novel autoantigens such as carbonic anhydrase.
Cabiralizumab, a colony-stimulating factor 1 receptor (CSF1R) inhibitor, may have contributed to our patient's presentation. This drug is designed to decrease the immunosuppressive effects of tumor-associated macrophages to facilitate more robust immunotherapy. Early data on cabiralizumab shows asymptomatic increases in creatine kinase at the beginning of treatment typically without sequelae, thought to be caused by metabolic effects (inhibition of hepatic Kuppfer cells which also express CSF1R) rather than autoimmunity.8, 9 Rash and pruritus are common reactions and have been described as maculopapular rather than autoimmune in nature.8 Autoimmune phenomena are much less common with CSF1R inhibitors, although induction of lupus-like reactions has been reported.10 In this case, cabiralizumab may have increased antigen presentation to an already activated immune system caused by PD-1 blockade. Another consideration is the association of Wong-type dermatomyositis with malignancy3; a paraneoplastic phenomenon is an additional risk factor for this patient's presentation. In the original report of Wong-type dermatomyositis, the risk of underlying neoplasm was reported as nearly 50%; however, more recent reviews have called this into question.3 We feel that although a paraneoplastic phenomenon may have contributed, this patient's presentation was more likely driven by his immunotherapy, given the resolution of his disease with drug cessation despite an unchanged cancer burden.
In the literature, immunotherapy-related eruptions are reported to be notably steroid responsive. The patient in our case, similar to several others, responded to treatment within 1 month.2, 11 Research on the pharmacodynamics of immunotherapy is ongoing and long-term data will be helpful in determining the natural history of cutaneous adverse effects from immunotherapy.12
The relative contributions of checkpoint inhibition, increased antigen presentation, and paraneoplastic mechanism in this patient's presentation cannot be definitively determined. However, this case is an excellent illustration of the multiple contributing factors that have led to increases in the presentation of rare connective tissue disease in the oncologic patient on immunotherapy.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
This case was presented as an oral presentation at the 2018 American Academy of Dermatology Annual Meeting; February 16, 2018; San Diego, California.
References
- 1.Hwang S.J.E., Carlos G., Wakade D. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(3):455–461. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Cho M., Nonomura Y., Kaku Y., Dainichi T., Otsuka A., Kabashima K. Generalized lichen nitidus following anti–PD-1 antibody treatment. JAMA Dermatol. 2018;154(3):367. doi: 10.1001/jamadermatol.2017.5670. [DOI] [PubMed] [Google Scholar]
- 3.Mutasim D.F., Egesi A., Spicknall K.E. Wong-type dermatomyositis: a mimic of many dermatoses. J Cutan Pathol. 2016;43(9):781–786. doi: 10.1111/cup.12733. [DOI] [PubMed] [Google Scholar]
- 4.Bourgeois Vionnet J., Joubert B., Bernard E. First case report of nivolumab-induced dermatomyositis. Neuromuscul Disord. 2017;27(Supplement 2):S152. [Google Scholar]
- 5.Kudo F., Watanabe Y., Iwai Y. A case of advanced lung adenocarcinoma with nivolumab-associated dermatomyositis. Intern Med. 2018 doi: 10.2169/internalmedicine.9381-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klade C.S., Voss T., Krystek E. Identification of tumor antigens in renal cell carcinoma by serological proteome analysis. Proteomics. 2001;1(7):890–898. doi: 10.1002/1615-9861(200107)1:7<890::AID-PROT890>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki Y., Jinno-Yoshida Y., Hamasaki Y., Ueki H. A novel autoantibody reactive with carbonic anhydrase in sera from patients with systemic lupus erythematosus and Sjögren's syndrome. J Dermatol Sci. 1991;2(3):147–154. doi: 10.1016/0923-1811(91)90060-b. [DOI] [PubMed] [Google Scholar]
- 8.Wainberg Z, Piha-Paul S, Luke J, et al. First-in-human phase 1 dose escalation and expansion of a novel combination, anti–CSF-1 receptor (cabiralizumab) plus anti–PD-1 (nivolumab), in Patients with advanced solid tumors. In: 32nd SITC Annual Meeting.
- 9.Cannarile M.A., Weisser M., Jacob W., Jegg A.-M., Ries C.H., Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5(1):53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassier P.A., Italiano A., Gomez-Roca C.A. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16(8):949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 11.Shao K., McGettigan S., Elenitsas R., Chu E.Y. Lupus-like cutaneous reaction following pembrolizumab: an immune-related adverse event associated with anti-PD-1 therapy. J Cutan Pathol. 2018;45(1):74–77. doi: 10.1111/cup.13059. [DOI] [PubMed] [Google Scholar]
- 12.Kohrt H.E., Tumeh P.C., Benson D. Immunodynamics: a cancer immunotherapy trials network review of immune monitoring in immuno-oncology clinical trials. J Immunother Cancer. 2016 doi: 10.1186/s40425-016-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]