Abstract
Purpose:
Maternal asthma increases adverse neonatal respiratory outcomes, and pollution may further increase risk. Air quality in relation to neonatal respiratory health has not been studied.
Methods:
Transient tachypnea of the newborn (TTN), asphyxia, and respiratory distress syndrome (RDS) were identified using medical records among 223,375 singletons from the Consortium on Safe Labor (2002–2008). Community Multiscale Air Quality models estimated pollutant exposures. Multipollutant Poisson regression models calculated adjusted relative risks of outcomes for interquartile range increases in average exposure. Maternal asthma and preterm delivery were evaluated as effect modifiers.
Results:
TTN risk increased after particulate matter (PM) less than or equal to 10-micron exposure during preconception and trimester one (9–10%), and whole-pregnancy exposure to PM less than or equal to 2.5 microns (PM2.5; 17%) and carbon monoxide (CO; 10%). Asphyxia risk increased after exposure to PM2.5 in trimester one (48%) and whole pregnancy (84%), CO in trimester two and whole pregnancy (28–32%), and consistently for ozone (34%–73%). RDS risk was associated with increased concentrations of nitrogen oxides (33%–42%) and ozone (9%–21%) during all pregnancy windows. Inverse associations were observed with several pollutants, particularly sulfur dioxide. No interaction with maternal asthma was observed. Restriction to term births yielded similar results.
Conclusions:
Several pollutants appear to increase neonatal respiratory outcome risks.
Keywords: TTN, Asphyxia, RDS, Criteria air pollutants, Maternal asthma
Introduction
Respiratory morbidity is common among newborns, affecting up to 15% of term and 29% of preterm neonates admitted into the neonatal intensive care unit (NICU) [1]. Neonatal respiratory distress can lead to hypoxia and respiratory acidosis that can then result in brain damage [2]. To prevent serious complications, neonates with respiratory distress are typically admitted to the NICU for treatment, making it a leading cause of NICU admission [2]. NICU admissions in the United States have increased 23% between 2007 and 2012 [3], and some of that increase may be due to increases in respiratory conditions [4].
Infants with neonatal respiratory complications also have higher risks of subsequent health sequelae including decreased lung function and higher prevalence of asthma and other respiratory illnesses during childhood [5, 6]. In addition to their direct health impact, neonatal respiratory complications can also lead to a significant financial burden on society. There are approximately 18,000 neonatal hospitalizations due to respiratory distress complications in the United States annually, costing the health care system approximately $2.3 billion [7]. Owing to the considerable health and economic impact of neonatal respiratory distress, it is important to examine modifiable risk factors for respiratory distress among neonates.
Prenatal exposure to air pollution has been consistently linked to adverse neonatal outcomes including preterm birth, low birth weight, small for gestational age, and intrauterine growth restriction [8–10]. More recently, studies have found that prenatal exposure to ambient air pollution may impact both lung development and lung function in the neonates [11–13], which can have lasting impacts into adulthood. For example, an infant's decreased lung function is associated with lower lung function in both childhood and early adulthood and with increased risk of chronic obstructive pulmonary disease [14, 15]. However, it is unclear whether prenatal exposure to pollution is associated with neonatal respiratory distress. According to the Developmental Origin of Health and Disease (DOHaD) framework, one potential pathway through which maternal exposure to air pollution may affect neonatal respiratory health is through changes in the intrauterine environment that can lead to an induction of epigenetic changes in the fetus [16]. With regard to the effects of preconception exposure to pollution, there is some evidence that pollution can alter cord blood phenotypes that can then affect organ development and fetal health [17, 18]. Our previous work has also suggested that preconception exposures can influence pregnancy complications [19] and adverse pregnancy outcomes such as preterm birth [8], which may be related to a chronic impact of oxidative stress and inflammation in early pregnancy that has long-term consequences related to placental development [20].
Maternal asthma has also been found to increase the risk of adverse neonatal outcomes including respiratory complications [21]. However, no studies have investigated whether prenatal exposure to air pollution is associated with the risk of neonatal respiratory complications or evaluated the potential for effect modification by maternal asthma on this risk.
To address this knowledge gap, we used a nationwide U.S. cohort to examine the potential effect of preconception and prenatal exposure to air pollution on neonatal respiratory outcomes and to evaluate whether maternal asthma would exacerbate this effect.
Methods
Study population
There were 223,385 singleton births greater than or equal to 23 weeks of gestation in the Consortium on Safe Labor (2002–2008), a retrospective cohort study using electronic medical records (EMRs) conducted across 12 U.S. clinical sites, which included 15 hospital referral regions with 19 hospitals [22]. Ten infants were excluded because of missing air pollution data, resulting in 223,375 singleton births available for analysis. Some women had more than one singleton delivery during the study period (n = 19,211, 8.6%), and maternal asthma status could change if women were diagnosed with asthma between pregnancies (i.e., mothers whose asthma status changed comprised both non-asthmatic and asthmatic pregnancies). The data were anonymized, and institutional review board approval was obtained from each study site.
Exposure assessment
We used modified Community Multiscale Air Quality (CMAQ) models to estimate ambient exposure to criteria air pollutants: carbon monoxide (CO), nitrogen oxides (NOx), ozone (O3), particulate matter less than or equal to 10 microns (PM10), particulate matter less than or equal to 2.5 microns (PM2.5), and sulfur dioxide (SO2). Briefly, the CMAQ was developed by the U.S. Environmental Protection Agency and is a regional air quality model that estimates hourly pollution exposures based on air pollution emissions, weather-related factors, and chemical reactions between pollutants. To improve the accuracy of modeled air pollution levels, CMAQ-modeled estimates were fused with observed data from local monitor stations using inverse distance weighting and weighted for population density [23]. Given that maternal address was unavailable, air pollution exposures were assigned based on average exposure for the 15 nonoverlapping hospital referral regions.
Pollutant concentrations were averaged and analyzed over several chronic time windows including 3 months' preconception (90 days before conception), trimester one (gestational weeks 1–13), trimester two (gestational weeks 14–28), and the whole pregnancy (conception to birth). Trimester three was not evaluated because preterm neonates (11.7%) would not have a complete third trimester. Acute exposures during the last 6 weeks of pregnancy were also explored in preliminary analyses, but results were not meaningful and were not pursued.
Outcome assessment
Transient tachypnea of the newborn (TTN), the most common cause of respiratory distress in term neonates, is the fast or labored breathing of neonates due to the presence of fluid in their lungs [2]. Asphyxia is the deprivation of oxygen in a neonate caused by suffocation due to mechanical issues or a lack of respiratory effort [24]. Respiratory distress syndrome (RDS) is the difficulty breathing caused by the lack of surfactants in the lungs of neonates [2]. Diagnoses of these three conditions were ascertained from newborn EMRs and/or discharge summaries using International Classification of Diseases, ninth edition (ICD-9). Codes for the outcomes are as follows: TTN, 770.6; asphyxia, 768.5, 768.6, 768.9; and RDS, 769.
Covariates
To control for potential confounding, several covariates were also assessed based on delivery records and/or discharge summaries using ICD-9 codes. Covariates were selected a priori based on our prior work on infant outcomes associated with maternal asthma [21]—specifically, route of delivery (vaginal, caesarean), gestational age, maternal age (<20, 20–24, 25–29, 30–34, ≥35, unknown), race (white/non-Hispanic, black/non-Hispanic, Hispanic, other/unknown), prepregnancy body mass index (BMI) (<18.5, 18.5–24.9, 25.0–29.9, 30–34.9, ≥35, unknown), marital status (divorced/widowed, married, single), region (West, Midwest, Northeast, South), parity (1, 2, 3 or more, unknown), smoking or alcohol use during pregnancy (yes, no), type of insurance (private, public, other), and season of conception (spring, summer, winter, fall). We also obtained diagnoses of gestational diabetes (yes, no), gestational hypertension (yes, no), preeclampsia (yes, no), and eclampsia (yes, no) from the EMRs and hospital discharge summaries.
Analysis
The neonate was the unit of analysis for statistical testing. Descriptive statistics and bivariate analysis were performed to compare individuals with and without each of the neonatal respiratory outcomes. Multipollutant Poisson regression models were used to calculate the relative risk (RR) and 95% confidence intervals (CI) for each of the three outcomes in relation to an interquartile range (IQR) increase in each pollutant. Separate models evaluated exposure during each of the acute and chronic time windows. Generalized estimating equations with an autoregressive covariance structure accounted for clustering within mothers who contributed more than one singleton pregnancy to the study. Final multipollutant models were adjusted for region, marital status, parity, maternal age, smoking/alcohol, comorbidities (i.e., HIV, chronic hypertension, pre-existing diabetes mellitus, or thyroid disease), cesarean delivery, type of insurance, prepregnancy BMI, race, and season of conception. We also tested for potential interaction between each air pollutant exposure and maternal asthma because asthmatics may be more susceptible to exposure.
Supplemental analyses included a model that adjusted for pregnancy complications (gestational diabetes mellitus [GDM], gestational hypertension, preeclampsia, and eclampsia) in addition to the other covariates. For each exposure window, we also ran a single pollutant model adjusting for exposures during other time windows in addition to the other covariates [25].
Given the length of exposure to pollution is not expected to be similar among births and the varying gestational ages, we also truncated the exposures of ongoing pregnancies in the whole pregnancy analyses to compare whole-pregnancy exposures for births at different gestational weeks (23–34; 35–38; 39–42) to ongoing pregnancies with a comparable exposure length. For example, all pollutant exposures for births delivered between 23 and 34 weeks were compared with that of ongoing pregnancies truncated to 27 weeks (midpoint). Because these conditions are more prevalent among preterm neonates, analyses were also restricted to term infants to assess the effect of air pollution independent of preterm birth. Finally, we ran the Benjamini-Hochberg procedure as a control for false discovery rate. All analyses were conducted using SAS 9.4 (Cary, NC).
Results
Characteristics of the clinical population are presented in Table 1. Overall, neonatal respiratory outcomes were diagnosed in 6.8% of neonates, with TTN being most prevalent at 3.6% (n = 8007), followed by RDS at 3.3% (n = 7325), and asphyxia at 0.3% (n = 590). Having a neonate with a respiratory outcome was more common among women who were non-Hispanic white, divorced/widowed, publicly insured, or lived in the South. Similarly, women who smoked and/or drank alcohol during pregnancy, had comorbidities, had a caesarean delivery, or delivered preterm were more likely to have a neonate with an adverse respiratory outcome. Maternal asthma was diagnosed in 7.6% of the deliveries (n = 17,034).
Table 1.
Characteristics of the study population of the Consortium on Safe Labor, 2002–2008 (n = 223,375)
| Characteristics | Neonatal respiratory outcomes, n (%) |
|||||
|---|---|---|---|---|---|---|
| TTN (n = 8007, 3.6%) |
No TTN (n = 215,368, 96.4%) |
Asphyxia* (n = 590, 0.3%) |
No asphyxia* (n = 222,785, 99.74%) |
RDS (n = 7325, 3.3%) | No RDS (n = 216,050, 96.7%) |
|
| Age (years) | ||||||
| <20 | 1164 (14.5) | 29,316 (13.5) | 87 (14.8) | 30,213 (13.6) | 1098 (15.1) | 29,202 (13.5) |
| 20–24 | 1600 (20.0) | 45,378 (21.1) | 144 (24.4) | 46,834 (21.0) | 1630 (22.3) | 45,348 (21.0) |
| 25–29 | 2096 (26.2) | 60,114 (27.9) | 159 (27.0) | 62,051 (27.9) | 1837 (25.1) | 60,373 (27.9) |
| 30–34 | 1755 (21.9) | 48,391 (22.5) | 95 (16.1) | 50,051 (22.5) | 1514 (20.7) | 48,632 (22.5) |
| ≥35 | 1379 (17.2) | 32,055 (14.9) | 105 (17.8) | 33,329 (15.0) | 1232 (16.8) | 32,202 (14.9) |
| Unknown | 13 (0.2) | 294 (0.1) | 0 (0.0) | 307 (0.1) | 14 (0.2) | 293 (0.1) |
| P-value† | 0.02 | .13 | .12 | |||
| Race | ||||||
| White/non-Hispanic | 3466 (43.3) | 106975 (49.7) | 271 (45.9) | 110170 (49.5) | 3172 (43.3) | 107269 (49.7) |
| Black/non-Hispanic | 1577 (32.2) | 47,657 (22.1) | 167 (28.3) | 50,067 (22.5) | 2309 (31.5) | 47,925 (22.2) |
| Hispanic | 1266 (15.8) | 37,768 (17.5) | 99 (16.8) | 28,935 (17.5) | 1162 (15.9) | 37,872 (17.5) |
| Other/unknown | 698 (8.7) | 22,968 (10.7) | 53 (9.0) | 23,613 (10.6) | 682 (9.3) | 22,984 (10.6) |
| P-value† | <.0001 | .60 | <.0001 | |||
| Prepregnancy BMI (kg/m2) | ||||||
| <18.5 | 238 (3.0) | 7757 (3.6) | 14 (2.4) | 7981 (3.6) | 262 (3.6) | 7733 (3.6) |
| 18.5–24.9 | 2305 (28.8) | 76,764 (35.6) | 210 (35.6) | 78,859 (35.4) | 2059 (28.1) | 77,010 (35.6) |
| 25.0–29.9 | 1230 (15.4) | 32,244 (15.0) | 99 (16.8) | 33,375 (15.0) | 997 (13.6) | 32,477 (15.0) |
| 30–34.9 | 645 (8.1) | 15,126 (7.0) | 54 (9.2) | 15,717 (7.1) | 596 (8.1) | 15,175 (7.0) |
| ≥35 | 620 (7.7) | 11,533 (5.4) | 42 (7.1) | 12,111 (5.4) | 554 (7.6) | 11,599 (5.4) |
| Unknown | 2969 (37.1) | 71,944 (33.4) | 171 (29.0) | 74,742 (33.6) | 2857 (39.0) | 72,056 (33.4) |
| P-value† | <.0001 | .56 | <.0001 | |||
| Parity (number of live births) | ||||||
| 1 | 2372 (50.4) | 66,019 (50.9) | 146 (46.1) | 68,245 (50.9) | 1975 (45.8) | 66,416 (51.1) |
| 2 | 1290 (27.4) | 35,335 (27.3) | 94 (29.7) | 36,531 (27.3) | 1210 (28.1) | 35,415 (27.2) |
| 3+ | 554 (11.8) | 15,922 (12.3) | 42 (13.3) | 16,434 (12.3) | 599 (13.9) | 15,877 (12.2) |
| Unknown | 487 (10.4) | 12,371 (9.5) | 35 (11.0) | 12,823 (9.6) | 626 (12.2) | 12,332 (9.5) |
| P-value† | .39 | .09 | <.0001 | |||
| Marital status | ||||||
| Married | 3919 (48.9) | 120883 (56.1) | 335 (56.8) | 130839 (58.7) | 3773 (51.5) | 127401 (59.0) |
| Divorced/widowed | 3806 (47.5) | 71,586 (33.2) | 241 (40.9) | 84,755 (38.0) | 3287 (44.9) | 81,709 (37.8) |
| Single | 282 (3.5) | 22,899 (10.6) | 14 (2.4) | 7191 (3.2) | 265 (3.6) | 6940 (3.2) |
| P-value† | <.0001 | .45 | <.0001 | |||
| Insurance | ||||||
| Private | 4018 (50.2) | 120883 (56.1) | 275 (46.6) | 124626 (55.9) | 3516 (48.0) | 121385 (56.2) |
| Public | 3223 (40.3) | 71,586 (33.2) | 275 (46.6) | 74,534 (33.5) | 3336 (45.5) | 71,473 (33.1) |
| Other | 766 (9.6) | 22,899 (10.6) | 40 (6.8) | 23,625 (10.6) | 473 (6.5) | 23,192 (10.7) |
| P-value† | <.0001 | .001 | <.0001 | |||
| Region | ||||||
| West | 1681 (21.0) | 65,551 (30.4) | 211 (35.8) | 67,021 (30.1) | 2036 (27.8) | 65,196 (30.2) |
| Midwest | 1697 (21.2) | 32,248 (15.0) | 92 (15.6) | 33,853 (15.2) | 1246 (17.0) | 32,699 (15.1) |
| Northeast | 1816 (22.7) | 52,807 (24.5) | 55 (9.3) | 54,568 (24.5) | 1113 (15.2) | 53,510 (24.8) |
| South | 2813 (35.1) | 64,762 (30.1) | 232 (39.2) | 67,343 (30.2) | 2930 (40.0) | 64,645 (29.9) |
| P-value† | <.0001 | 1.00 | <.0001 | |||
| Season of conception | ||||||
| Spring | 1684 (21.0) | 51,054 (23.7) | 129 (21.9) | 52,609 (23.6) | 1665 (22.7) | 51,073 (23.6) |
| Summer | 2070 (25.9) | 55,567 (25.8) | 185 (31.4) | 57,488 (25.8) | 1849 (25.2) | 55,788 (25.8) |
| Fall | 2278 (28.5) | 59,150 (27.5) | 185 (31.4) | 61,243 (27.5) | 1954 (26.7) | 59,474 (27.5) |
| Winter | 1975 (24.7) | 49,597 (23.0) | 127 (21.5) | 51,445 (23.1) | 1857 (25.4) | 49,715 (23.0) |
| P-value† | <.0001 | .57 | .0003 | |||
| Smoking/alcohol | ||||||
| No | 7154 (89.4) | 198521 (92.2) | 516 (87.5) | 205159 (92.1) | 6494 (88.7) | 199181 (92.2) |
| Yes | 853 (10.7) | 16,847 (7.8) | 74 (12.5) | 17,626 (7.9) | 831 (11.3) | 16,869 (7.8) |
| P-value† | <.0001 | <.0001 | <.0001 | |||
| Comorbidities‡ | ||||||
| No | 7233 (90.3) | 201895 (93.7) | 528 (89.5) | 208600 (93.6) | 6483 (88.5) | 202645 (93.8) |
| Yes | 774 (9.7) | 13,473 (6.3) | 62 (10.5) | 14,185 (6.4) | 842 (11.5) | 13,405 (6.2) |
| P-value† | <.0001 | <.0001 | <.0001 | |||
| Cesarean delivery | ||||||
| No | 4084 (51.0) | 156738 (72.8) | 257 (43.6) | 160565 (72.1) | 3223 (44.0) | 157599 (73.0) |
| Yes | 3923 (49.0) | 58,630 (27.2) | 333 (56.4) | 62,220 (27.9) | 4102 (56.0) | 58,451 (27.1) |
| P-value† | <.0001 | <.0001 | ||||
| Asthma | ||||||
| No | 7224 (90.2) | 199117 (92.5) | 527 (89.3) | 205814 (92.4) | 6633 (90.6) | 199708 (92.4) |
| Yes | 783 (9.8) | 16,251 (7.6) | 63 (10.7) | 16,971 (7.6) | 692 (9.5) | 16,342 (7.6) |
| P-value† | <.0001 | .005 | <.0001 | |||
| Term birth (≥37 weeks) | ||||||
| No | 2796 (34.9) | 23,330 (10.8) | 268 (45.4) | 25,858 (11.6) | 6059 (82.7) | 20,067 (9.3) |
| Yes | 5211 (65.1) | 192038 (89.2) | 322 (54.6) | 196927 (88.4) | 1266 (17.3) | 195983 (90.7) |
| P-value† | <.0001 | <.0001 | <.0001 | |||
No patients with asphyxia were missing data on maternal age.
Obtained from GEE models adjusting for repeated pregnancies.
HIV, chronic hypertension, diabetes mellitus, or thyroid disease.
The distribution (minimum, maximum, and IQR) of the concentrations of criteria air pollutants for each time window are available in Supplemental Table 1. Correlations between pollutants were consistent between time windows, and Spearman correlation coefficients over the whole pregnancy are presented in Supplemental Table 2. Ozone was consistently inversely correlated with each of the other five criteria air pollutants.
No significant interactions were observed between maternal asthma and any air pollutants, so we present only the main effects of air pollutants on neonatal respiratory outcomes adjusted for maternal asthma and other covariates previously described.
Transient tachypnea of the newborn
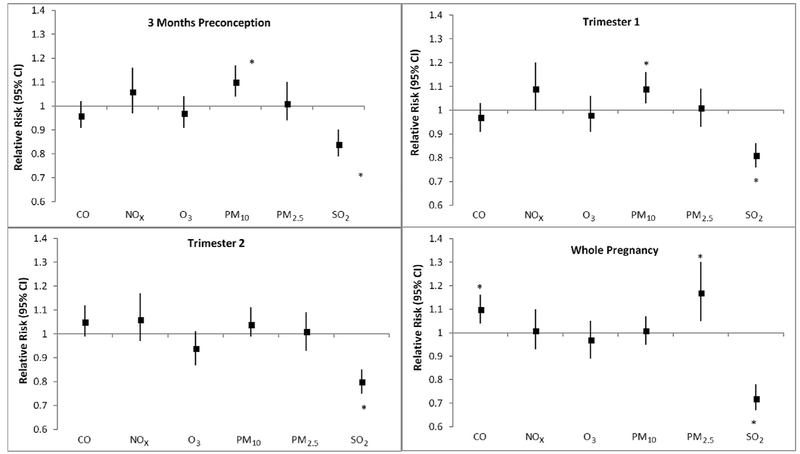
For each IQR increase in PM10 exposure in the 3 months' preconception and over trimester one, risk of TTN increased by 9%–10% (Fig. 1, Supplemental Table 3). PM2.5 and CO exposures over the whole pregnancy also increased risk by 17% and 10%, respectively. SO2 had a consistently significant inverse relationship (16–28%) with TTN in each of the time windows, whereas NOx and O3 were not associated with risk.
Fig. 1.
Adjusted relative risksa between criteria air pollutants and risk of transient tachypnea of the newborn (TTN; n = 8007 cases among 223,375 singleton births). Abbreviations: CO, carbon monoxide; NOx, nitrogen oxides; O3, ozone; PM10, particulate matter ≤10 microns; PM2.5, particulate matter ≤2.5 microns, SO2, sulfur dioxide.a Multipollutant models adjusted for region, marital status, parity, maternal age, maternal race, caesarean delivery, type of insurance, maternal BMI, smoking/alcohol, comorbidities (HIV, hypertension, diabetes, thyroid disease), season of conception, and asthma. Relative risks are estimated for an IQR increase in pollutants. * Denotes statistical significance at P < 0.05.
Asphyxia
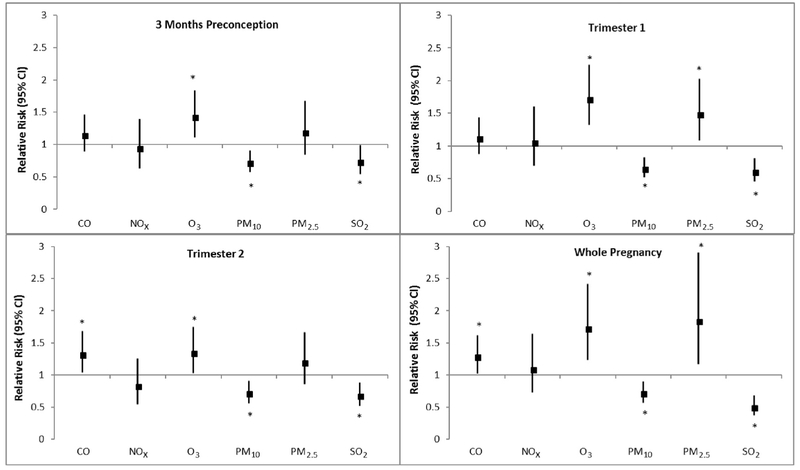
For each IQR increase in O3 exposure across all time windows, risk of asphyxia increased significantly by 34%–73% (Fig. 2, Supplemental Table 3). Elevated PM2.5 levels during trimester one and whole pregnancy also significantly increased the risk of asphyxia by 48% and 84%, respectively. CO exposure during trimester two and the whole pregnancy increased neonatal asphyxia risk by 28%–32%. PM10 was significantly inversely associated with asphyxia risk (28–35%) in each of the time windows. SO2 demonstrated a similar inverse trend, decreasing the risk of asphyxia by 27%–50%. NOx was not associated with asphyxia risk.
Fig. 2.
Adjusted relative risksa between criteria air pollutants and risk of asphyxia (n = 590 cases among 223,375 singleton births). Abbreviations: CO, carbon monoxide; NOx, nitrogen oxides; O3, ozone; PM10, particulate matter ≤10 microns; PM2.5, particulate matter ≥2.5 microns, SO2, sulfur dioxide.a Multipollutant models adjusted for region, marital status, parity, maternal age, maternal race, caesarean delivery, type of insurance, maternal BMI, smoking/alcohol, comorbidities (HIV, hypertension, diabetes, thyroid disease), season of conception, and asthma. Relative risks are estimated for an IQR increase in pollutants. * Denotes statistical significance at P < 0.05.
Respiratory distress syndrome
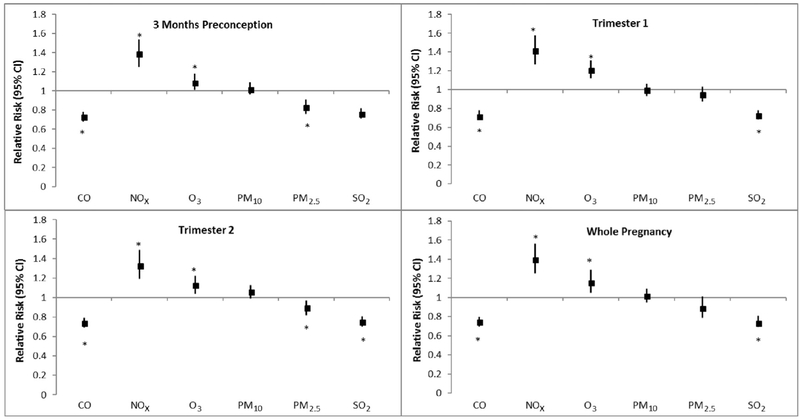
NOx exposure increased RDS in all time windows by 33%–42% (Fig. 3, Supplemental Table 3). Elevated exposure to O3 demonstrated a similar trend and significantly increased RDS risk by 9%–21% in each of the time windows. CO and SO2 exposure were inversely associated with the risk of RDS in each of the time windows. PM2.5 was associated with lower RDS risk for exposure in 3 months' preconception and in the second trimester. PM10 exposure was not significantly associated with RDS.
Fig. 3.
Adjusted relative risksa between criteria air pollutants and risk of respiratory distress syndrome (RDS; n = 7325 cases among 223,375 singleton births). Abbreviations: CO, carbon monoxide; NOx, nitrogen oxides; O3, ozone; PM10, particulate matter ≤10 microns; PM2.5, particulate matter ≤2.5 microns, SO2, sulfur dioxide.a Multipollutant models adjusted for region, marital status, parity, maternal age, maternal race, caesarean delivery, type of insurance, maternal BMI, smoking/alcohol, comorbidities (HIV, hypertension, diabetes, thyroid disease), season of conception, and asthma. Relative risks are estimated for an IQR increase in pollutants. * Denotes statistical significance at P < 0.05.
Additional analyses
Because the three neonatal respiratory outcomes are more prevalent among preterm neonates, additional analyses were restricted to term births and risk estimates were attenuated, in comparison to all births, but generally remained significant (Supplemental Table 4). Several pollutants lost statistical significance for term asphyxia cases, the outcome with the smallest sample, while the association of CO during trimester two with risk of TTN became significant. Results also remained generally consistent when pregnancy complications such as gestational hypertension, preeclampsia, eclampsia, and GDM were additionally included as covariates (Supplemental Table 5). In this model, SO2 was no longer significantly inversely associated with increased asphyxia risk in the 3 months' preconception window. O3 was no longer significantly associated with increased RDS risk for 3 months' preconception, whereas PM10 exposure gained significance to increase RDS risk for exposure during the second trimester of pregnancy.
In the single pollutant models adjusting for other time windows in addition to covariates, results generally remained similar, while some were attenuated (data not shown).
We also ran the Benjamini-Hochberg procedure to adjust p-values as a control for any falsely discovered significance (Supplemental Table 6), but we present the main results unadjusted for the false discovery rate because this is a novel hypotheses and most of the associations survived correction [26].
An additional analysis that truncated whole-pregnancy exposures between early preterm (gestational weeks 23–34), preterm and early term (gestational weeks 35–38), and term (gestational weeks 39–42) neonates also showed generally consistent findings (Supplemental Table 7). Of note, after adjustment for exposure length and covariates, whole-pregnancy PM2.5 seems to have stronger associations for TTN among only preterm and early term births.
Discussion
Results from this large nationwide U.S. cohort demonstrate that prenatal exposure to criteria air pollutants was associated with increased risk of TTN, asphyxia, and RDS in neonates, whereas maternal asthma did not modify this association. Although some effects were observed in association with preconception and trimester-specific exposure windows, the strongest increases in risk of neonatal respiratory outcomes were associated with chronic whole-pregnancy exposure. Although these respiratory conditions were more common among preterm births, results remained consistent when analyses were restricted to term infants, which suggests that air pollution likely affected neonates through a pathway that was not dependent on preterm birth status. The loss of significance for several criteria air pollutant exposures for asphyxia can be attributed to fewer infants with asphyxia at birth, which widened the confidence interval range. To our knowledge, this is the first study to examine prenatal exposures to air pollution in relation to neonatal respiratory outcomes despite many existing studies linking these exposures to respiratory outcomes in the general population [27]. These novel findings are important because neonatal respiratory conditions are a major contributor to NICU stays in the United States as well as worldwide [3], and incidence of neonatal respiratory conditions has been increasing substantially in recent years [4].
We have previously observed that asthma increases the risks of maternal and neonatal complications [17, 28]. We have also observed that air pollution can interact with asthma and increase the risk of certain outcomes, such as preterm birth [8] and preeclampsia [29], but not others [30]. We expected that maternal asthma would be associated with higher risk in the presence of air pollutants because asthmatic women may be more susceptible to these exposures, but none of the asthma–air pollutant interaction terms were significant. This finding indicates that regardless of maternal asthma status, prenatal air pollution exposure appears harmful to neonates both preterm and at term.
Although no previous studies investigated the relations between prenatal exposures to ambient air pollution and neonatal respiratory outcomes, some have examined the effect of air pollution on lung development and function [12, 13]. A review article observed that prenatal air pollution exposure can impair lung function, with long-term effects observed even during adulthood [13]. A clinical study in Switzerland found that higher PM10 levels during trimesters two and three increased the neonate's 1-minute ventilation, an indication of decreased lung function [12]. This finding is consistent with the results of our study, which showed that higher exposure to PM10 during 3 months' preconception and trimester one increased risk of TTN. We note that although the referenced study found no association between ozone and lung function [12], we found ozone to consistently increase the risk of asphyxia and RDS.
The biological mechanisms that underlie the observed associations between prenatal air pollution and neonatal respiratory conditions are uncertain, but air pollutants can induce systemic inflammation and oxidative stress in the mother. For example, PM10 has been found to trigger oxidative stress, which causes inflammation due to cytokine release in the lungs [31]. Similarly, other clinical studies have found NO2 to induce an inflammatory response in neonates [12, 32]. Through these pathways, markers of inflammation and oxidative stress may then pass the maternal fetal blood barrier and in turn negatively affect fetal development [33]. Animal studies in rats and mice have shown that maternal inflammation can result in morphofunctional changes in the lungs of offspring, which could negatively impact their respiratory health [13]. Clinical studies have also shown that air pollution affects human lungs and impairs lung function by disrupting alveorization during development [11]. This decreased alveorization can lead to inefficient gas exchange for the human fetus and a decreased production of surfactant phospholipids in the lungs [34]. Prenatal PM10 has also been linked with placental mitochondrial alterations in clinical populations, which can reflect and intensify oxidative stress production that can negatively affect fetal development [35].
The inverse associations we observed are counter to our hypotheses. They could be due to correlations between pollutants (Supplemental Table 3) but may also be related to compensatory mechanisms. In particular, SO2 was consistently inversely associated with the risk of TTN, asphyxia, and RDS in almost all pregnancy windows. One potential explanation for these findings could be that the increased SO2 in the environment led to increased SO2 within the body. A previous study conducted in rat models has shown that increased endogenous SO2 levels can inhibit oxidative stress, protecting rats against acute lung injuries [36]. Given our findings and those of the study on rat models, we speculate that ambient SO2 could be involved in a pathway that limits the generation of free radicals that can induce oxidative stress [36]. Another clinical study found CO and SO2 had an inverse association with preterm birth and hypothesized that neonates needed more gestational time to recover from the hypoxic damage they suffered due to the elevated SO2 [37].
This study has several limitations. First, air pollution exposure for each mother was estimated based on the hospital referral region, which varied in size. Although this method may introduce some nondifferential exposure misclassification, it also assesses a broader geographic area that helped account for residential mobility and daily activity patterns that were not available in our data. However, the model did adjust for region, which reduces variability and potentially bias our results toward the null. Although a strength of our study was our ability to evaluate different windows of exposure, the window-specific findings may be somewhat influenced by the correlation of exposure across time windows. Whole pregnancy averages avoid this concern when evaluating chronic exposure risks.
There were also few cases of asphyxia distributed across study sites, which limited certain analyses, such as the truncated whole-pregnancy exposure analysis for term neonates. Finally, we observed intermittent inverse associations, such as those between PM10 and asphyxia, which remain unexplained. These associations may be due to chance but might also be a function of the complex interrelationship of the pollutants studied. A strength of this study is the size and geographic diversity of the cohort along with the rich clinical medical record data that allowed for better determination of both outcome and covariate data. This is the first study to investigate the relations between air pollution, maternal asthma, and neonatal respiratory outcomes. If replicated in future studies, these findings can potentially inform strategies to reverse the increasing trend in neonatal respiratory complications by preventing cases associated with poor air quality.
Conclusion
In this large U.S. cohort, we found that prenatal exposure to most criteria air pollutants, particularly particulate matter, ozone, and nitrogen oxides, increased the risk of TTN, asphyxia, and RDS, whereas SO2 appeared to have inverse associations. Chronic whole-pregnancy exposures appeared to be most strongly related to risk of neonatal respiratory outcomes. If these findings are replicated, these findings may suggest that air pollution exposure is a potentially modifiable risk factor for serious neonatal respiratory morbidity.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. The Consortium on Safe Labor (contract no. HHSN26700603425 C) and Air Quality and Reproductive Health Study (contract no. HHSN275200800002I) were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Disclosure: The authors have no financial relationships relevant to this article to disclose. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the article for publication.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- [1].Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev 2014;35(10):417–28. quiz 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gallacher DJ, Hart K, Kotecha S. Common respiratory conditions of the newborn. Breathe (Sheff) 2016;12(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harrison W, Goodman D. Epidemiologic Trends in Neonatal Intensive Care, 2007–2012. JAMA Pediatr 2015;169(9):855–62. [DOI] [PubMed] [Google Scholar]
- [4].Ersch J, Roth-Kleiner M, Baeckert P, Bucher HU. Increasing incidence of respiratory distress in neonates. Acta Paediatr 2007;96(11):1577–81. [DOI] [PubMed] [Google Scholar]
- [5].Kotecha SJ, Watkins WJ, Paranjothy S, Dunstan FD, Henderson AJ, Kotecha S. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax 2012;67(1):54–61. [DOI] [PubMed] [Google Scholar]
- [6].Pike KC, Lucas JS. Respiratory consequences of late preterm birth. Paediatr Respir Rev 2015;16(3):182–8. [DOI] [PubMed] [Google Scholar]
- [7].Raj JU, Wright JR. Respiratory distress syndrome of the newborn In: Schraufnagel DE, editor. Breathing in America: Diseases, Progress, and Hope, American Thoracic Society; 2010. [Google Scholar]
- [8].Mendola P, Wallace M, Hwang BS, Robledo C, Männistö T, et al. Preterm birth and air pollution: Critical windows of exposure for women with asthma. J Allergy Clin Immunol 2016;138(2):432–440.e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology 2015;26(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Trasande L, Wong K, Roy A, Savitz DA, Thurston G. Exploring prenatal outdoor air pollution, birth outcomes and neonatal health care utilization in a nationally representative sample. J Expo Sci Environ Epidemiol 2013;23(3): 315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev 2017;21:38–46. [DOI] [PubMed] [Google Scholar]
- [12].Latzin P, Roosli M, Huss A, Kuehni CE, Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J 2009;33(3): 594–603. [DOI] [PubMed] [Google Scholar]
- [13].Veras MM, de Oliveira Alves N, Fajersztajn L, Saldiva P. Before the first breath: prenatal exposures to air pollution and lung development. Cell Tissue Res 2016. [DOI] [PubMed] [Google Scholar]
- [14].Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med 2016;375(9):871–8. [DOI] [PubMed] [Google Scholar]
- [15].Narang I, Bush A. Early origins of chronic obstructive pulmonary disease. Semin Fetal Neonatal Med 2012;17(2):112–8. [DOI] [PubMed] [Google Scholar]
- [16].Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis 2010;1(1):6–18. [DOI] [PubMed] [Google Scholar]
- [17].Baiïz N, Slama R, Béné MC, Charles MA, Kolopp-Sarda MN, Magnan A, et al. Maternal exposure to air pollution before and during pregnancy related to changes in newborn's cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth 2011;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cappon GD, Cook JC, Hurtt ME. Relationship between cyclooxygenase 1 and 2 selective inhibitors and fetal development when administered to rats and rabbits during the sensitive periods for heart development and midline closure. Birth Defects Res B Dev Reprod Toxicol 2003;68(1):47–56. [DOI] [PubMed] [Google Scholar]
- [19].Robledo CA, Mendola P, Yeung E, Männistö T, Sundaram R, Liu D, et al. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res 2015;137:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol 2017;77(5). [DOI] [PubMed] [Google Scholar]
- [21].Mendola P, Mannisto TI, Leishear K, Reddy UM, Chen Z, Laughon SK. Neonatal health of infants born to mothers with asthma. J Allergy Clin Immunol 2014;133(1). 85–90.e81–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 2010;203(4):326.e1–326.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen G, Li J, Ying Q, Sherman S, Perkins N, Sundaram R, et al. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ 2014;485–486:563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aslam HM, Saleem S, Afzal R, Iqbal U, Saleem SM, Shaikh MW, et al. Risk factors of birth asphyxia. Ital J Pediatr 2014;40:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for Bias When Estimating Critical Windows for Air Pollution in Children's Health. Am J Epidemiol 2017;186(11):1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rothman KJ. Writing for Epidemiology. Epidemiology 1998;9(3):333–7. [DOI] [PubMed] [Google Scholar]
- [27].Heinrich J, Wichmann H-E. Traffic related pollutants in Europe and their effect on allergic disease. Curr Opin Allergy Clin Immunol 2004;4(5):341–8. [DOI] [PubMed] [Google Scholar]
- [28].Mendola P, Laughon SK, Männistö TI, Leishear K, Reddy UM, Chen Z, et al. Obstetric complications among US women with asthma. Am J Obstet Gynecol 2013;208(2):127.e121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mendola P, Wallace M, Liu D, Robledo C, Mannisto T, Grantz KL. Air pollution exposure and preeclampsia among US women with and without asthma. Environ Res 2016;148:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ha S, Zhu Y, Liu D, Sherman S, Mendola P. Ambient temperature and air quality in relation to small for gestational age and term low birthweight. Environ Res 2017;155:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol 2010;126(3):453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fuchs O, Latzin P, Kuehni CE, Frey U. Cohort profile: the Bern infant lung development cohort. Int J Epidemiol 2012;41(2):366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 2004;103(3):546–50. [DOI] [PubMed] [Google Scholar]
- [34].Kajekar R Environmental factors and developmental outcomes in the lung. Pharmacol Ther 2007;114(2):129–45. [DOI] [PubMed] [Google Scholar]
- [35].Yurdakök K Ambient air pollution and the fetus. J Pediatr Neonatal Individual Med 2013;2(2):e020232. [Google Scholar]
- [36].Chen S, Zheng S, Liu Z, Tang C, Zhao B, Du J, et al. Endogenous sulfur dioxide protects against oleic acid-induced acute lung injury in association with inhibition of oxidative stress in rats. Lab Invest 2015;95(2):142–56. [DOI] [PubMed] [Google Scholar]
- [37].Capobussi M, Tettamanti R, Marcolin L, Piovesan L, Bronzin S, Gattoni ME, et al. Air Pollution Impact on Pregnancy Outcomes in Como. Italy J Occup Environ Med 2016;58(1):47–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.