Abstract
Study Design:
Retrospective study.
Objective:
There have been few reports of adjacent segment disease (ASD) after posterior lumbar interbody fusion (PLIF) with large numbers and long follow-up. The purpose of this study was to investigate (1) ASD incidence and time periods after primary PLIF, (2) repeat ASD incidence and time periods, and (3) ASD incidence and time periods by fusion length, age, and preoperative pathologies.
Methods:
A total of 1000 patients (average age 67 years, average follow-up 8.3 years) who underwent PLIF for degenerative lumbar disorders were reviewed. ASD was defined as a symptomatic condition in which revision surgery was required.
Results:
The overall ASD rate was 9.0%, and the average ASD period was 4.7 years after primary surgery. With respect to clinical features of ASD, degenerative spondylolisthesis at the cranial fusion segment was the most frequent. In terms of repeat ASD, second and third ASD incidences were 1.1% and 0.4%, respectively. As for ASD by fusion length, age, and preoperative pathologies, ASD incidence was increased by fusion length, while the time period to ASD was significantly shorter in elderly patients and those with degenerative lumbar scoliosis.
Conclusions:
In the present study, the overall ASD incidence was 9.0%, and the average ASD period was 4.7 years after primary operation. Second and third ASD incidences were 1.1% and 0.4%, respectively. Fusion length affected the ASD incidence, while aging factor and preoperative pathology affected the ASD time period.
Keywords: PLIF, adjacent segment disease, long follow-up, fusion length, elderly patients, degenerative lumbar scoliosis
Introduction
We have been using posterior lumbar interbody fusion (PLIF) with pedicle screw fixation to treat degenerative lumbar disorders with segmental instability. Whereas PLIF with pedicle screw fixation has shown satisfactory clinical results, solid fusion has been reported to accelerate degenerative changes at adjacent unfused levels.1–8 Several reports have described associations between aging and adjacent segment disease (ASD) after fusion surgery.6,9–13 ASD can develop as part of the normal aging and degenerative process, but it appears to be at least partly influenced by the alteration of stresses that occur as a consequence of lumbar fusion.
As the number of elderly individuals in the population is increasing, PLIF is becoming more common, and it has been predicted that ASD increases. However, there have been few reports of ASD after PLIF using the same technique with large numbers and long follow-up. To the best of our knowledge, there has been only 1 article with a large number (more than 1000 cases)14 and only 2 articles with follow-up periods of more than 10 years.13,15 These 3 reports noted that the ASD incidence would be changed by patient numbers, patients’ age, follow-up periods, fusion length, and pathologies. On the other hand, there have been few reports focused on the time period to ASD, although many reports have described the ASD incidence and risk factors for ASD. Furthermore, there have also been few reports of ASD incidence and time periods by age and pathologies, as well as repeat ASD. In order to obtain precise information about ASD incidence, time periods, and repeat ASD, data with large numbers and long follow-up are needed. The purpose of this study involving a series of 1000 cases with an average 8.3 years of follow-up was to investigate (1) ASD incidence and time periods after primary PLIF, (2) repeat ASD incidence and time periods, and (3) ASD incidence and time periods by fusion length, age, and preoperative pathologies.
Materials and Methods
Subjects
From 1996 to 2013, 1107 consecutive patients underwent PLIF for degenerative lumbar disorders. Of these patients, 1000 patients (443 men, 557 women) who were followed for at least 2 years were included in this study. The follow-up rate was 90.3%, and the average follow-up period was 8.3 years (range 2-21 years). The mean age at surgery was 67 years (range 16-87 years). The patients had one of the following diagnoses: degenerative spondylolisthesis (DS; N = 653), isthmic spondylolisthesis (SO; N = 145), lumbar spinal canal stenosis (LSS; N = 74), degenerative lumbar scoliosis (DLS; N=71), or lumbar disc herniation (LDH; N = 57). DLS was defined as lumbar scoliosis more than 20° or local disc wedging more than 10°. In the present study, there was no patient with DLS more than 40° or complained difficulty in standing due to sagittal or coronal imbalance. Patients who had infection, fracture-dislocation, rheumatoid arthritis, or destructive spinal arthropathy were excluded. A total of 945 patients had 1 level fused, and 55 patients had 2 levels fused. The average number of fused segments was 1.06 (Table 1). The protocol was approved by the institutional review board of the hospital.
Table 1.
Patients Demographics.
| Average age | 67 years (16-87) |
| Average follow-up | 8.3 years (2-21) |
| Male/female ratio | 451:549 |
| Fusion segment | |
| Single | 945 |
| Double | 55 |
| Average segment | 1.06 |
| Pathology | |
| DS | 653 |
| SO | 145 |
| LSS | 74 |
| DLS | 71 |
| LDH | 57 |
Abbreviations: DS, degenerative spondylolisthesis; SO, isthmic spondylolisthesis; LSS, lumbar spinal stenosis; DLS, degenerative lumbar scoliosis; LDH, lumbar disc herniation.
Surgical Indications and Procedures
All patients who underwent surgery had severe, disabling lower limb pain with or without low back pain unresponsive to conservative treatment such as medication, physical therapy, and root and/or epidural injection. The indications for PLIF were as follows: spondylolisthesis with slippage greater than 3 mm with posterior opening greater than 5° on flexion and extension lateral radiographs, LSS with foraminal stenosis of the same segment, or central huge LDH requiring wide decompression. All cases with ASD showed initial improvement of symptoms after the first operation, but they then developed gradual deterioration of neurological symptoms, which consisted of lower limb pain, sensory disturbance, and/or motor weakness. Magnetic resonance imagine and myelogram just before the second operation showed compression of the dural sac and/or nerve root at the adjacent fusion segment, although there was no significant compression detected before the first operation. Additional surgery was indicated when conservative treatment was not effective in the same way as the first operation. Similarly, additional surgical procedure was the same as the first operation.
All PLIF procedures were performed using the same technique described previously.4 PLIF procedure consisted of bilateral total facetectomy, subtotal discectomy, a large amount of autologous bone graft with 2 carbon cages, and pedicle screw fixation. Our PLIF technique was almost same procedure as bilateral transforaminal lumbar interbody fusion (TLIF). Our PLIF technique with bilateral total facetectomy provides wide posterior visualization and circumferential decompression of the neural elements in addition to rigid fixation.
Outcome Measures
Complete medical records of all patients were available for review. In the present study, outcome measure items that could be subject to measurement errors and interobserver errors, such as radiological measurements or clinical point systems, were excluded. Therefore, ASD was defined as a symptomatic condition in which additional surgery was required to treat neurological deterioration at the adjacent degenerative segment on the radiograph. Repeat ASD was defined as a condition in which additional surgery was required at the further adjacent segment of the secondary PLIF at the segment adjacent to the primary PLIF. The following were investigated: (1) ASD incidence, time periods, and postoperative clinical features after primary PLIF; (2) repeat ASD incidence, time periods, and postoperative clinical features; and (3) ASD incidence and time periods by fusion length, age, and preoperative pathologies.
Statistical Analysis
The χ2 test was used for categorical outcome variables. An α level of .05 was considered significant. SPSS (version 20; IBM, Armonk, NY, USA) was used for statistical analysis.
The incidence and prevalence of surgical intervention for ASD were calculated for each year, and a Kaplan-Meier survivorship curve with 95% confidence intervals was constructed. Incidence was defined as the percentage of patients who had not had revision surgery at the start of a given year and had had subsequent development of new disease that was treated surgically during that year.
Results
ASD Incidence and Time Period
ASD was observed in 90 patients (9.0%). The average period between the first and second operations was 4.7 years (range 0.3-18.7 years). The mean age of the ASD patients at the first operation was 66 years, with no difference between them and non-ASD patients (67 years; Table 2).
Table 2.
Repeated ASD Rates and Time Periods.
| Non-ASD | First ASD | Second ASD | Third ASD | |
|---|---|---|---|---|
| Numbers | 910 | 90 (9%) | 11 (1.1%) | 4 (0.4%) |
| Average age (years) | 67 | 66 | 67 | 65 |
| ASD period (years) | 4.7 | 3.5 | 1.5 |
Abbreviation: ASD, adjacent segment disease.
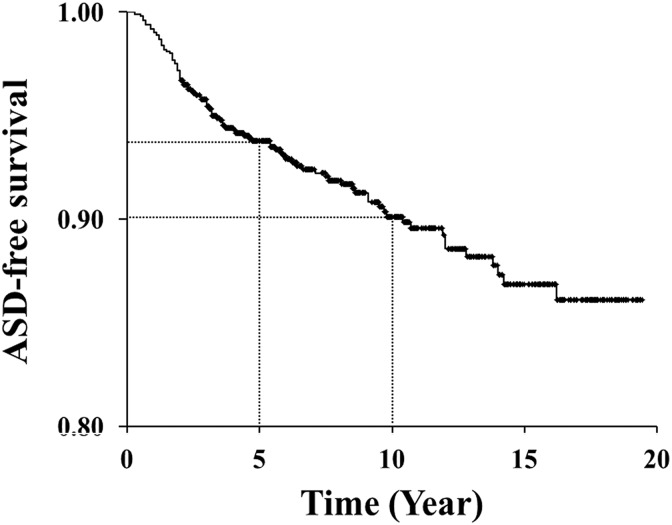
Kaplan-Meier survivorship analysis included all available data to the last follow-up point and predicted disease-free survivorship of the adjacent segments. Predicted survivorship of the adjacent segments was 93.8% at postoperative 5 years and 90.1% at 10 years. In other words, the probability of undergoing revision surgery for ASD was 6.2% at postoperative 5 years and 9.9% at 10 years. The annual incidence of ASD requiring surgery was relatively constant at 1.0% for 10 years after primary surgery (Figure 1).
Figure 1.
Kaplan-Meier survivorship analysis. Predicted survivorship of the adjacent segments is 93.8% at postoperative 5 years and 90.1% at 10 years.
ASD was observed at the cranial segment in 63 patients (70%), at the caudal segment in 20 patients (22%), and both cranial and caudal segments in 7 patients (8%). In terms of first ASD pathologies, DS was observed in 36 (40%), LSS in 34 (38%), LDH in 13 (14%), and DLS (foraminal stenosis) in 7 (8%). On the other hand, early-onset ASD within 1 year was observed in 8 patients. ASD was observed at the cranial segment in 2 patients (25%) and at the caudal segment in 6 patients (75%). In terms of first ASD pathologies, DS was observed in 1 (13%), LSS in 2 (25%), LDH in 2 (25%), and DLS (foraminal stenosis) in 3 (38%; Table 3). With regard to additional operations, 52 patients underwent PLIF, 29 patients underwent laminotomy, and 9 patients underwent discectomy. After the second operation, all 90 patients showed improvement of neurological symptoms.
Table 3.
Clinical Features of ASD.
| Overall ASD | Early-Onset ASD | |||
|---|---|---|---|---|
| n | % | n | % | |
| Segment | ||||
| Cranial | 63 | 70 | 2 | 25 |
| Caudal | 20 | 22 | 6 | 75 |
| Both | 7 | 8 | - | |
| Pathology | ||||
| DS | 36 | 40 | 1 | 13 |
| LSS | 34 | 38 | 2 | 25 |
| LDH | 13 | 14 | 2 | 25 |
| FS | 7 | 8 | 3 | 38 |
Abbreviations: ASD, adjacent segment disease; DS, degenerative spondylolisthesis; LSS, lumbar spinal stenosis; LDH, lumbar disc herniation; FS, foraminal stenosis.
Repeated ASD
In the present study, first ASD was observed in 90 patients (9.0%). In these patients, 11 patients (1.1% of overall and 12.2% of first ASD) developed second ASD. The average time period between the first and second ASDs was 3.5 years (range 1.1-6.2 years). The average age of second ASD patients was 67 years, with no difference in age between non-ASD and second ASD patients (Table 2). In terms of second ASD pathologies, DS was observed in 9, LSS in 1, and LDH in 1. With regard to additional operations, 9 patients underwent PLIF, 1 patient underwent laminotomy, and 1 patient underwent discectomy. The surgical procedure was selected in the same manner as for first ASD. After the additional operations, all 11 patients showed improved neurological symptoms, but 4 patients (0.4% of overall patients and 36.4% of second ASD) developed third ASD. The average time period between the second and third ASDs was 1.5 years (range 0.4-2.7 years). The average age of third ASD patients was 65 years, with no difference in age between non-ASD and third ASD patients (Table 2). In terms of third ASD pathologies, DS was observed in 2, LSS in 1, and vertebral collapse in 1. All 4 third ASD patients complained of standing disturbance due to sagittal imbalance with local kyphosis more than 20°, in addition to lower limb pain and gait disturbance. With respect to salvage operations, 2 patients underwent pedicle subtraction osteotomy, 1 patient underwent vertebral column resection, and 1 patient underwent laminotomy. All patients underwent long fusion with pedicle screws including more than 7 segments simultaneously.
ASD by Fusion Length
In terms of ASD by fusion length, the ASD incidence and time period were 8.6% and 4.6 years with single-segment PLIF, while they were 16.4% and 6.0 years with double-segment PLIF, respectively (Table 4). With regard to ASD by fusion length, there was a significant difference in the ASD incidence (P = .049), but not in the ASD time period.
Table 4.
ASD by Fusion Length.
| Total | ASD | |||
|---|---|---|---|---|
| Fusion Segment | N | N | Incidence (%) | Period (Years) |
| Single | 945 | 81 | 8.6 | 4.6 |
| Double | 55 | 9 | 16.4* | 6.0 |
| Total | 1000 | 90 | 9.0 | 4.7 |
Abbreviation: ASD, adjacent segment disease.
*P = .049.
ASD by Age
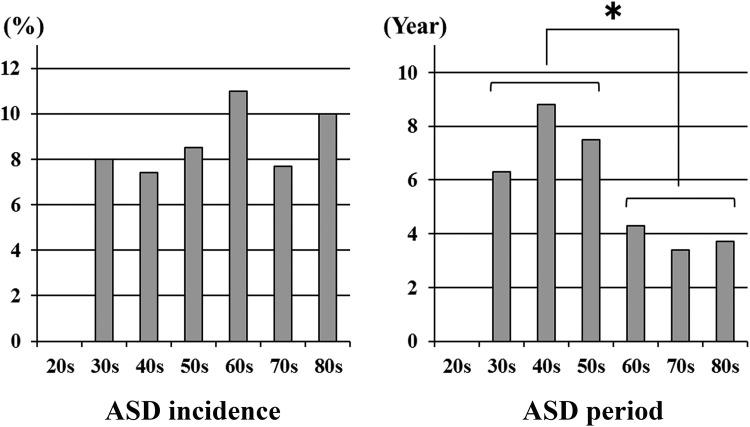
In terms of ASD incidence by age, there were no patients (0/10 patients) in their 20s, 8.0% (2/25 patients) in their 30s, 7.4% (4/54 patients) in their 40s, 8.5% (14/165 patients) in their 50s, 11.0% (38/344 patients) in their 60s, 7.7% (27/352 patients) in their 70s, and 10.0% (5/50 patients) in their 80s. With regard to ASD time periods by age, the period was 6.3 years in the 30s, 8.8 years in the 40s, 7.5 years in the 50s, 4.3 years in the 60s, 3.4 years in the 70s, and 3.7 years in the 80s. Although no difference was detected in the ASD incidence, the ASD time period was significantly shorter in patients more than 60 years old (P = .007; Figure 2).
Figure 2.
ASD by age. Although no difference is seen in the ASD incidence, the ASD time period is significantly shorter in patients more than 60 years old (*P = .007).
ASD by Preoperative Pathologies
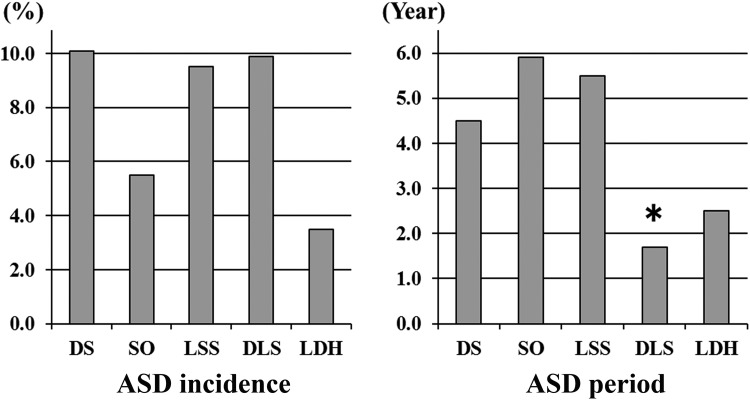
In terms of the ASD incidence by preoperative pathologies, it was 10.1% (66/653 patients) with DS, 5.5% (8/145 patients) with SO, 9.5% (7/74 patients) with LSS, 9.9% (7/71 patients) with DLS, and 3.5% (2/57 patients) with LDH. With regard to ASD time periods by preoperative pathologies, the period was 4.5 years with DS, 5.9 years with SO, 5.5 years with LSS, 1.7 years with DLS, and 2.5 years with LDH. Although no difference was observed in the ASD incidence, the ASD time period was significantly shorted in DLS patients (P < .001; Figure 3).
Figure 3.
ASD by preoperative pathologies. Although no difference is seen in the ASD incidence, the ASD time period is significantly shorter in DLS patients (*P < .001).
Discussion
ASD Incidence, Time Period, and Clinical Features
Although several studies have described ASD after lumbar arthrodesis, ASD incidence, and time period were affected by patient numbers and follow-up period.1–3,6–24 In the previous report, the largest study reported 1069 cases, with an average follow-up of 4 years, and the incidence and time period of symptomatic ASD with additional operation were 2.6% and 4.4 years,14 while the longest follow-up study had an average follow-up of 12.6 years, and included 111 cases; the radiological ASD incidence was 15%, with no information about the ASD time period.15
With respect to clinical features of ASD, previous reports described that cranial fusion segment was often observed.1–3,25 In the present study, overall DS and LSS at the cranial segment were often observed, while LDH and foraminal stenosis at the caudal segment were observed more often in early-onset ASD. Many reports have described the risk factors for ASD. In terms of risk factors for ASD, preexisting disc and facet joint degenerations was often reported as the morphological features, while segmental lordosis, sagittal imbalance, and excessive disc height distraction as the surgical features.1–3,5–24 In the present study, such risk factor analysis was not done; however, patients with early-onset ASD might have some risk factors for ASD.
Repeat ASD
To the best of our knowledge, there have been only 2 reports regarding repeat ASD, including our case report.5 Miwa et al reported the surgical outcomes of ASD after PLIF and found 11% of first ASD patients developed second ASD.24 This result was approximately equal to the present result. In the present study, 12.2% of the first ASD patients developed second ASD after an average of 3.5 years, and 36.4% of second ASD patients developed third ASD after an average of 1.5 years. The patients with repeat ASD might have some risk factors, similar to those of early onset ASD.
ASD by Fusion Length, Age, and Preoperative Pathologies
Many reports have described the contribution of fusion length and aging to ASD.6,9–13,21–23 From clinical and biomechanical aspects, fusion length was well reported the contribution to ASD. Similarly, Lee reported that patients older than 60 years were 2.5 times more likely to undergo revision operation than those younger than 60 years.11 In the present study, fusion length affected the ASD incidence and aging factor to the ASD time period. These results suggested that fusion length and aging appeared to be major risk factors for ASD in accordance with previous reports.
With regard to ASD by preoperative pathologies, no difference was observed in the ASD incidence, while the ASD time period was significantly shorter with DLS. Because there was no severe DLS patient such as more than 40° or complained difficulty in standing due to sagittal/coronal imbalance, single (67 patients) or double (4 patients) segment PLIF were selected for primary operation in the present series. Regarding ASD pathology in DLS patients, progression of foraminal stenosis at the adjacent fusion segment was more often observed as the ASD pathology. These symptoms, of which there were none before the primary operation, surfaced after PLIF. Precise evaluation of the foraminal lesion was difficult for DLS patients, because there were many asymptomatic cases when radiological foraminal stenosis was observed on magnetic resonance imaging or computed tomography. In the present series, all ASD patients with DLS showed initial improvement of symptoms after the first operation, but they developed deterioration of neurological symptoms rapidly. From the present results, surgical procedures such as multisegment PLIF or lateral interbody fusion with pedicle screw fixation should be considered as the primary operation, if radiological foraminal stenosis at the adjacent fusion segment was observed preoperatively.
Research Limitation
The present study had some limitations. First, there was a wide range of follow-up (2-21 years). The ASD rate and time period would change by the follow-up period, as mentioned above. However, the average follow-up period of 8.3 years was long compared with previous reports. Second, radiological risk factors for ASD such as spinopelvic parameters and preexisting disc degeneration were not investigated.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Okuda S, Iwasaki M, Miyauchi A, Aono H, Morita M, Yamamoto T. Risk factors for adjacent segment degeneration after PLIF. Spine (Phila Pa 1976). 2004;29:1535–1540. [DOI] [PubMed] [Google Scholar]
- 2. Okuda S, Miyauchi A, Oda T, Haku T, Yamamoto T, Iwasaki M. Surgical complications of posterior lumbar interbody fusion with total facetectomy. J Neurosurg Spine. 2006;4:304–309. [DOI] [PubMed] [Google Scholar]
- 3. Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am. 2006;88:2714–2720. [DOI] [PubMed] [Google Scholar]
- 4. Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt 2):310–320. [DOI] [PubMed] [Google Scholar]
- 5. Okuda S, Oda T, Yamasaki R, Maeno T, Iwasaki M. Repeated adjacent-segment degeneration after posterior lumbar interbody fusion. J Neurosurg Spine. 2014;20:538–541. [DOI] [PubMed] [Google Scholar]
- 6. Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938–1944. [DOI] [PubMed] [Google Scholar]
- 7. Lawrence BD, Wang J, Arnold PM, Hermsmeyer J, Norvell DC, Brodke DS. Predicting the risk of adjacent segment pathology after lumbar fusion: a systematic review. Spine (Phila Pa 1976). 2012;37(suppl 22):S123–S132. [DOI] [PubMed] [Google Scholar]
- 8. Xia XP, Chen HL, Cheng HB. Prevalence of adjacent segment degeneration after spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2013;38:597–608. [DOI] [PubMed] [Google Scholar]
- 9. Cheh G, Bridwell KH, Lenke LG, et al. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976). 2007;32:2253–2257. [DOI] [PubMed] [Google Scholar]
- 10. Chen BL, Wei FX, Ueyama K, Xie DH, Sannohe A, Liu SY. Adjacent segment degeneration after single-segment PLIF: the risk factor for degeneration and its impact on clinical outcomes. Eur Spine J. 2011;20:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee JC, Kim Y, Soh JW, Shin BJ. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion: comparison of posterior lumbar interbody fusion and posterolateral fusion. Spine (Phila Pa 1976). 2014;39:E339–E345. [DOI] [PubMed] [Google Scholar]
- 12. Heo Y, Park JH, Seong HY, et al. Symptomatic adjacent segment degeneration at the L3-4 level after fusion surgery at the L4-5 level: evaluation of the risk factors and 10-year incidence. Eur Spine J. 2015;24:2474–2480. [DOI] [PubMed] [Google Scholar]
- 13. Nakashima H, Kawakami N, Tsuji T, et al. Adjacent segment disease after posterior lumbar interbody fusion: based on cases with a minimum of 10 years of follow-up. Spine (Phila Pa 1976). 2015;40:E831–E841. [DOI] [PubMed] [Google Scholar]
- 14. Lee CS, Hwang CJ, Lee SW, et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ekman P, Möller H, Shalabi A, Yu YX, Hedlund R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bae JS, Lee SH, Kim JS, Jung B, Choi G. Adjacent segment degeneration after lumbar interbody fusion with percutaneous pedicle screw fixation for adult low-grade isthmic spondylolisthesis: minimum 3 years of follow-up. Neurosurgery. 2010;67:1600–1608. [DOI] [PubMed] [Google Scholar]
- 18. Kaito T, Hosono N, Mukai Y, Makino T, Fuji T, Yonenobu K. Induction of early degeneration of the adjacent segment after posterior lumbar interbody fusion by excessive distraction of lumbar disc space. J Neurosurg Spine. 2010;12:671–679. [DOI] [PubMed] [Google Scholar]
- 19. Anandjiwala J, Seo JY, Ha KY, Oh IS, Shin DC. Adjacent segment degeneration after instrumented posterolateral lumbar fusion: a prospective cohort study with a minimum five-year follow-up. Eur Spine J. 2011;20:1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakaura H, Yamashita T, Miwa T, Ohzono K, Ohwada T. Outcomes of 2-level posterior lumbar interbody fusion for 2-level degenerative lumbar spondylolisthesis. J Neurosurg Spine. 2013;19:90–94. [DOI] [PubMed] [Google Scholar]
- 22. Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86-A:1497–1503. [DOI] [PubMed] [Google Scholar]
- 23. Sears WR, Sergides IG, Kazemi N, Smith M, White GJ, Osburg B. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011;11:11–20. [DOI] [PubMed] [Google Scholar]
- 24. Miwa T, Sakaura H, Yamashita T, Suzuki S, Ohwada T. Surgical outcomes of additional posterior lumbar interbody fusion for adjacent segment disease after single-level posterior lumbar interbody fusion. Eur Spine J. 2013;22:2864–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malakoutian M, Volkheimer D, Street J, Dvorak MF, Wilke HJ, Oxland TR. Do in vivo kinematic studies provide insight into adjacent segment degeneration? A qualitative systematic literature review. Eur Spine J. 2015;24:1865–1881. [DOI] [PubMed] [Google Scholar]