Abstract
Background:
Frequent blood glucose readings are the most cumbersome aspect of diabetes treatment for many patients. The noninvasive TensorTip Combo Glucometer (CoG) component employs dedicated mathematical algorithms to analyze the collected signal and to predict tissue glucose at the fingertip. This study presents the performance of the CoG (the invasive and the noninvasive components) during a standardized meal experiment.
Methods:
Each of the 36 participants (18 females and males each, age: 49 ± 18 years, 14 healthy subjects, 6 type 1 and 16 type 2 patients) received a device for conducting calibration at home. Thereafter, they ingested a standardized meal. Blood glucose was assessed from capillary blood samples by means of the (non)invasive device, YSI Stat 2300 plus, Contour Next at time points –30, 0, 15, 30, 45, 60, 75, 90, 120, 150, and 180 minutes. Statistical analysis was performed by consensus error grid (CEG) and calculation of mean absolute relative difference (MARD) in comparison to YSI.
Results:
For the noninvasive (NI) CoG technology, 100% of the data pairs were found in CEG zones A (96.6%) and B (3.4%); 100% were seen in zone A for the invasive component and Contour Next. MARD was calculated to be 4.2% for Contour Next, 9.2% for the invasive component, and 14.4% for the NI component.
Conclusions:
After appropriate individual calibration of the NI technology, both the NI and the invasive CoG components reliably tracked tissue and blood glucose values, respectively. This may enable patients with diabetes to monitor their glucose levels frequently, reliably, and most of all pain-free.
Keywords: noninvasive glucose prediction, fingertip tissue, invasive device component, chaos theory, color sensor imaging
Diabetes patients encounter several struggles in daily life including secondary complications emerging out of microvascular, macrovascular and neuropathic deteriorations. These complications, directly associated with the disease, often lead to significant loss in quality of life, but also to immense treatment efforts to avoid the progression of the disorders. Consequently, bad glycemic control is not only an exclusive threat to individual patients, but also a challenge and burden for the whole health care system. The total number of people with diabetes is constantly on the rise, due to demographic changes, increasing access to excess of food, and a decline in mortality among people suffering from the disease. Thus, an increasing number of affected people is accompanied by an increasing number of diabetes complications.1
It is therefore of particular importance to reduce the incidence of new diabetes manifestations by means of targeted prevention measures, and by employing measures to avoid disease progression until the development of diabetes complications.2-4 One accepted measure is frequent glucose testing enabling targeted intervention and treatment intensification at the appropriate time-points.5-9 However, glucose measurement is the most cumbersome aspect of diabetes treatment for many patients and many of them fail to adhere to the corresponding recommendations over extended time periods. Therefore, commercially available invasive devices for glucose monitoring with test strips are commonly underutilized.10,11 There are a variety of reasons leading to this phenomenon including the physical pain of finger pricking to obtain the blood sample, the damage to skin sensitivity at the fingertip, risk of infection, and economic aspects such as cost constraints of the health care cost carriers.12,13 Moreover, invasive monitoring sensors do not provide continuous blood glucose information and are not capable of providing warnings of impending hypoglycemic and hyperglycemic excursions in advance.14
Noninvasive (NI) glucose monitoring devices may provide a solution, as they offer a simple and most of all pain-free way of measuring glucose levels multiplying glucose measurements per day. According to the United Kingdom Prospective Diabetes Study (UKPDS) and the Diabetes Control and Complications Trial (DCCT), frequent measurements help to reduce the incidence and progression of diabetes complications both in type 1 and type 2 diabetes,2,15 have sustaining benefits regarding vascular disease outcomes8,16 and help to reduce the amount of glycosylated hemoglobin.17-19 A device specifically designed to provide assistance to reach the frequent testing objective is the Cnoga TensorTip Combo Glucometer (CoG; Cnoga Medical Ltd, Caesarea, Israel). The CoG device provides NI pain-free prediction of fingertip capillary tissue glucose levels and is therefore believed to enhance the frequency of capillary tissue glucose determination.
The purpose of this study was to evaluate the accuracy of the CoG device in comparison to a standard point-of-care reference method for the assessment of capillary blood glucose concentrations (YSI 2300 Stat Plus) when tested during a standardized meal experiment in patients with type 2 diabetes, type 1 diabetes, and in healthy subjects.
Participants and Methods
Study Device
The CoG device (see Figure 1) is approved in Europe (CE), Brazil, China, and other countries and is intended for invasive and NI glucose monitoring for domestic use. This glucometer measures capillary glucose with invasive samples obtained from the fingertip in the range of 40-440 mg/dL (2.2-24.4 mmol/L), and noninvasively predicts tissue glucose also from the fingertip.
Figure 1.
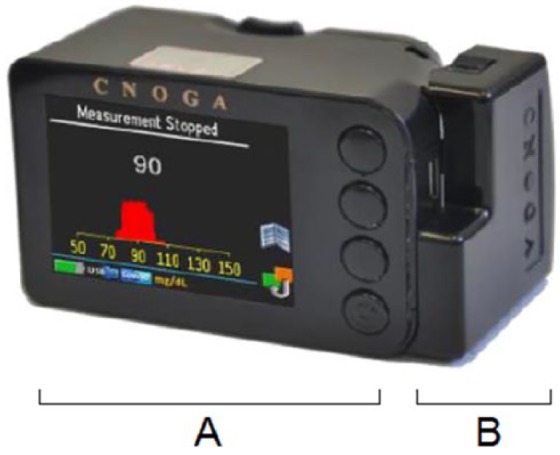
The Cnoga TensorTip Combo Glucometer. (A) Noninvasive component. (B) Add-on invasive glucometer.
For this purpose, the CoG is composed of two main units: an add-on module for the quantitative measurement of capillary whole blood samples identical to the approved Okmeter match device K090609 (OK Biotech Co, Ltd, Hsinchu City, Taiwan) using test strips similar to any ordinary glucometer for patient self-testing, and a component for the NI measurement of glucose levels in the fingertip tissue.
The technology underlying the NI measurements is presented in detail in the same issue of this journal.20 In brief, four light emitting diodes (LEDs) within the finger compartment emit a discrete wavelength from ~ 600 ~ 1000 nm through the fingertip. As the light wave passes through the tissue and blood capillaries, the light is partially absorbed and consequently, the light signal is changed. The traversed light is then projected onto a color image sensor (camera) of the device. The camera photographs in real time the light that traverses the fingertip tissue. The color images are stored in a digital memory for analysis by a dedicated algorithm executed in the digital processor unit. The six-dimensional signals (position [x, y], color [R, G, B], and time [t]) are associated with tissue glucose levels by means of adopted algorithms derived from brain neural mechanism to extract order out of disorder.
In practice, after the device is calibrated, a NI measurement is performed by inserting the finger in the finger compartment for about 30 seconds, until the results are being displayed on the color screen. Due to the noninvasiveness of these measurements, that is, no need for extracting any body fluid, the device does not require sterilization. Still, a frequent cleansing of the finger chamber is advised to guarantee reliability of results. For this purpose, it is also recommended to clean fingers before use of the CoG or to heat them by means of warm tap water beforehand to ensure the capillary blood supply, which is to be measured.
As a monitoring device, the CoG is not meant for diagnosis or determination of insulin doses. Medication intake or treatment decisions should not be based on the NI measurements obtained by the device, the approved invasive component may be used for this purpose.
Study Conduct and Objectives
This study was conducted as an open label, prospective, comparative, single-center trial and was performed in accordance with the ICH-GCP guidelines, the German Medical Device Act (Medizinproduktegesetz, MPG), and local ethical regulations. The Ethical Review Committee of the State of Rhineland-Palatinate approved the study protocol and participants signed informed consent prior to any study procedure.
All participants were men and women who were older than 18 years and who were either healthy subjects, or patients with type 1 or type 2 diabetes (HbA1c < 9.0%). Patients or healthy subjects were excluded if they had any anatomical abnormality at the fingertip, such as thick fingers not fitting inside the CoG chamber or damaged fingertips due to multiple finger pricking, as determined by the investigator. Further reasons for exclusion were end-stage renal failure, pregnancy, breast-feeding, known HIV or hepatitis C infections, or uptake of drugs or nutritional supplements known to interfere with the glucose-oxidase-based strip technology of the invasive device component (eg, uptake of acetyl-salicylic acid, Paracetamol, etc).
During the first visit, an instruction to the device and the necessary calibration procedure was given by the investigators. For calibration, 8 times a day the participants performed a NI reading immediately after two invasive measurements (double check) within 7 to 10 days (56 measurements per calibration, although the minimal requirement of the device is 25). The participants had to decide for one finger for the NI readings, while the capillary blood samples could be obtained from all other fingers. There was no need for any further blood glucose meter for this calibration procedure. For proper calibration, it is necessary to use two consecutive readings of the invasive component and a timely NI reading in parallel to ensure reliable correlation of fingertip tissue glucose values and blood glucose levels. After completing the calibration procedure, the patients were eligible for the meal experiment at visit 2, which normally happened at least 24 h after the last calibration measurement.
For the meal test, the participants came to the study site after an overnight fast for at least 6 hours. After assessment of glucose by means of several methods as indicated below, the participants ingested a standardized meal, which was composed of a bread roll with cheese and sausage, a cereal bar and orange juice (69 g carbohydrates, 27 g fat, 16 g proteins). Before and during the standardized meal visit (ie, at time points –30, 0, 15, 30, 45, 60, 75, 90, 120, 150, and 180 minutes) capillary blood was drawn for glucose measurement with the reference method (YSI 2300 Stat Plus, YSI Inc, Yellow Springs, OH), followed by readings with the invasive CoG component, the NI CoG component, the Contour Next device (Ascensia, Wuppertal, Germany), and a second and final additional YSI measurement. The coefficient of variation of the reference method was shown to be < 1% at all experimental days.
Statistical Methods
The primary endpoint of this study was to evaluate the accuracy of the NI CoG technology when predicting glucose levels in comparison to the YSI reference method. For this purpose, a consensus error grid (CEG) analysis,21,22 mean absolute relative differences (MARD, for values ≥100 mg/dL), and mean absolute differences (MAD, for values <100 mg/dL) were calculated in comparison to the YSI reference method. The secondary endpoint of this study included the evaluation of the invasive device component and the Contour Next device in comparison to YSI. All results were analyzed by means of descriptive statistics with appropriate parametric and nonparametric methods and were only interpreted in an exploratory sense. A P value < .05 was considered statistically significant.
Results
The finally enrolled 36 participants (15 healthy subjects, 6 type 1 and 15 type 2 patients) performed the study per protocol, and all subjects had either finished the calibration procedure by 100% or the missing calibration values were obtained prior to starting the meal experiments. During the experiment, one healthy subject was detected to have type 2 diabetes (male, 56 years old, BMI: 30.9 kg/m², HbA1c: 6.6%, fasting reference blood glucose: 127 mg/dL). This patient was therefore analyzed as a member of the type 2 cohort. The participant’s characteristics are provided in Table 1. If a subject had accomplished less than 90% of the calibration measurements, the subject was rescheduled with the request to perform the remaining calibration measurements at home. The majority of the calibration measurements (>95%), which were performed by the participants at home and in accordance with the instructions for use prior to the meal study experiments, were in the range > 100 mg/dL. During the second visit, a total of 36 paired data sets (one without NI readings because of technical problems in a type 2 patient) were collected with the 36 participants. YSI blood glucose levels indicated an observed blood glucose range from 59 mg/dL to 317 mg/dL.
Table 1.
Demographic Characteristics of All Participants Who Completed the Meal Study Experiment.
| Healthy subjects | Type 1 diabetes | Type 2 diabetes | |
|---|---|---|---|
| n | 14 | 6 | 16 |
| Gender (male/female) | 6/8 | 3/3 | 8/8 |
| Age (years) | 40 ± 17 | 41 ± 19 | 62 ± 6 |
| HbA1c (%) | 5.6 ± 0.5 | 8.1 ± 0.6 | 7.0 ± 1.3 |
| Disease duration (years) | — | 23 ± 17 | 13 ± 8 |
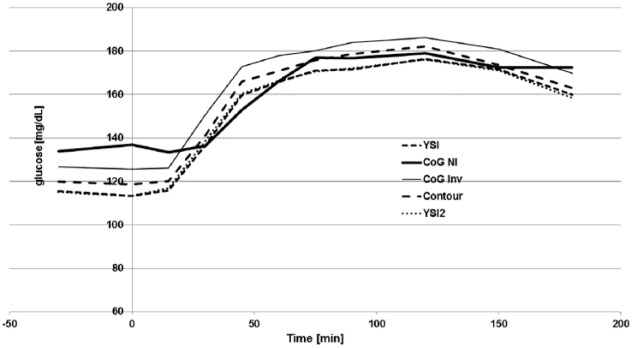
The mean glucose curves calculated for the entire study cohort as obtained during the standardized meal experiment are provided in Figure 2. Error bars were left out in this graph for better readability. Interindividual coefficients of variations were in the range of 30% with all devices. It can be seen that all employed glucose assessment technologies achieved a similar pattern for the glucose excursions in the course of the experiment. The NI CoG component started at a somewhat higher baseline level prior to meal ingestion. During the ascending part of the glucose excursion, the NI glucose prediction got in line with the other invasive measurement results. The invasive add-on CoG component presented with a systematic parallel shift to slightly higher values throughout the entire experiment that could also consistently be seen in the individual subject results (data not shown).
Figure 2.
Mean glucose values of the combined data set. YSI and YSI2, reference method before and after the measurements; CoG NI, NI study device component; CoG Inv, invasive study device component; Contour, Contour Next. Error bars were left out for better readability.
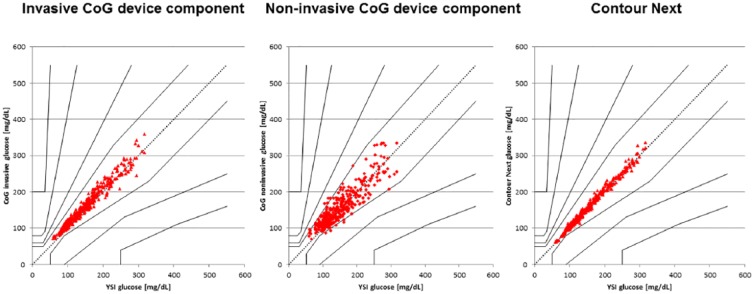
In the CEG analysis, 100% of the data pairs were found to be in the acceptable CEG zones A (96.6%) and B (3.4%) for the NI CoG component, while all values (100%) were found to be in zone A for the invasive CoG component and for the Contour Next device (see Figure 3). The MARD was calculated for values ≥ 100 mg/dL (NI CoG: 12.6%, invasive CoG: 8.2%, Contour Next: 4.1%) and the MAD was determined for values < 100 mg/dL in comparison to the YSI reference method (NI CoG: 19.8 mg/dL, invasive CoG: 14.4 mg/dL, Contour Next: 4.7 mg/dL). MARD over the entire measurement range (59-317 mg/dL) was calculated to be 4.2% for Contour Next, 9.2% for the invasive CoG meter, and 14.4% for the NI CoG technology. There were no major differences in all these results between the different participant subgroups tested in this study and the most accurate data set was obtained with Contour Next.
Figure 3.
Consensus error grid analysis of the different tested glucose monitoring devices.
In total, 385 NI measurements from 16 type 2 diabetes patients, 6 type 1 diabetes patients and 14 healthy subjects were performed and evaluated in this study. No adverse event, which is related to the frequent application of the NI device, was observed following the use of the CoG. In particular, no injuries or appearance of complications at the finger tips such as skin irritation, burning, or discomfort were reported.
Discussion
In this trial, we investigated the performance of a combined glucose monitoring device for conventional invasive capillary glucose measurement, and for additional optional prediction of glucose levels in the fingertip tissue by means of a noninvasive device component in comparison to several invasive point-of-care blood glucose measurement methods. The invasive component, which is employing an approved glucose oxidase-based strip technology, performed within the expected accuracy ranges as set forth in the regulatory acceptance criteria. The results for the NI component show that 100% of the performance evaluation data points were also within the clinically accepted zones A (96.6%) and B (3.4%) of the CEG, while compared to the YSI, which is accepted as point-of-care standard reference method for blood glucose monitoring by the regulatory agencies.23 The invasive CoG and the Contour Next showed also accurate device performance with all data sets in zone A of the CEG and MARD values < 10%.
When interpreting these results, it needs to be considered that the YSI reference and the invasive CoG component measure capillary blood glucose, while the NI CoG technology predicts tissue glucose in the fingertip using traversed light (visible to IR) that is detected by color image sensors. 20 Dynamic changes in the blood glucose content are known to be reflected in the tissue with a lag time induced by the shift of glucose from the vascular compartment to the interstitial fluid).24 This lag time has been described to last from less than 10 minutes to more than 45 minutes based on the diabetes stage, and in particular also related to the severity of secondary microcirculation disorders.25-27 This fact explains why NI glucose monitoring methods are considered to be of limited efficacy in emergency situations.14 However, the observed MARD of 14.5% for the NI CoG method indicates an acceptable performance of the NI technology during the meal study experiments.
The observed NI results were better for values larger than 100 mg/dL than for values in the low normal to hypoglycemic range, which may be related to the way the calibration was performed. The device requires an extensive calibration procedure with at least 25 calibration measurements with parallel assessment of capillary blood glucose by means of the invasive CoG component and with the NI CoG component over a period of few days, which are performed by the patients at home. The calibration is supposed to cover the entire range of the glucose prediction range. In the calibration time prior to the meal study experiments, which may also reflect daily patient life, the number of calibration assessments in the low normal and hypoglycemic range was very limited. It is tempting to speculate that a higher frequency of calibration measurements in the lower glucose range would have resulted in a better performance of the NI device in the glucose range below 100 mg/dL. Further studies with a special focus on calibration of the device in the hypoglycemic range are required to elucidate this phenomenon.
The NI device calibration was performed using the invasive component of the CoG device. Comparison of this add-on glucose-oxidase-based test method with the YSI reference showed a consistent parallel shift of the invasive results by about 10-20 mg/dL to higher glucose values. It may therefore be an option to improve the glucose prediction performance of the NI device by correcting this shift in the device software. Again, further studies need to be performed to test this hypothesis.
Our study has several limitations that need to be considered when interpreting the results in the context of routine daily practice: First, the study was a single-day meal study experiment, after home calibration of the device. The results indicate a tissue glucose prediction accuracy that seems to be suitable for regular daily meal situations, thus giving patients access to an unlimited number of pain-free daily glucose results. Further investigations whether more frequent NI glucose readings will lead to improved long-term glycemic control and potentially reduced development of secondary complications, as it has been shown for invasive blood glucose patient self- testing9 should be carried out in appropriately designed clinical studies. In addition, the study was performed with different subgroups of participants resulting in very small cohort sizes of 6 type 1 diabetes patients, 16 type 2 diabetes patients, and 14 healthy subjects. While the results are not different between the three groups, the low number of subjects and the observed variability do not allow for drawing final conclusions regarding differences in device performance in the three different groups tested. Finally, the device performance was tested by means of a standardized meal experiment with a defined food composition and food volume, which was equal in all participants. Daily individual variations in food uptake need to be considered before translating these results into daily routine.
Despite all these limitations, the prediction accuracy of this new NI device appears to be suitable for effective and safe use glucose monitoring in daily practice in people with prediabetes, type 1 diabetes and type 2 diabetes. According to our knowledge, MARD below 15% in a meal study experiment has not been described for any NI glucose prediction technology in the literature so far. If the underlying technology and mathematical algorithms can be further improved to meet even the acceptance criteria, such as for invasive glucose measurement in accordance with current regulatory guidelines, it may finally make the dream of pain-free glucose assessment even for patients with insulin treatment come true.
Conclusion
After appropriate individual calibration of the NI technology, both the NI and the invasive component of the CoG device were shown to reliably track tissue and blood glucose values, respectively. The measurements were taken during standardized meal experiments and provided acceptable accuracy in comparison to the YSI standard reference method. This may enable patients with diabetes to monitor their glucose levels frequently, reliably, and most of all pain-free.
If our results can be confirmed in daily routine, this new device represents a major step forward in facilitating patients’ life by providing convenient and pain-free access to unlimited glucose information in daily routine care.
Footnotes
Abbreviations: CEG, consensus error grid; DCCT, Diabetes Control and Complications Trial; ICH-GCP, International Conference on Harmonization - Good Clinical Practice; LED, light emitting diode; MAD, mean absolute difference; MARD, mean absolute relative difference; MPG, Medizinproduktegesetz; NI, noninvasive; CoG, TensorTip Combo Glucometer; UKPDS, United Kingdom Prospective Diabetes Study; YSI, Yellow Springs Instrument.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Andreas Pfützner has received speaker fees and travel support from CNOGA Medical. All other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a research grant provided by CNOGA Medical
ORCID iD: Andreas Pfützner  https://orcid.org/0000-0003-2385-0887
https://orcid.org/0000-0003-2385-0887
References
- 1. International Diabetes Federation. IDF diabetes atlas— 8th edition. 2017. Available at: www.diabetesatlas.org. [PubMed]
- 2. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 3. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 4. Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111(1):1-9. [DOI] [PubMed] [Google Scholar]
- 5. Elgart JF, Gonzalez L, Prestes M, Rucci E, Gagliardino JJ. Frequency of self-monitoring blood glucose and attainment of HbA1c target values. Acta Diabetol. 2016;53(1):57-62. [DOI] [PubMed] [Google Scholar]
- 6. Kempf K, Tankova T, Martin S. ROSSO-in-praxi-international: long-term effects of self-monitoring of blood glucose on glucometabolic control in patients with type 2 diabetes mellitus not treated with insulin. Diabetes Technol Ther. 2013;15(1):89-96. [DOI] [PubMed] [Google Scholar]
- 7. Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2(3):203-211. [DOI] [PubMed] [Google Scholar]
- 8. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589. [DOI] [PubMed] [Google Scholar]
- 9. Martin S, Schneider B, Heinemann L, et al. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49(2):271-278. [DOI] [PubMed] [Google Scholar]
- 10. Brindisi MC, Hahn J, Chiasson JL, Rabasa-Lhoret R. Under-utilization of capillary glucose monitoring by type 2 diabetic patients. Diabetes Res Clin Pract. 2007;75(1):123-125. [DOI] [PubMed] [Google Scholar]
- 11. Hansen MV, Pedersen-Bjergaard U, Heller SR, et al. Frequency and motives of blood glucose self-monitoring in type 1 diabetes. Diabetes Res Clin Pract. 2009;85(2):183-188. [DOI] [PubMed] [Google Scholar]
- 12. Mollema ED, Snoek FJ, Heine RJ, van der Ploeg HM. Phobia of self-injecting and self-testing in insulin-treated diabetes patients: opportunities for screening. Diabet Med. 2001;18(8):671-674. [DOI] [PubMed] [Google Scholar]
- 13. Wagner J, Malchoff C, Abbott G. Invasiveness as a barrier to self-monitoring of blood glucose in diabetes. Diabetes Technol Ther. 2005;7(4):612-619. [DOI] [PubMed] [Google Scholar]
- 14. Chen C, Zhao XL, Li ZH, Zhu ZG, Qian SH, Flewitt AJ. Current and emerging technology for continuous glucose monitoring. Sensors (Basel). 2017;17(1):E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48(5):643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haller MJ, Stalvey MS, Silverstein JH. Predictors of control of diabetes: monitoring may be the key. J Pediatr. 2004;144(5):660-661. [DOI] [PubMed] [Google Scholar]
- 18. Schutt M, Kern W, Krause U, et al. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384-388. [DOI] [PubMed] [Google Scholar]
- 19. Towfigh A, Romanova M, Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: a meta-analysis. Am J Manag Care. 2008;14(7):468-475. [PubMed] [Google Scholar]
- 20. Segman Y. Device and method for non-invasive glucose assessment. J Diabetes Sci Technol. 2018;12(6):1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfützner A, Klonoff DC, Pardo S, Parkes JL. Technical aspects of the Parkes error grid. J Diabetes Sci Technol. 2013;7(5):1275-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143-1148. [DOI] [PubMed] [Google Scholar]
- 23. Food and Drug Administration. FDA Self-monitoring blood glucose test systems for over-the-counter use. Guidance for industry and Food and Drug Administration staff; Available at: https://www.fda.gov/downloads/ucm380327.pdf. Accessed October 11, 2016. [Google Scholar]
- 24. Cobelli C, Schiavon M, Dalla Man C, Basu A, Basu R. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: insight from an in silico study. Diabetes Technol Ther. 2016;18(8):505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Basu A, Dube S, Veettil S, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9(1):63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chlup R, Krejci J, O’Connell M, et al. Glucose concentrations in blood and tissue—a pilot study on variable time lag. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(4):527-534. [DOI] [PubMed] [Google Scholar]
- 27. Koschinsky T, Jungheim K, Heinemann L. Glucose sensors and the alternate site testing-like phenomenon: relationship between rapid blood glucose changes and glucose sensor signals. Diabetes Technol Ther. 2003;5(5):829-842. [DOI] [PubMed] [Google Scholar]