Abstract
There is an important emerging role for the endogenous opioid dynorphin (DYN) and the kappa-opioid receptor (KOR) in the treatment of alcohol dependence. Evidence suggests a role for the DYN/KOR system in the bed nucleus of the stria terminalis (BNST) in maladaptive behavioral regulation related to alcohol dependence and withdrawal. The current experiments were designed to assess dysregulation of the BNST DYN/KOR system by evaluating alcohol dependence-induced changes in DYN/KOR gene expression (Pdyn and Oprk1, respectively), and the sensitivity of alcohol self-administration, negative affective-like behavior and physiological withdrawal to intra-BNST KOR antagonism during acute withdrawal. Wistar rats trained to self-administer alcohol, or not trained, were subjected to an alcohol dependence induction procedure (14h alcohol vapor /10h air) or air-exposure. BNST micropunches from airand vapor-exposed animals were analyzed using RT-qPCR to quantify dependence-induced changes in Pdyn and Oprk1 mRNA expression. In addition, vapor- and air-exposed groups received an intra-BNST infusion of a KOR antagonist or vehicle prior to measurement of alcohol self-administration. A separate cohort of vapor-exposed rats was assessed for physiological withdrawal and negative affective-like behavior signs following intra-BNST KOR antagonism. During acute withdrawal, following alcohol dependence induction, there was an upregulation in Oprk1 gene expression in alcohol self-administering animals, but not non-alcohol self-administering animals, that confirmed dysregulation of the KOR/DYN system within the BNST. Furthermore, intra-BNST KOR antagonism attenuated escalated alcohol self-administration and negative affective-like behavior during acute withdrawal without reliably impacting physiological symptoms of withdrawal. The results confirm KOR system dysregulation in the BNST in alcohol dependence, illustrating the therapeutic potential of targeting the KOR to treat alcohol dependence.
Keywords: alcohol dependence, alcohol withdrawal, bed nucleus of the stria terminalis (BNST), dynorphin, kappa opioid receptor, EPM, operant self-administration, Oprk1 mRNA, Pdyn mRNA, 22-kHZ ultrasonic vocalizations
1. Introduction
Approximately 6.2% of adults aged 18+ in the United States are diagnosable with an alcohol use disorder or AUD (National Survey on Drug Use and Health 2015). An inherent characteristic of alcohol dependence is its comorbidity with disorders of affect such as depression and anxiety (e.g., Williams et al. 2012). According to the self-medication hypothesis of addiction and dependence, these negative states likely contribute to continued alcohol use and relapse behaviors (Markou et al. 1998; Walker et al. 2012). Unfortunately, manipulation of neurotransmitter systems traditionally associated with anti-depressant efficacy rarely reduces alcohol consumption in dependent individuals (for review, see Walker et al. 2012). However, recent studies have found that alcohol dependence- and withdrawal-induced neuroadaptations within the endogenous dynorphin (DYN) / kappa-opioid receptor (KOR) system show potential as a target to treat alcoholism.
Consistent with the Opponent-Process Theory of Motivation (Solomon and Corbit et al. 1974), exposure to an acute dose of alcohol stimulates the release of β-endorphin that signals through the μ-opioid receptor (MOR) to produce reward, which is then followed by DYN release (Marinelli et al. 2003; Marinelli et al. 2006; Logrip et al. 2008) to mitigate the MOR-mediated deviation from a putative homeostatic mood set-point. Chronic alcohol-induced stimulation of the MOR receptor is incongruent with a tightly controlled homeostatic affective set-point in the brain. Given the oppositional nature of KOR’s (the receptor for endogenous DYNs; Chavkin et al., 1982) compared to the effects of MOR activation (Mucha and Herz 1985; Spanagel et al. 1992), the DYN/KOR system is well-situated to oppose the effects of chronic alcohol-induced perturbations in MOR signaling by alcohol. In addition to established MOR / KOR opposition, the endogenous opioid peptide nociceptin / orphanin FQ (N/OFQ) is putatively oppositional in nature to the DYN / KOR system and has been implicated in AUD-like phenotypes (Ciccocioppo et al. 1999; Economidou et al. 2008; Roberto et al. 2012; D’Addario et al. 2013; de Guglielmo et al. 2015; Aziz et al. 2016). Nevertheless, in the absence of alcohol during withdrawal once alcohol dependent, DYN / KOR system dysregulation can contribute to profound deficits in behavioral regulation that include escalated alcohol self-administration, depressive-and/or anxiety-like symptoms and the potential for compromised executive function during acute withdrawal (Berger et al. 2013; Kissler et al. 2014; Walker and Kissler 2013). These and other factors are thought to be primary features that promote continued excessive alcohol consumption during acute withdrawal and protracted abstinence (Walker et al. 2012; Kissler and Walker 2016).
DYN and KORs have been implicated in depressive and negative emotional phenotypes (Carlezon et al. 2006; Bruchas et al. 2007; Land et al. 2008; Berger et al. 2013) and have been shown to be up-regulated in the central nucleus of the amygdala (CeA) following chronic alcohol exposure (Kissler et al. 2014). Multiple human genetic studies have identified that changes in Oprk1 and Pdyn expression are linked with alcohol dependence (e.g., Xuei et al. 2006; Edenberg et al. 2008). Additionally, intragastric injections of alcohol in mature animals have been shown upregulate Pdyn mRNA expression in the amygdala (D’Addario et al. 2013), although when rats are prenatally exposed to alcohol and KOR densities measured during their pre-adolescent phase, downregulation of KOR expression in limbic nuclei was observed (Nizhnikov et al. 2014), although this dissociation putatively represents a developmental shift in KOR-mediated hedonics (Petrov et al. 2006).
Within the extended amygdala (i.e., CeA, nucleus accumbens shell (AcbSh) and bed nucleus of the stria terminalis (BNST); Alheid and Heimer 1988), previous analyses revealed dense levels of KORs and Oprk1 mRNA expression in the BNST (Mansour et al. 1987; Mansour et al. 1988). KORs in the BNST have been shown to disinhibit glutamatergic transmission (Li et al. 2012) which has been heavily implicated in symptoms of alcohol dependence (Spanagel 2009). Furthermore, the BNST has a role in negative affective-like signaling (Kash and Winder 2006), opioid-withdrawal-induced place aversions, and somatic signs of opiate withdrawal (Aston-Jones et al. 1999) involving noradrenergic afferents from the locus coeruleus that can be modulated by KORs (Al-Hasani et al. 2013), as well as stress-induced reinstatement of drug responding involving the CeA and BNST (Shaham et al. 2003; Shalev et al. 2002) that was recently shown to be contingent on KOR’s in the BNST (Le et al. 2017).
Given the BNST’s role in stress and withdrawal-related behaviors, the presence of KORs in the BNST and confirmed dysregulation of DYN/KORs by alcohol dependence in other extended amygdala nuclei (i.e., CeA and AcbSh; Nealey et al. 2011; Kissler et al. 2014; Kissler and Walker 2016), it is probable that DYN and/or KORs in the BNST are altered by alcohol dependence and contribute to maladaptive behavioral regulation. To test this hypothesis, male Wistar rats were trained to self-administer alcohol, or not, and then subjected to long-term intermittent alcohol vapor exposure to induce dependence (or air exposure), followed by the assessment of 1) alterations in Oprk1 and Pdyn mRNA expression in the BNST, 2) intra-BNST KOR antagonist effects on alcohol self-administration in non-dependent and alcohol-dependent rats, and 3) intra-BNST KOR antagonist effects on negative affective-like behavior and physiological withdrawal symptoms during acute withdrawal in alcohol-dependent rats.
2. Materials and Methods
2.1. Animals and Operant Conditioning
Male Wistar rats (N=40) were group housed (2–3 per cage) in a temperature-controlled (21±2 °C) vivarium on a 12hr reverse light cycle with ad libitum food and water. Animal care adhered to the National Research Council’s Guide for the Care and Use of Laboratory (National Research Council et al. 2011) with all procedures approved by the Washington State University Institutional Animal Care and Use Committee. In Experiment 1 (see section 2.3), animals were either untrained or trained to self-administer a 10% alcohol (wt/vol) solution according to a fixed-ratio 1 (FR-1) schedule of reinforcement in an operant paradigm using a sweetener-fade method for 30 minutes per day (Walker and Koob 2007; Walker and Koob 2008; Walker et al. 2011; Nealey et al. 2011; Smith et al. 2011; Williams et al. 2012; Kissler et al. 2014; Kissler and Walker 2016). In Experiments 2 and 3 (see sections 2.5 and 2.5), all animals were trained to self-administer alcohol. Standard operant chambers (Med Associates, St. Albans, Vermont) with custom drinking wells (Behavioral Pharma, La Jolla, CA) were utilized in which a lever press resulted in 0.1 mL of solution. For those animals trained to self-administer alcohol, once experimental cohorts of animals achieved stable levels of self-administration (<10% deviation over three sessions), experiment-specific manipulations were conducted. In all cases involving animals that self-administered alcohol, the groups of animals within each experiment were matched for alcohol consumption (either baseline or escalated depending on the experiment, see below) and therefore, within each experiment, there were no differences in the length of alcohol self-administration between groups. In all cases of self-administration, lever-presses were converted to g/kg alcohol to control for animal weight differences and used for statistical analyses (see section 3).
2.2. Intermittent Alcohol Vapor Exposure
Animals were exposed to air or intermittent 95% EtOH vapor (14h on, 10h off per day) which has been shown to induce alcohol dependence (O’Dell et al. 2004) as evidenced by dependence-like behavioral phenotypes such as escalated alcohol self-administration (O’Dell et al. 2004; Walker and Koob 2007) and negative affective-like behaviors (e.g., Williams et al. 2012) when tested at a timepoint corresponding to 6-h into acute withdrawal for EtOH vapor-exposed animals (i.e., 6-h after the daily vapor exposure terminated). Blood ethanol concentrations (BECs) were measured twice weekly by collecting blood (50 μl) from the tail prior to the end of daily alcohol vapor exposure and analyzed using the Analox AM1 (Analox Instruments, Lunenberg, MA). Target BECs of 175–225 mg% were maintained throughout the experiments and confirmed prior to the daily termination of alcohol vapor exposure for brain extractions (see Experiment 1 description) or prior to every acute withdrawal self-administration test day and prior to brain extractions (see Experiment 2 and 3 descriptions). The 6-h acute withdrawal timepoint used during each of the repeated acute withdrawal tests for self-administering animals was first adopted (Walker and Koob 2007) because the BECs of alcohol-dependent rats maintained at ~225 mg% to zero within 6-h (Gilpin et al. 2009) and therefore, the 6-h timepoint avoids a potential confound of alcohol presence at the time of brain extractions or acute withdrawal behavioral testing. It is important to note that within the current intermittent vapor exposure paradigm, once the initial alcohol vapor exposure period to induce alcohol dependence was completed, following each behavioral test that occurred during acute withdrawal, the animals were returned to the vapor chambers. Consequently, each subsequent behavioral test occurred under identical conditions of acute withdrawal. Furthermore, the repeated withdrawal aspect of the paradigm during dependence induction and subsequent behavioral testing is a benefit of the current approach that increases the face and construct validity of the model (O’Dell et al. 2004). In our previous investigations (e.g., Kissler et al. 2014; Kissler and Walker 2016) the total time required for vapor-induced dependence induction and progression through the acute withdrawal self-administration re-stabilization, sham infusion stabilization, and aCSF stabilization phases prior to pharmacological testing required ~3 – 4 months to complete. Based on this fact, we selected a 4-month exposure duration for the current experiments.
2.3. Experiment 1
Figure 1: Experiment 1 Timeline.
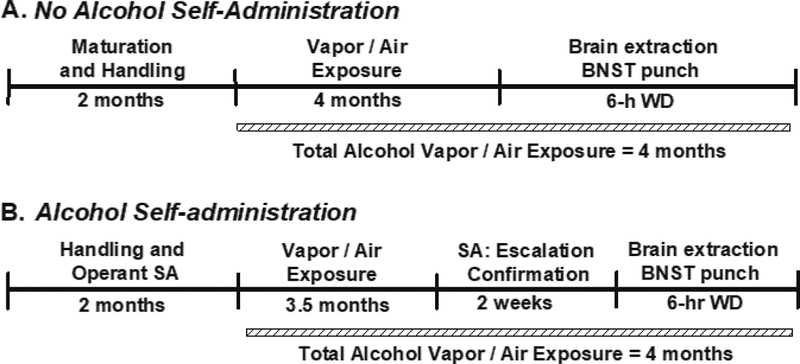
Rats trained to self-administer alcohol (B), or not trained to self-administer alcohol (A), had a total exposure duration of four months prior to brain extraction. SA = self-administration; WD = withdrawal.
Both untrained animals (matched for weight) and animals trained to self-administer alcohol (matched for baseline alcohol self-administration and weight) were subjected to a total of 4-mos intermittent alcohol vapor or air exposure. Therefore, a 2×2 experimental design was utilized with exposure (air or vapor) and self-administration experience (untrained or trained) as the between-group factors. Following the exposure period, the untrained animals (n=4/grp) were decapitated at a time-point equivalent to 6-h into withdrawal for later genetic analysis of Oprk1 and Pdyn mRNA expression in the BNST. Prior to brain extractions, those animals trained to self-administer alcohol (n=4/grp) were statistically confirmed for air- vs vapor exposure-induced escalation of alcohol self-administration. Operant sessions occurred twice weekly 6-h into withdrawal (see section 2.2).
Forty-eight hours following confirmation of escalation, at a time-point equivalent to 6-h of withdrawal, the SA animals were decapitated for later analysis of BNST Oprk1 and Pdyn mRNA expression. The 48-h extraction timepoint was selected to prevent the possibility of any confounding acute ‘hangover’ effects following the animals last escalation-confirmation self-administration session. The total air- or vapor-exposure duration of Experiment 1 (i.e., 4-mos) paralleled the duration of exposure in the site-specific studies of Experiments 2 and 3.
2.31. Post-Mortem Rat Brain Samples for RNA Extraction
At timepoint corresponding to 6-h into withdrawal for the alcohol vapor-exposed animals, air- and alcohol vapor-exposed animals were decapitated and had their brains extracted and snap-frozen, and micropunches (1.0 mm diameter) of the BNST were placed in 50 μl RNAlater in 4 °C for 24 hours, and then stored in −80 °C freezer until RNA extraction occurred. RNA extraction followed the Purelink RNA (Life Technologies, Carlsbad, CA) extraction protocol. BNST micropunches were homogenized in lysis buffer for isolation of total RNA according to the manufacturer directions. RNA quantity and quality were measured using the NanoDrop (ThermoFisher Scientific, Waltham, MA) and total RNA fragment analysis (Advanced Analytical Technologies, Ankeny, IA), respectively. Only samples with RNA quality number (RQN) > 8.0 were used for RT-qPCR.
2.32. RT-qPCR
Gapdh, Oprk1 and Pdyn mRNA primers (see Table 1) were taken from Vats et al. 2008 and D’Addario et al. 2013, or designed using Primer3 (Untergasser et al. 2012). Specificity of primers was confirmed using NCBI BLAST (Altschul et al. 1990). To evaluate primers, amplification efficiency was examined at five different concentrations in triplicate, as well as melt curves conducted to confirm a lack of primer-dimerization (see figure S1 for amplification and melt curves in addition to efficiency and R2 values for the primers). Total RNA was transcribed into cDNA using the Maxima First Strand cDNA Synthesis Kit (ThermoFisher Scientific, Waltham, MA). To provide precise quantification of initial target in each PCR, the amplification plot was examined, and the point of early log phase of product accumulation was defined by assigning a fluorescence threshold above background defined as the target gene value compared to endogenous control GAPDH (ΔCT). Differences in threshold cycle number were used to quantify the relative amount of PCR target contained within each sample. Relative expression of different gene transcripts was calculated from the relative ratio of ΔCT value of the target gene for the vapor exposed group to the air-exposed group (ΔΔCT method) and converted to relative expression ratio 2^ΔΔCT for statistical analysis.
Table 1.
| Gene | Forward (5’ to 3’) | Reverse (3’ to 5’) | Product Size |
|---|---|---|---|
| Gapdh | agacagccgcatcttcttgt | cttgccgtgggtagagtcat | 207 |
| Oprk1 | gccatccctgttatcatcac | ggtcttcatctttgtgtatcgg | 108 |
| Pdyn | cacggaactgaccaagctct | gtcagtgcccagtagctcag | 142 |
2.4. Experiment 2
Figure 2: Experiment 2 Timeline.
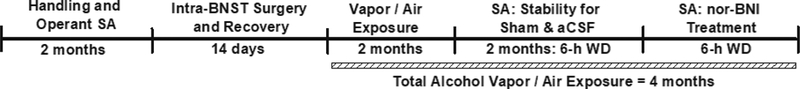
Rats trained to self-administer alcohol had a total exposure duration of four months that included the final pharmacological test session. aCSF = artificial cerebrospinal fluid; BNST = bed nucleus of the stria terminalis; nor-BNI = nor-binaltorphimine; SA = self-administration; WD = withdrawal.
Rats (N = 12) were trained to self-administer alcohol and once stability of responding was established (see section 2.1), were bilaterally cannulated and provided a 1-wk period of recovery before receiving an initial 2-mos of intermittent alcohol vapor or air exposure (see section 2.2). Following the air or vapor exposure period, animals were re-baselined for alcohol self-administration twice-weekly at a timepoint corresponding to 6-h into withdrawal for the vapor-exposed animals, and once stable responding was established (<10% deviation over three sessions), transitioned to testing under sham and aCSF infusion conditions until stability of alcohol self-administration was again achieved under each infusion condition (<10% deviation over two sessions) prior to their final pharmacological manipulation. The KOR antagonist norbinaltorphimine (nor-BNI) was bilaterally infused into the dorsolateral BNST prior to self-administration sessions for air- (n=6) and vapor-exposed (n=6) animals. Following a 5-minute wait period, animals were allowed to self-administer alcohol for 30-min. Therefore, the experiment utilized a mixed-model 2×2 design with exposure (air or vapor) as the between-group variable and treatment (aCSF or nor-BNI) as the within-subject variable. The total time of vapor-exposure prior to pharmacological challenges was ~4-mos to maintain exposure duration consistency with Experiment 1. Animals were subsequently decapitated, and histology was conducted to confirm cannula placement.
2.41. Surgical Procedures
Animals were anesthetized with isoflurane gas and bilaterally implanted with guide cannulae targeting the dorsolateral BNST; AP: −0.35, ML: +/−5.0 DV: −3.5, 29° from vertical (Paxinos and Watson, 2007). Guide cannulae were secured to four stainless steel machine screws (0/80 × 1/8; Fastenal, Moscow, ID) imbedded in the skull using dental acrylic and sealed with obturators. All animals received postoperative care for 5-d consisting of Baytril (antibiotic), flunixin (analgesic), and 0.9% sterile saline to prevent dehydration.
2.42. Intra-BNST KOR Antagonism and Alcohol Self-Administration
Following confirmation of stability under sham and aCSF (pH 7.2–7.4 composed of 145 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 5.4 mM d-Glucose, and 0.25 mM ascorbic acid; Nealey et al, 2011) infusions using 28-gauge internal cannulae (Plastics One; Roanoke, VA), a single dose of the KOR antagonist nor-BNI (4 μg/side for a total bilateral dose of 8 μg that was based on our previous dosing strategies; Nealey et al., 2011, Kissler et al. 2014, Kissler and Walker 2016; Tocris Bioscience, Minneapolis, MN) 5-min prior to alcohol self-administration sessions occurring at a timepoint corresponding to acute withdrawal for the vapor-exposed animals. All infusions were 0.5 μl per side over 2 min with the internal cannula left in place for 1 min to allow for diffusion of the solution. Following the completion of the experiment, all animals received a 0.5 μl cresyl violet infusion into the dorsolateral BNST, had their brains extracted, sliced, and mounted on slides to confirm BNST placement.
2.5. Experiment 3
Figure 3: Experiment 3 Timeline.
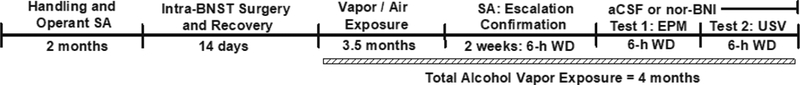
Rats trained to self-administer alcohol had a total vapor exposure duration of four months that included the EPM and USV test sessions. aCSF = artificial cerebrospinal fluid; BNST = bed nucleus of the stria terminalis; EPM = elevated plus-maze; nor-BNI = nor-binaltorphimine; SA = self-administration; USV = 22-kHZ ultrasonic vocalizations; WD = withdrawal.
Based on the results of Experiments 1 and 2, animals trained to self-administer alcohol (N=12) and displaying stable alcohol self-administration (<10% deviation over three sessions) were implanted with intra-BNST cannulae (see 2.41) and then subjected to chronic intermittent alcohol vapor exposure (see 2.2) for 3.5-mos followed by confirmation of acute withdrawal-induced escalated alcohol self-administration by statistically comparing the animal’s pre-exposure baseline responding to acute withdrawal responding. Following confirmation of escalated alcohol self-administration, the animals were split into two groups (matched for escalated alcohol self-administration levels and weight) and 48-h later assessed (see section 2.2) for physiological withdrawal signs and elevated plus-maze (EPM) performance (Test 1) following aCSF (n=6) or nor-BNI (n=6) infusions (as described in section 2.32) that occurred 6–8 h into acute withdrawal. Forty-eight hours following Test 1, physiological withdrawal symptoms and the number of air-puffs to induce 22-kHz USVs, as well as the number and duration of 22-kHZ USV’s emitted were measured 6–8 hours into acute withdrawal (Test 2). Therefore, a between-groups experimental design was used with aCSF and nor-BNI treatment as the between-groups variable. This repeated testing protocol (i.e., Test 2 occurring 48-h after Test 1) is viable due to nor-BNI’s long-term duration of action that we have previously shown lasts at least one month following a single injection in the CeA (Kissler and Walker 2016). The total time of vapor-exposure prior to pharmacological challenges was ~4-mos to maintain a consistent alcohol vapor exposure duration with Experiment 1 and 2, as well as maintain consistency with our previous investigations (see section 2.2). Animals were subsequently decapitated, and histology was conducted to confirm cannulae placement (see 2.42).
2.51. Physiological Withdrawal, Elevated Plus-Maze, and 22-kHz Ultrasonic Vocalizations
Physiological withdrawal signs indicative of alcohol dependence were assessed 6–8 h into acute withdrawal (see section 2.2). Four behaviors were assessed over 3 minutes and each given a ranking of 0–2; hyperirritability upon touch, presence of the ventromedial distal flexion response, tail stiffness/rigidity, and abnormal posture or gait for a total score that ranged from 0–8. Anxiety-like behavior was assessed using an EPM apparatus, as described previously (Williams et al., 2012), 6–8 h into withdrawal. The maze consists of a raised Plexiglas platform (50 cm high) with two open arms and two closed arms of equal length (47 cm × 10 cm each) and a 10 × 10 cm center platform. The floors of the EPM were black, but the Plexiglas walls of the closed arms were clear (40 cm high). Illumination in all arms was ~20 lux. Each animal tested in the EPM was placed in the center platform in an identical position and allowed to explore the maze for 5 minutes and recorded by video and performance assessed using AnyMaze video tracking software (Stoelting Co; Wood Dale, IL) to score the amount of time spent in the open arm, closed arm, and center platform, as well as open- and closed-arm entries and distance (cm) traveled. Percent time spent in the open arms was calculated and utilized as a measure of anxiety-like behavior in the EPM. The maze was cleaned with Quatricide® and dried between each animal. In addition, 22-kHz ultrasonic vocalizations (USVs) indicative of negative affect-like behavior were recorded by a microphone fixed above the animals’ head and assessed in a quiet room with dim lighting by administering repeated 60psi air-puffs to the nape of the animal’s neck (Knapp and Pohorecky 1995; Knapp et al. 1998; Williams et al. 2012) until vocalizations are produced. The number of air-puffs required to induce a 22-kHz USV, number of 22-kHz USV vocalizations, and duration of 22-kHz USV vocalizations were recorded. The sequence of behavioral tests was not counterbalanced, but instead selected to minimize potential test stress that could be an experimental confound. In general, there are multiple previous examples in the literature of repeated aversive stimuli recruiting the KOR system and given the nature of the 22-kHZ USV protocol we utilized, USV testing could be a confound for subsequent EPM testing.
3. Statistics
To determine group sizes for the various experiments, a priori power analyses for sample sizes were conducted (with α = 0.05 and β = 0.2) using effect sizes determined by previous molecular and site-specific behavioral characterizations (i.e., from Walker et al., 2011, Berger et al., 2013; Kissler et al., 2014 and Kissler and Walker, 2016). Experiment 1: Alcohol self-administration: In animals with a history of self-administration, independent-sample t-tests were used to assess changes in alcohol self-administration induced by air-exposure or EtOH vapor-exposure during acute withdrawal. Oprk1 and Pdyn mRNA expression: A between-groups 2×2 analysis of variance (ANOVA) was conducted on Oprk1 and Pdyn mRNA expression in animals with and without a history of alcohol self-administration that were air- or vapor-exposed. If a main effect or interaction was found, post-hoc univariate ANOVAs were conducted to identify differences between air- and vapor-exposed animals. Experiment 2: Baseline alcohol consumption: A univariate ANOVA was used to compare baseline alcohol self-administration between the air- and vapor-exposed groups. Baseline, aCSF- and nor-BNI treatment and alcohol self-administration: A 2×3 mixed-model ANOVA was used to compare animals exposed to air or vapor for baseline, aCSF-treated and nor-BNI-treated alcohol self-administration sessions. The between-groups variable was exposure (air or vapor) and the within-subject variable was session (baseline, aCSF, and nor-BNI). If main effects or interactions were found, post-hoc repeated-measures ANOVAs were utilized to identify differences between baseline and aCSF-treated alcohol consumption and between aCSF and nor-BNI in the different exposure conditions. Experiment 3: Alcohol self-administration: A paired-sample t-test was used to assess changes in alcohol self-administration for baseline and following vapor-exposure during acute withdrawal. Test 1 - Physiological withdrawal and EPM: Independent univariate ANOVAs were used to compare physiological withdrawal scores, percent open arm time, open-arm entries, closed-arm entries and distance traveled in vapor exposed animals following either aCSF or nor-BNI infusions. Test 2 - Physiological withdrawal and 22-kHz USVs: Independent univariate ANOVAs were used to compare physiological withdrawal scores, number of air-puffs required to elicit a 22-kHz USV, number of 22-kHz USVs and the duration of 22-kHZ USVs.
4. Results
4.1. Experiment 1: Oprk1 and Pdyn mRNA expression
Alcohol self-administration: In animals trained to self-administer alcohol and exposed to 4-mos air or alcohol vapor, acute withdrawal-induced escalation was confirmed (t (6) = −5.989, p < 0.001 when comparing air- and vapor-exposed alcohol self-administration) for the vapor-exposed group. Oprk1 and Pdyn mRNA expression: The 2×2 ANOVA identified main effects of Exposure (F (1, 12) = 7.934, p < 0.05) and Condition (F (1, 12) = 12.813, p < 0.01) and an Exposure × Condition interaction (F (1, 12) = 12.813, p < 0.01) for Oprk1 mRNA expression, but no differences in Pdyn mRNA expression were observed (see Figure 4). Post-hoc comparisons indicated that the Oprk1 mRNA expression was specifically increased in the vapor-exposed alcohol self-administering animals when compared to air-exposed alcohol self-administering controls (F (1, 6) = 13.036, p ≤ 0.01).
Figure 4: Oprk1 and Pdyn mRNA expression in the BNST following air or vapor exposure in rats with or without a history of alcohol self-administration.
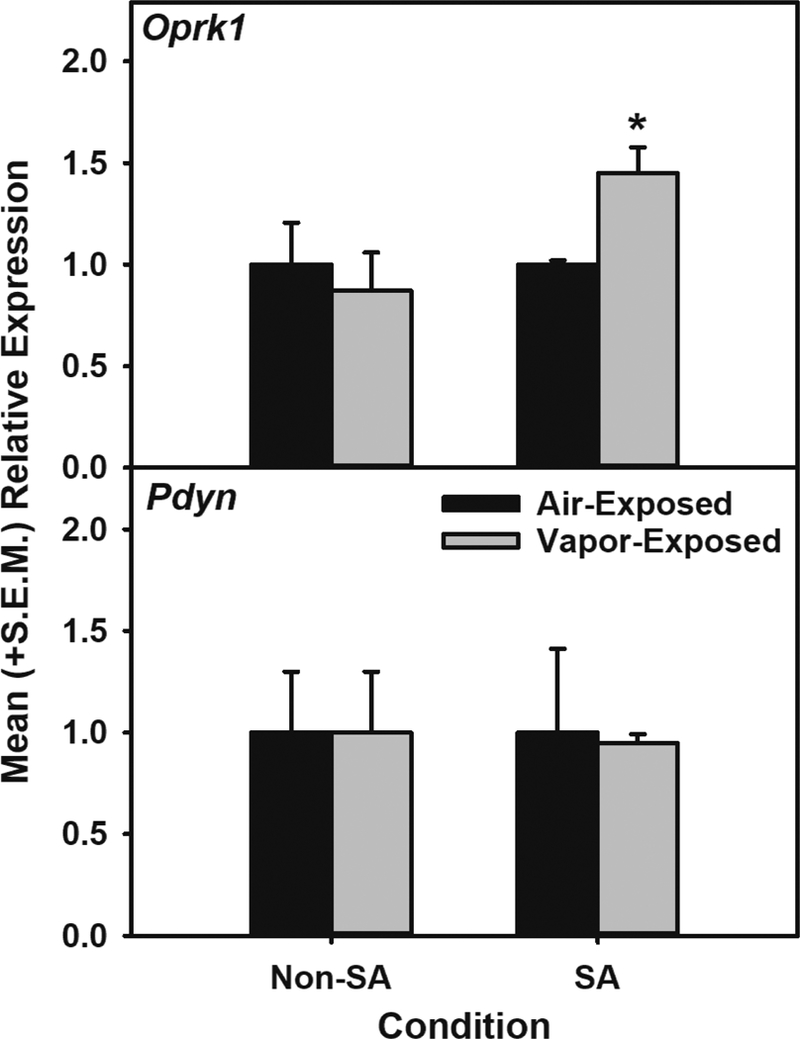
Top Panel: Mean (+S.E.M.) Oprk1 mRNA expression in the BNST is increased during acute withdrawal following vapor exposure exclusively in alcohol self-administering animals (n=4 / grp; * = p ≤ 0.01 when compared to air-exposed SA rats. Bottom Panel: Mean (+S.E.M.) Pdyn mRNA expression in the BNST was unchanged during acute withdrawal following vapor exposure. SA = self-administering.
4.2. BNST Histology
Only those animals having verified injection sites in the BNST were included in the statistical analyses for Experiments 2 & 3 (see Figure 5). One animal was removed due to cannula placement outside the dorsolateral BNST and another animal was viewed having abnormal tissue necrosis around the cannula. The animals removed due to histology were not included in the data analysis for Experiments 2 or 3.
Figure 5: Histological confirmation of infusion sites.
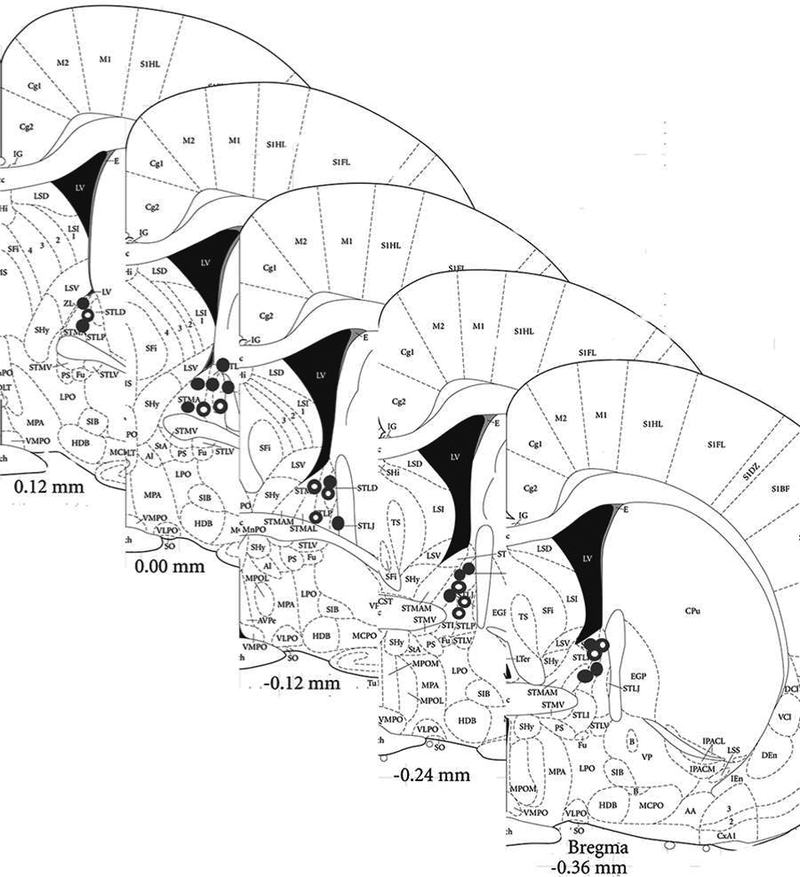
Histological confirmation of infusion sites targeting the BNST. Filled circles denote correct cannula placement for the self-administration experiment, whereas open circles denote correct cannula placement for the negative affective-like assessment.
4.3. Experiment 2: Site-Specific KOR Antagonism and Alcohol Self-administration
Baseline alcohol consumption: A univariate ANOVA indicated that there were no differences in baseline alcohol self-administration between the air- and vapor-exposed groups. Baseline, aCSF-and nor-BNI treatment and alcohol self-administration: The mixed-model ANOVA identified a main effect of Session (F (2, 18) = 4.765, p < 0.05) and an Exposure × Session interaction (F (2, 18) = 4.713, p < 0.05) which indicates that the different exposure conditions responded differently across the three sessions (see Figure 6). Post-hoc comparisons confirmed that vapor-exposed group showed escalated alcohol self-administration following aCSF treatment compared to baseline (F (1, 4) = 18.285, p ≤ 0.01) and that nor-BNI significantly attenuated alcohol self-administration compared to the escalated aCSF treatment condition in vapor-exposed animals (F (1, 4) = 35.374, p < 0.01).
Figure 6: Site-specific pharmacological validation of KOR dysregulation in the dorsolateral BNST during acute withdrawal in alcohol-dependence.
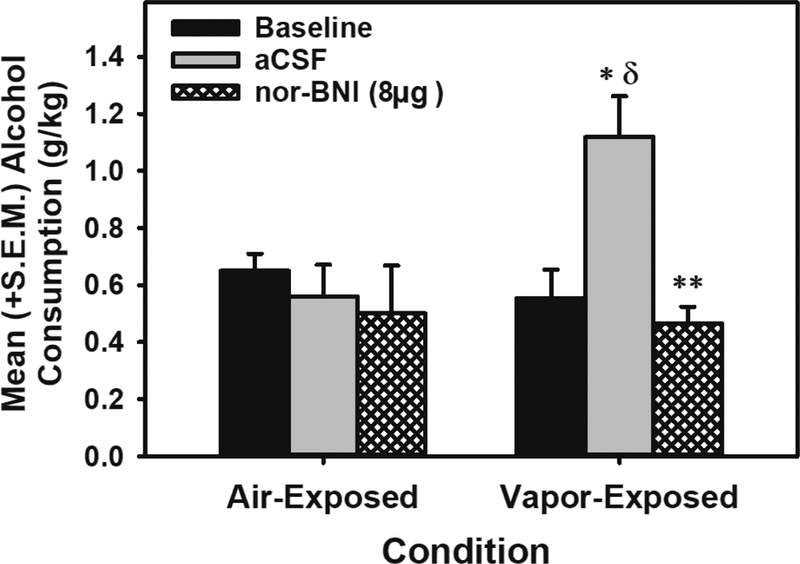
Mean (+S.E.M.) alcohol consumption (g/kg) during baseline, aCSF-treated or nor-BNI treatment in air- or EtOH vapor-exposed animals. During acute withdrawal, vapor-exposed animals displayed escalated alcohol self-administration under conditions of intra-dorsolateral BNST aCSF infusions (* = p<0.05 when compared to air-exposed aCSF-treated group; δ = p < 0.05 when compared to vapor-exposed baseline). The KOR antagonist nor-BNI (8.0 μg; n=5) attenuated vapor-induced escalation (** = p < 0.01), an effect not observed in air-exposed animals (n=6). aCSF = artificial cerebrospinal fluid.
4.4. Experiment 3: BNST KOR Antagonism and Physiological Withdrawal, EPM performance and 22-kHz USVs
Escalation of alcohol consumption: A paired sample t-test indicated that intermittent alcohol vapor exposure resulted in significantly escalated alcohol consumption following chronic intermittent vapor exposure when compared to pre-vapor baseline (t (10) = −2.87 p < 0.05 when comparing pre- and post-vapor alcohol (g/kg) self-administration). Test Day 1 - Physiological withdrawal and EPM: Independent univariate ANOVAs indicated that there was no change in physiological withdrawal scores or percent open-arm time, open-arm entries, closed-arm entries or distance traveled in the EPM during acute withdrawal when comparing aCSF to nor-BNI (F (1, 9) = 0.003 – 2.381, p > 0.05; see Figure 7).
Figure 7: Intra-dorsolateral BNST KOR antagonism on physiological withdrawal and anxiety-like behavior in the EPM.
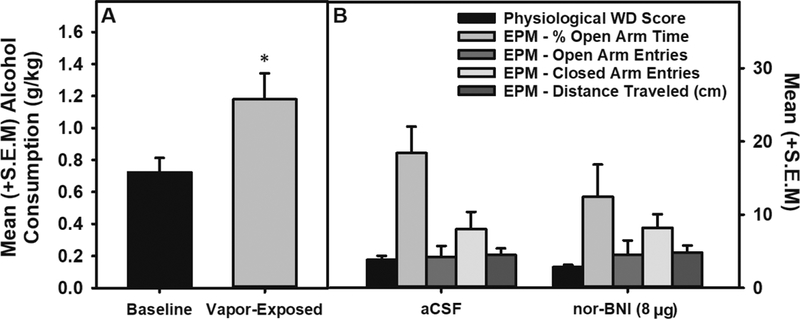
Panel A: Mean (+S.E.M.) alcohol consumption during acute withdrawal following EtOH vapor-exposure was escalated when compared to baseline consumption (n=11; * = p < 0.05). Panel B: Mean (+S.E.M.) physiological withdrawal scores and percent open-arm time, open-arm entries, closed-arm entries and distance traveled in the EPM were unaffected during acute withdrawal in vapor-exposed animals treated with aCSF (n=5) compared to those treated with nor-BNI (n=6).
Test Day 2 - Physiological withdrawal and 22-kHz USVs: Independent univariate ANOVAs indicated that there were main effects for the number of air-puffs to elicit a 22-kHz USV (F (1, 9) = 19.301, p < 0.01), as well as for number (F (1, 9) = 5.474, p < 0.05) and duration (F (1, 9) = 5.080, p ≤ 0.05) of 22-kHz USVs. Lastly, there was trend towards a reduction in physiological withdrawal scores (F (1, 9) = 4.072, p = .074; see Figure 8).
Figure 8: Intra-dorsolateral BNST KOR antagonism on physiological withdrawal and negative affective-like behavior.
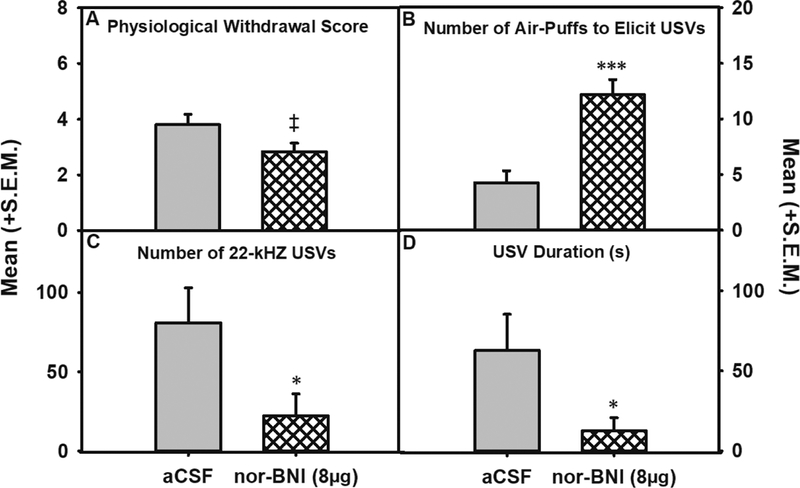
A trend towards a reduction in physiological withdrawal (Panel A) was observed following nor-BNI (‡ = p < 0.1) whereas nor-BNI significantly increased the number of air-puffs needed to elicit a 22-kHz USVs (Panel B; *** = p < 0.001) and decreased (* = p < 0.05) the number (Panel C) and duration (Panel D) of 22-kHz USV’s when comparing aCSF (n=5) and 8 μg nor-BNI (n = 6).
5. Discussion
The present results establish that in male Wistar rats demonstrating escalated alcohol self-administration during acute withdrawal following chronic intermittent alcohol vapor exposure, but not in animals without a self-administration history, Oprk1 mRNA expression is significantly upregulated in the BNST. Previous studies have found that the Oprk1 gene is associated with the risk for alcohol dependence in humans (Xuei et al. 2006; Edenberg et al. 2008). Differences in Oprk1 mRNA expression could lead to alterations in KOR density and activity. Consistent with upregulated Oprk1 expression contributing to maladaptive behavioral regulation (e.g., escalated alcohol self-administration) Oprk1 knockout (KO) mice show reduced alcohol intake (Kovacs et al. 2005). In humans, the Pdyn gene has been associated with the risk for alcohol dependence (Xuei et al. 2006; Karpyak et al. 2013) and the propensity to drink during negative emotional states (Karpyak et al. 2013), as well as shown alterations in regions of the prefrontal cortex in postmortem alcoholic brains when compared to non-alcoholic brains (Bazov et al. 2018). In the amygdala, an upregulation in Pdyn expression has been observed following short-term repeated intragastric alcohol administration in rats (D’Addario et al. 2013), however such changes appear to be transient. Although we have previously observed alterations in DYN A-like peptide expression, as well as increased KOR function in other regions of the extended amygdala (i.e, the CeA) in alcohol-dependent rats (Kissler et al. 2014), we did not observe consistent changes in BNST Pdyn expression levels during acute withdrawal in alcohol vapor-exposed animals in the present experiment.
An interesting caveat of Experiment 1 was the observation that only self-administering animals that were alcohol dependent showed increased Oprk1 mRNA expression during acute withdrawal. The behavioral and neurobiological ramifications of experimenter-delivered and self-administered drugs of abuse have been investigated previously (e.g., Dworkin et al. 1995; Mark et al. 1999; Stefanski et al. 1999; Robinson et al. 2002) using standard operant procedures and shown distinct differences in their effects. Further investigations into the appetitive and consummatory aspects of alcohol consumption have identified the importance of an appetitive, but not consummatory, component in alcohol self-administration (Walker and Ehlers 2009) that is consistent with the current results showing that Oprk1 mRNA is increased during acute withdrawal only in animals with a history of both alcohol self-administration and chronic intermittent alcohol vapor exposure. Given that we did not observe altered Oprk1 mRNA expression in air-exposed animals with a history of self-administration or in animals without a history of alcohol self-administration that were vapor-exposed, the collective evidence from Experiment 1 suggests that the ideal approach to investigate dysregulation of DYN / KOR systems in alcohol dependence is with the inclusion of a self-administration component.
KORs have been shown to pre- and post-synaptically modulate multiple neurotransmitter systems that that include DA, GABA, glutamate and serotonin, (Werling et al. 1988; Thompson et al. 2000; Hjelmstad and Fields 2003; Margolis et al. 2003; Margolis et al. 2006; Grilli et al. 2009; Land et al. 2009; Kang-Park et al. 2013; Kallupi et al. 2013; Tejeda et al. 2013; Gilpin et al. 2014; Tejeda et al. 2015; Karkhanis et al. 2016a; Siciliano et al. 2016), however, other than mesocortical DA originating from the ventral tegmental area (Margolis et al. 2003; Margolis et al. 2006), KOR actions are typically presynaptic in nature. While KORs have been shown to presynaptically inhibit GABA transmission and glutamatergic transmission in the BNST from projections originating in the CeA and BLA, respectively (Li et al. 2012; Crowley et al. 2016), it is unclear whether KORs also modulate local GABAergic interneurons in the BNST. Therefore, it is currently unknown whether the increased Oprk1 mRNA expression observed in the present study translates into local BNST effects or whether the increased expression occurs in BNST projection neurons (e.g., glutmatergic efferents; Spanagel 2009) that have been implicated in symptoms of alcohol dependence.
Given that Oprk1 mRNA expression was upregulated in the BNST during acute withdrawal exclusively in alcohol self-administering animals, a second objective of this study was to determine whether KOR-mediated dysregulation of BNST function contributes to escalated alcohol self-administration in alcohol-dependent rats during acute withdrawal. Compared to aCSF control infusions, site-specific pharmacological infusions of nor-BNI in the BNST of alcohol-dependent rats attenuated escalated self-administration during acute withdrawal. In non-dependent air-exposed animals that self-administered alcohol, there was no change in alcohol drinking behavior between baseline and post-exposure intra-BNST aCSF or nor-BNI infusions. These results are consistent with previous data showing increased KOR function in the CeA contributes to escalated alcohol self-administration during acute withdrawal that can be blocked by nor-BNI (Kissler et al. 2014) an effect also observed in the AcbSh (Nealey et al. 2011) that was also specific to those animals that were alcohol-dependent. Conversely, the N/OFQ receptor (NOP) shows reduced function in the CeA of Marchigian Sardinian alcohol-preferring (msP) rats (Economidou et al. 2008) and NOP agonists can suppress escalated alcohol consumption in alcohol-preferring and alcohol-dependent rats (e.g., Economidou et al. 2008; de Guglielmo et al. 2015), although such an effect was only observed in the CeA, but not the BNST, of msP rats (Economidou et al. 2008). However, given that NOP antagonists are also implicated as a potential therapeutic to treat AUDs (e.g., Rorick-Kehn et al. 2016; Post et al. 2016), the precise role of the N/OFQ in AUDs remains to be clarified. The present results are also supported by KOR system activation using the KOR agonist U50,488 or cues associated with U50,488 that can induce increased alcohol consumption in non-dependent animals (Anderson et al. 2016; Berger et al. 2013), as well as other manipulations that can increase KOR function, such as isolate-housing during adolescence, that also serve to increase alcohol consumption (Karkhanis et al. 2016b; Rose et al. 2016). In contrast to the present results is the interesting finding that in pre-adolescent rats (PND 14) subjected to low prenatal alcohol exposure, there was decreased KOR expression within extended amygdala circuitry (i.e., amygdala and NAc) and the HPC (Nizhnikov et al. 2014) that was accompanied by the demonstration that a KOR agonist could produce preferences whereas a MOR agonist could not. Generally, in adult animals, KOR agonists produce place aversions, whereas MOR agonists produce place preferences (e.g., Shippenberg and Herz 1986). This apparent discrepancy is potentially explained by the fact that there seems to be a developmental shift in KOR-mediated effects in which KOR activation is appetitive in young, pre-adolescent animals (Petrov et al. 2006) and aversive in mature animals (e.g., Shippenberg and Herz 1986; Land et al. 2008; Berger et al. 2013). Although not evaluated in the current study, intermittent alcohol vapor exposure does not appear to impact saccharin self-administration (O’Dell et al. 2004) and we have previously shown that within the extended amygdala, intra-CeA nor-BNI has no effect on saccharin self-administration (Kissler et al., 2014).
It is possible that dysregulation of the KOR/DYN system, and as a result, escalated alcohol consumption, is in part related to altered GABAergic neurotransmitter signaling between the three brain regions comprising the extended amygdala (e.g., Roberto et al. 2004). Neurons expressing dynorphin in the CeA send projections to the BNST (Marchant et al. 2007) and KOR activation in the BNST inhibits GABA transmission from the CeA (Li et al. 2012). Dampened GABA transmission and increased KOR activation are inherent characteristics of anxiety disorders and in the BNST, KOR activation can disinhibit glutamatergic neurotransmission that has been shown to be important in alcohol dependence and withdrawal (Spanagel, 2009; Li et al. 2012). More recent evidence has also shown that BNST KORs can presynaptically inhibit glutamatergic transmission originating in the BLA that contributes to an anxiogenic profile (Crowley et al. 2016). Therefore, while the present data clearly indicate that intra-BNST KOR antagonism can ameliorate escalated alcohol self-administration during acute withdrawal, it is unknown whether the effect occurs via blockade of KORs located on local interneurons or KORs that modulate afferents to the BNST. Several observations also indicate that corticotrophin releasing factor (CRF) contributes to the development of alcohol dependence and there are established CRF/KOR interactions (e.g., Land et al. 2008; Valdez et al. 2007). Upregulated CRF mRNA expression in nuclei of the extended amygdala in msP alcohol-preferring rats that translated into increased CRF receptors and was shown to be important for msP rat alcohol self-administration, but not unselected rat line alcohol consumption (Hansson et al. 2006) and interestingly, the upregulated CRF mRNA expression in msP rats was attenuated following ad libitum access to alcohol in extended amygdala nuclei (Hansson et al. 2007). Furthermore, CRF mRNA expression within the extended amygdala is upregulated in alcohol-dependent rats during withdrawal (Sommer et al. 2008) and increased CRF levels have been observed in the CeA and BNST during withdrawal from alcohol following the establishment of alcohol dependence (Funk et al. 2006; Olive et al. 2002; Roberto et al. 2010). Further evidence shows that reinstatement of drug-seeking behavior via activation of KOR’s is mediated by CRF and norepinephrine signaling in the CeA and BNST (Shaham et al. 2003; Shalev et al. 2002). Given that nor-BNI reduced alcohol consumption exclusively in vapor exposed animals, the current results support the hypothesis that enhanced KOR signaling within the BNST of dependent animals drives escalated alcohol consumption.
There are certain properties of nor-BNI that should be mentioned in relation to pretreatment times and the specificity of nor-BNI for the KOR immediately following administration. Evidence from mice tested in nociceptive assays has suggested that nor-BNI has mild affinity for the MOR immediately after administration that appears to last at least 2 h (Broadbear et al. 1994). Because of this, some researchers posit the use of extended pretreatment durations (e.g., 24-hrs). However, the transient MOR affinity of nor-BNI that has been observed in mice has not been replicated using rats (Picker et al. 1996). Furthermore, our own studies in male Wistar rats have confirmed that there are no observed differences in the effects of nor-BNI when administered immediately (i.e., 5-min), or 24 hours, prior to alcohol self-administration sessions in non-dependent and alcohol-dependent rats (Walker and Koob 2008; Walker et al. 2011; Nealey et al. 2011; Kissler et al. 2014; Kissler and Walker 2016). Most importantly, if nor-BNI did have MOR affinity that is functionally relevant when assessing motivational circuitry and behaviors, then one would predict that non-dependent alcohol self-administration should also be impacted as we have previously shown using antagonists with a specific MOR mechanism of action (Nealey et al. 2011; Kissler et al. 2014), an effect that was not observed in the present experiment. Thus, under the conditions used in the present study, there were no behavioral indications of an initial MOR mechanism of action for the KOR antagonist nor-BNI when administered immediately prior to testing.
Given the proposed role of the BNST in withdrawal and dependence (Aston Jones et al 1999; Kash and Winder 2006), there is a potential for either negative affective states or physiological withdrawal to contribute to excessive alcohol consumption during acute withdrawal. In rodents, withdrawal from alcohol is marked by aversive physiological symptoms as well as motivational, affective, and cognitive deficits (Williams et al. 2012). In addition to physiological withdrawal assessment, withdrawal-induced negative affect was confirmed in animals displaying escalated self-administration during acute withdrawal by measurement of 22-kHz USVs following intra-BNST aCSF or nor-BNI infusions. Rat 22-kHz ultrasonic vocalizations (USVs) provide a noninvasive means of characterizing affective states in rat models of drug dependence, with 22-kHz calls emitted during aversive states such as withdrawal from alcohol and opiates (Williams et al. 2012). Following two weeks of alcohol vapor exposure, intracerebroventricular nor-BNI has been shown to dose-dependently ameliorate the production of 22-kHz USVs indicative of negative affect (Berger et al. 2013) and 22-kHz USVs are increased in animals that have upregulated DYN A-like immunoreactivity and increased KOR function in the CeA (Kissler et al. 2014).
In the present study, acute administration of nor-BNI produced no significant change in EPM percent open arm time, open- or closed-arm entries, distance traveled or physiological symptoms of withdrawal. Although a lack of nor-BNI effect on percent open-arm time was unexpected given the role of the BNST in anxiety-like behavior, it has been noted that certain compounds common to treating anxiety and negative affect do not always lead to stable anxiolytic effects (Carobrez and Bertoglio 2005). In a recent study evaluating the role of KORs in BLA to BNST circuitry, it was shown that genetic ablation of KORs in amygdalar neurons projecting to the BNST produced an increase in open arm time in the EPM (Crowley et al. 2016), which is inconsistent with the present results. Numerous factors could contribute to this disparity, such as species differences, neuroanatomical specificity of genetic vs pharmacological manipulations, as well as the presence of alcohol dependence. In the present study, the percent open arm time displayed by the alcohol vapor-exposed animals during acute withdrawal was comparable to levels we have previously reported (Williams et al. 2012). The fact that nor-BNI produced no change in open- or closed-arm entries, as well as no change in distance traveled supports that nor-BNI attenuation of dependence-induced behaviors (i.e., escalated alcohol self-administration and 22-kHz USVs) is not confounded by locomotor effects. However, after infusion of an intra-BNST dose of nor-BNI, there was a significant increase in the number of air puffs needed to elicit 22-kHz USVs and a significant reduction in the number and duration of 22-kHz USVs produced during acute withdrawal in dependent animals. Therefore, the present data suggest that measurement of 22-kHz USVs may be a more sensitive index of negative affective-like behavior than other tests such as the EPM; a concept that was previously shown by an earlier formation of withdrawal-induced increases in 22-kHZ USVs compared to reduced open-arm time in the EPM following different lengths of alcohol vapor exposure to induce dependence (Williams et al. 2012). In the present experiment, we included an additional parameter to those previously utilized by our laboratory, namely number of air-puffs required to elicit a 22-kHz vocalization. This additional measure appeared to be a more sensitive metric for USV assessment than either USV number or duration and should contribute the assessment of negative affective-like behavior in future studies. These data show that in the BNST there is a dysregulation of the DYN/KOR system that promotes maladaptive behavioral regulation that can be rescued by KOR antagonist treatment. It is important to note that due to nor-BNI’s long duration of action, some might consider it to be unsuitable for clinical development, but more recently-developed short-acting antagonists need to be site-specifically evaluated in pre-clinical dependence models as they have been in AUD models (Domi et al. 2018).
The present data suggest that in the BNST, KOR antagonist efficacy for reducing escalated self-administration primarily stems from KOR antagonist effects on negative affective-like behavior and less so on aversive physiological withdrawal as evidenced by the nor-BNI-induced increase in the number of air-puffs required to elicit 22-kHZ USVs concomitant with reductions in escalated alcohol self-administration in vapor-exposed animals that did not reliably alter physiological withdrawal symptoms (although there was a trend towards a reduction). This dissociation between nor-BNIs efficacy for altering self-administration and physiological withdrawal has been reported previously in the CeA (Kissler and Walker, 2016), although in this particular situation, there was a trend towards a reduction of physiological withdrawal that could be evaluated in future experiments. Nor-BNI has shown efficacy in reducing physiological withdrawal signs following nicotine withdrawal (Tejeda et al. 2012). However, withdrawal signs in animals treated with nicotine and alcohol could be different as nicotine has stimulant affects. Additionally, the dose of nor-BNI that blocked spontaneous withdrawal signs was administered subcutaneously, versus site-specifically in the present study, and peripherally-administered nor-BNI could have impacted spinal KORs to produce an antinociceptive effect. KOR’s are widely expressed across the central nervous system as well as peripheral tissues and peripheral KOR antagonist actions could have contributed to a reduction in physiological withdrawal behavior and a possible explanation as to why an intra-BNST infusion only showed a trend towards reducing physiological symptoms of withdrawal. Nonetheless, the present data are consistent with a dissociation between physiological withdrawal and escalated alcohol self-administration in the extended amygdala that has also been observed in the CeA (Kissler and Walker, 2016). Studies have confirmed that after most acute physiological symptoms of alcohol dependence have subsided, the psychological discomfort of anxiety and depression experienced during abstinence plays a more important role in relapse and continued excessive alcohol use (Koob 2003; Driessen et al. 2001). The present data indicate that during the earliest phases of acute withdrawal, KOR antagonist mediation of maladaptive behavioral regulation is primarily within a negative affective domain. Given that the current study focused on the acute withdrawal phase of alcohol dependence, an important topic for future research is whether BNST KOR dysregulation persists into protracted abstinence, as has been initially reported in the CeA (Kissler and Walker, 2016), through systematic evaluations of the temporal characteristics of KOR-mediated neurobiological and behavioral dysregulation.
Previous studies investigating the role of DYN/KOR’s in alcohol dependence have primarily focused on the CeA and AcbSh, the present data reveals an important role of dorsolateral BNST dysregulation in acute withdrawal following dependence. More recent studies have also begun to highlight the BNST KOR system as contributing to an amygdalar anxiety circuit and reinstatement of alcohol seeking (Crowley et al. 2016; Le et al. 2017), but given the differential nature of the dorsal vs ventral BNST (e.g., Crestani et al. 2013) further research is still needed to elucidate the relative contributions of dorsal vs ventral BNST KORs in alcohol dependence-induced symptoms.
6. Conclusions
In the current study, Oprk1 mRNA expression was upregulated in the BNST of alcohol self-administering animals once alcohol dependent and in acute withdrawal. Furthermore, intradorsolateral BNST KOR antagonism attenuated escalated alcohol self-administration during acute withdrawal that was concomitant with KOR antagonist-induced amelioration of withdrawal-induced 22-kHz USVs. These data highlight KOR/DYN system dysregulation in the BNST as a driver of motivational and affective deficits in alcohol dependence. These findings supply evidence for the therapeutic potential of targeting the KOR through pharmacological and emerging gene therapies to assist individuals suffering from AUD’s.
Supplementary Material
Highlights:
Oprk1 mRNA expression was increased in the BNST of alcohol self-administering dependent animals
BNST KOR antagonism ameliorated escalated alcohol self-administration during acute withdrawal
BNST KOR antagonism did not reliably alter symptoms of physiological withdrawal
BNST KOR antagonism attenuated withdrawal-induced negative affective-like behavior
22-kHz USVs may be a more sensitive index of negative affective-like behavior than EPM performance
Acknowledgements
The authors would like to thank WSU Psychology and Integrative Physiology and Neuroscience Departments, as well as the WSU Honors College for assistance with the completion of this project. The authors also thank members of the Laboratory of Alcoholism and Addiction Neuroscience for their assistance and the vivarium staff for their continued support.
Funding and Declaration of Interest
The authors declare no conflict of interest. Support for this research was provided in part by R01AA020394 from the National Institute on Alcohol Abuse and Alcoholism (BMW) and grants from the WSU Alcohol and Drug Abuse Research Program according to the State of Washington Initiative Measure No. 17, the WSU Honors College and the WSU Psychology Department awarded to BMW and CME. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington.
Abbreviations
- BNST
bed nucleus of the stria terminalis
- DYN
dynorphin
- KOR
kappa-opioid receptor
- MOR
mu-opioid receptor
- nor-BNI
nor-binaltorphimine
- Oprk1
KOR gene
- Pdyn
prodynorphin
- USV
ultrasonic vocalization
- SA
self administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Hasani R, McCall JG, Foshage AM, Bruchas MR (2013). Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology 38: 2484–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alheid GF, Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27: 1–39. [DOI] [PubMed] [Google Scholar]
- 3.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 4.Aziz AM, Brothers S, Sartor G, Holm L, Heilig M, Wahlestedt C, et al. (2016). The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacology (Berl) 233: 3553–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazov I, Sarkisyan D, Kononenko O, Watanabe H, Karpyak VM, Yakovleva T, et al. (2018). Downregulation of the neuronal opioid gene expression concomitantly with neuronal decline in dorsolateral prefrontal cortex of human alcoholics. Transl Psychiatry 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger AL, Williams AM, McGinnis MM, Walker BM (2013). Affective Cue-Induced Escalation of Alcohol Self-Administration and Increased 22-kHz Ultrasonic Vocalizations during Alcohol Withdrawal: Role of Kappa-Opioid Receptors. Neuropsychopharmacology 38: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH (1994). Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 115: 311–319. [DOI] [PubMed] [Google Scholar]
- 8.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, et al. (2007). Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27: 11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. (2006). Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316: 440–447. [DOI] [PubMed] [Google Scholar]
- 10.Carobrez AP, Bertoglio LJ (2005). Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29: 1193–1205. [DOI] [PubMed] [Google Scholar]
- 11.Chavkin C, James IF, Goldstein A (1982). Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215: 413–415. [DOI] [PubMed] [Google Scholar]
- 12.Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M (1999). Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 141: 220–224. [DOI] [PubMed] [Google Scholar]
- 13.Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP (2013). Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol 11: 141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, et al. (2016). Dynorphin Controls the Gain of an Amygdalar Anxiety Circuit. Cell Rep 14: 2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Addario C, Caputi FF, Rimondini R, Gandolfi O, Del BE, Candeletti S, et al. (2013). Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol 18: 425–433. [DOI] [PubMed] [Google Scholar]
- 16.de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F (2015). MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol 20: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domi E, Barbier E, Augier E, Augier G, Gehlert D, Barchiesi R, et al. (2018). Preclinical evaluation of the kappa-opioid receptor antagonist CERC-501 as a candidate therapeutic for alcohol use disorders. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K (2001). The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol 36: 249–255. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin SI, Mirkis S, Smith JE (1995). Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 117: 262–266. [DOI] [PubMed] [Google Scholar]
- 20.Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, et al. (2008). Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry 64: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edenberg HJ, Wang J, Tian H, Pochareddy S, Xuei X, Wetherill L, et al. (2008). A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum Mol Genet 17: 1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funk CK, O’Dell LE, Crawford EF, Koob GF (2006). Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26: 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilpin NW, Roberto M, Koob GF, Schweitzer P (2014). Kappa opioid receptor activation decreases inhibitory transmission and antagonizes alcohol effects in rat central amygdala. Neuropharmacology 77: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN (2009). Operant Behavior and Alcohol Levels in Blood and Brain of Alcohol-Dependent Rats. Alcohol Clin Exp Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grilli M, Neri E, Zappettini S, Massa F, Bisio A, Romussi G, et al. (2009). Salvinorin A exerts opposite presynaptic controls on neurotransmitter exocytosis from mouse brain nerve terminals. Neuropharmacology 57: 523–530. [DOI] [PubMed] [Google Scholar]
- 26.Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M (2007). Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol 12: 30–34. [DOI] [PubMed] [Google Scholar]
- 27.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, et al. (2006). Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A 103: 15236–15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hjelmstad GO, Fields HL (2003). Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol 89: 2389–2395. [DOI] [PubMed] [Google Scholar]
- 29.Kallupi M, Wee S, Edwards S, Whitfield TW Jr., Oleata CS, Luu G, et al. (2013). Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biol Psychiatry 74: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD (2013). kappa-Opioid receptors in the central amygdala regulate ethanol actions at presynaptic GABAergic sites. J Pharmacol Exp Ther 346: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karkhanis AN, Huggins KN, Rose JH, Jones SR (2016a). Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacology 110: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karkhanis AN, Rose JH, Weiner JL, Jones SR (2016b). Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology 41: 2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karpyak VM, Winham SJ, Preuss UW, Zill P, Cunningham JM, Walker DL, et al. (2013). Association of the PDYN gene with alcohol dependence and the propensity to drink in negative emotional states. Int J Neuropsychopharmacol 16: 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kash TL, Winder DG (2006). Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology 51: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 35.Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, et al. (2014). The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry 75: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kissler JL, Walker BM (2016). Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala kappa-Opioid Receptors. Neuropsychopharmacology 41: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knapp DJ, Duncan GE, Crews FT, Breese GR (1998). Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res 22: 481–493. [PubMed] [Google Scholar]
- 38.Knapp DJ, Pohorecky LA (1995). An air-puff stimulus method for elicitation of ultrasonic vocalizations in rats. J Neurosci Methods 62: 1–5. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF (2003). Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27: 232–243. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs KM, Szakall I, O’Brien D, Wang R, Vinod KY, Saito M, et al. (2005). Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res 29: 730–738. [DOI] [PubMed] [Google Scholar]
- 41.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. (2009). Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A 106: 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le AD, Funk D, Coen K, Tamadon S, Shaham Y (2017). Role of kappa-Opioid Receptors in the Bed Nucleus of Stria Terminalis in Reinstatement of Alcohol Seeking. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, et al. (2012). Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry 71: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Logrip ML, Janak PH, Ron D (2008). Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J 22: 2393–2404. [DOI] [PubMed] [Google Scholar]
- 46.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1987). Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7: 2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 47.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1988). Anatomy of CNS opioid receptors. Trends Neurosci 11: 308–314. [DOI] [PubMed] [Google Scholar]
- 48.Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2003). Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 23: 9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL (2006). Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A 103: 2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C (2006). A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res 30: 982–990. [DOI] [PubMed] [Google Scholar]
- 51.Marinelli PW, Quirion R, Gianoulakis C (2003). A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 169: 60–67. [DOI] [PubMed] [Google Scholar]
- 52.Mark GP, Hajnal A, Kinney AE, Keys AS (1999). Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 143: 47–53. [DOI] [PubMed] [Google Scholar]
- 53.Markou A, Kosten TR, Koob GF (1998). Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18: 135–174. [DOI] [PubMed] [Google Scholar]
- 54.Mucha RF, Herz A (1985). Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 86: 274–280. [DOI] [PubMed] [Google Scholar]
- 55.National Research Council, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, National Academies Press (2011): Guide for the care and use of laboratory animals. National Academies Press: Washington, D.C. [Google Scholar]
- 56.Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM (2011). kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nizhnikov ME, Pautassi RM, Carter JM, Landin JD, Varlinskaya EI, Bordner KA, et al. (2014). Brief prenatal ethanol exposure alters behavioral sensitivity to the kappa opioid receptor agonist (U62,066E) and antagonist (Nor-BNI) and reduces kappa opioid receptor expression. Alcohol Clin Exp Res 38: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 58.O’Dell LE, Roberts AJ, Smith RT, Koob GF (2004). Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28: 1676–1682. [DOI] [PubMed] [Google Scholar]
- 59.Olive MF, Koenig HN, Nannini MA, Hodge CW (2002). Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav 72: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrov ES, Nizhnikov ME, Varlinskaya EI, Spear NE (2006). Dynorphin A (1–13) and responsiveness of the newborn rat to a surrogate nipple: immediate behavioral consequences and reinforcement effects in conditioning. Behav Brain Res 170: 1–14. [DOI] [PubMed] [Google Scholar]
- 61.Picker MJ, Mathewson C, Allen RM (1996). Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behav Pharmacol 7: 495–504. [PubMed] [Google Scholar]
- 62.Post A, Smart TS, Jackson K, Mann J, Mohs R, Rorick-Kehn L, et al. (2016). Proof-of-Concept Study to Assess the Nociceptin Receptor Antagonist LY2940094 as a New Treatment for Alcohol Dependence. Alcohol Clin Exp Res 40: 1935–1944. [DOI] [PubMed] [Google Scholar]
- 63.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. (2010). Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry 67: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberto M, Gilpin NW, Siggins GR (2012). The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med 2: a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004). Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24: 10159–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robinson TE, Gorny G, Savage VR, Kolb B (2002). Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse 46: 271–279. [DOI] [PubMed] [Google Scholar]
- 67.Rorick-Kehn LM, Ciccocioppo R, Wong CJ, Witkin JM, Martinez-Grau MA, Stopponi S, et al. (2016). A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin Exp Res 40: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, et al. (2016). Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaham Y, Shalev U, Lu L, De WH, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20. [DOI] [PubMed] [Google Scholar]
- 70.Shalev U, Grimm JW, Shaham Y (2002). Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1–42. [DOI] [PubMed] [Google Scholar]
- 71.Shippenberg TS, Herz A (1986). Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr 75: 563–566. [PubMed] [Google Scholar]
- 72.Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, et al. (2016). Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology (Berl) 233: 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith AW, Nealey KA, Wright JW, Walker BM (2011). Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem 96: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. (2008). Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry 63: 139–145. [DOI] [PubMed] [Google Scholar]
- 75.Spanagel R (2009). Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev 89: 649–705. [DOI] [PubMed] [Google Scholar]
- 76.Spanagel R, Herz A, Shippenberg TS (1992). Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89: 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR (1999). Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol 371: 123–135. [DOI] [PubMed] [Google Scholar]
- 78.Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Backman CM, et al. (2013). Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38: 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O’Donnell P (2015). Prefrontal Cortical Kappa Opioid Receptors Attenuate Responses to Amygdala Inputs. Neuropsychopharmacology 10.1038/npp.2015.138 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tejeda HA, Natividad LA, Orfila JE, Torres OV, O’Dell LE (2012). Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl) 224: 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson AC, Zapata A, Justice JB, Jr., Vaughan RA, Sharpe LG, Shippenberg TS (2000). Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci 20: 9333–9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. (2012). Primer3--new capabilities and interfaces. Nucleic Acids Res 40: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD (2007). Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. Journal of Pharmacology and Experimental Therapeutics 323: 525–533. [DOI] [PubMed] [Google Scholar]
- 84.Vats ID, Dolt KS, Kumar K, Karar J, Nath M, Mohan A, et al. (2008). YFa, a chimeric opioid peptide, induces kappa-specific antinociception with no tolerance development during 6 days of chronic treatment. J Neurosci Res 86: 1599–1607. [DOI] [PubMed] [Google Scholar]
- 85.Walker BM, Ehlers CL (2009). Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci 123: 926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walker BM, Kissler JL (2013). Dissociable effects of kappa-opioid receptor activation on impulsive phenotypes in wistar rats. Neuropsychopharmacology 38: 2278–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker BM, Koob GF (2007). The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 31: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walker BM, Koob GF (2008). Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology 33: 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walker BM, Valdez GR, McLaughlin JP, Bakalkin G (2012). Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol 46: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walker BM, Zorrilla EP, Koob GF (2011). Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol 16: 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Werling LL, Frattali A, Portoghese PS, Takemori AE, Cox BM (1988). Kappa receptor regulation of dopamine release from striatum and cortex of rats and guinea pigs. J Pharmacol Exp Ther 246: 282–286. [PubMed] [Google Scholar]
- 92.Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, et al. (2012). The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berl) 223: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, et al. (2006). Association of the kappa-opioid system with alcohol dependence. Mol Psychiatry 11: 1016–1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


