Abstract
Objective:
Several studies report a cross-sectional association between metabolic syndrome and depression. Possibly, metabolic syndrome promotes onset or chronicity of depression. However, such a longitudinal link has not yet been confirmed. This study examines whether metabolic syndrome or its components are associated with onset and chronicity of depression.
Method:
Secondary analyses were performed on data from 823 participants (≥ 65 years of age) in the InCHIANTI study, a prospective, population-based cohort study of older persons. From 1998 to 2000, the study sample was randomly selected from the population registry of 2 sites in Italy using a multistage stratified sampling method. Baseline data collection consisted of a home interview and a medical evaluation at the study clinic. Follow-up for each participant occurred after 3 years and 6 years. Metabolic syndrome at baseline was defined as ≥ 3 of the following: abdominal obesity, high triglycerides, low high-density lipoprotein cholesterol, high blood pressure, and high fasting glucose. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression scale (CES-D) at baseline and after 3 and 6 years. Sample characteristics were compared between persons with and without depression at baseline using χ2 and t statistics. Logistic regression analyses were conducted separately in persons with and without depression at baseline to test whether metabolic syndrome at baseline could predict onset and chronicity of depression at follow-up.
Results:
At baseline, 235 persons had metabolic syndrome, and 168 were depressed (CES-D score ≥ 20). Among persons not depressed at baseline, 26.0% developed depression. Higher waist circumference increased the odds of depression onset (adjusted OR per SD increase = 1.28; 95% Cl, 1.05–1.56), but there was no association between other metabolic syndrome components and onset of depression. Among persons depressed at baseline, depression had a chronic character in 69.0% of persons without and 88.5% of persons with metabolic syndrome. Metabolic syndrome was associated with an almost 3-fold increase in the odds of chronicity of depression (adjusted OR = 2.66; 95% Cl, 1.01–7.00), with almost every metabolic syndrome component contributing to this association.
Conclusion:
In late life, waist circumference, but not metabolic syndrome, predicted onset of depression. Depressed persons with metabolic syndrome were more likely to have persistent or recurrent depression. The latter may suggest that depression with metabolic abnormalities, which could be labeled metabolic depression, identifies a chronic subtype of depression.
Depression and cardiovascular disease are leading causes of disease burden worldwide, and their relative impact on public health will continue to increase.1 Substantial evidence supports an association between these harmful disorders.2,3 Gaining knowledge on the underlying mechanisms of comorbidity of disease could direct prevention and treatment strategies. Over the past years, metabolic syndrome—a combination of cardiovascular risk factors, including abdominal obesity, lipid abnormalities, hypertension, and hyperglycemia4—has become a key topic of interest as a possible link between depression and cardiovascular disease.5
Recent cross-sectional studies6–14 indicate a link between depression and metabolic syndrome, although some negative findings15–17 have been reported as well. Insight into cause and consequence between these disorders is still very limited, as only a few studies18–23 have assessed the temporal direction of their association. Three studies21–23 of middle-aged persons demonstrated that depressive symptoms predicted onset of metabolic syndrome. However, to improve both prevention and treatment of depression, it is essential to know whether metabolic syndrome could initiate and/or perpetuate depression. Two studies18,19 in middle-aged populations and 1 study20 in an older population showed an increased risk of depressive symptom onset for persons with the metabolic syndrome. We have previously shown24 that abdominal obesity, a central component in metabolic syndrome, predicted the new onset of significant depressive symptoms over 5 years. Even stronger associations were found with the onset of more persistent depressive symptoms. This last finding suggests that metabolic syndrome might continue to promote depressive symptoms after they have emerged, resulting in more chronic depression. To our knowledge, no previous study has examined whether metabolic syndrome is a prognostic factor of chronicity of depression in older persons.
The aims of the present study were to examine in a community-based sample of older persons whether metabolic syndrome and its components were associated with (1) onset of depression in persons who were depression-free at baseline and (2) chronicity of depression in persons who were depressed at baseline.
METHOD
Study Sample
Secondary analyses were performed on data from participants in the InCHIANTI Study, a prospective, population-based cohort study of older persons. Information on obtaining the data from the InCHIANTI Study may be found at http://www.inchiantistudynet/bindex.html.) From 1998 to 2000, the study sample was randomly selected from the population registry of 2 sites in Italy, Greve in Chianti and Bagno a Ripoli, using a multistage stratified sampling method. Baseline data collection consisted of a home interview and a medical evaluation at the study clinic, which took place within 21 days after the home interview. Follow-up assessments were performed 3 and 6 years after baseline. The ethics committee of the Italian National Institute of Research and Care on Aging approved the study protocol, and all respondents signed informed consent after procedures were fully explained. A more detailed description of the study design is given elsewhere.25
The InCHIANTI Study consisted of 1,155 participants aged 65 years and older, but, because of missing data on metabolic syndrome (n = 54), depression at baseline (n = 4), or depression during follow-up (n = 274), the present study included 823 participants. Excluded persons were significantly older (80.1 vs 73.3 years; P<.001), less educated (4.4 vs 5.6 years; P<.001), and more likely to have depression at baseline (28.5% vs 20.4%; P=.007) than included persons. No significant baseline differences with regard to sex, number of chronic diseases, or metabolic syndrome were found between included and excluded persons.
Depression
Depressive symptoms were assessed at baseline and after 3 and 6 years of follow-up using the Center for Epidemiologic Studies Depression scale (CES-D),26 a widely used self-report scale to assess depressive symptoms in the past week. The original 20-item version, ranging from 0 to 60 points, was filled in during the home interview. The 20-item CES-D has been shown to be a valid instrument for identifying depression in community-dwelling older adults27 and in Italian persons.28 In our study, the internal consistency was high: Cronbach α = 0.82. Onset of depression was defined as having no depression at baseline (CES-D score < 20) but having a CES-D score ≥ 20 after 3 or 6 years of follow-up. Chronicity of depression was defined as having a CES-D score ≥ 20 both at baseline and after 3 or 6 years.
A cut-off score of 20 on the CES-D has been used before in older populations.13,29 Although a cut-off score of 16 on the CES-D is more traditionally used, other studies with older samples30,31 and Italian samples28 have suggested that a higher cut-off might be more ideal, and the cut-off score of 20 has been shown to have greater specificity compared with the cut-off score of 16.27 In our sample, the proportion of persons scoring ≥ 16 on the CES-D was 31.1%, while 20.4% scored ≥20. The latter cut-off therefore seems more appropriate considering previously reported prevalences of clinically relevant depressive symptoms in older populations.32 Although scoring above a depressive symptom cut-off score does not necessarily imply depression, in this article, for convenience, we will refer to a CES-D score ≥ 20 as depression. Also, we cannot be sure whether a depression was persistent or recurrent. For convenience, we refer to a CES-D score ≥ 20 at both baseline and follow-up as chronic depression because this repeated score does suggest a chronic character of the depression.
Metabolic Syndrome
Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III guidelines4 as having 3 or more of the following criteria: (1) waist circumference ≥102 cm in men or ≥ 88 cm in women; (2) triglyceride level ≥150 mg/dL; (3) high-density lipoprotein cholesterol < 40 mg/dL in men or < 50 mg/dL in women; (4) systolic/diastolic blood pressure ≥ 130/85 mm Hg and/or currently using antihypertensive medication; and (5) fasting glucose ≥110 mg/dL and/or currently using antidiabetic medication.4
Fasting serum glucose, high-density lipoprotein cholesterol, and triglycerides were measured by standard laboratory methods. Waist circumference was measured by trained examiners at the largest midbody point. Three blood pressure measurements were taken using a standard mercury sphygmomanometer with the respondent in a supine position; the last 2 readings were averaged. Drugs taken in the previous 2 weeks were identified and coded using the Anatomic Therapeutic Chemical classification system33 to ascertain antidiabetic and antihypertensive medication use. In addition to a dichotomous indicator of metabolic syndrome and a count of the number of criteria met, analyses were also conducted with continuous measures of metabolic syndrome components in order to investigate the consistency over and importance of individual components. To incorporate medication use into these continuous measures, as done previously,13,34 10 and 5 mm Hg were added to systolic and diastolic blood pressure, respectively, for persons using antihypertensive medication, since these values represent the average decline in blood pressure in antihypertensive medication trials.35,36 Similarly, persons who used antidiabetic medication and had a fasting glucose level below 126 mg/dL (the usual cut-off to identify diabetes) were given a value of 126 for fasting glucose, as done before.13
Covariates
Covariates were selected a priori on the basis of previously reported associations with both metabolic syndrome and depression. Sociodemographic factors included age, sex, and years of education. Lifestyle and health factors included baseline smoking status (nonsmoker/former smoker/current smoker), alcohol intake (< 3 or ≥ 3 drinks per day), physical activity in the previous 12 months (sedentary [completely inactive or light physical activity, eg, walking], light [light physical activity for 2−4 hours per week], or moderate to intense [light physical activity for more than 4 hours per week or moderate physical activity, eg, swimming]37), and number of chronic diseases (including diabetes, cardiovascular disease, cancer, liver disease, gastrointestinal disease, congestive heart failure, Parkinsons disease, peripheral arterial disease, lung disease, hip fracture, herniated disc, arthritis, osteoporosis, and cognitive deterioration). Cardiovascular disease and diabetes were also considered separately and were adjudicated on the basis of information from self-reported history, medical examination data, hospital discharge records, and medication use. Other diseases were self-reported only. In addition, onset of new cardiovascular events during follow-up was assessed on the basis of similarly adjudicated information.
Statistical Analyses
Sample characteristics were compared between persons with and without depression at baseline using χ2 and t test statistics. Logistic regression analyses were conducted separately in persons without and with depression at baseline to test whether metabolic syndrome at baseline could predict onset and chronicity of depression at follow-up, respectively. Analyses were performed unadjusted, adjusted for sociodemographics (age, sex, years of education), and additionally adjusted for health indicators (smoking status, alcohol intake, physical activity, number of chronic diseases).
As some previous studies23,38 showed sex differences in the association between metabolic syndrome and depression, a sex-by-metabolic syndrome interaction term was entered into the fully-adjusted logistic regression analyses to assess whether findings were consistent for men and women. To test consistency of associations across all metabolic syndrome components, a series of fully adjusted logistic regression analyses was conducted, with each metabolic syndrome component entered separately as a predictor. In addition, logistic regression analyses tested the associations between number of metabolic syndrome components, as an indication of severity of metabolic disturbances, and onset or chronicity of depression. Finally, fully adjusted linear mixed-model analyses examined the association between metabolic syndrome and continuous CES-D score at 3 time points (baseline, after 3 years, and after 6 years) separately in persons without and with depression at baseline. Metabolic syndrome, time point (1–3), and all covariates were entered as fixed factors; subjects were treated as a random factor; and a random intercept was estimated. To examine whether the course of depressive symptoms was different for persons with and without metabolic syndrome, we added a metabolic syndrome-by-time interaction term.
RESULTS
Mean age of the participants was 73.3 years (SD = 6.2 years), 55.9% were women, 28.6% had metabolic syndrome, and 20.4% were depressed at baseline. Table 1 shows baseline characteristics for persons without and with depression at baseline. Persons with baseline depression were somewhat older, were more often women, were less often a smoker, drank less alcohol, were less physically active, had more chronic diseases, and had lower glucose levels, but they tended more often to have metabolic syndrome. During the 6-year follow-up, 26.0% of the initially nondepressed persons (n = 655) experienced onset of depression, and 75.0% of the initially depressed persons (n=168) had chronic depression.
Table 1.
Sample Characteristics According to Depression at Baseline (N = 823)
| Characteristic | No Depression at Baseline (n = 655) |
Depression at Baseline (n = 168) |
P Valuea |
|---|---|---|---|
| Sociodemographics | |||
| Age, mean (SD), y | 72.7 (6.0) | 75.3 (6.3) | <.001 |
| Sex, women, % | 50.7 | 76.2 | <.001 |
| Education, mean (SD), y | 5.7 (3.2) | 5.3 (3.3) | .08 |
| Health indicators | |||
| Smoking status, % | <.001 | ||
| Nonsmoker | 54.2 | 72.6 | |
| Former smoker | 30.4 | 16.7 | |
| Current smoker | 15.4 | 10.7 | |
| Alcohol use, ≥ 3 drinks per day, % | 12.5 | 6.0 | .02 |
| Physical activity, % | <.001 | ||
| Sedentary | 10.2 | 32.7 | |
| Light | 46.3 | 42.3 | |
| Moderate to intense | 43.5 | 25.0 | |
| Baseline cardiovascular disease, % | 12.2 | 10.1 | .45 |
| Baseline diabetes, % | 12.7 | 10.1 | .37 |
| No. of chronic diseases, mean (SD) | 1.2 (1.0) | 1.5 (1.1) | .02 |
| New cardiovascular event during follow-up, % |
11.0 | 9.5 | .58 |
| Metabolic syndrome | |||
| Waist circumference, mean (SD), cm | 93.2 (9.9) | 92.0(10.7) | .16 |
| Triglycerides, mean (SD), mg/dL | 130.2 (74.1) | 125.4 (62.3) | .43 |
| High-density lipoprotein cholesterol, mean (SD), mg/dL |
55.9 (14.5) | 56.8 (14.6) | .48 |
| Systolic blood pressure, mean (SD), mm Hg |
153.7 (20.4) | 154.7 (20.1) | .57 |
| Diastolic blood pressure, mean (SD), mm Hg |
85.8 (9.1) | 86.5 (9.1) | .40 |
| Glucose, fasting, mean (SD), mg/dL | 97.2 (24.9) | 91.4 (21.4) | .007 |
| Metabolic syndrome, % | 27.2 | 33.9 | .09 |
| No. of metabolic syndrome components, mean (SD) |
1.9 (1.1) | 2.0 (1.1) | .35 |
| Depression | |||
| Depressed during follow-up, % | 26.0 | 75.0 | <.001 |
Based on χ2 test for dichotomous and categorical variables and independent t test for continuous variables.
Onset of depression was assessed in persons nondepressed at baseline (n = 655; 94.2% had CES-D data available at year 3, and 83.5% at year 6). Of those without metabolic syndrome, 24.2% had developed depression at follow-up, compared to 32.0% of those with metabolic syndrome. Table 2 describes the results of logistic regression analyses assessing the association between metabolic syndrome components and onset of depression. Although metabolic syndrome tended to be associated with an increased likelihood of depression onset in the unadjusted model (OR =1.40; 95% Cl, 0.96–2.06), after adjustment was made for sociodemographics, lifestyle, and chronic diseases, this association completely disappeared (OR = 1.01; 95% Cl, 0.66–1.54). No indication of a sex-by-metabolic syndrome interaction was found (Pinteraction - −64). As for metabolic syndrome components, waist circumference was associated with increased odds of depression onset during follow-up (OR per SD increase = 1.28; 95% Cl, 1.05–1.56). This association was not mediated by new cardiovascular events during follow-up (n = 72), as adjustment for this variable did not change the OR (OR per SD increase = 1.28; 95% Cl, 1.05–1.56; P = .01). When we excluded persons with diabetes or cardiovascular disease at baseline (n= 144), the association between waist circumference and depression onset remained (OR per SD increase = 1.46; 95% Cl, 1.15– 1.86; P=.002). None of the other components, nor their sum, resulted in increased risks of depression onset. When we excluded persons using antihypertensive medication (n = 253) and antidiabetic medication (n = 47), respective findings for blood pressure and glucose levels remained similarly absent (results not shown). To verify that these findings were not distorted by persons who merely crossed the cut-off score of 20 on the CES-D from baseline to follow-up, the above analyses were repeated among persons with a CES-D score <16 (instead of <20) at baseline (n = 567), such that onset of depression represented a relevant increase in depressive symptoms. This analysis did not change the findings importantly (eg, metabolic syndrome: fully adjusted OR =1.12; 95% Cl, 0.70–1.80; P=.64; and waist circumference: fully adjusted OR = 1.21; 95% Cl, 0.97–1.52; P=.10).
Table 2.
Association Between Metabolic Syndrome and Onset of Depression in Persons Who Were Not Depressed at Baseline (n = 655)
| Metabolic Syndrome | Onset of Depression, OR (95% CI)a |
P Value |
|---|---|---|
| Metabolic syndromeb | 1.40 (0.96–2.06) | .08 |
| Metabolic syndromec | 1.20 (0.80–1.78) | .38 |
| Metabolic syndromed | 1.01 (0.66–1.54) | .96 |
| Waist circumferenced | 1.28 (1.05–1.56) | .01 |
| Triglyceridesd | 1.07 (0.90–1.28) | .46 |
| High-density lipoprotein cholesterold | 0.95 (0.78–1.15) | .58 |
| Systolic blood pressured | 0.91 (0.75–1.10) | .34 |
| Diastolic blood pressured | 0.90 (0.74–1.08) | .25 |
| Fasting glucosed | 1.03 (0.86–1.24) | .76 |
| No. of metabolic syndrome componentsd | 1.01 (0.85–1.21) | .89 |
ORs are given per SD increase: waist circumference, SD = 10.0 cm; triglycerides, SD = 71.9 mg/dL; high-density lipoprotein cholesterol, SD = 14.5 mg/dL; systolic blood pressure, SD = 20.4 mm Hg; diastolic blood pressure, SD = 9.1 mm Hg; and fasting glucose, SD = 24.3 mg/dL.
Based on unadjusted logistic regression analyses.
Based on logistic regression analyses adjusted for age, sex, and years of education.
Based on logistic regression analyses additionally adjusted for smoking status, alcohol intake, physical activity, and number of chronic diseases.
Figure 1 shows adjusted mean CES-D scores over the follow-up period for persons with and without metabolic syndrome. Among persons nondepressed at baseline, both those with and those without metabolic syndrome had an increase in CES-D score over time in a similar pattern (time: P=.004; metabolic syndrome-by-time: P=.61). In line with analyses for depression onset, no overall effect of metabolic syndrome was found (P=.22).
Figure 1.
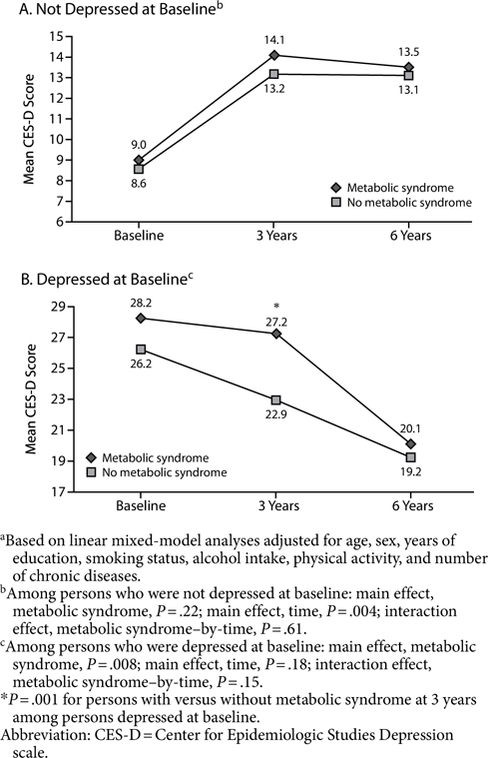
Adjusted Mean CES-D Scores Over the Follow-Up Period for (A) Persons Who Were Not Depressed at Baseline (n = 655) and (B) Persons Who Were Depressed at Baseline (n = 168)a
Chronicity of depression was assessed among persons depressed at baseline (n = 168; 95.8% and 73.8% had CES-D data available at year 3 and year 6, respectively). When metabolic syndrome was not present, 69.0% of persons experienced chronic depression compared to 88.5% when metabolic syndrome was present. Results of logistic regression analyses examining the association between metabolic syndrome components and chronicity of depression are presented in Table 3. Metabolic syndrome strongly predicted chronicity of depression (unadjusted OR = 3.29; 95% Cl, 1.36–7.98), even after adjustment for sociodemographics, lifestyle, and chronic diseases (OR = 2.66; 95% Cl, 1.01–7.00). Further adjustment for new cardiovascular events during follow-up (n = 16) did not change this finding (OR=2.66; 95% Cl, 1.01–6.99; P=.05). The association between metabolic syndrome and chronicity of depression was similarly present in both men and women (Pinteraction = −15). Exclusion of persons with diabetes or cardiovascular disease at baseline (n = 30) did not importantly affect findings (OR = 3.09; 95% Cl, 1.01–9.47; P=.05).
Table 3.
Association Between Metabolic Syndrome and Chronicity of Depression in Persons Who Were Depressed at Baseline (n = 168)
| Metabolic Syndrome | Chronicity of Depression, OR (95% CI)a |
P Value |
|---|---|---|
| Metabolic syndromeb | 3.29 (1.36–7.98) | .008 |
| Metabolic syndromec | 2.93 (1.18–7.26) | .02 |
| Metabolic syndromed | 2.66 (1.01–7.00) | .05 |
| Waist circumferenced | 1.35 (0.92–1.97) | .13 |
| Triglyceridesd | 1.67 (0.92–3.04) | .10 |
| High-density lipoprotein cholesterold | 0.69 (0.46–1.03) | .07 |
| Systolic blood pressured | 1.23 (0.82–1.85) | .32 |
| Diastolic blood pressured | 1.32 (0.89–1.94) | .17 |
| Fasting glucosed | 0.98 (0.61–1.58) | .92 |
| No. of metabolic syndrome componentsd | 1.62 (1.08–2.41) | .02 |
ORs are given per SD increase: waist circumference, SD = 10.0 cm; triglycerides, SD = 71.9 mg/dL; high-density lipoprotein cholesterol, SD = 14.5 mg/dL; systolic blood pressure, SD = 20.4 mm Hg; diastolic blood pressure, SD = 9.1 mm Hg; and fasting glucose, SD = 24.3 mg/dL.
Based on unadjusted logistic regression analyses.
Based on logistic regression analyses adjusted for age, sex, and years of education.
Based on logistic regression analyses additionally adjusted for smoking status, alcohol intake, physical activity, and number of chronic diseases.
No single metabolic syndrome component was specifically associated with chronicity of depression; all components, except glucose, showed an association with chronic depression in the expected direction, although not statistically significant. When we excluded persons using antihypertensive medication (n = 78) and antidiabetic medication (n = 9), respective findings for systolic blood pressure and glucose levels remained similar (results not shown). The OR per SD increase for diastolic blood pressure decreased to 1.12 (95% Cl, 0.61–2.08; P=.71). The odds of chronicity of depression increased by 62% for each additional component of metabolic syndrome a person had (OR= 1.62; 95% Cl, 1.08–2.41).
Figure 1 shows adjusted mean CES-D scores over the follow-up period for persons with and without metabolic syndrome. Among persons depressed at baseline, both those with and those without metabolic syndrome tended to show a decline in CES-D score over 6 years (time effect: P=.18). However, in line with analyses for depression chronicity (main effect, metabolic syndrome: P=.008), those with the metabolic syndrome continued to have high scores at the 3-year follow-up and declined only thereafter, in contrast to those without metabolic syndrome, who declined gradually from baseline onward. At the 3-year follow-up, CES-D scores of persons with metabolic syndrome were significantly higher than for persons without metabolic syndrome (27.2 vs 22.9, respectively; P=.001). The metabolic syndrome-by-time interaction showed a trend for significance (P=.15).
DISCUSSION
The present study, to our knowledge, is the first to examine both onset and chronicity of depression in relation to metabolic syndrome in a community-based sample of older persons. The results show that abdominal obesity, but not metabolic syndrome, predicts onset of depression. Once a person is depressed, metabolic syndrome increases the odds of that persons remaining depressed or having recurrent episodes by almost 3-fold. These results are indicative of the existence of a chronic depressive subtype associated with metabolic disturbances that could be labeled metabolic depression.
Onset of depression was predicted by large waist circumference, but not by metabolic syndrome and its other components. These results are not completely in line with 3 recent studies18–20 that did report a link between metabolic syndrome and new depression onset. Differences in sample and study characteristics (age range, general health status, duration of follow-up) might play a role in the found discrepancies. It is possible that, in this older population, those who have metabolic syndrome and are vulnerable for developing “metabolic depression” have already been affected, which might impede finding an association between metabolic syndrome and new depression onset. However, the specific association of waist circumference and depression is striking. Abdominal obesity is often regarded as a key component of the metabolic syndrome,39 and previous reports6,11,13,18 have suggested that waist circumference might be the most important metabolic syndrome feature in relation to depression. Our results are in agreement with these and other findings from an older population-based study,24 which showed that high levels of visceral fat are associated with onset of depressive symptoms. Moreover, results from that same study40 also indicated—in the opposite direction—an association between depressive symptoms and an increase in visceral fat over time, emphasizing that, in fact, a vicious cycle might exist between abdominal obesity and depression.
To our knowledge, it has not been examined before whether metabolic syndrome negatively affects depression prognosis. Our results clearly show that depression associated with metabolic syndrome is less likely to resolve. Although the rate of chronic depression in this older population was already high among persons without metabolic syndrome (about 70%), it was even noticeably higher among those with metabolic syndrome (about 90%). This finding was reflected in an almost 3-fold increased adjusted odds of persistent or recurrent depression, which strongly indicates the chronic character of depression in the presence of metabolic syndrome. More detailed analyses showed that the chronic character was mainly represented by a much later decline in depressive symptoms for persons with metabolic syndrome compared to those without.
These findings were not restricted to a specific metabolic abnormality. Although most metabolic syndrome components by themselves were not significantly associated with chronicity of depression, possibly due to limited power in the smaller depressed group, all of the metabolic syndrome components, except glucose, pointed in the direction of harmful disturbances. Moreover, the total count of metabolic syndrome components was strongly associated with chronicity of depression, indicating that almost every component contributed to heightened likelihood of a chronic depression. It is unclear why no effect for glucose was found, but previous studies6,11–13 have reported a lack of association between glucose levels and depressive symptoms. Nevertheless, our findings that metabolic disturbances are associated with a more chronic course of depression are in line with recent findings24 in older men showing that high visceral fat predicts onset of persistent depressive symptoms, even more strongly than onset of nonpersistent depressive symptoms. In addition, another recent study38 showed that men with metabolic syndrome were more likely to have experienced long-term depressive symptoms, although it was not examined whether metabolic syndrome was more strongly associated with longterm than with short-term depressive symptoms.
Considering both our results and earlier results, it seems that depression and metabolic syndrome are intertwined and represent a chronic depressive subtype. Depression and abdominal obesity might stimulate each others occurrence, but once both are present, multiple progressive metabolic abnormalities may arise, worsening the depression as well as the metabolic outcome more and more. These results are indicative of a distinct condition, which might be labeled metabolic depression. Metabolic abnormalities as a defining component in depressive disorders have been suggested before.41 The idea of a specific metabolic depressive subtype is further supported by a recent study42 that used data-driven techniques to identify depressive subtypes. Three depressive subtypes were distinguished, of which only 1, characterized by atypical symptoms, was associated with metabolic syndrome. The possible existence of a metabolic depression clearly opens up opportunities for prevention and treatment of both depression and metabolic syndrome. Recognizing that metabolic disturbances are hampering remission from depression raises the possibility that treating metabolic abnormalities could in fact stimulate remission.
What may underlie this interconnection between metabolic syndrome and chronic depression? Possible mechanisms include inflammatory processes, hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system dysfunction, and diminished functioning of the hypothalamic-pituitary-gonadal axis, as these have all been associated with both depression43–46 and metabolic syndrome.13,17,47–50 Further, the leptin hypothesis suggests that insufficiency or resistance of the adipose-derived hormone leptin forms a link between depression and obesity51 and might therefore also be involved in the relationship between depression and metabolic syndrome. Another possibility is the vascular depression hypothesis, which states that vascular damage in the brain might predispose to, precipitate, or perpetuate depression in the elderly.52 This vascular damage may be caused by the continuing influence of metabolic abnormalities, HPA-axis disturbances, autonomic nervous system dysfunction, or chronic low-grade inflammation. Our results show that metabolic syndrome and depression are also related in the absence of overt vascular disease, as adjustments for diabetes and intermediate cardiovascular events did not change our findings. Although subclinical vascular damage may be present, metabolic disturbances, while not yet causing profound vascular damage, might very well be sufficient on their own to produce depressive symptoms.
Our study has some important strengths. It prospectively examined the association between metabolic syndrome and depression. This study made use of a large community-based sample and was able to investigate both onset and chronicity of depression. Also, besides examining metabolic syndrome as one unified condition, association of depression with different metabolic syndrome components was explored as well.
Some limitations of the study have to be acknowledged, too. First, we used a self-report scale to assess depressive symptoms, which do not necessarily represent psychiatric diagnoses. However, the CES-D is a well-described and validated instrument in older populations.27,28 In addition, this study could not differentiate between recurrent and persistent depression, but the results do indicate a more chronic character of depression in persons who additionally have metabolic syndrome. Also, we had no information on history of depression; thus, some of the persons who were depression-free at baseline might have experienced depressive periods in the past.
To conclude, the results of the present study show that the central metabolic syndrome component, abdominal obesity, is an important risk factor for new onset of depression. Once a person is depressed, more widespread metabolic disturbances, such as present in metabolic syndrome, are highly indicative of persistent or recurrent depression. In concert with other research findings, our findings strongly indicate that depression with metabolic abnormalities, which could be labeled metabolic depression, identifies a chronic depressive subtype. It is yet to be examined whether treatment of metabolic disturbances could improve depression prognosis.
Clinical Points.
Depression with metabolic abnormalities, which could be labeled metabolic depression, may identify a chronic depressive subtype.
It is yet to be examined whether treatment of metabolic disturbances could improve depression prognosis.
Acknowledgments
Funding/support: These data analyses were supported through grant 1R01-HL972972 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland. The original InCHIANTI study was supported as a targeted project (ICS 110.1/RS97.71) by the Italian Ministry of Health and was supported in part by the US National Institute on Aging, Bethesda, Maryland (263 MD 916413 and 263 MD 821336).
Footnotes
Potential conflicts of interest: All authors report no conflicts of interest.
Previous presentation: Presented in part at the Gerontological Society of America 62nd Annual Scientific Meeting, November 18–22, 2009, Atlanta, Georgia.
Contributor Information
Nicole Vogelzangs, Department of Psychiatry and EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands.
Aartjan T. F. Beekman, Department of Psychiatry and EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands.
Ingrid G. Boelhouwer, Department of Psychiatry and EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands.
Stefania Bandinelli, Geriatric Rehabilitation, Azienda Sanitaria Firenze, Florence, Italy.
Yuri Milaneschi, Clinical Research Branch, National Institute on Aging, Baltimore, Maryland, Tuscany Health Regional Agency, Florence, Italy.
Luigi Ferrucci, Clinical Research Branch, National Institute on Aging, Baltimore, Maryland.
Brenda W. J. H. Penninx, Department of Psychiatry and EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands.
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3(ll):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006; 27(23):2763–2774. [DOI] [PubMed] [Google Scholar]
- 3.Van der Kooy K, van Hout H, Marwijk H, et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007;22(7):613–626. [DOI] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 5.Vitaliano PP, Scanlan JM, Zhang J, et al. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med 2002; 64(3):418–435. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar JA, Reddy P, Davis-Lameloise N, et al. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care 2008;31(12):2368–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil K, Radzillowicz P, Zdrojewski T, et al. Relationship between the prevalence of depressive symptoms and metabolic syndrome: results of the SOPKARD Project. KardiolPol 2006;64(5):464–469. [PubMed] [Google Scholar]
- 8.Kinder LS, Carnethon MR, Palaniappan LP, et al. Depression and the metabolic syndrome in young adults: findings from the Third National Health and Nutrition Examination Survey. Psychosom Med 2004;66(3):316–322. [DOI] [PubMed] [Google Scholar]
- 9.McCaffery JM, Niaura R, Todaro JF, et al. Depressive symptoms and metabolic risk in adult male twins enrolled in the National Heart, Lung, and Blood Institute twin study. Psychosom Med 2003;65(3):490–497. [DOI] [PubMed] [Google Scholar]
- 10.Skilton MR, Moulin P, Terra JL, et al. Associations between anxiety, depression, and the metabolic syndrome. Biol Psychiatry 2007;62(11):1251–1257. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi T, Nakao M, Nomura K, et al. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab 2009;35(1): 32–36. [DOI] [PubMed] [Google Scholar]
- 12.Vaccarino V, McClure C, Johnson BD, et al. Depression, the metabolic syndrome and cardiovascular risk. Psychosom Med 2008;70( 1):40–48. [DOI] [PubMed] [Google Scholar]
- 13.Vogelzangs N, Suthers K, Ferrucci L, et al. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology 2007;32(2): 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelzangs N, Beekman AT, Kritchevsky SB, et al. Psychosocial risk factors and the metabolic syndrome in elderly persons: findings from the Health, Aging and Body Composition study. J Gerontol A Biol Sei Med Sei 2007;62(5):563–569. [DOI] [PubMed] [Google Scholar]
- 15.Herva A, Räsänen P, Miettunen J, et al. Co-occurrence of metabolic syndrome with depression and anxiety in young adults: the Northern Finland 1966 Birth Cohort Study. Psychosom Med 2006;68(2):213–216. [DOI] [PubMed] [Google Scholar]
- 16.Hildrum B, Mykletun A, Midthjell K, et al. No association of depression and anxiety with the metabolic syndrome: the Norwegian HUNT Study. Acta Psychiatr Scand 2009;120(l):14–22. [DOI] [PubMed] [Google Scholar]
- 17.Vogelzangs N, Beekman AT, Dik MG, et al. Late-life depression, cortisol, and the metabolic syndrome. Am J Geriatr Psychiatry 2009; 17(8) :716–721. [DOI] [PubMed] [Google Scholar]
- 18.Akbaraly TN, Kivimäki M, Brunner EJ, et al. Association between metabolic syndrome and depressive symptoms in middle-aged adults: results from the Whitehall II study. Diabetes Care 2009;32(3):499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koponen H, Jokelainen J, Keinänen-Kiukaanniemi S, et al. Metabolic syndrome predisposes to depressive symptoms: a population-based 7-year follow-up study. J Clin Psychiatry 2008;69(2):178–182. [DOI] [PubMed] [Google Scholar]
- 20.Mast BT, Miles T, Penninx BW, et al. Vascular disease and future risk of depressive symptomatology in older adults: findings from the Health, Aging, and Body Composition Study. Biol Psychiatry 2008;64(4):320–326. [DOI] [PubMed] [Google Scholar]
- 21.Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med 2009;71(3):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care 2007;30(4): 872–877. [DOI] [PubMed] [Google Scholar]
- 23.Vanhala M, Jokelainen J, Keinänen-Kiukaanniemi S, et al. Depressive symptoms predispose females to metabolic syndrome: a 7-year follow-up study. Acta Psychiatr Scand 2009;119(2):137–142. [DOI] [PubMed] [Google Scholar]
- 24.Vogelzangs N, Kritchevsky SB, Beekman AT, et al. ; Health ABC Study. Obesity and onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J Clin Psychiatry 2010;71(4):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI Study. J Am Geriatr Soc 2000;48(12): 1618–1625. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1(3):385–401. [Google Scholar]
- 27.Beekman AT, Deeg DJ, Van Limbeek J, et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med 1997;27(1) :231–235. [DOI] [PubMed] [Google Scholar]
- 28.Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. J Clin Psychol 1983;39(2):249–251. [DOI] [PubMed] [Google Scholar]
- 29.Penninx BWJH, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA 1998; 279(21):1720–1726. [DOI] [PubMed] [Google Scholar]
- 30.Lyness JM, Noel TK, Cox C, et al. Screening for depression in elderly primary care patients: a comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med 1997;157(4):449–454. [PubMed] [Google Scholar]
- 31.Cheng ST, Chan AC. The Center for Epidemiologic Studies Depression Scale in older Chinese: thresholds for long and short forms. Int J Geriatr Psychiatry 2005;20(5):465–470. [DOI] [PubMed] [Google Scholar]
- 32.Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry 1999;174(4):307–311. [DOI] [PubMed] [Google Scholar]
- 33.WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical Classification Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 34.Licht CM, de Geus EJ, Seldenrijk A, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension 2009;53(4):631–638. [DOI] [PubMed] [Google Scholar]
- 35.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265(24):3255–3264. [PubMed] [Google Scholar]
- 36.Tannen RL, Weiner MG, Marcus SM. Simulation of the Syst-Eur randomized control trial using a primary care electronic medical record was feasible. J Clin Epidemiol 2006;59(3):254–264. [DOI] [PubMed] [Google Scholar]
- 37.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sei Sports Exerc 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 38.Viinamäki H, Heiskanen T, Lehto SM, et al. Association of depressive symptoms and metabolic syndrome in men. Acta Psychiatr Scand 2009; 120(1):23–29. [DOI] [PubMed] [Google Scholar]
- 39.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004;53(8):2087–2094. [DOI] [PubMed] [Google Scholar]
- 40.Vogelzangs N, Kritchevsky SB, Beekman AT, et al. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry 2008;65(12):1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntyre RS, Soczynska JK, Konarski JZ, et al. Should depressive syndromes be reclassified as “Metabolic Syndrome Type II”? Ann Clin Psychiatry 2007; 19(4):257–264. [DOI] [PubMed] [Google Scholar]
- 42.Lamers F, de Jonge P, Nolen WA, et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA) [published online ahead of print July 13,2010]. JClin Psychiatry 2010;71(12):1582–1589. [DOI] [PubMed] [Google Scholar]
- 43.Howren MB, Lamkin DM, Suis J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009;71 (2) : 171–186. [DOI] [PubMed] [Google Scholar]
- 44.Vreeburg SA, Hoogendijk WJ, van Pelt J, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 2009;66(6):617–626. [DOI] [PubMed] [Google Scholar]
- 45.Licht CM, de Geus EJ, Zitman FG, et al. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry 2008;65(12): 1358–1367. [DOI] [PubMed] [Google Scholar]
- 46.Morsink LF, Vogelzangs N, Nicklas BJ, et al. ; Health ABC Study. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC Study. Psychoneuroendocrinology 2007;32(8–10):874–883. [DOI] [PubMed] [Google Scholar]
- 47.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol 2009;20(3):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tentolouris N, Argyrakopoulou G, Katsilambros N. Perturbed autonomic nervous system function in metabolic syndrome. Neuromolecular Med 2008;10(3):169–178. [DOI] [PubMed] [Google Scholar]
- 49.Maggio M, Lauretani F, Céda GP, et al. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc 2006;54(12): 1832–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maggio M, Lauretani F, Céda GP, et al. Association of hormonal dysrégulation with metabolic syndrome in older women: data from the InCHIANTI study. Am J Physiol Endocrinol Metab 2007;292(1):E353–E358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 2007;7(6):648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression hypothesis. Arch Gen Psychiatry 1997;54(10):915–922. [DOI] [PubMed] [Google Scholar]


