Abstract
In this review, we present the growing literature suggesting, from a variety of angles, that the cerebellum contributes to higher-order cognitive functions, rather than simply sensorimotor functions, and more specifically to language and its development. The cerebellum’s association with language function is determined by the specific cortico-cerebellar connectivity to the right cerebellum from the left cortical hemisphere. The findings we review suggest that the cerebellum plays an important role as part of a broader language network, and also implies that the cerebellum may be a potential new therapeutic target to treat speech and language deficits, especially during development.
Keywords: cerebellum, language, speech, phonology, semantics, subtentorial
The cerebellum has been a mysterious structure for much of the history of neuroscientific inquiry, especially in the domain of language and higher-order cognition. Even Wernicke, in his earliest works on aphasia, conjectured about the function of the cerebellum with respect to language. However, despite this early interest, much of the research on cerebellar function focused on its role in the motor system (Baillieux, De Smet, Paquier, De Deyn, & Mariën, 2008). This changed significantly for the field of language research beginning about twenty-five years ago, and since then, interest in the “linguistic” cerebellum has continued to grow, with comprehensive reviews, special journal issues, and most recently an edited book (Mariën & Manto, 2016). In this paper, we review the cerebellum’s role in linguistic function, with a specific focus on development.
This review will highlight the cerebellum’s role in the broader language network, analyzing language function in both typical and atypical development, and the cortico-cerebellar connectivity that establishes this function. The organization of this review is guided by a developmental neuropsychological approach, where the topics of structure and function in relation to behavior will be the primary interest. We will be comprehensive and review work in animals, followed by human studies. The human studies will be organized first by examining typical populations (children and adults), followed by an analysis of disorders (both developmental and acquired). We conclude with a discussion of the possible mechanism of how the cerebellum might contribute to language processing and his development, and suggest directions for future research.
Cerebellar anatomy, structural, and functional connectivity in non-human primates and humans
In the cerebral cortex, there is a well-known cytoarchitecture and myeloarchitecture that differs across the cortical sheet. For example, the striate cortex of the calcarine fissure has a well-defined cortical layer IV such that, in histologically stained sections, it reveals a prominent “stripe.” The boundary where this prominent stripe ends defines the boundary of Brodmann Areas 17 and 18, or of primary and secondary visual cortex, which is defined by the region’s cytoarchitecture. This cortical heterogeneity of cellular and white matter morphology is a consistent feature across the entire cortical sheet, and has been used to define the functional boundaries of regions on the cortex (Brodmann 1909). In contrast, unlike the cerebral cortex, the cerebellar cortex has a consistent cytoarchitecture across its cortical sheet. Thus, the function of each region of the cerebellum is established by virtue of the connectivity that region has with the cerebral cortex, and not by any specific cyto- or myelo-architectonic signature.
This consistent architecture of the cerebellum is organized along three distinct cellular layers: a molecular layer, a layer of large Purkinje cells, and a compact layer of small granule cells. The molecular layer consists of two types of neurons, stellate cells and the basket cells located near the Purkinje cell bodies. The layer inferior to the molecular layer, the Purkinje layer, consists of a single layer of large Purkinje cells. Just beneath this is the granular layer, which consists of compact small granule cells, relatively large golgi cells, and a number of different glial cells. Due to the large amount of small granule cells, the cerebellum contains many more neurons than the cerebral cortex (~100 billion in the cerebellum compared to ~25 billion in the cerebral cortex; Andersen, Korbo, & Pakkenberg, 1992). This cytoarchitecture is consistent across the entire cerebellar cortex, and because of this it has been proposed that the cerebellum plays a similar functional role across cognitive and motor domains (i.e., it performs similar computations). However, as noted, the ultimate function of each cerebellar region is defined by its connectivity to different cortical and subcortical structures (Andersen et al., 1992; Naidich, Duvernoy, Delman, Sorensen, Kollias, & Haacke, 2009). Thus, sub-regions that connect to cortical language regions are likely to play a role in processing language within a broadly defined linguistic network.
This anatomical connectivity of the cerebellum with the cerebral cortex has been well-established in studies of both humans and non-human primates, and it is accomplished within a series of segregated cortico-cerebello-cortical loops consisting of the major afferent cortico-ponto-cerebellar pathways, and efferent cerebello-thalamo-cortical pathways (Naidich et al., 2009; Ramnani, 2006; Schmahmann & Pandya, 1995). Examination of the anatomical connectivity between the cerebellum and cortex provides a greater understanding of the cerebellum’s role in higher-order cognitive functions, such as language, and the precise regions of the cerebellum associated with different aspects of language.
Of course much of our understanding of cortico-cerebellar structural connectivity comes from research in non-human primates, and these provide a suggestion for the cerebellum’s involvement in higher cognitive functions. For example, results from tract-tracing studies in nonhuman primates reveal that projections from the prefrontal cortex have specific neuronal targets in regionally specialized sections of the cerebellar cortex. In particular, Crus II of the cerebellum receives projections from, and sends projections to, Area 46 of the primate prefrontal cortex that is involved in working memory (Naidich et al., 2009; Ramnani, 2006; Schmahmann & Pandya, 1995). Although non-human primates do not have language per se, this connectivity provides a phylogenetic foundation for the development of cerebello-cortical circuitry that might support higher-level cognition.
It is appropriate to be cautious though. Despite enthusiasm for what we can learn from non-human primate anatomy, not only do non-human primates not have language, but several studies have shown that the human cerebellum has undergone rapid expansion over the course of more recent evolutionary history. For example, a surface-based analysis comparing the cerebellum of a macaque monkey to a human’s cerebellum shows an expansion of the hemispheric cortex in the human, with the posterior lobe of the human showing more convolutions that are deeper than the macaque’s (Van Essen, 2002). A diffusion-weighted magnetic resonance imaging (MRI) study also noted this expansion in cortico-cerebellar connectivity. Thus, Ramnani et al (2006) reports that, in comparison to macaque monkeys, humans have an increased contribution from the prefrontal cortex to the pontine nuclei that target the cerebellum.
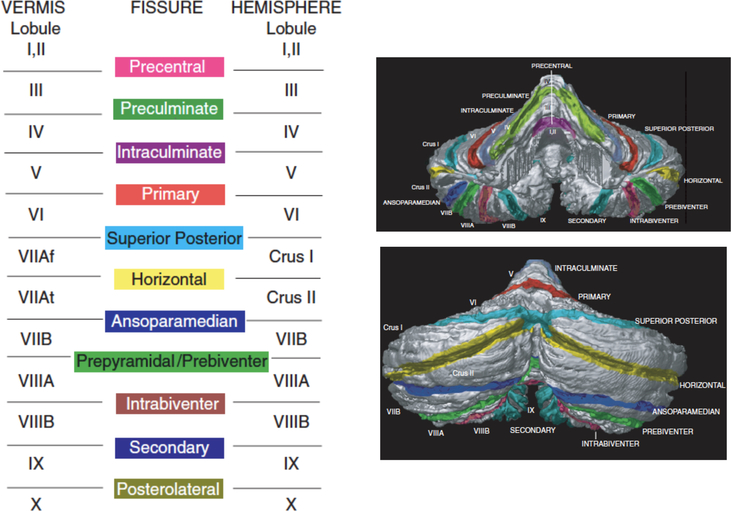
Like the cerebral cortex, the cerebellum has a folded cortical surface consisting of a thin layer of neurons, which covers a subcortical white matter. Also like the cerebral cortex, the cerebellum has a number of subcortical nuclei, which serve as the “output nuclei” of the cerebellum. The four paired deep cerebellar nuclei are the fastigial nucleus and the globose nucleus, which are collectively known as the interposed nuclei, and the emboliform nucleus and dentate nucleus. The cortical surface of the cerebellum has well-defined sulci (i.e., fissures), and these serve as anatomical boundaries to parse the cerebellar cortex into defined lobules (Schmahmann, Doyon, Petrides, Evans, & Toga, 2000).
The cerebellar atlas by Schmahmann and colleagues (2000) provides a recent and comprehensive definition of cerebellar anatomy, and while not the only one, it has become the most widely accepted definition of cerebellar anatomy. Recent papers, such as the surface-based atlases of Van Essen (2002) and Makris and colleagues (Makris et al., 2003), and the standardized MRI atlas of Diedrichsen and colleagues (Diedrichsen et al., 2006), make use of this nomenclature. According to the Schmahmann atlas, the cerebellum is organized into three major lobes: the anterior, posterior, and flocculonodular lobes. These can be further sub-divided into 13 distinct lobules, defined by sulcal landmarks, and into the medial vermis and lateral hemispheres. The anterior lobe is divided into lobules I-V, the posterior lobe is divided into lobules VI-IX, and the flocculonodular lobe consists of lobule X. Thus, 13 defined lobules in the lateral hemispheres (and vermis) are lobules I, II, III, IV, V, VI, Crus I (VIIAf), Crus II (VIIAt), VIIB, VIIIA, VIIIB, IX, X. The defining sulcal boundaries are the precentral, preculminate, intraculminate, primary, superior posterior, horizontal, ansoparamedian, prebiventer, intrabiventer, secondary, and posterolateral fissures. The anterior lobe is separated from the posterior lobe by the primary fissure, and the posterolateral fissure separates the posterior lobe from the flocculonodular lobe (see Figure 1; Schmahmann et al., 2000).
Figure 1.
The cerebellum is organized into three major lobes that can be further sub-divided into 13 distinct lobules, defined by sulcal landmarks, and into the medial vermis and lateral hemispheres. The anterior lobe is divided into lobules I-V, the posterior lobe is divided into lobules VI-IX, and the flocculonodular lobe consists of lobule X. The defining sulcal boundaries are the precentral, preculminate, intraculminate, primary, superior posterior, horizontal, ansoparamedian, prebiventer, intrabiventer, secondary, and posterolateral fissures. Reprinted from Schmahmann, J. D., Doyon, J., Petrides, M., Evans, A. C., & Toga, A. W. (2000). MRI atlas of the human cerebellum. San Diego: Academic Press.
The cerebellum is also defined functionally along a medial to lateral axis, largely based on its known connectivity with the spinal cord and cortex (Naidich et al., 2009). Thus there are median, intermediate, and lateral longitudinal zones. The median zone consists of the vermis and flocculonodular lobe; the intermediate zones consist of the medial portion of the cerebellar hemispheres; and the lateral zones consist of lateral portions of the cerebellar hemispheres. The spinal inputs are associated with proprioceptive, vestibular, and sensory function, and project to the median cerebellum, and the median cerebellum is associated with these functions. Intermediate and lateral cerebellum receive inputs via the cortico-ponto-cerebellar pathways and are historically associated with movement execution (intermediate) and movement planning (lateral). However, this historical focus on motor function of the cerebellum is giving way to an increasing interest in higher-order cortical function. This is driven, in part, by new information about cortico-ponto-cerebellar connections with regions outside of the motor cortex, such as connections to association cortical areas in the frontal, prefrontal, cingulate, and posterior parietal cortices (Naidich et al., 2009).
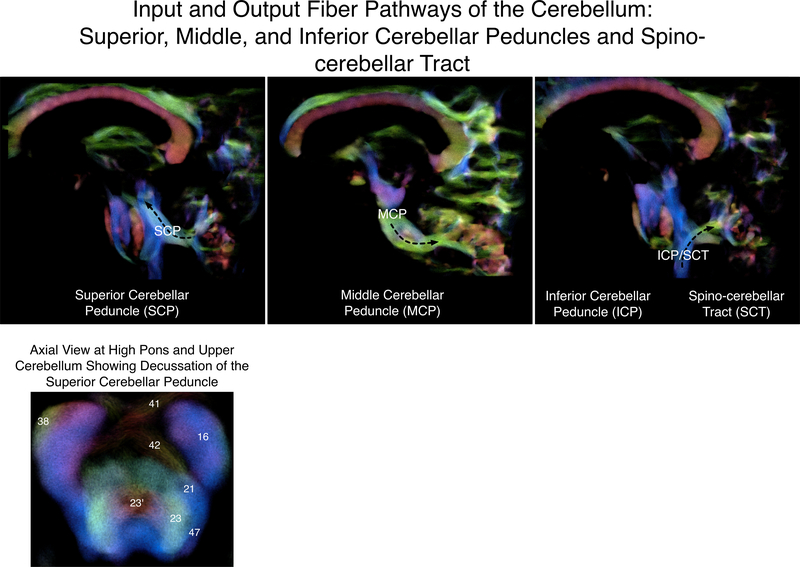
These afferent and efferent axonal fibers to and from the cerebellum travel through the three large cerebellar peduncles (see Figure 2). The major connection with the medulla and nuclei within the medulla comes from the inferior cerebellar peduncle. This pathway is mainly an input pathway to the cerebellum composed of the restiform body, carrying fibers from the inferior olivary nucleus, and more medially situated juxtarestiform body, carrying fibers from the vestibular nuclei. The juxtarestiform body also carries cerebellar efferents to the vestibular nuclei. A number of spinocerebellar fibers run through the inferior cerebellar peduncle, which include the dorsal spinocerebellar tract, cuneocerebellar tract, and trigeminocerebellar, and are associated with proprioceptive and sensory function. The dorsal spinocerebellar tract sends information from the lower parts of the body to the cerebellum, projecting from the posterior thoracic nucleus, whereas the cuneocerebellar tract sends information from the upper parts of the body to the cerebellum, projecting from the lateral cuneate nucleus in the medulla. The trigeminocerebellar fibers, arising from the spinal trigeminal nucleus in the medulla, transmit sensory information from the face to the cerebellum. These spinocerebellar fibers project to the fastigial and interposed nuclei and also send information to the vermis and intermediate zones of the anterior and posterior cerebellum lobes (Naidich et al., 2009).
Figure 2.
Input and output fiber pathways of the cerebellum, shown on a high-resolution diffusion-weighted image. Lower panel: Axial slice at the high pons/upper cerebellum showing the decussation of the superior cerebellar peduncle (crossing red fibers marked 23’). Numbering is from Naidich, T. P., Duvernoy, H. M., Delman, B. N., Sorenson, A. G., Kollias, S. S., & Haacke, E. M. (2009). Duvernoy’s atlas of the human brain stem and cerebellum. Vienna: Springer-Verlag/Wien. 16 = corticospinal tract; 21 = medial lemniscus; 23 = superior cerebellar peduncle; 23’ = decussation of the superior cerebellar peduncle; 38 = middle cerebellar peduncle; 41 = ventral pontine decussation; 42 = dorsal pontine decussation; 47 = lateral lemniscus.
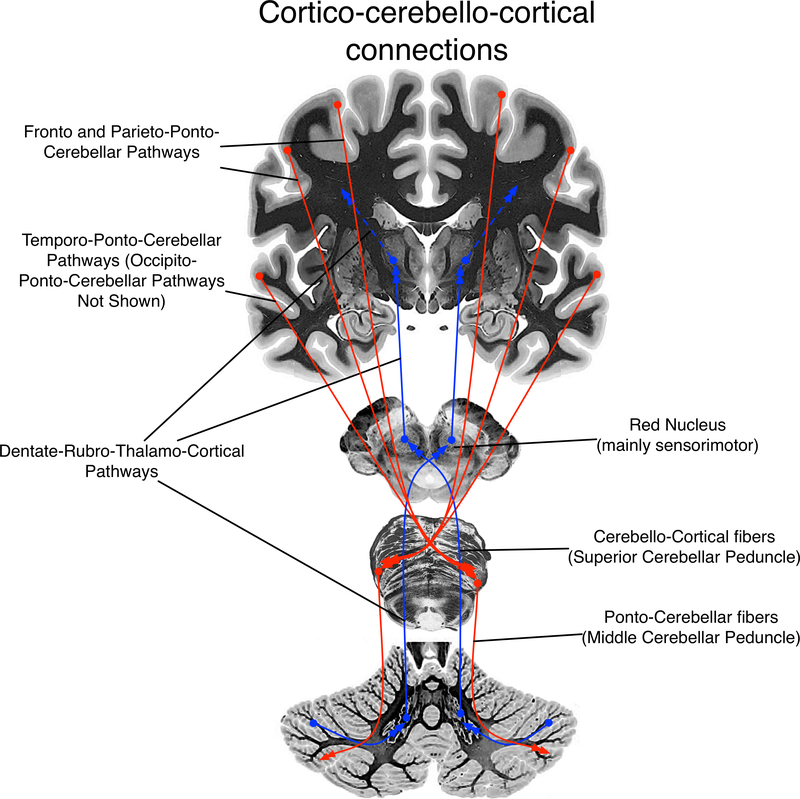
The largest cerebellar inputs arise through the middle cerebellar peduncle (brachium pontis) from the pontine nuclei that receive information from various cortical areas and form the cortico-ponto-cerebellar system. Motor, sensory, and association areas of the cerebral cortex project to the ipsilateral pontine nuclei, which then projects to the contralateral cerebellar cortex through the middle cerebellar peduncle. The major output pathway is the superior cerebellar peduncle (brachium conjunctivum), which sends efferents to the midbrain and thalamus. Most of the fibers of the superior cerebellar peduncle decussate (cross) in the midbrain, and pass via the contralateral red nucleus to the contralateral thalamus. Some afferents to the cerebellum also pass through the superior cerebellar peduncle. Neurons in the cerebellar cortex send information to the dentate nucleus, which then projects to both motor and association areas involved in higher-order cognitive functions through the thalamus. Therefore, the middle and superior cerebellar peduncles are the main cerebellar components of the cortico-cerebellar-cortical loop, where information travels from various cortical areas to the contralateral cerebellar cortex through the pontine nuclei, and projects to different areas in the cerebral cortex through various thalamic nuclei (Naidich et al., 2009; Figure 3).
Figure 3.
A rough schematic of the cortico-cerebello-cortical connections that might support developing language. Cortical outputs (red) from perisylvian regions cross the midline in the pons, and terminate in the lateral cerebellar cortex, travelling mainly via the middle cerebellar peduncle. Ascending pathways (blue) from cerebellar cortical regions project to subcortical cerebellar nuclei.
These descriptions from classical neuroanatomy are echoed in diffusion-weighted imaging of the cerebellar white matter (Salamon et al., 2007; Keser et al., 2015). For example, a diffusion-tensor imaging study of the human cerebellum revealed clear images of the inferior, middle, and superior cerebellar peduncles, as well as the fibers projecting to and from the cerebellum. The inferior cerebellar peduncle was the least detectable of the three, showing connections to the medial lemniscus. The superior cerebellar peduncle contained fibers that projected from the dentate nucleus, and decussated at the red nucleus, which showed to be the main efferent pathways of the cerebellum. Lastly, although it was not possible to differentiate the afferent and efferent fibers through the large middle cerebellar peduncles, these anteroposterior fibers showed connections to the pontine nuclei and dentate nucleus (Salamon et al., 2007).
A number of resting-state functional magnetic resonance imaging (rsfMRI) studies in humans have supported the diffusion-weighted imaging findings, revealing significant functional connectivity between the cerebellum and association areas in the frontal lobe and posterior parietal cortex (Bernard et al., 2012; Krienen & Buckner, 2009). Data from Bernard and colleagues (2012) suggest a functionally organized cerebellum, with distinct regions for motor and non-motor regions. Specifically, lobules I-VI of the anterior cerebellum were functionally correlated with motor cortical regions, while lobules of the posterior cerebellum such as lobules VI, Crus I, Crus II, and VIIb, were functionally correlated with prefrontal, temporal, and parietal cortices. In a similar study, Krienen and Buckner (2009) demonstrated functional connectivity between the posterior lobe of the cerebellum and the dorsolateral, medial, and anterior prefrontal cortex, revealing contralateral lateralization in the cerebellum for each circuit.
Taken together, the resting state studies provide suggestive evidence that cerebellar lobules VI, Crus I, Crus II, and VIIb receive projections from the prefrontal, posterior parietal, and superior temporal cortices known to be involved in language function (Bernard et al., 2012; Buckner, Krienen, Castellanos, Diaz, & Yeo, 2011; Krienen & Buckner, 2009; Stoodley & Schmahmann, 2009). These findings from anatomical and resting-state connectivity studies have been generally supported by meta-analytic studies of cerebellar connectivity (Riedel et al., 2015).
Cerebellar contributions to language and its development: Neuroimaging of humans
The functional and structural connectivity of the cerebellum with perisylvian language cortex suggests that the cerebellum may play a significant role in both receptive and expressive speech and language processing. Furthermore, patients with damage to the cerebellum can present with a cerebellar cognitive-affective syndrome (CCAS; Schmahmann 2001), and show deficits in planning, set-shifting, working memory, verbal fluency, visuospatial memory, grammatical processing, prosodic processing, and naming fluency. The syndrome also appears in children; in a review of 19 children’s records with cerebellar damage, Schmahmann (2001) found deficits in executive function, spatial cognition, and language appeared when damage was localized in the posterior lateral cerebellar hemisphere, and deficits in affect appeared when the cerebellar damage was localized in the vermis. The evidence for the cerebellum’s involvement in language has thus been available for quite some time, and it is additionally consistent with the connectivity revealed from resting state functional imaging studies and from the understanding of the known anatomical connectivity of cerebellar-cortical circuits. However, the specific functional contribution of the cerebellum to language is still unclear—to quote Fiez (2016), at this point current research focuses on how, not if, the cerebellum contributes to language processing. In this section, we review functional imaging and lesion deficit studies specifically addressing this question.
Although the CCAS findings point to a general association to cognitive and affective processing, to understand the more specific contribution of the cerebellum to language it is necessary to explore this in a more focused manner. For this reason, it has been helpful to employ in vivo functional imaging methods. In fact, there are a number of positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies that have provided a better understanding of the specific linguistic functions that are associated with the cerebellum. In particular, using fluency paradigms such as verb generation, verbal fluency, verbal working memory (Desmond, Gabrieli, Wagner, Ginier, & Glover, 1997; Frings et al., 2006; McDermott, Petersen, Watson, & Ojemann, 2003; Stoodley & Schmahmann, 2009), researchers have revealed that the posterior lateral right hemisphere (VI; Crus I and II) of the cerebellum is associated with phonological and semantic processing.
There are three types of tasks that have shown to elicit cerebellar activation: semantic fluency, phonemic fluency, and word (noun/verb) generation. Semantic and phonemic verbal fluency paradigms require that subjects overtly or covertly generate nouns from a specific semantic category (for semantic/category fluency), or words with initial consonant or vowel sounds (for phonemic/letter fluency). Word generation paradigms require the subject to overtly or covertly generate words (e.g., verbs) that are associated with a grammatical category (e.g., a list of nouns).
Verbal fluency paradigms often reveal cerebellar activations (Schlosser et al., 1998; Weiss et al., 2003). For example, Schlosser and colleagues found that a silent phonemic fluency task consistently revealed activation in the left prefrontal cortex and the right posterior lobe of the cerebellum (Schlosser et al., 1998). Similarly, Weiss et al., (2003) showed consistent activation across men and women in a silent phonemic fluency task in the left inferior frontal gyrus, anterior cingulate, and right posterior cerebellum. Both studies utilized a silent verbal fluency task to eliminate the motor aspects of speech, and suggested that across neuroimaging techniques the cerebellum is involved in verbal fluency tasks (Schlosser et al., 1998; Weiss et al., 2003).
Similarly, verb and word generation paradigms elicit activation in the right posterior lateral regions of lobules VI, Crus I, and VIIIA (Stoodley & Schmahmann, 2009; and in the left cerebellum, for those with atypical cortical language organization; Jansen et al., 2005). However, as with phonemic and semantic fluency, in these studies it is difficult to rule out the possibility that the cerebellar activity is due to the cerebellum’s well-known role in speech (motor) production (especially for overt verb generation). This confound was controlled, at least with respect to overt speech articulation, in a study by Frings and colleagues (Frings et al., 2006). In that study, the generation of verbs was compared with the reading of verbs in overt and covert speech. The findings showed that, after controlling for these effects of articulation, the cognitive act of verb generation still recruited the right lobule VI and Crus I, which supports the linguistic function of the right lateral cerebellar hemisphere, rather than a circumscribed motor function.
Researchers investigating verbal working memory also routinely report cerebellar activations (Desmond et al., 1997; E, Chen, Ho, & Desmond, 2012). Desmond and colleagues (1997) conducted a study using a verbal working memory task, and found consistent increased activation in the right posterior lobe of the cerebellum, specifically lobules VI, Crus I, VIIb (Desmond et al., 1997). These results were supported by Keren-Happuch and colleagues (2012) in a meta-analysis. An aspect of this meta-analysis focused on the regions similarly activated in verbal working memory tasks, specifically the Sternberg task and the n-back task, which showed consistent activation in bilateral lobules VI and Crus I, right VIIb, and left Crus II.
Several brain stimulation studies, using transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS), have illustrated the cerebellum’s contribution to linguistic function (Tomlinson, Davis, & Bracewell, 2013; Turkeltaub, Swears, D’Mello, & Stoodley, 2016). Both of these methods work by either enhancing or disrupting neural function under the site of stimulation. In the case of TMS, the stimulation is magnetic. In the case of tDCS, the stimulation is electrical.
When using TMS, cerebellar stimulation is found to impair phonemic fluency and lexical decision accuracy, while also enhancing lexical associative priming, specifically after stimulation of the right lateral cerebellar hemisphere (Arasanz et al., 2012; Tomlinson, Davis, & Bracewell, 2013). For example, Arasanz and colleagues (2012) studied the cerebellum’s role in both semantic and phonemic fluency tasks by testing the participant’s ability to switch categories within a word-generation task. In the semantic fluency task, there were no significant switching differences when the right cerebellar hemisphere was stimulated compared to the left cerebellar hemisphere, or compared to sham stimulation. However, for the phonemic fluency task, participants who received stimulation over the right posterior-lateral cerebellar hemisphere reported significantly lower switching scores than those who received stimulation over the left cerebellar hemisphere, and compared to the sham stimulation.
In two tDCS studies, investigators showed that stimulation applied over the right cerebellum facilitated performance on verb-generation tasks (Boehringer, Macher, Dukart, Villringer, & Pleger, 2013; Pope & Miall, 2012). Finally, using tDCS combined with fMRI, D’Mello and colleagues (2017) provided evidence that tDCS stimulation, relative to sham, increased activation of right Crus I/II during a semantic prediction task, and also enhanced cortical-cerebellar connectivity of regions involved in the task. Overall, the tDCS findings are consistent with functional neuroimaging studies that support the cerebellum’s role in linguistic functions, specifically in the right posterior lateral regions of lobules VI, Crus I, Crus II.
Meta-analyses of task-based functional imaging studies show that these findings are rather robust. For example, in a recent meta-analysis of cerebellar neuroimaging studies, Stoodley and Schmahmann (2009) reported cerebellar activation during a number of linguistic tasks, including word generation, word stem completion, semantic processing, phonological processing, and verbal fluency, even when reducing or eliminating the confounding motor aspects of speech. The meta-analysis revealed a functionally compartmentalized cerebellum, which is divided into general sensorimotor and cognitive regions where sensorimotor functions are related to the anterior lobe of the cerebellum and cognitive tasks are related to the posterior lobe of the cerebellum. Within the posterior lobe, cognitive functions are further localized into specific regions. Cerebellar activations during language tasks were generally lateralized to the right hemisphere, specifically in the right posterior lateral lobules VI, Crus I, Crus II, midline lobule VIIA, and a small lateral cluster in left hemisphere lobule VI. This was in contrast to the results of other cognitive functions, such as in spatial tasks that showed activation primarily in the left-hemisphere.
In summary, a large collection of neuroimaging and stimulation studies and metanalyses have demonstrated the cerebellum’s role in linguistic functions. Specifically, the right posterior lateral lobules VI, Crus I, and Crus II of the cerebellum are associated with phonological and semantic processes, as shown through verb generation tasks, verbal working memory tasks, verbal fluency tasks. However, the neuroimaging studies we have reviewed so far have not sufficiently addressed whether the cerebellum would be important for language development, or whether it is a critical component of the neural systems implementing language. These questions can be addressed by exploring acquired disorders, such those that result from stroke, tumor, or traumatic brain injury, and it is to these studies that we now turn.
Cerebellar contributions to language and its development: Acquired disorders affecting the cerebellum
A small but growing number of empirical studies suggest that the cerebellum impacts general cognitive development, including in the domain of language. For example, Davis and colleagues (2010) found that children who sustain injury to the cerebellum following tumor resection show both cognitive and motor deficits (Davis, Pitchford, Jaspan, McArthur, & Walker, 2010). One case study illustrated a 4-year-old girl who showed normal language development until suffering acute cerebellitis, which led to impairment in language sequencing and fluency. The child did not show phonetic impairment—she was still able to accurately produce the sounds of speech. However, her speech was slow, and she could only provide incomplete sentences and sequential dialogue under constant guidance (Riva 1998).
In addition to the small sample studies and single case studies, some research has shown that verbal fluency and other expressive language functions are affected following cerebellar injury at a young age. For example, Scott and colleagues (2001) showed, in a longitudinal study of children with cerebellar tumors, an association between right-handed children who had greater damage to their right cerebellar hemisphere and deficits in verbal and literacy skills measured by the Vocabulary, Similarities, and Information subtest scores of the Wechsler scales and the Single Word Reading and Spelling subtest scores taken from the Wechsler Objective Reading Dimensions. This of course fits with our understanding of cortico-ponto-cerebellar connectivity relevant for language, where the left cortical hemisphere projects to the right cerebellum. In contrast, greater damage to the left cerebellar hemisphere was associated with deficits in non-verbal and spatial skills (Scott et al., 2001). This also fits with the expected pattern—i.e., the right cortical hemisphere, which participates in the processing of non-verbal and spatial information, projects anatomically to the left cerebellum.
Similar results were found in a larger study of 26 children who had a cerebellar hemisphere or tumor of the vermis removed (Riva & Giorgi, 2000). Prior to the onset of the disease, all 26 children reported normal emotional, social, academic, and intellectual performances. However, following the removal of the tumor, the children showed differences in deficits depending on whether the tumor was located in the right hemisphere, left hemisphere, or vermis. Those with a right cerebellar lesion showed deficits in language processing and auditory sequential memory. Specifically, this group performed more poorly on tests of lexical naming, lexical comprehension, receptive syntax, formulation of sentences, verbal fluency, and executive function as assessed by the Wisconsin Cord Sorting Test (WCST).
In contrast, those with a left cerebellar lesion showed deficits in non-verbal performance of spatial and visual sequential memory. For those children with a vermis resection, six children presented with post-surgical mutism without any behavioral changes. Four of those children presented with severe speech anarthria, which returned to normal speech within 2-years, and two with severe language disturbances that resembled the agrammatical language seen in aphasic patients with left frontal lesions. These children had impairments in syntactic comprehension, auditory sequential memory, formulation of sentences, and these deficits lasted even three years post surgery. The other five children who had a tumor in the vermis presented post-surgical behavioral problems, including irritability and behaviors resembling autism. All of these children involved the removal of the lower portion of the vermis. This is consistent with studies in adults, in which patients with right-sided cerebellar lesions tend to show impairments in verbal working memory, naming, and verbal fluency, while patients with left-sided cerebellar lesions showed impairments in attention and visuo-spatial skills (Baillieux et al., 2010).
The small number of studies of acquired disorders of the cerebellum in children suggest that the cerebellum is an important contributor to language development. However, it has also been suggested that the cerebellum’s role in the development of non-motor, cognitive functions can be understood from studying the neuroanatomy of developmental disorders (Stoodley 2016). There are a number of these disorders that are associated with language or literacy dysfunction, namely autism spectrum disorder, dyslexia, attention deficit hyperactivity disorder (ADHD), and Williams syndrome. We review these disorders below with special emphasis on the evidence that the cerebellum might contribute to the co-morbid language impairments that often present in children with these disorders.
Cerebellar contributions to language and its development: Developmental disorders
Autism Spectrum Disorder.
Autism spectrum disorder is a developmental disorder associated with a constellation of deficits in the motor, cognitive, affective, social and linguistic domains. It is a “spectrum disorder”, which means that children can present along a wide continuum of impairment, and can also show spared function in one domain with impaired function in others. Furthermore, the disorder is characterized by wide inter-individual variability, which has made it both a difficult disorder to define and to treat. However, despite this wide variability, the presence of abnormal cerebellar anatomy is a point of consensus (Fatemi et al., 2012), and one of the consistent findings in MRI studies of people with autism is reduced cerebellar volume relative to typical children (Belmonte et al., 2004; Courchesne et al., 2001). For example, an analysis of brain growth patterns in children with autism revealed that, relative to typical controls, children with autism have reduced cerebellar grey matter, a smaller ratio of grey to white matter, and smaller vermis lobules VI-VII (Courchesne et al., 2001).
Some authors have speculated that this would lead to deficits across a variety of domains of function, including language (Belmonte et al., 2004). Indeed, a recent structural analysis of the cerebellum in children with autism revealed differences within the group when they were segregated according to whether those children did or did not have language impairment (Hodge et al., 2010). Specifically, the results showed differences in left posterior lateral cerebellar lobule VIIIA (which was larger for children with autism with language impairment), and in right cerebellar lobule VIIA (where children with autism without language impairment and typical controls had increased volume). These findings were mirrored in the cerebral cortex, where children with autism without a language impairment and typical controls had a larger left inferior frontal gyrus, a cortical region strongly associated with language function.
The asymmetry found in cerebellar volume VIIIA was also significantly correlated with language measures, as measured by the Clinical Evaluation of Language Fundamentals (CELF). Thus, higher scores on the language measures were related to larger VIIIA in the right hemisphere.
These anatomical findings are consistent with a meta-analysis of cerebellar grey matter volume in children and adults with autism, which revealed reduced grey matter in vermal lobule IX, left lobule VIIIb, and right Crus I (Stoodley 2014). However, in a more recent study, D’Mello and colleagues (D’Mello, Moore, Crocetti, Mostofsky, & Stoodley, 2016) showed that reduction in left cerebellar Crus I/II specifically differentiated children with autism who had early language delay from those children who did not have early language delay (D’Mello, Moore, Crocetti, Mostofsky, & Stoodley, 2016).
Other work investigating children with autism used a functional connectivity analysis of brain regions involved in verb generation. In addition to reduced cortico-cortico connectivity of these regions, the results also showed a reduction in functional connectivity between cortical language areas and the cerebellum. Specifically, in ASD patients there was a significant reduction in the connections between the right posterior lateral cerebellar hemisphere (lobules VI, Crus I, and Crus II) with the left dorsolateral prefrontal cortex, left premotor cortex, and left inferior frontal gyrus. This reduction of functional connectivity in the language network is proposed to be related to abnormal language function in children with autism spectrum disorder, although notably in this study the cortico-cerebellar connectivity was not significantly related to a verb generation task (Verly et al., 2014). The results from the small number of studies are promising, although more research is needed to establish a firm link between cerebellar function and language development in this population.
Attention Deficit Hyperactivity Disorder (ADHD).
Children diagnosed with ADHD, which is a developmental disorder characterized by behavioral symptoms such as inattention, hyperactivity, and impulsivity, also have reported cerebellar abnormalities. For example, smaller cerebellar volumes are reported in these individuals (Stoodley 2014), and these reductions are associated with symptom severity (Castellanos et al., 2002). ADHD is also associated with language difficulties, particular for pragmatic aspects of communication (Hawkins, Gathercole, Astle, The Calm Team, & Holmes, 2016). However, despite these associations, no study (to our knowledge) has directly assessed the relation between cerebellar dysfunction and language development in children with ADHD. This is a research gap that warrants further investigation.
Dyslexia.
Dyslexia is another developmental disorder associated with cerebellar abnormalities, and it is characterized by the failure to develop the linguistic skills of reading, writing, and spelling that are on par with other intellectual abilities. Nicolson, Fawcett, and colleagues (Mariën et al., 2014; Nicolson & Fawcett, 2011; Nicolson, Fawcett, & Dean, 2001) have postulated the cerebellar deficit hypothesis as a cause for the range of deficits associated with developmental dyslexia, including writing, reading, and spelling, as well as a general deficit in automated performance. Their detailed model of reading includes a central role for the cerebellum in the timing and coordination of speech and reading (e.g., in establishing fixation accuracy during reading), in more general linguistic abilities (e.g., phonological skill, articulation, grapheme-phoneme translation, verbal working memory), and in general adaptive plasticity that contributes to the emergence of skilled behavior (e.g., improvements in processing speed and automaticity).
A corollary of this cerebellar deficit theory of dyslexia is that the general deficits that lead to dyslexia are also associated with other disorders, such as ADHD, specific language impairment, dyspraxia, or more general learning disability. A key shared deficit in these disorders is the inability to develop skill automaticity (the process by which skills become fluent with practice and rely less on conscious control), and in fact the cerebellum’s role in literacy may have to do with the need to deal with increasing cognitive demands on speed and accuracy.
In support of this idea, in an early study Nicolson et al. (2001) showed that most of the children with dyslexia had cerebellar abnormalities and also showed deficits in other cerebellum-related functions, such as balance and skill automaticity. Further studies have revealed relatively consistent structural and functional differences in the cerebellum between children and adults with dyslexia and typical readers (Baillieux et al., 2009; Pernet, Poline, Demonet, & Rousselet, 2009). Cerebellar lobule VI is most commonly implicated. Reduced grey matter in the right hemisphere lobule VI is a significant biomarker for adult dyslexia, and rapid naming impairment is associated with abnormal activation in right lobule VI.
More focused cellular and connectivity analysis also supports a role for the cerebellum in dyslexia. For example, a post-mortem analysis revealed the neuroanatomical differences in the cerebellar cortex and within the olivo-cerebellar pathway of adults with dyslexia (Finch, Nicolson, & Fawcett, 2002). Specifically, in comparing the average cell area and cell density between adults with dyslexia and typical controls, those with dyslexia had larger Purkinje cells in the anterior and posterior lobes of the cerebellar cortex. Similarly, when analyzing the distribution of cell size in the inferior olive, there were a greater number of large cells in the dyslexic population in comparison to controls. The authors further showed that no differences were found in cell area and cell density in the dentate nucleus, leading them to conclude that the cause of dysfunction associated with dyslexia lies in the input fibers to the cerebellum, rather than the output from the dentate nucleus. This suggests a dysfunction in the processing or transfer of information within the cerebellar cortex.
These morphological findings are consistent with conclusions drawn from Baillieux and colleagues (2009) in their fMRI study in children with dyslexia. When performing a noun-verb association task, children with dyslexia showed widespread activation across hemispheric lobules VI-Crus II, and vermal lobules I-VII. In contrast, the control subjects showed activation in both left and right hemispheres, predominantly the right hemisphere, in the hemispheric lobules V-VII and vermal lobule III. Comparison of activation patterns revealed that children with dyslexia showed more widespread activation patterns, activating more regions in the left hemisphere, more vermal regions, and more inferior lobules than the control group. Given the widespread activation in children with dyslexia, the authors suggested that the dysfunction associated with developmental dyslexia lies in the processing of information within the cerebellar cortex (Baillieux et al., 2009). However, it is important to note that the sample size of this particular study was small, and so further replication is necessary.
As Stoodley (2016) notes, the three disorders—autism spectrum disorder, ADHD, and dyslexia—are associated with the dysfunction of different regions of the cerebellum, which suggests that different cerebro-cerebellar circuits are affected across the disorders. This is an important discovery because it allows for the possibility to focus and isolate the specific, distributed cortico-cerebellar loops that may be affected in each disorder, leading to better understanding of the etiology of each disorder, and better nosologic criteria for diagnosis.
Williams Syndrome.
Another developmental disorder linked with cerebellar abnormalities and cognitive dysfunction is Williams Syndrome. Williams Syndrome is a genetic disorder associated with a number of cognitive and physical disabilities, including delayed language development and impairments in semantic processing, reading comprehension, and pragmatics, although they have relative strengths in vocabulary, verbal working memory, and phonological processing (Mervis & Velleman, 2011). Structural abnormalities in the cerebral cortex and cerebellum have been observed in children and adults with Williams Syndrome. Specifically, patients with Williams Syndrome have shown to have an enlarged posterior lobe of the cerebellum and a smaller cerebellar cortex, compared to healthy controls (Jones et al., 2002). In a structural analysis of overall cerebellar volume in patients with Williams Syndrome, Down’s Syndrome, and healthy controls, patients with William Syndrome had relatively similar size cerebellar tonsils to healthy controls, but larger cerebellar tonsils to those with Down’s Syndrome. However, the authors controlled for cerebral cortical size, those with Williams Syndrome have larger cerebellar tonsils than healthy controls (Wang, Hesselink, Jernigan, Doherty, & Bellugi, 1992).
The neuroanatomical differences found in the studies involving patients with Williams Syndrome compared to patients with other developmental disorders and healthy controls may provide a better understanding of the cerebellum’s role in language function, given the strengths and weaknesses in language function observed in patients with Williams Syndrome. What is missing, though, is a more thorough explanation about the differential pattern of language disability. Thus, we do not have a coherent story about why cerebellar volume differences might explain relatively spared vocabulary, verbal working memory, and phonological processing in conjunction with impaired semantic processing, reading comprehension, and pragmatics, and other cognitive deficits observed in Williams Syndrome patients (Jones et al., 2002; Mervis & Velleman, 2011; Wang et al., 1992). Notably, the spared linguistic functions are those that are typically associated with cerebellar function. Thus, one possibility is that the cerebellum compensates during the course of development for impaired cortical function in children with Williams Syndrome relative to controls, and increased posterior cerebellar volume is the anatomical signature of this compensation. However, this remains speculative, as there are no direct, longitudinal studies of cerebellar contributions to language development in children with Williams Syndrome.
Summary.
The results of studies of typical children and adults support the notion of a functionally organized cerebellum due to its connectivity with regions in the contralateral cortex, where the right posterior lateral cerebellar hemisphere is associated with language function. The deficits in language function seen in both children and adults who sustained cerebellar injury and reduced functional connectivity also support the notion of the cerebellum’s role in language function and development (Baillieux et al., 2010; Riva 1998; Scott et al., 2001; Verly et al., 2014). Finally, there are some intriguing findings suggesting the importance of the cerebellum to language function in children with disordered language function, although these studies should be considered preliminary.
Cerebellar contributions to language and its development: Mechanism
We have reviewed evidence for the cerebellum’s importance to language in typical and atypical development, but we have not yet touched on the neurocomputational mechanisms supported by the human cerebellum that might contribute to language processing. A number of theories of cerebellar function have been proposed (Argyropoulos, 2016). The most influential class of models of cerebellar function, collectively called “internal models”, proposes that the cerebellum is involved in generating predictive, feed-forward “internal” models of potential consequences of enacted behaviors. Specifically, it is proposed that the cerebellum generates representations of the context-specific dynamics of the organism’s interactions with the environment (Moberget & Ivry, 2016). According to Ito (1993; 2008), the organization of the cerebellum supports this process by establishing individuated microcircuits that make feed-forward predictions about the next sensorimotor state of the organism. These individuated circuits can be modified by the activity of cerebellar climbing fibers or parallel fibers, which convey error signals about the differences between the intended action and the realized action. Feedback-error learning can occur to modify these circuits based on experience (Ito 1993, 2008).
The internal models theory (and similar models such as efference copy or corollary discharge models; Crapse & Sommer, 2008) have been applied to the study of voluntary motor control requiring precise sensorimotor prediction (Shadmehr, Smith, & Krakauer, 2010) and to classical reflex conditioning (Rasmussen & Hesslow, 2014). In this respect, the internal models hypothesis has provided a rich framework for understanding the cerebellum’s contribution to motor function, and has become the dominant model in this domain (Ebner 2013). Moberget and Ivry (2016) have recently applied this theory to language, and have proposed that internal models contribute to three components important to language processing: 1) timing; 2) adaptation; 3) prediction.
The cerebellum’s importance for timing is most apparent in the case of speech production. Speech production requires precise timing of the duration and speed of articulations, which are individuated movements of muscle groups and parts of the speech apparatus that engender intelligible speech sounds. Thus, online feedback and error correction about the intended speech act and the actual speech would, based on the theory of internal models, likely involve the cerebellum. This would explain why a common outcome of cerebellar damage in adults is ataxic dysarthria (resulting in slowed speech rate and syllabic timing; Ackermann & Hertrich, 1994). However, precise temporal processing is also important for speech perception. For example, some phonemic distinctions can be made purely on the basis of temporal characteristics (e.g., “BIDDEN” versus “BITTEN” are distinguished by differences in airway occlusion at the middle consonant), and people with cerebellar lesions are unable to make such distinctions (Ackermann, Gräber, Hertrich, & Daum, 1997). The cerebellum’s contribution to language and speech processing may thus depend on its importance in tasks that require the precise representation of temporal information.
Moberget and Ivry (2016) also argue that the cerebellum contributes to the process of adaptation during speech perception and language comprehension. For example, the listener must adapt online to cues from co-articulation, accent, and other paralinguistic cues such as prosody, visual gestures of speech movements, lexical information, and sentential and narrative-level semantic information. Error signals of the discrepancy between expected and actual sensory outcomes can guide adaptive adjustments in an online fashion. In a recent fMRI study, Guediche and colleagues (2015) investigated this adaptive function of the cerebellum. In a speech perception study, the authors controlled for temporal cues by using distorted and non-distorted speech, and showed that activity in the right Crus I was specifically correlated with performance on an adaptation task assessing word identification in distorted speech (Guediche, Holt, Laurent, Lim, & Fiez, 2015). This suggests that the cerebellum contributes to fairly-rapid adaptive plasticity in speech perception.
Finally, Moberget and Ivry (2016) suggested that the cerebellum might contribute to predictive processing during language comprehension in situations that involve temporal or sequential associations. There is emerging evidence in support of this hypothesis. For example, the cerebellum is involved in detecting deviations from predicted grammatical rules such as subject-verb agreement and canonical word order(Adamaszek & Kirkby, n.d.). Lesage and colleagues (2012) showed that language processing is delayed in a predictive language task when right cerebellar function is disrupted with rTMS (Lesage, Morgan, Olson, Meyer, & Miall, 2012). In a fMRI study, Moberget and colleagues (Moberget, Gullesen, Andersson, Ivry, & Endestad, 2014) showed that the right cerebellum was sensitive to the predictability, based on sentence context, of the final word of the sentence. In addition, D’Mello and colleagues (2017) recently reported in a combined tDCS and fMRI study that, relative to sham, anodal tDCS of the right posterolateral cerebellum increased activation in right Crus I/II during a semantic prediction task. These studies provide initial suggestion that predictive encoding in the cerebellum contributes to the speed and efficiency of language processing (D’Mello et al., 2017).
How would such a theory be applied to language development? Setting aside the more obvious relevance to speech production, there are some potential implications to the development of speech perception and language comprehension. The ability to accurately discriminate phonemic and syllabic contrasts is essential to the development of speech perception. Predictive encoding during the learning and processing of grammar may be important for its acquisition in a particular language, and for establishing automaticity in the acquisition of literacy. Finally, a recent study has suggested that the cerebellum might be involved in the acquisition of novel lexical items, which would suggest an important role for the right cerebellum in the consolidation of lexico-semantic associations (Lesage, Nailer, & Miall, 2016). Although the study was conducted in adults, this possibility has obvious implications for the acquisition of language in children. However, these studies have not yet been conducted in children, and so there is no literature base that could inform whether the internal models framework can be successfully applied to language development.
Cerebellar contributions to language and its development: Future directions
The body of research addressing the role of the cerebellum in non-motor higher-level cognition, including language, is sufficient to conclude that the cerebellum is an important component of the neural systems implementing a variety of cognitive processes. Progress is required, though, in a number of complementary domains. First, research on the neurobiology of language development should focus more on cerebellar contributions (and, indeed, on other subcortical structures). Because it is often the case that functional neuroimaging studies acquire whole-brain coverage, the lack of research in this area can, in part, be attributed to bias toward studying specific cortical regions and their contribution to language processing. The focus on the Broca-Wernicke-Geschwind language model, which has dominated brain-language research for the past 150 years, is certainly a cortio-centric model. A movement away from these models to theoretical models that take seriously the notion of networks is necessary to incentivize research on the cerebellum and language (Tremblay & Dick, 2016).
Second, more focused studies, in both typical and atypical populations, are required to pin down the specific domains in which the cerebellum contributes most significantly to language development. For example, evidence from cerebellar lesions suggests that sometimes the effects can be obvious (e.g., in the case of ataxic dysarthria), and other times the effects are more subtle (e.g., impairments in temporal discrimination of phonemes).
Third, progress needs to be made in understanding the cellular mechanisms at work in, and computations accomplished by, the cerebellum. Immense progress has been made in this area, but there are still significant gaps. Animal research can contribute in this area, but language is a uniquely human ability. Thus, researchers must become comfortable with the neural and computational models established in animal research, and apply those theories to their studies on humans. Recent papers (e.g., Moberget & Ivry, 2016) provide excellent examples of how work in understanding the cerebellum’s contribution to language must be conducted at multiple levels of analysis. A comprehensive theoretical framework, such as the internal model theory, is also necessary to focus these investigations.
Finally, it is equally important to understand which parts of the cerebellum are important for language development, and in what domains. Such knowledge necessary for hypothesis generation about the expected effects of behavioral interventions on neural function, and for establishing possible targets for non-invasive and invasive interventions at the neural level.
Conclusion
The studies and articles we have reviewed support an association between the cerebellum and higher-order cognitive functions, and more specifically for this review, language function. The cerebellum’s association with language function is determined by the specific cortico-cerebellar connectivity to the right cerebellum from the left cortical hemisphere. This functional association is supported by structural and functional connectivity analyses that reveal projections from higher-order association areas, including the prefrontal, posterior parietal, and superior temporal cortices, known to be involved in language function to posterior cerebellar lobules VI, Crus I, Crus II, and VIIb (Bernard et al., 2012; Buckner et al., 2011; Krienen & Buckner, 2009; Stoodley & Schmahmann, 2009). In typical development, the right hemisphere of the cerebellum has been associated with verb generation tasks, verbal working memory tasks, verbal fluency tasks and the processing of semantic relations (Desmond et al., 1997; Frings et al., 2006; McDermott et al., 2003; Stoodley & Schmahmann, 2009). In children and adults who have sustained cerebellar damage, both show deficits in verbal fluency tasks (Baillieux et al., 2010; Riva 1998; Scott et al., 2001). Electrostimulation studies have also revealed changes in tasks of verbal fluency, lexical decision-making, verb generation, and verbal working memory when undergoing TMS and tDCS (Arasanz et al., 2012; Argyropoulos & Muggleton, 2013; Boehringer et al., 2013; Pope & Miall, 2012; Turkeltaub et al., 2016). Examining typical and atypical cerebellar development and its association with linguistic tasks highlights the expanding view of the cerebellum’s role in higher-order cognitive functions. Specifically, it emphasizes the cerebellum’s role as part of a broader language network, and also implies that the cerebellum may be a potential new therapeutic target to treat speech and language deficits (Grimaldi et al., 2014), especially during development. Given the provided insight into the cerebellum’s role in language function and development, future studies should focus on the precise cerebellar regions associated with specific cerebellar functions in order to establish a functional topographic map that could lead to specific targets for therapeutic interventions.
References
- Ackermann H, & Hertrich I (1994). Speech rate and rhythm in cerebellar dysarthria: An acoustic analysis of syllabic timing. Folia Phoniatrica Et Logopaedica, 46, 70–78. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Gräber S, Hertrich I, & Daum I (1997). Categorical speech perception in cerebellar disorders. Brain and Language, 60, 323–31. [DOI] [PubMed] [Google Scholar]
- Adamaszek M, & Kirkby KC (2016). Cerebellum and grammar processing In The linguistic cerebellum (pp. 81–105). Cambridge, MA: Academic Press. [Google Scholar]
- Andersen BB, Korbo L, & Pakkenberg B (1992). A quantitative study of the human cerebellum with unbiased stereological techniques. Journal of Comparative Neurology, 326, 549–60. [DOI] [PubMed] [Google Scholar]
- Arasanz CP, Staines WR, Roy EA, & Schweizer TA (2012). The cerebellum and its role in word generation: A cTBS study. Cortex, 48, 718–24. [DOI] [PubMed] [Google Scholar]
- Argyropoulos GP, & Muggleton NG (2013). Effects of cerebellar stimulation on processing semantic associations. Cerebellum, 12, 83–96. [DOI] [PubMed] [Google Scholar]
- Argyropoulos GPD (2016). The cerebellum, internal models and prediction in ‘non-motor’ aspects of language: A critical review. Brain and Language, 161, 4–17. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Dobbeleir A, Paquier PF, De Deyn PP, & Mariën P (2010). Cognitive and affective disturbances following focal cerebellar damage in adults: A neuropsychological and SPECT study. Cortex, 46, 869–79. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, & Mariën P (2008). Cerebellar neurocognition: Insights into the bottom of the brain. Clinical Neurology and Neurosurgery, 110, 763–73. [DOI] [PubMed] [Google Scholar]
- Baillieux H, Vandervliet EJ, Manto M, Parizel PM, De Deyn PP, & Mariën P (2009). Developmental dyslexia and widespread activation across the cerebellar hemispheres. Brain and Language, 108, 122–32. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, & Webb SJ (2004). Autism and abnormal development of brain connectivity. Journal of Neuroscience, 24, 9228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, … Peltier SJ (2012). Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Frontiers in Neuroanatomy, 6,. doi: 10.3389/fnana.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer A, Macher K, Dukart J, Villringer A, & Pleger B (2013). Cerebellar transcranial direct current stimulation modulates verbal working memory. Brain Stimulation, 6, 649–53. [DOI] [PubMed] [Google Scholar]
- Brodmann K (1909). Vergleichende lokalisationslehre der grosshirnrinde in ihren prinzipien dargestellt auf grund des zellenbaues. Barth. [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, & Yeo BT (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, … Rapoport JL (2002). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA, 288, 1740–8. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Courchesne RY (2001). Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology, 57, 245–54. [DOI] [PubMed] [Google Scholar]
- Crapse TB, & Sommer MA (2008). Corollary discharge across the animal kingdom. Nature Reviews. Neuroscience, 9, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, Pitchford NJ, Jaspan T, McArthur D, & Walker D (2010). Development of cognitive and motor function following cerebellar tumour injury sustained in early childhood. Cortex, 46, 919–32. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, & Glover GH (1997). Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. Journal of Neuroscience, 17, 9675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J (2006). A spatially unbiased atlas template of the human cerebellum. NeuroImage, 33, 127–38. [DOI] [PubMed] [Google Scholar]
- D’Mello AM, Moore DM, Crocetti D, Mostofsky SH, & Stoodley CJ (2016). Cerebellar gray matter differentiates children with early language delay in autism. Autism Research, 9, 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Turkeltaub PE, & Stoodley CJ (2017). Cerebellar tDCS modulates neural circuits during semantic prediction: A combined tDCS-fMRI study. Journal of Neuroscience, 37, 1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner TJ (2013). Cerebellum and Internal Models: Handbook of the cerebellum and cerebellar disorders (pp. 1279–1295). Springer; Netherlands. [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, …Welsh JP (2012). Consensus paper: Pathological role of the cerebellum in autism. Cerebellum, 11, 777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA (2016). The cerebellum and language: Persistent themes and findings. Brain and Language, 161, 1–3. [DOI] [PubMed] [Google Scholar]
- Finch AJ, Nicolson RI, & Fawcett AJ (2002). Evidence for a neuroanatomical difference within the olivo-cerebellar pathway of adults with dyslexia. Cortex, 38, 529–539. [DOI] [PubMed] [Google Scholar]
- Frings M, Dimitrova A, Schorn CF, Elles H-G, Hein-Kropp C, Gizewski ER, … Timmann D (2006). Cerebellar involvement in verb generation: An fMRI study. Neuroscience Letters, 409, 19–23. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, … Ziemann U (2014). Non-invasive cerebellar stimulation--a consensus paper. Cerebellum, 13, 121–38. [DOI] [PubMed] [Google Scholar]
- Guediche S, Holt LL, Laurent P, Lim S-J, & Fiez JA (2015). Evidence for cerebellar contributions to adaptive plasticity in speech perception. Cerebral Cortex, 25, 1867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins E, Gathercole S, Astle D, The Calm Team, & Holmes J (2016). Language problems and ADHD symptoms: How specific are the links? Brain Sciences, 6,. doi: 10.3390/brainsci6040050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge SM, Makris N, Kennedy DN, Caviness VS, Howard J, McGrath L, … Harris GJ (2010). Cerebellum, language, and cognition in autism and specific language impairment. Journal of Autism and Developmental Disorders, 40, 300–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M (1993). Movement and thought: Identical control mechanisms by the cerebellum. Trends in Neurosciences, 16, 448–50; discussion 453–4. [DOI] [PubMed] [Google Scholar]
- Ito M (2008). Control of mental activities by internal models in the cerebellum. Nature Reviews. Neuroscience, 9, 304–13. [DOI] [PubMed] [Google Scholar]
- Jansen A, Flöel A, Van Randenborgh J, Konrad C, Rotte M, Förster A-F, … Knecht S (2005). Crossed cerebro-cerebellar language dominance. Human Brain Mapping, 24, 165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Hesselink J, Courchesne E, Duncan T, Matsuda K, & Bellugi U (2002). Cerebellar abnormalities in infants and toddlers with Williams Syndrome. Developmental Medicine and Child Neurology, 44, 688–94. [DOI] [PubMed] [Google Scholar]
- Keser Z, Hasan KM, Mwangi BI, Kamali A, Ucisik-Keser FE, Yozbatiran N, Francisco GE, & Narayana PA (2015). Diffusion tensor imaging of the human cerebellar pathways and their interplay with cerebral macrostructure. Frontiers in Neuroanatomy, 9, 41 10.3389/fnana.2015.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, & Buckner RL (2009). Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex, 19, 2485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG (2000). Phonological grouping is specifically affected in cerebellar patients: A verbal fluency study. Journal of Neurology, Neurosurgery, and Psychiatry, 69, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage E, Morgan BE, Olson AC, Meyer AS, & Miall RC (2012). Cerebellar rTMS disrupts predictive language processing. Current Biology, 22, R794–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage E, Nailer EL, & Miall RC (2016). Cerebellar BOLD signal during the acquisition of a new lexicon predicts its early consolidation. Brain and Language, 161, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Hodge SM, Haselgrove C, Kennedy DN, Dale A, Fischl B, … Schmahmann JD (2003). Human cerebellum: Surface-assisted cortical parcellation and volumetry with magnetic resonance imaging. Journal of Cognitive Neuroscience, 15, 584–99. [DOI] [PubMed] [Google Scholar]
- Mariën P, & Manto M (2016). The linguistic cerebellum. Cambridge, MA: Academic Press. [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, … Ziegler W (2014). Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum, 13, 386–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, & Ojemann JG (2003). A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia, 41, 293–303. [DOI] [PubMed] [Google Scholar]
- Mervis CB, & Velleman SL (2011). Children with Williams Syndrome: Language, cognitive, and behavioral characteristics and their implications for intervention. Perspectives on Language Learning and Education, 18, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, & Ivry RB (2016). Cerebellar contributions to motor control and language comprehension: Searching for common computational principles. Annals of the New York Academy of Sciences, 1369, 154–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, Gullesen EH, Andersson S, Ivry RB, & Endestad T (2014). Generalized role for the cerebellum in encoding internal models: Evidence from semantic processing. Journal of Neuroscience, 34, 2871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidich TP, Duvernoy HM, Delman BN, Sorenson AG, Kollias SS, & Haacke EM (2009). Duvernoy’s atlas of the human brain stem and cerebellum Vienna: Springer-Verlag/Wien. [Google Scholar]
- Nicolson RI, & Fawcett AJ (2011). Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex, 47, 117–27. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, & Dean P (2001). Developmental dyslexia: The cerebellar deficit hypothesis. Trends in Neurosciences, 24, 508–511. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, & Rousselet GA (2009). Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neuroscience, 10, 67. doi: 10.1186/1471-2202-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope PA, & Miall RC (2012). Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimulation, 5, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N (2006). The primate cortico-cerebellar system: Anatomy and function. Nature Reviews. Neuroscience, 7, 511–22. [DOI] [PubMed] [Google Scholar]
- Ramnani N, et al. , (2006). The evolution of prefrontal inputs to the cortico-pontine system: Diffusion imaging evidence from macaque monkeys and humans. Cerebral Cortex, 16, 811–818. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, & Hesslow G (2014). Feedback control of learning by the cerebello-olivary pathway. Prog Brain Res, 210, 103–19. [DOI] [PubMed] [Google Scholar]
- Riedel MC, Ray KL, Dick AS, Sutherland MT, Hernandez Z, Fox PM, … Laird AR (2015). Meta-analytic connectivity and behavioral parcellation of the human cerebellum. NeuroImage, 117, 327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D (1998). The cerebellar contribution to language and sequential functions: Evidence from a child with cerebellitis. Cortex, 34, 279–87. [DOI] [PubMed] [Google Scholar]
- Riva D, & Giorgi C (2000). The cerebellum contributes to higher functions during development: Evidence from a series of children surgically treated for posterior fossa tumours. Brain, 123, 1051–61. [DOI] [PubMed] [Google Scholar]
- Salamon N, Sicotte N, Drain A, Frew A, Alger JR, Jen J, … Salamon G (2007). White matter fiber tractography and color mapping of the normal human cerebellum with diffusion tensor imaging. Journal of Neuroradiology, 34, 115–28. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, … Brodie JD (1998). Functional magnetic resonance imaging of human brain activity in a verbal fluency task. Journal of Neurology, Neurosurgery, and Psychiatry, 64, 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (2001). The cerebellar cognitive affective syndrome: Clinical correlations of the dysmetria of thought hypothesis. International Review of Psychiatry, 13, 313–322. [Google Scholar]
- Schmahmann JD, & Pandya DN (1995). Prefrontal cortex projections to the basilar pons in rhesus monkey: Implications for the cerebellar contribution to higher function. Neuroscience Letters, 199, 175–8. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Petrides M, Evans AC, & Toga AW (2000). MRI atlas of the human cerebellum. Cambridge, MA: Academic Press. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Alexander MP, Susan Gillingham BA, Cusimano M, & Stuss DT (2010). Lateralized cerebellar contributions to word generation: A phonemic and semantic fluency study. Behavioural Neurology, 23, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RB, Stoodley CJ, Anslow P, Paul C, Stein JF, Sugden EM, & Mitchell CD (2001). Lateralized cognitive deficits in children following cerebellar lesions. Developmental Medicine and Child Neurology, 43, 685–91. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, & Krakauer JW (2010). Error correction, sensory prediction, and adaptation in motor control. Annual Review of Neuroscience, 33, 89–108. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ (2014). Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Frontiers in Systems Neuroscience, 8, 92 10.3389/fnsys.2014.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2016). The cerebellum and neurodevelopmental disorders.Cerebellum, 15, 34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, & Schmahmann JD (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage, 44, 489–501. [DOI] [PubMed] [Google Scholar]
- Tomlinson SP, Davis NJ, & Bracewell RM (2013). Brain stimulation studies of non-motor cerebellar function: A systematic review. Neuroscience and Biobehavioral Reviews, 37, 766–89. [DOI] [PubMed] [Google Scholar]
- Tremblay P, & Dick AS (2016). Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain and Language, 162, 60–71. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Swears MK, D’Mello AM, & Stoodley CJ (2016). Cerebellar tDCS as a novel treatment for aphasia? Evidence from behavioral and resting-state functional connectivity data in healthy adults. Restorative Neurology and Neuroscience, 34, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC (2002). Surface-Based atlases of cerebellar cortex in the human, macaque, and mouse. Annals of the New York Academy of Sciences, 978, 468–479. [DOI] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, … Sunaert S (2014). Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. NeuroImage. Clinical, 4, 374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PP, Hesselink JR, Jernigan TL, Doherty S, & Bellugi U (1992). Specific neurobehavioral profile of williams’ syndrome is associated with neocerebellar hemispheric preservation. Neurology, 42, 1999–2002. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Siedentopf C, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, … Delazer M (2003). Brain activation pattern during a verbal fluency test in healthy male and female volunteers: A functional magnetic resonance imaging study. Neuroscience Letters, 352, 191–194. [DOI] [PubMed] [Google Scholar]