Introduction
Iohexol is a non-ionic x-ray contrast medium of low osmolality, extensively used in clinical radiology and considered essentially free from side effects (1, 2, 3). Like other iodine-containing contrast media, it is eliminated from the body by excretion in the urine. These substances are therefore potential markers for renal function.
We designed a chemical method for measurement of iohexol concentrations (4) and characterized its pharmacokinetics in man in relation to a number of other contrast media (5, 6). We found that is indeed a suitable marker for glomerular filtration rate (GFR): after intravenous injection, it is quantitatively recovered in the urine; the elimination occurs by glomerular filtration, with no signs of tubular secretion or reabsorbtion.
In 1984, we introduced iohexol clearance as a method for the determination of glomerular filtration rate (GFR) in clinical work (4). Since then, we have used the method extensively in experimental medicine, and since 1988 it is the standard method for GFR measurements at our hospital. Iohexol clearance is now performed by at least 30 laboratories in Sweden, and has been adopted as a standard method, which has largely replaced Cr-EDTA clearance for the measurement of GFR. Numerous publications from other groups demonstrate that the method has proven useful also in an international perspective.
This paper summarizes our experiences from about 8000 GFR determinations of GFR by iohexol clearance in clinical routine, and presents some modifications of the original method with regard to the measurement of iohexol as well as to the design of GFR investigations.
Measurement of iohexol
Iohexol can be quantitated by high pressure liquid chromatography (HPLC) (4), by chemical measurement based on the determination of iodine (7), or by x-ray absorption (8). In clinical practice, HPLC has advantages due to its sensitivity and flexibility which responds well to the logistic demands of clinical medicine. The HPLC technique is based on separation and quantitation of iohexol by affinity chromatography after precipitation of plasma proteins (4). Iohexol is present in plasma as two isomers, both of which can be used for quantitation.
In the clinical setting, it was soon discovered that a number of endogenous and exogenous substances could interfere with the quantitation of iohexol. Especially, a metabolite of paracetamol (paracetamol glucuronide) tends to interfere with the chromatographic peaks of iohexol. A number of methodological modifications, aiming at combining different separative principles, have been introduce and have successfully eliminated or reduced this problem. Our present protocol for iohexol determination is given in appendix 1. With this method, it is extremely rare that the iohexol measurement is compromized by interfering substances, although our samples include those from patients with severe metabolic diseases, in intensive care, and on complex pharmacotherapy. The day-to-day variation in our hands is about 3% (CV), which is satisfactory when compared to the biological variation of GFR (see below).
In our hands, the method is stable and reliable. As a rule, samples obtained during the day are pretreated in the afternoon and analyzed overnight. When necessary, a GFR measurement can be performed in about 5 hrs (3 hrs for the clinical investigation and 1-2 hrs for the determination of iohexol and calculation of clearance).
Iohexol clearance determinations are now performed at numerous hospitals in Sweden. A national quality assurance program, now including 20 different laboratories, was initiated in 1996. All use versions of the HPLC technique. Evaluation of the program shows that the method has a satisfactory performance.
Dosage and sampling
Iohexol clearance was originally described (4) using a design similar to that employed for 51Cr-EDTA clearance, i. e. with four venous or capillary (9) samples drawn between 3 and 4 hrs after the injection of 2-5 mL iohexol (10). (This volume can be administered with negligible variation; the sensitivity of the analysis is by no means critical). However, in most subjects the area under the elimination curve, and hence clearance, can be accurately estimated from one single sample drawn 3 or 4 hours after injection (11). A prerequisite for this simplification is that the distribution volume (i e, the extracellular volume) can be accurately estimated from antropometric data (height, weight, age and sex), which in turn allows the calculation of theoretical initial concentration (injected dose/distribution volume). In practice, the calculated concentration at time 0 and the measured concentration at 3 or 4 hours have proven sufficient for accurate determination of GFR. In large patient materials, data from single-sample investigations have been shown to correlate closely with those from four-sample investigations (correlations >0,95). Thus, repeated sampling is necessary only in subjects where distribution volume cannot be accurately estimated from antropometric data, e g in pregnant women, in children and in patients with severe derangements in fluid balance. However, in these cases two samples are sufficient to accurately determine clearance.
Our standard protocol for the investigation is presented in appendix 2.
Measurement of GFR in subjects with reduced renal function
Although the measurement of GFR is clinically most important in subjects with moderately reduced renal function (where serum markers such as S-Creatinine and S-Cystatin C are insufficiently informative), accurate determinations of GFR are important in specific situations when severely reduced renal function is suspected or apparent. This requires modification of the protocol, since the elimination of the marker in such patients is so slow that iohexol concentrations 3 or 4 hrs after injection is not very different from that at time 0.
Moreover, the possible extrarenal elimination (which may be so slow that it is negligible for the determination of GFR in subjects with normal renal function) must be taken into consideration.
It is easily realized that, in patients with slow elimination of the exogenous marker, it is difficult or impossible to define the area under the elimination curve, and thus clearance, from samples obtained after 3 or 4 hours. The influence of the relevant sources of variation (dosage, measurement of iohexol, accuracy of sampling time) is minimized if the sample is drawn when the plasma concentration, in the semi-log elimination curve, has declined to a level about half-way between the initial concentration and zero (fig 1). By postponing sampling to up to 72 hrs after injection, accurate measurements of GFR can be performed in subjects with GFR as low as 2-3 mL/min (12). Furthermore, renal clearances and plasma clearances are virtually identical in such patients, demonstrating that the extrarenal elimination of iohexol is indeed negligible (12). Table 1 lists our present guidelines for sampling in patients with reduced renal function. A measurement of S-Creatinine is sufficient to choose a suitable protocol.
Figure 1:
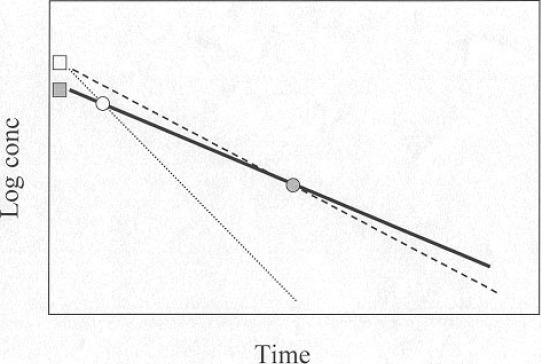
Rationale for differential sampling points, according to renal function, for single-sample determination of iohexol clearance. The theoretical concentration at time 0 is calculated from the given dose/distribution volume (estimated from antropometric data) and the sample is obtained at a time-point when the concentration, in the semilog representation, is about half the initial (filled symbols). Iohexol clearance is calculated as dose/area under the curve (solid line). With this format, the method is rather insensitive to a possible imprecision in the estimated initial concentration (dashed line), which is not the case if the sample is drawn at an earlier time-point (open symbols, dotted line). It is also evident that analytical imprecision in the measurement of iohexol will have a minor influence on the clearance value with this design.
Table I.
Time-points (hours after injection) for blood sampling recommended for the determination of GFR by iohexol clearance in subjects with reduced renal function.
| Estimated glomerular filtration rate (ml/min × 1,73 m 2) |
| >40 15-40 5-15 < 5 |
| Single sample 3-4 6-8 24-32 48-72 |
| Two-sample 3 and 4 6 and 8 24 and 32 48 and 72 |
* Two-sample investigations are needed only when the distribution volume of the marker can not be adequately predicted from antropometric data (see test).
Rationale for differential sampling points, according to renal function, for single-sample determination of iohexol clearance. The theoretical concentration at time 0 is calculated from the given dose/distribution volume (estimated from antropometric data) and the sample is obtained at a time-point when the concentration, in the semilog representation, is about half the initial (filled symbols). Iohexol clearance is calculated as dose/area under the curve (solid line). With this format, the method is rather insensitive to a possible imprecision in the estimated initial concentration (dashed line), which is not the case if the sample is drawn at an earlier time-point (open symbols, dotted line). It is also evident that analytical imprecision in the measurement of iohexol will have a minor influence on the clearance value with this design.
Clinical performance
The total variation of iohexol clearance, estimated from repeated investigations in subjects with normal renal function, is about 11% (4), which is similar to that of 51CRr-EDTA. Most of the variation (?10%) is accounted for by biological variation (4). This is considerably lower than what can be obtained, with reasonable precautions and patient instructions, for (renal) creatinine clearance. Reference intervals (13), as expected, reflect a decreasing GFR after age 50, and are similar to those reported for eg 51Cr-EDTA clearance.
GFR does not show any diurnal variations, and thus, investigations can be started in the morning or afternoon in order to avoid inconvenient sampling hours. Due to the possible short-term influence of protein intake on GFR (renal reserve capacity) we advice our patients to avoid extreme protein intake for two days before investigation.
We have found iohexol clearance a simple, precise method, suitable for the determination of GFR in all kinds of patients. Since no radioactivity is involved, the procedure is flexible and investigations can be performed outside the laboratory. In children, hemiplegic patients, patients in intensive care, women with pregnancy complications (14) etc, this flexibility has proven valuable.
In our hospital (with a population basis of about 300 000) the number of GFR measurements performed annually was, from 1976 to 1986, approximately 1000 (by Cr-EDTA clearance; measurements of creatinine clearance are not performed due to the poor precision). When iohexol clearance was introduced, the number of GFR investigations increased to about 1500 per year. We interpret this as a consequence of the superior flexibility and simplicity of the (non-radioactive) iohexol clearance, as well as a recruitment of patient groups in which radioactive techniques have, by tradition, been applied with some restriction (children, pregnant women).
Contraindications
Iohexol, also when used in radiographic doses (ie-10-50 times higher than in the GFR protocol), has an extremely low toxicity (1, 2, 3), probably due to its low osmolality and its negligible content of free iodine. Initially, we exerted precautions with iohexol clearance determinations in subjects who had a history of iodine hypersensitivity or a previous adverse reaction related to angiography or urography. However, with time we have realized that such anamnestic information has no relevance for the small doses of iohexol used in the GFR measurement. At present, we have no contraindications but keep all patients under supervision for 15 min after injection of iohexol. We have had no complications in 8000 investigations except for two patients who reported transient malaise and vomiting between 1 and 3 hours after injection of iohexol. We do not know whether this was indeed caused by our procedures.
Price
The total price for iohexol clearance is 400 SEK. If the investigation is performed locally and samples are sent to the laboratory for analysis and clearance calculation we charge 250 SEK. This is considerably lower than the cost of 51Cr-EDTA clearance (about 1500 >SEK); a major difference lies in the stricter routines which have to be adopted when isotopes are involved.
Conclusions
Iohexol clearance has now been in clinical use for 15 years; it has proven a simple, exact and reliable method for GFR determination. In about 8000 investigations, we have not recorded any side effects even in subjects with a history of iodine hypersensitivity or adverse reactions to x-ray contrast investigations. Our patients include children, pregnant women and patients with severely reduced renal function. In experimental medicine, the technique can be modified for specific purposes such as monitoring of short-term changes in renal function, determination of renal reserve capacity etc.
Acknowledgement
I am grateful to Anders Andersson, Ph D, for developing and summarizing the latest version of our HPLC method.
Appendix 1. Protocol for quantitation of iohexol by HPLC
Add 200 µL of perchloric acid (0,33 M) to 50 ml of serum/plasma and mix.
After centrifugation, iohexol in the supernatant is separated and quantified by reverse-phase HPLC using a 4,6 x 200 mm, C18, 5 m m particle size, analytical column (Hichrom, Theale, Reading, UK). The mobile phase contains 95 % (v/v) citric acid/citrate buffer, 20 mmol/L, pH 4,5 and 5 % acetonitrile and is maintained at a flow of 0,9 mL/min.
After elution of both iohexol isomers (10 minutes) the column is washed with 100% methanol during 1,5 minutes at a flow of 0,9 ml/min. This washing procedure eliminates interference, in the subsequent samples, from late eluting unknown peaks which may occur particularly in samples from patients with low renal function. The column is then equilibrated with mobile phase for 8,5 min.
The concentration of iohexol is calculated from the area under the largest iohexol peak as compared to suitable external standards of iohexol.
Appendix 2. Protocol for procedures for iohexol clearance investigation
Measure height and body mass.
Draw a 0-time sample (capillary or venous serum/plasma).
Inject exactly 5 ml of iohexol (Omnipaque, Nycomed Amersham, 300 mg I/L) intravenously.
After 15 min observation, the patient may leave the laboratory.
After 3-4 hours (record exact time) draw a capillary or venous sample. (Due to the risk of contamination, avoid to use the port of access to the circulation that was used for administration of iohexol).
Analyze the 0-time sample (to exclude interfering substances or previously given iohexol) and the test sample by HPLC.
Calculate clearance.
Iohexol clearance is reported as absolute value (ml/min) as well as corrected for body surface area (ml/min x 1,73 m2 body surface).
References
- 1.Almen T. Experimental investigations of iohexol and their clinical relevance. Acta Radiol 366: 9-19 (Suppl), 1983. [PubMed] [Google Scholar]
- 2.Schrott KM, Behrends B, Clauss W, Kaufmann J, Lehnert J. Iohexol in excretory urography. Fortschr Med 104:153-156, 1986. [PubMed] [Google Scholar]
- 3.Albrechtsson U, Hultberg B, Larusdottir H, Norgren L. Nefrotoxicity of ionic and non-ionic contrast media in aorofemoral angiography. Acta Radiol Diagnosis 26:615-618, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Krutzen E, Back SE, Nilsson-Ehle I, Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: A method for the determination of glomerular filtration rate. J Lab Clin Med 104:955-961, 1984. [PubMed] [Google Scholar]
- 5.Back SE, Krutzen E, Nilsson-Ehle P. Contrast media as markers for glomerular filtration: a pharmacokinetic comparison of four agents. Scand J Lab Clin Invest 48:247-253, 1988. [DOI] [PubMed] [Google Scholar]
- 6.Back S E, Krutzen E, Nilsson-Ehle P. Contrast media and glomerular filtration: Dose dependance of clearance for three agents. J Pharmaceutical Sci 77: 765-767, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Back S E, Masson P, Nilsson-Ehle P. A simple chemical method for the quantitation of the contrast agent, iohexol, applicable for GFR measurements. Scand J Clin Lab Invest 48: 825-829, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Gronberg T, Sjoberg S, Almen T, Golman K, Mattson S. Non-invasive estimation of kidney function by x-ray fluorescence analysis. Invest radiol 18:445-452, 1983. [PubMed] [Google Scholar]
- 9.Krutzen E, Back SE, Nilsson-Ehle P. Determination of glomerular filtration rate using iohexol clearance and capillary sampling. Scand J Clin Lab Invest. 50: 279-283, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Lab Clin Invest 30:271-274, 1972. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsson L: A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 3:297-305, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson-Ehle P, Grubb A. New markers for the determination of GFR: Iohexol clearance and cystatin C serum concentration. Kidney Int 46, suppl 47:S-17-S-19, 1994. [PubMed] [Google Scholar]
- 13.Back SE, Ljungberg B, Nilsson-Ehle I, Borga O, Nilsson-Ehle P. Age dependence of renal function: Clearance of iohexol and p-aminohippurate in a healthy male population. Scand J Clin Lab Invest 49:641-646, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Krutzen E, Olofsson P, Back S E, Nilsson-Ehle P. 1992. Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand J Clin Lab Invest 52, 387-392. [DOI] [PubMed] [Google Scholar]


