Summary
There is a lack of well-established criteria for the specific measurement of fibrinolytic parameters. On behalf of the SSC, the subcommittee on Fibrinolysis started a process to develop criteria for the specific measurement of fibrinolytic variables. This report describes the criteria for the specific measurement of plasmin inhibitor activity. In summary, a plasma deficient in plasmin inhibitor should show an activity close to 0%. Plasma containing only the non-plasminogen binding form of plasmin inhibitor should show an activity nearby the activity of a plasma deficient for plasmin inhibitor. Other inhibitors of plasmin, like a 2 -macroglobulin, antithrombin in the presence of heparin, and C1-esterase inhibitor should not interfere in the assay at the level usually found in pathological conditions or at the higher normal level.
Specific measurement of Plasmin Inhibitor activity
Introduction
Plasmin Inhibitor, previously known as a 2 -plasmin inhibitor or a 2 -antiplasmin (1), occurs in blood partially as a very fast-acting inhibitor of plasmin and therefore is an important regulator of the fibrinolytic system (2). The glycoprotein, plasmin inhibitor, is a serine protease inhibitor of molecular weight 65-70 kD, present in plasma at a concentration of approximately 1 m mol/l (3). The circulating glycoprotein is mainly synthesized by the liver and has a catabolism corresponding to a plasma half-life of about 2.5 days (2). The human gene is constituted of 16 Kb, 10 exons, 9 introns, and is located on chromosome 17 (4,5).
Reduced plasma levels of plasmin inhibitor can occur due to congenital deficiencies I and II (6). These deficiencies can be associated with bleeding occurring some hours after the initial injury.
Clotting and wound healing are usually normal, but the haemostatic plug breaks down prematurely (7). Decreased concentrations are known for thrombolytic therapy, severe chronic liver diseases, nephrotic syndrome, disseminated intravascular coagulation, amyloidosis, leukaemia (specially acute promyelocytic leukaemia), L-asparaginase therapy, the postoperative period and extracorporeal circulation (6,8-11). Elevated levels of plasmin inhibitor have been observed in some cases with thrombotic complications and in cases with type II hyperlipoproteinemia and progressive renal failure (12-17).
The plasmin inhibitor occurs in blood mainly in two molecular forms: a plasminogen-binding (PB) and a non-plasminogen binding (NPB) form (18). On average the ratio PB:NPB is 2:1(19). The PB form is a very fast-acting plasmin inhibitor; NPB reacts at least 20 times more slowly (20-25). The PB form of plasmin inhibitor is responsible for the rapid plasmin inactivation observed in plasma. the inhibitor further can lose its N-terminal 12 amino acid peptide in the circulation (26,27) and acquires the capacity to cross-link to fibrin catalysed by coagulation factor XII (26,28). In blood other molecules can also complex with plasmin, e.g. a 2 - macroglobulin, antithrombin and C1-esterase inhibitor (29).
For the quantitative analysis of the fast-acting PB form of plasmin inhibitor in plasma, several chromogenic methods have been developed (30-34). Recently it has been shown that some of the commercially available activity methods have restricted specificity, especially at low concentrations. This effect results in values of 10-30% in the analysis of plasmin inhibitor deficient plasmas (35-37). It was shown that the apparent plasmin inhibitor values for deficient plasmas will increase with increased levels of added plasmin (37). The reason could be an increased effect of a 2 -macroglobulin. It is reported that at low plasmin concentrations a 2 -macroglobulin can play an important role in the inhibition of plasmin (25,38). Using selected low plasmin concentrations (37) a new commercially available method has been developed with enhanced specificity (39).
This report describes the criteria for a specific method of the functional measurement of the fast-acting form of plasmin inhibitor in plasma.
Kinetics
In the measurement of plasmin inhibitor three reactions of plasmin are of importance. First is the reaction with the PB-form of the plasmin inhibitor. Second is the reaction with the NPB-form of the plasmin inhibitor and third the reaction with a 2 - macroglobulin. Using a small excess of plasmin (approx. 1 m M), all three reactions take place as a second order reaction ([plasmin inhibitor] total approx. [plasmin] total). At a plasmin inhibitor concentration of 1 m M gives this a velocity for the plasmin-PB-plasmin inhibitor complex formation of 27 s -1. For the plasmin-NPB-plasmin inhibitor complex formation this is 0.2 s -1 and for the plasmin- a 2 -macroglobulin complex formation this is 1.0 s -1. Expressed in ratios of the complexes this is 95.7% for the plasmin-PB-plasmin inhibitor complex, 0.7% for the plasmin-NPB-plasmin inhibitor complex and 3.6% for the plasmin- a 2 - macroglobulin complex.
At a level of 0.1 m M of plasmin inhibitor the ratios of the complexes will be 72.6% for the plasmin-PB-plasmin inhibitor complex, 0.0% for the plasmin-NPB-plasmin inhibitor complex and 26.9% for the plasmin- a 2 -macroglobulin complex (25, 38).
The increased importance of the influence of a 2 - macroglobulin at low plasmin inhibitor concentrations indicates the need for inhibition of this effect. The effect of a 2-macroglobulin could be abolished by the addition of methylamine to the assay system (40,41).
Principle of the assay procedure
The assay of the fast-acting form of plasmin inhibitor involves two reaction steps illustrated in fig 1
Figure 1.
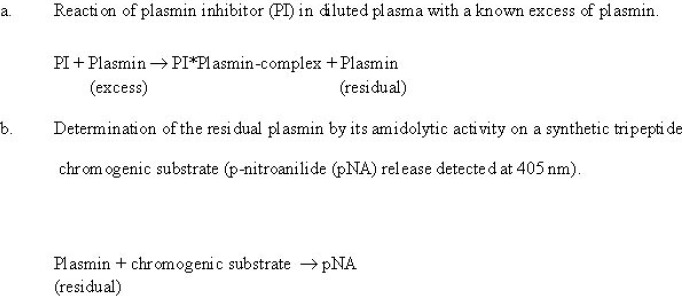
The rate of pNA release is compared to similar data of a calibration curve constructed by using different dilutions of pooled plasma standard. The content of the pooled plasma standard is set at 1 arbitrary unit/ml (= 100%).
The amount of plasma used in the test and in relation to that the concentration of the added plasmin and the incubation time is important for the specificity of the method (37).
Since plasmin and plasmin inhibitor forms a 1:1 molar complex the excess of plasmin added to the test should be minimised to avoid the influence of other inhibitors.
The preincubation time should be as short as possible (< 60 seconds) to avoid the participation of slow- acting inhibitors in the reaction.
Manufacturers of commercially available tests should have stated these variables in their kit insert, expressing the final plasmin concentration (nKat) and the time of the different reaction steps (preincubation and reading).
The measurement of plasmin inhibitor can be influenced by a 2 -macroglobulin. This effect can be abolished by the addition of methylamine to the dilution buffer (40).
In a specific test for plasmin inhibitor no interference was observed for _-amino caproic acid (4 mmol/l), Heparin (2 IU/ml), Fragmin (2 IU/ml) and Lysine (2000 m mol/l) (43).
Manuals
The determination of plasmin inhibitor is described in a laboratory manual.
ECAT assay procedures: Plasma Plasmin Inhibitor activity by C. Kluft and P. Meijer (34)
Criteria for specificity
Plasma deficient in plasmin inhibitor, both for the PB and NPB form, should show an activity close to 0%.
Plasma containing only the NPB form of plasmin inhibitor should show an activity near to the activity of a plasma deficient in plasmin inhibitor.
a 2 -macroglobulin, antithrombin in the presence of heparin, and C1-esterase inhibitor should not interfere in the assay at the level usually found in pathological conditions or at the higher normal level.
Test method for criteria
A method for the detection of plasmin inhibitor should be tested for specificity by measuring the following samples:
Plasma naturally deficient in or immuno-depleted for plasmin inhibitor should show a residual activity less then 5%.
The plasmin inhibitor in plasma containing only the NPB form of plasmin inhibitor should be equal to plasma deficient in plasmin inhibitor (residual activity less then 5%, see point a).
Plasma charged with 3 times higher level of a 2 - macroglobulin, C1-esterase inhibitor or antithrombin in the presence of heparin (2 IU/ml), in normal plasma should have the same plasmin inhibitor as the plasma without the addition of an excess of these inhibitors.
Standardisation and quality assurance
No reference material is available at present. Calibrator plasma should be obtained by pooling plasma of apparently healthy volunteers (at least 20 donors), using a sex ratio of approximately 1. Users of oral contraceptives or hormone replacement therapy should be excluded. The value of this calibrator plasma is set at 1 arbitrary unit (AU) (= 100%). A calibration curve should cover the whole reference range and exists of minimal 5 points.
Two control plasmas should be included in each set of measurements, including a normal range value (0.80-1.00 AU) and a low range value (0.20-0.40 AU). Repeatability and reproducibility should allow preferably less than 6% of variation coefficient on 10 consecutive determinations.
Remarks
1. Collection of the blood sample
Since no diurnal rhythm for plasmin inhibitor is known, blood sampling can take place at any time of the day.
To avoid variability in haematocrit, select either the sitting or lying position of the patient during venepuncture (42).
2. Processing of the blood sample
Storage of blood for a longer time and at higher temperatures promotes the conversion of the PB-form to the NPB-form (19).
3. Instrumentation
The test for the determination could be done by a manual method as well as with automated analysers with the possibility of photometric measurements (405 nm).
Since analysers from different suppliers have their own specifications and limitations, the criteria for specificity should be tested for all type of equipments separately, or made available from the reagent manufacturers.
4. Reference ranges
In 25 apparently healthy volunteers, aged between 20 and 50 years and sex ratio approximately 1, plasmin inhibitor, assayed with a method fulfilling the criteria described above, showed a narrow range: 83-108%.
References
- 1.Blomback M, Abildgaard U, van den Besselaar AM, Clementson KJ, Dahlback B, Exner T, et al. Nomenclature of quantities and units in thrombosis and haemostasis (recommendation 1993). A Collaborative project of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (ISTH/SSC) and the Commission/Committee on Quantities and Units (in Clinical Chemistry) of the International Union of Pure and Applied Chemistry International Federation of Clinical Chemistry (IUPAC IFCC/CQU(CC)). Thromb Haemost 1994;71:375-394. [PubMed] [Google Scholar]
- 2.Collen D, Wiman B. Fast-acting plasmin inhibitor in human in plasma. Blood 1978; 51: 563-569 [PubMed] [Google Scholar]
- 3.Wiman B. Human a2-antiplasmin. Methods Enzymol 1981; 80: 395-408 [Google Scholar]
- 4.Hirosawa S, Nakamura Y, Miura O, Sumi Y, Aoki N. Organization of the a2-plasmin inhibitor gene. Proc Natl Acad Sci 1988; 85: 6839-6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch SK, Francke U. Assignment of the human a2-plasmin inhibitor gene (PLI) to chromosome 17, region pter-p12, by PCR analysis of somatic cell hybrids. Genomics 1982; 13: 213-214 [DOI] [PubMed] [Google Scholar]
- 6.Saito H. a2-Plasmin inhibitor and its deficiency states. J Lab Clin Med 1988; 112: 671-678 [PubMed] [Google Scholar]
- 7.Booth NA. The natural inhibitors of fibrinolysis. In: Bloom AL, Forbes CH, Thomas D P, Tuddenham EGD, eds. Haemostasis and Thrombosis, vol 1, 3rd ed.,1994: 699-717 [Google Scholar]
- 8.Aoki N. Hemostasis associated with abnormalities of fibrinolysis. Blood Rev 1989; 3: 11-17 [DOI] [PubMed] [Google Scholar]
- 9.Avvisati G, Ten Cate JW, Sturk A, Lamping RJ, Petti MC, Mandelli F. Acquired a2-antiplasmin deficiency in acute promyelocytic leukaemia. Br J Haematol 1988;70:43-48 [DOI] [PubMed] [Google Scholar]
- 10.Vellenga E, Kluft C, Mulder NH, Wijngaards G, Nieweg HO. The influence of L-asparaginase therapy on the fibrinolytic system. Br J Haematol 1984; 57:247-254 [PubMed] [Google Scholar]
- 11.Kluft C. Fibrinolytic shut-down after surgery. Sawaya R, ed. Fibrinolysis and the central nervous system, Hanley & Belfus; 1990:127-140 [Google Scholar]
- 12.Engesser L. Thrombophilia. Disorders of blood coagulation and fibrinolysis. Thesis. 1988, Leiden University. [Google Scholar]
- 13.Kluft C, Leebeek FWG. a2-Antiplasmin and thrombosis: results of a questionnaire. Fibrinolysis 1988; 2, suppl.2: 47-48 [Google Scholar]
- 14.Brommer EJP, Gevers Leuven JA, Kluft C, Wijngaards G. Fibrinolytic inhibitor in type II hyperlipoproteinaemia. Lancet 1982;1:1066. [DOI] [PubMed] [Google Scholar]
- 15.Ratnoff OD. The role of haemostatic mechanisms. Clin Haematol 1981;10:261-281 [PubMed] [Google Scholar]
- 16.Lowe GDO, Stromberg P, Forbes CD, McArdle BM, Lorimer AR, Prentice CRM. Increased blood viscosity and fibrinolytic inhibitor in type Hhyperlipoproteinaemia. Lancet 1982;1:472. [DOI] [PubMed] [Google Scholar]
- 17.Gordge MP, Faint RW, Rylance PB, Kluft C, Nield GH. Abnormal fibrinolysis in progressive renal failure due to a reduction in circulating tissue plasminogen activator. Br J Haematol 1988;69:133 [Google Scholar]
- 18.Clemmensen I. Different molecular forms of a2-antiplasmin. Collen D, Wiman B, Verstraete M, eds. The physiological inhibitors of coagulation and fibrinolysis. Amsterdam: Elsevier/North-Holland; 1979:131-136 [Google Scholar]
- 19.Kluft C, Los P, Jie AFH, van Hinsbergh VWM, Vellenga E, Jespersen J, Henny C. The mutual relationship between the two molecular forms of the major fibrinolysis inhibitor a2-antiplasmin in blood. Blood 1986;67:616-622 [PubMed] [Google Scholar]
- 20.Christensen U, Clemmensen I. Kinetic properties of the primary inhibitor of plasmin from human plasma. Biochem J 1977;163:389-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen U, Clemmensen I. Purification and reaction mechanisms of the primary inhibitor of plasmin from human plasma. Biochem J 1978;175:635-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiman B, Collen D. On the kinetics of the reaction between human antiplasmin and plasmin. Eur J Biochem 1978;84:573-578 [DOI] [PubMed] [Google Scholar]
- 23.Wiman B, Boman L, Collen D. On the kinetics of the reaction between human antiplasmin and a low-molecular-weight form of plasmin. Eur J Biochem 1978;87:143-146 [DOI] [PubMed] [Google Scholar]
- 24.Petersen LC, Clemmensen I. Kinetics of plasmin inhibition in the presence of synthetic tripeptide substrate. Biochem J 1981;199:121-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen U, Bangert K, Thorsen S. Reaction of human a 2 -antiplasmin and plasmin stoppedflow fluorescence kinetics. FEBS Letters 1996;387:58-62 [DOI] [PubMed] [Google Scholar]
- 26.Koyama T, Koike Y, Toyota S, Miyagi F, Suzuki N, Aoki N. Different NH2-terminal form with 12 additional residues of a2-plasmin inhibitor from human plasma and culture media of Hep G2 cells. Biochem Biophys Res Commun 1994; 200: 417-422. [DOI] [PubMed] [Google Scholar]
- 27.Bangert K, Johnsen AH, Christensen U, Thorsen S. Different N-terminal forms of alpha 2 plasmin inhibitor in human plasma. Biochem J 1993; 291:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumi Y, Ichikawa Y, Nakamura Y, Miura O, Aoki N. Expression and characterization of pro a2-plasmin inhibitor. J Biochem 1989;106:703-707. [DOI] [PubMed] [Google Scholar]
- 29.Levi M, Roem D, Kamp AM, de Boer JP, Hack CE, ten Cate J W. Assessment of the relative contribution of different protease inhibitors to the inhibition of plasmin in vivo. Thromb Haemost 1993; 69:141-146 [PubMed] [Google Scholar]
- 31.Teger-Nilsson AC, Friberger P, Gyzander E. Determination of a rapid plasmin inhibitor in human blood by means of a plasmin specific tripeptide substrate. Scand J Clin lab Invest 1977;37:403-409 [PubMed] [Google Scholar]
- 31.Naito K, Aoki N. Assay of a2-plasmin inhibitor activity by means of a plasmin specific tripeptide substrate. Thromb Res 1978;12:1147-1156 [DOI] [PubMed] [Google Scholar]
- 32.Gallimore MJ, Amundsen E, Aasen AO, Larsbraaten M, Lyngaas K, Svendsen L. Studies on plasma antiplasmin activity using a new plasmin speciific chromogenic tripeptide substrate. Tromb Res 1979;14:51-60 [DOI] [PubMed] [Google Scholar]
- 33.Friberger P. Chromogenic peptide substrates. Their use for the assay of factors in the fibrinolytic and the plasma kallikrein-kinin systems. Scand J Clin Lab Invest 1982;42, suppl.262:41-47 [PubMed] [Google Scholar]
- 34.Kluft C, Meijer P. Plasma Plasmin Inhibitor activity. Jespersen J, Bertina RM, Haverkate F, eds. ECAT assay procedures. A Manual of laboratory techniques. Dordrecht Kluwer, 165-171. [Google Scholar]
- 35.Meijer P, Westerveld W, Huisman LGM. An improved method for the detection of a-antiplasmin on the automated coagulation analyzer MLA Electra 1000C. Fibrinolysis 1994; 8, suppl.2:160-162 [Google Scholar]
- 36.Woodham B. Letter to the Editor. Fibrinolysis 1995; 9:129-131 [Google Scholar]
- 37.Meijer P, Kluft C. Criteria for an automated specific plasmin inhibitor test (SPIT) in human plasma. Fibrinolysis 1996;10, suppl.2:121-123 [Google Scholar]
- 38.Christensen U, Sottrup-Jensen L. Mechanism of alpha2-macroglobulin-proteinase interactions. Studies with trypsin and plasmin. Biochemistry 1984;23:6619-6626 [DOI] [PubMed] [Google Scholar]
- 39.Billing-Clason S, Meijer P, Kluft C, Ersdal E. A novel method, Coamatic Plasmin Inhibitor, for the specific determination of plasmin inhibitor activity in plasma. Fibrinolysis 1996;10, suppl.2:165-167 [DOI] [PubMed] [Google Scholar]
- 40.Matsuda T, Ogawara M, Miura R, Seki T. Selective determination of a2-plasmin inhibitor activity in plasma using chromogenic substrate. Thromb Research 1984;33:379-388 [DOI] [PubMed] [Google Scholar]
- 41.Christensen U, Simonsen M, Harrit N, Sottrup-Jensen L. Pregnancy Zone Protein, a proteinasebinding macroglobulin. Interactions with proteinases and methylamine. Biochemistry 1989;28:9324-9331 [DOI] [PubMed] [Google Scholar]
- 42.Kluft C, Meijer P. Update 1996: Blood collection and handling procedures for assessment of plasminogen activators and inhibitors (Leiden Fibrinolysis Workshop). Fibrinolysis 1996;10, suppl.2:171-179 [Google Scholar]
- 43.Billing Clason S, Meijer P, Kluft C, Ersdal E. Specific determination of plasmin inhibitor activity in plasma: documentation of specificity of manual and automated procedures. Blood Coag and Fibr 1999;10:487-494 [DOI] [PubMed] [Google Scholar]


