Abstract
Objectives
Phosphoribosylpyrophosphate synthetase (PRPS1) superactivity is an X-linked disorder characterized by urate overproduction Online Mendelian Inheritance in Man (OMIM) gene reference 300661. This condition is thought to rarely affect women, and when it does, the clinical presentation is mild. We describe a 16-year-old African American female who developed progressive tophi, nephrolithiasis and acute kidney failure due to urate overproduction. Family history included a mother with tophaceous gout who developed end-stage kidney disease due to nephrolithiasis and an affected sister with polyarticular gout. The main aim of this study was to describe the clinical manifestations of PRPS1 superactivity in women.
Methods
Whole exome sequencing was performed in affected females and their fathers.
Results
Mutational analysis revealed a new c.520 G > A (p.G174R) mutation in the PRPS1 gene. The mutation resulted in decreased PRPS1 inhibition by ADP.
Conclusion
Clinical findings in previously reported females with PRPS1 superactivity showed a high clinical penetrance of this disorder with a mean serum urate level of 8.5 (4.1) mg/dl [506 (247) μmol/l] and a high prevalence of gout. These findings indicate that all women in families with PRPS1 superactivity should be genetically screened for a mutation (for clinical management and genetic counselling). In addition, women with tophaceous gout, gout presenting in childhood, or a strong family history of severe gout should be considered for PRPS1 mutational analysis.
Keywords: hereditary gout, PRPS1 superactivity, PRPS1, X-linked inheritance, gouty arthritis, rare disease, chronic kidney disease, polyarticular gout, hyperuricaemia
Rheumatology key messages
Phosphoribosylpyrophosphate synthetase superactivity is an X-linked disorder that increases enzyme activity.
Unlike other X-linked disorders, female carriers of phosphoribosylpyrophosphate synthetase are likely to be clinically affected.
Females with early onset hyperuricemia and gout should be screened for PRPS1 mutations.
Introduction
Phosphoribosylpyrophosphate synthetase (PRPS1) superactivity (OMIM 300661) is a rare X-linked disorder resulting in increased activity of PRPS1. PRPS1 is the gateway enzyme for entrance of ribose into the purine pathway, catalysing the conversion of adenosine triphosphate (ATP) and ribose-5-phosphate to 5-phospho-α-D-ribosyl, 1-pyrophosphate. PRPS1 superactivity results in increased purine production, leading to increased urate production and gout. Most X-linked disorders result in loss of activity or decreased translation of the affected protein, leading to absence of activity in males and partially preserved function by the alternate X allele in females due to X inactivation. In contrast, in PRPS1 superactivity, there is increased function of the mutated protein. This unregulated increased function can result in clinical effects in females. The extent of this condition in women is poorly characterized [1–9]. In this report we characterize severe gouty arthritis in three women affected with this disorder and review other cases that have been reported.
Case report
The index case was a 16-year-old African American female referred for tophaceous gouty arthritis. The patient had a normal birth and development. At 12.5 years, the patient passed a kidney stone. At 13.5 years the patient presented with polyarticular arthritis. On physical examination, the patient’s height was 153 cm and weight 77.7 kg. Laboratory studies revealed a serum urate level of 14.5 mg/dl (860 μmol/l) normal range (2–5.5 mg/dl, 53–327 μmol/l) and a serum creatinine concentration of 0.9 mg/dl (79.6 μmol/l) (normal range 0.5–1.5 mg/dl, 44–133 μmol/l). Physical examination revealed arthritis of the left fourth and second PIP joints and bilateral ankles. Minimal fluid was obtained on arthrocentesis of the left second PIP joint and the right ankle; fluid analysis revealed no crystals. A diagnosis of gout was considered but not made due to the patient’s age, gender and lack of crystals. The patient was started on treatment for suspected JIA with etanercept and MTX, with minimal improvement. At 14.8 years, the patient presented with a serum urate level of 19.9 mg/dl (1183 μmol/l), a serum creatinine concentration of 2.1 mg/dl (185 μmol/l) and bilateral nephrolithiasis. The patient was given intravenous fluids and was started on allopurinol, 200 mg daily, with improvement of the serum urate level to 9.8 mg/dl (582.9 μmol/l) and the serum creatinine level to 1.2 mg/dl (106 μmol/l). The patient was non-compliant with allopurinol, and at age 15.5 years presented with a serum urate level of 18.2 mg/dl (1083 μmol/l) and serum creatinine of 1.3 mg/dl (114.9 μmol/l). A 24 h urine collection revealed a urinary volume of 1.02 l, a urinary creatinine of 0.73 g (8.3 mg/kg, with 15–20 mg/kg suggesting an adequate collection), and, despite a substantial undercollection, a urinary urate content of 890 mg/day (normal <750 mg/day), with a fractional excretion of urate of 8.7% (normal 6–15%) The allopurinol dosage was increased to 500 mg daily, and the serum urate level improved to 6.9 mg/dl (410 μmol/l). The patient was eventually changed to febuxostat, 80 mg daily, with a decline in the serum urate level to 4.8 mg/dl (285 μmol/l).
Family history (see Fig. 1A and supplementary Table S1, available at Rheumatology online) revealed that the mother was 157.5 cm and 78.02 kg. She suffered from severe tophaceous gouty arthritis with tophi of the feet and both hands. She had uric acid nephrolithiasis and required haemodialysis at age 39 years. The patient’s father did not suffer from gout. III-3 was a child born with hypotonia who died of sudden death at 6 months, consistent with prior reports of some affected males dying in infancy [2].
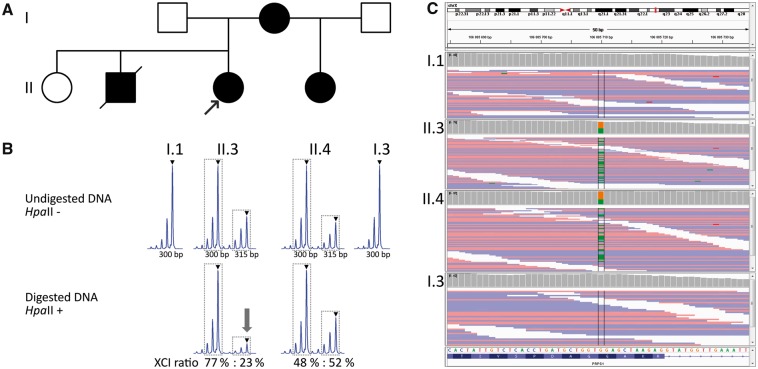
Fig. 1.
Genetic studies
(A) Family pedigree. Black symbols denote affected individuals and open unaffected individuals. (B) X-inactivation analysis in the proband (II.3) and her half-sister (II.4). The XCI ratio was calculated as the ratio of the peak areas of two alleles of the analysed polymorphic repeat in the digested sample, and the result was corrected by the ratio of the peak areas of two alleles in the undigested sample to avoid an error caused by preferential amplification of the shorter allele. The main peaks are denoted by triangle; peak areas used in the X-inactivation ratio formula are designated by dotted lines. The arrow highlights the evident reduction of the maternal allele in the digested sample. (C) The integrative genome viewer (IGV) displays heterozygous mutation chrX: 106885710 NM_002764; c. G>A; p.G174R) of PRPS1 in affected individuals II.3 and II.4 and normal genotype in their fathers I.1 and I.3. XCI: X-chromosome inactivation; chrX: chromosome X.
The patient’s half-sister presented at age 11 with marked swelling and tenderness of the right fourth PIP and MCP joints. The serum uric acid level was 14.5 mg/dl (862 μmol/l), with a serum creatinine of 0.6 mg/dl (53.1 μmol/l).
From a literature review, we identified 14 other females from families with PRPS1 superactivity (supplementary Table S1, available at Rheumatology online). Serum urate levels were elevated in 13 individuals, with a mean serum urate level of 8.5 (4.2) mg/dl (510 (250) μmol/l). Several women had extremely high urinary urate values >1000 mg/day. Only one female had neurologic findings (sensorineural hearing impairment similar to her affected son.)
Methods
The study was approved by the institutional review boards of Wake Forest School of Medicine and the First Faculty of Medicine of Charles University. Informed consent was provided by each patient in the study.
DNA analysis
Genomic DNA isolation and whole exome sequencing was performed in affected females and their fathers using the SOLiD 4 System at the Institute for Inherited Metabolic Disorders (Prague, Czech Republic). Genetic variants of interest were genotyped by direct Sanger sequencing as previously described [10]. The X-inactivation ratios in the affected females were assessed by analysis of differential methylation of promoter regions of connector enhancer of kinase suppressor of Ras 2 (CNKSR2), transmembrane protein 185A (TMEM185A) and high mobility group box 3 (HMGB3) [11].
Structural studies
A figure of the 3 D structure of PRPS1 including known mutations was created [Research Collaboratory for Structural Bioinformatics (RCSB) Protein Database ID 2H06]. Structural models were visualized using Pymol Viewer (DeLano Scientific Palo Alto, CA, USA).
Functional studies
The cDNA coding for wild-type (wt) and mutated 174 R PRPS1 were cloned into the expression vectors pMAL-c2 (NEB, Frankfurt am Main, Germany) and pcDNA™4/myc-HisB (Life Technologies, USA) and corresponding proteins were expressed in Escherichia coli and HeLa cells, respectively. Fusion proteins were affinity purified as previously described [12, 13]. PRPS1 activities were assayed at 37°C in 50 mM Tris pH 7.4, 10 mM magnesium chloride, 1.25 mM EDTA, 0.75 mM ATP, 1 mM dithiothreitol, 0.5 mM hypoxanthine, 1 mM ribose-5-P, 37.5 mM potassium phosphate, with 1-2 μg of human recombinant hypoxanthine-guanine phosphoribosyltransferase. The assays were performed simultaneously either with 1 mM adenosine diphosphate (ADP) (E.coli) or 3 mM ADP (HeLa). The reaction product inosine monophosphate was quantified by HPLC analysis.
Results
DNA investigation
Exome sequencing revealed a c.520 G > A (p.G174R) mutation in the proband (III-1) and her sister (III-4) that was not found in either father (II-1 or II-3). PCR amplification and PRPS1 sequence analysis confirmed these findings (Fig. 1C).
The standard assay for assessing the X-inactivation ratios (human androgen receptor, HUMARA) was not informative in the studied samples. The methylation status of the HpaII restriction sites near the polymorphic short tandem repeats in the promoter regions of CNKSR2, TMEM185A and HMGB3, which has been shown to correlate with inactivation of the HUMARA gene [11], revealed moderate skewing of X-inactivation (ratio of 77: 23%; s.d. 0.3%; in favour of the maternal mutated allele) in the blood. X-inactivation in the proband’s sister was more balanced, with the results of the HMGB3 assay being 48: 52% (s.d. 6.4%) (Fig. 1B), and the CNKSR2 assay 35: 65% (s.d. 0.7%) (data not shown).
Structural studies
Structural analysis (Fig. 2A and B) revealed that the G174R mutation is localized at the dimeric interface close to both the catalytic pocket and the allosteric binding site for ADP (guanine diphosphate, GDP) [14]. Replacement of G174 likely affects inter-subunit interactions and also alters the architecture of the catalytic pocket, as the arginine moiety would now be oriented towards the phosphate group of ATP, with electrostatic interactions increasing the affinity of the enzyme for this substrate. Interestingly, mutations of the G174 residue were described in relapsed acute lymphoblastic leukaemia, causing PRPS1 hyperactivity due to abolished response to allosteric regulators [15]. Moreover, the dimeric interface has been previously identified as a mutational hotspot for PRPS1 superactivity implicated in inherited purine overproduction [16] and in relapsed acute lymphoblastic leukaemia [15]. These data suggest that the G174R mutation induces constitutive hyperactivity due to structural changes in catalytic and regulatory sites of PRPS1 (Fig. 2C).
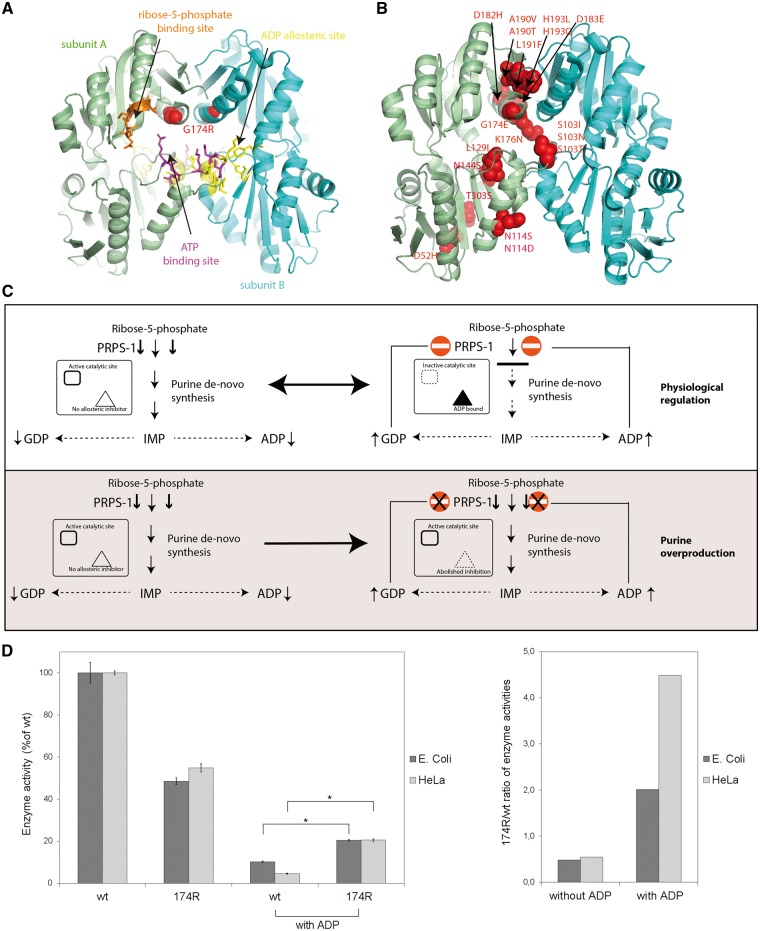
Fig. 2.
Structural and functional studies
(A) Structural mapping of the G174R mutation (red spheres) shows its localization close to PRPS1 binding sites. The ATP binding site (magenta) forms a catalytic pocket jointly with the ribose-5-phosphate site (orange). The ADP allosteric site (yellow) binds allosteric inhibitors of PRPS1. Two subunits of the PRPS1 hexamer are highlighted in a different color. Coordinates of PDB entry 1H06 are used for illustration. (B) Localization of all described PRPS1 mutations causing enzyme superactivity, with most mutations clustered at the dimeric interface. (C) Molecular mechanism of PRPS1 superactive mutations. The mutant enzyme has abolished feedback regulation inducing constitutive activity and purine overproduction. (D) Enzymatic activities of affinity purified recombinant wild-type (wt) PRPS1 and PRPS1 p.174R proteins expressed in E. coli and HeLa cells. Under standard conditions, the PRPS1 p.174R had reduced activity compared with the wt PRPS1. Addition of ADP reduced the activity of the wt PRPS1 much more than the p.174R mutant. Affected feedback regulation of the PRPS1 activity by ADP was demonstrated by increased enzyme activity ratios of PRPS1 p.174R compared with wt PRPS1. Enzyme activities were normalized to that of the wt PRPS1and displayed as mean values of three independent expression experiments; *P < 0.05.
Functional studies
The active site of PRPS1 has binding sites that are regulated by ADP (inhibition) and ribose-5 phosphate (upregulation). To test the hypothesis that the regulation by ADP is affected by the mutation, we measured the enzyme activities of wt and p.174 R recombinant proteins expressed in E. coli and HeLa cells in the presence of ADP. The PRPS1 enzyme activity was inhibited in both wt and mutant recombinant proteins, but the enzymatic activity of mutant protein was higher than the enzymatic activity of wt protein (Fig. 2D). These results showed that the allosteric regulatory site for ADP is impaired in the mutant protein and the regulation of the enzymatic activity by ADP inhibition is affected. Specifically, the wt protein retains <5% of its activity in the presence of ADP, whereas the mutant retains 20% of activity. Thus, it is more active in the presence of ADP, and it constitutively leads to more purine synthesis.
Discussion
We have described a new mutation in PRPS1, resulting in severe hyperuricaemia and gout in three African American women. PRPS1 superactivity has been described in <20 families, and a detailed clinical description has only been provided in two females [3, 7].
There are several factors that should be considered and may be helpful in the diagnosis of hereditary gout. First, the presence of severe gout in a child or female is highly suggestive of a genetic disorder, especially when a strong family history is obtained. The presence of urate nephrolithiasis points to urate overproduction as the cause of hyperuricaemia. Orange urine in infancy is also a clue to urate overproduction.
A first diagnostic step is to perform a 24 h urine collection to assess for urate overproduction (24 h urine urate > 500 mg) or underexcretion (fractional excretion of urate < 6%). Patients with overproduction should be checked for PRPS1 mutations or hypoxanthine phosphoribosyl transferase mutations in males. Patients with a reduced fractional excretion and a positive family history should undergo mutational analysis for UMOD [17] and REN genes [18].
The hyperuricaemia present in the three affected females was quite severe, and these findings may have been related to the mutation type or genetic or environmental factors. As PRPS1 superactivity in women has rarely been described, significant skewing of X-inactivation was suspected. X-inactivation studies showed a mildly increased prevalence of the maternal (affected) chromosome in the proband but not in her sister. This finding likely could explain the increased severity of gout that was found in the proband compared with in her sister.
Women are less likely to be affected by PRPS1 superactivity due to random X inactivation and decreased expression of the mutant enzyme relative to men. In addition, women normally have a higher renal fractional excretion of urate than men, resulting in lower serum urate levels. Despite this, there are a significant number of affected females briefly described in the literature (see supplementary Table S1, available at Rheumatology online). Most affected women have been found to suffer from hyperuricaemia, nephrolithiasis and gout, with only one female being affected with neurologic changes (deafness). These findings indicate that all women in families with PRPS1 superactivity should be screened for a mutation (both for clinical management and genetic counselling). In addition, women with tophaceous gout, gout presenting in childhood, or a strong family history of severe gout should be considered for PRPS1 mutational analysis.
The mutation p.G174R affects the phosphate-binding loop of the catalytic pocket (residues Asp171–Gly174). The enzymatic activity of PRPS1 is regulated by both ribose-5 phosphate, which activates PRPS1, and ADP, which is an inhibitor. Experimental studies, consistent with structural mapping, revealed that the mutant PRPS1 had increased activity in the setting of elevated ADP concentrations compared with the wt PRPS1. Thus the mutated enzyme is locked in a constitutively active state, exhibiting lower activity but lacking responsivity toward physiological feedback inhibitors.
Fortunately, there are increasing therapeutic options for gout, making prompt diagnosis important. Both allopurinol and febuxostat decrease serum urate and urinary urate levels by xanthine oxidase inhibition. Lesinurad and probenecid increase urinary urate excretion but are not indicated in urate overproduction disorders as they will further increase urate excretion and could lead to worsening uric acid excretion [17].
The authors have a specific interest in identifying the genetic causes of pediatric and hereditary gout and can provide genetic analysis for these conditions.
Supplementary Material
Acknowledgements
We thank The National Center for Medical Genomics (LM2015091) for their technical support with exome and gene panel sequencing. Institutional support was provided by the UNCE 204011, UNCE 204064, PROGRES-Q26/LF1 and SVV 260367/2017 programmes of Charles University in Prague and by the Ministry of Education, Youth and Sports of CR [LQ1604 National Sustainability Program II]. AH was supported by grant 15-06582 S from the Czech Science Foundation. The authors acknowledge Vladimir Cermak for providing us with p6H vector.
Funding: This work was partially supported by NIH-NIDDK grant R21 DK106584 and by grants AZV 15-28979 A and AZV 17-29786 A from the Ministry of Health of the Czech Republic.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Porrmann J, Betcheva-Krajcir E, Di DN. et al. Novel PRPS1 gain-of-function mutation in a patient with congenital hyperuricemia and facial anomalies. Am J Med Genet A 2017;173:2736–42. [DOI] [PubMed] [Google Scholar]
- 2. Simmonds HA, Webster DR, Wilson J, Lingham S.. An X-linked syndrome characterised by hyperuricaemia, deafness, and neurodevelopmental abnormalities. Lancet 1982;2:68–70. [DOI] [PubMed] [Google Scholar]
- 3. Becker MA, Raivio KO, Bakay B, Adams WB, Nyhan WL.. Superactive phosphoribosylpyrophosphate synthetase with altered regulatory and catalytic properties. Adv Exp Med Biol 1980;122A:387–92. [DOI] [PubMed] [Google Scholar]
- 4. Nyhan WL, James JA, Teberg AJ, Sweetman L, Nelson LG.. A new disorder of purine metabolism with behavioral manifestations. J Pediatr 1969;74:20–7. [DOI] [PubMed] [Google Scholar]
- 5. Becker MA, Meyer LJ, Wood AW, Seegmiller JE.. Purine overproduction in man associated with increased phosphoribosylpyrophosphate synthetase activity. Science 1973;179:1123–6. [DOI] [PubMed] [Google Scholar]
- 6. Becker MA, Losman MJ, Itkin P, Simkin PA.. Gout with superactive phosphoribosylpyrophosphate synthetase due to increased enzyme catalytic rate. J Lab Clin Med 1982;99:495–511. [PubMed] [Google Scholar]
- 7. García-Pavía P, Torres RJ, Rivero M. et al. Phosphoribosylpyrophosphate synthetase overactivity as a cause of uric acid overproduction in a young woman. Arthritis Rheum 2003;48:2036–41. [DOI] [PubMed] [Google Scholar]
- 8. Sperling O, Boer P, Brosh S, Zoref E, De VA.. Superactivity of phosphoribosylpyrophosphate synthetase, due to feedback resistance, causing purine overproduction and gout. Ciba Found Symp 1977;48:143–64. [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi F, Hanaoka F, Yano E. et al. The mode of genetic transmission of gouty family with increased phosphoribosylpyrophosphate synthetase activity. Hum Genet 1981;58:322–30. [DOI] [PubMed] [Google Scholar]
- 10. Hartmannova H, Piherova L, Tauchmannova K. et al. Acadian variant of Fanconi syndrome is caused by mitochondrial respiratory chain complex I deficiency due to a non-coding mutation in complex I assembly factor NDUFAF6. Hum Mol Genet 2016;25:4062–79. [DOI] [PubMed] [Google Scholar]
- 11. Musalkova D, Minks J, Storkanova G, Dvorakova L, Hrebicek M.. Identification of novel informative loci for DNA-based X-inactivation analysis. Blood Cells Mol Dis 2015;54:210–6. [DOI] [PubMed] [Google Scholar]
- 12. Kmoch S, Hartmannová H, Stribůrková B. et al. Human adenylosuccinate lyase (ADSL), cloning and characterization of full-length cDNA and its isoform, gene structure and molecular basis for ADSL deficiency in six patients. Hum Mol Genet 2000;9:1501–13. [DOI] [PubMed] [Google Scholar]
- 13. Zikanova M, Skopova V, Hnizda A, Krijt J, Kmoch S.. Biochemical and structural analysis of 14 mutant adsl enzyme complexes and correlation to phenotypic heterogeneity of adenylosuccinate lyase deficiency. Hum Mutat 2010;31:445–55. [DOI] [PubMed] [Google Scholar]
- 14. Li S, Lu Y, Peng B, Ding J.. Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site. Biochem J 2007;401:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li B, Li H, Bai Y. et al. Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat Med 2015;21:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Brouwer AP, van BH, Nabuurs SB. et al. PRPS1 mutations: four distinct syndromes and potential treatment. Am J Hum Genet 2010;86:506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bleyer AJ, Kidd K, Živná M, Kmoch S.. Autosomal dominant tubulointerstitial kidney disease. Adv Chronic Kidney Dis 2017;24:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zivná M, Hůlkova H, Marignon M. et al. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am J Human Genet 2009;85:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.