Abstract
Herpes zoster (HZ) is a viral infection believed to be caused by the re activation of varicella zoster virus (VZV) or human herpes virus type 3 (HHV 3) that persists in the posterior nerve root ganglion. HZ is rarely reported in the pediatric age group with an intact immunity. Past infection with VZV and immunization with chickenpox vaccine are key markers in the onset of varicella zoster in children. Our aim was to study the clinicoepidemiological pattern of HZ infection in children aged less than 12 years and to start an early management to prevent long term complications. A prospective observational study over a total duration of 2 years was conducted in a tertiary hospital, and all children less than 12 years of age with diagnosed HZ were included in the study. A total of 39 children were diagnosed to have pediatric HZ infection during the study period. The children were followed up over 4 weeks post diagnosis and were treated with oral acyclovir therapy along with symptomatic management. All children had an uneventful benign course, and their siblings and close pediatric contacts were also screened for the development of HZ or chickenpox during the incubation period. All children were screened for an underlying immunodeficiency and two cases of HIV co infection were detected. HZ is a rare disease in childhood. Varicella in early childhood is a risk factor for HZ in both immunocompromised and immunocompetent children. The appearance of HZ in a young child does not always imply an underlying immunodeficiency or malignancy, but the children should be screened for immunodeficiency. In general, the prognosis is good in healthy children.
Keywords: Herpes zoster, pediatrics, varicella zoster virus
Introduction
Herpes zoster (HZ) infection, also known as “shingles,” is caused by the reactivation of endogenous latent varicella zoster virus (VZV) that resides in a sensory dorsal root ganglion usually after primary infection with VZV which causes chickenpox.[1] It usually develops after several years of the primary infection of chickenpox or vaccination with chickenpox vaccine. As varicella vaccine is a live attenuated virus, there is a possibility that a vaccine recipient can develop HZ. The vaccine comprises the live-attenuated Oka seed stock of VZV which is used for the monovalent varicella vaccine, however, the infectious dose for this vaccine is at least 14 times higher. As is the case with the wild-type VZV, which can establish latent infection in dorsal root ganglia following primary varicella infection in susceptible hosts and subsequently reactivate years later to cause herpes zoster (HZ), latency and HZ due to the Oka strain of VZV (Oka VZV) can occur following varicella vaccination; however, the risks are considerably lower. This phenomenon has been well documented in both healthy and immunocompromised individuals, although HZ due to vOka appears to be quite uncommon.[2,3,4] The incidence of HZ among vaccine recipients is approximately 14 cases per 100,000 person-years.[5] HZ has been mentioned to account for 8.6% of the adverse effects noted due to varicella vaccination, and most cases have been accounted within the first 2 weeks of vaccination.[6]
In general, the disease reactivation occurs in the adult population when resistance against this virus declines due to selective cell-mediated immune suppression or there is a generalized immune suppression. The cumulative lifetime incidence among the general population is approximately 10%–30%, with increased risk after 50 years of age. The age-adjusted incidence rate in children below 12 years is only 0.45 per 1000 persons whereas in the age group of 75 years and beyond it raises significantly up to 4.5 per 1000 persons.[7] The re-activated VZV tracks down the affected cutaneous nerve, causing a painful, unilateral vesicular eruption in a restricted dermatomal distribution or contagious dermatomes. Although HZ is predominantly an infection of the adult population, the chances of pediatric infection are more if the patient had chickenpox infection in the first year of life or had an in-utero exposure to the virus.[8] The objective of our study is to review clinicoepidemiological data for HZ in the pediatric population for early diagnosis and treatment to minimize long-term complications.
In general, the course of herpes is milder in children, and the mean duration of the disease is 1–3 weeks. Though lesional pain and itching may be present, post herpetic neuralgia has been rarely reported. The first line of therapy in childhood HZ is oral acyclovir given at a dose of 20–40 mg/kg body weight four times a day.[9]
Materials and Methods
A prospective cohort study over a duration of 2 years was conducted in a tertiary care hospital. All clinically diagnosed cases of HZ in children up to 12 years of age were included in the study. Our study was planned to review clinicoepidemiological data for HZ in the pediatric population for early diagnosis and treatment to minimize long-term complications. The study was reviewed and approved by an institutional ethical committee, and all patients gave their voluntary informed consent to participate in the study.
Anti-varicella virus antibodies were not estimated in the study as the facilities were not available at the study center, and the diagnosis was based on clinical presentation alone.
Results
A total of 39 patients were diagnosed to have HZ in the age group of less than 12 years over the study duration of 2 years. Out of these, 2 patients had an underlying known diagnosis of HIV infection; however, both children did not report any history of chickenpox. All the diagnosed cases were subjected to a Tzank smear, and HIV infection was ruled out in the remaining 37 cases using a screening enzyme-linked immunosorbent assay (ELISA) test. One patient developed painful fluid-filled lesions in a dermatomal distribution while he was being treated with chemotherapeutic agents for acute lymphocytic leukemia. Out of the 39 cases, 22 (56%) were girls and 17 (44%) were boys. The youngest and oldest patient was aged 3 years and 11 years, respectively [clinical pictures are shown in Figures 1 and 2]. The median age of the study population was 4.5 years with 26 patients in the age group of 2–6 years and 13 patients were in the age group of 6–12 years.
Figure 1.
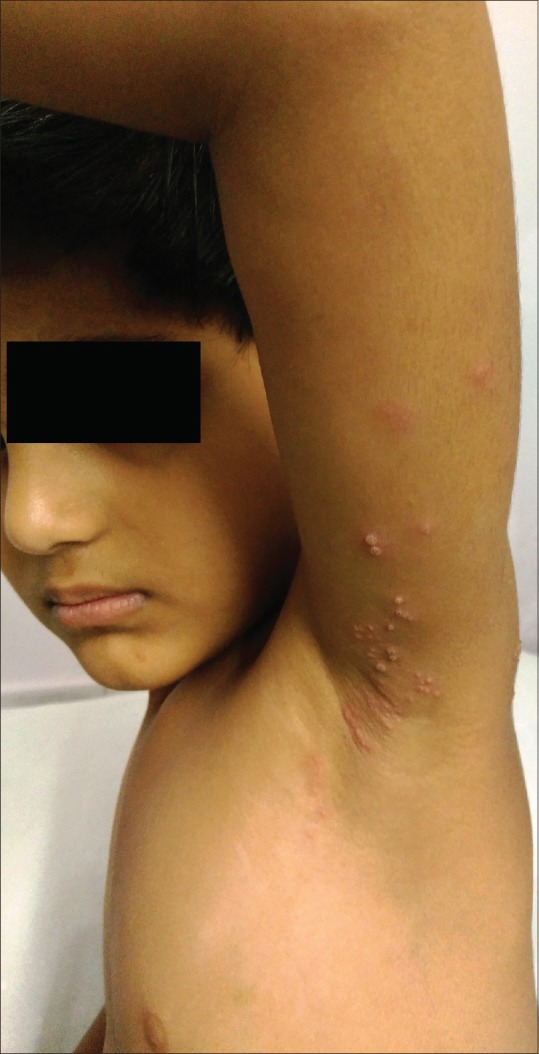
Herpes zoster in a 2-year-old child with grouped vesicles and erythematous papules distributed along the left T-1 dermatome
Figure 2.
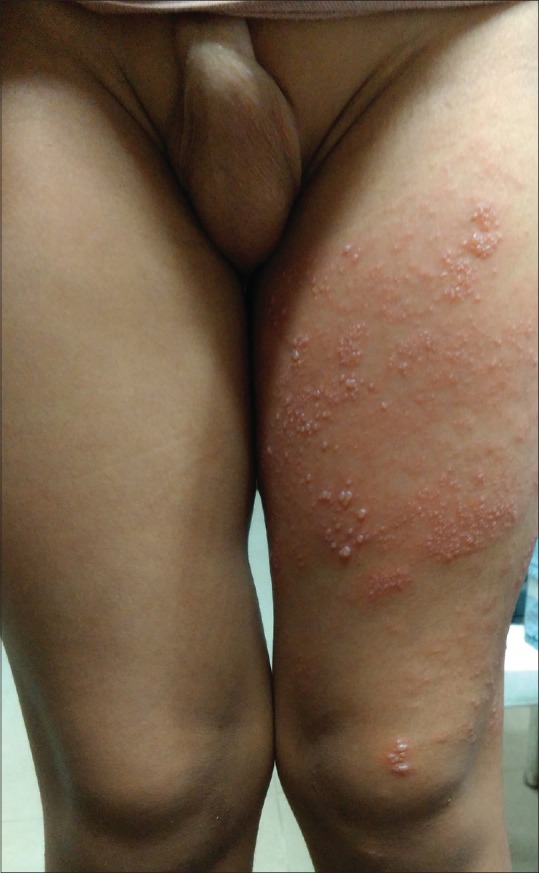
Herpes zoster in an 11-year-old child showing grouped vesicles over an erythematous base along the left L3-L4 dermatomes
Out of the 39 children, 12 children (31%) were vaccinated against chickenpox and 20 children (51%) were not vaccinated, and for the remaining 7 (18%), the vaccination status was not known. Both the children with a positive HIV status were not vaccinated with a live attenuated chickenpox virus. The vaccination status of the patients is shown in Figure 3. There was a documented or a reliable history of chickenpox infection in only 11 (28%) of the patients, and the remaining 28 (72%) did not have any history of chickenpox. Out of the 11 children with a documented history of chickenpox, the average time period between the onset of chickenpox and the HZ lesions was 4.3 years and the minimum gap was 1 year. Out of the 12 vaccinated children, 2 children gave a history of having developed chickenpox, and the average duration of development of chickenpox was approximately 15 months after the vaccination.
Figure 3.
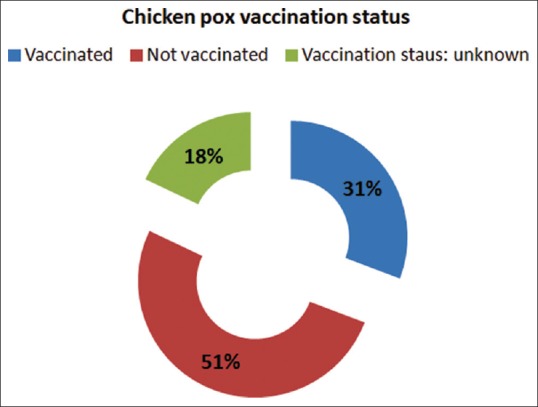
Vaccination status of the affected children
Chickenpox was documented in 3 children in the first year of life, and two out of these three had a history of chickenpox in the neonatal period, with a positive history of maternal chickenpox in the perinatal period.
There were no prodromal symptoms in the children, and all the children had de-novo appearance of grouped vesicles in a dermatomal pattern. The lesions were associated with mild burning sensation and pain along the affected dermatome. The average duration between the onset of rash and reporting to the study center was 1.3 days, and 32 (82%) children reported within the first 72 hours of the rash. Thoracic dermatomes were affected in 20 (51%) children, upper limbs in 9 (23%), head and neck in 6 (15%), and lower limbs in 4 (10%) of the children [Figure 4]. Tzank smear stained with methylene blue revealed multinucleated giant cells. The histopathological findings are shown in Figure 5, which showed multinucleated and acantholytic keratinocytes with distinct nuclear inclusions and dense perinuclear lymphocytic infiltrate. The two patients with HIV infection had CD4 counts of 340 and 560 cells/ml. All children were treated with acyclovir at a dose of 20 mg/kg/qid for 7 days. The patients were also given symptomatic management in the form of oral paracetamol and topical calamine lotion. There was complete resolution of skin lesions in all the patients, and no post herpetic neuralgia was reported after 4 weeks of observation. During the entire period of observation, none of the children developed any painful fluid filled lesions, signifying a recurrence of HZ.
Figure 4.
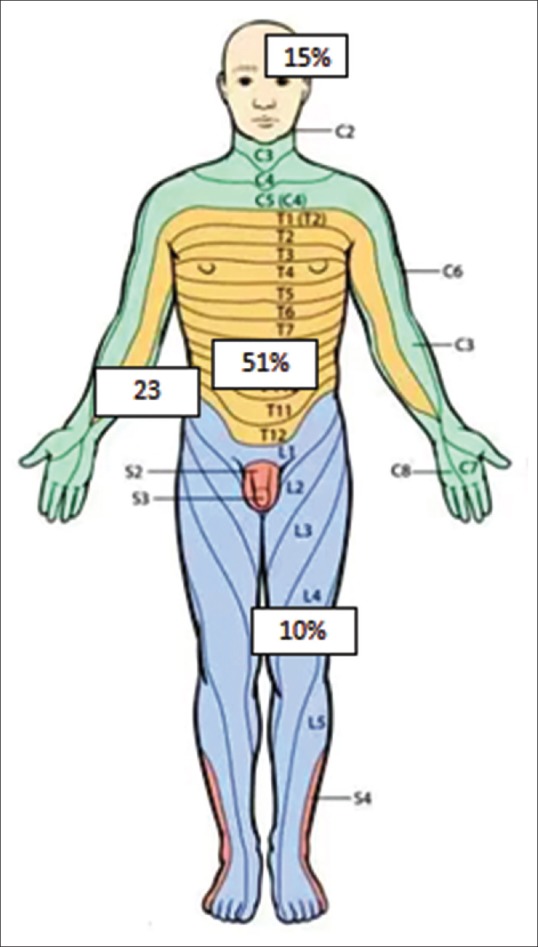
Distribution of dermatomes affected (percentage)
Figure 5.
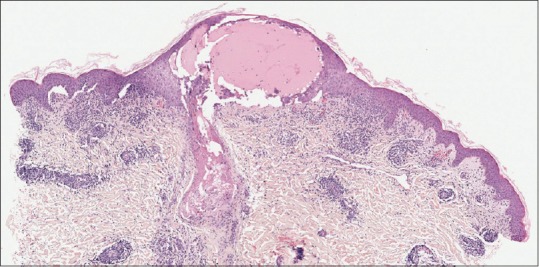
Skin biopsy, H and E, ×40 magnification showing multinucleated and acantholytic keratinocytes with distinct nuclear inclusions with dense perinuclear lymphocytic infiltrate
Discussion
In the past, childhood HZ was believed to be an indicator for an underlying malignancy, especially acute lymphatic leukemia; however, contrary to these findings, recent studies have shown that there is no increase in the incidence of malignancy in children with HZ. Approximately 3% of the pediatric HZ cases occur in children with malignancies.[10] There has been a recent trend in the rise of HZ cases in childhood. Vaccination with live attenuated virus could be one of the reasons for this rise in the number of cases.[11] Children developing HZ de novo without the development of chickenpox can be possibly explained by the fact that the episode of chickenpox is mild and goes unnoticed by the parents and the treating physician. Mild episode of chickenpox is attributed to the general course of mild chickenpox in children, and the symptoms and disease duration is further ameliorated by immunizing the child. Also, the episode of chickenpox in childhood, if not associated with classical signs and symptoms, can be diagnosed as a different viral exanthema and is not documented.
This rising incidence of HZ in otherwise healthy children may be due to acquiring primary varicella infection in utero or in infancy, wherein the body's immunity is not fully developed. Bhushan et al.[12] stated that the immunological status at the time of acquiring the primary infection is the most important factor in childhood HZ. A low level of natural killer (NK) cells, lymphocytes, and cytokines are seen in children along with virus-specific immunoglobulins that may result in an inability to maintain the latency of VZV, leading to early appearance of zoster in children. In a study by Federer and Hoss, it has been mentioned that the incidence of HZ increases with advancing age, although those children who have had varicella during the first year of life (or in utero) are at an increased risk of developing HZ.[13]
A diagnosis of HZ is made mostly by a detailed clinical examination, and the characteristic dermatomal distribution of grouped vesicles clinches the diagnosis. The dilemma arises in cases of skip lesions or multidermatomal distribution. The diagnosis can be confirmed by a Tzanck smear of scrapings from the floor of the vesicles that reveal multinucleated giant cells on direct microscopy. The other methods are skin biopsy and histopathological examination and direct fluorescent antibody tests. Serological tests for antibody against the virus and viral culture studies are also available for diagnosis.[14] Ideally, in the pediatric age group, absolute lymphocyte counts, CD4/CD8 ratio, and serum immunoglobulin levels also need to be estimated to rule out undetected concurrent immunosuppression in cases of HZ.
In a study by Wood et al. on primary varicella and HZ among HIV-infected children it has been noted that the incidence of HZ in the pediatric population infected with HIV infection has decreased since 1989. This decline that occurred after 2000 likely represents the combined effect of highly-active antiretroviral therapy and immunization against varicella.[15]
The relative risk of developing childhood HZ is much more in children who have a history of childhood chickenpox than that in children who have been vaccinated and do not have a definitive history of chickenpox.[16]
Blaschkitis is an important differential diagnosis and can be a clinical challenge in the pediatric population. However, Blaschkitis does not typically follow dermatomes, and is along the proposed lines of Blaschko. Pain and constitutional symptoms are usually absent in Blaschkitis, and the resolution of lesions in zoster helps in easy clinical differentiation between the two conditions.
In general, the course of the disease is milder in children with complete resolution in approximately 2–3 weeks. In our study, in children between the age group of 2 and 12 years, the acute sharp shooting pain was not observed, which is the hallmark of HZ in adults. Though lesional pruritus and pain were present, the incidence of post herpetic neuralgia is negligible, which is the most common complication of HZ in adults. So far, almost all the reported series and isolated case reports have stressed upon the fact that childhood zoster is a relatively mild disease with negligible prodromal symptoms, post herpetic neuralgia, or other significant complications. Antiviral drugs such as acyclovir are the mainstay of treatment of childhood herpes zoster. Symptomatic treatment and allaying the anxiety of parents is mandatory. Complications such as post herpetic neuralgia or other significant complications such as secondary infections or disseminated viremia is rarely reported.
The closest differential diagnoses to be ruled out are insect bite reaction and irritant dermatitis, however, the group vesicles in a dermatomal distribution are sufficient to diagnose the cases.
Although this condition may resolve in weeks without any sequelae, complications such as secondary bacterial infection, motor nerve involvement, and effect on vital organs like the eyes in the form of HZ ophthalmicus, should be monitored and treated at the earliest.
There have been very few studies highlighting the prevalence of HZ in the pediatric population. Most published articles have highlighted case reports and post vaccination incidences. A literature search with the key words “herpes zoster” and “children/pediatrics” yielded a few studies, and findings of the same have been documented in Table 1.
Table 1.
All published studies on herpes zoster in children with relevant findings

Conclusions
HZ is rare in children. Varicella infection in early childhood is a risk factor for HZ in early childhood. The appearance of HZ in children is not a cutaneous marker for immunodeficiency or underlying malignancy. The prognosis is quite good and post herpetic neuralgia, as reported in the adult population, was absent in our study. Chickenpox in children can manifest as subclinical infection or with atypical mild features and can go unnoticed. HZ can be the first manifestation of varicella infection in the pediatric age group, which is evident even in children vaccinated against varicella virus.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Leung AK, Robson WL, Leong AG. Herpes zoster in childhood. J Pediatr Health Care. 2006;20:300–3. doi: 10.1016/j.pedhc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Weinmann S, Chun C, Schmid DS, Roberts M, Vandermeer M, Riedlinger K, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859–68. doi: 10.1093/infdis/jit405. [DOI] [PubMed] [Google Scholar]
- 3.Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, et al. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc. 2012;123:17–33. [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng HF, Schmid DS, Harpaz R, LaRussa P, Jensen NJ, Rivailler P, et al. Herpes zoster caused by vaccine-strain varicella zoster virus in an immunocompetent recipient of zoster vaccine. Clin Infect Dis. 2014;58:1125–8. doi: 10.1093/cid/ciu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med. 1991;325:1545–50. doi: 10.1056/NEJM199111283252204. [DOI] [PubMed] [Google Scholar]
- 6.Willis ED, Woodward M, Brown E, Popmihajlov Z, Saddier P, Annunziato PW, et al. Herpes zoster vaccine live: A 10 year review of post-marketing safety experience. Vaccine. 2017;35:7231–9. doi: 10.1016/j.vaccine.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Katakam BK, Kiran G, Kumar U. A prospective study of herpes zoster in children. Indian J Dermatol. 2016;61:534–9. doi: 10.4103/0019-5154.190121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakourou T, Theodoridou M, Mostrou G, Syriopoulou V, Papadogeorgaki H, Constantopoulos A, et al. Herpes zoster in children. J Am Acad Dermatol. 1998;39:207–10. doi: 10.1016/s0190-9622(98)70076-3. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu S, Sripathi H, Gupta S, Prabhu M. Childhood herpes zoster: A clustering of ten cases. Indian J Dermatol. 2009;54:62–4. doi: 10.4103/0019-5154.48991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guffey DJ, Koch SB, Bomar L, Huang WW. Herpes zoster following varicella vaccination in children. Cutis. 2017;99:207–11. [PubMed] [Google Scholar]
- 12.Bhushan P, Sardana K, Mahajan S. Dermatomal vesicular eruption in an asymptomatic infant. Dermatol Online J. 2005;11:26. [PubMed] [Google Scholar]
- 13.Feder HM, Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451–7. doi: 10.1097/01.inf.0000126901.88982.32. [DOI] [PubMed] [Google Scholar]
- 14.Solomon AR. New diagnostic tests for herpes simplex and varicella zoster infections. J Am Acad Dermatol. 1988;18:218–21. doi: 10.1016/s0190-9622(88)70032-8. [DOI] [PubMed] [Google Scholar]
- 15.Wood SM, Shah SS, Steenhoff AP, Rutstein RM. Primary varicella and herpes zoster among HIV-infected children from 1989 to 2006. Pediatrics. 2008;121:e150–6. doi: 10.1542/peds.2007-0564. [DOI] [PubMed] [Google Scholar]
- 16.Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: A population-based study. Pediatrics. 2015;135:e565–71. doi: 10.1542/peds.2013-4037. [DOI] [PubMed] [Google Scholar]


