Abstract
Many drugs are known to cause systemic lupus erythematosus (SLE), however there are no well defined criteria for drug induced lupus erythematosus (DILE). We present a rare case of lamotrigine induced lupus presenting as acute syndrome of apoptotic pan epidermolysis (ASAP).
Keywords: Acute syndrome of apoptotic pan epidermolysis, lamotrigine, Systemic Lupus erythematosus
Introduction
Many drugs are known to cause systemic lupus erythematosus (SLE).[1] Lamotrigine is a newer anticonvulsant drug that selectively blocks voltage-dependent sodium channels, preventing excitatory neurotransmitter release. It has various neurologic, gastrointestinal, and cutaneous adverse reactions. It has been reported to cause Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), and rarely SLE.
Case Report
A 22-year-old female, known case of generalized tonic-clonic seizures since three—and—a--half years, presented to us with complaint of erythematous rash on face, trunk, and upper limbs since 6 weeks with exacerbation since five days. There was history of photosensitivity since six weeks and oral ulceration on palate since five days. There was no history of fever, joint pains, and dyspnea. Lamotrigine 50 mg once daily was started eight weeks ago for relapse of generalized tonic--clonic seizures and the dose was increased to 50 mg twice daily one week before the presentation. She was previously treated with sodium valproate for three years. On examination, diffuse erythematous maculopapular rash associated with vesiculation, crusting, and erosions was present on face, bilateral upper limbs, and back [Figures 1 and 2]. Periungual erosions and splinter hemorrhages were present [Figure 3]. The buccal, ocular, and genital mucosa were characteristically spared. Systemic examination was unremarkable. Routine blood counts, urine analysis, ECG, and chest X-ray were normal and viral markers for hepatitis B, C, and HIV were nonreactive. Her antinuclear antibodies antinuclear antibodies (ANA) were strongly positive (++++) by immunofluorescence (coarse speckled pattern) and anti--SS-A/Ro60, SS-A/Ro 52, and SS-B/La (++) were also positive, while antihistone and anti--DsDNA antibodies were negative. A skin biopsy was done which showed necrosis of the epidermis with basal layer vacuolar degeneration and presence of interface lymphocytic infiltrate [Figure 4]. In view of sparing of mucosa, photosensitivity and positive ANA, and histopathological findings of interface dermatitis, a diagnosis of SLE presenting as SJS/TEN also known as acute syndrome of apoptotic pan epidermolysis (ASAP) triggered by lamotrigine was made. The patient was started on prednisolone 40 mg and lamotrigine was stopped. Levetiracetam was added to control the seizures. Erythema and edema subsided and the skin started desquamating by the fifth day [Figure 5].
Figure 1.
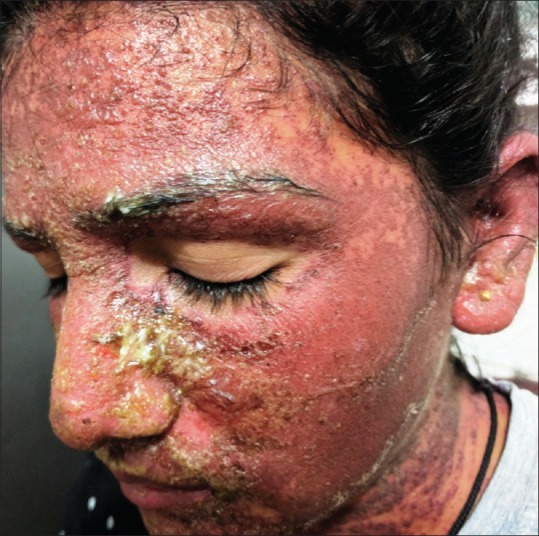
Erythematous maculopapular rash involving the entire face and neck with erosions, crusting, and vesiculation
Figure 2.
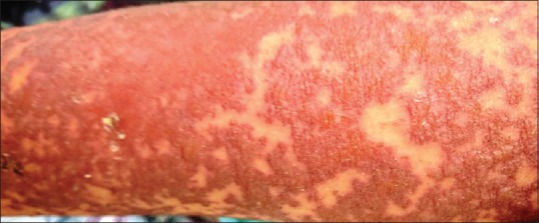
Diffuse maculopapular erythema on right arm
Figure 3.
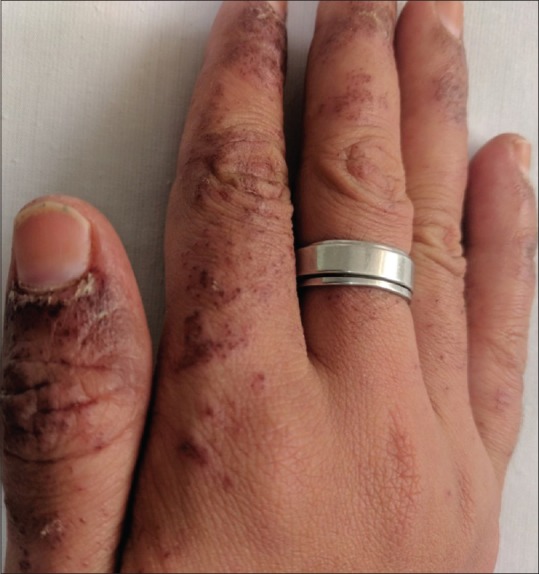
Periungual erosions and splinter hemorrhage
Figure 4.
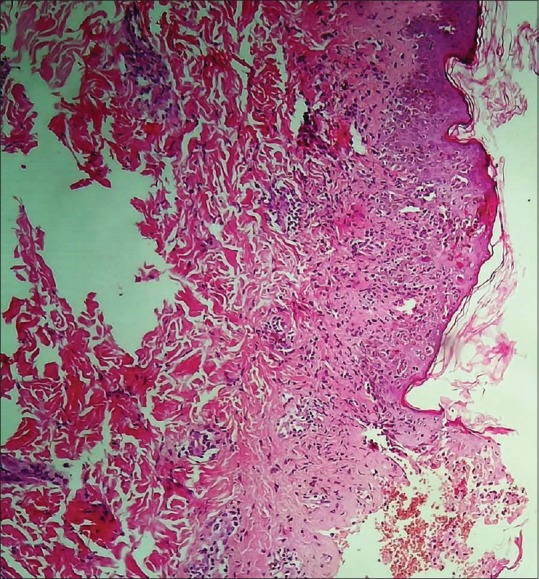
Basal layer vacuolar degeneration with presence of necrotic keratinocytes and mild superficial perivascular lympho-histiocytic infiltrate [hematoxylin and eosin (H and E 400×)]
Figure 5.
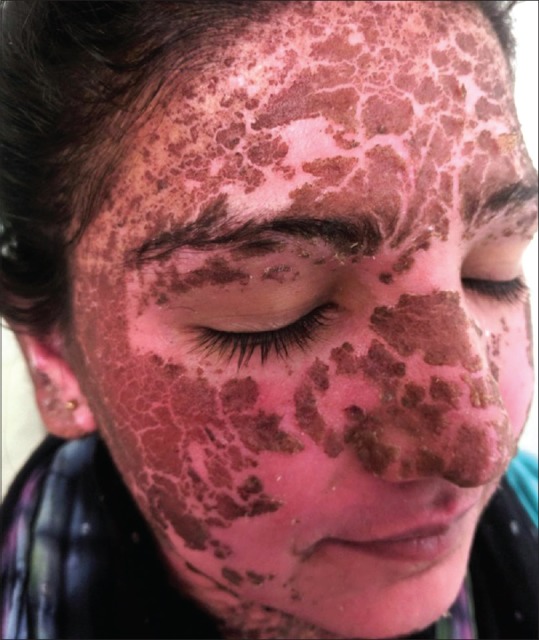
Resolution of lesions within 1 week
Discussion
Certain drugs have been known to cause SLE.[1,2] Drugs causing SLE can be divided into different groups based on the level of evidence. The first group of drugs such as isoniazid and quinidine has been demonstrated in well-controlled prospective clinical trials. The second group consists of drugs such as sulfasalazine and several anticonvulsants are probably associated with drug-induced lupus erythematous (DILE), for which evidence is available in the form of case reports, case series, or small studies. For the drugs in the third group (such as lithium and captopril), only anecdotal reports are available. The fourth group contains recently reported drugs. Although there are no universally agreed criteria for DILE, the following proposed criteria[2,3,4] are generally used-: presence presence of at least one clinical symptom of SLE plus positive ANA or other lupus serology, administration of the suspect drug over a period of time, roughly from 33 weeks to 2 years before development of any sign or symptom of SLE, prompt improvement in clinical signs after discontinuation of suspected drug, and recurrence of symptoms upon re--challenge.
Lamotrigine is a rare cause of drug-induced SLE. Sarzi-Puttini et al.[5] reported a case of 57-year-old woman woman with malar rash, photosensitivity, and positive ANA on lamotrigine since 3 3 years, which resolved on its withdrawal. Ravindran[6] reported an an 18-year-old female taking lamotrigine for 18 months developed facial rash, painless oral ulcers, and small joint arthralgia's arthralgia. She had positive ANA and proteinuria and she improved on stopping lamotrigine. The patient reported by Chang et al. et al.[7] had no skin lesions but recurrent arthralgia and positive ANA. Rarely SLE may present with TEN-like features which has been labeled as ASAP or TEN--like acute cutaneous lupus erythematosus (ACLE). Ting et al.[8] proposed the acronym ASAP for acute syndrome of apoptotic pan epidermolysis to designate a clinical constellation having several causes characterized by acute and massive cleavage of the epidermis resulting from hyperacute epidermal basal cell apoptotic injury. They hypothesized that their patient had an underlying SLE predisposition that remained subclinical until she had oral nonsteroidal antiinflammatory drug intake and tanning bed exposure. ASAP can present from drug hypersensitivity (TEN), following graft versus host disease graft versus host disease, pseudoporphyria, or as TEN-like ACLE. Most cases of ASAP occurring in patients of SLE are drug-induced TEN.[9] Rarely, SLE may present with a presentation similar to TEN but with a subacute onset with lack of systemic involvement and sparing of mucosa. Such patients have an autoimmune serology consistent with SLE that helps reach a diagnosis of SLE. Our patient presented with cutaneous features mimicking SJS/TEN following lamotrigine ingestion and fulfilled four clinical (malar rash, alopecia, photosensitivity, and oral ulcers) and one immunological criteria (positive ANA) according to the Systemic Lupus International Collaborating Clinics criteria for SLE. To the authors’ knowledge, this is the first case in literature of lamotrigine-induced SLE presenting as ASAP. Antihistone antibodies have been reported to be positive in only about 75% of the cases of drug-related lupus.[2,4] According to Naranjo Probability Scale (score = 5), it is probable that lamotrigine in our case is the triggering drug.[10] Certain features that distinguish this case from SJS/TEN are lack of any characteristic mucosal involvement, presence of periungual lesions and splinter hemorrhages, and biopsy findings of vacuolar interface dermatitis and positive ANA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess E. Drug-related lupus. N Engl J Med. 1988;318:1460–2. doi: 10.1056/NEJM198806023182209. [DOI] [PubMed] [Google Scholar]
- 3.Pramatarov KD. Drug-induced lupus erythematosus. Clin Dermatol. 1998;16:367–77. doi: 10.1016/s0738-081x(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 4.Antonov D, Kazandjieva J, Etugov D, Gospodinov D, Tsankov N. Drug-induced lupus erythematosus. Clin Dermatol. 2004;22:157–66. doi: 10.1016/j.clindermatol.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Sarzi-Puttini P, Panni B, Cazzola M, Muzzupappa S, Turiel M. Lamotrigine-induced lupus. Lupus. 2000;9:555–7. doi: 10.1177/096120330000900715. [DOI] [PubMed] [Google Scholar]
- 6.Ravindran V. Lamotrigine-induced lupus: A case report. Int J Rheum Dis. 2011;14:e47–8. doi: 10.1111/j.1756-185X.2011.01634.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang RS, Cole AJ. Lamotrigine-induced lupus-like syndrome: A case report and literature review. Am J Ther. 2014;21:e85–7. doi: 10.1097/MJT.0b013e3182491c31. [DOI] [PubMed] [Google Scholar]
- 8.Ting W, Stone MS, Racila D, Scofield RH, Sontheimer RD. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic pan-epidermolysis (ASAP): A case report, concept review and proposal for new classification of lupus erythematosus vesiculobullous skin lesions. Lupus. 2004;13:941–50. doi: 10.1191/0961203304lu2037sa. [DOI] [PubMed] [Google Scholar]
- 9.Paradela S, Martínez-Gómez W, Fernández-Jorge B, Castiñeiras I, Yebra-Pimentel T, Llinares P, et al. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus. Lupus. 2007;16:741–5. doi: 10.1177/0961203307079498. [DOI] [PubMed] [Google Scholar]
- 10.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


