Pemphigus is a group of autoimmune blistering disorders, clinically characterized by mucocutaneous blisters and erosions and histopathologically by intraepidermal acantholysis. It is caused due to autoantibodies directed against the cell surface proteins, desmogleins. Pemphigus was traditionally associated with high mortality and morbidity. However, advent of corticosteroids dramatically changed the outlook of this invariably fatal disease and reduced the mortality rate to <10%.[1] Another milestone in the therapeutics of pemphigus in India was the use of dexamethasone cyclophosphamide pulse (DCP) therapy by Pasricha and Ramji in 1984.[2] Since then, DCP and oral corticosteroids have been the backbone of pemphigus treatment in India.[3] This has been reiterated by a number of publications.[4,5,6] However, long-term corticosteroid intake is associated with various metabolic complications, global immunosuppression, and an antecedent risk of serious infections. Thus, there was a continuous search for a safer, more targeted therapeutic option, especially in patients in whom corticosteroids were contraindicated. Thus came the use of intravenous immunoglobulin (IVIg) and plasmapheresis, which differed from the commonly used corticosteroids by their immunomodulatory action compared to the global immunosuppression achieved by corticosteroids.[7,8]
The next major development in pemphigus treatment was the use of rituximab in 2001 by Heizmann et al.[9] This serendipitous discovery of improvement in mucocutaneous lesions of paraneoplastic pemphigus when rituximab was used to treat non-Hodgkin's lymphoma dawned upon a new era of targeted therapy to treat autoimmune blistering diseases. Rituximab, a chimeric monoclonal antibody, selectively acts on the CD20 expressing B cells, which are known to secrete autoantibodies targeting the epidermal desmogleins. In the Indian scenario, rituximab was first used by Kanwar and colleagues in 2010 and the promising findings were first published in 2012.[10] Since then its use has increased exponentially with reports from various parts of the country. In this review, we briefly discuss the road covered, way ahead, and future challenges in the biological treatment of pemphigus.
Rituximab in Pemphigus
Indian scenario
Rituximab is a chimeric monoclonal antibody, which acts against the cell surface CD20 antigen (a calcium channel in cell membrane) expressed on B cells. It acts by causing direct induction of apoptosis, complement-dependent cytotoxicity (CMC), and antibody-dependent cellular cytotoxicity (ADCC).[11] The usage of rituximab has increased many folds over the recent years with availability of rituximab biosimilars, which has drastically cut down the marketing cost of the drug.[12] Though concerns have been raised on the efficacy of these biosimilars vis-à-vis the reference molecule,[13] these have now been allayed with biosimilars showing similar efficiency as the reference molecule.[14]
Rituximab has been used in various protocols and in combination with other immunomodulators in treatment of pemphigus. Currently, the two commonly used protocols in India are the lymphoma protocol (LP) and the rheumatoid arthritis (RA) protocol. The various regimes were summarized in a previous review.[15] Kanwar et al.[10] treated 10 pemphigus patients by RA protocol [Table 1]. At a mean follow-up of 33.4 weeks, three patients had achieved complete remission off all treatment [CR(off)] and four patients had achieved complete remission on minimal therapy [CR(on)]. One patient died of sepsis. In this study the mean time to disease control (TDC) was 8 weeks. In a retrospective review, Sharma et al.[16] reported the treatment outcome of 25 pemphigus patients treated with rituximab mostly by RA protocol [Table 1]. At a mean follow-up of 18 months, CR was noted in 22 patients and PR in 3 patients with a mean TDC of 5 weeks. Relapse was seen in four patients after a mean duration of 11.75 months. Adverse events included disease exacerbation in two patients, acute respiratory distress syndrome and cellulitis in one patient each.
Table 1.
Overview of the major Indian studies reporting rituximab use in pemphigus
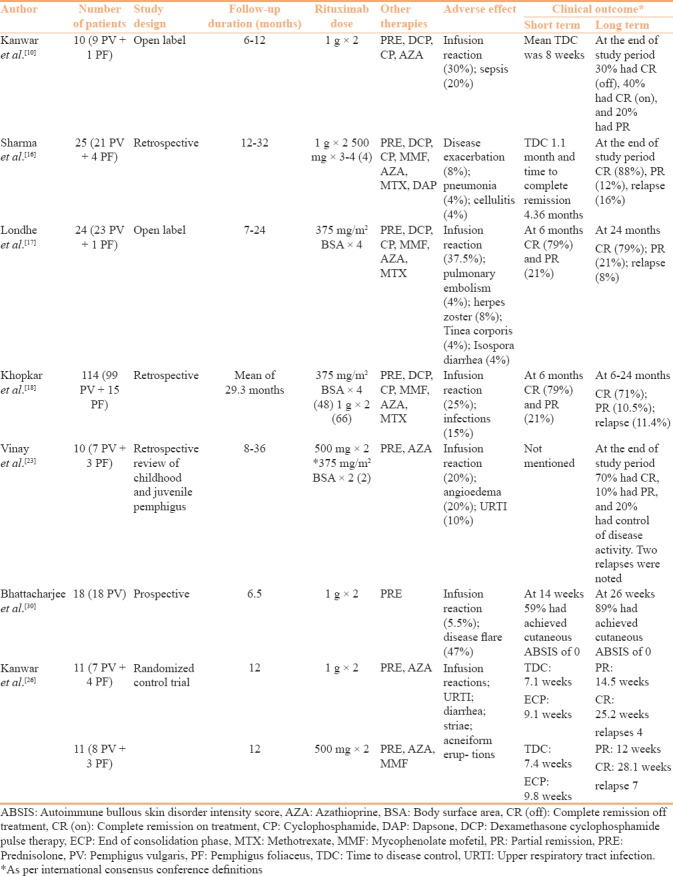
Londhe et al.[17] treated 24 pemphigus patients with a modified version of LP [Table 1]. At a mean follow-up of 18 months, all 24 patients had responded to treatment with 9 patients achieving CR(off), 10 achieving CR(on), and 5 patients achieving partial remission (PR). Adverse effects were limited to infusion reactions. In a follow-up publication of this cohort Khopkar and colleagues reported the outcome of 114 pemphigus patients (including the 24 cases reported by the authors in 2014) receiving rituximab.[18] Fourty-nine (43%) cases had achieved CR(off), 32 (28%) patients had achieved CR(on), and 12 patients had achieved PR at the end of 24 months. Relapse was noted in 13 (11.4%) patients. There was no remarkable difference in the clinical outcome between the patients treated with RA protocol (n = 66) and LP (n = 48). In the systematic analysis of published literature by Ahmed and Shetty, the authors found CR in a statistically higher number of patients receiving RA protocol.[19] Also, patients receiving RA protocol were more likely to be off all treatment during post-treatment follow-up.[19]
The common variation in the RA protocol was the high- and low-dose rituximab administration. The high-dose regimen involved administration of two doses of 1000 mg of rituximab 2 weeks apart. Whereas, in low-dose regimen, two doses of 500 mg rituximab was administered 2 weeks apart. In a prospective study, Gupta and colleagues treated 50 pemphigus patients with low-dose RA protocol. At 6 months follow-up 20/50 (40%) patients were in CR(off).[20] In a randomized control trial, Kanwar et al. compared the clinical and immunological outcome of pemphigus patients treated with high- and low-dose RA protocol.[21] The clinical response as evident by the fall in the disease severity scale was significantly more in the high-dose group. Additionally, the immunological parameters assessed by fall in the anti-desmoglein antibody titer and B cell repopulation was significantly better in patients receiving the high-dose regimen. The meta-analysis of low- and high-dose regimen by Wang and colleagues also reported longer duration of CR with high-dose regimen.[22]
In a retrospective review of patient records, Vinay et al.[23] reported the encouraging results of rituximab treatment (two doses of 500 mg 15 days apart) in childhood and juvenile pemphigus patients. CR(off) treatment was achieved in 7/10 patients at a median follow-up period of 16 months. Relapse was seen in six patients by a mean of 13 months, which showed good treatment response to repeat infusions of rituximab and/or conventional immunosuppressants. Oral lesions of pemphigus show treatment refractoriness in comparison to cutaneous lesions.[24] Vinay et al.[25] treated three pemphigus patients with refractory oral ulcers using intralesional rituximab (5 mg/cm2 two injections 15 days apart) with a good response in all. Rituximab has also been used in special situations in treating paraneoplastic pemphigus and in pemphigus patients with hepatitis B and C infection.[26,27,28]
Various studies have analyzed the immunological changes after rituximab treatment. Post-rituximab treatment, a gradual fall in anti-desmoglein antibody titers is generally observed.[10,17,21] In the study by Kanwar et al.[10] the clinical response paralleled the fall in anti-desmoglein 1 antibody indices, whereas there was only a partial reduction in anti-desmoglein 3 titers. The fall in CD19 cell count is dramatic after rituximab infusion and is seen as early as 2 weeks.[21] Even low-dose RA protocol and intralesional rituximab injection successfully reduced CD19 cell count.[21,25] However, CD19 cell repopulation is earlier in patients receiving low-dose rituximab regimens compared to patients receiving high-dose regimen.[21] Since relapses are associated with B cell repopulation, low-dose regimens may have a higher relapse rate compared to high-dose regimens.[29] Bhattacharjee et al.[30] studied the effect of rituximab on circulating T regulatory cells in 18 pemphigus patients. No direct relationship was found between the disease severity/clinical response and circulating T regulatory cells. In the seminal study by Colliou et al.[31] increased CD19+CD27 − naïve B cells to CD19+CD27+ memory B cells ratio, increased transitional B cells and interleukin-10 – secreting regulatory B cells were associated with complete remission. Delayed appearance of memory B cells and the disappearance of desmoglein-specific circulating immunoglobulin G-positive (IgG+) B-lymphocytes were also associated with long-lasting remission with rituximab.
Global scenario
In a landmark randomized controlled trial, Joly and colleagues compared clinical outcome of patients receiving rituximab and low-dose corticosteroids compared to corticosteroids alone.[32] The study recruited 91 treatment naïve pemphigus patients and randomized them in 1:1 ratio to rituximab or corticosteroid group. At the end of 36 months of follow-up, 41/46 (86%) of patients in rituximab arm were in CR compared to 15/44 (34%) patients in prednisolone only arm. The adverse effects were common and more severe in the prednisolone only group.
The noted deviation by Joly et al. was the use of rituximab as a first line adjuvant in treatment naïve patients.[32] Though many authors have previously suggested using rituximab as a first line adjuvant,[30,33,34] most of the current treatment guidelines recommend rituximab as a second or third line drug after failing conventional immunosuppressants.[35] The trial by Joly et al.[32] has paved way for considering rituximab treatment earlier in the disease course. Using rituximab early in the disease course has added advantage. Cho et al.[36] suggested that relapse after rituximab treatment was associated with prior long-term use of conventional immunosuppressive agents. Also, the probability of achieving CR(off) is more in pemphigus patients receiving rituximab within 6 months of disease onset.[37,38,39] The United States Food and Drug Administration has now approved rituximab for the treatment of adults with moderate-to-severe pemphigus vulgaris, which makes the drug the first biologic approved for the treatment of pemphigus vulgaris. The most recent guidelines by the international panel of experts recommend rituximab as a first line treatment option for pemphigus.[40]
Questions Unanswered
Though rituximab has now been firmly established as a treatment modality of pemphigus, many questions still remain unanswered. Important among these is the indication to use rituximab. Should rituximab be the first line therapy for all pemphigus patients irrespective of disease severity or disease duration? Should rituximab treatment be guided by immunological parameters like desmoglein indices, CD19, and CD4 cell counts? Is there a sub-set of patients who benefit from starting rituximab early in the disease course? Future studies are required to answer these questions for a patient-tailored treatment approach.
Rituximab is generally used in combination with low-dose corticosteroids. Ahmed and colleagues strongly advocate using IVIg in combination with rituximab.[41,42] Few authors have used azathioprine, cyclophosphamide, and mycophenolate mofetil as adjuvants in addition to rituximab. However, there is no consensus on use of other immunosuppressants and immunomodulators along with rituximab.[40] Questions regarding optimal dose, frequency, total number of maintenance infusions to use, and treatment schedule for relapses also needs to be answered.
The literature on vaccination for patients receiving rituximab is blurred. Live vaccines such as influenza and varicella-zoster vaccine are contraindicated while on immunosuppression.[43] Whereas killed vaccine, sub-unit vaccine, and other non-live inactivated vaccines can be safely administered. The literature-based immunization recommendations for immunosuppressed autoimmune bullous dermatoses patients recommend vaccination with non-live vaccines of pneumococcal, hepatitis B, and inactivated influenza vaccine (annually).[44] The same can be currently followed for patients receiving rituximab; however, specific data on immune conversion and complications after vaccination are required.
Future Prospects
Rituximab for maintenance therapy
Many long-term case series and a few randomized control trials have now clearly established the efficacy of rituximab to induce remission.[10,32,45] However, these studies and systematic analysis consistently report a relapse rate of 40–60%.[19,22,45] Interestingly, in their randomized control trial, Joly et al.[32] administered 500 mg rituximab at 12 and 18 months irrespective of the disease activity. This was based on the author's observation that the desmoglein indices increase 12 months after rituximab infusion following the initial fall in its titers.[32] It is also supplemented by the observation that the CD19 repopulation and relapses are common after 12 months and usually occur at a median of 15 months.[32,45] Therefore, few authors recommend additional rituximab infusions every 6 monthly to maintain clinical remission.[46,47] A previous study by Gregoriou et al.[48] found no additional benefit from prophylactic infusions of rituximab. However, many recent studies have reported low or no relapse rate with maintenance rituximab infusions.[32,49] However, there is uncertainty on the optimal dose (500 mg or 1 g) to be used and frequency of administration (every 6 months or 1 year) when used for maintenance therapy. Many immunologic markers can be used to predict disease relapse including desmoglein indices, CD19, and CD4 cell counts. Future studies are needed to assess these markers as criteria to administer or withdraw rituximab maintenance.[29,50]
Ultra low-dose rituximab
Rituximab acts by depletion of CD20 expressing circulating B cells, but has no action on CD20 negative early pre B cells and terminally differentiated plasma cells.[15] The B cell burden in autoimmune blistering diseases is much lower than in lymphoproliferative diseases. Recent studies have found 97% of circulating B cell depletion with rituximab dose as less as 1 mg/m2 (contrasting to 375 mg/m2 in lymphoma).[51] We previously reported similar findings with intralesional injection of ultra low-dose rituximab injection (30–40 mg) wherein CD19 B cell suppression was seen within 2 weeks.[22] There has been a suggestion that 100 mg rituximab may be sufficient to induce depletion of B cells for 3 months and, consequently, two doses of 100 mg every 3 months could deplete the B cell population for 6 months.[52] However, well-designed clinical trials are warranted to determine its efficacy in the context of treating autoimmune blistering disorders.
Future strategies beyond rituximab
Use of newer generation anti-CD20 monoclonal antibodies are being explored to treat B cell mediated diseases including pemphigus.[53] Anti-CD20 antibodies are categorized into Type I (including rituximab, ofatumumab, veltuzumab, and ocrelizumab) and Type II (including tositumomab or obinutuzumab), depending on mechanism of action.[54] Type I antibodies cause a clustering of CD20 that enhances the recruitment and activation of complement for a potent CDC response. On the other hand, Type II antibodies exhibit stronger homotypic adhesion and induction of direct cell death but with a minimal CDC response.
The newer generation anti-CD20 monoclonal antibodies have added advantage.[55] Humanized monoclonal antibodies are less immunogenic than mouse-derived proteins. Few of these antibodies can be injected subcutaneously, obviating the need for hospitalization for intravenous infusions. Increased binding to the affinity effector cells leads to increased B cell depletion, which may translate to better/prolonged clinical efficacy. Veltuzumab, a second generation Type 1 anti-CD20 antibody has been reported useful in inducing remission in a treatment resistant case of pemphigus.[56] Phase III studies are currently being conducted for ofatumumab and anti-BAFF antibodies in pemphigus patients.[53] Monoclonal antibodies targeting CD19 and CD22 are being explored in multiple sclerosis and systemic lupus erythematosus, which may in future be evaluated in treating autoimmune blistering diseases. Another interesting strategy is the antigen-specific B cell depletion using chimeric autoantibody receptor (CAAR) T cells.[47,51,55] In this strategy, biochemically engineered T cells specifically recognize and deplete anti-desmoglein 1 and anti-desmoglein 3 secreting B cells.[57] CAAR T cells have the ability to proliferate and expand in vivo, which may lead to long-lasting effect.
Conclusion
In the era of evidence-based medicine, it is essential to provide customized treatment options, balancing its efficacy, tolerance, adverse effect profile, and patients co-morbidity. It is true in the therapeutics of pemphigus too. The established use of rituximab has heralded a new era in this regard and the horizon looks bright with an armory of new monoclonal antibodies. Future studies will pave way in providing the tailor made patient care for this orphan disease.
References
- 1.Lever WF, Schaumburg-Lever G. Immunosuppressants and prednisone in pemphigus vulgaris: Therapeutic results obtained in 63 patients between 1961 and 1975. Arch Dermatol. 1977;113:1236–41. [PubMed] [Google Scholar]
- 2.Pasricha JS, Gupta R. Pulse therapy with dexamethasone- cyclophosphamide in pemphigus. Indian J Dermatol Venereol Leprol. 1984;50:199–203. [Google Scholar]
- 3.Kanwar AJ, Vinay K. Treatment of pemphigus: An Indian perspective. Indian J Dermatol Venereol Leprol. 2014;80:285–8. doi: 10.4103/0378-6323.136828. [DOI] [PubMed] [Google Scholar]
- 4.Parmar NV, Kanwar AJ, Minz RW, Parsad D, Vinay K, Tsuruta D, et al. Assessment of the therapeutic benefit of dexamethasone cyclophosphamide pulse versus only oral cyclophosphamide in phase II of the dexamethasone cyclophosphamide pulse therapy: A preliminary prospective randomized controlled study. Indian J Dermatol Venereol Leprol. 2013;79:70–6. doi: 10.4103/0378-6323.104672. [DOI] [PubMed] [Google Scholar]
- 5.Kanwar AJ, Kaur S, Thami GP. Long-term efficacy of dexamethasone-cyclophosphamide pulse therapy in pemphigus. Dermatology. 2002;204:228–31. doi: 10.1159/000057886. [DOI] [PubMed] [Google Scholar]
- 6.Kanwar AJ, De D. Pemphigus in India. Indian J Dermatol Venereol Leprol. 77:439–49. doi: 10.4103/0378-6323.82396. [DOI] [PubMed] [Google Scholar]
- 7.Ruocco V, Astarita C, Pisani M. Plasmapheresis as an alternative or adjunctive therapy in problem cases of pemphigus. Dermatologica. 1984;168:219–23. doi: 10.1159/000249707. [DOI] [PubMed] [Google Scholar]
- 8.Amagai M, Ikeda S, Shimizu H, Iizuka H, Hanada K, Aiba S, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60:595–603. doi: 10.1016/j.jaad.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 9.Heizmann M, Itin P, Wernli M, Borradori L, Bargetzi MJ. Successful treatment of paraneoplastic pemphigus in follicular NHL with rituximab: Report of a case and review of treatment for paraneoplastic pemphigus in NHL and CLL. Am J Hematol. 2001;66:142–4. doi: 10.1002/1096-8652(200102)66:2<142::AID-AJH1032>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Kanwar AJ, Tsuruta D, Vinay K, Koga H, Ishii N, Dainichi T, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:1–7. doi: 10.1111/j.1468-3083.2011.04391.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiner GJ. Rituximab: Mechanism of action. Semin Hematol. 2010;47:115–23. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta V, Khaitan BK. Therapeutic potential of biosimilars in dermatology. Indian J Dermatol Venereol Leprol. 81:451–6. doi: 10.4103/0378-6323.163706. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh A, Das A. Biologics or biosimilars: What is the difference? Indian J Dermatol Venereol Leprol. 2016;82:683–4. doi: 10.4103/0378-6323.191537. [DOI] [PubMed] [Google Scholar]
- 14.Gota V, Karanam A, Rath S, Yadav A, Tembhare P, Subramanian P, et al. Population pharmacokinetics of Reditux™, a biosimilar Rituximab, in diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2016;78:353–9. doi: 10.1007/s00280-016-3083-x. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar AJ, Vinay K. Rituximab in pemphigus. Indian J Dermatol Venereol Leprol. 2012;78:671–6. doi: 10.4103/0378-6323.102354. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V, Bhari N, Gupta S, Sahni K, Khanna N, Ramam M, et al. Clinical efficacy of rituximab in the treatment of pemphigus: A retrospective study. Indian J Dermatol Venereol Leprol. 2016;82:389–94. doi: 10.4103/0378-6323.174379. [DOI] [PubMed] [Google Scholar]
- 17.Londhe P, Khopkar U, Kalyanpad Y. Intermediate doses of rituximab used as adjuvant therapy in refractory pemphigus. Indian J Dermatol Venereol Leprol. 2014;80:300. doi: 10.4103/0378-6323.136832. [DOI] [PubMed] [Google Scholar]
- 18.Khopkar US, Chadha AA, Nitya MS, Lahoria V. Follow-up data of rituximab as adjuvant therapy in pemphigus. Indian J Dermatol. 2017;62:671–3. doi: 10.4103/ijd.IJD_303_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed AR, Shetty S. A comprehensive analysis of treatment outcomes in patients with pemphigus vulgaris treated with rituximab. Autoimmun Rev. 2015;14:323–31. doi: 10.1016/j.autrev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Gupta J, Raval R, Shah A, Solanki RB, Patel DD, Shah KB, et al. Low-dose rituximab as an adjuvant therapy in pemphigus. Indian J Dermatol Venereol Leprol. 2017;83:317–25. doi: 10.4103/ijdvl.IJDVL_1078_14. [DOI] [PubMed] [Google Scholar]
- 21.Kanwar AJ, Vinay K, Sawatkar GU, Dogra S, Minz RW, Shear NH, et al. Clinical and immunological outcomes of high- and low-dose rituximab treatments in patients with pemphigus: A randomized, comparative, observer-blinded study. Br J Dermatol. 2014;170:1341–9. doi: 10.1111/bjd.12972. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Liu C, Li Y, Huang Y. Efficacy of rituximab for pemphigus: A systematic review and meta-analysis of different regimens. Acta Derm Venereol. 2015;95:928–32. doi: 10.2340/00015555-2116. [DOI] [PubMed] [Google Scholar]
- 23.Vinay K, Kanwar AJ, Sawatkar GU, Dogra S, Ishii N, Hashimoto T. Successful use of rituximab in the treatment of childhood and juvenile pemphigus. J Am Acad Dermatol. 2014;71:669–75. doi: 10.1016/j.jaad.2014.05.071. [DOI] [PubMed] [Google Scholar]
- 24.Bystryn J-C, Rudolph JL. Pemphigus. Lancet. 2005;366:61–73. doi: 10.1016/S0140-6736(05)66829-8. [DOI] [PubMed] [Google Scholar]
- 25.Vinay K, Kanwar AJ, Mittal A, Dogra S, Minz RW, Hashimoto T. Intralesional rituximab in the treatment of refractory oral pemphigus vulgaris. JAMA Dermatol. 2015;151:878–82. doi: 10.1001/jamadermatol.2014.3674. [DOI] [PubMed] [Google Scholar]
- 26.Kanwar AJ, Vinay K, Varma S, Koga H, Ishii N, Hashimoto T. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2014;54:576–9. doi: 10.1111/ijd.12053. [DOI] [PubMed] [Google Scholar]
- 27.Kanwar AJ, Vinay K, Heelan K, Walsh S, Shear NH, Dhiman RK. Use of rituximab in pemphigus patients with chronic viral hepatitis: Report of three cases. Indian J Dermatol Venereol Leprol. 2014;80:422–6. doi: 10.4103/0378-6323.140301. [DOI] [PubMed] [Google Scholar]
- 28.Jain A, Prakash G, Nampoothiri RV, De D, Bal A, Khadwal A, et al. Peri-anal paraneoplastic pemphigus heralding the relapse of follicular lymphoma and its successful management by rituximab: A short correspondence. Indian J Hematol Blood Transfus. 2016;32:519–21. doi: 10.1007/s12288-016-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albers LN, Liu Y, Bo N, Swerlick RA, Feldman RJ. Developing biomarkers for predicting clinical relapse in pemphigus patients treated with rituximab. J Am Acad Dermatol. 2017;77:1074–82. doi: 10.1016/j.jaad.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharjee R, De D, Handa S, Minz RW, Saikia B, Joshi N. Assessment of the effects of rituximab monotherapy on different subsets of circulating t-regulatory cells and clinical disease severity in severe pemphigus vulgaris. Dermatology. 2016;232:572–7. doi: 10.1159/000448031. [DOI] [PubMed] [Google Scholar]
- 31.Colliou N, Picard D, Caillot F, Calbo S, Le Corre S, Lim A, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30. doi: 10.1126/scitranslmed.3005166. [DOI] [PubMed] [Google Scholar]
- 32.Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): A prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031–40. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 33.Craythorne EE, Mufti G, DuVivier AW. Rituximab used as a first-line single agent in the treatment of pemphigus vulgaris. J Am Dermatol. 2011;65:1064–5. doi: 10.1016/j.jaad.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 34.Nigam R, Levitt J. Where does rituximab fit in the treatment of autoimmune mucocutaneous blistering skin disease? J Drugs Dermatol. 2012;11:622–5. [PubMed] [Google Scholar]
- 35.Hertl M, Jedlickova H, Karpati S, Marinovic B, Uzun S, Yayli S, et al. Pemphigus. S2 Guideline for diagnosis and treatment-guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol. 2015;29:405–14. doi: 10.1111/jdv.12772. [DOI] [PubMed] [Google Scholar]
- 36.Cho HH, Jin SP, Chung JH. Clinical experiences of different dosing schedules of rituximab in pemphigus with various disease severities. J Eur Acad Dermatol Venereol. 2014;28:186–91. doi: 10.1111/jdv.12080. [DOI] [PubMed] [Google Scholar]
- 37.Vinay K, Cazzaniga S, Amber KT, Feldmeyer L, Naldi L, Borradori L. Rituximab as first-line adjuvant therapy for pemphigus: Retrospective analysis of long-term outcomes at a single center. J Am Acad Dermatol. 2018;78:806–8. doi: 10.1016/j.jaad.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Lunardon L, Tsai KJ, Propert KJ, Fett N, Stanley JR, Werth VP, et al. Adjuvant rituximab therapy of pemphigus: A single-center experience with 31 patients. Arch Dermatol. 2012;148:1031–6. doi: 10.1001/archdermatol.2012.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amber KT, Hertl M. An assessment of treatment history and its association with clinical outcomes and relapse in 155 pemphigus patients with response to a single cycle of rituximab. J Eur Acad Dermatol Venereol. 2015;29:777–82. doi: 10.1111/jdv.12678. [DOI] [PubMed] [Google Scholar]
- 40.Murrell DF, Peña S, Joly P, Marinovic B, Hashimoto T, Diaz LA, et al. Diagnosis and management of pemphigus: Recommendations by an international panel of experts. J Am Acad Dermatol. 2018 doi: 10.1016/j.jaad.2018.02.021. In Press. doi: 10.1016/j.jaad.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355:1772–9. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed AR, Nguyen T, Kaveri S, Spigelman ZS. First line treatment of pemphigus vulgaris with a novel protocol in patients with contraindications to systemic corticosteroids and immunosuppressive agents: Preliminary retrospective study with a seven year follow-up. Int Immunopharmacol. 2016;34:25–31. doi: 10.1016/j.intimp.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Recommendations of the Advisory Committee on Immunization Practices (ACIP): Use of vaccines and immune globulins for persons with altered immunocompetence. MMWR Recomm Rep. 1993;42:1–18. [PubMed] [Google Scholar]
- 44.Laniosz V, Lehman JS, Poland GA, Wetter DA. Literature-based immunization recommendations for patients requiring immunosuppressive medications for autoimmune bullous dermatoses. Int J Dermatol. 2016;55:599–607. doi: 10.1111/ijd.13140. [DOI] [PubMed] [Google Scholar]
- 45.Heelan K, Al-Mohammedi F, Smith MJ, Knowles S, Lansang P, Walsh S, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703–8. doi: 10.1001/jamadermatol.2013.6739. [DOI] [PubMed] [Google Scholar]
- 46.Leshem YA, Hodak E, David M, Anhalt GJ, Mimouni D. Successful treatment of pemphigus with biweekly 1-g infusions of rituximab: A retrospective study of 47 patients. J Am Acad Dermatol. 2013;68:404–11. doi: 10.1016/j.jaad.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Cianchini G, Lupi F, Masini C, Corona R, Puddu P, De Pità O, et al. Therapy with rituximab for autoimmune pemphigus: Results from a single-center observational study on 42 cases with long-term follow-up. J Am Acad Dermatol. 2012;67:617–22. doi: 10.1016/j.jaad.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Gregoriou S, Giatrakou S, Theodoropoulos K, Katoulis A, Loumou P, Toumbis-Ioannou E, et al. Pilot study of 19 patients with severe pemphigus: Prophylactic treatment with rituximab does not appear to be beneficial. Dermatology. 2014;228:158–65. doi: 10.1159/000357031. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez J, Ingen-Housz-Oro S, Chosidow O, Antonicelli F, Bernard P. Rituximab as single long-term maintenance therapy in patients with difficult-to-treat pemphigus. JAMA Dermatol. 2018;154:363. doi: 10.1001/jamadermatol.2017.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleh MA. A prospective study comparing patients with early and late relapsing pemphigus treated with rituximab. J Am Acad Dermatol. 2018;79:97–103. doi: 10.1016/j.jaad.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 51.Schoergenhofer C, Schwameis M, Firbas C, Bartko J, Derhaschnig U, Mader RM, et al. Single, very low rituximab doses in healthy volunteers - a pilot and a randomized trial: Implications for dosing and biosimilarity testing. Sci Rep. 2018;8:124. doi: 10.1038/s41598-017-17934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alaibac M. Ultra-low dosage regimen of rituximab in autoimmune blistering skin conditions. Front Immunol. 2018;9:810. doi: 10.3389/fimmu.2018.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musette P, Bouaziz JD. B cell modulation strategies in autoimmune diseases: New concepts. Front Immunol. 2018;9:1–5. doi: 10.3389/fimmu.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De A, Ansari A, Sharma N, Sarda A. Shifting focus in the therapeutics of immunobullous disease. Indian J Dermatol. 2017;62:282–90. doi: 10.4103/ijd.IJD_199_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollmann R, Schmidt T, Eming R, Hertl M. Pemphigus: A comprehensive review on pathogenesis, clinical presentation and novel therapeutic approaches. Clin Rev Allergy Immunol. 2018;54:1–25. doi: 10.1007/s12016-017-8662-z. [DOI] [PubMed] [Google Scholar]
- 56.Ellebrecht CT, Choi EJ, Allman DM, Tsai DE, Wegener WA, Goldenberg DM, et al. Subcutaneous veltuzumab, a humanized anti-CD20 antibody, in the treatment of refractory pemphigus vulgaris. JAMA Dermatol. 2014;150:1331–5. doi: 10.1001/jamadermatol.2014.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–84. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]


