Abstract
Background:
Itraconazole (ITZ) is widely used for cutaneous and systemic mycoses. Its bioavailability is inconsistent and shows interindividual variations. The quality of ITZ pellets is an important factor determining its absorption profile and thereby the therapeutic effect. Analysis of morphometric characteristics is a surrogate method to determine the same. Pellet number and size are the most important parameters in this regard.
Aim:
We aimed to delineate few low-cost brands, the assessed variables of which fell within the formulated criteria.
Materials and Methods:
In all, 22 (100 mg) formulations of ITZ were included. The pellet number was calculated manually and pellet size was determined using a dermoscope with an inbuilt measurement tool. Furthermore, size variation with respect to the innovator US Food and Drug Administration (FDA)-approved brand was determined.
Results:
There is a large variation in pellet number and average pellet size among different brands. Pellet number ranged from 121 to 820 and average size from 959 to 1845 μm. Few brands had dummy pellets and loose powder within the capsule. Two brands within the price range of less than Rs. 20 per capsule fulfilled the three formulated criteria: of a good pellet count, small pellet size, and low size variation. Two other brands also satisfied these criteria but were priced between Rs. 20 and 30 per capsule. The innovator US FDA-approved brand had the highest number of pellets and minimum size variation but is the costliest of all assessed brands.
Limitations of the Study:
We cannot comment on inter-batch variation as ours was a one-point assessment. Advanced techniques such as scanning electron microscopy and drug release profile, which would have given further useful information on pellet quality, were beyond our scope.
Conclusion:
There is marked variation in the assessed characteristics of ITZ formulations. These morphometric characteristics may have a significant bearing on the quality of ITZ. And thus analysis of these can help clinicians make an informed decision in choosing an ITZ formulation to achieve optimal efficacy.
Keywords: Dermatophytoses, itraconazole, pellets, recalcitrant, tinea
Introduction
Recalcitrant dermatophytoses have reached an epidemic proportion in India.[1] There are documentations of inadequate responses and recurrences despite seemingly adequate treatment.[2,3] This has driven many dermatologists toward using itraconazole (ITZ) as the first-line agent and in higher dosages, beyond the US Food and Drug Administration (FDA) approved dose. This has also led to an unregulated influx of higher dose ITZ formulations (200 and 400 mg) by many pharmaceutical companies. Although persistent and recalcitrant dermatophytoses may possibly be explained by many factors including host immunity, or in vitro resistance to systemic antifungals (AFs),[4] one cause could be the drug quality. The quality of an ITZ formulation can have a significant influence on its AF efficacy and is expected to strongly affect the final therapeutic outcome. There are documented reports of treatment failure related to the proprietary formulation of ITZ used, with subsequent positive results on changing to another.[5,6] Not all countries enforce quality regulation and bioequivalence to innovator product rigidly and this may lead to inadequate and erratic responses with different formulations. The scenario becomes even more critical for a drug like ITZ which also has numerous inherent pharmacokinetic (PK) issues in relation to its absorption and bioavailability. It exhibits very low water solubility, strong food effect, and high inter-subject oral bioavailability and hence is manufactured as a nanoemulsion and is available as capsules with drug-coated pellets inside. The pellets provide a large surface area for absorption. The drug is mixed with special solvents and then coated onto beads using a complex technology. The solvent determines the drug release within the gut.[7]
A quality product should have small pellet size, and consequently large number of pellets to provide a large surface area for absorption.[7] Furthermore, pellet size variability should be minimal, as this reflects an optimum drug: solvent ratio and is a surrogate marker of the manufacturing quality.[8] A recent article has studied the effect of these variables on the drug dissolution rate, using two different bead–solvent pairs, although in most places only the sucrose beads’ formulation is currently available.[9]
Aims and Objectives
We performed this study to assess the physical characteristics of 22 ITZ brands available in the Indian market with direct observation and dermoscopy. The characteristics analyzed were pellet number, average pellet size, size variability, and cost.
Secondly, we aimed to perform a cost analysis of brands with a pellet count more than 500, average pellet size within 15%, and mean size variation within 25% of the innovator US FDA-approved brand.
Materials and Methods
As the study did not involve human subjects, ethical clearance was not required to conduct this work. We have no conflicts of interest with regard to any of the brands assessed in this study. The study was conducted in the month of September 2017. We chose 22 brands of ITZ 100 mg capsules for the analysis. The selection was based on all the brands that were being commonly prescribed in our hospital. The 200-mg and higher dosage capsules were deliberately omitted as only 100-mg ITZ capsules have US FDA clearance for treating cutaneous dermatophytosis. One capsule from a strip was used for pellet counting and another one for pellet size estimation with dermoscope. The pellets were taken out of the capsules by the first assessor, followed by dermatoscopy and manual counting by the second and third, respectively. The second and third assessors were unaware of the brand being assessed. The number of pellets was counted by naked eye examination. This procedure did not require any special apparatus/magnifying lens to perform. Dermoscopy was done using a Dinolite (AM 4113 ZT-R4) video-dermoscope with inbuilt measurement tools, at 50× and 220× magnification, using polarized light. The whole field was photographed with an attempt to cover the maximum number of pellets [Figure 1]. The photographs were then analyzed to determine the mean size (in μm). This was done by measuring the size of 50 different beads (the dimension of a straight line joining the distant-most points on the pellet, while passing through its center), including only those beads with all edges clearly visible in the photographed field, and then taking an average of these. The size variation was assessed as the mean of size variation of 50 individual pellets from the average pellet size of the formulation. Furthermore, the difference in the size of the smallest and the largest measured pellet was recorded. The presence of loose powder and dummy particles (much larger than the average pellet and with a different color) was also noted. The assessment of mean difference in average size among brands with >500, 300–500, and <300 pellets per capsule was done by one-way analysis of variance (ANOVA) with post hoc Bonferroni.
Figure 1.

Measurement of pellet size. Dummy particles seen as perfectly smooth round pellets with a different color compared with the surrounding drug-coated pellets
Results
The results are tabulated in Table 1. The size of the outer capsule shell was comparative for all assessed brands. The pellet count of different brands ranged between 121 (brand 7) to 820 (brand 1). The majority (11) had a count between 300 and 500, whereas 3 had a very low count of less than 300 [Figure 2]. Four brands had a good count of more than 600 pellets in a capsule (brands 1,3,12, and 19).
Table 1.
Analyzed characteristics of the 22 Itraconazole brands
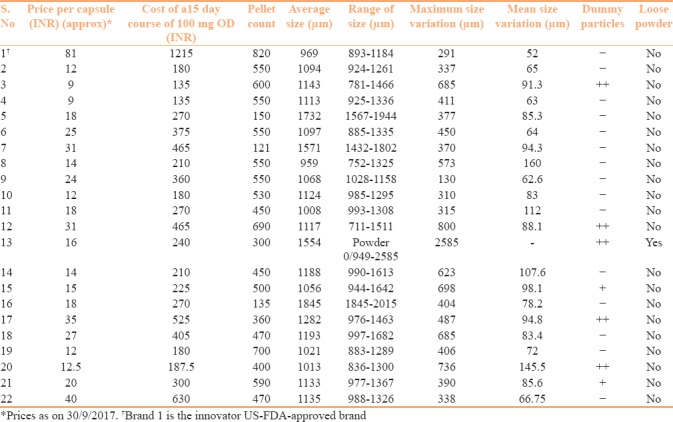
Figure 2.
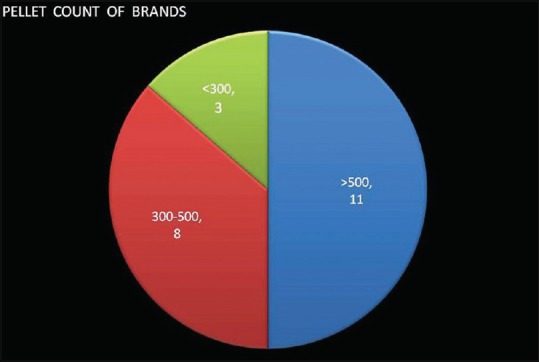
Pellet count distribution among different brands
The average size of pellets varied between 959 μm (brand 8) and 1845 μm (brand 16). The brands with a pellet number of >500 had a lower mean pellet size when compared with the other brands [Table 2]. Brands 5,7,13, and 16 had very large sized pellets, all more than 1500 μm. Nine brands (2,4,6,8,9,11,15,19, and 20) had a pellet size within 15% (up to 1114 μm) of the innovator brand. Two of these (brands 11 and 20), however, had a poor pellet count of less than 500.
Table 2.
Average size among the three pellet groups

There was a significant negative correlation between the pellet count and average pellet size [Figure 3] (Pearson's coefficient 0.848, P < 0.0001). This is expected as within the outer shell of the same size, a larger number of smaller pellets can be filled than larger ones.
Figure 3.
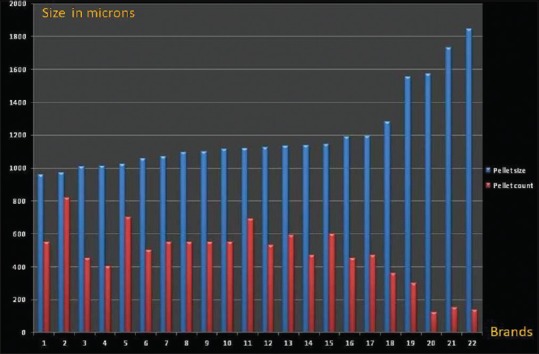
Comparison of increasing pellet size with pellet count
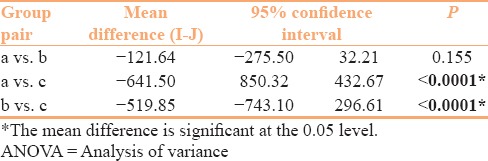
Furthermore, on comparison of brand groups with >500, 300–500, and <300 pellets, there was a significant mean difference in the average pellet size between the first (>500) and third (<300) and the second (300–500) and third (<300) groups (with P < 0.0001) [Table 3]. Loose powder was found in one brand (brand 13) and dummy particles in seven (brands 3, 12, 13,15,17,20, and 21). The difference between the smallest and the largest pellet ranged between 130 μm (brand 9) and 2585 μm (brand 13 with loose powder).
Table 3.
Assessment of mean difference in average size among brands with >500 (a), 300-500 (b), and <300 (c) pellets per capsule (by one-way ANOVA with post hoc Bonferroni)
As per the cost (as on 30/9/2017), a majority of the included formulations had a per-capsule price ranging between Rs. 10 and 20 (11), whereas 9 were priced above Rs. 20 per capsule. Only two had a price of less than Rs. 10 per capsule.
The mean size variation (assessed as the mean of size variation in 50 individual pellets from the average pellet size of the formulation) varied from 52 μm in the innovator brand to 160 μm in brand 16. Four brands (brands 2,4,6, and 9) had mean size variation within 25% of the mean size variation in the innovator brand, one each had that between 25%–30% (brand 22) and 30%–50% (brand 19) of the innovator brand, whereas most (15) had a mean size variation more than 50% of the value of the innovator brand [Figure 4].
Figure 4.
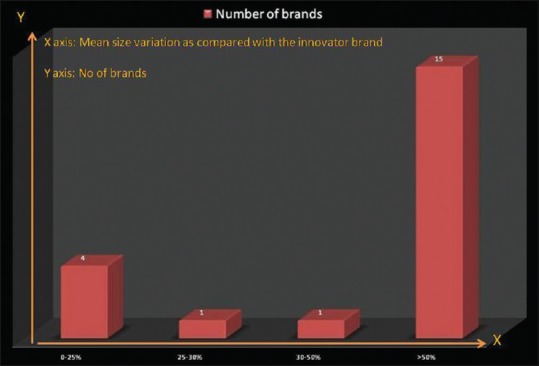
Mean size variation when compared with that of the innovator brand
Discussion
Analysis of the data revealed that using the criterion of ≥500 pellets and low size variability, brands 2,4,6, and 9 were comparable to the innovator brand, although the innovator brand had by far the highest number of pellets.
There are numerous factors leading to erratic absorption and efficacy of ITZ, mainly poor aqueous solubility, incomplete drug release, significant food effects, and pH-dependent dissolution.[10] Among the various measures of improving ITZ's bioavailability, pelletization is the method currently used by most manufacturers.[11] The pellet technology has been a major breakthrough in the pharmaceutical industry providing many therapeutic advantages over single-unit drug delivery systems. When administered orally, pellets pass the pylorus even in the closed state and disperse freely throughout the gastrointestinal tract and maximize the drug absorption.[7] Pellets provide less risk of dose dumping, reduce peak plasma fluctuations, and improve drug bioavailability.[7] Their use is associated with a reduced variation in gastric emptying rate and transit time which is then less dependent on the state of nutrition, reducing inter- and intrapatient variability.[7] However, preparation of pellets is a high-cost, complicated, and time-consuming process raising quality concerns. Pellet number and size are the most important visually measurable characteristics of ITZ capsules, acting as surrogate indicators of the quality of the formulation. These beads are made of sucrose or cellulose and should ideally be between 600 and 700 μm in size.[11] A size less than 600 μm cannot be coated properly, whereas larger diameter particles offer lesser surface area for drug dissolution. Hydroxypropyl methylcellulose (HPMC) and copovidone are the usual coating polymers used. The beads are coated with the drug–polymer mixture by a complex layering technique. ITZ is in a molecular dispersion in amorphous HPMC polymer in the capsule. The fast-dissolving polymers provide a supersaturated solution of ITZ, from which enhanced absorption is expected.[8,10] For ITZ–HPMC mixture, the ideal ratio of drug to polymer is about 40:60.[11] A higher ratio may hamper release of the drug, whereas a lower may cause ineffective and unstable binding.[12] A secondary coating by PEG 20000 is added to prevent agglutination in the gut.
It has been experimentally demonstrated that as the pellets in each unit increase, the dissolution rate also increases.[12] Pellet number within a capsule would directly depend on the pellet size and determines the surface area presented for absorption in the gastrointestinal (GI) tract. Thus, smaller pellets would result in larger pellet numbers as seen in our analysis (r = −0.848, P < 0.0001). On the same lines, we observed a significant difference (with P < 0.0001) in average pellet size of brands with >500 pellets versus those with <300 pellets, with significantly smaller pellets in the former [Table 3]. A similar statistically significant difference was also found in the average sizes of pellets among the brands with 300–500 pellets and those with <300 pellets. Brands 7 and 16 had unusually big pellets (1571 and 1845 μm) leading to very low pellet counts of 121 and 135, respectively.
Only three of the included brands had a pellet number of more than 600, whereas three had a disappointing figure of less than 300 pellets per capsule [Figure 2]. The majority (11) had a count between 300 and 500, which may be considered as average. Brands 1, 3, 12, and 19 fared the best in terms of pellet number, although brands 3 and 12 had high mean size variation.
Low size variability is a determinant of quality of the manufacturing process.[8] The presence of inert dummy particles and powder within the capsule also reflects on poor quality control as it does not match the parameters of the innovator US FDA-approved brand.[11] Brands 1, 2,4,6,9, and 22 had low mean size variation (<70 μm), whereas brands 8, 11, 13, 14, and 20 had high mean size variation (>100 μm). It has been seen that optimum HPMC concentration leads to less variation from the predetermined size range of ITZ pellets.[13] Thus, size variation may be taken as an indirect marker of optimal HPMC concentrations, which is a stabilizer and is an important factor for drug release and absorption. In simple terms, we may say that a drug with less size variation may have a better GI absorption profile.
There is a wide variation in the prices of available ITZ brands. However, it was an interesting and important finding that some brands measuring well on the assessed parameters (brands 2,4, and 19) were much cheaper than some poorly formulated ones (brands 5,7,12,14,16, and 17) [Table 1]. Furthermore, we observed that capsules from some big multinational pharmaceutical companies (brands 5 and 16) fared poorly on the measured factors, compared with certain homegrown brands (brands 2, 4, and 19). Thus, cost cannot be taken as a surrogate maker of superiority.
As per the cost, nine brands had a per-capsule price of more than Rs. 20 rupees, whereas 11 were between Rs. 10 and 20 and only 2 below Rs. 10. Considering a dosage of 100 mg once a day, the cost of a standard 15 day treatment would fall between Rs. 180 and Rs 1215. And the treatment dosages and durations being given by many are much higher than the approved doses. Furthermore, use of 200 mg and higher dose capsule formulations (amply available in the Indian market) in this regard may produce suboptimal response. This is because keeping the same pellet size and technology, a 200-mg drug load formulation would require a much larger capsule size. The change in pellet size by altering the drug–polymer ratio or removing PEG 20000 layer leads to instable formulations with a negative impact on bioavailability.[14]
Taking brands in the low/medium price range of less than Rs. 20 per capsule, and considering a good pellet count (>500), small pellet size (within 15% of the innovator brand, i.e., <111 4μm), and mean size variation within 25% of the innovator brand, brands 2 and 4 fare the best. Brands 6 and 9 fulfill the last two criteria but are priced at Rs. 25 and Rs. 24 per capsule, respectively. The innovator brand (brand 1) tops in most characteristics assessed except the very high cost.
There are certain limitations to our work. First, there may be batch-to-batch variation in the brands assessed. Ours was a one-time analysis, hence variations thereof cannot be ruled out. Second, there are other parameters that are needed to compare the various brands, including type of base, type of bead used, layering technology, size variation using scanning microscopy, and coating polymer-to-drug ratio.
In conclusion, our primary goal was to address the marked variation in quality of ITZ brands, through analysis of certain well-described parameters that have a proven effect on the drug's PK and bioavailability. It may be beneficial in optimal utilization of existing brands and might address one aspect of recalcitrant dermatophytosis. Only the 100-mg dosage capsule formulations of ITZ are approved by US FDA and Drug Controller General of India (DCGI)/Central Drugs Standard Control Organisation (CDSCO),[15,16] and higher dose formulations (200 and 400 mg) may have quality issues as highlighted previously. Our assessment of 100 mg ITZ shows wide variability in the quality of the available formulations reflecting poor quality control. This also reiterates the fact that if ITZ pellets meet the desired specifications, there is probably no need to updose and prescribe ITZ in doses exceeding 100 mg/day, which mirrors largely the skin PK of ITZ.[17]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bishnoi A, Vinay K, Dogra S. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:250–1. doi: 10.1016/S1473-3099(18)30079-3. [DOI] [PubMed] [Google Scholar]
- 2.Majid I, Sheikh G, Kanth F, Hakak R. Relapse after oral terbinafinetherapy in dermatophytosis: A clinical and mycological study. Indian J Dermatol. 2016;61:529–33. doi: 10.4103/0019-5154.190120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma R, Adhikari L, Sharma RL. Recurrent dermatophytosis: A rising problem in Sikkim, a Himalayan state of India. Indian J Pathol Microbiol. 2017;60:541–5. doi: 10.4103/IJPM.IJPM_831_16. [DOI] [PubMed] [Google Scholar]
- 4.Hau CS, Tada Y, Kanda N, Watanabe S. Immunoresponsesin dermatomycoses. J Dermatol. 2015;42:236–44. doi: 10.1111/1346-8138.12718. [DOI] [PubMed] [Google Scholar]
- 5.Pasqualotto AC, Denning DW. Generic substitution of itraconazole resulting in sub-therapeutic levels and resistance. Int J Antimicrob Agents. 2007;30:93–4. doi: 10.1016/j.ijantimicag.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Pierard-Franchimont G, De Doncker P, Van de Velde V, Jacqmin P, Arrese JE, Pierard GE. Paradoxical response to itraconazole treatment in a patient withonychomycosiscaused by Microsporum gypseum. Ann Soc Belg Med Trop. 1995;75:211–7. [PubMed] [Google Scholar]
- 7.Sirisha KVR, Sri KV, Suresh K, Reddy GK. Devanna N.A review of pellets and pelletization process—A multiparticulate drug delivery system. Int J Pharm Sci Res. 2013;4:2145–58. [Google Scholar]
- 8.Kapoor BS, Subbaro GV. Formulation development and characterization of itraconazole granules. J Pharm Sc. 2013;102:3966–77. [Google Scholar]
- 9.Parmentier J, Tan EH, Low A, Möschwitzer JP. Downstream drug product processing of itraconazole nanosuspension: Factors influencing drug particle size and dissolution from nanosuspension-layered beads. Int J Pharm. 2017;30(524):443–53. doi: 10.1016/j.ijpharm.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Allegra S, Fatiguso G, De Francia S, Favata F, Pirro E, Carcieri C, et al. Pharmacokineticevaluation of oralitraconazole for antifungalprophylaxis in children. Clin Exp Pharmacol Physiol. 2017;44:1083–8. doi: 10.1111/1440-1681.12822. [DOI] [PubMed] [Google Scholar]
- 11.De Doncker P, Pande S, Richarz U, Garodia N. Itraconazole: What clinicians should know? Indian J Drugs Dermatol. 2017;3:4–10. [Google Scholar]
- 12.Swaminathan S, Sangwai M, Wawdhane S, Vavia P. Solubleitraconazolein tablet formusing disordered drug delivery approach: Criticalscale-upconsiderations and bio-equivalencestudies. AAPS PharmSciTech. 2013;14:360–74. doi: 10.1208/s12249-012-9918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor BS, Subbarao GV. Formulation development and characterization of itraconazole granules. Int J Pharm Sci Nanotech. 2011;4:1497–500. [Google Scholar]
- 14.Baert LEC, Verreck G, Thoné D. Antifungal compositions with improved bioavailability. US Patent 6,509,038 B2. 2013 [Google Scholar]
- 15. [Last accessed on 2017 Dec 20]. Available from: https://www.pharmacompass.com/fda-orangebook/itraconazole .
- 16. [Last accessed on 2017 Dec 20]. Available from: https://cdscoonline.gov.in/CDSCO/Drugs .
- 17.Sardana K, Arora P, Mahajan K. Intracutaneous pharmacokinetics of oral antifungals and their relevance in recalcitrant cutaneous dermatophytosis: Time to revisit basics. Indian J Dermatol Venereol Leprol. 2017;83:730–2. doi: 10.4103/ijdvl.IJDVL_1012_16. [DOI] [PubMed] [Google Scholar]


