Abstract
Background:
Recently, with better understanding of the immunology of warts, immunotherapeutic approaches have emerged as an effective treatment option for the management of cutaneous warts. Intralesional immunotherapy with MMR vaccine is one such modality but there are still lack of enough placebo-controlled studies.
Aim:
To evaluate the efficacy of intralesional MMR in patients of extragenital warts in a double-blinded manner using normal saline as control.
Patients and Methods:
One hundred patients of extragenital cutaneous warts were randomly allocated into two groups, the interventional (MMR) group and control (normal saline) group. MMR vaccine was injected intralesionally in the patients belonging to interventional group, a similar volume of normal saline (NS) was injected in the control group. The outcome in terms of treatment response, adverse effects, and recurrences were evaluated and compared.
Results:
Eighteen of thirty (60%) patients in the interventional group achieved complete response as against 7 (23.3%) in the control group (P = 0.01). Distant warts cleared in 69.5% patients in the interventional groupcompared to none in the control group. Adverse effects seen in both groups were injection site pain and mild erythema. A total of 57.1% patients showed recurrences in the control group compared to 16.6% in the interventional group.
Conclusion:
Intralesional MMR vaccine is an effective treatment option in patients with multiple extragenital warts. It is suggested that it should be used as first-line therapy for multiple warts and a second-line therapy for warts recalcitrant to standard therapies.
Keywords: Cutaneous wart, immunomodulator, intralesional MMR
Introduction
Human papilloma viruses (HPV) are small DNA viruses that infect both keratinized and nonkeratinized squamous cell epithelia and cause the development of warts.[1,2,3] There are various morphological types of warts caused by different serotypes of HPV. Warts occur with equal frequency in both males and females and transmission occurs by direct or indirect contact.[1,4] There are several destructive and immunotherapeutic treatment options available for extragenital warts but no single treatment has yet proven to be 100% effective. Destructive therapies include either topical chemoablative agents, such as salicylic acid, podophyllotoxin, and trichloroacetic acid or surgical methods such as cryosurgery, laser ablation, electrocautery, and surgical excision. These destructive treatment modalities are usually painful and associated with recurrences rate as high as 30%.[5] Lack of development of specific immune response to target HPV serotype underlie the high recurrence associated with these methods. Therefore, a simple, safe, inexpensive, and effective modality is needed to address these gaps in the management of warts. One such modality is immunotherapy using different intralesional antigens.[6] In the recent past, various intralesional antigens have been tried, but intralesional immunotherapy using MMR vaccine has attracted the interest of clinicians and researchers. The principle underlying immunotherapy in warts is mounting of delayed-type hypersensitivity reaction against intralesional antigens as well as the viruses causing warts; this stimulated immune response is necessary for regression of warts and further prevention of recurrences.[2,3,7] Previous studies have revealed the effectiveness of intralesional immunotherapy in the treatment of extragenital cutaneous warts.[7,8,9,10] One such study was conducted by our group in 2012–2013[11] using intralesional MMR vaccine in the treatment of cutaneous warts. The study suggested that intralesional MMR is an effective and safe treatment modality for cutaneous warts. As there is scarcity of placebo-controlled blind studies in this domain, we aimed to carry out a randomized, double-blind, placebo-controlled study to further establish the effectiveness of intralesional MMR in the management of cutaneous warts among immunocompetent patients.
Patients and Methods
Before initiating the study, due approval was taken from the institutional review board. The study included 100 patients who were recruited over a period of one and half years. Patients had either single or multiple extragenital cutaneous warts of more than 1 year duration. Few patients also had distant warts which were defined as those present in anatomic sites, different from the wart receiving intralesional therapy. The diagnosis of extragenital warts was made clinically and patients were advised not to use any additional or alternate treatment for warts during the study period. Patients were randomly assigned to two groups using computer-generated random number tables. The group receiving intralesional MMR vaccine was designated as the interventional group and the other group receiving intralesional normal saline was designated as the control group. Neither the patient nor the dermatologist who evaluated the treatment response in both the groups, were aware of the treatment given. In this manner, we could ensure the double blind nature of the study. Thirty patients in each group completed the study, and 40 patients were lost to follow-up. These dropouts were excluded from the final analysis.
Following patients were excluded from the trial; patients with a history of prior allergic response to MMR vaccine, patients with acute febrile illness, patients who received any other treatments for their warts in the last month before enrolment, past history of asthma or allergic skin disorders, past history of meningitis or convulsions, pregnancy, lactation, iatrogenic, or primary immunosuppression.
After obtaining a written informed consent from all patients, baseline characteristics of the warts including number, site, size, duration, and presence or absence of distant warts were noted at the start of the study and thereafter at each follow-up visit. A total of 0.3 ml of either intralesional MMR or normal saline was injected into the warts by a fellow dermatologist colleague using insulin syringe at an interval of 3 weeks for a maximum of three injections. Large warts were selected for intralesional therapy in patients having multiple warts. Response to treatment was observed by the dermatologist other than the one who carried out the procedure. Responses were recorded as decrease in size or number of warts with photographic documentation of the same [Figures 1–3]. The response was considered complete if there was disappearance of the wart(s) and return of the normal skin markings, partial if the wart(s) had regressed in size by 50–99%, and no response if there was 0–49% decrease in wart size. Immediate and late adverse effects of MMR vaccine were observed at each treatment session and follow-up visits. Only patients who achieved complete responses in both the groups were observed for 6 months post treatment to detect any recurrences.
Figure 1.
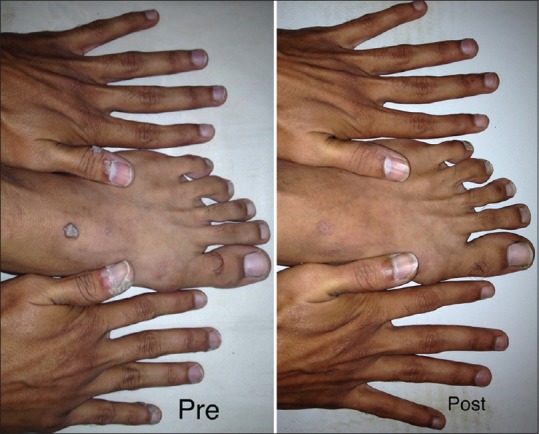
Photograph showing improvement in warts after three doses of the intralesional MMR
Figure 3.
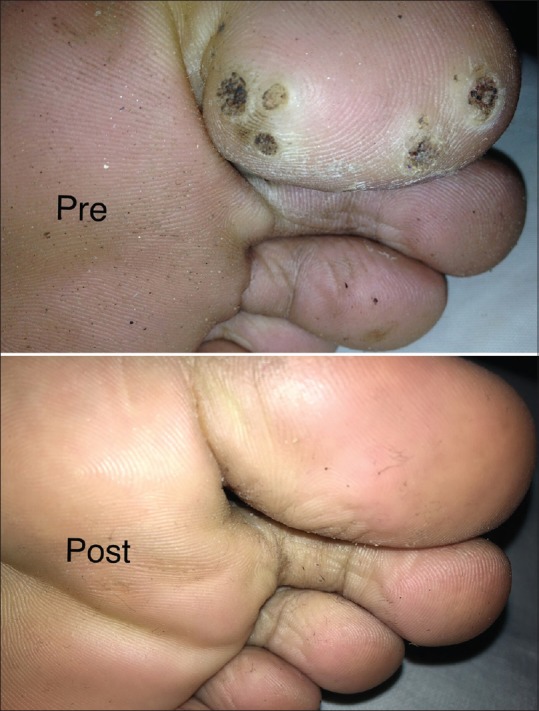
Plantar wart in a patient after first follow-up visit post intralesional MMR
Figure 2.
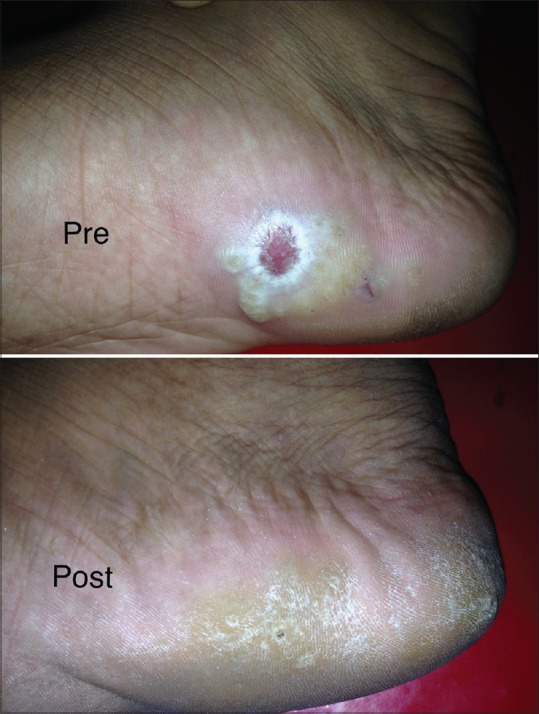
Photograph showing complete resolution of warts following three doses of intralesional MMR
Statistical analysis
Data were entered, checked, and analyzed using the SPSS/PC* (Statistical package for social science for personal computers Ver. 16) package. Data were expressed as mean ± SD for quantitative variables, and number and percentage for qualitative variables. Chi-square test and F-test were used as appropriate. P values of <0.05 were considered significant.
Results
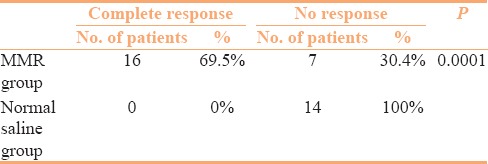
Sixty patients completed the study, 30 each in interventional (MMR) and control (normal saline) group. Forty patients were lost to follow-up and were excluded from the final analysis. Baseline characteristics of the patients in both groups are presented in Table 1. Both groups were comparable in terms of demographic and clinical profile. Table 2 compares the treatment responses in both groups at the end of study period, showing that 60% of the patients in the MMR group achieved complete response compared to only 23.3%in the control group. The differences observed in the treatment responses between the groups was statistically significant (P value = 0.01). Complete clearance of distant warts was observed in 69.5% of the patients who presented with multiple warts in the MMR group in contrast to 0% in the control group [Table 3]. The therapeutic response to intralesional MMR was independent of the age and gender of the patients or different determinants of the warts. Side-effects included injection site pain in most of the patients (60%) and erythema in 4 patients (13.3%) of the MMR group. Recurrence was observed at distant site in 4 out of 7 patients (57.1%) in the control group while only 3 out of 18 patients (16.6%) had recurrence in the MMR group after completion of the 6 month follow-up period.
Table 1.
Baseline clinicodemographic profile of patients in interventional and control groups (n=30 in each groups)
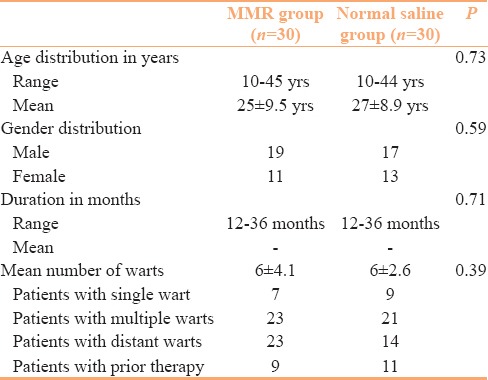
Table 2.
Treatment responses in interventional and control groups (n=30 in each groups)

Table 3.
Clearance of distant warts in interventional and control groups (n=30 ineach groups)
Discussion
Treating extragenital warts is a considerable challenge for a clinician and it is often frustrating because the results of the available therapeutic modalities are usually disappointing. Many treatment modalities are available for the treatment of warts. Destructive modalities are the most commonly used treatment for warts but most of them are painful, associated with disfiguring scarring, and have a high recurrence rate. Several immunotherapeutic agents with variable efficacy have been used for the treatment of different types of warts.[3] Among these agents is the recently used intralesional immunotherapy which has been shown to be an effective and safe modality. It has the potential advantages of clearance of both treated and untreated distant warts without scarring, with a presumed lower rate of recurrence and a high safety profile.[4] There are various isolated antigens available for intralesional therapy, however, triple antigens in composition as in MMR vaccine have added advantage of producing boosted immune response due to adjuvant effects of different antigens on each other.[7]
In a previous open label study we have demonstrated the utility of intralesional MMR in clearing extragenital warts.[11] The present work is an extension of the previous study where we have compared the treatment response of intralesional MMR with normal saline in a double-blind manner. The results demonstrated a highly significant difference between the therapeutic response of extragenital warts to MMR vaccine compared to normal saline. Similar findings have also been reported by other studies that compared intralesional antigen therapy with normal saline as the control group.[7,8,9,10,12]
Nofal et al.[7] observed complete response in 81.4% patients and Mohamad et al.[10] observed complete response in 82% patients with MMR vaccine. This figure is higher than what was achieved in the present study. A possible explanation of this could be that Nofal et al. tested all patients for the presence of existing immunity by injecting 0.1 ml of MMR vaccine intradermally into the skin of the forearm, and those patients who did not react to the skin test were excluded from the study. This probably minimized the number of patients who were less likely to respond to MMR vaccine. In addition, the treatment session carried out by Nofal et al.were also higher than in our study (five treatment sessions at 2-week intervals until complete clearance of warts).
The number of patients showing complete response in this study was relatively less as achieved in an earlier open study conducted in the same department.[11] The possible reason could be the inclusion of warts with lesser duration in the previous study. Spontaneous resolution is known to occur in warts. Factors that determine spontaneous resolution are duration, type of warts, and age of the patients.[13] Eighty percent patients (24/30) with intralesional MMR achieved either partial or complete response in this study. This was higher than what was reported by other researchers who have used different test antigen or single antigen in the form of vaccine.[6,8,9,14] The higher response could be attributed to the presence of the three synergistic viral antigens in MMR vaccine as well as better immunogenicity of a vaccine compared to other test antigens.
Sixteen patients (69.5%) had complete clearance of distant warts, albeit MMR was injected only into largest wart, while in the normal saline group not a single patient showed clearance of distant warts. Similar observation of the clearance of all anatomically distant wart was also made by Nofal et al.[7] (17/20, 85%) and Gamil[15] (5/6, 83.3%). Johnson et al.[16] also observed clearance of all anatomically distant warts in 14 (78%) out of 18 patients. The clearance of untreated distant wart strongly suggests the development of a widespread HPV-targeted immunity as a response to antigen injection and may represent a major advantage of the intralesional immunotherapy.
We did not find any statistically significant association between the therapeutic response to MMR vaccine and the different clinical variables such as age and gender of patients and the clinical type of warts. Similar observations were also made by Nofal et al.[7] Horn et al.[9] and Signore et al.[17] reported better response for intralesional immunotherapy in patients with age group of <40 years. This can be attributed to relatively robust immune response of younger patients.
Recurrence of warts was seen in 3 out of 18 patients (16.6%) in the MMR group compared to 4 out of 7 patients (57.1%) in the control group. The difference between recurrence rates of two groups was statistically significant (P < 0.05). This suggests that intralesional MMR leads to long-lasting immunity directed against HPV virus. Induction of memory T cell could be one of the explanations. A similar observation of absent or low rates of recurrence have also been reported by other studies of similar nature.[7,10,16,17] This finding represents another important advantage of intralesional immunotherapy over traditional treatments. Although theoretically accepted, this advantage needs longer follow-up periods and more number of patients for further validation.
Seven patients (23.3%) who received intralesional normal saline also showed complete response. This can be explained by the fact that trauma alone can activate a strong nonspecific inflammatory response against the HPV-infected cells through an interaction of stimulated macrophages, T-helper cells, neutrophils, and natural killer cells.[16,17] However, none of the patient in intralesional normal saline showed clearance of distant warts. This could mean, that widespread HPV targeted immunity cannot be achieved with trauma alone.
Intralesional immunotherapy is usually associated with mild insignificant side-effects such as injection site pain, erythema, and post inflammatory hyperpigmentation.[18] More aggressive side-effects such as infection, wounding, and scarring were not observed in our study and other studies utilizing intralesional immunotherapy. A suggested association between MMR vaccine and autism has been a controversial issue, particularly within the public; however, rigorous scientific studies have not identified such an association.[19]
Recently, few studies[20,21] have measured the level of IL-4 and IL-12 following intralesional Purified Protein Derivative (PPD) and MMR. However, high serum levels of these interleukins are usually not associated with wart clearance. It would be worthwhile to carry out further studies using immunological parameters such as lesional and systemic level of cytokines following intralesional MMR vaccine.
Conclusion
Intralesional MMR immunotherapy is a promising treatment modality for cutaneous extragenital warts, particularly the multiple ones. The induction of HPV-directed immunity through the establishment of a delayed-type hypersensitivity reaction to an otherwise unrelated immunogen is a suggested mechanism of action of MMR vaccine and other lesional immunotherapeutic antigen. The result of this controlled study confirms our earlier observation[11] that intralesional MMR vaccine is an inexpensive, effective, and safe option that has the potential advantage of widespread and sustained effects against HPV. The limitations of our study were small sample size, short follow-up period, and lack of immunological measurements. Despite these limitations, the results of our study suggest that intralesional MMR may be used as a first-line therapy for multiple warts and a second-line therapy for warts recalcitrant to standard therapies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sterling JC. Virus infections. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology of Dermatology. 8th ed. Oxford: Blackwell publishing; 2010. pp. 33.37–60. [Google Scholar]
- 2.De Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Androphy EJ, Lowy DR. Warts. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. New York: Mc Graw-Hill; 2008. pp. 1914–23. [Google Scholar]
- 4.Kilkenny M, Marks R. The descriptive epidemiology of warts in the community. Australas J Dermatol. 1996;37:80–6. doi: 10.1111/j.1440-0960.1996.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 5.Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4:273–93. doi: 10.3121/cmr.4.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089–93. doi: 10.1111/j.1468-3083.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 7.Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166–70. doi: 10.1111/j.1468-3083.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- 8.Kus S, Ergun T, Gun D, Akin O. Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol. 2005;19:515–6. doi: 10.1111/j.1468-3083.2004.01176.x. [DOI] [PubMed] [Google Scholar]
- 9.Horn TD, Johnson SM, Helm RM, Roberson PR. Intralesional immunotherapy of warts with mumps, Candida and trichophyton skin test antigens: A single-blinded, randomized and controlled trial. Arch Dermatol. 2005;141:589–94. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- 10.Mohamad NS, Badran F, Yakout E. Evaluation of the efficacy of a combination-measles, mumps and rubella vaccine in the treatment of plantar warts. Our Dermatol Online. 2013;4:463–7. [Google Scholar]
- 11.Saini P, Mittal A, Gupta LK, Khare AK, Mehta S. Intralesional mumps, measles and rubella vaccine in the treatment of cutaneous warts. Indian J Dermatol Venereol Leprol. 2016;82:343–5. doi: 10.4103/0378-6323.175920. [DOI] [PubMed] [Google Scholar]
- 12.Brunk D. Injection of Candida antigen works on warts. Skin Allergy News. 1999;30:5. [Google Scholar]
- 13.Choi H, Kim HR, Na CH, Kim MS, Shin BS, Choi KC. Intralesional immunotherapy of warts with mumps, measles and rubella vaccine. J Dermatol. 2012;39:63–64. doi: 10.1111/ced.12369. [DOI] [PubMed] [Google Scholar]
- 14.Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional Mumps or Candida antigens. Pediatr Dermatol. 2003;20:268–71. doi: 10.1046/j.1525-1470.2003.20318.x. [DOI] [PubMed] [Google Scholar]
- 15.Gamil H. Elgharib I. Intralesional Immunotherapy of Plantar Warts: Report of a New Antigen Combination. J Am Acad Dermatol. 2010;63:40–3. doi: 10.1016/j.jaad.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SM, Roberson PK, Horn TD. Intralesional injection of Mumps or Candida skin test antigens: A novel immunotherapy for warts. Arch Dermatol. 200l;l37:45l–5. [PubMed] [Google Scholar]
- 17.Signore RJ. Candida albicans intralesional injection immunotherapy of warts. Cutis. 2002;70:185–92. [PubMed] [Google Scholar]
- 18.Lichon V, Khachemoune A. Plantar warts: A focus on treatment modalities. Dermatol Nurs. 2007;19:372–5. [PubMed] [Google Scholar]
- 19.Miller L, Reynolds J. Autism and vaccination - the current evidence. J Spec Pediatr Nurs. 2009;14:166–72. doi: 10.1111/j.1744-6155.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- 20.Shaheen MA, Salem SAM, Fouad DA, El-Fatah AAA. Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2015;28:194–200. doi: 10.1111/dth.12230. [DOI] [PubMed] [Google Scholar]
- 21.Abd-EIazeim FMA, Mohammed GFA, Fathy A, Mohamed RW. Evaluation of IL-12 serum level in patients with recalcitrant multiple common warts, treated by intralesional tuberculin antigen. J Dermatol Treat. 2014;25:264–7. doi: 10.3109/09546634.2013.768760. [DOI] [PubMed] [Google Scholar]


