Abstract
Background:
The incidence of dermatophytosis is increasing over the last few years and there are many cases which are recurrent and chronic.
Aim:
The aim was to study the host and pathogen factors in dermatophytosis, to identify the species responsible, and to study the histopathological features of chronic dermatophytosis.
Materials and Methods:
It was a descriptive study conducted in the Department of Dermatology for a period of 1 year and all patients who were clinically diagnosed as dermatophytosis were included. Isolated hair, and nail involvement were excluded from the study. Epidemiological parameters and treatment history were analyzed, scrapings, and fungal culture were done in all patients. Histopathological examination was done in patients with chronic dermatophytosis who had applied topical steroids.
Results:
Chronic dermatophytosis was seen in 68%; tinea corporis was the most common presentation; topical steroid application was seen in 63%; azoles were the most common antifungals used; varied morphologies such as follicular and nonfollicular papules, arciform lesions, pseudoimbricata were seen in steroid modified tinea. Trichophyton rubrum and Trichophyton mentagrophytes were the most common species isolated in culture, but rare species such as Trichophyton tonsurans, Trichophyton schoenleinii, Epidermophyton floccosum, and Microsporum audouinii were also isolated from chronic cases. Histopathology showed perifolliculitis in steroid modified tinea. Minimal inhibitory concentration was lowest for itraconazole in susceptibility studies.
Conclusion:
Chronicity in dermatophytosis is due to various factors such as topical steroid application, noncompliance, and change in predominant species.
KEY WORDS: Chronic dermatophytosis, perifolliculitis, pseudoimbricata
Introduction
Dermatophytic infections are one of the most common diseases encountered by dermatologists in outpatient departments and there has been an increasing trend of dermatophytosis over the last few years in different parts of India.[1,2] There is a changing trend in the dermatophytic infections in that the cases are presenting as chronic, treatment unresponsive and recurrent cases.[3] This superficial fungal infection was an easily treatable condition for the practitioners previously with the commonly used antifungals. There are no validated definitions for chronic and recurrent dermatophytosis. Chronic dermatophytosis refers to patients who continue to have disease for 6 months to 1 year with or without recurrence in spite of being treated. Recurrent dermatophytosis is cutaneous dermatophytosis in which the infection recurred within 6 weeks of stopping the adequate antifungal treatment with at least two such episodes in last 6 months.[4] The resistance to treatment may be due to use of over-the-counter (OTC) topical steroids or due to host factors such as noncompliance and immunosuppression. There have been reports of antifungal resistance also for commonly used antifungals fluconazole and terbinafine.[3] Hence, this study was done to assess the current pattern of dermatophytosis, and antifungal susceptibility.
Materials and Methods
The study was conducted in the Department of Dermatology, Government Medical College, Kottayam, over a period of 1 year after obtaining permission from the Institutional Review Board. It was a descriptive study with the participation of the Department of Microbiology and Pathology, Government Medical College Kottayam and Department of Mycology PGI Chandigarh. The sample size was calculated using the formula 4pq/d2 (p is the prevalence, q is 100 - p, d is precision of reference study) and the calculated sample size was 106.
In the study, a total of 120 consecutive patients with dermatophytosis of glabrous skin and who gave consent were enrolled in the study. Tinea capitis and tinea unguium cases were excluded. A thorough history was taken regarding the duration, treatment taken, OTC application, compliance of treatment, affection of other family members, type of clothing, sharing of towels and soaps, intake of immunosuppressant, history of diseases such as diabetes, asthma, connective tissue diseases, and other skin diseases. General examination, systemic examination, and thorough dermatological examination were done in all cases with special attention to sites involved, morphology of lesions. Blood sugar, retrovirus screening, scraping for fungus, and fungal culture were done in all cases. In cases where fungal species identification was difficult specimens were sent to PGI Chandigarh, the reference mycology center in India. Antifungal susceptibility testing was done in selected cases by Microbroth dilution method (M38-A) standardized by Clinical Laboratory Standards Institute. In patients with chronic dermatophytosis who had applied topical steroids and who gave consent for biopsy, a histopathological examination with H and E stain and special stain with Grocott's methenamine silver (GMS) was done.
Results
Among 120 cases studied, 32% had first episode of dermatophytosis and in 68% it was chronic. Male-to-female ratio was 1:1.1 in first episode and 1.5:1 in recurrent cases. Male preponderance was seen in chronic cases. The most common age group affected was 10–20 years in the first episode and 5th decade (40–50 years) in chronic cases. Fifty-two percent were manual laborer, 27% were homemaker, and 16% were sedentary worker. Duration of illness was 1–6 months in 47% and more than 6 months in 53%. Tinea corporis was the most common presentation in first episode cases (28.7%) followed by tinea cruris in 17.2% and tinea faciei in 2.6%. In chronic cases, tinea corporis was seen in 38.2%, tinea cruris in 20.8%, and tinea faciei in 4.3%. Associated diseases were diabetes in 2.6%, thyroid diseases in 2.6% in first episode and in chronic cases diabetes was associated in 11% (P > 0.5). Immunosuppressant intake was noted in 2.6% of first episode and 3.6% in chronic cases. Family history of dermatophytosis was found in 21%, with the habit of sharing towels in 20% and use of synthetic dress material in 14% in first episode. In chronic cases family history was present in 28%, towel sharing in 22% and use of synthetic dress material in 50%. The drug history of chronic dermatophytosis revealed the use of topical-steroid antifungal combination in 42%, topical steroid in 21%, azoles in 28% and allylamines in 19% cases. Among the oral antifungals, fluconazole was used by 22%, allylamines in 12.5%, griseofulvin in 7.5%, and itraconazole in 5.8%. Compliance of topical medication was assessed and found that 53% applied it regularly and 47% had irregular application. The amount of application was also inadequate in 37%.
In 48% of patients with topical steroid-modified tinea, erythematous patches were present. Follicular and nonfollicular papules were seen in 40% [Figure 1]. Other atypical features seen were pustules in 7%, pseudoimbricata 1% [Figure 2], arciform lesions in 3%, and a case of erythroderma.
Figure 1.
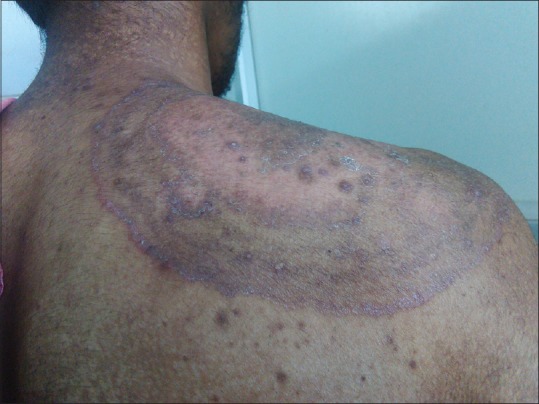
Hypopigmentation and follicular papules in steroid modified tinea
Figure 2.
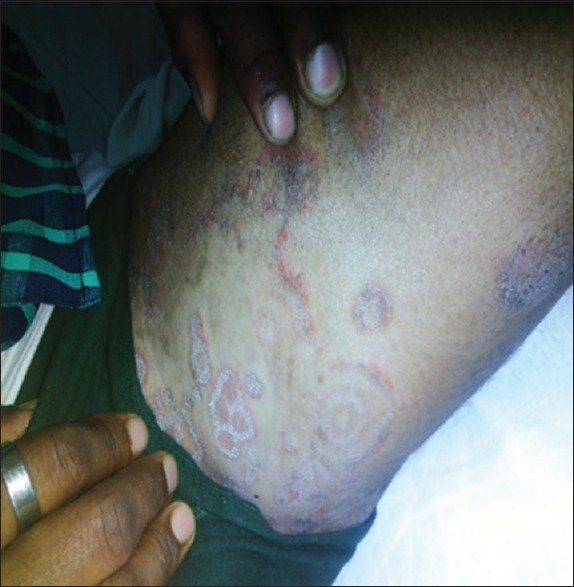
Tinea pseudoimbricata - multiple concentric circles with intermittent areas of clearing seen in steroid modified tinea
Scraping for fungus was positive in 79% in first episode and 34% in chronic cases. Fungal species identification was done by culture and in first episode cases, Trichophyton rubrum was isolated in 21%, Trichophyton mentagrophytes in 10% and mixed growth in 3%. In chronic dermatophytosis, 45% of isolates were T. rubrum, 18% was T. mentagrophytes, 2% was Trichophyton tonsurans, and single isolates of Trichophyton schoenleinii, Epidermophyton floccosum, and Microsporum audounii were obtained.
Antifungal susceptibility testing was done in two isolates of T. mentagrophytes. Minimal inhibitory concentrations (MIC) were found to be increased for fluconazole and griseofulvin, and MIC was low for itraconazole.
Histopathology was done in 10 chronic cases who had applied steroids. Inflammatory infiltrates in dermis were seen in all cases and included, neutrophils and lymphocytes in all cases, eosinophils in 30% and in another 30% all types of cells were seen. Acanthosis and parakeratosis were seen in 75% and hyperkeratosis in 37% cases. Perifolliculitis and perivascular inflammatory infiltrate were seen in 87% cases. In special stains, sandwich sign was positive in 37%. Hyphae were seen in stratum corneum in 87%, dermis in 37%, around follicles in 37%, and in all sites in 37% [Figure 3].
Figure 3.
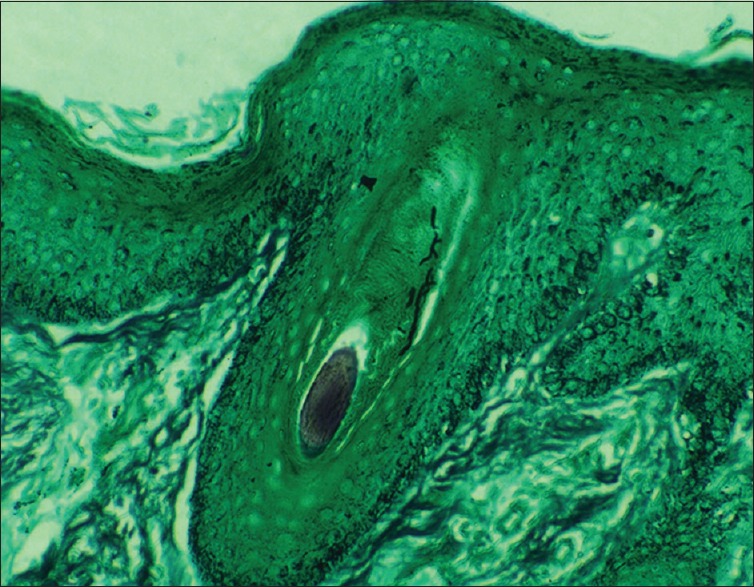
Grocott's methenamine silver stain (×400) showing fungal hyphae around hair follicle
A total of 30 chronic cases were treated with oral itraconazole 200 mg once daily for 1 month, and only one had recurrence with few lesions. Repeat fungal scraping was negative in these patients after treatment for 2 weeks.
Discussion
The recurrence rate of dermatophytosis in our study was 68% compared to 32% by Bindu and Pavithran in 2002 conducted at Kozhikode, Kerala.[3] This increasing trend of chronic dermatophytosis has been observed in various studies. Male-to-female ratio was approximately equal in first episode, but a slight male preponderance was seen in chronic cases. This may be related to more outdoor activities in males. However, Sudha et al. and Bindu et al noted a male predominance in their studies on the first episode of dermatophytosis.[3,5] In chronic cases, we noted a male predominance, but Sivaprakasam et al. noted more female cases.[6] The age group commonly affected in our study was second decade in first episode, but Agarwal et al. noted most cases in 3rd decade.[7] Bindu et al. noted the maximum number of cases in the second decade. In chronic cases, the age group affected was 5th decade in the present study and 4th decade in the study by Sivaprakasam et al. Hence, chronic dermatophytosis is more common in late middle age and this may be due to waning immunity in them and other comorbidities. Most common clinical type we found was tinea corporis in both first episode and chronic, and this was in concordance with studies conducted by Bindu et al., Sudha et al.,[5] and Sivaprakasam et al. Association with diabetes was 2.6% and 11% in first episode and chronic dermatophytosis respectively; however, this difference was not statistically significant. Bindu et al. noted diabetes in 11% in first episode and Sivaprakasam et al. noted 30% in recurrent cases. Family history of similar illness was 21% in our study and this was not in concordance with 48% in the study by Ghosh et al.[8] However, in chronic cases, a slight increase in percentage of affected family members was seen. Affected family member was always a source of reinfection in chronic cases. Manual laborers and home makers were more commonly involved and this in concordance with the study by Ghosh et al. This might be due to increased sweating associated in them. Use of synthetic dress material was found to be 50% in our study and in the study by Bindu et al. it was 70%. The dressing patterns have changed over years and tight fitting synthetic dressings, leggings, and jeans which are not suited for our climate are contributing factors to recurrence.
On analyzing the treatment history of our patients, it was found that 63% used topical steroids and in a study by Mahajan et al. topical steroid antifungal combinations were used by 70.6%.[9] Among the topical antifungals azoles were the commonly used ones in 28% and allylamines in 19% in our study, but in study by Soniya et al., topical antifungals were used by 5.7% only. In this study, fluconazole was found to be the most common oral antifungal used in 22% followed by allylamines in 12.5%, griseofulvin in 7.5% and itraconazole in 5.8%. In the study by Mahajan et al. fluconazole was the most common antifungal used (26.8%) followed also by terbinafine in 8.7% and griseofulvin in 2.3%. The dermatophytic clearance from the skin is secondary to the activation of cell-mediated immunity (CMI) and is Th1/Th17-dependent.[10] The cutaneous inflammatory response that the skin mounts to resist and limit the fungal infection is majorly suppressed by topical as well as systemic steroids. However, this effect of topical steroids is said to be more profound than with the other routes. Concomitantly, there is local suppression of T-cell mediated immune response to the dermatophyte. The use of OTC topical steroid antifungal combination and fixed drug combinations including steroid, antifungal, and antibacterial agents might be the cause for the chronicity and recurrences.
Azoles were the most common antifungal used orally and topically; however, the compliance of application was poor regarding duration and amount applied. However, recurrences were also seen in patients who were compliant and this might be due to antifungal resistance. Clinical resistance has been defined as the persistence or progression of infection despite appropriate antimicrobial therapy.[11] In other words, it is the failure to eradicate a fungal infection despite the administration of an antifungal agent with in vitro activity against the organism. A successful clinical response to antimicrobial therapy depends not only on the susceptibility of the pathogenic organism but also relies on the host immune system, drug penetration and distribution, patient compliance, and absence of a protected or persistent focus of infection.[12]
T. rubrum was the most common species isolated in the first episode and in chronic dermatophytosis followed by T. mentagrophytes in the present study. Rare species, such as T. tonsurans and single cases of T. schoenleinii, E. floccosum and M. audouni, were isolated from chronic cases. This was in concordance with the study conducted by Jain et al.,[13] Agarwal et al. and Bindu et al. in which T. rubrum was the most common species, but Soniya et al. and Sahai et al. reported T. mentagrophytes to be the most common species. T. tonsurans, E. floccosum, and T. schoenleinii were isolated in studies by Mahajan et al. and Sahai et al.[14] Isolation of the species which usually infect the scalp hair may be a reason for chronicity as they need to be treated for longer duration due to invasion of hair. About 90% of cases of chronic dermatophytosis have been attributed to T. rubrum infection. Widespread T. rubrum dermatophytosis has often been described as T. rubrum syndrome, generalized chronically persistent rubrophytia, and tinea corporis generalisata.[15]
In first-episode cases, we could isolate fungus in 48% by fungal culture, but Surendran et al.[16] noted 39%, Agarwal et al. 80% and Bindu et al. 45% isolate in first episode. In chronic cases, we could isolate only 12% and this was not in concordance with Sivaprakasam et al. who could isolate fungus in 52% in their study. This sparse isolate may be due to previous antifungal and topical steroid application causing deeper penetration.
About 63% of the patients in this study used topical steroid/steroid antifungal combination and this may be the reason for chronicity of the disease due to suppression of CMI. Many morphological patterns were seen in the cases who applied topical steroids and erythema around the lesion was the most common feature followed by follicular and nonfollicular papules. Shyam Verma and Reghu reported many atypical presentations of steroid modified tinea and it included erythema, eczematous lesions, pseudoimbricate, and pustules as in our study. The centrifugal spread of dermatophytosis is because of the CMI clearing the fungus in the center of the lesion and the dermatophyte moving radially further out at a rate that is faster than the rate of shedding of the outer corneocytes to survive.[17] Use of topical steroids, especially intermittently, would lead to the suppression of inflammation and therefore promote survival of the dermatophyte which not only spreads centrifugally but also remains in the center due to inadequate clearance. If this happens repeatedly, it would lead to multiple active borders with intermittent clearing in areas where the organism has been cleared forming circles concentrically leading to “tinea pseudoimbricata.”
Skin biopsy was taken in cases of steroid-modified tinea, who were willing for the procedure and special stains were used. Dermatophytes cannot be easily identified by routine histopathological stains such as H and E. Visualization of fungal elements in histological slides is with fungal-specific stainings, such as periodic acid–Schiff (PAS) and PAS-d (PAS-diastase) which stain hyphae red as well as GMS that stains them black.[18] The presence of fungal elements in the epidermis induces tissue inflammatory reactions which vary from almost undetectable responses to severe reactions. The histologic reaction patterns highly depended on the fungi, immune status of the host and local factors. Various histopathological patterns seen included perivasculitis, spongiotic/eczematous, psoriasiform, folliculitis and perifolliculitis and granulomatous reaction patterns.[19] In our study, neutrophils and lymphocytes were seen in epidermis in all cases.
According to Ackerman, the presence of neutrophils and/or their fragments in the corneal layer, in association with compact orthokeratosis and/or parakeratosis, should be considered a positive symptom of dermatophytosis. Since 1986, it has been termed the diagnostic “clue to dermatophytoses.”[20] Other histopathological clues include sandwich sign resulting from the presence of fungal hyphae between two zones of cornified cells.[21] Superficially, there is a orthokeratotic lamella, partially, or completely parakeratotic lamellae beneath, with formation of fissure in between, basket-weave pattern or compact hyperkeratosis, and prominent papillary dermal edema. In a study by Gocev et al., reaction pattern was the most common type but in our study, it was perifolliculitis which might be because all cases were steroid modified tinea.[22] Sandwich sign was positive in 25% in their study and 37% in our study. The presence of hyphae around hair follicles and in dermis in 37% each may be due to steroid application leading to deeper penetration of fungus.
Antifungal susceptibility testing was done in two cases, and MIC was found to be increased for fluconazole, terbinafine, and griseofulvin and lower for itraconazole. This was in concordance with the study by Mahajan et al. Microbiological resistance refers to nonsusceptibility of a fungus to an antifungal agent as determined by in vitro susceptibility testing, in which the MIC exceeds the susceptibility breakpoint for that organism. However, till now, the breakpoints have not been defined for the dermatophytes due to lack of data on the clinical correlation, pharmacokinetic/pharmacodynamic studies, or epidemiological cutoff MIC values. Various biochemical mechanisms contribute to the phenotype of drug resistance in fungi. The most frequent ones involve a decrease in drug uptake, structural alterations in the target site, and an increase in drug efflux or in intracellular target levels. The overexpression of the drug efflux pump transporters Tru MDR1 and Tru MDR2 has been seen in the dermatophytes in the presence of azoles, and this might contribute to drug resistance. Another mechanism of resistance has been attributed to biofilm production by the dermatophytes.[23] A study from north India showed that there were nonresponders to gold standard drug griseofulvin among the tinea capitis patients. In 2003, Mukherjee et al. found a T. rubrum strain exhibiting primary resistance to terbinafine. This resistance was attributed to a single missense amino acid substitution at L398F.[24] In a study of 100 isolates of onychomycosis, Sarifakioglu et al. found itraconazole and fluconazole having the greatest variation in MIC.[25] Azambuja et al. found high MIC value for fluconazole and itraconazole (66.7% and 25%, respectively) in 100 isolates of T. rubrum from patients with onychomycosis.[26]
Conclusion
Chronicity is a major problem encountered in treating a case of dermatophytosis. Injudicious use of topical steroid application was found in alarming numbers both OTC and prescribed by practitioners. Noncompliance is another major factor. Topical steroid application can also enhance the penetration of fungus to dermis as proved by histopathological examination which can lead to treatment failure. The isolation of species which can invade hair T. tonsurans, T. schonleini, E. floccosum and M. audouni also contribute to chronicity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Narasimhalu CR, Kalyani M, Somendar S. A cross-sectional, clinico-mycological research study of prevalence, aetiology, speciation and sensitivity of superficial fungal infection in Indian patients. J Clin Exp Dermatol Res. 2016;7:324. [Google Scholar]
- 2.Grover S, Roy P. Clinico-mycological profile of superficial mycosis in a hospital in North-East India. Med J Armed Forces India. 2003;59:114–6. doi: 10.1016/S0377-1237(03)80053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bindu V, Pavithran K. Clinico-mycological study of dermatophytosis in Calicut. Indian J Dermatol Venereol Leprol. 2002;68:259–61. [PubMed] [Google Scholar]
- 4.Panda S, Verma S. The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol. 2017;83:281–4. doi: 10.4103/ijdvl.IJDVL_224_17. [DOI] [PubMed] [Google Scholar]
- 5.Sudha M, Ramani CP, Anandan H. Prevalence of dermatophytosis in patients in a tertiary care centre. Int J Contemp Med Res. 2016;3:2399–401. [Google Scholar]
- 6.Sivaprakasam K, Govindan B. A clinico-mycological study of chronic dermatophytosis of more than years duration. Int J Sci Res. 2016;5:551–4. [Google Scholar]
- 7.Agarwal US, Saran J, Agarwal P. Clinico-mycological study of dermatophytes in a tertiary care centre in Northwest India. Indian J Dermatol Venereol Leprol. 2014;80:194. doi: 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh RR, Ray R, Ghosh TK, Ghosh AP. Clinico-mycological profile of dermatophytosis in a tertiary care hospital in West Bengal, an Indian scenario. Int J Curr Microbiol Appl Sci. 2014;3:655–66. [Google Scholar]
- 9.Mahajan S, Tilak R, Kaushal SK, Mishra RN, Pandey SS. Clinico-mycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care center. Indian J Dermatol Venereol Leprol. 2017;83:436–40. doi: 10.4103/ijdvl.IJDVL_519_16. [DOI] [PubMed] [Google Scholar]
- 10.de Sousa Mda G, Santana GB, Criado PR, Benard G. Chronic widespread dermatophytosis due to Trichophyton rubrum: A syndrome associated with a Trichophyton-specific functional defect of phagocytes. Front Microbiol. 2015;6:801. doi: 10.3389/fmicb.2015.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev. 2007;20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain N, Sharma M, Saxena VN. Clinico-mycological profile of dermatophytosis in Jaipur, Rajasthan. Indian J Dermatol Venereol Leprol. 2008;74:274–5. doi: 10.4103/0378-6323.41388. [DOI] [PubMed] [Google Scholar]
- 14.Sahai S, Mishra D. Change in spectrum of dermatophytes isolated from superficial mycoses cases: First report from central India. Indian J Dermatol Venereol Leprol. 2011;77:335–6. doi: 10.4103/0378-6323.79718. [DOI] [PubMed] [Google Scholar]
- 15.Dogra S, Uprety S. The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J. 2016;7:73–6. doi: 10.4103/2229-5178.178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surendran K, Bhat RM, Boloor R, Nandakishore B, Sukumar D. A clinical and mycological study of dermatophytic infections. Indian J Dermatol. 2014;59:262–7. doi: 10.4103/0019-5154.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227–36. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinshaw M, Longley BJ. Elder DE, Elenitsas R, Johnson BL, Murphy GF. Lever's Histopathology of the Skin. Philadelphia: Lippincott Williams and Wilkins; 2004. Fungal diseases; p. 603. [Google Scholar]
- 19.Tani K, Adachi M, Nakamura Y, Kano R, Makimura K, Hasegawa A, et al. The effect of dermatophytes on cytokine production by human keratinocytes. Arch Dermatol Res. 2007;299:381–7. doi: 10.1007/s00403-007-0780-7. [DOI] [PubMed] [Google Scholar]
- 20.Ackerman AB, editor . Histologic Diagnosis of Inflammatory Skin Diseases. Philadelphia: Lea and Febiger; 1978. When you see-think; p. 830. [Google Scholar]
- 21.Gottlieb GJ, Ackerman AB. The “sandwich sign” of dermatophytosis. Am J Dermatopathol. 1986;8:347–50. doi: 10.1097/00000372-198608000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Gocev D, Damevska K. The role of histopathology in the diagnosis of dermatophytoses. Serbian J Dermatol Venereol. 2010;2:45–53. [Google Scholar]
- 23.Nigam PK. Antifungal drugs and resistance: Current concepts. Our Dermatol Online. 2015;6:212–21. [Google Scholar]
- 24.Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA, et al. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother. 2003;47:82–6. doi: 10.1128/AAC.47.1.82-86.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarifakioglu E, Seçkin D, Demirbilek M, Can F. In vitro antifungal susceptibility patterns of dermatophyte strains causing tinea unguium. Clin Exp Dermatol. 2007;32:675–9. doi: 10.1111/j.1365-2230.2007.02480.x. [DOI] [PubMed] [Google Scholar]
- 26.Azambuja CV, Pimmel LA, Klafke GB, Xavier MO. Onychomycosis: Clinical, mycological and in vitro susceptibility testing of isolates of Trichophyton rubrum. An Bras Dermatol. 2014;89:581–6. doi: 10.1590/abd1806-4841.20142630. [DOI] [PMC free article] [PubMed] [Google Scholar]


