Significance
Calmodulin is a ubiquitous Ca2+-sensing protein that can bind to more than a 100 different targets. In doing so, it endows many of these with Ca2+-dependent modulation. As one of the most conserved proteins throughout evolutionary history, all calmodulin genes in vertebrates are identical. However, several disease-associated mutations have been uncovered in human calmodulin genes, all of which are linked to inherited cardiac arrhythmia syndromes. This report shows high-resolution glimpses into calmodulin disease mutations and their effect on binding the L-type voltage-gated calcium channel, a channel involved in the cardiac action potential that receives calcium-dependent feedback. Although each mutant is able to affect calcium-dependent inactivation, the structures show that they adopt different mechanisms.
Keywords: calcium signaling, X-ray crystallography, NMR, calcium channels, inactivation
Abstract
Calmodulin (CaM) represents one of the most conserved proteins among eukaryotes and is known to bind and modulate more than a 100 targets. Recently, several disease-associated mutations have been identified in the CALM genes that are causative of severe cardiac arrhythmia syndromes. Although several mutations have been shown to affect the function of various cardiac ion channels, direct structural insights into any CaM disease mutation have been lacking. Here we report a crystallographic and NMR investigation of several disease mutant CaMs, linked to long-QT syndrome, in complex with the IQ domain of the cardiac voltage-gated calcium channel (CaV1.2). Surprisingly, two mutants (D95V, N97I) cause a major distortion of the C-terminal lobe, resulting in a pathological conformation not reported before. These structural changes result in altered interactions with the CaV1.2 IQ domain. Another mutation (N97S) reduces the affinity for Ca2+ by introducing strain in EF hand 3. A fourth mutant (F141L) shows structural changes in the Ca2+-free state that increase the affinity for the IQ domain. These results thus show that different mechanisms underlie the ability of CaM disease mutations to affect Ca2+-dependent inactivation of the voltage-gated calcium channel.
Calmodulin (CaM) is an essential Ca2+ sensor that plays a pivotal role in many signaling pathways (1). It has a simple architecture, consisting of two domains (N-lobe and C-lobe) that can each bind two Ca2+ ions in a highly cooperative manner. The binding of Ca2+ results in the exposure of hydrophobic residues, which affects the ability of CaM to bind other target proteins (2, 3). CaM thus confers Ca2+ sensitivity to a large list of both cytosolic and membrane-embedded proteins (2–5).
The human genome contains three individual CALM genes that encode identical proteins. The sequence of CaM has been highly conserved throughout evolution, with no variation found among vertebrates (1). Given this degree of sequence conservation, it was long thought that any mutation in the CALM genes would be fatal, because CaM is known to bind hundreds of target proteins and any mutation would thus likely affect multiple pathways simultaneously. However, several studies have recently reported mutations in the CALM genes of human patients (6–11). The finding that any CaM mutations are viable is already of note, but even more surprising is that they are linked to cardiac phenotypes, suggesting that other pathways involving CaM are less affected. The clinical phenotypes include long-QT syndrome (LQT), catecholaminergic polymorphic ventricular tachycardia (CPVT), and idiopathic ventricular fibrillation (IVF).
Subsequent studies have shown that the mutations can alter the functional behavior of several cardiac ion channels (10–16). This includes the cardiac ryanodine receptor (RyR2), a Ca2+ release channel located in the sarcoplasmic reticulum (17). Mutations in this channel are often associated with CPVT, causing a gain-of-function phenotype with excessive Ca2+ release (18). The first two reported CaM mutations (N53I, N97S) were found in patients with CPVT (6) and were subsequently found to affect the function of RyR2 (13, 14).
In addition, several CaM mutations have been found to affect an L-type voltage-gated calcium channel (CaV1.2). This channel has the intriguing property to undergo Ca2+-dependent inactivation (CDI), a process whereby Ca2+ accelerates the kinetics of inactivation (19, 20). This process plays a role in shaping the action potential in cardiac myocytes and slowing of inactivation can lead to LQT (12, 21). CDI requires the preassociation of CaM to the channel in low Ca2+ conditions, and upon binding Ca2+, a conformational change is transmitted to the channel to impose inactivation. A region of prime importance is the IQ domain, located within the channel’s proximal C-terminal tail. CaM can bind this region in both Ca2+-free and Ca2+-loaded states (22–24), and mutations in the IQ domain can diminish and even abolish CDI (19, 24). Accordingly, LQT-associated mutations in the C-lobe of CaM (D95V, N97S, F141L) have been shown to either obliterate or decrease the extent of CDI (12).
Despite the substantial impact of CaM disease mutations on the functions of various channels, we still lack insights into their mechanism of action. Do they merely affect Ca2+ affinity or do they alter structure and dynamics? Do they affect target recognition? Here we report an integrated structural biology approach, combining NMR and X-ray crystallography. Together with quantitative binding assays, we describe the mechanism of action for four different CaM disease mutants. Although each mutant is able to reduce CaV1.2 CDI, their mechanisms of action are highly divergent. We observe at least three distinct primary effects, indicating that the disease mutations act in different ways to affect CDI of L-type calcium channels.
Results
Throughout this report we utilize the numbering for mature CaM in which the initial methionine (residue 0) is removed.
Effect of CaM Mutations on Binding to the CaV1.2 IQ Domain.
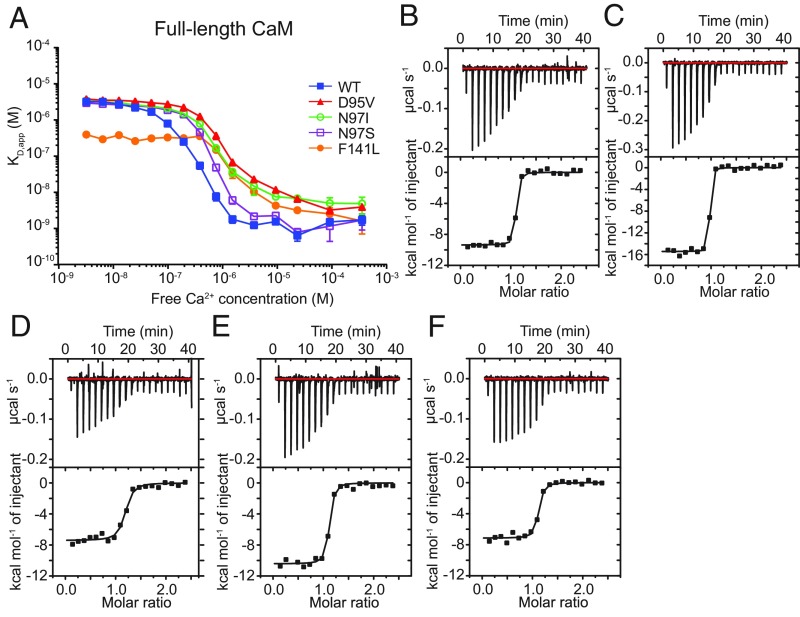
It has been shown previously that several CaM disease mutants, linked to LQT, have a dominant effect on CDI of CaV1.2 (12). We therefore started by analyzing their effect on the ability to bind to the IQ domain of CaV1.2, a prerequisite for normal CDI. We covered a range of free Ca2+ concentrations and measured the affinities using fluorescence anisotropy, making use of a TAMRA-labeled IQ domain (SI Appendix, Fig. S1 G–K). The overall plot of KD vs. Ca2+ concentration for each mutant is displayed in Fig. 1A. These data show that several mutants have a decreased affinity for the IQ domain in the range of 100 nM–10 μM Ca2+. In this range, the affinity is the weakest for D95V and N97I CaM, whereas N97S CaM has a smaller effect on the affinity and is closer to wild-type.
Fig. 1.
Ca2+/CaM- and Ca2+/C-lobe-CaV1.2 IQ domain binding affinities. (A) Binding affinity (KD,app) of full-length CaM variants to the IQ domain at various free Ca2+ concentrations by TAMRA fluorescence anisotropy. Error bars represent the SD of three replicates. Isothermal titrations of 75 μM (B) wild-type Ca2+/C-lobe, (C) D95V Ca2+/C-lobe, (D) N97I Ca2+/C-lobe, (E) N97S Ca2+/C-lobe, and (F) F141L Ca2+/C-lobe into the 11-μM IQ domain. Isotherms were fit to a one-site binding model. Panels show injections of 2 μL of titrant into IQ domain (Upper) and binding isotherms (Lower). Experiments were conducted in triplicates; representative curves are shown.
A peculiar observation is made for F141L CaM, which has a >10-fold higher affinity for the IQ domain than wild-type under low Ca2+ concentrations (SI Appendix, Table S1). At concentrations near 10−6 M free Ca2+, the affinity of F141L CaM is similar to those of D95V and N97I CaM. Although both D95V and F141L CaM have previously been shown to abolish CDI of CaV1.2 (12), these results suggest that there are differences in the inherent mechanisms for each mutant. To confirm the peculiar observation for F141L CaM, we also performed affinity measurements on the isolated C-lobes (SI Appendix, Fig. S1 and Table S1). These confirm that the F141L mutant has an increased affinity for the IQ domain at low Ca2+ concentrations. These results suggest substantial changes in the Ca2+-free form of CaM, due to the F141L mutation. Indeed, native PAGE experiments show a different mobility of F141L CaM, relative to wild-type, under Ca2+-free but not Ca2+-loaded conditions (SI Appendix, Fig. S2).
At high Ca2+ concentrations, the affinities remain high for each mutant. To confirm this, and to obtain further thermodynamic insights, we also performed isothermal titration calorimetry (ITC) experiments on the individual C-lobes at saturating (10 mM) Ca2+ levels (Fig. 1 B–F). These confirm that the binding affinities remain high (KD in the low nanomolar range). However, the D95V mutant has a more negative ΔH (−15 vs. −10 kcal⋅mol−1). The N97I mutant binds the weakest, with an affinity ∼3.5-fold lower compared with wild-type CaM (SI Appendix, Table S2).
These changes in affinity for the IQ domain have implications on the inherent affinity of the CaM:IQ complexes for Ca2+. To obtain experimental estimates of the CaM affinity for Ca2+ when bound to the IQ domain, we monitored the fluorescence anisotropy development of the TAMRA-labeled IQ domain as a function of the free Ca2+ concentration at the highest CaM concentration (SI Appendix, Fig. S3). We find an approximately threefold reduction in Ca2+ affinity for the D95V and N97I mutants compared with wild-type. The F141L mutation affected affinity the most, ∼5.5-fold lower than wild-type CaM, reflecting the increase in affinity for the IQ domain in the apo-state and the maintained high affinity for the IQ domain in the Ca2+-saturated state. Thus, all three mutations display a reduced Ca2+-sensing ability when in complex with the Cav1.2 IQ domain compared with wild-type CaM.
Crystal Structure of D95V Ca2+/CaM Bound to the CaV1.2 IQ Domain.
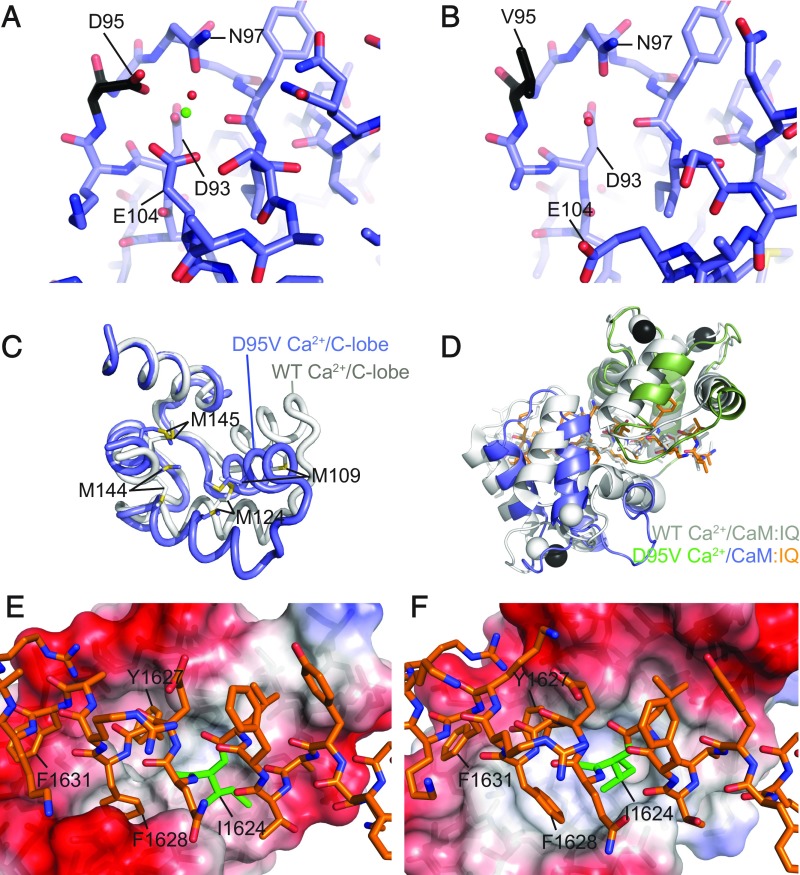
Because the D95V mutation did not seem to abolish CaM binding to the IQ domain at high Ca2+ concentrations, we solved a crystal structure of this complex at 2.0-Å resolution. The structure contains two complexes per asymmetric unit, which are very similar. The figures and analysis are shown for complex A.
We found no electron density that could fit a fully occupied Ca2+ ion in EF hand 3 (EF3). At longer wavelengths, Ca2+ has an anomalous signal, so we collected an anomalous dataset. The corresponding anomalous density map shows the positions of Ca2+ in the structure. We show this map around EF3 and EF4 in the C-lobe (SI Appendix, Fig. S4A). Whereas the density for Ca2+ in EF4 is clearly visible, no density is found for EF3. This shows that even under saturating Ca2+ conditions (10 mM in the crystallization condition), the D95V mutation completely disrupts Ca2+ binding to EF3.
This loss of Ca2+ results in major allosteric changes, most readily observed in animations (Movies S1 and S2). Within EF3, Glu104, which normally coordinates the Ca2+ ion, has now relaxed to a different position, with its side chain shifting up to 6.6 Å (Fig. 2 A and B). This has long-range allosteric consequences, as it completely rearranges the α-helical packing in the C-lobe (Fig. 2C). Methionine residues in the C-lobe form major contributions to the binding to the IQ domain (22, 23). The effect of the structural changes on these methionine residues is most easily observed for Met109 and Met144: their side chains are ∼15 Å apart in the wild-type Ca2+/CaM:IQ complex structure but are in Van der Waals contact (3.8 Å) in the D95V mutant.
Fig. 2.
Comparisons of the wild-type Ca2+/CaM- and D95V Ca2+/CaM:CaV1.2 IQ domain structures. Stick representation of EF3 of (A) wild-type Ca2+/CaM (PDB ID code 2BE6) and (B) D95V Ca2+/CaM. Residue 95 is shown in black, calcium ion as a green sphere, and water molecule as a red sphere. (C) Superpositions of C-lobes of wild-type (PDB ID code 2BE6, gray) and D95V Ca2+/CaM (blue). The superposition is based on the first helix of the lobes, highlighting the different conformation. Methionine residues are labeled, showing how M109 and M144, far apart in wild-type, form Van der Waals interactions in the mutant. (D) Superposition of WT Ca2+/CaM (PDB ID code 2BE6, gray) and D95V Ca2+/CaM (N-lobe in green, C-lobe in blue, Ca2+ ions in black) based on the IQ domain. Interactions of the C-lobe of (E) wild-type Ca2+/CaM (PDB ID code 2BE6) or (F) D95V Ca2+/CaM with the CaV1.2 IQ domain. The IQ domain is shown in stick representation with I1624 colored in green; CaM C-lobe is shown in surface representation with the colors indicating the electrostatic potential (red: negative; blue: positive).
The wild-type CaM lobes are known to undergo large conformational changes upon binding or unbinding Ca2+, so we wondered whether the conformation of D95V Ca2+/CaM is similar to that of a Ca2+-free lobe. We superposed the D95V C-lobe with Ca2+-free and Ca2+-occupied wild-type C-lobes and with CaM1234 (SI Appendix, Fig. S4). This shows that the helical packing in D95V CaM is different from either, and thus represents a conformation that has not been reported before.
To check whether the altered conformation also occurs in the absence of the IQ domain, we compared HSQC spectra of WT and D95V Ca2+/CaM in the absence of IQ peptide (SI Appendix, Fig. S5). These show that the C-lobe resonances display great shifts, whereas the N-lobe resonances superpose well. Overlaying the NMR spectra of wild-type apoCaM and D95V Ca2+/CaM (SI Appendix, Fig. S6) shows that all C-lobe resonances are altered, also at positions remote from the mutation site. Thus, we conclude that the structure of the C-lobe of D95V Ca2+/CaM neither resembles the wild-type apo nor the wild-type Ca2+-loaded form, and that large changes also occur in the absence of the IQ domain.
Despite the large conformational changes in the C-lobe, the D95V mutant is still able to bind to the IQ domain with nanomolar affinity at saturating Ca2+ concentrations (Fig. 1). However, because the surface of the D95V C-lobe has altered dramatically, the mode of the interaction has changed, as shown by a direct comparison with the wild-type complex structure (22, 23) (Fig. 2 E and F). In one such structure (PDB ID code 2BE6), three complexes are present in the asymmetric unit. In each of these, the N-lobe adopts different positions, supporting the notion that the N-lobe is bound weakly to the IQ domain and may have another binding site in full-length channels (25, 26). However, the C-lobe is bound in identical ways in all published structures, with three aromatic IQ domain residues binding to hydrophobic pockets within the C-lobe. Ile1624, which defines the “I” in the canonical IQ motif, is at the periphery, partially exposed to solvent. In the D95V CaM, however, the structural distortions lead to a new hydrophobic pocket that fully sequesters Ile1624. The three aromatic anchors in the IQ domain (Y1627, F1628, F1631), which contribute most to the binding in the wild-type CaM structure, have altered environments.
These altered interactions result in a rotation of the C-lobe relative to the IQ domain (Fig. 2D), with positional shifts in the main chain up to 11 Å. There are smaller shifts in the relative position of the N-lobe, but because this lobe can adopt multiple conformations around the IQ domain (22, 23), this change in the N-lobe position is not necessarily the result of the disease mutation.
The Ile1624 residue has previously been shown to be crucial for CDI (24). Its altered interactions with the D95V C-lobe, along with the complete loss of Ca2+ binding to EF3, may both contribute to the loss of CDI observed for D95V CaM (12).
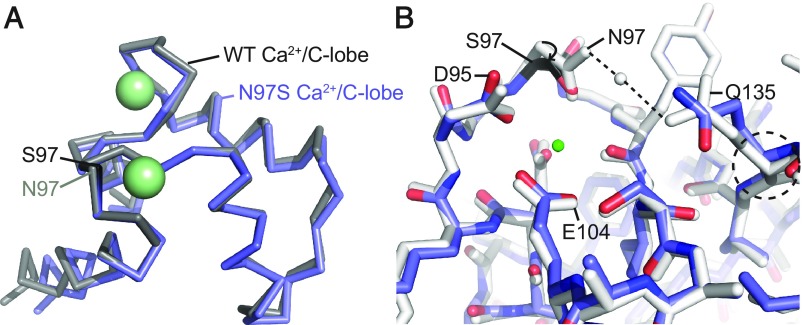
Structure of N97S Ca2+/CaM.
CaM Asn97 is another residue in EF3. The N97S mutation has been linked to both CPVT and LQT (6, 7). Because it coordinates the same Ca2+ ion as CaM Asp95, we wondered whether it also results in large conformational changes, similar to the D95V mutation. We were unable to obtain a crystal structure of a complex with the IQ domain, but were able to solve a 2.5-Å crystal structure of Ca2+-saturated N97S CaM, in the absence of any target peptide. Fig. 3A shows a superposition of the region in EF3 with a wild-type CaM structure obtained in the absence of a target peptide (PDB ID code 4BW8). In this case, N97S CaM is still able to bind Ca2+, and the conformational changes are highly localized and much smaller than for D95V CaM (Fig. 3B and Movie S3). Interestingly, the Ser97 side-chain hydroxyl oxygen is able to compensate for Ca2+ coordination. However, because this oxygen is one position closer to the main chain compared with the Asn97 side-chain oxygen, the residue needs to move closer to the Ca2+ ion, with changes in the conformation of the main chain. We hypothesize that this results in a less-favorable conformation, which would impact the affinity for Ca2+. Indeed, it has previously been found that the N97S mutation leads to a reported 1.5- to 4-fold reduction in the affinity for Ca2+ (6, 13, 14, 27).
Fig. 3.
Comparisons of the wild-type Ca2+/CaM and N97S Ca2+/CaM structures. (A) Overall superposition of the C-lobes of wild-type Ca2+/CaM (PDB ID code 4BW8, gray) and N97S Ca2+/CaM (blue). C-lobes are shown in ribbon representation and calcium ions as green spheres. S97 is colored black. (B) Superposition of wild-type Ca2+/CaM (gray) and N97S Ca2+/CaM (blue) based on residues 92–102; shown are details around EF3. CaM is in stick representation, with the mutated S97 residue in black, and calcium ion as a green sphere. The dashed lines indicate hydrogen bonds. To maintain a similar Ca2+-coordination, the main chain and side chain of Ser97 move closer, likely resulting in strain that reduces the Ca2+ affinity. Due to the absence of hydrogen bonds with Gln135 in the mutant, the Gln135 conformation is altered, resulting in small changes in EF4.
Another feature of the N97S structure is an altered conformation of Gln135, located in EF4. In wild-type CaM, this residue makes water-mediated hydrogen bonds with Asn97, but not with Ser97 in the N97S structure. As a result, the side-chain conformation is different, with small shifts in the main chain, showing that changes in EF3 can be transmitted to EF4.
A full superposition of the wild-type and N97S C-lobes shows that the two structures superpose well, with an RMSD of 0.38 Å for 55 Cα atoms (Fig. 3A). The main effect of the mutation is thus a local change in the energetics.
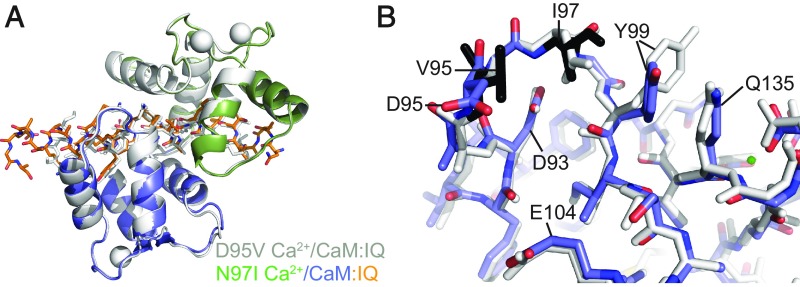
Crystal Structure of N97I Ca2+/CaM Bound to the CaV1.2 IQ Domain.
Residue 97 in CaM is the target for another mutation (N97I), linked to LQT (7). Because the D95V and N97S mutations have such different effects on the structure of CaM, despite coordinating the same Ca2+ ion, we wondered whether this is due to the exact position, or due to the nature of the substitution. We therefore solved a structure of the N97I Ca2+/CaM:IQ domain complex at 1.65-Å resolution (Fig. 4).
Fig. 4.
Comparison of the D95V Ca2+/CaM - and N97I Ca2+/CaM:CaV1.2 IQ domain structures. (A) Superposition of D95V Ca2+/CaM:IQ (gray) and N97I Ca2+/C-lobe:IQ (N-lobe in green, C-lobe in blue, IQ in orange) based the IQ domain. CaM is shown in cartoon representation, IQ domain as sticks, and calcium ions as spheres. The overall conformation of N97I is similar to D95V, and thus very different from wild-type Ca2+/CaM. (B) Superposition of D95V Ca2+/CaM (gray) and N97I Ca2+/CaM (blue) based on residues 92–102; shown are details around EF3. CaM is in stick representation, with the mutated residues in black, and calcium ion as a green sphere. In both mutants, no Ca2+ is visible in EF3, leading to a conformational switch, mediated by Glu104. A noticeable difference is the stacking of Tyr99 against the hydrophobic Ile97 residue. This conformation of Tyr99 is unique, and not found in wild-type Ca2+/CaM or any of the other disease mutant structures. Because Tyr99 now stacks against EF4, small changes in the positions of EF4 residues can be observed.
In contrast to the N97S mutation, N97I results in large conformational changes reminiscent of the D95V mutant. Ca2+ binding to EF3 is abolished, resulting in relaxation of Glu104 to a position different from the wild-type. This causes a distortion of the C-lobe and a rotation of the C-lobe relative to the IQ domain. Fig. 4A shows a superposition of the D95V and N97I Ca2+/CaM:IQ domain complexes, showing that these two CaM mutants share similar overall conformations, thus both very different from the wild-type complex.
Although both the N97I and D95V mutations decrease the affinity of the C-lobe for Ca2+, N97I CaM, in the absence of the IQ domain, still binds Ca2+ with a twofold higher affinity compared with D95V CaM (7, 8, 27). We therefore compared the area around the mutation site for possible clues. Fig. 4B shows a local superposition in EF3. In addition to small changes in main-chain conformation, Tyr99 is seen to adopt a different conformation, with its side chain stacking with Ile97 and Gln135. This conformation is present in both complexes of the asymmetric unit, whereas it is not observed for any of the D95V or wild-type CaM complexes. We postulate that this additional stabilizing interaction, not observed for D95V CaM, results in a more stable Ca2+-bound state, and hence a higher Ca2+ affinity.
Overall, these results show that not only the exact location, but also the identity of the substitution determines the effect on the structure. Given the similar impact of the N97I and D95V mutations on the conformation of Ca2+/CaM, we predict that N97I CaM would also abolish CDI of CaV1.2.
Crystal Structure of F141L Ca2+/CaM Bound to the CaV1.2 IQ Domain.
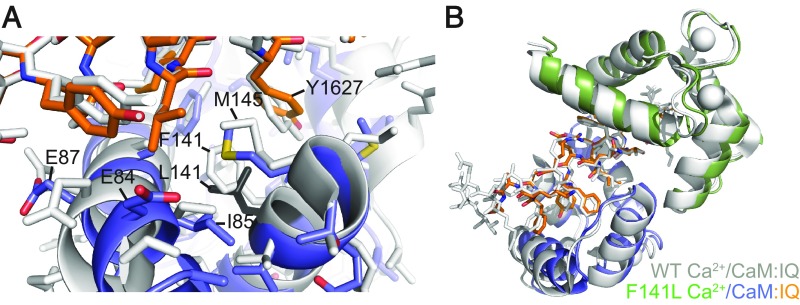
The three mutations described above affect residues directly involved in Ca2+ binding. F141L is a mutation originally linked to LQT (8) and has been shown to abolish CDI of CaV1.2 (12). Phe141 is not directly involved in Ca2+ coordination, but nevertheless, its mutation to Leu was shown to decrease the affinity of the C-lobe for Ca2+ approximately fivefold in the absence of any binding partner (8). We crystallized the F141L mutant in the presence of the CaV1.2 IQ domain.
Fig. 5A shows a superposition of wild-type (PDB ID code 2BE6) and F141L Ca2+/CaM bound to the IQ domain around the site of the mutation. In wild-type CaM, Phe141 is involved in interactions with the IQ domain, but also in forming hydrophobic interactions with other Ca2+/C-lobe residues. It interacts with Tyr1627 of the IQ domain, a residue that forms one of the main anchor points for the Ca2+/C-lobe (22). In the mutant, Leu141 is more than 4 Å away from the Tyr1627 side chain, and so contributes minimally to the binding. In addition, there is an overall reduction in hydrophobic interactions with other Ca2+/C-lobe residues for the F141L mutant, suggesting a less-stable Ca2+-bound conformation. So although Phe141 is not directly involved in Ca2+ coordination, and the Leu141 mutation does not lead to a visible change in coordination of the Ca2+ ions in EF3 and EF4, a decreased stability of the F141L Ca2+/C-lobe may explain the reduction in Ca2+ affinity.
Fig. 5.
Comparisons of the wild-type and F141L Ca2+/CaM:IQ domain structures. (A) Superposition of WT Ca2+/CaM (PDB ID code 2BE6) (gray) and F141L Ca2+/CaM (N-lobe in green, C-lobe in blue) based on the IQ domain (orange IQ corresponds to the F141L Ca2+/CaM structure). CaM is shown in cartoon representation, IQ domain as sticks, and calcium ions as spheres. (B) Details around the F141L mutation site. IQ domain is shown in stick representation, and CaM in cartoon and stick representation. Residue L141 is colored black. Because Phe141 is part of a hydrophobic stacking interaction, multiple side chains have different conformations in the F141L mutant. This leads to altered interactions that result in the shift of C-lobe residues up to 2.3 Å.
Despite the less-favorable packing, the overall conformation of the F141L Ca2+/C-lobe and its relative position to the IQ domain are very similar to wild-type (Fig. 5B). As discussed earlier, relative positional changes of the Ca2+/N-lobe are less likely to be relevant due to the relative mobility of this lobe in the wild-type structure (22). Overall, the minor structural changes observed for the F141L Ca2+/CaM:IQ complex seem insufficient to explain its ability to obliterate CDI, suggesting that there are changes in dynamics, not captured with the crystal structure, or that there are changes in the apo-form of this mutant.
NMR Analysis of F141L apoCaM.
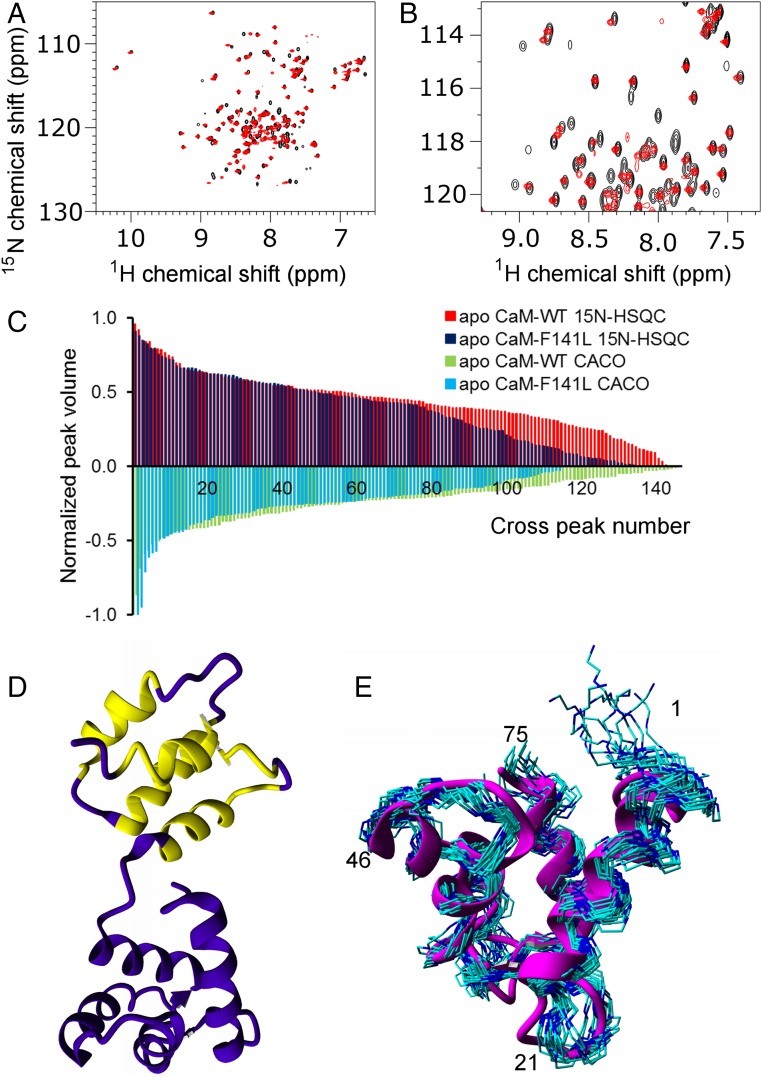
The F141L mutation shows the puzzling effect of increasing the affinity for the IQ domain, relative to wild-type CaM, in the absence of Ca2+. We therefore expect substantial structural changes in the Ca2+-free state. Because our efforts to crystalize a F141L apoCaM were unsuccessful, we resorted to NMR (Fig. 6). The NMR spectra reveal major changes in F141L vs. wild-type apoCaM, because F141L apoCaM displays fewer peaks than wild-type apoCaM. Fig. 6 A and B show an overlay of [1H-15N]-HSQC spectra, demonstrating that there are several peaks missing in the mutant spectrum. However, for the peaks that are present, the chemical shifts are very similar to wild-type. These belong to the N-lobe, suggesting this lobe is minimally impacted by the mutation. A peak intensity histogram (Fig. 6C) shows an almost identical intensity profile of the first 77 peaks (corresponding roughly to the N-lobe), while the remaining amino acids display significantly weaker NMR peaks and the overall number of peaks is reduced in the mutant. The direct carbon-detected CACO also show fewer than the 148 expected peaks. While signal intensities in the N-lobe allowed for a full assignment, the weak signals almost exclusively come from C-lobe residues, making resonance assignment of large stretches of the C-lobe residues impossible. Those that could be assigned are all located in positions where the wild-type apo C-lobe is flexible (Fig. 6D). Those chemical shifts that could be assigned for F141L apoCaM display very little difference to the wild-type apoCaM (SI Appendix, Fig. S7). Correspondingly, the 3D structure of the N-lobe of F141L apoCaM is indistinguishable from the structure of the wild-type (Fig. 6E), whereas no structure could be calculated for the C-lobe.
Fig. 6.
NMR analysis of F141L apoCaM. (A and B) [1H-15N]-HSQC spectra of wild-type (black) and F141L apoCaM (red) overlaid. (A) Whole spectrum; (B) region of interest. (C) Comparison of the peak volumes (normalized to the strongest peak) found in the [1H-15N]-HSQC spectra of both wild-type (red bars) and F141L (blue bars) apoCaM along the positive y axis. In each spectrum, each visible backbone amide peak was picked, integrated, and then normalized. The negative y axis shows the corresponding values (multiplied by −1) for the directly C-detected CACO spectrum of wild-type (green) and F141L apoCaM (light blue). The values are sorted by descending volume (i.e., their x coordinate does not correspond to any specific residue number). It is seen that the peak volume profile of both HSQC and CACO spectra is very similar for the most intense ∼80 peaks, slightly more than half the number of amino acid residues. However, the remaining peaks are much less intense in F141L apoCaM and there is an overall smaller numer of peaks in the mutant. Also the C-detected CACO yields a significantly smaller number of peaks for F141L CaM than for the wild-type. (D) Structure of WT apoCaM (first model of 1DMO) color-coded according to the assignment status of F141L apoCaM: amino acids shown in purple could be assigned in the mutant, amino acids shown in yellow could not. (E) Bundle of 20 NMR structures of F141L apoCaM (PDB ID code 6GDL, backbone only, carbon atoms in cyan, nitrogen atoms in blue) overlaid with a ribbon drawing (magenta) of the first model of the NMR structure of wild-type apoCaM (PDB ID code 1DMO) (28). Numbers indicate residue numbers.
An important observation is that the F141L apo C-lobe is not fully disordered, because in such a case we would easily have been able to detect all resonances. Instead, these observations suggest it is likely in an equilibrium between multiple states exchanging at rates that correspond roughly to the chemical-shift differences, making it difficult to observe NMR resonances. This is corroborated by T2 relaxation dispersion data showing only few residues with relaxation dispersion in the N-lobe, but significant relaxation dispersion throughout the C-lobe (SI Appendix, Fig. S8). We also tried to alter both exchange rates (by changing temperature) and resonance frequency (by recording NMR spectra at higher field strength) to see whether this would improve the spectra. Only minute changes could be observed within the permissible temperature range (277–310 K) and at accessible field strengths (14.1 and 22.3 T) (SI Appendix, Fig. S9). NMR structures of wild-type apoCaM show that F141 is fully buried (28), forming part of a hydrophobic core involving Leu105, Met124, Met144, and Met145. Destabilization of these interactions by introducing of a shorter Leu side chain most likely underlies the conformational changes.
In conclusion, the F141L mutation imparts substantial conformational changes in the apo C-lobe, resulting in a mixture of states. This most likely underlies the ability of this mutant to bind the IQ domain with 10-fold higher affinity at low Ca2+ concentrations.
NMR Analysis of F141L Ca2+/CaM.
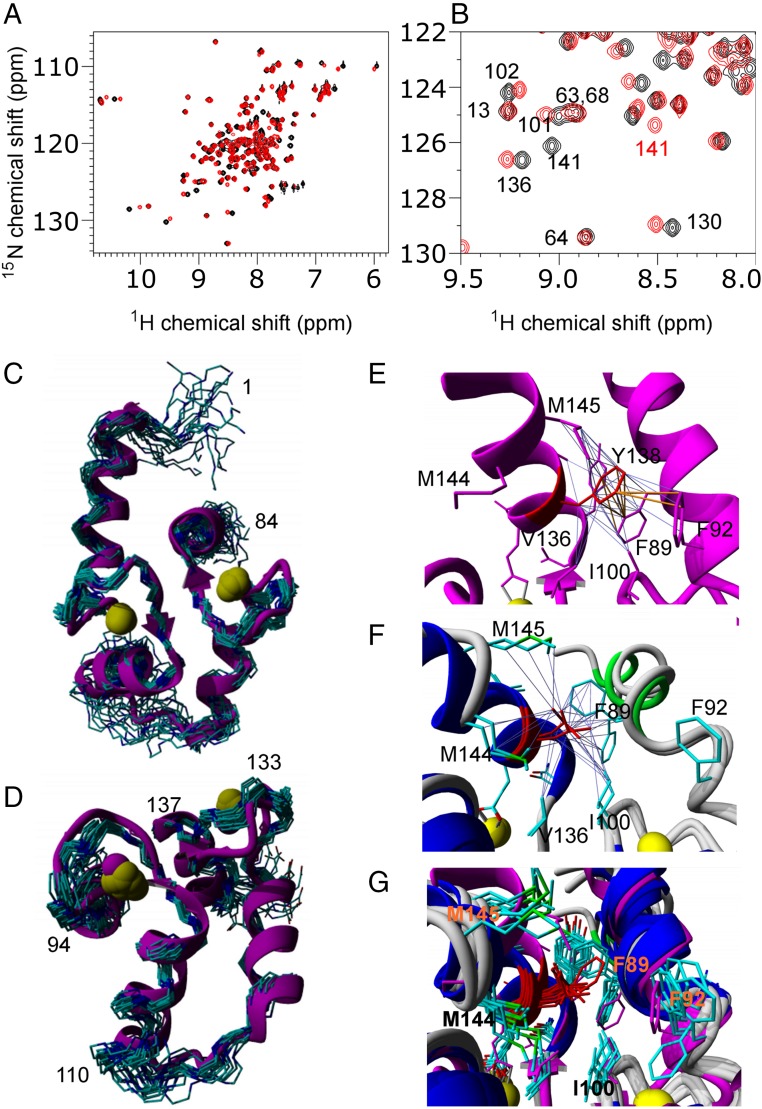
F141L imparts minimal changes to the Ca2+/CaM:IQ complex, but this may be due to the presence of the IQ domain, which might stabilize the folding of CaM. We therefore analyzed the F141L Ca2+/CaM, in the absence of IQ domain, with NMR. In contrast to the apoCaM data, the NMR spectra of F141L Ca2+/CaM are more similar to wild-type (Fig. 7 A and B). A similar number of signals is seen in the [1H-15N]-HSQC spectrum and structure determination shows that the N-lobe structure resembles the wild-type closely (Fig. 7C). The C-lobe displays the same overall fold as the wild-type, but there are distinct differences around the site of mutation and notably in EF4 (Fig. 7D). The final α-helix from residues 138–146 has a slightly changed orientation, with the helical axis tilted 18 ± 3.6° closer toward residue 128. As has been shown for wild-type Ca2+/CaM (29), the relative orientations of N-lobe and C-lobe for F141L Ca2+/CaM are entirely dynamic in solution and thus undefined. Residues 75–87, linking the two lobes, are undefined and adopt a variety of conformations. In the wild-type, F141 is involved in several π–π interactions to both F92 and F89. In addition, strong hydrophobic interactions exist with M145 and I100, as well as weaker interactions with Y138, V142, and V136. Leucine cannot form π–π interactions and its side chain protrudes less far from the backbone, thus leaving an unfavorable void in the hydrophobic packing. This could be the reason why the terminal α-helix is bent toward the hydrophobic core, thus allowing the shorter leucine side chain to form hydrophobic interactions and avoiding the formation of a void volume. Fig. 7 E–G show the environment of residue 141 in both wild-type and mutant. Interestingly, F89 adopts two different orientations in the bundle of NMR structures, both satisfying the NOE data available and both allowing for hydrophobic interactions between F89 and L141, as well as π–π interactions between F89 and Y138. Hydrophobic and π–π interactions between residue 141 and its surroundings are highlighted in Fig. 7E (wild-type) and Fig. 7F (F141L). F92, which is close enough to F141 in the wild-type to form both hydrophobic and π–π interactions, is too far away for hydrophobic interactions with L141 in the mutant. L141 still interacts with I100, V136, and M145. In addition, the side chain of L141 interacts with the side chain of M144, which is not the case in the wild-type. The final turn of the terminal α-helix is distorted to allow for this interaction to happen.
Fig. 7.
NMR analysis of F141L Ca2+/CaM. (A and B) [1H-15N]-HSQC spectra of WT (black) and F141L (red) Ca2+/CaM overlaid. (A) Whole spectrum; (B) region of interest with selected peaks labeled with the corresponding amino acid number. (C and D) Structures of the N-lobe and C-lobe, respectively, of F141L Ca2+/CaM (PDB ID code 6GDK, backbone only, carbon atoms in cyan, nitrogen atoms in blue, calcium in yellow) overlaid with a ribbon drawing (magenta) of wild-type Ca2+/CaM lobes (PDB ID code 1CLL) (44). Numbers refer to the position of selected amino acids. (E) Close-up of residue F141 (shown in red) in wild-type Ca2+/CaM. YASARA was used to identify possible π–π interactions of F141 with other amino acids (orange) as well as its hydrophobic interactions with other amino acids (purple). (F) Same information as E, but for the F141L Ca2+/CaM structure: two representative structures of the the F141L Ca2+/CaM bundle are shown with each their orientation of F89. YASARA was used to identify possible hydrophobic interactions of L141 with other amino acids (purple). (G) Superposition of the wild-type structure (magenta) overlaid with a bundle of 10 structures of F141L Ca2+/CaM (PDB ID code 6GDK, blue and gray ribbon). The side chain of residue 141 is shown in red. Side chains of other residues no more than 4.5 Å away from any atom in the side chain of residue 141 are shown (carbon atoms in cyan, sulfur in green).
Overall, the changes in Ca2+/CaM, induced by F141L, are much smaller compared with apoCaM, even in the absence of the IQ domain. However, because differences may also arise from a change in dynamics, we performed {1H}-15N-NOE measurements. In rigid parts of a folded protein, the NOE is on the order of 0.7–0.8, while smaller values indicate intramolecular mobility, typically found in loops, termini, or disordered regions. For F141L apoCaM, NOE data show a well-ordered N-lobe with some disordered loops (SI Appendix, Fig. S10). Only few NOEs can be observed in the C-lobe, but all of them display a higher degree of intramolecular mobility than what can be expected from a stable folded protein. Although only 50% of the C-lobe resonances could be detected, and only one-third of these assigned, this further supports the observation that F141L imparts significant changes to the apoCaM structure.
For Ca2+/CaM, NOE data show no significant differences between wild-type and F141L (SI Appendix, Fig. S11). In both cases, the region around amino acid 43 in the N-lobe displays higher mobility, and lower NOE values are also observed for the linker regions (approximately residues 75–86), and the loop from residues 111–118 in the C-lobe. Similarly, 15N-T1 and T2 data (SI Appendix, Figs. S12 and S13) do not show any significant differences in molecular mobility between wild-type and F141L Ca2+/CaM. Although these relaxation experiments only probe the nanosecond to picosecond mobility range and cannot detect changes in processes occurring on the microsecond timescale, our inability to find significant differences between wild-type and F141L Ca2+/CaM further highlight that the cause for loss of CDI for this mutant lies in the Ca2+-free state.
Discussion
CaM is one of the most conserved proteins throughout evolution, but genetic linkage in a large family (6), followed by sequence analysis of several patients, have revealed mutations in the CALM genes that are linked to cardiac arrhythmias, including CPVT, LQT, and IVF (6–11). The disease-associated mutations have a dominant effect, and with three different CALM genes encoding identical CaM proteins, only one of six alleles is found to be mutated in each of the patients, enough to create a disease phenotype.
CaV1.2 is a major voltage-gated calcium channel isoform expressed in cardiac myocytes. Because CaV1.2 contributes to depolarization, it is imperative that it inactivates to allow a timely repolarization. Indeed, a delayed inactivation will result in a prolonged plateau phase of the action potential in ventricular myocytes, and may lead to LQT (30). Inactivation of CaV1.2 carries both a voltage- and a Ca2+-dependent component. CaM, an essential component for CDI, is known to preassociate with the channel under resting Ca2+ concentrations and undergoes conformational changes upon saturation with Ca2+. Importantly, the C-lobe has previously been shown to be crucial for CDI of CaV1.2, as site-directed mutations that knock out Ca2+ binding in both EF3 and EF4 can obliterate CDI (31). It is therefore interesting to note that all CaM variants, known to affect CDI of CaV1.2, are located in the C-lobe.
The major interaction site for CaM in CaV1.2 is the IQ domain, located in the cytoplasmic C-terminal tail of the channel. The CaV1.2 IQ domain can associate with CaM under both Ca2+-occupied and Ca2+-free conditions (24, 31). In addition, it has been found that the N-lobe can associate with an N-terminal fragment, previously termed NSCaTE, under Ca2+-saturating conditions (25, 26). Although the NSCaTE segment can modulate the degree of CDI, it is not essential for robust CDI to occur. Mutations in the IQ domain, however, can completely knock out CDI (24). We therefore utilized the CaM:IQ domain interaction as a probe to test the impact of several disease variants on the structure, dynamics, and binding properties using a combination of biochemical, X-ray crystallography, and NMR approaches.
We investigated four different CaM variants, all of which affect CDI. N97S was first identified as a causative variant in CPVT (6), where an affected individual presented with cardiac arrest at age 4 y. A later study also linked it to a case of prolonged QT interval (∼480 ms), with the first report at age 5 y (7). The exact phenotype may thus depend on the genetic makeup of the patient or environmental factors. In contrast, the D95V, F141L, and N97I mutations were found in cases of a more severe LQT (QT interval > 600 ms) (7, 8, 10), where the first symptoms were found as early as 17 mo (N97I) (7), right after birth (F141L) (10), or even prenatally (D95V) (10). F141L, D95V, and N97S all prolonged the action potential in transfected ventricular myocytes, with N97S showing a milder effect (12).
The variants linked to LQT are scattered throughout the C-lobe, and we therefore wondered whether they affect CDI via different mechanisms. Because CaM binds CaV1.2 at different Ca2+ concentrations, the mutations could affect binding of either Ca2+/CaM or apoCaM to the IQ domain. They could interfere with binding directly through altering the interaction interface, or indirectly by causing conformational changes or by altering the Ca2+ affinity. The main observations for each mutant are summarized in SI Appendix, Table S5.
The mutant with the subtlest effect on CDI of CaV1.2, N97S, decreases the affinity for Ca2+ 1.5- to 4-fold (6, 13, 14, 27). Our crystallographic analysis shows that N97S indeed still binds Ca2+, but because the Ser97 hydroxyl group is closer to the main chain than the Asn97 amide group, the side chain needs to move in closer to the Ca2+, thus introducing strain. This is in stark contrast to two other mutants, D95V and N97I, which completely obliterate Ca2+ binding to EF3. Surprisingly, these two mutants display a highly distorted conformation of the C-lobe that resembles neither a Ca2+-occupied nor a Ca2+-free wild-type C-lobe. This results in a complete reorganization of the hydrophobic surface that binds target peptides, but despite this both mutants retain the ability to bind the IQ domain with high affinity under saturating Ca2+ concentrations. However, the interactions between the C-lobe and IQ domain are altered, a direct effect of the distorted C-lobe conformation. A new hydrophobic pocket is formed, which binds the canonical Ile1624 of the IQ motif. In the wild-type structure, Ile1624 is partially exposed to solvent. Because this residue is crucial for CDI (24), its sequestration by the mutant C-lobe may contribute to loss of CDI observed for D95V (12). Although both mutants have a complete loss of Ca2+ binding in EF3, they have a different effect on the affinity for Ca2+ in EF4, with N97I binding twofold stronger compared with D95V (7). Our structures suggest that this is due to an additional stabilizing interaction unique to N97I, whereby the Tyr99 side chain stacks against the hydrophobic Ile97 side chain. This is not possible with Asn97 in D95V.
A fourth mutant, F141L, seems to adopt a completely different mechanism. Although it has also been found to abolish CDI (12), this mutation does not affect a Ca2+-coordinating residue, but instead interferes with hydrophobic packing within the C-lobe, as shown by both X-ray and NMR structures. Although the mutation directly affects the interaction with Y1627 in the IQ domain, F141L CaM retains high-affinity binding to the IQ domain in Ca2+-saturating conditions. However, there appears a significant effect of this mutation in Ca2+-free conditions, because this mutant binds 10-fold stronger to the IQ domain than wild-type CaM. This is corroborated by NMR experiments on this mutant. Although the N-lobe of F141L apoCaM is well defined, very few peaks are visible for the C-lobe. Importantly, the C-lobe is not a completely random coil, because this would have resulted in clearly visible peaks. Instead, the data suggest that the F141L apo C-lobe adopts multiple conformations. Most likely, one or more of these result in increased affinity for the IQ domain. The affinity of Ca2+ for the CaM:IQ complex is determined by the relative affinities of apoCaM or Ca2+/CaM for the IQ domain, and a stronger apoCaM interaction is predicted to decrease the Ca2+ affinity. Indeed, the fluorescence experiments suggest at an ∼5.5-fold decrease in Ca2+ affinity for the F141L CaM:IQ complex.
Our data also explain the ability of these mutants to have a dominant effect on CDI in the presence of wild-type CaM. Because they have very minimal effects on structure and binding to the IQ domain in low Ca2+ conditions, or even enhance affinity (F141L), all four mutants can preassociate with CaV1.2, thus occupying a significant fraction of the binding sites. However, because of large structural changes in the Ca2+-bound state, altered Ca2+ affinities, different interactions with the IQ domain, or a combination thereof, these mutants are unable to adopt the proper interactions with CaV1.2 to mediate CDI. Although the channels occupied with wild-type CaM can still undergo CDI, having a significant fraction of channels (e.g., one-sixth) incapable of CDI is sufficient to affect the overall inactivation profile of the CaV1.2 population in a cell (12). In this regard, the higher affinity of F141L apoCaM for the IQ domain would result in a better competition compared with wild-type CaM, and thus a higher occupancy of mutant CaM bound to CaV1.2 than expected from its expression relative to wild-type CaM.
Importantly, both F141L and N97S CaM display only minor structural differences in the Ca2+-bound state, implying that the reduction in CDI is a result from the altered interactions in the Ca2+-free state (F141L CaM) or the reduction in Ca2+ affinity. The latter implies a reduction in the on-rate or increase in off-rate for Ca2+ binding, resulting in a delayed or reduced CDI, thus prolonging the plateau phase of the cardiac myocyte action potential and contributing to LQT. Interestingly, our estimated Ca2+ affinities for the CaM:IQ complexes suggest a KD of ∼130 nM for wild-type CaM. If this affinity holds true for CaM bound to full-length CaV1.2, which remains to be confirmed, this implies that a significant amount of Ca2+/CaM could preassociate with the channel in resting conditions, in addition to apoCaM preassociation. As several mutations decrease the Ca2+ affinity, they would affect both the degree of preassociated Ca2+/CaM, and affect Ca2+ binding to preassociated apoCaM, resulting in a more complex effect on overall CDI.
These structures provide snapshots of disease-causing mutations in CaM. Because CaM regulates multiple ion channels, it is likely that the same mutations perturb the action of other cardiac channels, with the structural distortions observed in this study forming the same cause. For example, the cardiac RyR2 is also regulated by CaM (32–36), and several studies show that some mutations in the present study also affect its function (11, 13–16). In the presence of Ca2+, D95V CaM is still able to bind either full-length RyR2 or the major CaM binding peptide of RyR2 with high affinity (13, 16), indicating that the inherently distorted D95V Ca2+/CaM structure can also interact with peptides other than the IQ domain. Interestingly, F141L was found as the divergent mutant, as one study found it to confer an extra inhibitory effect on RyR2, in contrast to other CaM disease mutations (16). Further studies with either RyR2 CaM-binding peptides (37) or full-length RyR2 will be required to determine the precise structural impact of the disease mutations on RyR2 (38).
As these studies are performed on isolated IQ domains, not intact channels, it is likely that several factors, such as the inherent affinities between CaM, Ca2+, and IQ, are altered, as well as the precise interaction between CaM and the channel. Cryoelectron microscopy (cryo-EM) studies on full-length channels can shed more light on this, but thus far no reconstructions are available for full-length CaV1.2 and most cytosolic elements, including the IQ domain, have remained unresolved in cryo-EM reconstructions of the related CaV1.1 (39, 40). High-quality cryo-EM studies on CaV1.2 in complex with CaM will be required to enhance our insights into the mechanism of CDI and the impact of disease mutations on this process.
Methods
Cloning, Expression, and Purification.
Full-length human CaM, CaM C-lobe (residues 79–148), and the IQ domain of human CaV1.2 (CaVα1c77; residues 1611–1641) were cloned as previously described (22) into a modified pET28 vector containing an N-terminal hexahistidine tag, maltose-binding protein (MBP), and cleavage site for the tobacco etch virus (TEV) protease. Full-length human CaM for cocrystallization with the IQ domain was cloned into pEGST without affinity tag. The D95V, N97I, N97S, and F141L mutations were produced by QuikChange (Stratagene).
Proteins were expressed at 37 °C in Escherichia coli Rosetta (DE3) pLacI (Novagen) grown in 2×YT media and induced at OD600 of ∼0.6 by addition of 0.4 mM isopropyl-β-d-thiogalactoside (IPTG) for 3 h. For cocrystalization, the complex of CaM and IQ domain was coexpressed and copurified. Cells were lysed by sonication in buffer A (250 mM NaCl, 10 mM Hepes, pH 7.4, and 10 mM CaCl2) with 25 μg mL−1 DNase I, 25 μg mL−1 lysozyme, and 1 mM phenylmethylsulphonyl fluoride. The lysate was applied to a 10 mL HisTrap FF column (GE), washed with 10 column volumes (CV) of buffer A, and eluted with 30% (vol/vol) buffer B (250 mM NaCl, 500 mM imidazole, pH 7.4, and 10 mM CaCl2). The protein was dialyzed against buffer C (10 mM NaCl, 10 mM Hepes, pH 7.4, and 10 mM CaCl2) for ∼12 h at 4 °C and simultaneously cleaved with recombinant TEV protease. The sample was applied to a 25-mL amylose column (New England Biolabs), and the flow-through applied to a Hiload HQ column (GE) with a linear gradient of 0–45% buffer D (1 M NaCl, 10 mM Hepes, pH 7.4, and 10 mM CaCl2). Residual MBP was removed by passage of the purified material through a 5-mL talon column (GE), and the flow-through applied to a HiLoad 16/60 Superdex 75 prep grade (GE) in buffer A. For crystallography, the protein buffer was exchanged to 25 mM NaCl, 10 mM Hepes, pH 7.4, and 10 mM CaCl2. For ITC, the protein buffer was exchanged to 10 mM Hepes, pH 7.4, and 10 mM CaCl2.
TAMRA Fluorescence Anisotropy Experiments.
A CaV1.2 IQ peptide with an N-terminal 5-TAMRA label was obtained from Proteogenix (1659-DEVTVGKFYATFLIQEYFRKFKKRKEQGLVGKPS-1692). Ca2+-buffered solutions contained 50 mM Hepes, 100 mM KCl, 0.5 mM EGTA, and 2 mM NTA at pH 7.2, 25 °C. The total amount of CaCl2 and MgCl2 needed to attain a desired free Ca2+-concentration and a free Mg2+ concentration at 1 mM, were calculated using pCa Calculator (39).
Using a liquid-handling robot (Microlab STARlet; Hamilton), we employed a 2D titration assay in which CaM was serially diluted in different Ca2+-buffered solutions containing a constant amount of TAMRA-labeled CaV1.2 IQ peptide. The interaction between CaM and CaV1.2 IQ was monitored by changes in the fluorescence anisotropy at 25 °C, using a fluorescence polarization plate reader (Infinite M1000; Tecan) and excitation and emission wavelengths at 530 and 582 nm (5- and 20-nm bandwidths, respectively). The use of 384-well plates (Cat. #3575; Corning) allowed for 24 titration points for CaM at 16 different [Ca2+]free, resulting in 16 protein–peptide titration curves for each plate (SI Appendix, Fig. S1). Curve fitting is described in SI Appendix, Supplementary Methods.
Crystallization, Data Collection, and Structure Solution.
Crystallization conditions are shown in SI Appendix. After flash-freezing, diffraction data were collected at the Stanford Synchrotron Radiation Lightsource beamline 9-2 (N97S Ca2+/CaM) and the Advanced Photon Source beamline 23ID-B (D95V, N97I, and F141L Ca2+/CaM:IQ complexes) and processed using XDS (41). Structures were solved by molecular replacement using Phenix (42); and using, as search models, wild-type Ca2+/CaM (PDB ID code 1CLL) for N97S Ca2+/CaM, Ca2+/N-lobe (PDB ID code 2BE6), and apo/C-lobe (PDB ID code 4CDK) for D95V Ca2+/CaM:IQ domain, D95V Ca2+/CaM:IQ domain for N97I Ca2+/CaM:IQ domain, and wild-type Ca2+/CaM:IQ domain (PDB ID code 2BE6) for F141L Ca2+/CaM:IQ domain. The structures were refined using COOT (43) and Phenix (42). No residues are in disallowed regions of the Ramachandran plot. Final models contain four molecules (N97S Ca2+/CaM) or two complexes (D95V, F141L, and N97I Ca2+/CaM:IQ) in the asymmetric unit. Figures were prepared using PyMOL (Schrödinger). Statistics are shown in SI Appendix, Table S3. Structures were deposited in the PDB database with ID codes 6DAD, 6DAE, 6DAF, and 6DAH.
Isothermal Titration Calorimetry.
Samples were dialyzed against 10 mM Hepes, pH 7.4, and 10 mM CaCl2. The IQ peptide (GenScript) corresponds to human CaV1.2 (CaVα1c77) residues 1611–1644. Experiments were conducted on an ITC200 (MicroCal) at 25 °C. Titrations consisted of 20 injections of 2 μL of 75-μM C-lobe into the cell containing 11 μM IQ peptide. Data were processed with MicroCal Origin 7.0. For data fitting, the active IQ peptide concentration was adjusted by a factor of 0.6 to achieve a binding stoichiometry of ∼1. Control experiments injecting titrant into buffer were used to adjust the baseline of each experiment.
Protein expression and purification for NMR.
Wild-type, D95V, and F141L CaM were expressed from a pMAL vector, containing an N-terminal MBP and a TEV cleavage site, in E. coli Rosetta-2 (DE3) cells (Novagen) first grown in LB media to an OD600 of ∼0.5. The LB culture was centrifuged at 4,000 × g for 10 min, and pelleted cells added to standard M9 minimal media (Cold Spring Harbor protocols) containing 15N-ammonium sulfate and d-[U-13C] glucose to an OD600 of ∼0.05. The M9 culture was induced at OD600 ∼0.5 by addition of 1 mM IPTG and incubated at 25 °C for 18 h. The cells were lysed by sonication in lysis buffer (20 mM Tris⋅HCl, 50 mM NaCl, pH 7.5, and 1 mM β-mercaptoethanol) with 1 mg/mL lysozyme and 0.34% (vol/vol) protease inhibitor mixture (Sigma Aldrich). The lysate was centrifuged at 30,000 × g at 4 °C for 45 min and the supernatant applied to a 120-mL amylose column (New England Biolabs), washed with 5 CV buffer A (20 mM Tris⋅HCl, 200 mM NaCl, pH 7.5), and eluted with 100% (vol/vol) buffer B (buffer A and 10 mM maltose). The fusion protein was cleaved with recombinant TEV protease overnight at 4 °C. The sample was then applied to a 50-mL Q-Sepharose anion exchange column equilibrated in buffer C (20 mM Tris⋅HCl, 50 mM NaCl, pH 7.5), washed with 3 CV 30% (vol/vol) buffer D (20 mM Tris⋅HCl, 500 mM NaCl, pH 7.5), and then eluted with a linear gradient from 30 to 100% (vol/vol) buffer D. The eluted protein was concentrated to 2 mL. Apo samples were produced by adding EDTA to a final concentration of 10 mM, whereas Ca2+ loaded samples were obtained by adding CaCl2 to a final concentration of 5 mM. The sample was applied to a HiLoad 16/60 Superdex 200 pg (GE Healthcare) using buffer E (2 mM Hepes, 100 mM KCl, pH 6.5). The molecular weight of the purified protein was verified by MALDI-TOF mass spectrometry and the concentration was determined by absorption at 280 nm. The protein buffer was exchanged to 2 mM Hepes, 10 mM KCl, pH 6.5.
NMR experiments.
All NMR spectra, if not specified otherwise, were recorded on a BRUKER AVIII-600 MHz spectrometer equipped with a CPP-TCI probe. Spectra were acquired at 298.1 K. BRUKER TopSpin 3.5pl6 was used for recording and processing. A full description is provided in SI Appendix, Supplementary Methods.
Structures and restraints are deposited in the PDB databank (PDB ID codes 6GDL and 6GDK). NMR assignments and relaxation data were deposited in the BioMagResBank (accession nos. 34263 and 34262).
Supplementary Material
Acknowledgments
We thank Oleksandr Grachov, Oscar Mejias Gomez, and Morten Bjerring for expert technical assistance; and Frans A. Mulder for valuable discussions. Use of the 950-MHz spectrometer at the Danish Center for Ultrahigh-Field NMR Spectroscopy, funded by the Danish Ministry of Higher Education and Science Grant AU-2010-612-181, is acknowledged. We also thank the support staff at the Advanced Photon Source (Chicago) GM/CA-CAT beamline 23-ID-D, the Stanford Synchrotron Radiation Lightsource (Menlo Park), and at the Canadian Light Source (Saskatoon, SK, Canada), which is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan. This work is funded by Canadian Institutes of Health Research Operating Grant PJT-148632 (to F.V.P.). M.T.O. was supported by research grants from the Obel Family Foundation, Novo Nordisk Foundation Grants NNF15OC0012345 and NNF16OC0023344, Lundbeck Foundation Grant R151-2013-14432, and Danish Council for Independent Research Grant DFF-4181-00447. C.H. and R.W. acknowledge Grant DFF-1323-00344 from the Danish Council for Independent Research and Grant NNF15OC0016186 from the Novo Nordisk Foundation. The NMR laboratory at Aalborg University is supported by the Obel Family Foundation, SparNord, and Carlsberg Foundations.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 6GDL, 6GDK, 6DAE, 6DAH, 6DAF, and 6DAD). The NMR chemical shifts have been deposited in the BioMagResBank, www.bmrb.wisc.edu (accession nos. 34263 and 34262).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808733115/-/DCSupplemental.
References
- 1.Friedberg F, Rhoads AR. Evolutionary aspects of calmodulin. IUBMB Life. 2001;51:215–221. doi: 10.1080/152165401753311753. [DOI] [PubMed] [Google Scholar]
- 2.Kursula P. The many structural faces of calmodulin: A multitasking molecular jackknife. Amino Acids. 2014;46:2295–2304. doi: 10.1007/s00726-014-1795-y. [DOI] [PubMed] [Google Scholar]
- 3.Tidow H, Nissen P. Structural diversity of calmodulin binding to its target sites. FEBS J. 2013;280:5551–5565. doi: 10.1111/febs.12296. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen AB, Søndergaard MT, Overgaard MT. Calmodulin in a heartbeat. FEBS J. 2013;280:5511–5532. doi: 10.1111/febs.12337. [DOI] [PubMed] [Google Scholar]
- 5.Berchtold MW, Villalobo A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim Biophys Acta. 2014;1843:398–435. doi: 10.1016/j.bbamcr.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Nyegaard M, et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–712. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makita N, et al. Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circ Cardiovasc Genet. 2014;7:466–474. doi: 10.1161/CIRCGENETICS.113.000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crotti L, et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–1017. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsman RF, et al. A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. J Am Coll Cardiol. 2014;63:259–266. doi: 10.1016/j.jacc.2013.07.091. [DOI] [PubMed] [Google Scholar]
- 10.Boczek NJ, et al. Spectrum and prevalence of CALM1-, CALM2-, and CALM3-encoded calmodulin variants in long QT syndrome and functional characterization of a novel long QT syndrome-associated calmodulin missense variant, E141G. Circ Cardiovasc Genet. 2016;9:136–146. doi: 10.1161/CIRCGENETICS.115.001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Hurtado N, et al. Novel CPVT-associated calmodulin mutation in CALM3 (CALM3-A103V) activates arrhythmogenic Ca waves and sparks. Circ Arrhythm Electrophysiol. 2016;9:e004161. doi: 10.1161/CIRCEP.116.004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limpitikul WB, et al. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca(2+) currents and promote proarrhythmic behavior in ventricular myocytes. J Mol Cell Cardiol. 2014;74:115–124. doi: 10.1016/j.yjmcc.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang HS, et al. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ Res. 2014;114:1114–1124. doi: 10.1161/CIRCRESAHA.114.303391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Søndergaard MT, et al. Calmodulin mutations causing catecholaminergic polymorphic ventricular tachycardia confer opposing functional and biophysical molecular changes. FEBS J. 2015;282:803–816. doi: 10.1111/febs.13184. [DOI] [PubMed] [Google Scholar]
- 15.Søndergaard MT, et al. Arrhythmogenic calmodulin mutations affect the activation and termination of cardiac ryanodine receptor-mediated Ca2+ release. J Biol Chem. 2015;290:26151–26162. doi: 10.1074/jbc.M115.676627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Søndergaard MT, et al. The arrhythmogenic calmodulin p.Phe142Leu mutation impairs C-domain Ca2+ binding but not calmodulin-dependent inhibition of the cardiac ryanodine receptor. J Biol Chem. 2017;292:1385–1395. doi: 10.1074/jbc.M116.766253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Petegem F. Ryanodine receptors: Allosteric ion channel giants. J Mol Biol. 2015;427:31–53. doi: 10.1016/j.jmb.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Priori SG, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Johny M, Yue DT. Calmodulin regulation (calmodulation) of voltage-gated calcium channels. J Gen Physiol. 2014;143:679–692. doi: 10.1085/jgp.201311153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brehm P, Eckert R. Calcium entry leads to inactivation of calcium channel in paramecium. Science. 1978;202:1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- 21.Splawski I, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallon JL, Halling DB, Hamilton SL, Quiocho FA. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Ca(v)1.2 calcium channel. Structure. 2005;13:1881–1886. doi: 10.1016/j.str.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Zühlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 25.Dick IE, et al. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–834. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Vogel HJ. Structural basis for the regulation of L-type voltage-gated calcium channels: Interactions between the N-terminal cytoplasmic domain and Ca(2+)-calmodulin. Front Mol Neurosci. 2012;5:38. doi: 10.3389/fnmol.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassilakopoulou V, et al. Distinctive malfunctions of calmodulin mutations associated with heart RyR2-mediated arrhythmic disease. Biochim Biophys Acta. 2015;1850:2168–2176. doi: 10.1016/j.bbagen.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Tanaka T, Ikura M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat Struct Biol. 1995;2:758–767. doi: 10.1038/nsb0995-758. [DOI] [PubMed] [Google Scholar]
- 29.Anthis NJ, Doucleff M, Clore GM. Transient, sparsely populated compact states of apo and calcium-loaded calmodulin probed by paramagnetic relaxation enhancement: Interplay of conformational selection and induced fit. J Am Chem Soc. 2011;133:18966–18974. doi: 10.1021/ja2082813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betzenhauser MJ, Pitt GS, Antzelevitch C. Calcium channel mutations in cardiac arrhythmia syndromes. Curr Mol Pharmacol. 2015;8:133–142. doi: 10.2174/1874467208666150518114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 32.Ikemoto T, Iino M, Endo M. Enhancing effect of calmodulin on Ca(2+)-induced Ca2+ release in the sarcoplasmic reticulum of rabbit skeletal muscle fibres. J Physiol. 1995;487:573–582. doi: 10.1113/jphysiol.1995.sp020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathy A, Xu L, Mann G, Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor) Biophys J. 1995;69:106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buratti R, Prestipino G, Menegazzi P, Treves S, Zorzato F. Calcium dependent activation of skeletal muscle Ca2+ release channel (ryanodine receptor) by calmodulin. Biochem Biophys Res Commun. 1995;213:1082–1090. doi: 10.1006/bbrc.1995.2238. [DOI] [PubMed] [Google Scholar]
- 35.Fuentes O, Valdivia C, Vaughan D, Coronado R, Valdivia HH. Calcium-dependent block of ryanodine receptor channel of swine skeletal muscle by direct binding of calmodulin. Cell Calcium. 1994;15:305–316. doi: 10.1016/0143-4160(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 36.Fruen BR, Bardy JM, Byrem TM, Strasburg GM, Louis CF. Differential Ca(2+) sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am J Physiol Cell Physiol. 2000;279:C724–C733. doi: 10.1152/ajpcell.2000.279.3.C724. [DOI] [PubMed] [Google Scholar]
- 37.Lau K, Chan MM, Van Petegem F. Lobe-specific calmodulin binding to different ryanodine receptor isoforms. Biochemistry. 2014;53:932–946. doi: 10.1021/bi401502x. [DOI] [PubMed] [Google Scholar]
- 38.Peng W, et al. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science. 2016;354:aah5324. doi: 10.1126/science.aah5324. [DOI] [PubMed] [Google Scholar]
- 39.Wu J, et al. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 2015;350:aad2395. doi: 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, et al. Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 Å resolution. Nature. 2016;537:191–196. doi: 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- 41.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyaya R, Meador WE, Means AR, Quiocho FA. Calmodulin structure refined at 1.7 A resolution. J Mol Biol. 1992;228:1177–1192. doi: 10.1016/0022-2836(92)90324-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.