Abstract
Understanding intraspecific variation in sociality is essential for characterizing the flexibility and evolution of social systems, yet its study in nonhuman animals is rare. Here, we investigated whether chimpanzees exhibit population-level differences in sociality that cannot be easily explained by differences in genetics or ecology. We compared social proximity and grooming tendencies across four semiwild populations of chimpanzees living in the same ecological environment over three consecutive years, using both linear mixed models and social network analysis. Results indicated temporally stable, population-level differences in dyadic-level sociality. Moreover, group cohesion measures capturing network characteristics beyond dyadic interactions (clustering, modularity, and social differentiation) showed population-level differences consistent with the dyadic indices. Subsequently, we explored whether the observed intraspecific variation in sociality could be attributed to cultural processes by ruling out alternative sources of variation including the influences of ecology, genetics, and differences in population demographics. We conclude that substantial variation in social behavior exists across neighboring populations of chimpanzees and that this variation is in part shaped by cultural processes.
Keywords: chimpanzees, animal culture, sociality, behavioral diversity, social learning
What is a typical chimpanzee like? How is it similar to or different from a typical human? To answer these questions, it is fundamental to consider and account for the variability across individuals and populations within each species. While this logic is recognized for humans, there is a paucity of scientific focus on intraspecific variation in nonhuman animals. In this paper, we provide an account of population-level variation of social behavior in one of humans’ closest living relatives—the chimpanzee.
Intraspecific, population-level variation in sociality may stem from a variety of factors, including genetic differences at the subspecies level, differences in ecological environments, differences in demographic makeup, and differences in individual temperaments (1–4). For nonhuman primates (henceforth primates) in particular, socioecological theory was developed to understand and predict variation in social organization and behavior. This theory postulates that the structure of primate social organizations, emerging from the relationships among their members, can be understood as ecologically and phylogenetically determined (5–11).
With the advent of cultural primatology (12–16), and the identification of numerous learned behavioral differences across groups in great apes (17–21) and monkeys (22–24), the propensity of primates to develop population-specific behaviors has been well-established (but see ref. 25). These behavioral differences extend beyond material culture to “social conventions” or “traditions” without apparent function (20, 21, 24, 26–30). Consequently, it seems conceivable that intraspecific, population-level differences in general sociality (e.g., interaction tendencies) could also emerge by means of learning processes, hence extending the ecological and phylogenetic determinism of sociality postulated by socioecological theory. Based on the current status of cultural primatology, or the study of culture in nonhuman animals more generally, we view this as a pressing question in the study of psychological and behavioral diversity: Beyond isolated accounts of tradition formation in nonhuman animals, is there any indication that nonhuman animals exhibit intraspecific population-level variation in their everyday social interactions that might be instigated by cultural processes?
One seminal case demonstrating the plausibility of learned, population-level differences in sociality was reported by Sapolsky and Share (31) in their study of olive baboons (Papio anubis). When a substantial portion of dominant baboon males had died from tuberculosis, the remaining troop was characterized by atypically low levels of aggression and high levels of affiliation (ref. 31 and also see ref. 32). If the baboons’ interaction styles would have been merely contingent on genetics, ecology, and individual learning, the sudden alteration in troop-level behavioral characteristics would have converged back to olive baboon-typical behavioral phenotypes over time. Instead, the atypical interaction style became the new troop-level phenotype, which led the authors to argue for the existence of nonhuman primate social culture (31, 32).
The possibility that intraspecific variation in primate sociality may in part emerge through social learning has been explored experimentally in marmosets (3, 33) and chimpanzees (28). In response to prerecorded affiliative calls of familiar conspecifics, marmosets were found to temporarily increase their overall levels of affiliative behavior (33). In another study, the same species was shown to exhibit group-level differences in individual boldness produced by social effects (3). Chimpanzees were observed to differ at a population level in the extent to which they tolerated each other’s presence around valuable food resources (28). These experimental studies opened up the possibility that the observed behavioral patterns might be best explained in terms of local cultures, although alternative explanations could not be ruled out.
Here we investigate differences in sociality across neighboring chimpanzee populations with the specific purpose of identifying a cultural signature in naturally occurring variation in sociality by ruling out several alternative explanations. Specifically, we examine behaviors representative of chimpanzees’ general level of sociality (i.e., spatial proximity and grooming) for possible population-level differences and assess the likelihood that any observed differences could be traced back to socially learned templates of within-group interaction styles. In doing so, we acknowledge the intricate connection between ecology and culture (e.g., ref. 34) but follow the reasoning that (i) this connection is less clear in the realm of social interaction patterns (cf. socioecological theory) compared to the technological domain (i.e., tool use) (20) and (ii) when ecology can be controlled for adequately, any remaining population-level variation requires an explanation.
First, we describe a unique testbed comprising several chimpanzee populations within the same ecological environment (i.e., ruling out ecological influences on behavior such as food availability and predation risk). Second, for two populations, we consider subspecies for each individual and assess the scope of its potential influence on inducing population differences in social behavior (i.e., ruling out genetically anchored subspecies-typical behavior). Third, we employ the same data-collection procedure across all four neighboring chimpanzee populations (i.e., ruling out methodological interference of the population difference analysis; ref. 35) and control for key demographic variables affecting chimpanzees’ social dynamics in our statistical models (e.g., population size and number of kin). Finally, we use generalized linear mixed models and social network analysis to assess the nature of dyadic and population-level sociality, respectively.
Results
Party Size.
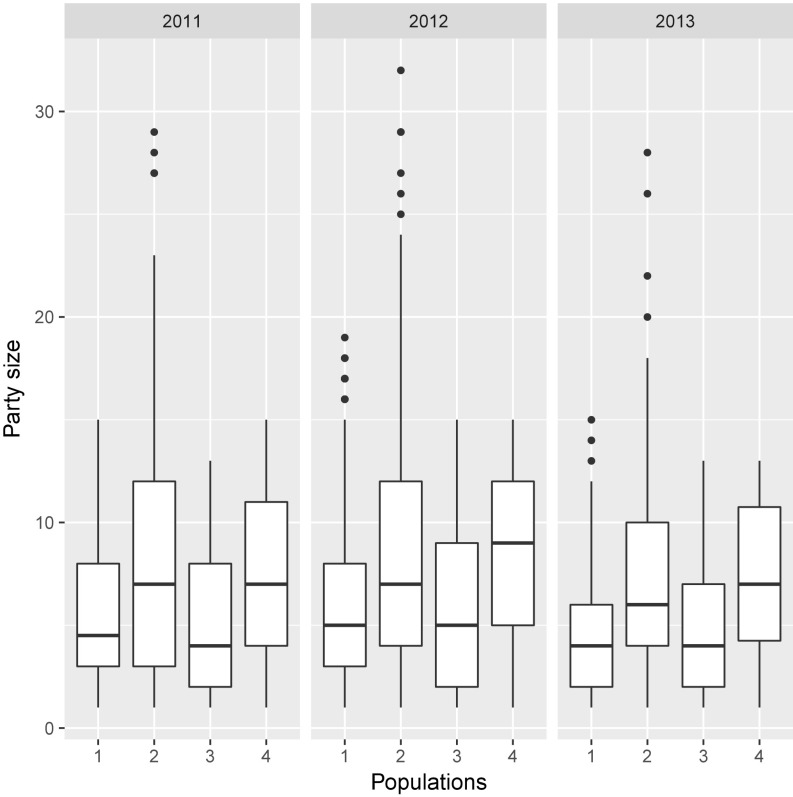
The size of congregations was significantly different between the four chimpanzee populations across the sampling period [likelihood ratio tests (LRT) population: χ2 = 32.4, df = 1, P < 0.0001; Fig. 1]. Note that this effect emerged after controlling for population size, which did not significantly influence party size (χ2 = 0.16, df = 1, P = 0.69). Mean ± SD party sizes for populations 1–4 are given in SI Appendix, Table S1. The tendency to congregate in parties of specific sizes was a stable population-level feature across time, as indicated by the absence of a significant interaction between year and population (LRT year|population: χ2 = 2.82, df = 2, P = 0.244). None of the other variables predicted party size [all nonsignificant (NS)].
Fig. 1.
Party size across four neighboring populations of semiwild chimpanzees, 2011–2013 (populations 1–4: n = 765, 911, 635, and 691 observations, respectively). Medians are represented by the bold, horizontal lines within the boxes. The boxes represent the interquartile range (IQR); the vertical lines attached to the boxes represent Q1 − 1.5 IQR (lower) and Q3 + 1.5 IQR (upper).
Populations 3 and 4 closely matched in demography and subspecies (SI Appendix, Table S2) yet organized themselves in congregations of different sizes each year, with population 4 consistently congregating in larger parties than group 3 (LRT; 2011: χ2 = 11.25, df = 1, P < 0.0008; 2012: χ2 = 19.16, df = 1, P < 0.0001; 2013: χ2 = 9.40, df = 1, P < 0.003; Fig. 1).
Association Indices.
Proximity.
The probability for two population members to have associated in close proximity over the course of the study period significantly differed across populations (binomial part LRT for “population”: χ2 = 37.29, df = 1, P < 0.0001). Whereas in the two smaller populations each possible dyad was observed to be in proximity at least once, in the two larger populations there were dyads who never associated (population 1: ∼15% of possible dyads; population 2: ∼41% of possible dyads). To understand this pattern better, we reran the analysis with population size as fixed effect (instead of offset term), finding that the population difference in proximity probability could be explained by differences in population size (LRT “population size”: χ2 = 10.29, df = 1, P < 0.002; estimate ± SD = −4.30 ± 2.73; LRT “population”: χ2 = 0.01, df = 1, P = 0.98). This may be interpreted in terms of an inability to form social bonds with all individuals in large populations, not necessarily in terms of relatively low propensities to be in proximity to others. Furthermore, dyads’ age (χ2 = 6.69, df = 2, P = 0.035) and family configuration (χ2 = 44.59, df = 1, P < 0.0001) significantly affected subjects’ likelihood to associate, with adults being more likely to associate than dyads including subadults (estimate ± SD: adult–adult versus adult–subadult: −1.08 ± 0.45, P = 0.016; adult–adult versus subadult–subadult: −1.45 ± 0.79, P = 0.067), and relatives being more likely to associate than nonrelatives (estimate ± SD: 3.15 ± 0.60, P < 0.0001). Dyads consisting of different configurations with respect to “origin” (wild- or captive-born) and “sex” did not vary in their probability to be in proximity.
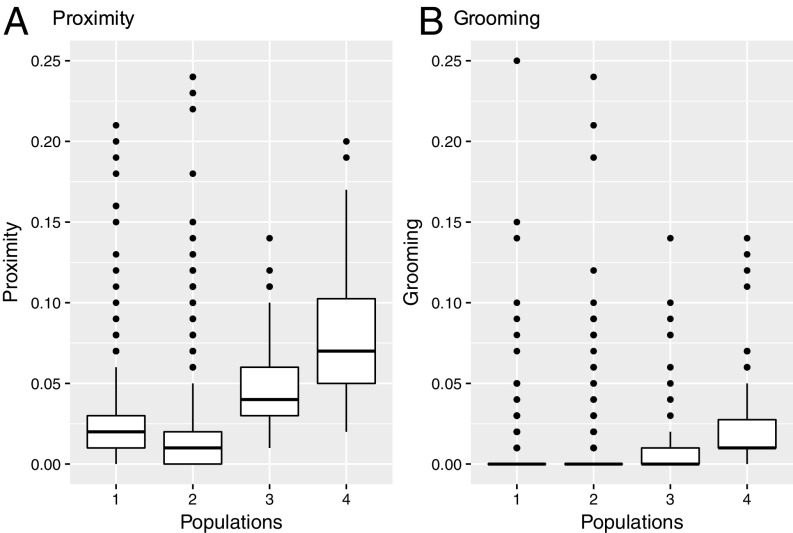
The extent of associating within dyads was significantly affected by population identity, while controlling for population size (χ2 = 27.60, df = 1, P < 0.0001; Fig. 2A; see SI Appendix, Fig. S1 for temporal consistency of proximity propensities across 3 y). Population size (χ2 = 6.33, df = 1, P = 0.012) and family (χ2 = 49.78, df = 1, P < 0.0001) significantly affected the extent of associating as well, with smaller populations (estimate ± SD: −0.28 ± 0.07) and relatives (estimate ± SD: 0.50 ± 0.02) associating more prominently than larger populations and nonrelatives, respectively. Variation in dyadic configurations with respect to sex, age, and origin did not influence the extent of associating (all NS).
Fig. 2.
Dyadic (A) proximity and (B) grooming associations per population. The association values (black dots) are the twice-weight indices [x/(x + 2yAB + yA + yB)] for all dyads (populations 1–4: n = 300, 1,081, 91, and 78, respectively). Medians are represented by the bold, horizontal lines within the boxes. The boxes represent the interquartile range (IQR); the vertical lines attached to the boxes represent Q1 − 1.5 IQR (lower) and Q3 + 1.5 IQR (upper).
Proximity matched population comparison.
Populations 3 and 4 were both characterized by the absence of nonassociated dyads (i.e., all possible dyads spent more or less time in close proximity), yet they significantly differed from each other in terms of the extent to which dyads associated (permutation test: χ2 = 50.24, P < 0.001; mean ± SD twice-weight association index group 3: 0.055 ± 0.066; group 4: 0.084 ± 0.054).
Grooming.
The probability of two population members to engage in grooming with each other significantly differed across populations (binomial part LRT for “population”: χ2 = 35.94, df = 1, P < 0.0001). In populations 1–4, the following proportions of all possible dyads had a higher than 0 probability to be observed in a grooming interaction: 21.7, 8.7, 47.25, and 75.64%, respectively. This population-level difference could again be explained by differences in population size (LRT “population size”: χ2 = 8.41, df = 1, P < 0.004; estimate ± SD = −2.82 ± 0.53; LRT “population”: χ2 = 0.14, df = 1, P = 0.713). Dyads’ age (LRT χ2 = 23.01, df = 2, P < 0.0001) and family configuration (LRT χ2 = 127.38, df = 1, P < 0.0001) significantly affected subjects’ likelihood to engage in grooming, with adults being more likely to groom than dyads including subadults (estimate ± SD: adult–adult versus adult–subadult: −1.33 ± 0.35, P < 0.001; adult–adult versus subadult–subadult: −3.08 ± 0.68, P < 0.0001), and relatives being more likely to groom than nonrelatives (estimate ± SD: 3.77 ± 0.42, P < 0.0001). Dyads consisting of different configurations with respect to “sex” also differed in their probability to engage in grooming (χ2 = 6.03, df = 1, P = 0.049), with male–male dyads grooming with higher probability than female–female dyads (estimate ± SD: 1.16 ± 0.42, P = 0.006) and female–male dyads (estimate ± SD: 1.16 ± 0.42 0.81 ± 0.36, P = 0.025). Female–male dyads and female–female dyads did not differ in their probabilities to groom (estimate ± SD: 0.35 ± 0.27, P = 0.203). Variation in dyadic configuration with respect to “origin” did not influence the probability of dyads to engage in grooming.
The extent to which dyads groomed was not significantly influenced by population size (χ2 = 0.092, df = 1, P = 0.761; estimate ± SD: −0.079 ± 0.27), yet it was significantly different for the four populations (χ2 = 20.50, df = 3, P < 0.0002; Fig. 2B; also see SI Appendix, Fig. S2 for temporal consistency of grooming propensities across 3 y). Relatives engaged in grooming more markedly than nonrelatives (χ2 = 29.71, df = 1, P < 0.0001; estimate ± SD: 3.77 ± 0.417). Different dyadic configurations with respect to sex, age and origin did not influence grooming magnitude (all NS).
Grooming matched population comparison.
Populations 3 and 4 were characterized by significantly different probabilities to engage in grooming (LRT χ2 = 6.39, df = 1, P = 0.012; group 3: 47.3%, group 4: 75.6% of all possible dyads established a grooming association). Moreover, the populations significantly differed from each other in terms of the extent to which dyads engaged in grooming (permutation test: χ2 = 26.82, P = 0.004; mean ± SD twice-weight association index group 3: 0.013 ± 0.025; group 4: 0.026 ± 0.032).
Taken together, the generalized linear mixed model (GLMM) analyses yield the results depicted in Table 1.
Table 1.
Overview of tests assessing differences in sociality across four neighboring chimpanzee populations, while statistically controlling for influential behavioral determinants using GLMMs
| Measure | Test variable | What it means | Population differences? | What we can infer |
| Party size | Number of group members near focal subject | Size of social congregations individuals spend time in | P < 0.0001 | Population-specific preferences for individuals’ tendency to group together (within eye sight) |
| Association index | Probability to be in proximity to others | Having established a 1-m association with partners (yes/no) | P < 0.0001 | Populations differ due to differences in population size* |
| Association index | Extent of being in proximity to others | Time spent within 1-m distance of associated partners | P < 0.0001 | Population-specific preferences in magnitude by which individuals seek close proximity to others |
| Association index | Probability to groom others | Having established a grooming association with partners (yes/no) | P < 0.0001 | Populations differ due to differences in population size* |
| Association index | Extent of grooming others | Time spent engaged in grooming with associated partners | P < 0.0002 | Population-specific preferences in magnitude by which individuals engage in grooming with others |
After controlling for population size, the P values for population identity were 0.98 and 0.71, respectively (Results). All P values are Bonferroni–Holm-corrected.
Social Network Metrics.
Individual attributes.
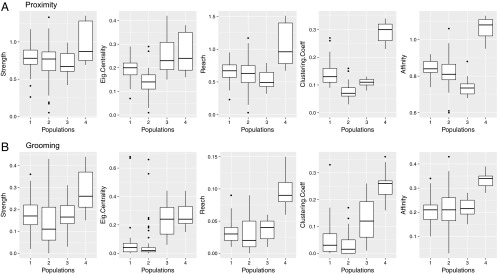
We permuted (n = 1,000) the individually derived social network attributes (SNas) across populations to assess whether individuals could be characterized as belonging to distinct populations by their magnitudes of network integration. The most obvious population differences, also taking into account the specific comparisons between the two populations matched in demography, were found for “reach” (grooming), “clustering” (proximity and grooming), and “affinity” (proximity). Fig. 3 depicts the observed variation across all four populations (SI Appendix, Tables S3 and S4).
Fig. 3.
(A and B) Social network attributes across the four study populations. Significant differences (P < 0.0001) were found for all attributes except “strength,” some of which were between the larger and smaller populations (e.g., eigenvector centrality) and others independent of population size (e.g., clustering coefficient); also see SI Appendix, Tables S3 and S4. Ranges are represented by the boxes (IQR); medians are indicated by the bold, horizontal lines within the boxes.
Population-level properties.
Population-level social network measures were calculated to investigate population differences beyond measures of individual social integration and dyadic interactions. Clustering coefficients, modularity, and social differentiation scores can be viewed as indicators of population cohesion that are relatively robust against variation in population size (35). The network properties showed substantial variation across the four populations, with the highest clustering coefficient (i.e., cohesion) being approximately three times (proximity) and approximately eight times (grooming) as large as the lowest one. The highest modularity (i.e., fragmentation) score was approximately two times (proximity) and ∼1.7 times as large as the lowest one. The highest social differentiation (i.e., inequality of associations) score was ∼2.8 times (proximity) and ∼3.6 time as large as the lowest one (SI Appendix, Figs. S3 and S4 and Tables S5 and S6).
Taken together, the individual- and population-level network metrics reveal significant differences in sociality between the study populations. On the level of individual integration, these differences are most pronounced with respect to reach, clustering coefficient, and affinity. On the population level, the differences are salient for all metrics assessed. (The population-level metrics cannot be statistically compared because they represent single values per population.)
Discussion
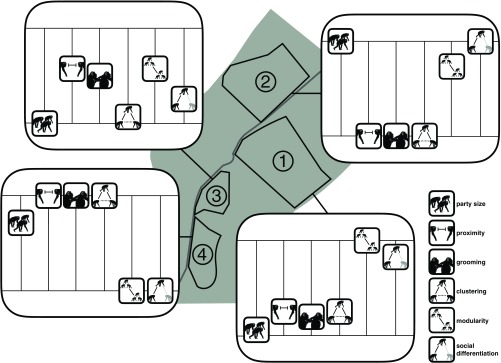
Our investigation reveals the existence of substantial differences in sociality across neighboring populations of semiwild chimpanzees that are not easily explained by socioecological theory and suggest the presence of culturally learned interaction styles. Specifically, some populations proved more gregarious than others in terms of the size of their typical congregations throughout the day and their proclivities to associate and positively interact with others. Moreover, the relatively gregarious populations were characterized by network properties indicative of high social cohesion (Fig. 4). Notably, neither ecology nor subspecies could be identified as explanatory mechanisms for the observed diversity, given that all chimpanzees lived in similar environments and populations did not systematically differ in ratios of subspecies (nota bene: the most stringent comparison between populations 3 and 4 only comprised one subspecies). Furthermore, the population-level differences in gregariousness could not be fully explained by variation in population size and number of kin present, and the standardized method of data collection across populations precluded interference of group-specific procedural biases that have hindered previous comparative work. In conjunction, these results are consistent with the evolutionary anthropological conception of “culture” [i.e., larger between-group than within-group variation (36)], also because of their temporal stability (24), and thus suggest that at least part of the observed diversity in sociality across the studied chimpanzee populations might best be explained in terms of population-specific cultural styles of interacting.
Fig. 4.
Visual representation of population differences in sociality across the four populations at the Chimfunshi Wildlife Orphanage. The icons refer to the following characteristics of each group: party size, proximity, grooming, clustering, modularity, and social differentiation. Proximity and grooming represent the aggregation of all dyadic twice-weight association indices. Clustering, modularity, and social differentiation represent population structure in terms of social cohesiveness, based on proximity. The height of the grids indicates the range of a given characteristic across the four populations. The position of each icon on the grid’s vertical axis indicates the relative position of the group in relation to the total range of the characteristic across all populations.
The population differences with respect to individuals’ propensity to congregate in variable party sizes were striking, especially for the populations closely matched in population size and demographic composition. Given the large enclosure spaces (averaging ∼20,000 m2 per individual), spatial proximity out of necessity seems highly unlikely. Nonetheless, based on socioecological theory, we opted to control for population size in the estimation of the party size differences across populations, finding no indication to that effect. This suggests that individuals spontaneously exhibit population-specific tendencies to place themselves in contact with others, which was substantiated by corresponding population differences in the extent to which individuals associate with others, both in terms of proximity and grooming. The results from the social network analyses corroborate the findings following from the linear models, both concerning party size differences and rates of association. In particular, “affinity” [i.e., the extent to which one’s neighbors associate themselves, thus representing a form of social embeddedness (35)] was highest in the population with large average party sizes and strongest prevalence of associating. Furthermore, “reach” is a relevant measure for all sorts of transmission given that this measure captures the likelihood that individuals will interact with all population members. As such, high reach represents increased probability for transmission to occur, acknowledging that transmission (e.g., of information) requires spatial proximity (37). Based on our study, we hypothesize that some populations at Chimfunshi will have higher rates of information transmission than others. This hypothesis is warranted by the accompanying levels of population cohesion (clustering coefficients and modularity). Overall, the alignment of the results concerning party size, association tendencies, and network metrics provides credibility to the existence of significant population differences in chimpanzee sociality. Given that social closeness lies at the heart of many fitness-affecting behaviors, like cooperation (e.g., refs. 38 and 39) and social learning (e.g., refs. 17 and 19), we consider the reported differences in social interaction styles (representing social closeness) meaningful and encourage the study of the interplay between social climate and tangible behaviors more generally. Notably, the improbability of explanations in terms of ecological, subspecies, or demographic variation additionally lends support to the conclusion that at least part of the documented variation in sociality is cultural in nature.
The search for cultural behaviors in primates has mainly been guided by the so-called method of exclusion (13, 21, 40). By ascertaining that population-specific behavioral phenotypes cannot be explained by noncultural determinants (most prominently ecology and genetics), causation in terms of social learning or culture is derived. This method has been criticized for its limited scope [i.e., populations often live in different ecological environments, and hence ecology is difficult to “rule out” as explanatory factor (41)] and for the reason that culture should not be seen as a residual product of an elimination process but as intricately connected with other determinants of behavior, like ecological affordances (34, 40). For instance, the selection of hammers for nut cracking in chimpanzees may be afforded by the presence of suitable materials in their habitat, yet the choice for particular tools over others may be governed by population-specific custom (18). Nonetheless, when multiple, intraspecific populations are present in the same ecological environment, such as in this study, the method of exclusion gains power (21). In such a context, it presents a conservative approach to the identification of the cultural phenomenon.
An important challenge would be to explore whether socially learned interaction patterns affect fitness at the individual or group level. In humans, multilevel selection has been invoked to explain within-group convergence in cooperative interaction styles which enhance group survival in the context of between-group competition (42–44). Given its potential to align behavioral tendencies more rapidly than genetic evolution, culture plays a crucial role in this account (44, 45). The extent to which a similar explanation could hold for chimpanzees should be explored in light of the present study and the evidenced cultural potential of chimpanzees more generally (15, 18, 27). For common marmosets, the emergence of population-specific behavioral styles (i.e., “group personality”) was interpreted as a proximate mechanism to promote group-level cooperation, which in turn could boost individual-level fitness (3). The tendency for chimpanzees to cooperate in large parties (46, 47), even for targeted competition with neighboring groups (48), supports a multilevel selection explanation of population-level variation in chimpanzee interaction patterns. However, multilevel selection is considered to be one of the main drivers of the unique extent of human cooperation and prosociality (42, 45, 49), which should thus warrant a thorough scrutiny of its potential role in the evolution of the chimpanzee phenotype.
Proximately, the population differences in sociality could have emerged through the adoption of observed and/or experienced interaction patterns, in combination with variation in the social behavior of the largest bases of attraction (e.g., the alpha males). Social learning has been robustly identified as within the range of capacities of chimpanzees (e.g., refs. 15, 19, 20, and 50), and the exact mechanisms by which chimpanzees would learn from observed interaction patterns need not be cognitively demanding (refs. 51 and 52, although see ref. 53). A similar case of interaction-style adoption has been reported with respect to reconciliation rates in a translocation experiment with macaques (ref. 54; also see ref. 55). In general, by means of associative learning, chimpanzees could become psychologically predisposed to interact with future partners in line with previously experienced interaction styles (e.g., with respect to the degree of gregariousness or tolerance) (56). The alternative explanation that the observed population differences are an artifact of management practices is unlikely for the reasons that the populations have not been formed discriminatively on the basis of gregariousness, and the handling procedures including food provisioning and interfering protocols (i.e., only when infants are very sick will there be interventions in the populations) are the same across populations. Moreover, all populations encompass both individuals with likely early trauma (wild-born) and individuals that were born in the sanctuary, deeming the existence of population differences in sociality not easily explained in terms of variation in traumatized individuals (nota bene: in the linear models, the effect of “origin” was controlled for). Furthermore, our findings would not support the a posteriori hypothesis that variation in sociality emerges due to variation in levels of social familiarity, as operationalized in terms of the length of population existence (Fig. 4; the order of population identity number corresponds to the order of population formation; also see Materials and Methods and ref. 28). The influence of individual personality differences, however, has not been assessed in the current study. It may well be that the composition of personalities affects social network structure (e.g., see ref. 57). However, both the multilevel regression and social network analyses pointed in the same direction with respect to the population-level differences in sociality, presupposing the workings of mechanisms that facilitate convergence from individual-level variation to population-level homogeneity (3).
Our findings are consistent with the identification of spontaneously emerged social climates in sperm whales (58). Based on measures of coordinated activity and association quality, sympatric sperm whales could be characterized by their clan-specific social interaction styles. Similar to the findings of the present study, some “clans” showed higher levels of dyadic sociality than others, which was associated with more homogenous relationships across dyads (58). A recent examination of intraspecific variation in social structure and dynamics in vervet monkeys reported population differences with respect to individuals’ tendencies to preferentially interact with well-connected group members, and with respect to the stability of dyadic relationships (59). Despite the fact that these studies did not explicitly focus on identifying cultural variation (cf. ref. 60), in conjunction with the present study these reports should spark further investigation of the presence of culturally induced social climates in nonhuman animals. For instance, longitudinal studies are needed to examine whether such social climates persist, despite repeated changes in population composition (through, e.g., migrations, births, and deaths). Similarly, translocation experiments in captivity could shed light on the extent to which local cultures influence the behavior of immigrants (e.g., see ref. 54). The latter approach would simultaneously enable the opportunity to study the mechanisms by which population-level homogeneity in interaction patterns could ensue [e.g., by means of conformity (18, 23, 61, 62), although see refs. 63–65].
The topic of behavioral diversity in nonhuman animals in general, and chimpanzees in particular (being one of humans’ closest living relatives), is both timely and pressing. Recent accounts have hinted at the possibility of substantial between-group variation in chimpanzees (28, 66, 67), despite the lingering species-typical view of “the chimpanzee” (see ref. 46). Notably, this variation need not be restricted to isolated traditions, like nut cracking (18) or hand clasping (20), but may be more fundamentally embedded in the very fabric of social interactions. Here, we show that neighboring chimpanzee populations can differ significantly in their social interaction patterns, while controlling for many factors that are hard to account for in a comparison of spatially distinct field sites (e.g., food availability, climate, and predation risk but also influential scientific methods like data-collection protocols and sampling rates). Such population-specific interaction dynamics are important to recognize not only for acknowledging that results from experimental studies tapping into social behavior (e.g., social learning, prosociality, and cooperation) may be biased by their particular study population, but also for pressing the need to incorporate a multipopulation approach for obtaining an accurate species representation for phylogenetic studies (ref. 68; also see ref. 59). For instance, in tracing the evolutionary origins of humans’ extended forms of prosociality, based on the findings of the current study it may be warranted to assess prosocial behavior in chimpanzee populations with differing magnitude of social dynamics, like the populations at Chimfunshi.
In more detail, as our closest living relatives chimpanzees (and bonobos) are often studied with the aim of learning whether certain human behaviors (e.g., cooperation and prosociality) might be derived or otherwise rooted in deeper phylogenetic history (69). This comparative approach—identifying contingencies and changes in evolutionary history by pinpointing similarities and differences across extant species—relies crucially on a correct characterization of any of the compared species. While the last years have witnessed a renewed interest in variation between human populations, and an increased recognition of the relevance to include this variation in any account of the human species as a whole, comparative psychology still often assumes the existence of a typical exemplar of a species without accounting for within-species variation (66). For example, there has been a series of opposing results concerning whether chimpanzees and humans vary in their active prosociality (70–74). One possible, as of today unexplored, explanation for these conflicting results is that the different groups of chimpanzees studied, ceteris paribus, vary in their tendencies to behave prosocially. Our data, we argue, promote a cultural comparative psychology that embraces within-species variation as a characteristic of the respective species, both in an aim to compare species fairly and as a phenomenon worth studying comparatively in its own right.
Materials and Methods
Study System.
Data were continuously collected from March 2011 to March 2013 at the Chimfunshi Wildlife Orphanage Trust, a chimpanzee sanctuary in Zambia. Subjects comprised 89 chimpanzees across four populations, living in forested enclosures ranging in size from 47 to 190 acres (SI Appendix, Fig. S5). Chimpanzees at Chimfunshi stay outside overnight and only come indoors for supplemental feeding between 1130 and 1330 hours. Except for a few meters along the fence line between groups 3 and 4, the chimpanzees do not have visual access to each other. Approximately half the chimpanzees were wild-born and integrated into peer groups at the sanctuary; the other half were mother-reared at the sanctuary. Groups 1–4 were formed between 1984–1989, 1990–1994, 1995–1999, and 2000–2002, respectively. For demographic details of the chimpanzees under study see SI Appendix, Table S2.
Data Collection and Operational Measures.
Data collection across all populations was standardized by adhering to one focal follow protocol (75). Subjects were quasirandomly selected as focal subject by a trained observer (E) starting at one of four (one of seven in the two larger groups) preassigned locations surrounding the enclosure and selecting the subject closest to the start location. Subjects were video-recorded (centered with a 2-m radius) continuously for 10 min. If the focal moved out of sight, data were only included when the total time the focal was in view exceeded 5 min. At the end of each focal follow, one scan sample was obtained by E panning from left to right. All chimpanzees observed during the focal follow and scan sample were counted to belong to the focal’s party composition. The next focal chosen was the closest chimpanzee to the previously recorded focal. Observations were done for 1 h every day, alternatingly between 0830 and 1100 hours and 1400 and 1630 hours. Only one video per subject per week was randomly selected to increase data independency, resulting in a total of 3,002 focal follow videos for analysis (groups 1–4, n = 765, 911, 635, and 691, respectively). Data collection procedures were approved by the Chimfunshi Research Advisory Board (the overarching committee who evaluates research proposals in light of chimpanzee safety and welfare, among other factors) prior to the start of the study.
From the videos, we derived party size and coded proximity, grooming, play, aggression, and copulation using a standard chimpanzee ethogram (adapted from ref. 76). Party size was defined as the sum of individuals within a focal’s party composition (including the focal). Proximity was defined as being in a 1-m radius of the focal individual; direct passings within a 1-m radius (without a moment of paused locomotion), grooming, or aggressive encounters were excluded from this category. Grooming was defined sensu Nishida et al. (76) and counted both when the focal provided or received grooming (i.e., directionality not considered here). Play, aggression, and copulation were also defined sensu Nishida et al. (76), with the restrictions that interactions required physical contact (to minimize ambiguity). Per day, a 1/0 sampling method was used (for each behavior coded) to further maximize data independency (35). Before coding the videos, all members of the coding team demonstrated high interobserver reliability with a lead coder (Cohen’s kappa ≥ 0.85). Videos were coded in INTERACT (Mangold International GmbH) and Excel. Party size, proximity, and grooming (given and received collapsed) were measures with sufficient data for analysis (n = 3,002, 6,064, and 946, respectively); play (n = 246), aggression (n = 10), and copulation (n = 17) were observed too infrequently for reliable between-population comparison.
Social network indices were calculated with SOCPROG (77). First, we extracted twice-weight association indices (35), both for the proximity and grooming data. The twice-weight index was chosen as it is the least biased when there is an increased possibility of observing individuals who were associated over those alone (ref. 78; also see ref. 79). The twice-weight association index (AI) is calculated as
where x is the number of sampling periods (days) in which individual A and individual B were associated, yA is the number of sampling periods in which only A was identified, yB is the number of sampling periods in which only B was identified, and yAB is the number of sampling periods in which both A and B were identified but not associated with each other. “Identified” refers to an individual being captured on video that day, either as a focal subject or as present in the subgroup of another focal subject.
Second, for their relevance to individuals’ social integration, the following SNas per individual were extracted, both for the proximity and grooming data: strength, eigenvector-centrality, reach, clustering-coefficient, and affinity (SI Appendix, Table S7). Additionally, for their relevance to group sociality beyond the dyad, and comparability across groups when sampling methods are identical (35), as in our case, the following population-level social network measures were extracted: clustering coefficient, modularity [based on eigenvector method, calculated from gregariousness (80)], and social differentiation. Clustering coefficient is a measure of group cohesiveness, encapsulating the extent to which connected individuals are themselves connected to others (81). A relatively large clustering coefficient corresponds to high group cohesion. Modularity represents group fragmentation and can be viewed as a measure of subgroup division (81). As such, a relatively large modularity score corresponds to low group cohesion. Social differentiation is a measure of variability in probability of association among dyads (35). Hence, a relatively large value corresponds to a relatively unequal distribution of associations across group members.
Finally, given that socioecological theory predicts that social behavior could potentially vary depending on population size (82, 83), and that results from social network analysis may be affected by the number of individuals interacting (35), we present all results separately for two populations highly matched in demography (e.g., group size and composition in terms of sex and age; SI Appendix, Table S2), but also in enclosure size and subspecies.
Data Analysis.
First, party size differences between populations were analyzed with GLMM with Poisson error distribution and log link function (lme4 package; ref. 84). The full model consisted of the fixed effects origin (wild-/sanctuary-born), rank (z-transformed), age, and sex. Additionally, to account for potentially meaningful differences in population demography, we included population size and number of family units (both log-transformed) as fixed effects (i.e., assuming direct link with party size). Subspecies variation was minimal (i.e., almost all chimpanzees were found to belong to the subspecies troglodytes schweinfurthii; SI Appendix, Table S2) and thus could not be modeled for its effect on party size. (For the same reason, we excluded subspecies information from all further analyses.) Focal follow duration was included as offset term to control for observation effort. We included the random intercepts for focal, day, and population identity and the random slopes for rank and age nested in day. To test the temporal stability of any population effect, we further included the random slopes for year (two dummy coded and centered variables derived from the years 2011 and 2012 + 2013) within population. The null model resembled the full model, except for the omission of the random effects for population identity. The effects of population identity (including “year within population”) were tested with LRT (85).
Second, social network indices were analyzed with Hurdle models (for AIs, to accommodate the numerous zeros reflecting absence of association) and permutation tests (for SNas). The Hurdle models consisted of a binomial part (logit link function) to model the likelihood of presence/absence of association, and a Gamma part (log link function) to model the nonzero AIs. Both model types consisted of the fixed effects dyad.sex (female–female, male–female, or male–male), dyad.age (subadult–subadult, subadult–adult, or adult–adult), and dyad.origin (wild–wild, wild–sanctuary, or sanctuary–sanctuary). For its potential effect on the tendency of two group members to associate, we included population size (log-transformed) as inverse offset term (i.e., offsetting the decreased opportunity to associate with each individual with increasing population size). Instead of number of family units, here we added a variable denoting whether or not the dyad was between family members (same.matriline yes/no) as fixed effect. Furthermore, we included the random intercepts of population identity, focal, and partner, including all possible random slopes within focal and partner (86, 87). The full models were compared with reduced models (LRT; ref. 85) to assess the effect of population identity. For the SNas, we permuted (n = 1,000) population identity across individuals to test the likelihood that obtained network indices were indistinguishable from a random distribution across populations. Given the complexity of social dynamics in chimpanzees, and our decision to use only one focal follow per subject per week for increased data independency, to obtain reliable SNas, we used all data for computing the respective social network metrics (SI Appendix, Table S7) instead of parsing the data across the three data-collection years, hence precluding any stability-across-time analysis.
All models were fitted in R (version 3.3.3; ref. 88) using the functions lmer and glmer of the R package lme4 (version 1.1–12; ref. 84). We considered P values less than 0.05 as significant and corrected for multiple testing using Bonferroni–Holm corrections (89).
Supplementary Material
Acknowledgments
We thank the Social Climate Coding Team led by Vivian Vreeman, including Maddalena Tacchetti, Karoline Kneist, Marjolein van Ginneken, Marloes van der Goot, Lisa-Marie Bossen, Clara Dubois, and Becky Koomen; Innocent Mulenga for facilitating the research; the Zambian research team, including Patrick Chambatu, Thomson Mbilishi, Albert Mulembo, Goodson Muletele, Felix Chinyama, Patrick Mwika, Mumba Kawele, Misheck Kasongo, John Kayuya, and Joseph Kasongo; the Zambia Wildlife Authority; the Chimfunshi Board of Trustees; and the Chimfunshi Research Advisory Board. E.J.C.v.L. was supported by the European Research Council (Grant Agreement 609819, project Constructing Social Minds: Coordination, Communication, and Cultural Transmission) and the Research Foundation Flanders (FWO).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Pressing Questions in the Study of Psychological and Behavioral Diversity,” held September 7–9, 2017, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/pressing-questions-in-diversity.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722614115/-/DCSupplemental.
References
- 1.Schradin C. Intraspecific variation in social organization by genetic variation, developmental plasticity, social flexibility or entirely extrinsic factors. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120346. doi: 10.1098/rstb.2012.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lott DF. Intraspecific variation in the social systems of wild vertebrates. Behaviour. 1984;88:266–325. [Google Scholar]
- 3.Koski SE, Burkart JM. Common marmosets show social plasticity and group-level similarity in personality. Sci Rep. 2015;5:8878. doi: 10.1038/srep08878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappeler PM, Barrett L, Blumstein DT, Clutton-Brock TH. Constraints and flexibility in mammalian social behaviour: Introduction and synthesis. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120337. doi: 10.1098/rstb.2012.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
- 6.Sterck EHM, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol. 1997;41:291–309. [Google Scholar]
- 7.van Schaik CP. Why are diurnal primates living in groups? Behaviour. 1983;87:120–144. [Google Scholar]
- 8.van Schaik CP. The ecology of social relationships amongst female primates. In: Standen V, Foley R, editors. Comparative Socioecology: The Behavioral Ecology of Humans and Other Mammals. Blackwell Scientific; Oxford: 1989. pp. 195–218. [Google Scholar]
- 9.Schulke O, Ostner J. Ecological and social influences on sociality. In: Mitani JC, Call J, editors. The Evolution of Primate Societies. Univ of Chicago Press; Chicago: 2012. pp. 195–219. [Google Scholar]
- 10.Clutton-Brock T, Janson C. Primate socioecology at the crossroads: Past, present, and future. Evol Anthropol. 2012;21:136–150. doi: 10.1002/evan.21316. [DOI] [PubMed] [Google Scholar]
- 11.Thierry B. Primate socioecology, the lost dream of ecological determinism. Evol Anthropol. 2008;17:93–96. [Google Scholar]
- 12.de Waal FBM. Cultural primatology comes of age. Nature. 1999;399:635–636. doi: 10.1038/21310. [DOI] [PubMed] [Google Scholar]
- 13.Wrangham RW, de Waal FBM, McGrew WC. The challenge of behavioral diversity. In: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG, editors. Chimpanzee Cultures. Harvard Univ Press; Cambridge, MA: 1994. pp. 1–18. [Google Scholar]
- 14.McGrew WC. The Cultured Chimpanzee: Reflections on Cultural Primatology. Cambridge Univ Press; Cambridge, UK: 2004. [Google Scholar]
- 15.Whiten A, et al. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 16.Whiten A, Horner V, Marshall-Pescini S. Cultural panthropology. Evol Anthropol. 2003;12:92–105. [Google Scholar]
- 17.van Schaik CP, et al. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- 18.Luncz LV, Mundry R, Boesch C. Evidence for cultural differences between neighboring chimpanzee communities. Curr Biol. 2012;22:922–926. doi: 10.1016/j.cub.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Hobaiter C, Poisot T, Zuberbühler K, Hoppitt W, Gruber T. Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 2014;12:e1001960. doi: 10.1371/journal.pbio.1001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Leeuwen EJC, Cronin KA, Haun DBM, Mundry R, Bodamer MD. Neighbouring chimpanzee communities show different preferences in social grooming behaviour. Proc Biol Sci. 2012;279:4362–4367. doi: 10.1098/rspb.2012.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Leeuwen EJC, Cronin KA, Haun DBM. A group-specific arbitrary tradition in chimpanzees (Pan troglodytes) Anim Cogn. 2014;17:1421–1425. doi: 10.1007/s10071-014-0766-8. [DOI] [PubMed] [Google Scholar]
- 22.Santorelli CJ, et al. Traditions in spider monkeys are biased towards the social domain. PLoS One. 2011;6:e16863. doi: 10.1371/journal.pone.0016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate’s foraging decisions. Science. 2013;340:483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- 24.Perry S, et al. Social conventions in wild white-faced capuchin monkeys–Evidence for traditions in a neotropical primate. Curr Anthropol. 2003;44:241–268. [Google Scholar]
- 25.Tennie C, Call J, Tomasello M. Ratcheting up the ratchet: On the evolution of cumulative culture. Philos Trans R Soc Lond B Biol Sci. 2009;364:2405–2415. doi: 10.1098/rstb.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry S. Social traditions and social learning in capuchin monkeys (Cebus) Philos Trans R Soc Lond B Biol Sci. 2011;366:988–996. doi: 10.1098/rstb.2010.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Leeuwen EJC, Mundry R, Cronin KA, Bodamer M, Haun DBM. Chimpanzee culture extends beyond matrilineal family units. Curr Biol. 2017;27:R588–R590. doi: 10.1016/j.cub.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Cronin KA, van Leeuwen EJC, Vreeman V, Haun DBM. Population-level variability in the social climates of four chimpanzee societies. Evol Hum Behav. 2014;35:389–396. [Google Scholar]
- 29.McGrew WC, Tutin CEG. Evidence for a social custom in wild chimpanzees? Man (Lond) 1978;13:234–251. [Google Scholar]
- 30.Nakamura M, McGrew WC, Marchant LF, Nishida T. Social scratch: Another custom in wild chimpanzees? Primates. 2000;41:237–248. doi: 10.1007/BF02557594. [DOI] [PubMed] [Google Scholar]
- 31.Sapolsky RM, Share LJ. A pacific culture among wild baboons: Its emergence and transmission. PLoS Biol. 2004;2:E106. doi: 10.1371/journal.pbio.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapolsky RM. Social cultures among nonhuman primates. Curr Anthropol. 2006;47:641–656. [Google Scholar]
- 33.Watson CFI, Buchanan-Smith HM, Caldwell CA. Call playback artificially generates a temporary cultural style of high affiliation in marmosets. Anim Behav. 2014;93:163–171. [Google Scholar]
- 34.Koops K, Visalberghi E, van Schaik CP. The ecology of primate material culture. Biol Lett. 2014;10:20140508. doi: 10.1098/rsbl.2014.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitehead H. Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. Univ of Chicago Press; Chicago: 2008. [Google Scholar]
- 36.Richerson PJ, Boyd R. Not by Genes Alone: How Culture Transformed Human Evolution. Univ of Chicago Press; Chicago: 2005. [Google Scholar]
- 37.CoussiKorbel S, Fragaszy DM. On the relation between social dynamics and social learning. Anim Behav. 1995;50:1441–1453. [Google Scholar]
- 38.Melis AP, Hare B, Tomasello M. Engineering cooperation in chimpanzees: Tolerance constraints on cooperation. Anim Behav. 2006;72:275–286. [Google Scholar]
- 39.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr Biol. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 40.Koops K, Schöning C, Isaji M, Hashimoto C. Cultural differences in ant-dipping tool length between neighbouring chimpanzee communities at Kalinzu, Uganda. Sci Rep. 2015;5:12456. doi: 10.1038/srep12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laland KN, Janik VM. The animal cultures debate. Trends Ecol Evol. 2006;21:542–547. doi: 10.1016/j.tree.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Boyd R, Richerson P. Culture and the Evolutionary Process. Univ of Chicago Press; Chicago: 1985. [Google Scholar]
- 43.Bowles S, Gintis H. A Cooperative Species : Human Reciprocity and Its Evolution. Princeton Univ Press; Princeton: 2011. [Google Scholar]
- 44.Boyd R, Richerson PJ. Culture and the evolution of human cooperation. Philos Trans R Soc Lond B Biol Sci. 2009;364:3281–3288. doi: 10.1098/rstb.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richerson P, et al. Cultural group selection plays an essential role in explaining human cooperation: A sketch of the evidence. Behav Brain Sci. 2016;39:e30. doi: 10.1017/S0140525X1400106X. [DOI] [PubMed] [Google Scholar]
- 46.Mitani JC. Cooperation and competition in chimpanzees: Current understanding and future challenges. Evol Anthropol. 2009;18:215–227. [Google Scholar]
- 47.Suchak M, et al. How chimpanzees cooperate in a competitive world. Proc Natl Acad Sci USA. 2016;113:10215–10220. doi: 10.1073/pnas.1611826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson ML, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513:414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- 49.Bell AV, Richerson PJ, McElreath R. Culture rather than genes provides greater scope for the evolution of large-scale human prosociality. Proc Natl Acad Sci USA. 2009;106:17671–17674. doi: 10.1073/pnas.0903232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiten A, et al. Transmission of multiple traditions within and between chimpanzee groups. Curr Biol. 2007;17:1038–1043. doi: 10.1016/j.cub.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 51.Subiaul F, Vonk J, Okamoto-Barth S, Barth J. Do chimpanzees learn reputation by observation? Evidence from direct and indirect experience with generous and selfish strangers. Anim Cogn. 2008;11:611–623. doi: 10.1007/s10071-008-0151-6. [DOI] [PubMed] [Google Scholar]
- 52.Wittig RM, Crockford C, Langergraber KE, Zuberbühler K. Triadic social interactions operate across time: A field experiment with wild chimpanzees. Proc Biol Sci. 2014;281:20133155. doi: 10.1098/rspb.2013.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelmann JM, Herrmann E, Tomasello M. Five-year olds, but not chimpanzees, attempt to manage their reputations. PLoS One. 2012;7:e48433. doi: 10.1371/journal.pone.0048433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Waal FB, Johanowicz DL. Modification of reconciliation behavior through social experience: An experiment with two macaque species. Child Dev. 1993;64:897–908. [PubMed] [Google Scholar]
- 55.de Waal FBM. Macaque social culture: Development and perpetuation of affiliative networks. J Comp Psychol. 1996;110:147–154. doi: 10.1037/0735-7036.110.2.147. [DOI] [PubMed] [Google Scholar]
- 56.Schino G, Aureli F. Reciprocal altruism in primates: Partner choice, cognition, and emotions. Adv Study Behav. 2009;39:45–69. [Google Scholar]
- 57.Pike TW, Samanta M, Lindström J, Royle NJ. Behavioural phenotype affects social interactions in an animal network. Proc R Soc B. 2008;275:2515–2520. doi: 10.1098/rspb.2008.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cantor M, Whitehead H. How does social behavior differ among sperm whale clans? Mar Mammal Sci. 2015;31:1275–1290. [Google Scholar]
- 59.Borgeaud C, Sosa S, Bshary R, Sueur C, van de Waal E. Intergroup variation of social relationships in wild vervet monkeys: A dynamic network approach. Front Psychol. 2016;7:915. doi: 10.3389/fpsyg.2016.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantor M, et al. Multilevel animal societies can emerge from cultural transmission. Nat Commun. 2015;6:8091. doi: 10.1038/ncomms9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aplin LM, et al. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature. 2014;518:538–541. doi: 10.1038/nature13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cantor M, Whitehead H. The interplay between social networks and culture: Theoretically and among whales and dolphins. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120340. doi: 10.1098/rstb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Leeuwen EJC, Cronin KA, Schütte S, Call J, Haun DBM. Chimpanzees (Pan troglodytes) flexibly adjust their behaviour in order to maximize payoffs, not to conform to majorities. PLoS One. 2013;8:e80945. doi: 10.1371/journal.pone.0080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vale GL, et al. Lack of conformity to new local dietary preferences in migrating captive chimpanzees. Anim Behav. 2017;124:135–144. doi: 10.1016/j.anbehav.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Leeuwen EJC, Acerbi A, Kendal RL, Tennie C, Haun DBM. A reappreciation of “conformity”. Anim Behav. 2016;122:e5–e10. [Google Scholar]
- 66.Boesch C. Wild Cultures: A Comparison Between Chimpanzee and Human Cultures. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 67.Watts D. The apes; Taxonomy, biogeography, life history, and behavioral ecology. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Univ of Chicago Press; Chicago: 2015. pp. 113–142. [Google Scholar]
- 68.Sponheimer M, Lee-Thorpe JA, Reed KE, Ungar P. Early Hominin Paleoecology. Univ Press of Colorado; Boulder, CO: 2013. [Google Scholar]
- 69.Cronin KA. Comparative studies of cooperation: Collaboration and prosocial behavior in animals. In: Call J, Burghardt GB, Pepperberg I, Snowdon CT, Zental T, editors. APA Handbook of Comparative Psychology. Am Psychol Assoc; Washington, DC: 2017. pp. 915–929. [Google Scholar]
- 70.Jensen K, Hare B, Call J, Tomasello M. What’s in it for me? Self-regard precludes altruism and spite in chimpanzees. Proc Biol Sci. 2006;273:1013–1021. doi: 10.1098/rspb.2005.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tennie C, Jensen K, Call J. The nature of prosociality in chimpanzees. Nat Commun. 2016;7:13915. doi: 10.1038/ncomms13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Claidière N, et al. Selective and contagious prosocial resource donation in capuchin monkeys, chimpanzees and humans. Sci Rep. 2015;5:7631. doi: 10.1038/srep07631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horner V, Carter JD, Suchak M, de Waal FBM. Spontaneous prosocial choice by chimpanzees. Proc Natl Acad Sci USA. 2011;108:13847–13851. doi: 10.1073/pnas.1111088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silk JB, et al. Chimpanzees are indifferent to the welfare of unrelated group members. Nature. 2005;437:1357–1359. doi: 10.1038/nature04243. [DOI] [PubMed] [Google Scholar]
- 75.Martin PR, Bateson PPG. Measuring Behaviour: An Introductory Guide. Cambridge Univ Press; Cambridge, UK: 2007. [Google Scholar]
- 76.Nishida T, Kano T, Goodall J, McGrew WC, Nakamura M. Ethogram and ethnography of Mahale chimpanzees. Anthropol Sci. 1999;107:141–188. [Google Scholar]
- 77.Whitehead H. SOCPROG programs: Analysing animal social structures. Behav Ecol Sociobiol. 2009;63:765–778. [Google Scholar]
- 78.Cairns SJ, Schwager SJ. A comparison of association indices. Anim Behav. 1987;35:1454–1469. [Google Scholar]
- 79.Wakefield ML. Social dynamics among females and their influence on social structure in an East African chimpanzee community. Anim Behav. 2013;85:1303–1313. [Google Scholar]
- 80.Newman MEJ. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kasper C, Voelkl B. A social network analysis of primate groups. Primates. 2009;50:343–356. doi: 10.1007/s10329-009-0153-2. [DOI] [PubMed] [Google Scholar]
- 82.Snaith TV, Chapman CA. Primate group size and interpreting socioecological models: Do folivores really play by different rules? Evol Anthropol. 2007;16:94–106. [Google Scholar]
- 83.Maldonado-Chaparro AA, Hubbard L, Blumstein DT. Group size affects social relationships in yellow-bellied marmots (Marmota flaviventris) Behav Ecol. 2015;26:909–915. [Google Scholar]
- 84.Bates D, Maechler M, Bolker B. 2013 lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-2. Available at CRANR-project.org/package=lme4.
- 85.Dobson AJ. An Introduction to Generalized Linear Models. Chapman & Hall/CRC; Boca Raton, FL: 2002. [Google Scholar]
- 86.Forstmeier W, Schielzeth H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol. 2011;65:47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J Mem Lang. 2013;68:255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.R Core Team 2017. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna)
- 89.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.