Significance
Finding that “innate” natural killer cells possessed T cell-like immune memory was an unprecedented discovery that rocked our understanding of the divide between innate and adaptive immunity. In this report we demonstrate that Ly49 receptors on natural killer cells, which engage the class I antigen-presenting platform on target cells, are critical components of adaptive natural killer cell memory. We also demonstrate the clinical potential of natural killer cell memory, using it to mediate a cancer vaccine against melanoma. These findings offer insights into how adaptive natural killer cell memory might function and what clinical applications this mysterious immune response might have.
Keywords: natural killer cells, immune memory, adaptive memory, cancer vaccines, Ly49 receptors
Abstract
Adaptive natural killer (NK) cell memory represents a new frontier in immunology. Work over the last decade has discovered and confirmed the existence of NK cells with antigen-specific memories, which had previously been considered a unique property of T and B cells. These findings have shown that antigen-specific NK cells gain their specificity without the use of RAG proteins, representing a novel mechanism for generating antigen specificity, but the details of this mechanism have remained a mystery. We have discovered that members of the Ly49 family of surface receptors are critically involved in both the sensitization and the challenge phases of an NK cell memory response, as is antigen presentation from their binding partner, the class I MHC. Moreover, we demonstrate that the Ly49-interacting component of a presented antigen dictates the specificity of the NK cell memory response, implicating Ly49 receptors themselves in antigen-specific recognition. Finally, we demonstrate that adaptive NK cell memories can protect against an otherwise lethal melanoma without T cell or B cell support. These findings offer insight into the mechanism behind NK cell antigen specificity and demonstrate the clinical potential of this adaptive immune cell.
Adaptive immunity allows immune cells to remember a specific pathogen and direct a powerful immune response upon reexposure. It is the basis for all vaccines and is responsible for many medical success stories from the past two centuries. T and B cells comprise the adaptive immune system and were considered the only immune cells able to form adaptive immune memories. Recent findings challenge this paradigm (1), suggesting that natural killer (NK) cells—innate immune cells responsible for virus and cancer immunity—also possess adaptive immune features, from enhanced recall responsiveness (2) and memory pool formation (3) to a full, T cell-like ability to mediate antiviral vaccination (4).
The NK cell’s enhanced recall responsiveness upon cytokine treatment and ability to form a memory pool in response to cytomegalovirus infection are both fairly well understood. However, these phenomena depend on germline-encoded receptors with defined cytokine or antigen-binding partners. In contrast, adaptive NK memory cells are able to recognize chemical haptens (5, 6) or virus peptides such as HIV glycoprotein (4), which are not mouse pathogens and so likely have no germline receptor in mice. Unlike T and B cells, NK cells do not use the recombination-activating gene (RAG) complex to generate diverse, antigen-specific receptors (5) and so must use a novel method of antigen specificity. In the absence of a recombining receptor in NK cells, we hypothesized that the Ly49 family of class I major histocompatibility complex (MHC-I) receptors (encoded by the Klra genes) could offer insight into the mechanisms behind NK cell antigen specificity, since MHC-I is a binding partner of the antigen-specific T cell receptor as well. Moreover, adaptive NK cells from C57BL/6 mice always express Ly49C or Ly49I (5), the only two Ly49 receptors that engage MHC-I in this mouse (7).
Results
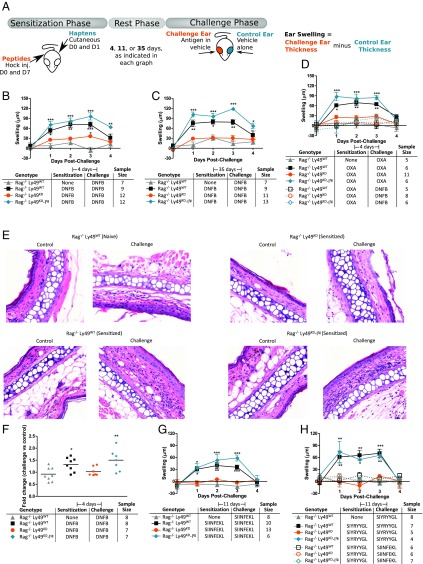
We crossed two lines of Ly49-modulated mice (8) onto the Rag1−/− background (9) to remove T and B cells. The resulting Rag1−/− Ly49KD mice have dramatically reduced expression of all Ly49 family members (8) in addition to lacking all T and B cells, while the Rag1−/− Ly49KD-Itg mice have been transgenically rescued with Ly49I, making all NK cells in these T and B cell-deficient mice express Ly49I alone (SI Appendix, Fig. S1). We then tested adaptive memory in these mice using the mouse ear swelling test (Fig. 1A). Rag1−/− mice reacted to the chemical hapten 2,4-dinitrofluorobenzene (DNFB) only if they had been previously exposed to the hapten, indicating memory (Fig. 1 B and C). This memory was lost in Rag1−/− Ly49KD mice but was completely rescued by restoring Ly49I. This Ly49-dependent memory was also antigen-specific, as memories to another hapten, oxazolone (OXA), did not cross-react with memories to DNFB (Fig. 1D).
Fig. 1.
Ly49I expression is required for adaptive, T cell- and B cell-independent memory. (A) Schematic of the experimental ear swelling test. (B and C) Ear swelling following hapten challenge of T cell- and B cell-deficient mice with the indicated Ly49 modulations. Short-term (B) and long-term (C) memory is indicated. (D) Ear swelling for mice challenged with either the sensitizing hapten (solid lines) or an irrelevant hapten (dotted lines). (E and F) H&E staining of infiltrating cells into ears taken at day 2 of the ear swelling response as in B. (Magnification: E, 20×.) Each point in F represents the median value obtained across seven fields in E. Horizontal bars represent the mean value of these medians. (G and H) Ear swelling results using the indicated peptide antigens. *P < 0.05; **P < 0.01; ***P < 0.001.
To rule out technical bias in these results, we correlated the ear swelling data with the degree of cellular infiltration into challenged ears (Fig. 1 E and F). Similarly, to rule out artifacts introduced by using nonphysiological antigens such as chemical haptens, we repeated these ear swelling experiments using two peptide antigens, SIINFEKL and SIYRYYGL, with similar MHC-I affinity (10), again finding that NK cells display an antigen-specific, Ly49I-dependent memory for several peptides (Fig. 1 G and H).
Although some CD8+ T cells do express Ly49C/I (11), these cells are absent from our T cell-deficient mice, so our results are consistent with an NK-mediated phenomenon. Moreover, numerous adoptive transfer studies in which NK cells from a sensitized mouse are introduced into a naive mouse, which is then tested for a transferred memory, have shown that NK cells are responsible for adaptive memory in the absence of T and B cells (3–6, 12). Nevertheless, we proceeded to corroborate these findings and demonstrate that our Ly49I-dependent memory was indeed NK cell mediated. Antibody-mediated depletion of NK cells from Rag1−/− mice completely abrogated the ear swelling response, while treatment with an irrelevant antibody had no effect (Fig. 2A and SI Appendix, Fig. S2). Because the Rag1−/− mice represent a severe immune remodeling that could have unanticipated effects on NK cells (13), we repeated this experiment in CD8α−/− mice, which lack CD8+ T cells but have normal CD4+ T cell and B cell levels and display normal humoral immune responses (14). Again, NK depletion in CD8α−/− mice was sufficient to eliminate memory responses to DNFB, while depletion of CD4+ cells had no effect (Fig. 2B), consistent with previous findings that CD8+ and not CD4+ T cells mediate the response to DNFB in mice (15). We also confirmed in both CD4−/− and MHC-II−/− mice that the DNFB ear swelling response is intact, as expected (SI Appendix, Fig. S3).
Fig. 2.
NK cells mediate Ly49-dependent adaptive immunity. (A and B) Ear swelling in Rag1−/− (A) or CD8+ T cell-deficient (B) mice after depletion of NK cells (NK1.1) or all CD4+ cells (CD4). (C) Ear swelling in Rag1−/− or WT mice after depletion of all Ly49C/I+ cells, or Ly49G+ cells as a control. (D) Ear swelling following IFNγ neutralization (using XMG1.2 antibody) in Rag1−/− and WT mice. (E) Ear swelling in Rag1−/− mice lacking perforin. Results are pooled from two experiments. (F and G) Ear sections collected as in Fig. 1 were stained with anti-NKp46 to reveal NK infiltration. (Magnification: G, 20×; G Inset, 60×.) Each point in F represents the sum count of stained cells across five fields. Horizontal bars represent the mean value of these sums. Arrowheads in G represent NK cells. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
We also performed antibody-mediated depletions of all Ly49C/I+ cells or all Ly49G+ cells in Rag1−/− or normal WT mice (Fig. 2C and SI Appendix, Fig. S2). As expected, Ly49C/I depletion throughout the sensitization and challenge phases was sufficient to prevent memory in Rag1−/− mice, while depleting the irrelevant Ly49G+ cells had no effect. Neither depletion was effective in WT mice, indicating that this Ly49C/I-dependent memory is distinct from classical T cell-mediated memory, and so residual or contaminating T cells cannot account for the Ly49-dependent memory we observe in Rag1−/− mice. Similarly, we found that NK memory responses fully depended on IFNγ, in agreement with previous genetic work (12), while T cell memory did not (Fig. 2D). IFNγ appears to be the principle mediator of adaptive NK responses, because perforin was dispensable for NK-mediated ear swelling (Fig. 2E). Finally, we repeated the histological examination of inflamed ears described above but this time stained specifically for infiltrating NK cells and confirmed that NK cells are significantly enriched in inflamed vs. nonresponsive challenged ears (Fig. 2 F and G), again implicating NK cells as mediators of the adaptive memory response in the absence of T cells.
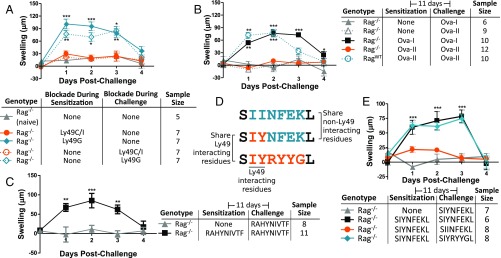
We next sought to determine why Ly49C/I are required for the memory response. Ly49C/I mediate a developmental maturation process known as “NK cell education” or “NK cell licensing” (16–18). It was possible that Ly49C/I were required for their ability to license an NK cell and not for a direct role during the sensitization or challenge phases of the memory response. We repeated the ear swelling assay using nondepleting F(ab′)2 fragments of the Ly49C/I and Ly49G antibodies, which would interfere with their target Ly49’s binding ability but would not deplete the Ly49-expressing, licensed NK cells (SI Appendix, Fig. S4). To our surprise, antibody blockade of Ly49C/I—but not Ly49G—during either the sensitization or challenge phase separately was sufficient to prevent NK cell memory responses, indicating that Ly49C/I play some direct role in the adaptive immune response beyond NK licensing (Fig. 3A).
Fig. 3.
Ly49C/I are involved in direct antigen recognition. (A) Ear swelling following nondepleting antibody-mediated blockade of Ly49C/I or Ly49G during sensitization or challenge phases as indicated. All groups were sensitized and challenged with DNFB except the naive group, which was only challenged. (B) Ear swelling in Rag1−/− or WT mice following challenge with either the MHC-I–restricted ovalbumin peptide SIINFEKL (Ova-I) or the MHC-II–restricted peptide ISQAVHAAHAEINEAGR (Ova-II). (C) Ear swelling in Rag1−/− mice following challenge with the H-2Db–restricted HPV-E7 peptide RAHYNIVTF. (D and E) Schematic demonstrating the hybrid peptide design (D) used to sensitize mice in a repeat of the ear swelling test (E). Mice were then challenged with the indicated peptide and examined for cross-reactivity with the hybrid peptide. *P < 0.05; **P < 0.01; ***P < 0.001.
Because Ly49C/I interact with MHC-I, which is itself required for classical antigen presentation, we repeated the ear swelling test in mice lacking MHC-I expression but with normal Ly49, confirming that NK cells, like their T cell counterparts, require MHC-I expression for adaptive memory responses (SI Appendix, Fig. S5). Unfortunately, this MHC-I–deficient model also results in developmentally unlicensed NK cells (17). To correct for this and to determine whether MHC-I must present antigen during the NK cell memory response, we sensitized mice to MHC-I–restricted or MHC-II–restricted ovalbumin peptides (Fig. 3B). The MHC-I–restricted peptide provoked NK cell memory as expected, but the MHC-II–restricted peptide did not, despite being able to provoke T cell memory. Taken together, these results indicate that NK cell memory depends on Ly49’s ability to bind MHC-I and on MHC-I’s ability to present antigen. Along similar lines, we tested the ability of an H-2Db restricted peptide, RAHYNIVTF (19), to provoke NK memory responses and confirmed that H-2Db is just as capable as H-2Kb at presenting peptide to NK cells (Fig. 3C).
Because Ly49C/I binding and MHC-I antigen presentation were both required during adaptive NK cell memory responses, and because Ly49C/I interact with MHC-I, it was possible that Ly49C/I were directly involved in antigen recognition. Ly49C+ cells have been reported to be sensitive to residues 2 and 3 of an MHC-I–presented peptide (20). We hypothesized that if Ly49C/I were responsible for the NK cell antigen specificity, then peptides that shared residues 2 and 3 would cross-react. Since we had already shown that the peptides SIINFEKL and SIYRYYGL do not cross-react, we sensitized mice to a hybrid peptide with the sequence SIYNFEKL and then challenged them with either of the two peptides (Fig. 3D). SIYRYYGL cross-reacted with the hybrid peptide, indicating that the antigen specificity of adaptive NK responses depends on the Ly49-sensitive residues of the presented peptide (Fig. 3E ). This result implicates Ly49 directly in the antigen-recognition process, suggesting an Ly49-centric mechanism for adaptive NK cell memory.
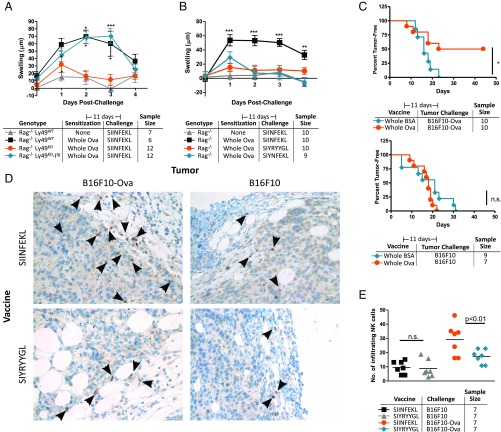
Finally, a recent publication has identified a hapten that can provoke NK cell memory to melanoma (6). We sought to confirm our findings and recapitulate these results using a more clinically relevant protein vaccine. To date, our experiments have used preprocessed antigenic peptides, so it is possible that the NK cell responses we observed were an artificial reaction to processed peptides and not the result of a physiological adaptive response. However, we found that NK cells sensitized to whole ovalbumin could react to SIINFEKL challenge (Fig. 4A) but not to SIYNFEKL or SIYRYYGL (Fig. 4B), indicating that an active immune process was converting ovalbumin to SIINFEKL and presenting it to NK cells in these mice and that protein vaccines could provoke adaptive NK cell responses.
Fig. 4.
NK memory can mediate conventional cancer vaccines. (A and B) Ear swelling in Rag1−/− mice following sensitization with whole ovalbumin (Whole Ova) protein and challenge with the MHC-I–restricted fragment of whole ovalbumin, SIINFEKL (A), or variant or irrelevant peptides as in Fig. 3 (B). (C) Tumor growth in Rag1−/− mice following vaccination with the indicated whole protein and flank challenge with 1 × 105 B16F10 melanoma cells transgenically expressing whole ovalbumin or parental, non–antigen-expressing cells as a control. Results are pooled from two experiments. (D) Immunohistology of tumors at end point, performed and quantified as in Fig. 2. (Magnification: D, 20×.) (E) Statistical analysis for the tumor infiltration was performed by one-way ANOVA followed by a Bonferroni post hoc test comparing members of the same challenge group. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
We then vaccinated Rag1−/− mice with whole ovalbumin as a surrogate tumor antigen or with bovine albumin as a negative control and challenged them with B16F10 melanoma either expressing the ovalbumin antigen or not (Fig. 4C). Amazingly, 50% of the vaccinated T cell-deficient mice were completely protected from the antigen-expressing cancer, remaining tumor-free throughout the experiment. Curiously, the tumor growth rate of the vaccinated but unprotected mice was not different from that of the unvaccinated groups, suggesting that protection was either complete or nonexistent (SI Appendix, Fig. S6). Vaccines using single peptides were less effective, showing only a modest reduction in cancer growth (SI Appendix, Fig. S7). However, this allowed histological examination of the tumors, confirming that NK cells from vaccinated mice were significantly enriched in antigen-expressing tumors (Fig. 4 D and E).
Discussion
In this report, we show by genetic and antibody disruption that Ly49C/I receptors are required for and are intimately involved in adaptive NK cell memory and may be directly responsible for the observed NK cell antigen specificity. Indeed, primates display adaptive NK memory (21), and killer cell Ig-like receptors—the human analog to Ly49—are also peptide sensitive (22); a similar phenomenon may well exist in humans. We also show that adaptive NK cell memory can mediate potent cancer vaccines. This finding is particularly exciting for cancer immune therapies, because NK cells are less prone than T cells to autoimmune reactions and so may be safer targets when provoking immune responses against self-antigens (23).
Our discovery that Ly49C/I are directly implicated in adaptive antigen specificity is only the first piece of the NK antigen-specificity puzzle. However, it raises the possibility that NK specificity arises from fine-tuning Ly49C/I’s antigen sensitivity and not from any as yet undiscovered NK cell recombining receptor. One intriguing possibility is that something akin to the specific self/nonself discrimination employed by the tunicate Botryllus schlosseri is at play. This tunicate is hypothesized to assemble splice variants of the fester receptor in specific microclusters on its surface, so that each microcluster has higher affinity for self than for nonself histocompatibility molecules and therefore can distinguish between the two (24). While Ly49 receptors are not known to exist in many alternately spliced forms, the memory NK cell may fine-tune its sensitivity by adjusting the expression of either Ly49C/I or MHC-I so that the Ly49 receptors are more likely to bind to self-peptide–presenting cis MHC-I molecules than to non–self-presenting trans MHC-I, identifying their target antigen by its relative affinity for Ly49 compared with self-peptide–bound MHC-I. However, this assumes that nonself peptides have lower Ly49C/I affinity than self peptides, which requires further investigation. Alternatively, Ly49C/I may use the same activating pathways that induce the increased responsiveness observed in licensed NK cells following Ly49C/I stimulation. In either case, evaluating whether Ly49 peptide affinities can explain the observed antigen specificity in adaptive NK cell memory is now a top priority if we are to uncover how this surprising immune response functions.
Methods
Complete methods can be found in SI Appendix.
Mice.
Age-matched males and females were used in all experiments. Mice were from the C57BL/6 background, either generated on that background or backcrossed to it at least 10 times. For the Ly49KD-Itg mice, the Ly49I transgene was maintained as a heterozygote, and transgene-negative animals were used as Ly49KD controls. All breeding and manipulations were approved by the University of Ottawa Animal Care Committee and Dalhousie University’s University Committee on Laboratory Animals.
Mouse Ear Swelling Test.
For contact hypersensitivity, mice were sensitized to 0.5% DNFB (MP Biomedicals) in acetone or 5% 4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one (Oxa; Sigma-Aldrich) in 1:1 acetone/methanol. Naive controls were sensitized to the appropriate vehicle. For peptide reactions, mice were sensitized to the indicated peptides or proteins or to the adjuvant alone. Mice were sensitized by hock injection of 25 nM of peptide in Complete Freund’s Adjuvant (Sigma-Aldrich). Challenges were performed with the appropriate hapten carrier or sterile PBS for peptides in the ear, far from the site of sensitization.
Tumor Vaccine and Challenge.
Mice were vaccinated using the peptide or whole protein in Complete Freund’s Adjuvant emulsion as above and were challenged with 1 × 105 B16F10 cells, with or without ovalbumin expression, by s.c. flank injection.
Statistical Analysis.
Unless otherwise indicated, ear swelling data were analyzed by two-way repeated-measures ANOVA followed by Bonferroni post hoc test. Cell infiltration counts were analyzed by Kruskal–Wallis test followed by Dunnett’s post hoc test comparing all groups with the naive control. Tumor-free survival was analyzed by the rank-sum test. Data are plotted as mean ± SE or as individual data points with means indicated by a solid bar.
Supplementary Material
Acknowledgments
We thank Yizhou Situ, the University of Ottawa Animal Care and Veterinary Services, the University of Ottawa Flow Cytometry and Virometry Core, and the University of Ottawa Department of Pathology and Laboratory Medicine Histology Facility for technical assistance; Drs. Simon Bélanger, Lee-Hwa Tai, Jeanette Boudreau, Brent Johnston, David Hoskin, and Craig McCormick for editorial comments; Drs. James Carlyle, Charles Sentman, and Jean-Simon Diallo for contributing cell lines; and IMV Inc. for contributing a peptide. This work was funded by Canadian Institutes of Health Research Grant MOP 62841 and by Canadian Cancer Society Research Institute Innovation Grant 702450 and Innovation to Impact Grant 703935-1. A.W. is supported by a Queen Elizabeth II-Graduate Scholarship in Science or Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.C. is a guest editor invited by the Editorial Board.
See Commentary on page 11357.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722374115/-/DCSupplemental.
References
- 1.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: Walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 6.van den Boorn JG, et al. Inflammasome-dependent induction of adaptive NK cell memory. Immunity. 2016;44:1406–1421. doi: 10.1016/j.immuni.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Hanke T, et al. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11:67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 8.Bélanger S, et al. Impaired natural killer cell self-education and “missing-self” responses in Ly49-deficient mice. Blood. 2012;120:592–602, and erratum (2013) 122:2525. doi: 10.1182/blood-2012-02-408732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 10.Engels B, et al. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell. 2013;23:516–526. doi: 10.1016/j.ccr.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coles MC, McMahon CW, Takizawa H, Raulet DH. Memory CD8 T lymphocytes express inhibitory MHC-specific Ly49 receptors. Eur J Immunol. 2000;30:236–244. doi: 10.1002/1521-4141(200001)30:1<236::AID-IMMU236>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Majewska-Szczepanik M, Paust S, von Andrian UH, Askenase PW, Szczepanik M. Natural killer cell-mediated contact sensitivity develops rapidly and depends on interferon-α, interferon-γ and interleukin-12. Immunology. 2013;140:98–110. doi: 10.1111/imm.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karo JM, Schatz DG, Sun JC. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell. 2014;159:94–107. doi: 10.1016/j.cell.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung-Leung WP, et al. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 15.Bour H, et al. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 16.Bix M, et al. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 17.Höglund P, et al. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: Nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci USA. 1991;88:10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu YYL, et al. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4:67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 19.Feltkamp MCW, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 20.Marquez EA, Kane KP. Identities of P2 and P3 residues of H-2Kb-bound peptides determine mouse Ly49C recognition. PLoS One. 2015;10:e0131308. doi: 10.1371/journal.pone.0131308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves RK, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16:927–932. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malnati MS, et al. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267:1016–1018. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 23.Olson JA, et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taketa DA, De Tomaso AW. Botryllus schlosseri allorecognition: Tackling the enigma. Dev Comp Immunol. 2015;48:254–265. doi: 10.1016/j.dci.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.