Significance
Alzheimer’s disease (AD) is the most common cause of age-related neurodegeneration. Damage initially occurs in the hippocampus, a neurogenic brain region essential in forming memories. Since there is no cure for AD, therapeutic strategies that may aid to slow hippocampal dysfunction are necessary. We describe the precocious hippocampal stem cell loss of a mouse model that mimics the onset of pathological AD-like neurodegeneration. The loss is due to an increase in BMP6 that limits neurogenesis. We demonstrate that blocking BMP signaling by means of Noggin administration is beneficial to the hippocampal microenvironment, restoring stem cell numbers, neurogenesis, and behavior. Our findings support further development of BMP antagonists into translatable molecules for the rescue of stem cells and neurogenesis in neurodegeneration/aging.
Keywords: neural stem cells, adult neurogenesis, aging, Alzheimer’s disease, BMP signaling
Abstract
Increasing age is the greatest known risk factor for the sporadic late-onset forms of neurodegenerative disorders such as Alzheimer’s disease (AD). One of the brain regions most severely affected in AD is the hippocampus, a privileged structure that contains adult neural stem cells (NSCs) with neurogenic capacity. Hippocampal neurogenesis decreases during aging and the decrease is exacerbated in AD, but the mechanistic causes underlying this progressive decline remain largely unexplored. We here investigated the effect of age on NSCs and neurogenesis by analyzing the senescence accelerated mouse prone 8 (SAMP8) strain, a nontransgenic short-lived strain that spontaneously develops a pathological profile similar to that of AD and that has been employed as a model system to study the transition from healthy aging to neurodegeneration. We show that SAMP8 mice display an accelerated loss of the NSC pool that coincides with an aberrant rise in BMP6 protein, enhanced canonical BMP signaling, and increased astroglial differentiation. In vitro assays demonstrate that BMP6 severely impairs NSC expansion and promotes NSC differentiation into postmitotic astrocytes. Blocking the dysregulation of the BMP pathway and its progliogenic effect in vivo by intracranial delivery of the antagonist Noggin restores hippocampal NSC numbers, neurogenesis, and behavior in SAMP8 mice. Thus, manipulating the local microenvironment of the NSC pool counteracts hippocampal dysfunction in pathological aging. Our results shed light on interventions that may allow taking advantage of the brain’s natural plastic capacity to enhance cognitive function in late adulthood and in chronic neurodegenerative diseases such as AD.
Decades of research have firmly established that new neurons are produced in the postnatal and adult hippocampus of most mammals, including humans, due to the existence of a stem cell reservoir that confers regenerative capacity to this brain area essential in forming memories (1, 2). Despite the long-lasting plastic potential of the hippocampus, defects in hippocampal neurogenesis emerge during aging (3–6) and are exacerbated in pathological conditions such as Alzheimer’s disease (AD) (7–9). Previous studies indicated that the newly generated cells in aged mice develop at a slower pace (6), have a reduced survival (10, 11), and differentiate less into neurons and more into astrocytes (10–13), but why this defective fate choice occurs and how we might manipulate stem cells or their niche to effectively counteract hippocampal dysfunction upon healthy and pathological aging remains largely unknown.
The neurogenic process involves several sequential steps. First, quiescent neural stem cells (NSCs) with radial morphology located in the subgranular zone (SGZ) of the dentate gyrus (DG) are activated and enter the cell cycle; next, the NSCs asymmetrically divide and produce nonradial (NR) proliferative progenitors that generate the new neurons. Finally, the young neurons mature and integrate into preexisting networks. Extracellular short-range niche signals regulate neurogenesis at all these different stages (14–16) and alterations in the niche microenvironment may be linked to the age-related neurogenic decline (17). A relevant family of secreted factors whose function in the aged SGZ remains poorly defined is the bone morphogenetic protein (BMP) family (18). The expression of several BMPs is dysregulated in the hippocampus of old mice, in transgenic mouse models of familial AD, and in the brains of patients with early and severe AD compared with nondemented controls (19–24), pointing to a causative role of BMP signaling in the neurogenic defects found during aging and in AD.
We here analyze the causes underlying the decline in adult NSCs and neurogenesis during pathological aging, taking advantage of the senescence-accelerated mouse strain SAMP8. This nontransgenic short-lived strain precociously and progressively develops learning and memory deficits and a multisystemic aging phenotype (25–27). SAMP8 also exhibits increased amyloid precursor protein (APP) and amyloid β (Aβ) levels, oxidative stress, tau phosphorylation, astrogliosis, and many of the biochemical findings of AD starting at the age of 6 mo (28–30). Due to the spontaneous onset of pathology similar to that of human disease, SAMP8 is considered a good animal model for research into the transition from healthy aging to the onset phase of sporadic AD (28–31). SAMP8 is evaluated in reference to SAMR1, a genetically related strain that is resistant to accelerated senescence (25). We herein show that SAMP8 mice display an accelerated depletion of the hippocampal NSC pool compared with SAMR1 that coincides in space and time with an increase in astroglial differentiation, a reduction in neurogenesis, an increase in BMP6 protein level, and a dysregulation of the BMP signaling pathway. We also demonstrate that blocking the enhanced BMP signaling of the SAMP8 strain restores normal NSC numbers by counteracting the BMP-mediated NSC astroglial differentiation and simultaneously ameliorates the neurogenic and behavioral deficits of the senescent strain.
Results
SAMP8 Mice Show an Accelerated Depletion of the Adult Hippocampal NSC Pool.
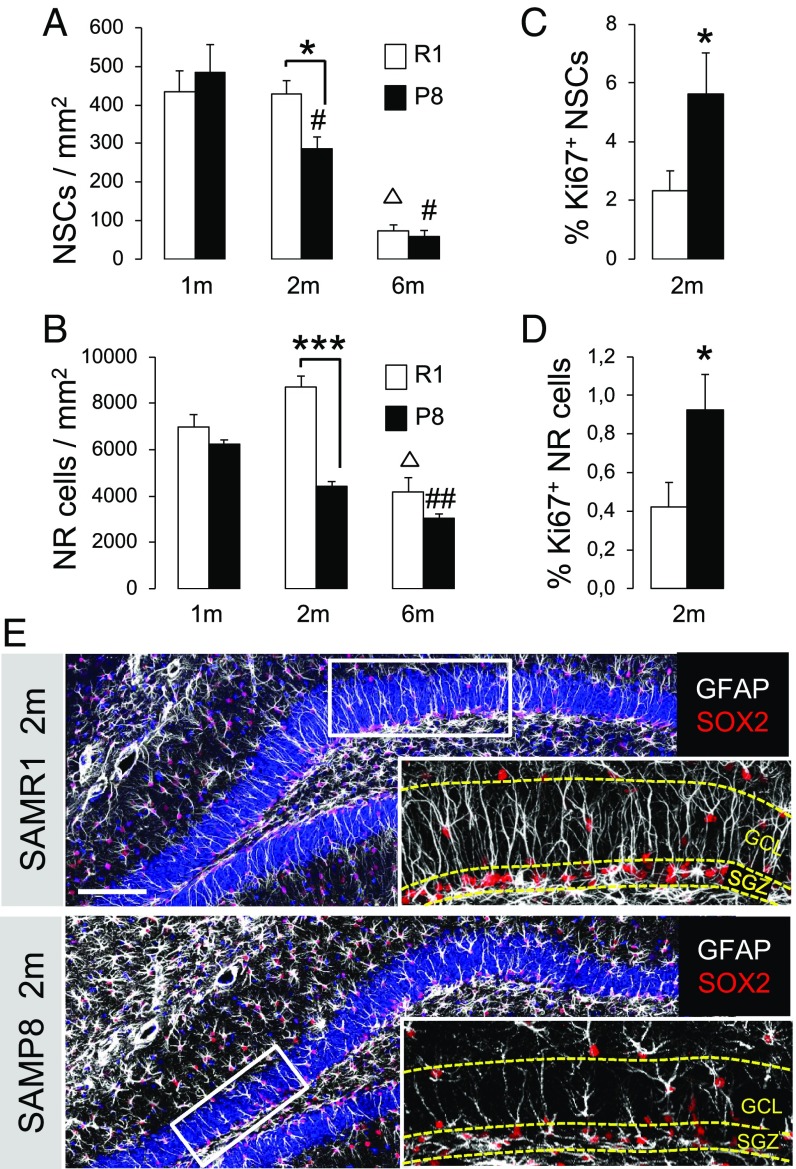
We first measured the time course of stem cell decay in the SGZ of mice with pathological aging (SAMP8) and age-matched control animals with healthy aging (senescence accelerated-resistant mice, SAMR1). We found a progressive age-dependent decrease in SOX2+GFAP+ NSCs with radial morphology in both strains; however, SAMP8 mice exhibited lower radial NSC numbers compared with SAMR1 mice already at the age of 2 mo (Fig. 1 A and E), evidencing the loss of the stem cell population before the onset of AD symptoms (28–30). The total number of SOX2+ cells (SI Appendix, Fig. S1) and SOX2+GFAP− NR progenitors (Fig. 1B) was also lower in 2-mo SAMP8 vs. SAMR1. The proportion of NSCs and NR cells that stained for the cell-cycle marker Ki67 was higher in SAMP8 (Fig. 1 C and D). No statistically significant differences were found in the proportion of NSCs that incorporated the thymidine analog BrdU during S phase (2 h after BrdU injection, P = 0.24) and, consequently, the BrdU/Ki67 ratio [which is inversely proportional to cell-cycle length (32)] was lower in SAMP8 vs. SAMR1. In summary, the stem and progenitor cell pools become precociously depleted in the hippocampus of the senescence model SAMP8 but the remaining NSCs remain proliferative and apparently lengthen their cell cycle.
Fig. 1.
NSCs and progenitor (NR) cells are precociously depleted in the hippocampus of the SAMP8 model. (A and B) SAMP8 animals show a decreased number of SOX2+GFAP+ radial NSC (A) and SOX2+GFAP− NR cells (B) compared with 2-mo SAMR1 (*P < 0.05 and ***P < 0.001, respectively). Both strains show an age-related reduction of these cell populations (SAMR1: ΔP < 0.05; SAMP8: #P < 0.05, ##P < 0.01). (C and D) Two-month SAMP8 animals exhibit a higher percentage of actively dividing Ki67+ radial NSCs (C) and NR cells (D) vs. SAMR1 (*P < 0.05). (E) Representative coronal micrograph showing the reduction of the number of radial NSCs and NR cells in 2-mo SAMP8 strain vs. SAMR1. (Scale bar, 100 μm.) (Magnification: E, Insets, 5×.)
The Newly Generated Hippocampal Cells Fail to Undergo Normal Differentiation and Survival in SAMP8 Mice.
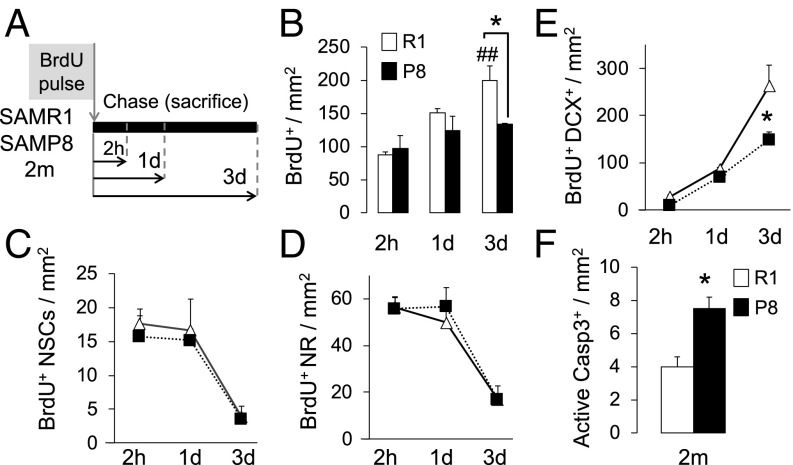
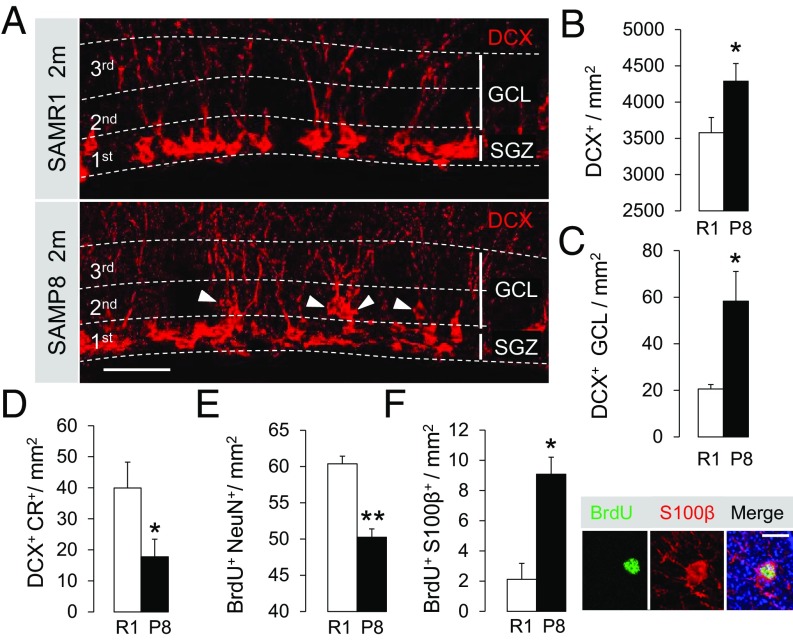
To analyze the dynamics of neuronal production in 2-mo SAMP8 and SAMR1 animals, we employed a BrdU pulse-chase approach. BrdU incorporates into the newly synthesized DNA during cell division, enabling the labeling of dividing cells at the time of injection and their progenies after prolonged chase periods (33). We performed a single injection (pulse) of BrdU and chased the analog at 2 h, 1 d, and 3 d postpulse (Fig. 2A). The total number of BrdU+ cells in the SGZ was similar between both strains at the 2-h and 1-d chase time points but decreased in SAMP8 animals later on (Fig. 2B). The number of BrdU+ NSCs and BrdU+ NR cells was similar for both strains and there were no differences in their temporal dynamics, suggesting that the cells give rise to new progeny at a similar pace (Fig. 2 C and D). When we examined the DCX+ immature neuron compartment, we found equal numbers of newly generated BrdU+DCX+ cells at 2 h and 1 d postpulse but a significant reduction at 3 d, pointing to a loss of the newborn neurons as they progress toward maturation (Fig. 2E). In accordance with this finding, we detected a rise in the number of active Caspase3+ apoptotic cells in SAMP8 mice (Fig. 2F). Concomitant with the loss of the newborn BrdU+DCX+ neurons, we found an abnormal accumulation of the DCX+ cell population (Fig. 3 A and B). Not only was the total number of DCX+ cells abnormally increased in SAMP8 animals but also their location was altered (Fig. 3 A–C). In animals with pathological aging, DCX+ cells migrated deeper into the granule cell layer (GCL) and were aberrantly positioned. Moreover, the number of DCX+ cells that acquired the more mature neuronal marker calretinin (CR) was decreased (Fig. 3D). Overall, the data suggest that the increase in cell death of the newly generated cells is accompanied by a failure to undergo a normal neuronal differentiation program in the SAMP8 microenvironment. To confirm whether this defect finally results in a reduction in the number of new granule neurons reaching maturity, we injected another cohort of animals with BrdU and killed it 3 wk later. The number of newly generated NeuN+BrdU+ neurons that had matured and integrated into the DG was significantly reduced in the SAMP8 strain (Fig. 3E). Notably, concomitant with the decrease in neurogenesis at 2 mo, we also detected a fourfold increase in the number of newly generated S100b+BrdU+ astrocytes (Fig. 3F), suggesting that the loss of the stem cell population in SAMP8 mice could be due to their preferential differentiation into mature astrocytic cells. This cellular output is in accordance with previous findings in aged animals (10–13).
Fig. 2.
Newborn neurons are lost in 2-mo SAMP8 animals. (A) Pulse-chase experimental design using BrdU. Animals were killed at 2 h (2h), 1 d (1d) and 3 d (3d) after BrdU injection. (B) BrdU+ cells at the chase time points after injection. SAMP8 animals show a reduction at 3 d vs. SAMR1 (*P < 0.05). SAMR1 animals show an increase over time (##P < 0.01). (C and D) BrdU+ radial NSCs (C) and BrdU+ NR progenitors (D) at different chase time points. Neither radial NSCs nor NR cells show differences in temporal dynamics. (E) BrdU+DCX+ newborn neurons at all chase time points. SAMP8 strain shows a reduction in this population at 3 d compared with SAMR1. (F) Active Casp3+ cells at 3 d after BrdU injection, showing an increase in cell death in the DG of 2-mo SAMP8 vs. SAMR1.
Fig. 3.
Newborn neurons of 2-mo SAMP8 animals fail to undergo proper maturation. (A) Representative images of the altered number and location of DCX+ cells in 2-mo SAMP8 vs. SAMR1. Arrows indicate DCX+ cells with altered location in SAMP8 GCL. (Scale bar, 25 μm.) (B and C) Number of DCX+ cells (B) and analysis of their location (C). In control animals, DCX+ cells were mainly confined to the innermost (first) third of the DG. SAMP8 animals showed an increased number and abnormal location of this cell population. (D) Number of DCX+CR+ cells at 2 mo, showing a decrease in SAMP8. (E and F) Number of NeuN+BrdU+ mature neurons (E) and S100β+BrdU+ mature astrocytes (F) 3 wk after BrdU injection. SAMP8 animals generate fewer mature neurons and more mature astrocytes. (Right) Image showing a SAMP8 S100β+BrdU+ mature astrocyte, as an example. (Scale bar, 10 µm.) *P < 0.05, **P < 0.01.
BMP6 Levels Are Elevated in the Hippocampal DG of SAMP8 Mice.
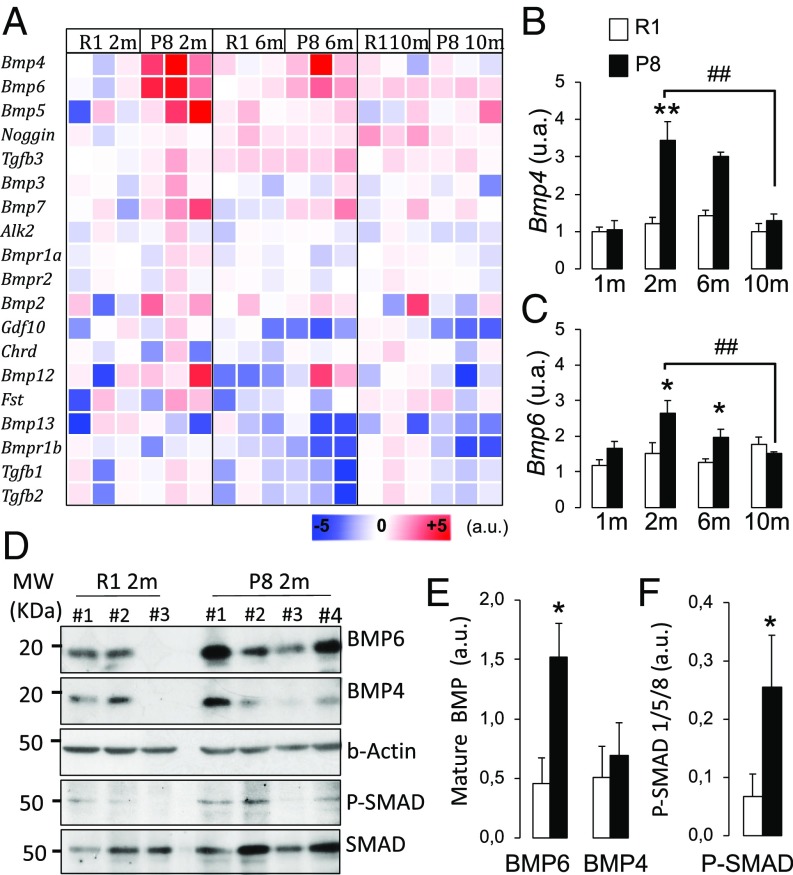
The signals that regulate the age-related depletion of the adult hippocampal stem cells and their conversion to astroglia have not yet been identified. Given the progliogenic role of BMPs at late developmental stages (34), and since the expression of BMP family members is dysregulated in the aging and AD murine and human hippocampus (19–24), we speculated that an early rise in BMP ligands and BMP signaling could underlie the SAMP8 defects. We screened the gene expression of BMPs and BMP-related signaling components in the SAMP8 and SAMR1 DG tissue (Fig. 4A). We found a significant age-dependent increase in Bmp4 and Bmp6 mRNAs in SAMP8 that peaked at the age of 2 mo (Fig. 4B) and was specific for the DG (SI Appendix, Fig. S2). We selected these two genes to further analyze their expression at the protein level (Fig. 4 D and E). We also measured the amount of P-SMAD1/5/8 relative to total SMAD1/5/8 as an estimate of the canonical BMP signaling pathway activity (Fig. 4 D–F). We confirmed a significant threefold rise in the mature BMP6 protein, but not in the mature BMP4 protein, as well as an increase in canonical BMP signaling in the DG of 2-mo SAMP8 animals vs. age-matched SAMR1 animals. A similar rise in BMP6 has been described in AD patients (22).
Fig. 4.
BMP6 levels are increased in the DG of SAMP8 mice. (A) Heat map showing the mRNA levels (a.u.) of BMP-related signaling components in the DG of 2-mo, 6-mo, and 10-mo SAMR1 and SAMP8. (B) Bmp4 and (C) Bmp6 mRNA expression is significantly increased in 2-mo SAMP8 vs. SAMR1. (D–F) Western blot and densitometry analysis of 2-mo DG lysates (D) showing an increased content in BMP6 normalized to β-actin (E) and P-SMAD1/5/8 normalized to total SMAD1/5/8 (F) in 2-mo SAMP8 vs. SAMR1. *P < 0.05, **P < 0.01, ##P < 0.01.
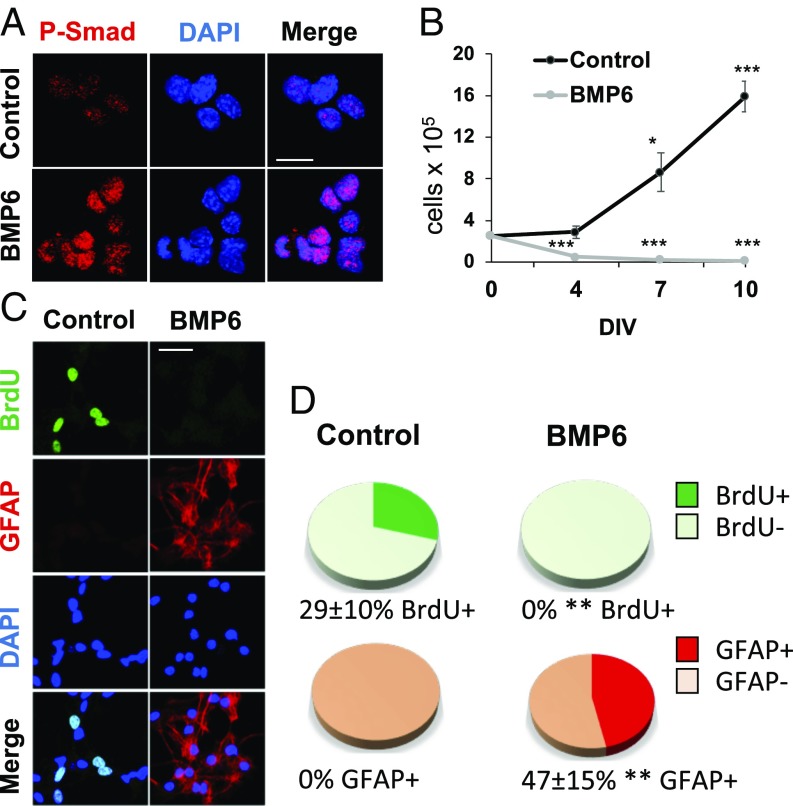
BMP6 Blocks the Expansion of Adult Hippocampal Stem and Progenitor Cell Cultures by Promoting Astroglial Differentiation.
To directly evaluate the effect of BMP6 on adult hippocampal neural stem and progenitor cells (NSPCs) we turned to an in vitro assay. We isolated mouse primary NSPC cultures from wild-type Crl:CD1 2-mo-old animals and expanded them with mitogens in the presence or absence of 50 ng/mL BMP6. The purity of the NSPC cultures was confirmed before the treatment (SI Appendix, Fig. S3). As shown in Fig. 5A, BMP6 treatment effectively induced canonical signaling in adult hippocampal NSPCs. Despite the continuous mitogenic stimulation, BMP6 severely impaired the expansion of the cultures (Fig. 5B) and promoted their differentiation into postmitotic GFAP+BrdU− astroglial cells (Fig. 5 C and D). We did not observe signs of increased apoptosis (TUNEL+ cells) or cellular senescence (SA-b-gal+ cells) (SI Appendix, Fig. S4). To elucidate whether the BMP6 effects are cell-autonomous or not, we analyzed BMP6 expression in NSPCs isolated from the DG of 2-mo SAMP8 and SAMR1 animals (SI Appendix, Figs. S3 and S5). BMP6 mRNA and preprotein levels were very low and the amount was reduced in SAMP8 NSPCs compared with SAMR1 NSPCs (SI Appendix, Fig. S5). BMP6 production in its mature functional form was negligible in both cultures. Of note, we found that SAMP8 NSPCs expanded more slowly (n = 9, P < 0.01) and had a decreased CldU/Ki67 ratio compared with SAMR1 NSPCs (79% lower, n = 3, P < 0.05); no significant differences in apoptosis were encountered (SI Appendix, Fig. S6). Treating the cells with the BMP natural antagonist Noggin had no effect on the growth of the cultures (SI Appendix, Fig. S7). Furthermore, SAMP8 NSPCs and SAMR1 NSPCs responded similarly to exogenously added BMP6 ligand (SI Appendix, Fig. S8). Together, the in vitro data suggest that the loss of the adult hippocampal stem cell population in mice with accelerated aging could be due to a noncell autonomous (niche) progliogenic effect of BMP6 canonical signaling. Moreover, NSPCs from SAMP8 mice display a cell-cycle lengthening trend that seems rather an intrinsic feature of the cells, in line with recent aging studies in the subependymal zone niche (35).
Fig. 5.
BMP6 promotes astroglial differentiation of cultured adult hippocampal NSPCs. (A) Canonical signaling activation in cultured NSPCs after BMP6 treatment. (B) In vitro cell growth kinetics at 0, 4, 7, and 10 d (DIV). BMP6 impaired the expansion of NSPC cultures (*P < 0.05, ***P < 0.001). (C) Postmitotic astroglial differentiation (GFAP+BrdU−) of primary NSPC cultures after BMP6 treatment. (D) Percentage of GFAP+ and BrdU+ cells after BMP6 treatment. BMP6 impairs proliferation (0% BrdU+, **P < 0.01) and induces astroglial differentiation (% GFAP+, **P < 0.01). Data correspond to the average ± SEM, n = 3. (Scale bars, 10 μm in A and 20 μm in B.)
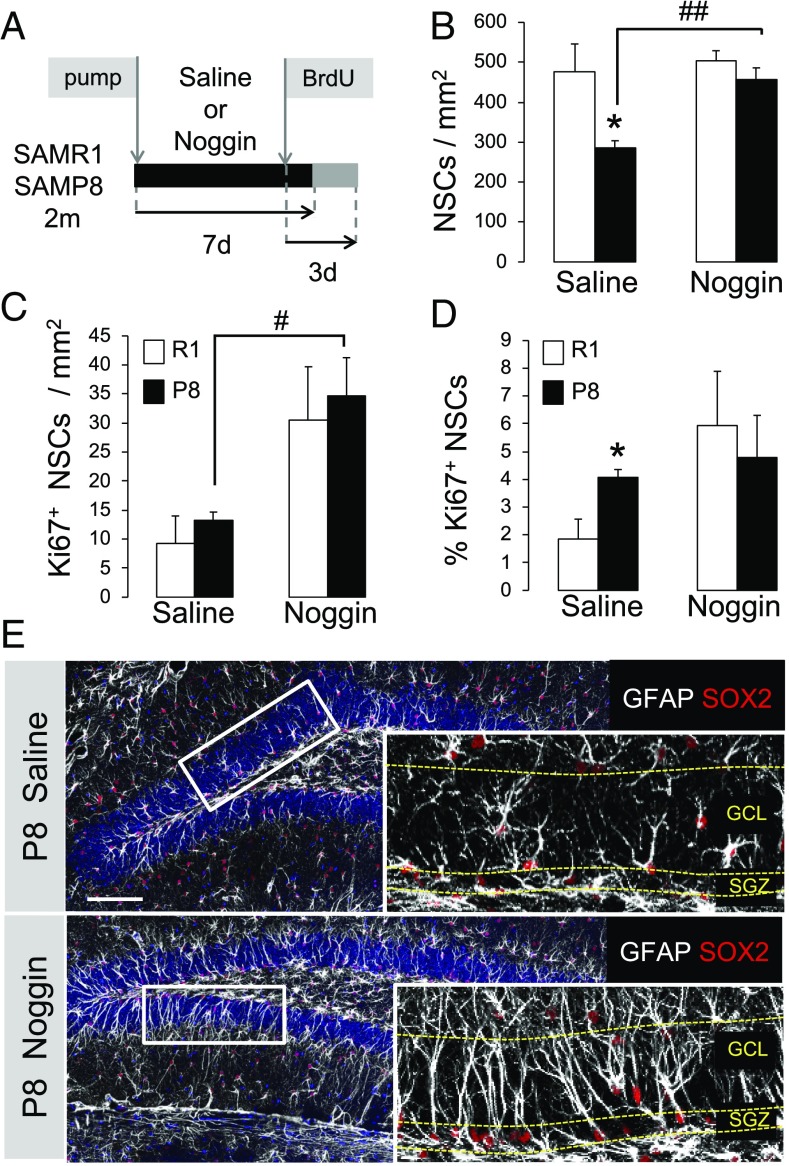
Intracranial Infusion of the BMP Antagonist Noggin Rescues Hippocampal Stem Cell Loss in SAMP8 Mice and Ameliorates the Neurogenic Deficits.
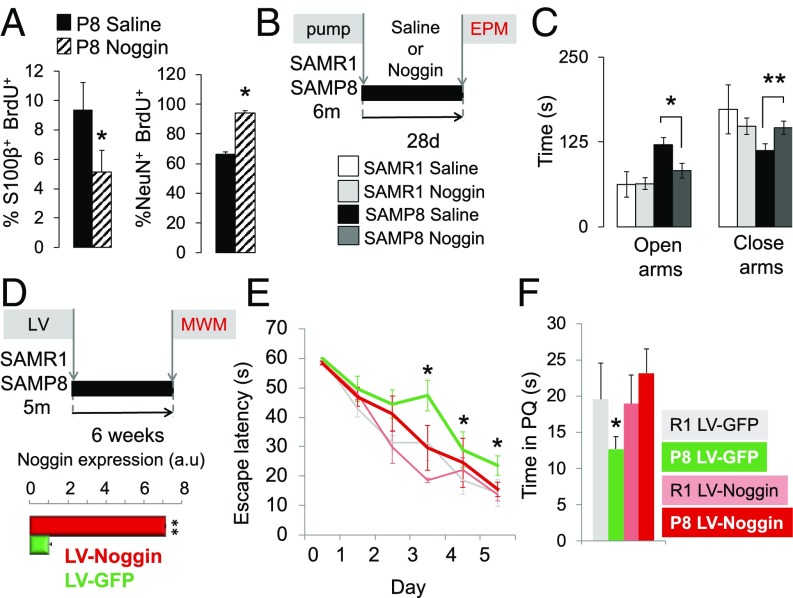
To test whether increased BMP signaling plays a role in the stem cell depletion and neurogenic defects in mice with pathological aging we analyzed the impact of administering the antagonist Noggin. For this purpose, we implanted osmotic minipumps in SAMP8 and SAMR1 animals and infused Noggin or saline solution as a control into the brain. Mice were injected with BrdU on the last day of infusion and were either killed 3 d (Fig. 6) or 3 wk later (Fig. 7). As shown in Fig. 6, Noggin efficiently rescued the precocious loss of hippocampal radial NSCs in SAMP8 (Fig. 6 B–E). The total number of stem cells increased almost twofold in SAMP8 animals and reached the values of the SAMR1 strain. As previously reported by our group (18), Noggin did not change the total number of radial NSCs in control animals (Fig. 6B), although it increased their cycling activity (Fig. 6 C and D), suggesting that under normal healthy conditions Noggin enhances the asymmetric division of NSCs. In contrast, although Noggin augmented the total number of radial NSCs in SAMP8 animals, it did not further increase their cell cycle, pointing to a specific effect of Noggin exposure in animals with pathological aging, which are endowed with higher DG levels of BMP6 and BMP signaling compared with control mice. We next explored whether Noggin administration rescued the progliogenic phenotype of the SAMP8 animals and/or ameliorated the neurogenic defects. Noggin infusion diminished the differentiation of the NSC pool toward the astrocytic lineage and increased the production of fully mature neurons (Fig. 7A). It also significantly reduced the aberrant accumulation and ectopic location of the immature DCX+ cells (SI Appendix, Fig. S9). Together, the data suggest that blocking the enhanced BMP signaling of the SAMP8 strain restores normal hippocampal NSC numbers, most likely by counteracting the BMP-mediated astroglial differentiation, and simultaneously improves the neurogenic defects of the senescent strain.
Fig. 6.
Intracranial infused Noggin rescues hippocampal NSC loss in SAMP8. (A) Minipump infusion and pulse-chase experimental design using Noggin and BrdU. Two-month animals were infused with Noggin for 7 d and injected with BrdU the last day of infusion. Animals were killed 3 d after BrdU injection (21 d for Fig. 7A). (B) SAMP8 animals show an increase in the number of radial NSCs when treated with Noggin compared with control saline-treated animals (##P < 0.01). (C and D) The number of proliferating Ki67+ radial NSCs is increased in SAMP8 animals treated with Noggin (C, #P < 0.05). The percentage of proliferating radial NSCs is restored to SAMR1 levels (D, *P < 0.05). (E) Representative images showing the increase in radial NSCs after intracranial Noggin infusion in 2-mo SAMP8 animals. (Scale bar, 100 μm.) (Magnification: E, Insets, 5×.)
Fig. 7.
Noggin infusion counteracts the NSC astroglial differentiation and the neurogenic and behavioral defects in SAMP8. (A) Percentage of S100β+BrdU+ mature astrocytes (Left) and NeuN+BrdU+ mature neurons (Right) after treating 2-mo SAMP8 with saline or Noggin (7-d infusion as in Fig. 6A) at 21 d after BrdU injection. SAMP8 show an increased number of mature neurons at the expense of mature astrocytes (*P < 0.05). (B) Experimental design for the Noggin 28-d infusion employing minipums and elevated plus maze (EPM). (C) Total time spent in the open and closed arms of the elevated plus maze. Noggin restored SAMP8 anxiety behavior to control levels (*P < 0.05, **P < 0.01). (D–F) Experimental design for the Morris water maze (MWM). (D) Experimental design of the lentiviral injection and MWM. Relative Noggin mRNA expression by RT-qPCR is also shown in fold (a.u.) (*P < 0.01). (E) Learning curve calculated as daily mean escape latency during five consecutive days (*P < 0.05; LV-Noggin-SAMP8 vs. LV-GFP-SAMP8). A habituation trial (60 s without platform was performed on day 0; see SI Appendix, SI Materials and Methods). (F) Probe trial showing the absolute time spent at the platform quadrant (PQ) of the pool (*P < 0.05; LV-Noggin-SAMP8 vs. LV-GFP-SAMP8).
Behavioral Deficits in SAMP8 Mice Are Rescued by Noggin.
SAMP8 mice show age-associated behavioral impairments at 6 mo, such as learning and memory deficits (36) and reduced anxiety (37), so next we analyzed the behavioral phenotype of both SAMR1 and SAMP8 6-mo animals infused with Noggin or saline (Fig. 7B). We found that SAMP8 animals spent significantly longer times at the open arms of the elevated plus maze compared with SAMR1 mice, pointing to clear differences in anxiety (Fig. 7C). Interestingly, Noggin normalized the anxiety behavior of SAMP8 animals to the levels of SAMR1 animals (Fig. 7C). To test their learning and memory capabilities, SAMR1 and SAMP8 5-mo animals were injected with a lentivirus (LV) overexpressing Noggin or GFP as a control and their performance in the Morris water maze test was evaluated (Fig. 7D). Noggin improved the acquisition phase of the test in SAMP8 animals; learning of the LV-Noggin-SAMP8 group was superior to that of LV-GFP-SAMP8 group across a number of days of testing (Fig. 7E). A clear effect was also found when the animals were tested in the probe trial to analyze the quality of the learning process or short-term memory retention (Fig. 7F and SI Appendix, Fig. S10). SAMP8 mice obtained a lower score, pointing to worse learning. This difference was fully restored by Noggin in SAMP8 animals, which spent similar times in the platform quadrant compared with SAMR1 mice.
Discussion
Age-related neurodegenerative disorders such as AD slowly undermine cognitive function and behavioral abilities. Although AD is not a part of normal healthy aging, the rate of the disease doubles every decade after the age of 60. Alterations in hippocampal neurogenesis, which have been extensively documented both during normal aging and in AD (7–9), possibly contribute to the age- and AD-related hippocampal dysfunction, but the mechanistic causes underlying this phenomenon remain poorly understood. Hence, unraveling the changes affecting the hippocampal neurogenic niche and the hippocampal stem cell dynamics during aging and, most importantly, at early presymptomatic AD stages, may provide new insights into the progression of the disease.
We herein show that BMP6 accumulates very early in the hippocampal niche of SAMP8 animals, a senescent strain that has been used to model some age-related aspects of the onset of sporadic AD. BMP6 is also significantly increased at the mRNA and protein levels in the hippocampus of patients with early and severe AD compared with nondemented controls and in a transgenic mouse model of familial AD (22). BMP6 accumulates around Aβ plaques in the hippocampus and, as in SAMP8, the accumulation in human tissue is accompanied by reduced hippocampal neurogenesis and SOX2+ cells. Moreover, at least in vitro, hippocampal stem cells exposed to Aβ overexpress BMP6 (22). An increase in BMP6 has been also reported during healthy aging in wild-type mice, possibly associated to microglial cells, the resident immune cells of the brain (23). Thus, although the available data suggest that BMP6 accumulates during healthy aging, the process may be exacerbated in pathological conditions such as AD that lead to increased Aβ levels, the activation of microglia, and an unfavorable inflammatory microenvironment (38, 39). Increased Aβ levels and inflammation have been detected in the SAMP8 brain (40–42) and could therefore contribute to the rise of BMP6 in this mouse model.
Our results indicate that the increase in BMP6 and in canonical BMP signaling mainly results in the precocious depletion of the adult hippocampal NSCs due to their BMP-mediated differentiation into astroglial cells. Interestingly, in 2-mo SAMP8 mice the remaining hippocampal NSCs proliferate and continue to generate new immature neurons, but these cells have a reduced survival and display an aberrant delay in differentiation, leading to a reduction in the number of NeuN+ granule neurons reaching maturity, as reported elsewhere (43). A similar aberrant hindrance to the normal development of the immature neuronal population has been described in the aging brain of wild-type mice (6), in the brain of AD patients, and in some transgenic AD models of the familial early-onset forms of the disease (7–9, 22, 44).
Increasing evidence suggests that the aged and AD neurogenic niche is no longer optimal for neurogenesis (7–9); however, taking advantage of the SAMP8 model and employing the BMP antagonist Noggin we demonstrate that it is still possible to influence the niche microenvironment, to rescue stem cell numbers, and to revert the pathological neurogenic phenotype by means of blocking the aberrant BMP signaling. The treatment with Noggin also normalized the behavioral phenotype of SAMP8 animals, making the scores similar to those of control SAMR1 animals. The SAMP8 anxiety phenotype, the spatial learning defects, and the short-term memory retention capacity were improved by Noggin. In line with our results, hippocampal neurogenesis has been also rescued in transgenic AD models with Noggin (20) and it has been shown that increased BMP signaling results in cognitive impairments, while its reduction ameliorates hippocampus-dependent cognitive function in young and aged mice (24, 45, 46).
In summary, age-related stem cell depletion and dysfunction may result from a combination of several factors, both intrinsic and extrinsic to the stem cell compartment, that are dysregulated with increasing age and that may be exacerbated in pathological conditions such as AD. Our data show that the temporal increase of BMP6 expression in the DG may be one of such altered extrinsic factors. The cell cycle lengthening of NSPCs may be rather a cell intrinsic feature (35). The loss of the stem cell population in SAMP8 mice is due to their differentiation into mature astrocytic cells, which is in accordance with previous findings in aged animals (19–24). We have uncovered why this defective fate choice occurs and how we might mitigate it by manipulating the stem cell niche. The increased glial differentiation of the NSCs can further help to explain the hippocampal astrocytosis that is detected in the SAMP8 strain starting at 6 mo of age (26) and that characterizes the late stages of AD (47). Our findings, combined with previous reports in which the in vivo delivery of lentiviral shRNA against downstream effectors of the BMP pathway partially rejuvenated NSC function in the aged brain (23, 24), support further development of BMP antagonists into translatable molecules for the rescue of stem cell depletion and neurogenesis during healthy and pathological aging.
Materials and Methods
Animals.
SAMP8 and SAMR1 males were used at 1, 2, 6, and 10 mo. Crl:CD1 males were used at 2 mo (SI Appendix). Mice were maintained under specific-pathogen-free conditions and all manipulations were approved by the Animal Care Committee of the Instituto de Salud Carlos III.
Immunostaining.
Antibody stainings were performed as described in SI Appendix.
DG NSPC Culture.
NSPCs were isolated from dissected hippocampi of 2-mo-old Crl:CD1, SAMR1, and SAMP8 mice and cultured in FGF2 and EGF (SI Appendix).
Intracerebroventricular Infusion.
Alzet osmotic minipumps were implanted in SAMR1 and SAMP8 mice for Noggin or vehicle solution infusion. Different experimental paradigms of infusion and BrdU administration were applied (SI Appendix).
Lentiviral Injections.
Five-month-old SAMP8 and SAMR1 males underwent stereotaxic surgery, and a 1.5-μL suspension of lentiviral ORF particles expressing Noggin or GFP (Origene Technologies) was injected into both hemispheres at DG coordinates (SI Appendix).
Behavioral Assessments.
Animals’ general activity was tested with a VersaMax Animal Activity Monitoring System. For anxiety measurement and spatial learning and memory tests, elevated plus maze and Morris water maze were performed as described in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. Gerd Kempermann and members of his laboratory for technical guidance in the NSPC isolation and the ISCIII Confocal Microscopy Core and of Lucía Casares-Crespo at the Instituto de Biomedicina de Valencia for assistance. This work was supported by a predoctoral fellowship from the Spanish Ministerio de Educación (to M.D.-M.), Spanish Ministerio de Economía y Competitividad Grant BFU2013-48907-R (to J.L.T.), and Grants PI12/101 and SAF2015-70433-R (to H.M.). We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813205115/-/DCSupplemental.
References
- 1.Aimone JB, et al. Regulation and function of adult neurogenesis: From genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempermann G, et al. Human adult neurogenesis: Evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drapeau E, Nora Abrous D. Stem cell review series: Role of neurogenesis in age-related memory disorders. Aging Cell. 2008;7:569–589. doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SW, Clemenson GD, Gage FH. New neurons in an aged brain. Behav Brain Res. 2012;227:497–507. doi: 10.1016/j.bbr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchero MF, et al. High plasticity of new granule cells in the aging hippocampus. Cell Rep. 2017;21:1129–1139. doi: 10.1016/j.celrep.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 7.Lazarov O, Marr RA. Neurogenesis and Alzheimer’s disease: At the crossroads. Exp Neurol. 2010;223:267–281. doi: 10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7:a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 13.Encinas JM, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 15.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choe Y, Pleasure SJ, Mira H. Control of adult neurogenesis by short-range morphogenic-signaling molecules. Cold Spring Harb Perspect Biol. 2015;8:a018887. doi: 10.1101/cshperspect.a018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolando C, Taylor V. Neural stem cell of the hippocampus: Development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol. 2014;107:183–206. doi: 10.1016/B978-0-12-416022-4.00007-X. [DOI] [PubMed] [Google Scholar]
- 18.Mira H, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Li D, et al. Decreased hippocampal cell proliferation correlates with increased expression of BMP4 in the APPswe/PS1DeltaE9 mouse model of Alzheimer’s disease. Hippocampus. 2008;18:692–698. doi: 10.1002/hipo.20428. [DOI] [PubMed] [Google Scholar]
- 20.Tang J, et al. Noggin and BMP4 co-modulate adult hippocampal neurogenesis in the APP(swe)/PS1(DeltaE9) transgenic mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2009;385:341–345. doi: 10.1016/j.bbrc.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, et al. The function of BMP4 during neurogenesis in the adult hippocampus in Alzheimer’s disease. Ageing Res Rev. 2013;12:157–164. doi: 10.1016/j.arr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Crews L, et al. Increased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci. 2010;30:12252–12262. doi: 10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousef H, et al. Age-associated increase in BMP signaling inhibits hippocampal neurogenesis. Stem Cells. 2015;33:1577–1588. doi: 10.1002/stem.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers EA, et al. Increased bone morphogenetic protein signaling contributes to age-related declines in neurogenesis and cognition. Neurobiol Aging. 2016;38:164–175. doi: 10.1016/j.neurobiolaging.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. 2009;34:639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- 26.Díaz-Moreno M, et al. Aβ increases neural stem cell activity in senescence-accelerated SAMP8 mice. Neurobiol Aging. 2013;34:2623–2638. doi: 10.1016/j.neurobiolaging.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Sousa-Victor P, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 28.Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp Gerontol. 2005;40:774–783. doi: 10.1016/j.exger.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Pallas M, et al. From aging to Alzheimer’s disease: Unveiling “the switch” with the senescence-accelerated mouse model (SAMP8) J Alzheimers Dis. 2008;15:615–624. doi: 10.3233/jad-2008-15408. [DOI] [PubMed] [Google Scholar]
- 30.Ito K. Frontiers of model animals for neuroscience: Two prosperous aging model animals for promoting neuroscience research. Exp Anim. 2013;62:275–280. doi: 10.1538/expanim.62.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng XR, Zhou WX, Zhang YX. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer’s disease animal model. Ageing Res Rev. 2014;13:13–37. doi: 10.1016/j.arr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Falcão AM, et al. Topographical analysis of the subependymal zone neurogenic niche. PLoS One. 2012;7:e38647. doi: 10.1371/journal.pone.0038647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima K, Taga T. Mechanisms underlying cytokine-mediated cell-fate regulation in the nervous system. Mol Neurobiol. 2002;25:233–244. doi: 10.1385/MN:25:3:233. [DOI] [PubMed] [Google Scholar]
- 35.Apostolopoulou M, et al. Non-monotonic changes in progenitor cell behavior and gene expression during aging of the adult V-SVZ neural stem cell niche. Stem Cell Reports. 2017;9:1931–1947. doi: 10.1016/j.stemcr.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto M, et al. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM) Physiol Behav. 1986;38:399–406. doi: 10.1016/0031-9384(86)90112-5. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto M, Kiyota Y, Nishiyama M, Nagaoka A. Senescence-accelerated mouse (SAM): Age-related reduced anxiety-like behavior in the SAM-P/8 strain. Physiol Behav. 1992;51:979–985. doi: 10.1016/0031-9384(92)90081-c. [DOI] [PubMed] [Google Scholar]
- 38.Mrak RE. Microglia in Alzheimer brain: A neuropathological perspective. Int J Alzheimers Dis. 2012;2012:165021. doi: 10.1155/2012/165021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuster-Matanzo A, Llorens-Martín M, Hernández F, Avila J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: Therapeutic approaches. Mediators Inflamm. 2013;2013:260925. doi: 10.1155/2013/260925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morley JE, Farr SA, Kumar VB, Armbrecht HJ. The SAMP8 mouse: A model to develop therapeutic interventions for Alzheimer’s disease. Curr Pharm Des. 2012;18:1123–1130. doi: 10.2174/138161212799315795. [DOI] [PubMed] [Google Scholar]
- 41.Torregrosa-Muñumer R, et al. Reduced apurinic/apyrimidinic endonuclease 1 activity and increased DNA damage in mitochondria are related to enhanced apoptosis and inflammation in the brain of senescence- accelerated P8 mice (SAMP8) Biogerontology. 2016;17:325–335. doi: 10.1007/s10522-015-9612-x. [DOI] [PubMed] [Google Scholar]
- 42.Griñan-Ferré C, et al. Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp Gerontol. 2016;80:57–69. doi: 10.1016/j.exger.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Gang B, et al. Limited hippocampal neurogenesis in SAMP8 mouse model of Alzheimer’s disease. Brain Res. 2011;1389:183–193. doi: 10.1016/j.brainres.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 44.Ekonomou A, et al. Medical Research Council Cognitive Function and Ageing Neuropathology Study Stage-specific changes in neurogenic and glial markers in Alzheimer’s disease. Biol Psychiatry. 2015;77:711–719. doi: 10.1016/j.biopsych.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Fan X-T, Cai W-Q, Yang Z, Xu H-W, Zhang J-H. Effect of antisense oligonucleotide of noggin on spatial learning and memory of rats. Acta Pharmacol Sin. 2003;24:394–397. [PubMed] [Google Scholar]
- 46.Gobeske KT, et al. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One. 2009;4:e7506. doi: 10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamowicz DH, Mertens J, Gage FH. Alzheimer’s disease: Distinct stages in neurogenic decline? Biol Psychiatry. 2015;77:680–682. doi: 10.1016/j.biopsych.2015.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.