Significance
The age of puberty in humans is changing via unknown mechanisms, although metabolic alterations in childhood are blamed as a major contributing factor. Perturbations in pubertal timing are posed with increased risk of later cardiometabolic diseases and reduced life expectancy, urging a better understanding of the molecular basis for these phenomena. We describe a mechanism whereby the main cellular energy sensor, AMPK, operates in a population of hypothalamic neurons, named Kiss1, which produce the puberty-activating signal, kisspeptin, to metabolically control puberty onset. This neuroendocrine circuit provides a molecular link between conditions of negative energy balance and delayed pubertal timing, via a repressive AMPK–Kiss1 pathway, which may become a druggable target in conditions of disordered puberty, especially of metabolic origin.
Keywords: AMPK, Kiss1, puberty, energy balance, undernutrition
Abstract
Conditions of metabolic distress, from malnutrition to obesity, impact, via as yet ill-defined mechanisms, the timing of puberty, whose alterations can hamper later cardiometabolic health and even life expectancy. AMP-activated protein kinase (AMPK), the master cellular energy sensor activated in conditions of energy insufficiency, has a major central role in whole-body energy homeostasis. However, whether brain AMPK metabolically modulates puberty onset remains unknown. We report here that central AMPK interplays with the puberty-activating gene, Kiss1, to control puberty onset. Pubertal subnutrition, which delayed puberty, enhanced hypothalamic pAMPK levels, while activation of brain AMPK in immature female rats substantially deferred puberty. Virogenetic overexpression of a constitutively active form of AMPK, selectively in the hypothalamic arcuate nucleus (ARC), which holds a key population of Kiss1 neurons, partially delayed puberty onset and reduced luteinizing hormone levels. ARC Kiss1 neurons were found to express pAMPK, and activation of AMPK reduced ARC Kiss1 expression. The physiological relevance of this pathway was attested by conditional ablation of the AMPKα1 subunit in Kiss1 cells, which largely prevented the delay in puberty onset caused by chronic subnutrition. Our data demonstrate that hypothalamic AMPK signaling plays a key role in the metabolic control of puberty, acting via a repressive modulation of ARC Kiss1 neurons in conditions of negative energy balance.
Ensuring sufficient energy stores to face the diversity of bodily functions is a major homeostatic priority, which engages numerous brain pathways (1). In turn, body energy and metabolic status have a clear impact on all physiological systems, with a discernible influence on key maturational events and energy-demanding functions. Puberty is a critical transitional period, when attainment of reproductive capacity and sexual maturity occurs (2–4), together with key somatic, metabolic, psychological, and behavioral changes. Recent studies have reported trends for altered (mostly earlier) puberty onset in girls, and also in boys (5–7). This is worrisome, considering the adverse health outcomes seemingly associated with disturbed pubertal timing, including higher risk of cardiometabolic disorders, such as type 2 diabetes and hypertension (8), and reduced life expectancy (9). The molecular underpinnings of such associations are yet to be elucidated.
One of the most important determinants of puberty onset is the nutritional and metabolic state (10, 11). Conditions of metabolic stress, from cachexia to early-onset obesity, are linked to perturbed pubertal timing (12, 13). Indeed, while the higher frequency of child obesity has been related to the trends of earlier puberty (5, 6), conditions of persistent energy deficit, ranging from eating disorders to strenuous exercise, are known to severely delay pubertal maturation (13). The mechanisms for such a tight metabolic–pubertal connection are yet to fully surface, but appear to be largely brain centered. Notably, gonadotropin-releasing hormone (GnRH) neurons, the indispensable output pathway for the brain control of reproductive axis and puberty, are seemingly devoid of appropriate sensing mechanisms for directly detecting some key metabolic modifiers of puberty, such as leptin (12). Thus, although the presence of metabolic sensors in immortalized, GnRH-related, GT1–7 cells has been suggested by expression and pharmacological analyses (14), the above evidence points to the existence of intermediary pathways whose nature remains largely unknown.
The search for neural afferents to GnRH neurons, transmitting (among others) the regulatory actions of metabolic signals, has been revolutionized by the identification of the puberty-activating gene, Kiss1, and its receptor, Gpr54 (15, 16). Kiss1 neurons, mostly located in the arcuate nucleus (ARC) and rostral [mainly, the anteroventral periventricular nucleus (AVPV) in rodents] hypothalamic areas, play essential roles in the timing of puberty and mediate the modulatory actions of numerous puberty-regulating signals, from sex steroids to circadian rhythms (15, 16). Conclusive evidence in preclinical models has demonstrated that the Kiss1 system is sensitive to, and a (direct or indirect) transmitter for, conditions of metabolic distress impacting puberty, from subnutrition to overfeeding (12). However, the molecular substrate for such a function remains ill defined.
AMPK is a highly conserved serine/threonine kinase, with a catalytic α-subunit (with two isoforms, α1 and α2), and two regulatory subunits, β (β1 and β2) and γ (γ1–3) (17, 18). AMPK is activated by phosphorylation at Thr-172 of the α-subunit, which is promoted by increased AMP/ATP and ADP/ATP ratios (17). Upon AMPK phosphorylation, ATP-producing catabolic processes are activated, while ATP-consuming anabolic phenomena are inhibited, thereby restoring the AMP/ATP balance, essential for cellular energy homeostasis (18). Accordingly, AMPK is regarded as the genuine (and indispensable) cellular energy sensor (17). In fact, in contrast to other putative metabolic gauges, AMPK is able to directly sense changes in availability of the real energy sources of the cells, namely, adenine nucleotides (17). Besides its fundamental role in driving cellular metabolism, AMPK signaling at specific brain (hypothalamic) centers is essential for whole-body energy homeostasis. Accordingly, hypothalamic AMPK activity is tightly coupled to changes in feeding and modulated by food intake-controlling signals (17, 19); fasting and orexigenic hormones activate AMPK, thereby inducing feeding, whereas refeeding and anorectic factors inhibit AMPK, resulting in suppression of food intake (17). Central AMPK signaling participates also in the control of energy expenditure, since AMPK activation in the ventromedial hypothalamus (VMH) suppresses the thermogenic program of brown adipose tissue, while its inhibition at this nucleus promotes energy expenditure (17, 20–22). Thus, AMPK operates as an integral hypothalamic regulator of energy homeostasis, acting on feeding and thermogenesis in a nucleus-specific manner.
Because of its prominent role in whole-body weight and metabolic homeostasis, brain AMPK is likely to connect energy balance and other metabolically gated functions (17). These seemingly include reproduction, which is highly sensitive to nutritional and metabolic cues, and dynamically controlled by hormones, such as leptin and ghrelin (12, 23). In fact, as yet fragmentary evidence suggests that AMPK may transmit a negative valence to the elements of the reproductive brain in adulthood (14, 24–26). However, the putative physiological relevance of these phenomena is yet to be fully defined. Moreover, the roles, if any, of brain AMPK in the regulation of puberty remains virtually unexplored to date. In this work, we aimed to address the potential role of brain AMPK signaling in connecting metabolic/nutritional status and pubertal timing. Our data disclose a regulatory pathway of puberty onset, involving an AMPK-mediated repressive control of Kiss1 in ARC kisspeptin neurons, which becomes activated in conditions of energy deficit and contributes to the metabolic gating of puberty.
Results
Hypothalamic AMPK Is Activated by Chronic Subnutrition and Negatively Regulates Puberty Onset.
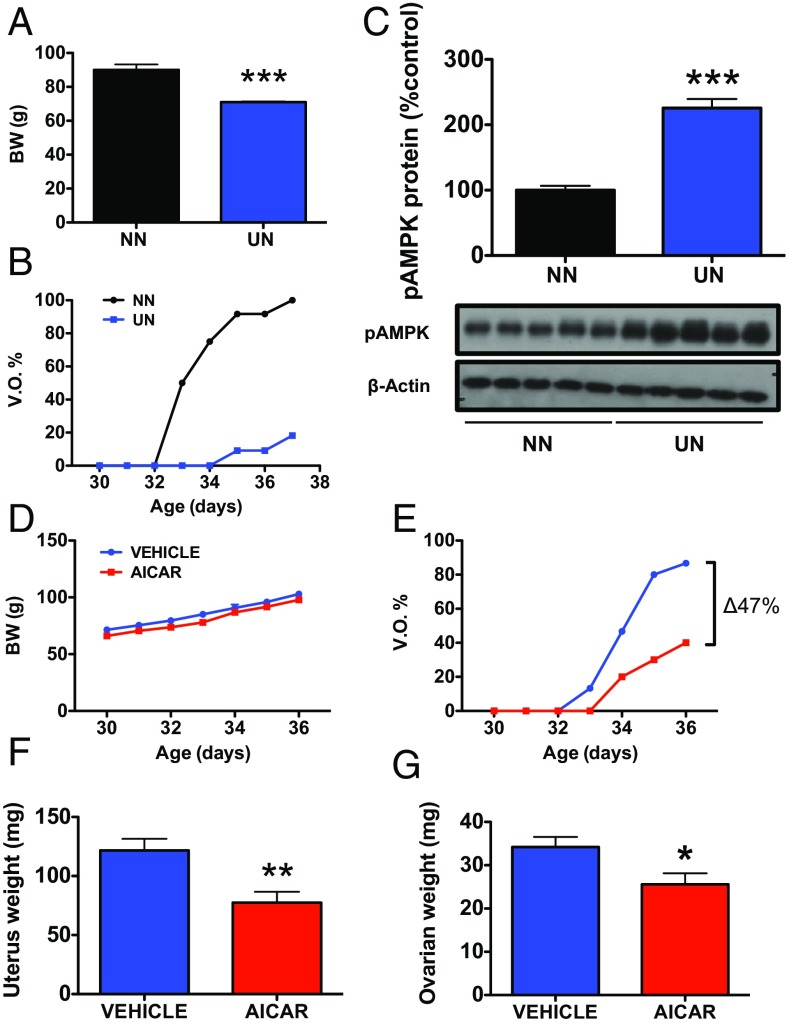
The ability of hypothalamic AMPK to sense changes in energy availability was tested in pubertal female rats subjected to chronic 30% peripubertal undernutrition (UN), from weaning [at postnatal day 23 (PND23)] onwards. This protocol of UN caused a significant 22% decrease in body weight (BW) (Fig. 1A) and a substantial delay in the age of vaginal opening (VO), as a phenotypic sign of puberty onset (VO in UN at PND37: 20% vs. 100% in pair-aged female rats fed ad libitum; Fig. 1B). Chronic UN at puberty resulted also in a significant increase (more than twofold) in hypothalamic pAMPK levels (Fig. 1C).
Fig. 1.
Metabolic and pharmacological activation of brain AMPK delays puberty onset. In the Upper panels, the impact of chronic subnutrition (UN) on BW (A), time course of VO, as phenotypic sign of puberty onset (B), and hypothalamic pAMPK levels (C) are shown. Peripubertal female rats were subjected to 30% reduction in daily food ration, from PND23 onwards. Age-paired rats fed ad libitum [normal nutrition (NN)] were used as controls. Group sizes were for BW: NN = 20, UN = 10 animals per group; for Western blot: NN = 5, UN = 5 animals per group. ***P < 0.001 vs. corresponding controls (Student t tests). In the Lower panels, the effects of chronic icv infusion of the pharmacological activator of AMPK, AICAR, on BW (D), VO (E), uterus weight (UW) (F), and ovarian weight (OW) (G) (at PND36) in pubertal female rats are presented. Females chronically treated with vehicle served as controls. Group sizes were: n = 16 animals per group for vehicle treatment; n = 10 animals per group for AICAR. *P < 0.05; **P < 0.01 vs. corresponding control groups.
The consequences of hypothalamic AMPK activation at puberty were addressed pharmacologically, by intracerebroventricular (icv) infusion of the AMPK activator, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), between PND30 and PND36 into female rats. While this regimen did not alter BW gain during the pubertal transition (Fig. 1D), it profoundly delayed the course of VO, with an overall reduction of ∼50% in the total number of females displaying VO at PND36 (Fig. 1E). AICAR infusion also induced a significant reduction in uterus weight (UW) and ovarian weight (OW) at PND36, as additional indices of deferred pubertal maturation (Fig. 1 F and G). Efficient activation of hypothalamic AMPK in our model was demonstrated by Western blot, which showed increased pAMPK/AMPKα1 and pAMPK/AMPKα2 ratios and enhanced phosphor-Acetyl-CoA carboxylase (pACC) content, as a downstream target of pAMPK (17), in the hypothalami of AICAR-treated female rats (SI Appendix, Fig. S1 A–C). In contrast, no significant elevation of pAMPK levels was detected in other brain areas of AICAR-treated animals, such as the cortex or hippocampus, taken for reference purposes (SI Appendix, Fig. S2). The lack of increase in pAMPK levels after AICAR in the hippocampus, despite its circumventricular location, might be due to low basal pAMPK content vs. the hypothalamus, as denoted by immunohistochemistry (SI Appendix, Fig. S2).
Activation of AMPK in the ARC Negatively Regulates Puberty Onset.
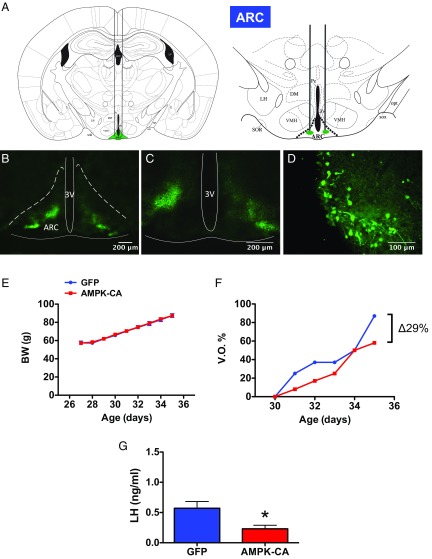
Pharmacological experiments of central activation of AMPK were complemented by virogenetic gain-of-function studies involving overexpression of a constitutively active form of AMPKα (AMPK-CA) in the ARC of (pre)-pubertal female rats; a similar tool has previously been used by our team to address the metabolic role of central AMPK in the control of energy homeostasis (20, 22). For reference purposes, stereotaxic targeting of AMPK-CA in the VMH, which is relevant for the thermogenic actions of AMPK (17), was also conducted in parallel. Efficient targeting of ARC and VMH was verified by coadministration of fluorescein, while efficiency of viral-induced expression was confirmed by stereotaxic delivery of adenoviruses encoding GFP (Fig. 2 A–D). Overexpression of AMPK-CA in the ARC did not modify BW gain along puberty (Fig. 2E), but tended to delay the course of VO; on PND35, female rats injected with adenoviruses encoding AMPK-CA displayed an accumulated VO frequency that was ∼30% lower than females injected with mock vectors (Fig. 2F). In addition, circulating luteinizing hormone (LH) levels (as a hormonal biomarker and major driver of gonadal maturation) in pubertal female rats injected with AMPK-CA in the ARC were significantly lower than in corresponding controls (Fig. 2G). In clear contrast, overexpression of AMPK-CA in the VMH, which also failed to change BW, did not delay VO (actually, an opposite trend was detected), nor did it decrease LH levels at PND35 (SI Appendix, Fig. S3).
Fig. 2.
Virogenetic overexpression of AMPK in the arcuate nucleus delays puberty onset. In A, a schematic representation of the bilateral stereotaxic injection of viruses inducing overexpression of AMPK-CA in the ARC, is shown. Representative images, at two different magnifications, of bilateral targeting of the ARC, as denoted by fluorescein labeling, are also presented in B and C, while efficiency of adenoviral-mediated infection of ARC neurons by stereotaxic delivery is documented by GFP labeling, as shown in D. In addition, the impact of AMPK-CA overexpression on BW (E), the percentage of VO (F), and serum LH levels (as marker of activation of the reproductive axis) (G) is documented. Animals injected with viral vectors overexpressing only the marker, GFP, were used as controls. Due to coadministration of fluorescein isothiocyanate in both GFP and AMPK-CA–treated animals, the injection site was evaluated postmortem, and animals with evidence for off-target injections were not considered for further analyses. Final group sizes (i.e., animals with proper viral injections) were: control-GFP = 8 animals per group; AMPK-CA = 9 animals per group. *P < 0.05 vs. control-GFP group (Student t tests).
AMPK Suppresses Kiss1 in the ARC and Is Coexpressed in Kiss1 Neurons.
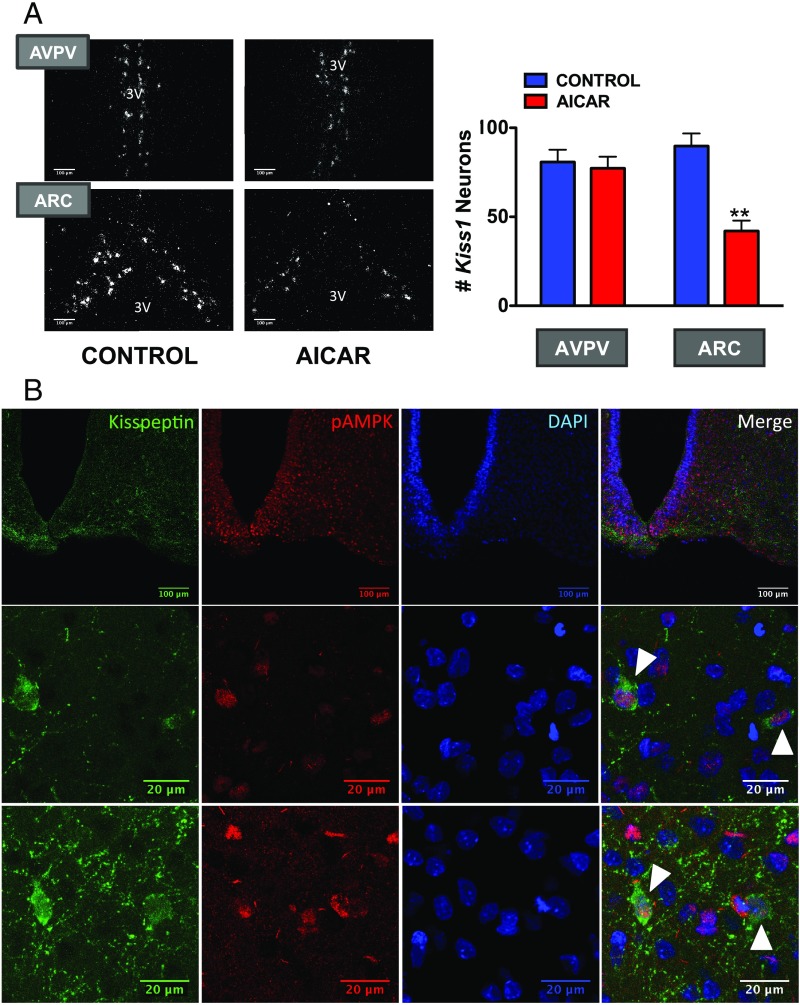
Since the ARC holds a prominent population of Kiss1 neurons (27), we explored the potential AMPK–Kiss1 interplay by a combination of expression and functional analyses. A protocol of central activation of AMPK using AICAR was applied to pubertal female rats, and expression of Kiss1 was evaluated by in situ hybridization (ISH) in the ARC and AVPV, as major hypothalamic sites harboring Kiss1 neurons. To control for the confounding influence of fluctuations in endogenous sex steroid levels on Kiss1 expression, a model of pubertal ovariectomized (OVX) rats clamped to have a fixed physiological level of circulating estradiol (E2) was used (28). Prominent expression of Kiss1 was detected at the ARC and AVPV, in line with previous studies (27). However, while central administration of AICAR failed to modify Kiss1 mRNA in the rostral (AVPV) population, Kiss1 expression in the ARC was significantly blunted by AICAR treatment, with a mean reduction of >50% in the number of Kiss1-expressing neurons (Fig. 3A).
Fig. 3.
Activation of AMPK suppresses Kiss1 expression in the arcuate nucleus. In the Upper panels (A), dark-field photomicrographs showing Kiss1 mRNA expression (white clusters of silver grains) in representative sections of the AVPV and the ARC in the hypothalamus of pubertal female rats (PND33) chronically treated with vehicle or AICAR are presented. In addition, we display data from the quantification of Kiss1 expression in the above experimental groups. **P < 0.01 vs. corresponding control group and nucleus (ANOVA followed by Student–Newman–Keuls multiple range test) (n = 4–5 animals per group). 3V, third ventricle. In the Lower panels (B), confocal images are shown of the expression of kisspeptin and pAMPK in the ARC of pubertal female rats (PND36). In the Top row, low magnification images are shown for Kisspeptin (Left), pAMPK (Central Left), and DAPI (nuclear staining; Central Right); the image on the Right corresponds to the final merge of the three signals. Two different examples at higher magnification are shown in the additional two rows below. Colocalization is denoted by white arrowheads.
Immunohistochemical (IHC) colocalization experiments were also implemented to address whether (activated) AMPK is coexpressed in Kiss1 neurons. Based on our ISH data, and considering the documented fact that very few discernible immunoreactive kisspeptin cell bodies are identifiable in the AVPV of female rats (29), unless axonal transport is inhibited, particular attention was given to the ARC Kiss1 neuron population. IHC analyses revealed a set of kisspeptin-immunoreactive cells in the ARC of pubertal female rats, in line with previous references (27), with clearly distinguishable cell bodies and a dense network of fibers. Similar IHC approaches using an antibody against phosphorylated AMPK permitted identification of pAMPK-positive cells in the ARC, with a wider and more scattered pattern of distribution than Kiss1 neurons. Notably, while kisspeptin immunoreactivity was only cytoplasmic, pAMPK staining was also nuclear, as revealed by DAPI colabeling, in keeping with previous references in other cell types (30–32). Merging of kisspeptin and pAMPK IHC signals revealed coexpression of activated AMPK in Kiss1 neurons in the ARC of pubertal female rats (Fig. 3B). This was further documented by digital reconstruction and orthogonal 3D analyses of the IHC sections, which unambiguously documented the colocalization of kisspeptin and pAMPK-positive neurons in the ARC of pubertal female rats (SI Appendix, Fig. S4).
Conditional Ablation of AMPK in Kiss1 Neurons Prevents the Impact of Energy Deficit on Puberty Onset.
Our expression and functional data suggested that AMPK is well suited for transmitting metabolic information to Kiss1 neurons controlling puberty onset. To further address this putative role in a physiological setting, we generated a Kiss1 cell-specific AMPKα1 null mouse line by crossing a well-validated Kiss1-Cre/GFP mouse line (33), with the AMPKα1flox/flox mouse (34), thereby allowing congenital ablation of AMPKα1 in Kiss1 neurons. Allele-specific PCR in null animals demonstrated the successful deletion of AMPKα1 in the hypothalamic regions, namely preoptic area (POA) and the ARC, which hold the two prominent populations of Kiss1 neurons (SI Appendix, Fig. S5). In contrast, no Cre-mediated recombination was observed in the cortex of null mice, used as negative control, nor was it found in any of the tissues analyzed in the corresponding control (Cre negative) animals (SI Appendix, Fig. S5).
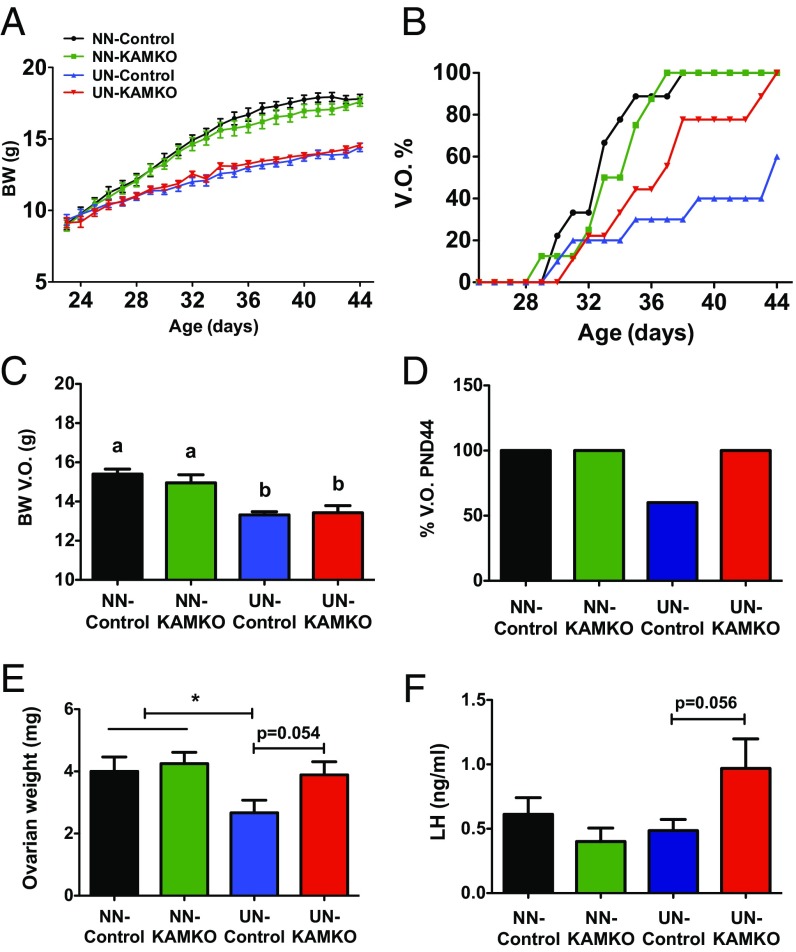
Kiss1-Cre/AMPKα1flox/flox [termed Kiss1-specific AMPK knockout (KAMKO)] mice were born at a Mendelian rate and females (KO and their littermate controls) were assessed for somatic and pubertal maturation postnatally. No differences in terms of BW gain were detected between genotypes before and after weaning, when animals were fed ad libitum (Fig. 4A). Likewise, fed animals did not display overt differences in relevant indices of puberty onset, including VO, BW at VO, percentage of animals with complete VO at PND44, ovarian weights, and LH levels, as surrogate marker of activation of the hypothalamic–pituitary–gonadal (HPG) axis (Fig. 4 B–F).
Fig. 4.
Genetic ablation of AMPKα1 in Kiss1 neurons prevents pubertal delay induced by subnutrition. A summary is presented of different markers of pubertal onset in mice with genetic ablation of the AMPKα1 subunit in Kiss1-expressing cells (Kiss1-Cre+/−; AMPKα1 loxP/loxP, named KAMKO), and their corresponding Kiss1-Cre−/−; AMPKα1 loxP/loxP controls. The animals were explored in two metabolic conditions: fed ad libitum (normal nutrition; NN) and after chronic subnutrition (20% reduction in daily food ration from PND23 onwards; UN). Data on evolution of BW (A) and percentage of VO (B), as well as final BW (C), VO (D), ovarian weight (OW) (E), and circulating LH levels (F) on PND44, are also shown. Group sizes were: NN-control = 9; NN-KAMKO = 8; UN-control = 10; UN-KAMKO = 9 animals per group. *P < 0.05 vs. corresponding control group (ANOVA followed by Student–Newman–Keuls multiple range test).
Since AMPK becomes activated in conditions of chronic negative energy balance (Fig. 1C), similar analyses were conducted in a model of pubertal UN, previously validated by our group to cause pubertal delay in rodents (35, 36). As expected, 20% reduction in the daily chow ration from PND23 onwards caused a decline in the BW gain curves, with an approximately 20% suppression in overall BW during the pubertal transition; no differences were detected between genotypes in response to UN (Fig. 4A). In control females subjected to UN, a marked delay in the course of VO and a significant decrease in ovarian weight at PND44 were observed, in line with a phenotype of deferred puberty. In clear contrast, and despite having similar BW as underfed controls (Fig. 4C), female mice with congenital ablation of AMPKα1 in Kiss1 neurons were partially protected from the delay in the course of VO caused by UN (Fig. 4B). In fact, all UN KAMKO mice displayed complete vaginal canalization at PND44, whereas only 60% of UN controls showed VO at this age (Fig. 4D). Of note, completion of VO in UN KAMKO mice occurred at a mean BW lower than that of control mice fed ad libitum. Furthermore, KAMKO mice subjected to UN showed a strong trend for increased ovarian weights and LH levels (P = 0.05), compared with control mice under the same feeding regimen (Fig. 4 E and F).
Discussion
The timing of puberty is fitted to metabolic and nutritional conditions of the organism. The translational relevance of such metabolic influence on puberty onset is epitomized by the dramatic impact of conditions of metabolic stress, including malnutrition, on the age of puberty in humans (12, 13). AMPK is a fundamental cellular energy gauge, which acting on discrete neuronal populations at different hypothalamic nuclei, plays an essential role in whole-body metabolic homeostasis, and its coordination with multiple vital functions and hormonal pathways (17). By a combination of gain- and loss-of-function studies, we disclose here the relevant role of AMPK signaling in ARC Kiss1 neurons for the metabolic control of puberty and its inhibition by conditions of energy insufficiency.
In line with its role as master cellular sensor of energy deficit (18), our data document that hypothalamic AMPK becomes activated in conditions of chronic energy deficiency at puberty, whereas central activation of AMPK, either pharmacological or virogenetic, suffices to delay pubertal onset to a variable extent, possibly depending on the effectiveness of AMPK targeting, thus partially mimicking what is observed in subnutrition. Of note, documentation of the delay in puberty onset was achieved by a combination of phenotypic and hormonal markers that, admittedly, did not include first estrus. This was mainly due to the fact that the delay of vaginal opening, as a robust and reliable sign of puberty onset in rodents (37), which was associated with activation of AMPK, caused a substantial number of animals to not undergo complete canalization of the vagina by the time of termination of some experiments. In any event, our findings disclose a pubertal dimension of brain AMPK signaling, which is compatible with previous fragmentary evidence suggesting that activation of AMPK might repress adult reproductive function, eventually via inhibition of key components of the reproductive brain (e.g., GnRH neurons) in adverse metabolic conditions, such as extreme glucoprivation, as suggested by electrophysiological studies (24). While the physiological connection between AMPK signaling and reproduction in adulthood still remains unsolved, our present data conclusively demonstrate that pubertal activation of the HPG axis is sensitive to the regulatory actions of central AMPK, suggesting a conserved repressive role of this key metabolic pathway in gating the acquisition and maintenance of reproductive capacity. Notably, central activation of AMPK at the time of puberty failed to alter food intake or body weight, a phenomenon that excludes the potential confounder impact of changes in body energy reserves on puberty onset and resembles the lack of feeding- or body weight-promoting actions of other orexigenic signals at the time of puberty, such as ghrelin (38, 39). A tenable explanation is that puberty is a physiological state of accelerated weight gain, in which the superimposed actions of orexigenic signals, such as AMPK, on body weight would be difficult to detect. However, it can drive a discernible inhibitory input to puberty onset.
Our results unveil also the neuroendocrine circuit underlying the repressive action of AMPK, which seemingly involves Kiss1 neurons in the ARC. This neuronal population is known to be sensitive to sex steroids and responsible for the pulsatile secretion of GnRH (16, 40). ARC Kiss1 neurons are also responsive to adverse metabolic conditions, ranging from subnutrition to obesity (12, 16). Moreover, recent optogenetic studies revealed that this population makes contacts and provides glutamatergic inputs to ARC proopiomelanocortin and agouti-related protein neurons, which operate as a key first-order neuronal hub for energy homeostasis (41). Although the precise percentage of colocalization could not be accurately established due to limitations in detecting the whole set of Kiss1 cell bodies in the ARC against the dense network of kisspeptin fibers, our data conclusively document that ARC Kiss1 neurons coexpress pAMPK, whose pharmacological activation causes not only pubertal delay but also a suppression of Kiss1 expression. Kiss1 has emerged in the last decade as master puberty-activating gene, whose hypothalamic expression in adulthood is congruently regulated by metabolic signals acting as permissive (e.g., leptin) or repressive (e.g., ghrelin) factors on puberty onset, which activate or suppress Kiss1 expression, respectively (42–44). A previous in vitro study, using the immortalized GnRH cell line, GT1–7, showed that pharmacological activation of AMPK was capable of suppressing Kiss1 expression (25); yet, the physiological relevance of such data is dubious, as GnRH neurons themselves do not express Kiss1 (16). Our results, showing that activation of AMPK in a physiological setting can suppress Kiss1 expression in the ARC, suggest this is a tenable pathway for suppression of puberty onset in metabolically compromised conditions. The lack of detectable effects of AMPK activation on Kiss1 expression in the AVPV, together with the genuine limitations of immunodetection of discernible cell bodies of kisspeptin-immunoreactive neurons in this rostral hypothalamic area in rats (29), prevented us from further exploring the potential contribution of AVPV Kiss1 neurons to this phenomenon, which nonetheless seems to be less tenable.
We have previously documented that hypothalamic signaling via the mammalian target of rapamycin (mTOR) interplays with Kiss1 in the metabolic control of puberty (28, 45). However, in line with its putative role as sensor that becomes activated in conditions of nutritional/energy surplus (46, 47), it was the blockade (not the activation) of mTOR that evoked a suppression of ARC Kiss1 levels and delayed puberty (28). Given the reciprocal interplay between mTOR and AMPK in numerous cellular systems (48, 49), it is plausible that the AMPK/mTOR duo may operate as a molecular conduit for the metabolic control of hypothalamic Kiss1 expression and puberty: conditions where AMPK activation prevails would suppress puberty onset and vice versa. Interestingly, the regulatory actions of mTOR seem to occur indirectly, as suggested by the fact that Kiss1 neurons do not express the downstream mediator of mTOR, pS6 (50). In contrast, at least a subset of Kiss1 neurons appears to be equipped with a functional AMPK signaling pathway, as denoted by our IHC and functional genomic studies, therefore suggesting a more proximal role of AMPK in transmitting metabolic information to Kiss1 cells. The putative upstream regulators of AMPK signaling in Kiss1 neurons warrant future investigation, but these might include the adipose hormone, adiponectin, which is known to modulate AMPK in GT1–7 cells (51, 52), as well as other hormonal signals, such as leptin and ghrelin, known to reciprocally regulate pubertal timing and AMPK in other neuronal systems (17, 53). Moreover, the possible involvement of ovarian steroids, such as estrogen and progesterone, which have been shown to modulate AMPK and fatty acid metabolism at the VMH, cannot be excluded (20, 54).
The notion that AMPK modulates puberty onset via regulation of Kiss1 is fully supported by our pubertal studies in KAMKO mice, with conditional ablation of AMPKα1 in Kiss1 cells. Genetic invalidation of AMPKα1 in Kiss1 neurons did not affect the timing of puberty in favorable/neutral nutritional conditions (i.e., animals fed ad libitum), but clearly attenuated the detrimental effects of chronic subnutrition on pubertal progression, as KAMKO females subjected to undernutrition displayed preserved vaginal opening and elevated ovarian weights and LH levels, as surrogate markers of pubertal activation, despite decreased body weight. This is fully compatible with a permissive role of AMPK signaling in Kiss1 neurons as a gating mechanism for puberty to proceed only in optimal metabolic conditions. Consequently, while suppression of AMPK signaling (e.g., by nutritional surplus) would not overtly alter pubertal timing, situations leading to AMPK activation, such as subnutrition, would result in delay or blockade of puberty, via repression of the pubertal heightening of Kiss1. In our genetic approach, we targeted the AMPKα1 subunit, since it is widely expressed in different hypothalamic areas and it has recently been shown to play a prominent role in the metabolic actions of AMPK, including the hypothalamic control of the thermogenic program of brown adipose tissue (22). In any event, we cannot rule out the possibility of partial developmental compensation of the lack AMPKα1 by AMPKα2 or alternative pathways. In fact, a recent report suggested that AMPKα2 in Kiss1 cells might also participate, albeit modestly, in reproductive adaptations to conditions of acute metabolic distress in adulthood (55). Moreover, the eventual contribution of AMPK signaling in non-Kiss1 neurons, including GnRH, to modulation of puberty remains a distinct possibility (24, 26), which is yet to be proven. Nonetheless, any eventual compensation in KAMKO mice did not abrogate the protective phenotype against chronic undernutrition imposed by congenital ablation of AMPKα1 in Kiss1 neurons, therefore stressing the functional relevance of this particular pathway.
In recent years, perturbations in the age of puberty have become more prevalent and a source of concern due to their potential long-term consequences, especially in terms of cardiometabolic health. Not only conditions of earlier puberty, putatively linked to child obesity, are showing escalating incidence, but also forms of pubertal delay, associated with nutritional or metabolic disorders ranging from anorexia to strenuous exercise or wasting diseases, may have deferred detrimental effects on the health span of the individual (8). Our data disclose a neuroendocrine circuit linking negative energy balance and pubertal timing, via a repressive AMPK–Kiss1 pathway. Besides its potential pathophysiological relevance, our data are of considerable translational interest, as they identify a tenable molecular target for refined strategies of central modulation of the onset of puberty. Indeed, early therapies with drugs known to activate AMPK, such as metformin (56), have been reported to delay menarche in girls at risk for precocious puberty (57). Moreover, metformin treatment in girls with precocious pubarche has been shown to reduce androgen excess and to ameliorate insulin resistance (58). While those clinical studies did not address the underlying mechanisms of metformin, nor did they dissect out central vs. peripheral sites of action, our current data pinpoint hypothalamic AMPK signaling in Kiss1 neurons as a putative pharmacological target of intervention to normalize/delay pubertal timing in conditions of increased risk of precocity, such as child obesity.
Methods
Animals.
Wistar rats and genetically modified C57Bl6 mice, engineered for conditional ablation of AMPKα1 in Kiss1 cells (namely, KAMKO mice; see below), bred in the vivarium of the University of Cordoba, were used. The animals were housed under constant conditions of light (12-h light/dark cycles) and temperature (22 ± 2 °C). The day the litters were born was considered day 1 of age (PND1). Animals were weaned at PND21 and provided with free access to tap water and fed ad libitum with a standard soy-free diet, unless otherwise indicated. The experiments and animal protocols included in this study were approved by the Ethical Committee of the University of Cordoba; all experiments were conducted in accordance with European Union (EU) normative for the use and care of experimental animals (EU Directive 2010/63/UE, September 2010).
Drugs.
The cell-permeable activator of AMPK, AICAR, was purchased from Phoenix Pharmaceuticals, Inc. 17β-estradiol (E2) was purchased from Sigma Chemical Co. AICAR was dissolved in physiological saline (0.9% NaCl), whereas E2 was dissolved in olive oil.
Treatments and Experimental Design.
The experimental studies included herein were implemented to investigate the function of AMPK signaling in the control of puberty onset in female rodents and to explore its potential interaction with Kiss1 neurons. When required, central administration of the different compounds was implemented using standard procedures of cannulation, following previously published procedures (28, 36). In brief, rats were subjected to icv cannulation 24 h before the beginning of the experiments. To this end, cannulas (INTRAMEDIC polyethylene tubing; Becton Dickinson) were inserted to a depth of 2 mm beneath the surface of the skull, with an insert point at 1 mm posterior and 1.2 mm lateral to Bregma, according to a rat brain atlas (59). For continuous central administration, an icv cannula was connected to a miniosmotic pump (ALZET osmotic pumps, model 2001; DURECT Corporation), which was implanted s.c. at the back of the neck. After cannulation, the animals were housed in individual cages until the end of the experiments. Correct positioning of icv cannulas was confirmed postmortem. Blood samples for hormone assays were obtained by decapitation at the end of the experiments. While grossly similar time windows for conducting the various experiments addressing pubertal maturation were applied, the actual dates for termination of each experiment were minimally adjusted depending on the specific conditions of the studies, as indicated in detail below. In any event, in all experiments, appropriate controls were run in parallel, thus allowing proper interpretation of data.
As an initial approach, in experiment 1, we evaluated the impact of conditions of negative energy balance on the hypothalamic levels of pAMPK in pubertal female rats. To this end, hypothalamic pAMPK content was analyzed in pubertal female rats (PND36) submitted to a protocol of chronic subnutrition. The animals were subjected to continuous caloric restriction of 30% (vs. littermate females fed ad libitum) between PND23 and PND36, by reducing their daily food ration proportionally. This protocol is fully validated and has been extensively used in our laboratory for studies on the neuropeptide and metabolic control of puberty (28, 35, 36). Dissection of hypothalamic blocks for protein analyses was conducted immediately upon killing of the animals, following previously published protocols (35, 60).
Based on initial results, in experiment 2, the impact of central pharmacological activation of AMPK signaling on puberty onset was assessed in peripubertal female rats. To this end, continuous infusion of AMPK activator, AICAR (1.5 mg/mL), was applied icv from PND30 to PND36 using a miniosmotic pump at a flow rate of 1 μL/h. Animals treated with vehicle served as control. BW and VO were daily monitored during the treatment. Animals were killed at PND36, and trunk blood, uterus, and ovaries were collected, on the basis of previous references (61). In addition, hypothalami were dissected out and processed for protein analysis of the AMPK pathway, as described in detail elsewhere (20, 22). Extrahypothalamic areas, namely, the cortex and hippocampus, were also dissected and included in protein analysis of AMPK activation (pAMPK), for comparative purposes.
The nucleus-specific intervention of AMPK signaling in the regulation of puberty onset was assessed in experiment 3. To this end, prepubertal rats were subjected to bilateral injection of adenoviruses expressing AMPK-CA or GFP (as control) into the ARC or VMH, as these two hypothalamic areas are well recognized to be involved in AMPK actions on energy balance (17). Viruses expressing GFP and AMPK-CA were obtained from ViraQuest. In brief, for expression of activated AMPK or GFP, 1 μL of virus (2.4 × 1010 particles per milliliter) was injected bilaterally in female rats at PND27 in the ARC [from Bregma anteroposterior (AP): −2.0 mm; lateral (L): ±0.3 mm; and dorsoventral (DV): −9.4 mm) or in the VMH (from Bregma AP: −2.0 mm; L: ±0.5 mm; and DV: −9.0 mm]. BW and VO were monitored daily and trunk blood was collected at the end of the experiment for measurement of LH. In addition, brains were harvested for verification of the injection. As a general procedure, visualization of the location of viral injections was achieved by coadministration of fluorescein isothiocyanate (Sigma Aldrich), in both Ad-GFP and Ad–AMPK-CA treated animals, the injection site being evaluated postmortem under a fluorescence microscope. Assessment of GFP in an additional set of animals was used to further confirm the effective infection capacity of our viral vector. Systematic analysis of fluorescein labeling allowed discrimination of proper hits (in the ARC or VMH) from off-target injections; the latter were excluded from further analyses.
As a consequence of the results from experiment 3, the involvement of Kiss1 neurons in mediating AMPK effects on puberty onset was evaluated in experiment 4. To this end, Kiss1 mRNA expression was analyzed by in situ hybridization in brain slices of rats submitted to continuous AICAR infusion, using the same dosing protocol as in experiment 2, between PND29 and PND33. To avoid the possible confounding effect of fluctuations of endogenous estrogen levels due to the pharmacological treatment upon Kiss1 mRNA expression, female rats were OVX at PND25 and replaced with a fixed dose of estradiol (E2) known to cause restoration of endogenous estrogen levels to physiological (low) range, in keeping with previous references (28). To this end, SILASTIC brand silicon tubing (i.d. 1.98 mm, o.d. 3.18 mm, and 12.5-mm length; Dow Corning) containing 10 µg/mL E2, dissolved in olive oil was implanted s.c. At the end of the treatment (PND33), animals were killed and brains were collected, frozen on dry ice, and stored at −80 °C for posterior in situ hybridization analyses, as described in detail below.
In an additional set of experiments, the potential direct action of AMPK signaling in Kiss1 neurons for mediating the metabolic control of puberty was evaluated. First, in experiment 5, we evaluated whether Kiss1 neurons coexpress the phosphorylated (active) form of AMPK, namely pAMPKα1/2 (Thr-172), by double immunofluorescence of Kisspeptin and phosphor-AMPK in pubertal (PND36) female rats (see details of the immunofluorescence protocol below). In addition, to further examine the potential direct contribution of AMPK signaling in Kiss1 neurons to control puberty onset, a genetically modified mouse line with congenital ablation of the catalytic domain of the AMPKα1 subunit specifically in Kiss1 neurons was generated, using a Cre-loxP strategy (see next section). The pubertal phenotype of this mouse was characterized in experiment 6, in which daily BW and VO were monitored from PND23 to PND44 in female mice fed ad libitum or subjected to 20% caloric restriction after weaning; the latter model was included as a means to assess the eventual role of AMPK signaling in Kiss1 neurons in mediating the negative impact of undernutrition on pubertal onset. At the end of the experiment, animals were killed, and trunk blood and ovaries were collected for specific analyses.
Generation of a Kiss1 Neuron-Specific AMPKα1 Null Mouse Line.
To address the potential role of AMPK signaling on Kiss1 neurons (see experiment 6), mice lacking the functional AMPKα1 catalytic subunit specifically in Kiss1-expressing cells were generated by crossing a well-validated mouse line carrying Cre-recombinase expression under the Kiss1 promoter, the Kiss1-Cre+/− mouse (33), with mice harboring LoxP sites flanking exon 3 of the Prkaa1 gene (AMPKα1loxP/loxP mouse, Prkaa1tm1.1Sjm/J; stock no. 014141; The Jackson Laboratory). This ensures specific Cre-dependent ablation of AMPKα1 signaling on Kiss1 neurons. Of note, the Kiss1-Cre mouse harbors a knockin dual Cre/GFP cassette, allowing location of Cre-expressing cells (33).
Kiss1-Cre+/− mice were initially mated with AMPKα1loxP/loxP animals. The resulting genotypes, Kiss1-Cre+/; AMPKα1loxP/−, were self-crossed to generate all of the possible genotypic combinations. For the experiments, only Kiss1-Cre+/−; AMPKα1 loxP/loxP (referred as KAMKO mouse) and Kiss1-Cre−/−; AMPKα1 loxP/loxP (referred as controls) were used.
Mice were genotyped using the following combination of primers for Kiss1-Cre: 13746, 5′-GAC CTA GGC TCT GGT GAA G-3′; 11493, 5′-GGC AAA TTT TGG TGT ACG GTC AG-3′; 13747, 5′-GAG CCT CCA GTG CTC ACA GC-3′; resulting in a DNA fragment of 337-bp for the endogenous wild type, and a 250-bp amplicon for the Cre allele. Determination of AMPKα1loxP alleles was done using the following primers: 11528, 5′-CCC ACC ATC ACT CCA TCT CT-3′; 11529 5′-AGC CTG CTT GGC ACA CTT AT-3′; resulting in a 334-bp amplicon for wild type, and a 450-bp fragment for the floxed allele (SI Appendix, Fig. S5).
Validation of the Specificity of KAMKO Mice.
To assess the specificity of the conditional ablation of exon 3 of the AMPKα1 (Prkaa1) gene in our mouse model, two complementary approaches were carried out. First, we demonstrated that Cre expression is restricted to Kiss1 neurons. To this end, we performed double immunohistochemical analyses in Kiss1-Cre+/− mice for the detection of kisspeptin and the GFP-tagged Cre (see details of immunohistochemistry below). To reduce the dense network of kisspeptin fibers in the ARC, as a means to facilitate visualization of Kiss1 cell bodies, colchicine (20 μg/5 μL/mouse) was administrated icv 24 h before the killing of the animals, following previously published recommendations (62). Representative examples are shown in SI Appendix, Fig. S5; analysis of the histochemical data demonstrated almost exclusive GFP-tagged Cre location in Kiss1-expressing areas (namely, AVPV and ARC), with an average 98% colocalization of GFP and kisspeptin signals.
In addition, effective recombination, denoting Cre activity and, subsequently, ablation of the exon 3 sequence of the AMPKα1 gene flanked by the loxP sites, was assessed by PCR in different brain areas, including those of high Kiss1 expression, namely areas containing the AVPV and the ARC, vs. areas devoid of endogenous Kiss1 expression, namely the cortex. To this end, an additional primer (delete allele 5′-CCC ACA TAG GAA AGC GTG TT-3′) was used in combination with the 11528 primer (see previous section) to detect the presence of a recombination event. A 530-bp amplicon was detected following recombination, while a 1,300-bp DNA fragment was obtained if recombination did not occur. As a note, the last band is amplified in all of the samples since non-Cre cells are present everywhere and it was used as loading control of DNA. For further details, see SI Appendix, Fig. S5.
Protein Analysis by Western Blot.
Total protein was extracted from hypothalamic blocks, as previously described (20). Briefly, total protein lysates (20 μg) were subjected to SDS/PAGE on 7% polyacrylamide gels, electrotransferred on polyvinylidene difluoride (PVDF) membranes (Millipore) and probed overnight at 4 °C in the presence of the following primary antibodies: Anti-phosphor-AMPKα (Thr-172, 1:1,000; ref. no. 2535; Cell Signaling); anti-phosphor-acetyl-CoA carboxylase (Ser-79); (1:1,000; ref. no. 3661; Cell Signaling); anti-acetyl-coA carboxylase 1 (1:1,000; ref. no. 04–322; Merck Millipore); anti-AMPKα1 (1:1,000; ref. no. 07–350; Merck Millipore); and anti-AMPKα2 (1:1,000; ref. no. 07–363; Merck Millipore). For protein detection, we used horseradish peroxidase (HRP)-conjugated secondary antibodies and chemiluminescence (anti-rabbit, 1:5,000; ab6721; Abcam). A total of 5–6 samples per group were assayed; protein levels were normalized to β-actin (1:5,000, A5060; Sigma Aldrich). Densitometric analysis of protein bands was conducted using the open source image processing software, ImageJ (https://imagej.net/ImageJ).
ISH.
For analysis of Kiss1 mRNA expression in the hypothalamus, brains were collected from selected experimental groups and ISH analyses were performed, following previous references (63). In brief, five sets of coronal brain sections (20 μm thick) encompassing from rostral to caudal hypothalamus were generated, mounted on Super-Frost Plus slides (Thermo Fisher Scientific, Inc.), and stored at −80 °C until ISH analyses.
A specific antisense-specific riboprobe for Kiss1 rat mRNA, spanning 83–371 nt of the cDNA sequence (GenBank NM_181692.1) was generated according to a previous protocol (63). A single set of sections was used for ISH (adjacent sections 100 μm apart). These tissue sections were fixed in 4% PFA, acetylated in triethanolamine buffer, dehydrated in increasing concentrations of ethanol, and delipidated with chloroform. After these steps, hybridization with Kiss1 riboprobe was performed during 16 h at 55 °C. Kiss1 riboprobe was diluted in hybridization buffer to a final concentration of 0.03 pmol/mL along with yeast tRNA. After hybridization, slides were washed, treated with RNase-A, and dehydrated in increasing ethanol series as previously described (63). Finally, slides were dipped in Kodak autoradiography emulsion type NTB (Eastman Kodak) and exposed for 2 wk at 4 °C in the dark. After this period, the sections were developed and fixed following manufacturer instructions (Kodak). Then, slices were coverslipped with Sub-X mounting medium (Leica). For analysis, 54–60 sections from each animal (9–10 slides, 6 sections per slide) were evaluated. Five animals per group were included in the analysis. Slides were read under dark-field illumination with custom-designed software and enabled to count the total number of cells (grain clusters). Cells were counted as Kiss1 mRNA-positive when the number of silver grains in a cluster exceeded that of background.
Double Immunohistochemistry.
For preparation of the brain for immunohistochemistry, rats were anesthetized with an i.p. injection of ketamine/xylazine mixture and perfused intracardially with 0.9% saline followed by 4% PFA (pH 7.6). After removal, brains were postfixed in the same fixative (4 °C, 24 h), washed in PBS (4 °C for 24 h), and dehydrated in sucrose (30% in 0.1 M PBS for 24–48 h). Brains were cut and divided in three sets of coronal sections (30 μm) in a freezing microtome Leica CM1850 UV. One set of free-floating sections was used for incubation with different combinations of antibodies against phosphorylated AMPKα1/2 (Thr-172) (sc-33524; Santa Cruz Biotechnology, Inc.), sheep anti-rat/mouse Kisspeptin no. 017, rabbit anti-rat/mouse Kisspeptin no. 566 (both kisspeptin antibodies were a generous gift from A. Caraty, Physiologie de la Reproduction et des Comportements-Institut National de la Recherche Agronomique, Nouzilly, France), or GFP (Ab13970; Abcam).
Antigen retrieval was performed in 10 mM sodium citrate buffer, pH 6, for 10 min at 90 °C. Then, sections were removed from heat and allowed to cool down at room temperature (20 min). After washing, sections processed for immunofluorescence were incubated for 48 h at 4 °C with rabbit anti-pAMPKα1/2 (Thr-172) polyclonal antibody (1:100) and sheep anti-rat/mouse Kisspeptin polyclonal antibody (1:5,000) in incubation buffer (0.25% donkey serum and 0.3% Triton X-100 in TBS). Previously, Kisspeptin antibody was preincubated with thyroglobulin for 24 h at 4 °C to decrease the background, since the immunogen used to obtain the antibody consisted of 10 amino acids from rat Kisspeptin sequence attached to thyroglobulin to increase the immunogenicity. After washing the slices three times in TBS (10 min per wash), they were incubated with a biotinylated donkey anti-rabbit secondary antibody (Jackson Immunoresearch, Inc.) at 1:200 in incubation buffer for 90 min at room temperature. After three more washes, the sections were incubated with FITC-conjugated donkey anti-sheep (Jackson Immunoresearch, Inc.) at 1:100 and Texas Red streptavidin (Vector Laboratories) at 1:200 in incubation buffer, for 2 h at room temperature. Finally, the sections were washed thoroughly in TBS, mounted on gelatin-coated slides, air dried, and coverslipped with Vectashield mounting medium (Vector Laboratories).
For double Nickel-3,3′-Diaminobenzidine (nickel-DAB)/DAB staining, the tissue sections were subjected to a protocol similar to that indicated above for immunofluorescence, with slight modifications. Sections were incubated sequentially for 72 h at 4 °C with chicken anti-GFP polyclonal antibody (1:40,000) and rabbit anti-rat/mouse Kisspeptin polyclonal antibody (1:10,000). For detection of GFP (black; using Nickel-DAB as chromogen) and Kisspeptin (brown; using DAB as chromogen), Vector Elite ABC peroxidase kit (Vector Laboratories) was used after incubation with biotinylated donkey anti-chicken secondary antibody (1:500) and biotinylated donkey anti-rabbit secondary antibody (1:500), respectively. Single pAMPK immunostaining was achieved using the above Nickel-DAB protocol for the detection of pAMPK-positive cells, using rabbit anti-pAMPKα1/2 (Thr-172) polyclonal antibody (1:5,000).
As a general procedure, negative controls, generated by omission of primary antibodies, were routinely included and processed in parallel; these analyses verified the specificity of immunodetection, as no immunoreactivity was detected in those samples.
Image Processing.
For double immunofluorescence, immunoreactivity was visualized on a Zeiss LSM 710 confocal system. Confocal images were taken, and Z stacks were condensed to maximum intensity 2D projections using ImageJ (NIH). When relevant, the resulting images were processed for 3D reconstructions using Imaris Software (Bitplane, Oxford Instruments).
For quantification of pAMPK immunoreactivity in different brain areas, images were taken with a Leica DM2500 microscope and processed using ImageJ (NIH). The content of pAMPK was presented as the percentage of the specific brain area occupied by pAMPK cells. Brain areas were delimited using as reference the mouse coronal section from the Allen Brain Atlas (mouse.brain-map.org/static/atlas).
Hormone Measurements.
Serum LH levels in mice and rats were determined using the double-antibody method and radioimmunoassay kits supplied by the National Institute of Health, NIH (A. F. Parlow National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program, Bethesda, MD). Hormone provided by NIH (LH-I-10) was labeled with 125I using the Iodo-gen method (Pierce). The hormone concentrations were expressed using reference preparations of LH-RP-3. The sensitivity of the assay was 75 pg/mL. Intra- and interassay coefficients of variation (CV) were lower than 8%. The reliability of the hormone determinations was confirmed by measurement of rat serum samples with known concentrations of LH.
Presentation of Data and Statistics.
Data of BW, absolute organ weights, ISH, hormonal levels, and protein quantification values are expressed as the mean ± SEM. Hormonal determinations were conducted with a minimal total number of 8–16 determinations per group. ISH and IHC assays were conducted with a minimum of four to five animals per group. Results were analyzed using Student t test or ANOVA followed by Student–Newman–Keuls multiple range tests (Prism GraphPad 5.0 software; GraphPad Software, Inc.). Significance level was set at P ≤ 0.05 and different letters or asterisks indicate statistical significance.
Supplementary Material
Acknowledgments
This work was supported by Grants BFU2011-025021 and BFU2014-57581-P (to M.T.-S.), and SAF2015-71026-R (to M.L.) from the Ministerio de Economía y Competitividad, Spain, cofunded with EU funds from the Fondo Europeo de Desarrollo Regional (FEDER) Program; Project PIE14-00005 (to M.T.-S.) Flexi-Met, Instituto de Salud Carlos III, Ministerio de Sanidad; Projects P08-CVI-03788 and P12-FQM-01943 (to M.T.-S.) Junta de Andalucía, Spain; Projects 2015-CP079 and 2016-PG068 (to M.L.) Xunta de Galicia; and EU Research Contracts DEER FP7-ENV-2007-1 (to M.T.-S.) and FP7/2007-2013 (ObERStress). M.P. and M.T.-S. are recipients of a grant from the Finnish Distinguished Professorship (FiDiPro) Program (Academy of Finland). J.R. was a recipient of a postdoctoral research contract from the Spanish Ministerio de Economía y Competitividad (Juan de la Cierva Program; JCI-2012-12681). T.I. was supported by the Scientific and Technological Research Council of Turkey. Centro de Investigación Biomédica en Red (CIBER), Fisiopatología de la Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III. Senior authors are indebted to R. A. Steiner (University of Washington, Seattle) for provision of relevant mouse lines, essential for conducting some of the experiments included in this study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802053115/-/DCSupplemental.
References
- 1.Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: Beyond the hypothalamus and feeding. Cell Metab. 2015;22:962–970. doi: 10.1016/j.cmet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. 2015;38:73–88. doi: 10.1016/j.yfrne.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomniczi A, Wright H, Ojeda SR. Epigenetic regulation of female puberty. Front Neuroendocrinol. 2015;36:90–107. doi: 10.1016/j.yfrne.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parent AS, et al. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 5.Aksglaede L, Juul A, Olsen LW, Sørensen TI. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4:e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Leonibus C, et al. Timing of puberty and physical growth in obese children: A longitudinal study in boys and girls. Pediatr Obes. 2014;9:292–299. doi: 10.1111/j.2047-6310.2013.00176.x. [DOI] [PubMed] [Google Scholar]
- 7.Herman-Giddens ME, et al. Secondary sexual characteristics in boys: Data from the Pediatric Research in Office Settings network. Pediatrics. 2012;130:e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- 8.Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: The UK Biobank study. Sci Rep. 2015;5:11208. doi: 10.1038/srep11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakshman R, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- 10.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci. 2013;70:841–862. doi: 10.1007/s00018-012-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roa J. Role of GnRH neurons and their neuronal afferents as key integrators between food intake regulatory signals and the control of reproduction. Int J Endocrinol. 2013;2013:518046. doi: 10.1155/2013/518046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellano JM, Tena-Sempere M. Metabolic control of female puberty: Potential therapeutic targets. Expert Opin Ther Targets. 2016;20:1181–1193. doi: 10.1080/14728222.2016.1212015. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz-Calvo MT, Argente J. Nutritional and pubertal disorders. Endocr Dev. 2016;29:153–173. doi: 10.1159/000438884. [DOI] [PubMed] [Google Scholar]
- 14.Coyral-Castel S, et al. The effect of AMP-activated kinase activation on gonadotrophin-releasing hormone secretion in GT1-7 cells and its potential role in hypothalamic regulation of the oestrous cyclicity in rats. J Neuroendocrinol. 2008;20:335–346. doi: 10.1111/j.1365-2826.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 15.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 17.López M, Nogueiras R, Tena-Sempere M, Diéguez C. Hypothalamic AMPK: A canonical regulator of whole-body energy balance. Nat Rev Endocrinol. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 18.Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–37. doi: 10.1016/j.ceb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez de Morentin PB, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittle AJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Sanchez N, et al. Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 2017;26:212–229.e12. doi: 10.1016/j.cmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roa J, Tena-Sempere M. Connecting metabolism and reproduction: Roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014;397:4–14. doi: 10.1016/j.mce.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Roland AV, Moenter SM. Glucosensing by GnRH neurons: Inhibition by androgens and involvement of AMP-activated protein kinase. Mol Endocrinol. 2011;25:847–858. doi: 10.1210/me.2010-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen JP, et al. Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1-7 neurons. J Endocrinol. 2012;214:177–189. doi: 10.1530/JOE-12-0054. [DOI] [PubMed] [Google Scholar]
- 26.Minabe S, et al. Pharmacological and morphological evidence of AMPK-mediated energy sensing in the lower brain stem ependymocytes to control reproduction in female rodents. Endocrinology. 2015;156:2278–2287. doi: 10.1210/en.2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: Developmental roles and major regulatory actions. J Neuroendocrinol. 2012;24:22–33. doi: 10.1111/j.1365-2826.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 28.Roa J, et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150:5016–5026. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- 29.Overgaard A, et al. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides. 2013;45:85–90. doi: 10.1016/j.peptides.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Ju TC, et al. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. J Cell Biol. 2011;194:209–227. doi: 10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ríos M, et al. AMPK activation by oncogenesis is required to maintain cancer cell proliferation in astrocytic tumors. Cancer Res. 2013;73:2628–2638. doi: 10.1158/0008-5472.CAN-12-0861. [DOI] [PubMed] [Google Scholar]
- 32.Doménech E, et al. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nat Cell Biol. 2015;17:1304–1316. doi: 10.1038/ncb3231. [DOI] [PubMed] [Google Scholar]
- 33.Gottsch ML, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castellano JM, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 36.Navarro VM, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397. doi: 10.1523/JNEUROSCI.4288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaytan F, et al. Development and validation of a method for precise dating of female puberty in laboratory rodents: The puberty ovarian maturation score (pub-score) Sci Rep. 2017;7:46381. doi: 10.1038/srep46381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Fernández R, et al. Effects of chronic hyperghrelinemia on puberty onset and pregnancy outcome in the rat. Endocrinology. 2005;146:3018–3025. doi: 10.1210/en.2004-1622. [DOI] [PubMed] [Google Scholar]
- 39.Roa J, et al. Metabolic control of puberty onset: New players, new mechanisms. Mol Cell Endocrinol. 2010;324:87–94. doi: 10.1016/j.mce.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12:452–466. doi: 10.1038/nrendo.2016.70. [DOI] [PubMed] [Google Scholar]
- 41.Nestor CC, et al. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30:630–644. doi: 10.1210/me.2016-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 43.Castellano JM, et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 44.Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett. 2009;460:143–147. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 45.Roa J, Tena-Sempere M. Energy balance and puberty onset: Emerging role of central mTOR signaling. Trends Endocrinol Metab. 2010;21:519–528. doi: 10.1016/j.tem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Martínez de Morentin PB, et al. Hypothalamic mTOR: The rookie energy sensor. Curr Mol Med. 2014;14:3–21. doi: 10.2174/1566524013666131118103706. [DOI] [PubMed] [Google Scholar]
- 47.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 50.Quennell JH, et al. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen JP, et al. Globular adiponectin inhibits GnRH secretion from GT1-7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun. 2008;371:756–761. doi: 10.1016/j.bbrc.2008.04.146. [DOI] [PubMed] [Google Scholar]
- 52.Cheng XB, et al. GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine. 2011;39:6–12. doi: 10.1007/s12020-010-9375-8. [DOI] [PubMed] [Google Scholar]
- 53.Avendaño MS, Vazquez MJ, Tena-Sempere M. Disentangling puberty: Novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum Reprod Update. 2017;23:737–763. doi: 10.1093/humupd/dmx025. [DOI] [PubMed] [Google Scholar]
- 54.Martínez de Morentin PB, et al. Pregnancy induces resistance to the anorectic effect of hypothalamic malonyl-CoA and the thermogenic effect of hypothalamic AMPK inhibition in female rats. Endocrinology. 2015;156:947–960. doi: 10.1210/en.2014-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torsoni MA, et al. AMPKα2 in Kiss1 neurons is required for reproductive adaptations to acute metabolic challenges in adult female mice. Endocrinology. 2016;157:4803–4816. doi: 10.1210/en.2016-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Ibáñez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. Early metformin therapy to delay menarche and augment height in girls with precocious pubarche. Fertil Steril. 2011;95:727–730. doi: 10.1016/j.fertnstert.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 58.Ibáñez L, López-Bermejo A, Díaz M, Marcos MV, de Zegher F. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab. 2011;96:E1262–E1267. doi: 10.1210/jc.2011-0555. [DOI] [PubMed] [Google Scholar]
- 59.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic; San Diego: 2001. [Google Scholar]
- 60.Navarro VM, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 61.Watanobe H, et al. Further evidence for a significant participation of the melanocortin 4 receptor in the preovulatory prolactin surge in the rat. Brain Res Bull. 2001;54:521–525. doi: 10.1016/s0361-9230(01)00442-7. [DOI] [PubMed] [Google Scholar]
- 62.Marshall CJ, Desroziers E, McLennan T, Campbell RE. Defining subpopulations of arcuate nucleus GABA neurons in male, female, and prenatally androgenized female mice. Neuroendocrinology. 2017;105:157–169. doi: 10.1159/000452105. [DOI] [PubMed] [Google Scholar]
- 63.Manfredi-Lozano M, et al. Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol Metab. 2016;5:844–857. doi: 10.1016/j.molmet.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.