Significance
Although millions of procedures are performed under general anesthesia every year, we do not fully understand the mechanisms underlying anesthesia. Orexin neurons in the hypothalamus form one of the centers in the central nervous system involved in sleep–wake control. The orexin system has also been implicated in anesthesia. In this study, we specifically activated orexin neurons via a designer receptor that is exclusively activated by a designer drug, to test how activation of orexin neurons affects anesthesia recovery. We found that orexin neuronal activation can speed up wakeup and improve pain control as well. Our study suggests that the orexin system has the potential as a target for drug discovery to facilitate postprocedural recovery.
Keywords: orexin, hypocretin, DREADD, anesthesia, pain
Abstract
Orexin (also known as hypocretin) neurons in the hypothalamus play an essential role in sleep–wake control, feeding, reward, and energy homeostasis. The likelihood of anesthesia and sleep sharing common pathways notwithstanding, it is important to understand the processes underlying emergence from anesthesia. In this study, we investigated the role of the orexin system in anesthesia emergence, by specifically activating orexin neurons utilizing the designer receptors exclusively activated by designer drugs (DREADD) chemogenetic approach. With injection of adeno-associated virus into the orexin-Cre transgenic mouse brain, we expressed the DREADD receptor hM3Dq specifically in orexin neurons and applied the hM3Dq ligand clozapine to activate orexin neurons. We monitored orexin neuronal activities by c-Fos staining and whole-cell patch-clamp recording and examined the consequence of orexin neuronal activation via EEG recording. Our results revealed that the orexin–DREADD mice with activated orexin neurons emerged from anesthesia with significantly shorter latency than the control mice. As an indication of reduced pain sensitivity, these orexin–DREADD mice took longer to respond to the 55 °C thermal stimuli in the hot plate test and exhibited significantly less frequent licking of the formalin-injected paw in the formalin test. Our study suggests that approaches to activate the orexin system can be beneficial in postoperative recovery.
Millions of procedures are performed under general anesthesia every year. However, many questions regarding the mechanism for anesthesia remain to be answered. Both anesthesia and sleep involve the orexin/hypocretin system, first identified in 1998 (1, 2). In addition to sleep–wake control and feeding, orexin is involved in autonomic regulation, thermoregulation, neuroendocrine regulation, and memory formation (3–7). As expected from the broad range of physiological functions, orexin neurons project widely throughout the brain and the spinal cord (8–10). Orexin neurons may activate histaminergic tuberomammillary neurons (11), cholinergic basal forebrain neurons, ventral tegmental area (VTA) dopaminergic neurons (12), and noradrenergic locus coeruleus (LC) neurons (13) to modulate sleep–wake control. Among the neurons involved in sleep–wake modulation, the orexin system is unique for its use of peptidergic transmission.
The orexin system has been implicated in sleep and metabolic disorders. Canine narcolepsy is caused by a mutation in the orexin receptor-2 gene (14). Orexin knockout mice have narcoleptic symptoms, whereas mice with their orexin neurons ablated display additional symptoms like hypophagia and severe obesity. Some human narcoleptic patients have undetectable levels of orexin in their cerebrospinal fluid (15), though the plasma levels of orexin remain normal (16). Suvorexant, a selective dual orexin receptor antagonist, was approved by the US Food and Drug Administration for treatment of insomnia in 2014 (17). Although small molecule agonists of orexin receptor-2 have been reported to ameliorate narcolepsy–cataplexy symptoms in mouse models (18), there has been no clinical trial thus far.
Orexin neurons may play a role in the process of general anesthesia, especially during the recovery phase and the transition to wakefulness. With intracerebroventricular (ICV) injection or direct microinjection of orexin into certain brain regions, previous studies have shown that local infusion of orexin can shorten the emergence time from i.v. or inhalational anesthesia (19–23). In addition, the orexin system is involved in regulating upper airway patency, autonomic tone, and gastroenteric motility (24). Orexin-deficient animals show attenuated hypercapnia-induced ventilator response and frequent sleep apnea (25). ICV injections of orexin peptides have been shown to increase respiration rate, heart rate, blood pressure, and gastric motility (3, 4, 26). The emergence speed, autonomic stability, airway patency, and gastric motility are all important aspects during the recovery from anesthesia.
Orexin neurons not only innervate neurons in the hypothalamus, but also send projections to remote sites throughout the brain and the spinal cord. To study how anesthesia may be affected upon the specific activation of orexin neurons, we utilized designer receptors exclusively activated by designer drugs (DREADD) as a chemogenetic tool to activate orexin neurons. DREADD receptors are engineered muscarinic receptors that are no longer sensitive to any native ligands such as acetylcholine, but can be activated by the inert small molecule ligand clozapine-N-oxide (CNO). A recent study revealed that clozapine, an antipsychotic drug and a metabolite of CNO, has an even higher affinity to the DREADD receptor than CNO (27). Although clozapine is not a true inert drug, only subtherapeutic doses are needed to activate the DREADD receptors. With this technology, we investigated the function of orexin neuronal activities in two important aspects of anesthesia recovery: emergence speed and pain control.
Results
Expression of the DREADD Receptor hM3Dq in Orexin Neurons.
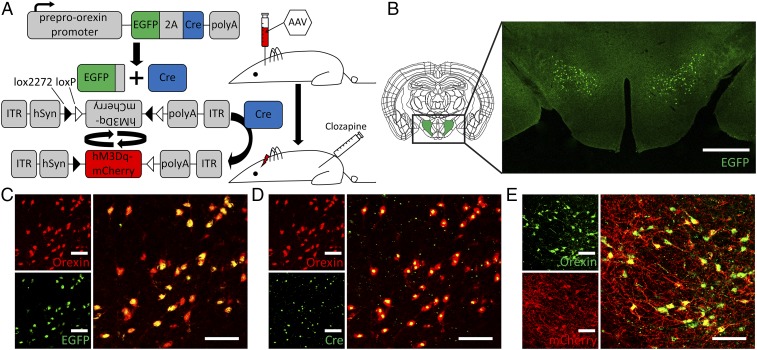
In the orexin-Cre mouse line generated from a frozen embryo provided by Akihiro Yamanaka, Nagoya University, Nagoya, Japan (28), the Cre recombinase was fused with a 2A peptide from the Thosea asigna virus and EGFP, and the expression was under the control of the human preproorexin promoter (Fig. 1A). The 2A peptide is separated from EGFP and the Cre recombinase via self-cleaving after translation. Our immunohistochemical studies revealed that EGFP and the Cre recombinase were exclusively expressed in the perifornical area of lateral hypothalamus (Fig. 1B). We found colocalization of EGFP, Cre, and orexin in orexin neurons (Fig. 1 C and D).
Fig. 1.
hM3Dq DREADD expression in orexin-Cre mice. (A) Transgenic mice expressing EGFP and Cre recombinase under the preproorexin promoter were stereotaxically injected with AAV containing a flipped hM3Dq DREADD gene fused with mCherry [AAV-hSyn-DIO-hM3D(Gq)-mCherry]. This experimental setup allowed specific expression of the hM3Dq DREADD in orexin neurons. (B) The cell bodies of orexin neurons are located at the lateral hypothalamic perifornical area, as revealed by the immunostaining of EGFP. Double labeling of the orexin neuropeptide with EGFP (C), Cre (D), or mCherry (E) indicates specific expression of the hM3Dq receptor in orexin neurons. The enlarged image is the merge of the two panels on the Left. C and D are images from the same animal. We found that 93.8 ± 4.3% (n = 5) orexin neurons express the DREADD receptor. The native fluorescence of EGFP or mCherry cannot be detected after the fixation and staining process. The signals shown here are from fluorescently labeled antibodies recognizing EGFP or mCherry. The different staining pattern for mCherry reflects the fusion of mCherry with the hM3Dq receptor that is expressed on the cell membrane. (Scale bars: 500 μm in B, 100 μm in C–E.)
To express the DREADD receptor hM3Dq in orexin neurons, we used the Cre-dependent adeno-associated viral (AAV) vector, pAAV-hSyn-DIO-hM3D(Gq)-mCherry, from Addgene. This AAV construct contains a FLEX switch, which includes loxP and lox2272, to ensure stable expression of hM3Dq only in Cre-expressing cells (Fig. 1A). AAV-hSyn-DIO-hM3D(Gq)-mCherry was stereotaxically injected into the lateral hypothalamus of 8-wk-old orexin-Cre male mice. The mCherry fused with hM3Dq allowed identification of orexin neurons expressing the DREADD receptor hM3Dq, with strong mCherry fluorescence in the processes and the soma of orexin neurons (Fig. 1E). A control group of wild-type animals that also received the AAV injection showed no expression of DREADD receptors (SI Appendix, Fig. S1).
Activation of Orexin Neurons via the DREADD Receptor hM3Dq.
After confirming the expression of hM3Dq in orexin neurons, we tested whether activation of hM3Dq led to increased orexin neuronal activities, both by monitoring orexin neuronal activity via whole-cell patch-clamp recording and by using c-Fos expression as a reporter of neuronal activities. After a minimum of 2 wk following viral injection, the mice were intraperitoneally injected with CNO (1 mg/kg) or clozapine (0.01 mg/kg) and killed 45 min later. Taking into account the diurnal activities of orexin neurons, we injected the DREADD ligand at 10:00 AM (zeitgeber time 4), when the orexin neuronal activities are low, so as to induce a greater change of neuronal activity.
Given recent studies showing that clozapine is the true high-affinity ligand of hM3Dq, we compared clozapine with CNO in their abilities to activate neurons and elevate c-Fos expression. We found that both can activate c-Fos, but clozapine at 0.01 mg/kg generated stronger c-Fos signal compared with CNO at 1 mg/kg (SI Appendix, Fig. S2). Therefore, we chose to use clozapine to activate the DREADD receptor hM3Dq in our experiments.
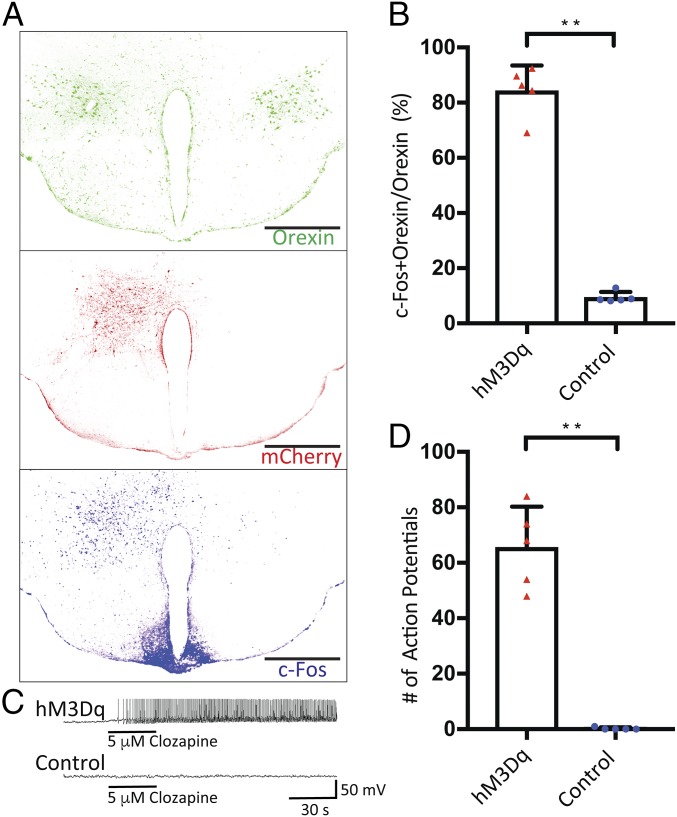
We injected the AAV into only the hypothalamus in one hemisphere, which resulted in unilateral expression of hM3Dq-mCherry (Fig. 2A), so that the hypothalamus in the other hemisphere provides an internal control. After clozapine injection to activate hM3Dq, c-Fos expression was higher on the hemisphere with hM3Dq expression compared with the other hemisphere without the receptor (Fig. 2A); 84.4 ± 9.1% of orexin neurons in the injected hemisphere (304 orexin neurons counted in five mice) were positive for c-Fos and orexin, whereas only 9.3 ± 1.7% of orexin neurons in the uninjected hemisphere (323 orexin neurons counted in the same five mice) were positive for c-Fos and orexin (Fig. 2B). The strong c-Fos signal in the arcuate nuclei likely arose from the dense innervation by orexin neurons (6). In contrast to the DREADD group of transgenic mice with Cre expression in orexin neurons, the control group of wild-type animals also received the AAV injection but yielded little c-Fos signal (SI Appendix, Fig. S1).
Fig. 2.
Pharmacogenetic activation of orexin neurons through hM3Dq. (A) Unilateral expression of hM3Dq tagged with mCherry in animals with AAV-hSyn-DIO-hM3D(Gq)-mCherry injected into one hemisphere. c-Fos staining revealed neuronal activation near the injection site after the i.p. administration of 0.01 mg/kg of clozapine. The other hemisphere provides internal control for clozapine activation of hM3Dq receptor specifically expressed in the hemisphere with AAV injection. This demonstrates that only the orexin neurons with the DREADD receptor can be activated through the administration of clozapine. Five animals were unilaterally injected with the AAV. Each image is a composite image of 3 × 3 tiles stitched by Leica SP8 confocal microscope software. (Scale bars: 500 μm.) (B) A total of 84.4 ± 9.1% of orexin neurons in the injected hemisphere (304 orexin neurons counted in five mice) were positive for c-Fos and orexin, whereas only 9.3 ± 1.7% of orexin neurons in the uninjected hemisphere (323 orexin neurons counted in the same five mice) were positive for c-Fos and orexin. (C) Whole-cell patch-clamp recordings from cells with positive mCherry fluorescence (hM3Dq) and negative mCherry flurorescence (control). Local application of 5 μM clozapine in aCSF solution near the cell under recording-induced rapid firing of action potentials in the hM3Dq-positive cells. (D) A total of 65.6 ± 14.7 (n = 5) action potentials were recorded during the first minute following the clozapine application, whereas 0.2 ± 0.45 were recorded in the control cells. All data are expressed as mean ± SD. Significance was analyzed using two-tailed t test, **P < 0.01.
Having examined c-Fos expression to reveal increased neuronal activities in orexin neurons and their downstream neurons in the DREADD group, we next tested for the specific activation of orexin neurons, by recording from acute brain slices expressing DREADD receptors. We selected neurons that were positive for the mCherry fluorescence for whole-cell patch-clamp recordings. Application of 5 μM clozapine locally to the orexin neuron under recording induced rapid and sustained firing of action potentials (Fig. 2C); 65 ± 14 (n = 5) action potentials were recorded during the first minute following the clozapine application in the hM3Dq-positive cells, whereas 0.2 ± 0.45 were recorded in the control cells (Fig. 2D). Cells that did not express the hM3Dq receptors exhibited no change in action potential firing upon the application of clozapine.
Activation of Orexin Neurons Facilitates Emergence from Anesthesia.
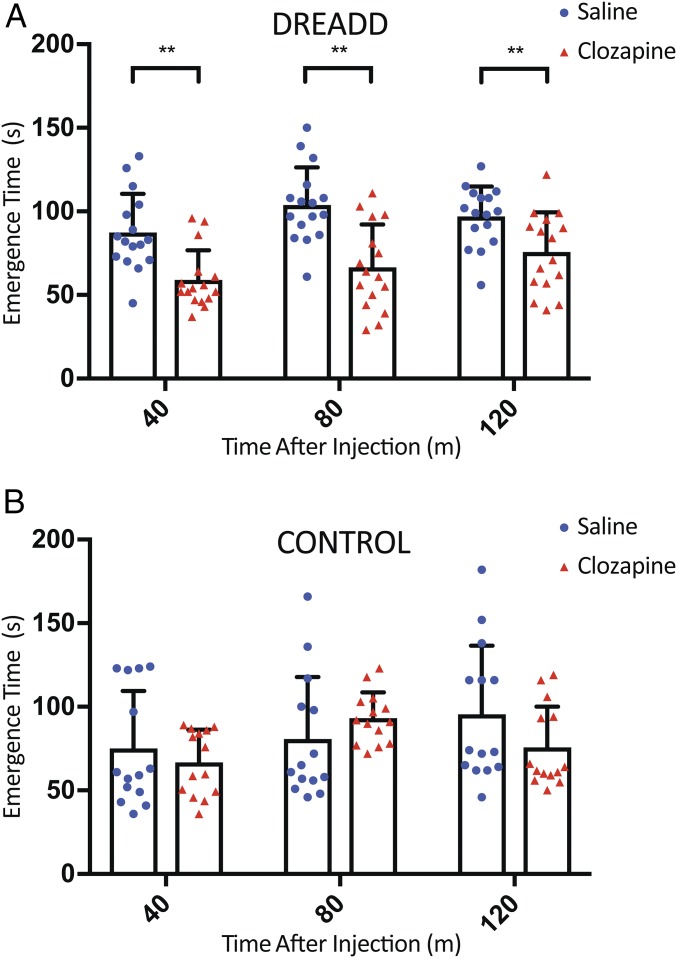
To test whether activation of orexin neurons could change the speed of emergence from anesthesia, we performed the emergence test of mice under isoflurane anesthesia. Similar to the timing for the c-Fos staining, the experiment was performed during the day when the orexin neuronal activity was low. Clozapine or saline was intraperitoneally injected into orexin-Cre mice that had received bilateral stereotaxic injections of AAV-hSyn-DIO-hM3D(Gq)-mCherry at least 2 wk before the i.p. injection (Fig. 3A). After 10 min of recovery following the i.p. injection, the animals were exposed to 2% isoflurane for 30 min. The duration from the end of the isoflurane exposure to the return of the righting reflex (RoRR) was recorded (emergence time). This procedure was repeated two more times with 10 min of recovery in between, so the emergence time was assessed at 40, 80, and 120 min after the i.p. injection. Transgenic orexin-Cre mice without AAV injections received the same dose of clozapine or saline and served as control, revealing no significant effect of clozapine on emergence from anesthesia (Fig. 3B).
Fig. 3.
Activation of orexin neurons facilitates the emergence from anesthesia. Eight-week-old transgenic orexin-Cre mice were injected bilaterally with AAV-hSyn-DIO-hM3D(Gq)-mCherry and allowed a minimum of 2 wk for viral expression. Ten minutes before the anesthesia exposure, the mice received i.p. injection of clozapine (0.01 mg/kg) or saline. (A) The time it took for the righting reflex to return after 30 min of 2% isoflurane exposure. Each mouse was subjected to three repetitions of the test. The emergence time for the clozapine group (59.1 ± 17.7, 66.5 ± 25.7, 75.8 ± 23.6 s; n = 16) was shorter than that of the saline group (87.4 ± 23.0, 103.9 ± 22.5, 97.1 ± 17.8 s; n = 16) at 40, 80, and 120 min following the i.p. injection, respectively. (B) There was no significant difference in the emergence time between the clozapine group (66.8 ± 19.5, 93.3 ± 15.4, 75.8 ± 24.3 s; n = 14) and the saline group (75.0 ± 34.5, 80.9 ± 37.0, 95.6 ± 41.0 s; n = 14) for the control animals without AAV injection. All data are expressed as mean ± SD. Significance was analyzed using two-tailed t test, **P < 0.01.
The clozapine group of DREADD animals that received AAV injection took significantly less time to emerge from anesthesia compared with the saline group (59.1 ± 17.7, 66.5 ± 25.7, 75.8 ± 23.6 s vs. 87.4 ± 23.0, 103.9 ± 22.5, 97.1 ± 17.8 s; n = 16) at 40, 80, and 120 min following the i.p. injection, respectively (Fig. 3A). Two-way ANOVA multiple comparisons (Prism 7) did not show significant difference among the three average emergence times at 40, 80, and 120 min within the saline group or the clozapine group.
Faster emergence was also observed for the DREADD mice injected with CNO. Since a metabolite of CNO is expected to be the ligand for the DREADD receptor, we initiated the 30 min of isoflurane exposure at 30, 70, and 110 min after CNO injection. Of the three time points tested, the emergence was accelerated 100 and 140 min after CNO injection, but not 60 min (SI Appendix, Fig. S3), likely reflecting the fact that the DREADD ligand clozapine is the metabolite of CNO. Taken together, these results suggest that activation of orexin neurons in mice facilitates emergence from isoflurane anesthesia.
Activation of Orexin Neurons Increases Pain Tolerance.
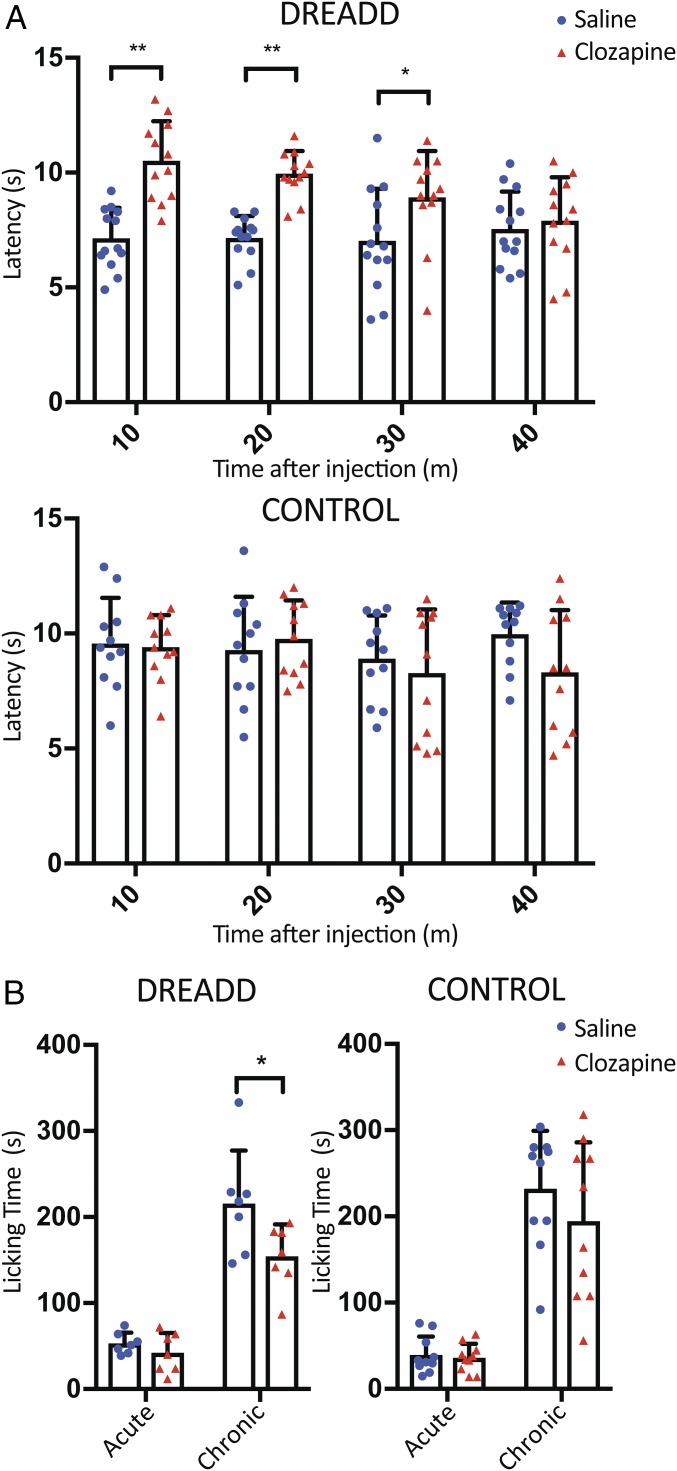
Previous studies have shown that orexin applied via intrathecal injection has analgesic effects (29). To test for the effect of orexin neuronal activation in analgesia, we subjected DREADD mice to the hot plate test and the formalin test. In the hot plate test, 10 min after receiving clozapine or saline, transgenic orexin-Cre mice were placed on the 55 °C hot plate and assessed for the latency to respond to the heat stimulus. The first time when animals showed any response (licking, fanning, or jumping) was recorded as the latency. The same test was repeated three more times with 10-min intervals in between. Statistical analysis revealed that there is a significant difference in latency between the two groups of DREADD mice with bilateral stereotaxic AAV injections (Fig. 4A). The clozapine group displayed significantly longer latencies than the saline group at 10, 20, and 30 min following the injection, respectively.
Fig. 4.
Activation of orexin neurons improves pain tolerance. (A) Ten minutes before the hot plate test, orexin-Cre mice with bilateral stereotaxic AAV injection were injected with clozapine (0.01 mg/kg) or saline intraperitoneally. Latency to the first response (licking, fanning, or jumping) to the 55 °C thermal stimulus was measured. The hot plate test was repeated three more times with 10-min intervals. The latency was significantly longer for the clozapine group (10.5 ± 1.7, 10.0 ± 1.0, 8.9 ± 2.0, 7.9 ± 1.9 s; n = 12) than the saline group (7.1 ± 1.3, 7.1 ± 1.0, 7.0 ± 2.3, 7.6 ± 1.6 s; n = 13) at 10, 20, and 30 min after i.p. injection (Top). The control orexin-Cre mice without AAV injection for DREADD expression revealed no difference in the latency between the clozapine (9.4 ± 1.4, 9.8 ± 1.7, 8.3 ± 2.8, 8.3 ± 2.7; n = 11) and saline treatment (9.6 ± 2.0, 9.3 ± 2.3, 8.9 ± 1.9, 10.0 ± 1.4; n = 11) (Bottom). (B) Ten minutes after i.p. injection of 0.01 mg/kg of clozapine or saline into orexin-Cre mice that had received bilateral stereotaxic AAV injection, 10 μL of 5% formalin was injected into the left hind paw. The total licking time was recorded for 60 min. No significant difference was observed during the acute phase (0–10 min). However, during the chronic phase (10–60 min), the clozapine group spent less time tending to their wounds (154.4 ± 36.8 s, n = 7) than the saline group (212.7 ± 61.2 s, n = 7) (Left). In controls with orexin-Cre mice without AAV injection for DREADD expression, the licking time was comparable between the clozapine group (194.7 ± 91.3 s, n = 10) and the saline group (232.0 ± 67.1 s, n = 10) (Right). All data are expressed as mean ± SD. Significance was analyzed using two-tailed t test, *P < 0.05, **P < 0.01.
In the hot plate assay, the clozapine effect lasted for about 30 min. However, clozapine seems to have longer lasting effect on the emergence assay as shown by the significant difference at 40, 80, and 120 min after clozapine injection. Besides the difference between the neural circuits involved in emergence and pain, the different effects of clozapine could be influenced by the experimental design used in the hot plate test; the animals were retested in relatively short periods of time (every 10 min) which may affect the pain perception. Control orexin-Cre mice without AAV injections demonstrated no difference between the clozapine and saline treatments (Fig. 4A).
Additionally, the formalin test was performed to further evaluate the effect of activating orexin neurons on inflammatory pain. Orexin-Cre mice with or without stereotaxic AAV injection for DREADD expression were injected intraperitoneally with clozapine or saline 10 min before injection of formalin into the left hind paw of the mouse. The total paw licking time for the clozapine group was 27.4% shorter than that of the saline group (Fig. 4B). Further analysis showed that the significant difference arose mainly from the chronic phase (10–60 min). Taken together, the hot plate and formalin tests indicated that activation of the orexin system increases pain tolerance of the mice.
EEG Change Caused by the Activation of Orexin Neurons.
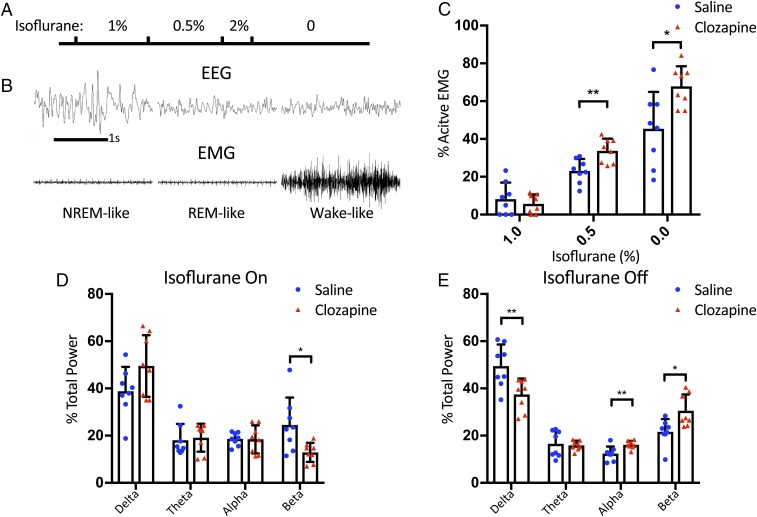
Having performed behavior tests to reveal enhanced anesthesia recovery and pain tolerance of mice with elevated orexin neuron activities, we next explored the impact of orexin neuron activation on the brain activities of mice under anesthesia, by conducting EEG and electromyogram (EMG) analysis. EEG/EMG recording headsets were surgically implanted onto the skull of orexin-Cre mice 1 wk after the stereotaxic injection of AAV virus for DREADD expression. On the day of anesthesia test, the animals received i.p. injection of either clozapine or saline and underwent anesthesia exposure to 1% isoflurane for 25 min, then 0.5% isoflurane for 25 min, followed by 2% isoflurane for 10 min and subsequent recovery (Fig. 5A).
Fig. 5.
EEG/EMG changes upon the activation of orexin neurons. (A) The graphical representation of the timeline for the experiments. Orexin-Cre mice with bilateral stereotaxic AAV injection for DREADD expression were implanted with a four-channel EEG/EMG tethered system and subjected to 1% isoflurane for 25 min followed by 0.5% for 25 min. Then these mice were exposed to 2% isoflurane for 10 min before the discontinuation of anesthesia. Clozapine (0.01 mg/kg) or saline were injected into the mice 10 min before the test. (B) Representative NREM-like, REM-like, and wake-like EEG/EMG traces under isoflurane anesthesia. (C) The percentage of active EMG increases with decreasing dose of isoflurane. There was no significant difference between the clozapine group (5.7 ± 5.0%, n = 8) and the saline group (8.2 ± 8.7%, n = 8) under 1% isoflurane. However, the clozapine group had significantly more active EMG than the saline group (33.8 ± 6.5% vs. 23.1 ± 6.3%) under 0.5% isoflurane and immediately after isoflurane was turned off (67.8 ± 10.6% vs. 45.4 ± 19.5%). (D and E) EEG power spectrum analysis of data from 5-min window before (D) and after (E) turning off isoflurane revealed the significant changes in relative δ-, α-, and β-powers during emergence. The animals with clozapine treatment showed a significant decrease of δ-power (37.5 ± 6.7% vs. 49.5 ± 9.1%) and increase of α- (16.1 ± 1.7% vs. 12.4 ± 3.0%) and β-powers (30.5 ± 6.9% vs. 21.6 ± 5.4%) in the first 5 min after turning off isoflurane. All data are expressed as mean ± SD. Significance was analyzed using two-tailed t test, *P < 0.05, **P < 0.01.
The EEG/EMG recordings revealed three different states under isoflurane anesthesia: nonrapid eye movement (NREM)-like (low-frequency, high-amplitude EEG with low-amplitude EMG), rapid eye movement (REM)-like (theta-dominated EEG and EMG atonia), and wake-like (low-amplitude, mix-frequency EEG and high-amplitude, active EMG) (Fig. 5B). Because mice under anesthesia display waveforms that differ from those waveforms characteristic of natural sleep, we have focused on analyzing the percentage of wake-like epochs with active EMG signals. We found that the animals injected with saline had on average 23% active EMG epochs with saline, whereas those injected with clozapine had 34% active EMG epochs while they were exposed to 0.5% isoflurane. During the 2-h recovery period, the saline group had on average 45%, whereas the clozapine group had 68% active EMG epochs (Fig. 5C). Thus, the activation of orexin neurons produced a change in EMG signal even when the mice were still under 0.5% isoflurane, thereby allowing these mice to emerge from anesthesia sooner with more numerous wake-like epochs of EEG/EMG activity.
We next analyzed power spectrum densities. There was no significant difference in the spectrum between the two groups while they were under anesthesia. We then shifted our focus to the emergence. Under 0.5% isoflurane, mice were lightly anesthetized as shown by the EMG recording so we had to use 2% isoflurane to resedate the mice to suppress their EEG activities to a similar basal level to compare the changes during emergence. Power spectrum analysis showed that the percent distributions of the four frequency bands (δ, θ, α, and β) are similar between the clozapine group and the saline group while the mice are under 2% isoflurane, except that the clozapine group had somewhat less β-power (Fig. 5D). Importantly, upon switching off the anesthesia, the clozapine group showed significantly greater decrease of δ-power and increase of α- and β-powers in the first 5 min (Fig. 5E). Vazey and Aston-Jones have shown that the power spectrum changes involve mainly the δ- and θ-powers when the locus coeruleus noradrenergic system is activated with DREADD even under isoflurane anesthesia (30). It appears that the orexin system and locus coeruleus play different roles in the emergence of anesthesia (31). Our results revealed that the faster emergence from the clozapine group is consistent with the changes of the EEG spectrum.
Discussion
In this study, we investigated whether activation of mouse orexin neurons has any effect on anesthesia related behavior. Our results revealed that the specific activation of orexin neurons by hM3Dq DREADD receptors can facilitate emergence from isoflurane anesthesia and increase pain tolerance of mice subjected to 55 °C thermal stimulation and 5% formalin inflammatory stimulation.
We chose the DREADD approach in this study because, unlike local application of orexin, activation of DREADD receptors in orexin neurons simulates the physiological activation of the orexin circuits. Because DREADD provides more widespread and long-lasting effects without the need to implant an optical fiber as in the case of optogenetics, it is a relatively simple yet powerful tool for studying the role of the orexin system in anesthesia. However, it does lack the spatial and temporal precision provided by optogenetics. Therefore, further studies with optogenetics are desirable for analyzing a spectrum of specific roles of the arousal circuits.
Recently Gomez et al. (27) reported that clozapine, a metabolite of CNO and a blood–brain barrier permeable drug, has an even higher affinity than CNO for DREADD activation. Only subthreshold doses of clozapine (clozapine: 0.01–0.1 mg/kg vs. CNO: 1–10 mg/kg) are required for receptor activation, because of the higher affinity of hM3Dq for clozapine (Ki = 7 ± 2 nM) compared with CNO (Ki = 3.8 ± 0.3 μM). In our study, a comparison of the effects by CNO (1 mg/kg) versus clozapine (0.01 mg/kg) on c-Fos expression and emergence from isoflurane anesthesia consistently showed that CNO even at a relatively high dose is slower to generate the effect (SI Appendix, Fig. S3).
The exact mechanisms by which orexin facilitates emergence from anesthesia can be multifactorial. GABA is the main inhibitory neurotransmitter in the central nervous system and GABAA receptors (GABAARs) have been shown to be a major target of general anesthetics (32–34). The crosstalk between the excitatory orexin receptors (OxRs) and the inhibitory GABAAR remains to be an open question. The subunit composition, the extrasynaptic tonic inhibition, and the membrane trafficking of GABAAR are important aspects of their functional modulation (35, 36). The β-subunit of GABAAR has been known to be a key element in the receptor membrane trafficking (37) and anesthetic binding (38, 39). In HEK293 cells cotransfected with orexin receptor-1 (OxR1) and GABAAR (α1β1γ2s), Sachidanandan et al. (40) showed that activation by orexin peptide-A (OX-A) can inhibit GABA-induced currents through increased phosphorylation of β1 subunit of GABAAR. In a separate study using the human SH-SY5Y cells, Andersson et al. (41) showed that the activation of OxR1 by OX-A can decrease the surface expression of GABAAR through phosphorylation of the β2 subunit. These studies suggest that the local modulation of GABAAR activity level by orexin may play a role in the arousal control and anesthesia emergence.
Orexin neurons project widely in the central nervous system so that activation of orexin neurons may change the activity level of downstream nuclei involved in arousal control, such as the dopaminergic VTA (12), adrenergic LC (42), histaminergic ventral tuberomammillary nuclei (VTM) (11), serotonergic raphe nuclei (43), and cholinergic basal forebrain (44). Anesthesia states are known to be modulated by activation of VTA and LC (30, 45). The comparatively milder effect of DREADD activation of the orexin system on anesthesia states is consistent with the current understanding of arousal being affected by the orexin system as the master regulator of VTA, LC, and other downstream nuclei (46). In addition to these brain regions, orexin neurons also innervate the region in the spinal cord involved in autonomic regulation and pain sensation (9). Orexin intracerebroventricular injection induces increase of heart rate, blood pressure, respiration rate, and tidal volume (4), which will also indirectly facilitate the recovery by affecting the pharmacokinetics of the anesthetics. It will be important to examine the specific effects generated by anesthetics on the different arousal and nociceptive nuclei and how these effects may be modulated by the orexin system.
We did not investigate the effects of activating orexin neurons on the rate of anesthesia induction in this study. Whereas a previous study with intracerebroventricular injection of orexin peptide did not show any difference in the induction speed (19), the question regarding the possible modulation of anesthesia induction by the orexin system awaits future studies employing more sensitive assays.
Currently, there is no active “wakeup” step in anesthesia management. After the discontinuation of anesthetics, recovery from the anesthetic state toward full consciousness simply relies on the ability of the human body to metabolize and eliminate the drug. As we have learned from research regarding sleep, waking up from sleep is an active, highly regulated process. Hopefully, with better understanding of the arousal control in the brain, we can design ways for a more active reversal of anesthesia to minimize the duration of the inactivated state caused by general anesthetics and to bring the activity levels back to their baselines as quickly as possible. In addition to the operating room setting, it will also be helpful to target delirium in the intensive care units. Besides the reversal from hypnosis, pain management in the recovery room after surgery is also a key element of recovery. It is widely accepted that multimodal pain management is required to minimize opioid consumption. Our results show that the orexin neuronal circuit, as part of a stress response system, has potential in the multimodal pain management besides the facilitation of emergence from anesthesia. Our findings support the concept that the orexin system can be a potential drug target for anesthesia reversal to reduce emergence time and to facilitate pain control.
Materials and Methods
Orexin-Cre Mouse Line.
We generated the transgenic C57BL/6 orexin-Cre mouse line, with EGFP and Cre recombinase under the preproorexin promoter, from frozen embryos provided by Akihiro Yamanaka, Nagoya University, Japan. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee, University of California, San Francisco. Mice were maintained in a strictly controlled environment with food and water freely available. The light cycle starts at 6:00 AM and ends at 6:00 PM and dark cycle runs from 6:00 PM to 6:00 AM. The temperature was controlled between 20 °C to 22 °C. Altogether 134 mice were used in the experiments for emergence, hot plate, formalin, and EEG/EMG recording.
Adeno-Associated Virus.
The AAV was purchased from Addgene. The AAV-hSyn-DIO-hM3D(Gq)-mCherry (44361-AAV8; Addgene) is a double-floxed Gq-coupled hM3D DREADD receptor coupled with the mCherry fluorescence marker under the control of a human synapsin promoter. We chose AAV-8 because it was more effective than AAV-2 and AAV-5 at transfecting orexin neurons. The viral titer was ∼4 × 1012 vg/mL.
Stereotaxic AAV Injection.
Surgeries were performed under balanced anesthesia using an automated stereotaxic apparatus (Neurostar). Ketamine and xylazine (80 mg/kg, i.p. injection), carprofen (5 mg/kg, s.c. injection), buprenorphine (0.1 mg/kg, s.c. injection), and isoflurane were used. Eight-week-old animals were injected with 500 nL of the AAV-hSyn-DIO-hM3D(Gq)-mCherry per side using a 5-μL glass syringe with a 33-gauge needle (syringe: 87943, needle: 7762–06; Hamilton Company). The coordinates were Bregma −1.46 mm, lateral ± 0.85 mm, and ventral +5.1 mm. All injections are made bilaterally except the initial experiment to test the c-Fos signal with a unilateral injection and using the contralateral side as the internal control. After a period of at least 2 wk following the injection to allow for expression of the transgene, the mice were subjected to behavior assays.
Immunohistochemistry.
The mice were anesthetized with isoflurane (Henry Schein Animal Health) before transcardial perfusion with cold PBS followed by cold solution of 4% paraformaldehyde in PBS (Electron Microscopy Services). After perfusion, the brain was removed for postfixation in 4% paraformaldehyde solution at 4 °C overnight and then transferred to 40% sucrose in PBS solution for a minimum of 2 d. A series of 30-μm coronal brain slices was generated using a cryostat (Leica CM 3050S; Leica Microsystems).
The coronal brain slices were mounted on microscope slides, then exposed to blocking solution (5% donkey serum, 3% BSA, and 0.3% Triton-X in PBS) for 1 h at room temperature. Subsequently, they were incubated with primary antibodies at 4 °C overnight. The brain slices were then washed three times with PBS, 5 min each time. After the wash, the slices were incubated with secondary antibodies for 1 h at room temperature. Finally, the slices were washed three times with PBS, 5 min each.
The primary antibody solution was diluted in the blocking buffer as follows: mouse c-Fos antibody (ab208942; Abcam) at 1:300, chicken mCherry antibody (NBP2-25158; Novus Biologicals) at 1:400, goat orexin-A antibody (sc-8070; Santa Cruz Biotechnology) at 1:100, rabbit Cre antibody (Poly9080; BioLegend) at 1:200, and chicken GFP (GFP-1020; Aves Lab) at 1:400.
Similarly, the secondary antibody solution was diluted in the blocking buffer as follows: donkey anti-mouse conjugated with Cy3 (715–165-151; Jackson ImmunoReseach) at 1:300, donkey anti-chicken conjugated with Alexa-647 (703–605-155; Jackson ImmunoReseach) at 1:400, donkey anti-goat conjugated with Alexa-488 (A-11055; Invitrogen) at 1:250, donkey anti-rabbit conjugated with Alexa-647 (711–605-152; Jackson ImmunoResearch) at 1:300, and donkey anti-chicken conjugated with Alexa-488 (703–545-155; Jackson ImmunoResearch) at 1:400.
Electrophysiology.
The transgenic animals were anesthetized with isoflurane and transcardially perfused with ice-cold cutting solution containing: 93 mM NMDG, 2.5 mM KCl, 1.2 mM NaH2PO4, 30 mM NaHCO3, 20 mM Hepes, 25 mM glucose, 5 mM sodium ascorbate, 2 mM thiourea, 3 mM sodium pyruvate, 10 mM MgSO4, 0.5 mM CaCl2, 300–310 mOsm, and adjusted with HCl to pH 7.4. The solution was bubbled with carbogen (95% O2/5% CO2). The mouse was decapitated, and the brain was removed. The brain was then cut into 300-μm-thick sections with a microtome (Leica Microsystems). The brain sections were allowed to recover in cutting solution for 15 min at 34 °C and artificial cerebrospinal fluid (aCSF) recovery solution for 45 min at room temperature. The aCSF solution contained: 125 mM NaCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 12.4 mM glucose, 300–310 mOsm, and bubbled with carbogen. The final pH was 7.4.
Neurons with mCherry fluorescence were identified and subjected to electrophysiological recordings. The recordings were performed with a MultiClamp 700B Amplifier (Molecular Devices), and the electrodes were prepared with a glass micropipette puller (Sutter Instruments). During the recordings, the neurons were perfused with the aCSF solution with a peristaltic pump. Internal solution contains: 135 mM potassium methanesulfonate, 6.5 mM KCl, 2 mM MgCl2, 4 mM Na-ATP, 0.2 mM EGTA, and 10 mM Hepes at pH 7.2 with KOH, 304 mOsm. Clozapine solution diluted in aCSF was administered directly onto the target cell using micropipette with a 100-μm opening placed right above the neuron (Columbus Instruments). The data were recorded and analyzed with the Axon pClamp software (Molecular Devices).
Isoflurane Emergence Test.
The RoRR, when the animal has all four paws on the ground, was used as the indicator for the emergence from anesthesia. The emergence time from turning off the isoflurane to the RoRR was recorded for analysis. All animals received 5 min of 2% isoflurane (Henry Schein Animal Health) through an isoflurane vaporizer (Kent Scientific) to facilitate i.p. injections, and they recovered for 10 min in their own cages before the 30-min anesthesia test. Each animal received an i.p. injection of clozapine (0.01 mg/kg), or CNO (1 mg/kg) (30), or saline. The same animals with DREADD expression were used for both saline and clozapine treatments, but on different days with at least 3 d apart between experiments. We always performed the saline treatments first. After several days recovery, we then finished the clozapine treatments. Clozapine was dissolved in 100% DMSO to 50 μM stock solution. The final injectant has 0.001 μg/μL clozapine and 0.006% DMSO. All anesthesia chambers were placed on a temperature-controlled heatpad. A separate temperature probe connected to a temperature controller was placed underneath the animal body to automatically regulate the temperature between 35–37 °C. After 30-min exposure to isoflurane, the animals were quickly removed and placed on their backs to test how quickly the righting reflex returned. The animals were allowed a 10-min recovery period from the anesthesia before repeating the experiment. Mice without AAV injections were used as control and received the same treatment of clozapine, CNO, or saline. All sets of trials were age matched with similar animals.
Hot Plate Test.
The temperature used in the hot plate test was 55 °C. An acrylic container was used as an enclosure to prevent the animal from escaping. Animals were injected with either clozapine (0.01 mg/kg) or saline intraperitoneally 10 min before the hot plate test. The animal was placed on the hot plate until the first sign of pain (licking of the hind paw, fanning, or jumping), at which point the animal was immediately removed from the hot plate. The latency time is the time it took for the animal to elicit the first response and it was recorded for analysis. The animal was removed if there was no response within 45 s. The trials were repeated several times with a 10-min rest in between. Both transgenic and wild-type animals were tested. All sets of trials were age matched with similar animals.
Formalin Test.
With minimum restraint, the animals were injected with 10 μL of 5% formalin or saline under the skin of the dorsal surface of the left hind paw. Animals received an injection of clozapine intraperitoneally (0.01 mg/kg) 10 min before the injection of formalin. Immediately following the formalin injection, the animals were placed onto the recording platform where each animal was individually placed in an acrylic cylinder with a mirror underneath. The behavior was videotaped for 60 min. The video was analyzed later and the licking time of the left hind paw was recorded.
Electroencephalography.
Eight-week-old male mice received bilateral stereotaxic injections of AAV-hSyn-DIO-hM3D(Gq)-mCherry. After 1 wk, the animals were subjected to EEG device placement (Pinnacle Technologies). Four guide holes were made using a 23-gauge surgical needle epidurally over the frontal cortical area (1 mm anterior to Bregma, 1 mm lateral to the midline) and over the parietal area (3 mm posterior to Bregma, 2.5 mm lateral to midline). One ground screw and three screws with leads were placed into the skull through the holes. The screws with leads were then soldered onto a six-pin connector EEG/EMG headstage. For EMG recordings, EMG leads from the headstage were placed into the neck muscle. The headstage was then covered with black dental cement to form a solid cap atop the mouse’s head. The incision was then closed with VetBond (3M; Santa Cruz Biotechnology) and animals were given a s.c. injection of marcaine (0.05 mg/kg) before recovery on a heating pad. Behavioral experiments were conducted 2 wk (1 wk of recovery and another week of acclimation) later to allow for sufficient recovery. Mice were anesthetized with isoflurane under a nose cone. On day 1, the mice received i.p. saline injections and the baseline EEG/EMG without hM3Dq activation at 1.0%, 0.5% isoflurane were recorded for 25 min at each dose. At the same time on day 2, the mice received an i.p. clozapine (0.01 mg/kg) injection. After 30 min, the mice were exposed to isoflurane following the same protocol as day 1 with continuous EEG/EMG recording.
For EEG/EMG recording, mice were singly housed and habituated to the recording cable for 7 d in light/dark 12:12 conditions. Tethered preamplifiers were attached to the headstage of the mice. The signals were relayed through the commutators which allowed the animals to be freely moving. Data were acquired through the Sirenia software package (Pinnacle Technologies). EEG signal was sampled at 500 Hz. Data were scored automatically via the Sirenia Sleep Pro software using the cluster scoring algorithm, and then subsequently a small number of unscored epochs were hand picked by a blinded researcher to tag more epochs with active EMG signals. Cluster scoring utilizes the plot of EEG-delta versus EMG. All of the epochs with active EMG signals were used for calculating the percentage of epochs with active EMG. Four-frequency band power analyses were calculated by Sirenia (δ, 0.4–2.7 Hz; θ, 2.7–8.5 Hz; α, 8.5–13.0 Hz; β, 13–30 Hz, 10-s epochs).
Statistical Significance.
Student’s t test (two tailed) and two-way ANOVA were used to analyze the significance of the data, with P < 0.05 indicating a significant difference. All means were followed by SDs.
Supplementary Material
Acknowledgments
We thank Dr. Akihiro Yamanaka for sharing the frozen embryo of the orexin-Cre mouse line; Yuan-Hung Lin, Tong Cheng, Marena Tynan-La Fontaine, and Eirish Sison for their technical support; and Drs. Chao Chen, Cindy Li, Chin Fen Teo, and Tongfei Wang for their helpful discussions. L.Y.J. is an investigator of the Howard Hughes Medical Institute. This study is supported by NIH Grant NS069229 (to L.Y.J.), NIH Grant NS100801 (to Z.G.), and the International Anesthesia Research Society Mentored Research Award (to W.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808622115/-/DCSupplemental.
References
- 1.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Mavanji V, et al. Orexin/hypocretin treatment restores hippocampal-dependent memory in orexin-deficient mice. Neurobiol Learn Mem. 2017;146:21–30. doi: 10.1016/j.nlm.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Date Y, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belanger-Willoughby N, Linehan V, Hirasawa M. Thermosensing mechanisms and their impairment by high-fat diet in orexin neurons. Neuroscience. 2016;324:82–91. doi: 10.1016/j.neuroscience.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Pol AN. Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer L, et al. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- 12.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath TL, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 14.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 15.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 16.Dalal MA, et al. Normal plasma levels of orexin A (hypocretin-1) in narcoleptic patients. Neurology. 2001;56:1749–1751. doi: 10.1212/wnl.56.12.1749. [DOI] [PubMed] [Google Scholar]
- 17.Mieda M, Sakurai T. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders. Rationale for development and current status. CNS Drugs. 2013;27:83–90. doi: 10.1007/s40263-012-0036-8. [DOI] [PubMed] [Google Scholar]
- 18.Irukayama-Tomobe Y, et al. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114:5731–5736. doi: 10.1073/pnas.1700499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelz MB, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci USA. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong H, et al. Activation of orexin signal in basal forebrain facilitates the emergence from sevoflurane anesthesia in rat. Neuropeptides. 2009;43:179–185. doi: 10.1016/j.npep.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LN, et al. Orexin-A facilitates emergence from propofol anesthesia in the rat. Anesth Analg. 2012;115:789–796. doi: 10.1213/ANE.0b013e3182645ea3. [DOI] [PubMed] [Google Scholar]
- 22.Tose R, et al. Orexin A decreases ketamine-induced anesthesia time in the rat: The relevance to brain noradrenergic neuronal activity. Anesth Analg. 2009;108:491–495. doi: 10.1213/ane.0b013e31819000c8. [DOI] [PubMed] [Google Scholar]
- 23.Shirasaka T, Yonaha T, Onizuka S, Tsuneyoshi I. Effects of orexin-A on propofol anesthesia in rats. J Anesth. 2011;25:65–71. doi: 10.1007/s00540-010-1071-6. [DOI] [PubMed] [Google Scholar]
- 24.Gestreau C, Bévengut M, Dutschmann M. The dual role of the orexin/hypocretin system in modulating wakefulness and respiratory drive. Curr Opin Pulm Med. 2008;14:512–518. doi: 10.1097/MCP.0b013e32831311d3. [DOI] [PubMed] [Google Scholar]
- 25.Kuwaki T. Hypothalamic modulation of breathing. Adv Exp Med Biol. 2010;669:243–247. doi: 10.1007/978-1-4419-5692-7_49. [DOI] [PubMed] [Google Scholar]
- 26.Bülbül M, Babygirija R, Ludwig K, Takahashi T. Central orexin-A increases gastric motility in rats. Peptides. 2010;31:2118–2122. doi: 10.1016/j.peptides.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Gomez JL, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inutsuka A, et al. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology. 2014;85:451–460. doi: 10.1016/j.neuropharm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Nozaki-Taguchi N, Chiba T. Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol. 2002;137:170–176. doi: 10.1038/sj.bjp.0704851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci USA. 2014;111:3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–952. doi: 10.1038/npp.2017.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonin RP, Orser BA. GABA(A) receptor subtypes underlying general anesthesia. Pharmacol Biochem Behav. 2008;90:105–112. doi: 10.1016/j.pbb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 34.Antkowiak B. Closing the gap between the molecular and systemic actions of anesthetic agents. Adv Pharmacol. 2015;72:229–262. doi: 10.1016/bs.apha.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Wang DS, et al. Dexmedetomidine prevents excessive γ-aminobutyric acid type A receptor function after anesthesia. Anesthesiology. 2018;129:477–489. doi: 10.1097/ALN.0000000000002311. [DOI] [PubMed] [Google Scholar]
- 36.Bai D, et al. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 37.Jacob TC, et al. GABA(A) receptor membrane trafficking regulates spine maturity. Proc Natl Acad Sci USA. 2009;106:12500–12505. doi: 10.1073/pnas.0903943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belelli D, Pistis M, Peters JA, Lambert JJ. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends Pharmacol Sci. 1999;20:496–502. doi: 10.1016/s0165-6147(99)01405-4. [DOI] [PubMed] [Google Scholar]
- 39.Jurd R, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 40.Sachidanandan D, Reddy HP, Mani A, Hyde GJ, Bera AK. The neuropeptide orexin-A inhibits the GABAA receptor by PKC and Ca2+/CaMKII-dependent phosphorylation of its β1 subunit. J Mol Neurosci. 2017;61:459–467. doi: 10.1007/s12031-017-0886-0. [DOI] [PubMed] [Google Scholar]
- 41.Andersson H, Björnström K, Eintrei C, Sundqvist T. Orexin a phosphorylates the γ-aminobutyric acid type A receptor β2 subunit on a serine residue and changes the surface expression of the receptor in SH-SY5Y cells exposed to propofol. J Neurosci Res. 2015;93:1748–1755. doi: 10.1002/jnr.23631. [DOI] [PubMed] [Google Scholar]
- 42.van den Pol AN, et al. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eggermann E, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 45.Taylor NE, et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci USA. 2016;113:12826–12831. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Lecea L. Optogenetic control of hypocretin (orexin) neurons and arousal circuits. Curr Top Behav Neurosci. 2015;25:367–378. doi: 10.1007/7854_2014_364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.