Significance
Substance-use disorders damage individuals and communities, providing large societal costs. For stimulant-use disorders, there are no Food and Drug Administration-approved medications. Human genetic associations with common variants in the gene encoding the receptor-type protein tyrosine phosphatase D (PTPRD) make this cell-adhesion molecule/synaptic specifier gene an interesting target for new addiction therapeutic agents. We now report results of cocaine self-administration in heterozygous PTPRD-KO mice, discovery that 7-butoxy illudalic acid analog (7-BIA) inhibits PTPRD’s phosphatase with in vitro potency and specificity, and discovery that 7-BIA reduces cocaine reward in self-administration and conditioned place-preference models. PTPRD’s phosphatase is a target for antiaddiction medication development, and 7-BIA is a lead compound.
Keywords: Post-GWAS, drug discovery, cell adhesion molecule, addiction, stimulant use disorder
Abstract
Receptor-type protein tyrosine phosphatase D (PTPRD) is a neuronal cell-adhesion molecule/synaptic specifier that has been implicated in addiction vulnerability and stimulant reward by human genomewide association and mouse cocaine-conditioned place-preference data. However, there have been no reports of effects of reduced expression on cocaine self-administration. There have been no reports of PTPRD targeting by any small molecule. There are no data about behavioral effects of any PTPRD ligand. We now report (i) robust effects of heterozygous PTPRD KO on cocaine self-administration (These data substantially extend prior conditioned place-preference data and add to the rationale for PTPRD as a target for addiction therapeutics.); (ii) identification of 7-butoxy illudalic acid analog (7-BIA) as a small molecule that targets PTPRD and inhibits its phosphatase with some specificity; (iii) lack of toxicity when 7-BIA is administered to mice acutely or with repeated dosing; (iv) reduced cocaine-conditioned place preference when 7-BIA is administered before conditioning sessions; and (v) reductions in well-established cocaine self-administration when 7-BIA is administered before a session (in WT, not PTPRD heterozygous KOs). These results add to support for PTPRD as a target for medications to combat cocaine use disorders. 7-BIA provides a lead compound for addiction therapeutics.
Even though more than seven million Americans reported illicit stimulant use during a recent year (1), there are no Food and Drug Administration (FDA)-approved medications for stimulant-use disorders. Clues about novel targets for antiaddiction medications have come from molecular-genetic studies of human individual differences in addiction-related phenotypes (2). Data from mouse models support several of these human molecular-genetic findings (3–5).
Receptor type protein tyrosine phosphatase D (PTPRD) is a target for addiction therapeutic agent development that has been identified by modest-magnitude but replicated human genetic associations and supported by initial conditioned place-preference studies in mouse models (5). PTPRD is a single-transmembrane cell-adhesion molecule/synaptic specifier that can make homomeric and/or heterologous dimers with binding partners expressed on membranes of adjacent neurons (6). Binding of PTPRD by its extracellular ligands alters phosphatase activity of its intracellular phosphatase domain (7). Phosphoprotein changes with KO of ptp-3, PTPRD’s Caenorhabditis elegans homolog, support PTPRD effects on phosphorylation of conserved tyrosines in human proteins (8) that include the addiction-associated kinase cyclin-dependent protein kinase 5 (Cdk5) (9).
PTPRD has been identified in human genomewide SNP and CNV association studies (10–12) of several addiction-related phenotypes, usually by clusters of nearby SNPs or CNVs that each display association with nominal significance (10−8 < P < 10−2). Such associations began with 10K SNP studies of polysubstance dependence (1 of 38 SNPs reported; now known to map to PTPRD’s 3′ flank) (13) and continued with studies that included 600K SNP associations with polysubstance dependence (14) (1 of 119 genes), 1M SNP associations for illegal substance abuse in African American subjects from two independent samples (15) (1 of 97 genes), 1M SNP studies of alcohol disorders in an epidemiological sample* (1 of 9 genes identified in both European- and African-American samples), and genes identified by SNP and CNV associations for opiate dependence (16) (1 of 3 genes). Ability to quit smoking in clinical-trial settings displayed PTPRD associations in several studies (1 of 33 genes in ref. 17 and 1 of 116 genes in ref. 18). PTPRD was identified by one of the top two genomewide association peaks in a study of acute responses to amphetamine (19). It was one of 156 genes identified by association with responses to drinking during the “first five” occasions for alcohol consumption (20).
PTPRD variants that reduce expression are associated with reduced vulnerability. Rare CNVs likely to reduce PTPRD expression are more frequent in controls than in addicted individuals (16). There are robust genetic associations with individual differences in levels of expression of PTPRD mRNA in postmortem brain samples (5), with 70% differences in levels of PTPRD expression in brains from major vs. minor allele carriers for common PTPRD intron 10 SNPs. Common haplotypes marked by the SNPs associated with lower PTPRD brain expression are associated with reduced addiction vulnerabilities (5).
These observations in humans have received initial support from studies of mouse cocaine-conditioned place preference. Heterozygous PTPRD-KO mice that express half of WT levels of PTPRD display reduced preference for places paired with effects of the normally highly rewarding 10-mg/kg doses of cocaine (5). Tests of strength, motor function, anxiety, and mnemonic function reveal no obvious confounding effects (5). However, we are aware of no reported data concerning effects on self-administration of cocaine, a face-valid model in which to test drug reward. In the first part of the present report, we thus describe effects of heterozygous PTPRD KO on cocaine self-administration under fixed and progressive ratio schedules. These results provide substantial additional support for PTPRD as a target for novel antiaddiction drugs.
PTPRD is a member of a “DSF” subfamily of receptor type protein tyrosine phosphatases that also includes PTPRS and PTPRF. Natural product library screening has identified illudalic acid ligands that display micromolar IC50 values (21, 22) in inhibiting PTPRF’s phosphatase. Interest in the phosphatase “business end” of PTPRD as a drug target is increased by nonconservative amino acid differences between PTPRF, PTPRD, and PTPRS phosphatase domains (23) that could allow specificity for PTPRD phosphatase inhibitors. Although these illudalic acid analog PTPRF ligands provide clues, no PTPRD ligand has been previously identified.
In the second part of this report, we provide in vitro and in vivo characterization of an illudalic acid analog as a PTPRD phosphatase inhibitor and document this compound’s PTPRD-dependent effects on cocaine reward. We describe: (i) synthesis and identification of a 7-butoxy illudalic acid analog (7-BIA) as an in vitro inhibitor of recombinant human PTPRD phosphatase fusion protein with some selectivity vs. the activity of the PTPRS phosphatase fusion protein; (ii) failure of 7-BIA to display IC50 < 10−5 M at any of many tested receptors, transporters, or sites of action of other drugs; (iii) tolerability of acute and repeated 7-BIA administration by WT C57 mice; (iv) reduction of cocaine-conditioned place preference when 7-BIA is administered before each cocaine conditioning session in WT mice; (v) failure of 7-BIA administration to cause preference or aversion for places in which mice experience this compound’s effects; (vi) the greater numbers of WT mice (but not PTPRD heterozygous KOs) who fail to self-administer the maximal amount of cocaine available when well-established cocaine self-administration sessions are preceded by administration of 7-BIA; and (vii) overall reductions in cocaine self-administration in 7-BIA–treated WT (but not 7-BIA–treated PTPRD heterozygous KO) animals. These results each contribute to proof of principle that PTPRD is a druggable site for addiction therapeutic agents and that acute modulation of PTPRD’s phosphatase can alter stimulant reward. 7-BIA provides an attractive scaffold for further development of PTPRD phosphatase-inhibitor antiaddiction medications.
Materials and Methods
Animals.
Heterozygous PTPRD-KO mice and WT littermates were bred from heterozygote × heterozygotes crosses and genotyped as described with New Mexico VA Healthcare System (NMVAHCS) Institutional Animal Care and Use Committee approval (5). Mice underwent self-administration testing under auspices of the National Institute on Drug Abuse (NIDA) Animal Care and Use Committee. Euthanasia used rapid cervical dislocation (at NVMAHCS) or pentobarbital overdose (NIDA Intramural Research Program).
i.v. Cocaine Self-Administration by WT and PTPRD-KO Mice.
Mice were catheterized via external jugular veins and allowed to press a lever for 1-mg/kg cocaine infusions over 42 s, paired with light and sound cues under fixed-ratio 1 (FR1) schedules during the first week (SI Appendix). Mice were later provided with access to cocaine on a progressive ratio schedule that delivered 1-mg/kg cocaine infusions on the following schedule: 2, 4, 5, 6, and 9 presses per infusion. The numbers of presses of the active lever and numbers of infusions received were noted.
In Vitro Assays for Characterization of PTPRD and PTPRS Phosphatase Activities and Actions at Other Brain Sites.
Production of recombinant human PTPRD and PTPRS phosphatase fusion proteins from expressing Escherichia coli began with PCR amplification of human PTPRD and PTPRS D1 phosphatase domain sequences by using appropriate oligonucleotides and cDNA prepared from human cortical polyA+ RNA as described previously (8). These sequences were cloned into pET-43.1 Ek/LIC inserts confirmed by sequencing, and fusion protein sequences expressed in BL21 cells. Transformed BL21 cells were grown, plasmid over-expression was induced, and cells were washed and lysed to extract phosphatase domain fusion proteins (24) (SI Appendix). Phosphatase domain fusion protein activity was tested by using OD405 spectrophotometric assessment of p-nitrophenyl phosphate dephosphorylation to p-nitrophenolate. In some experiments, p-nitrophenyl phosphate and 7-BIA concentrations were varied for Lineweaver–Burke analyses. A total of 90 µL of assay mixture and preincubated enzyme-inhibitor mix was incubated at 22 or 27 °C with OD405 assessed for 20–60 min (SI Appendix).
Receptor binding and uptake assays for 77 brain sites of action of known drugs were performed by EUROFINS (https://www.eurofins.com) and Aaron Janowsky under NIDA contracts as described (SI Appendix).
Synthesis of 7-BIA and Precursors.
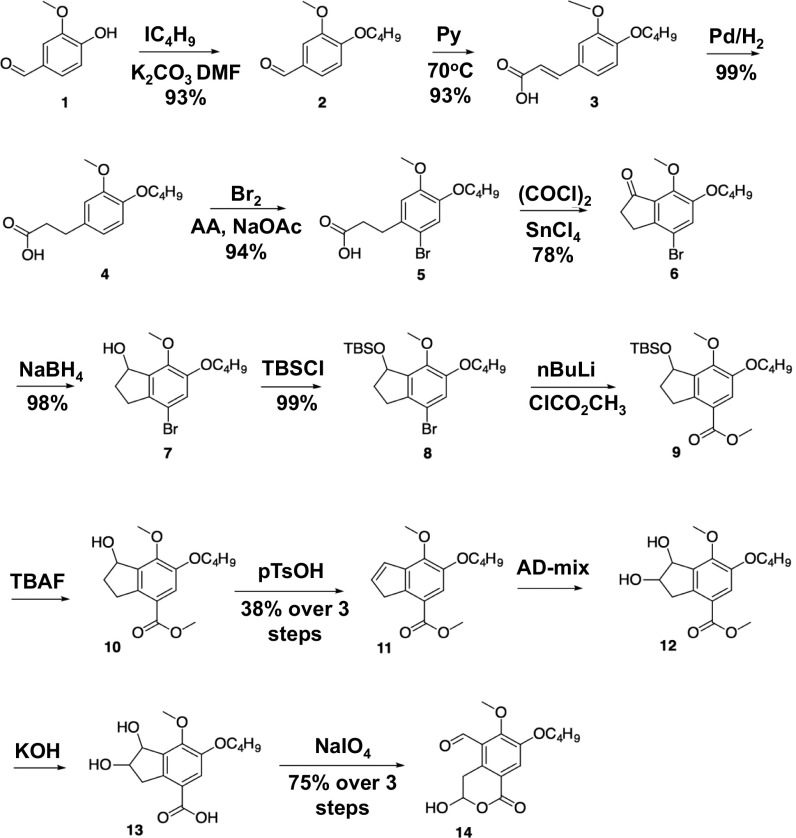
We synthesized the 7-butoxy analog of illudalic acid [14 (boldface type added to indicate a structure), Fig. 2; synthesized previously in 13 steps (22)] with modifications to three steps as a result of low yields for bromination of acid 4, the Friedel–Crafts acylation of 5, and the deprotection of TBS ether 9 (SI Appendix). We brominated acid 4 under buffered conditions in the presence of sodium acetate in acetic acid solution, yielding compound 5 as the sole product. We used oxalyl chloride in the preparation of the acyl chloride intermediate in Friedel–Crafts acylation of 5, resulting in fewer byproducts. We applied mild deprotection with tetra-n-butylammonium fluoride to yield a quantitative conversion of 9 to alcohol 10, eliminating chromatographic purification. We purified the final product by crystallization from diethyl ether, affording the 7-butoxy analog of illudalic acid (i.e., 14) as white crystals with melting point of 116–117 °C. Analytical data for all compounds agreed with reported values (22).
Fig. 2.
Synthetic scheme and structures, including structure for the illudalic acid analog PRPRD phosphatase inhibitor 7-BIA (i.e., 14; further detail provided in SI Appendix).
Acute and Repeated 7-BIA Dosing to WT C57b Mice.
C57bl mice were first exposed to acute repeated ascending i.p. dosing every 20 min, starting from 6-mg/kg doses and ascending to 60-mg/kg doses. Other mice were administered alternate-day doses of 60-mg/kg doses in 2-wk chronic exposure experiments. Mice were observed to note any behavioral alterations and killed acutely and 1 wk following the last 7-BIA dose. Locomotor activity, mnemonic function (Morris water maze), and somatosensation (pain) were also tested as described previously (5, 25, 26). Gross pathologic evaluations were performed by a veterinarian. Histopathological analyses were evaluated by veterinary pathologists with special pathologic attention to the kidney, one of the nonneuronal/endocrine tissues that expresses the most PTPRD mRNA in humans (27).
Effects of 7-BIA on Cocaine-Conditioned Place Preference.
We administered 7-BIA 90 min before each of the two 10-mg/kg cocaine conditioning sessions. On each of two preconditioning days, mice were allowed to explore both of the two CPP box chambers for 20 min. On the two subsequent conditioning days, mice were injected with vehicle or 6 or 60 mg/kg 7-BIA 90 min before administration of 10 mg/kg cocaine. A postconditioning session performed on the day following the last conditioning session tested changes in preference for previously cocaine-paired places. Mouse positions were tracked and stored by using ANYmaze. We also tested preference for places paired with effects of 7-BIA alone (SI Appendix).
Effects of 7-BIA on Established Cocaine Self-Administration.
WT or heterozygous PTPRD-KO mice were prepared by external jugular vein catheterization and allowed to self-administer 0.5–1 mg/kg per infusion of cocaine for at least 20 prior sessions on FR1 and/or progressive ratio schedules. Mice that were self-administering at least 40 (of 50 possible) times per 3-h session on FR1 schedules were studied. Ninety minutes before the start of the subsequent self-administration session, mice were pretreated with vehicle or 10 or 20 mg/kg 7-BIA (i.p.) and allowed to self-administer 1 mg/kg per infusion of cocaine for a 3-h session. During the next sessions, mice were allowed to self-administer 1 mg/kg per infusion of cocaine without pretreatment. The numbers of cocaine infusions self-administered were noted.
Results
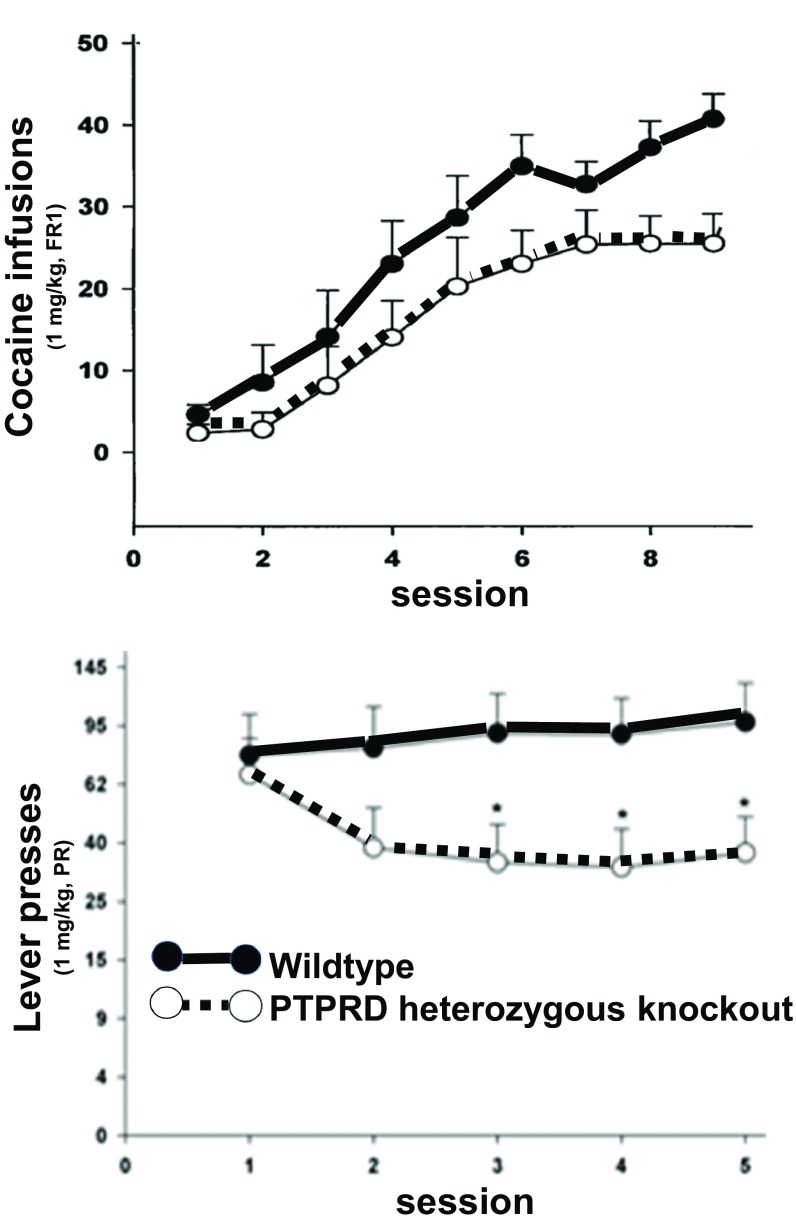
Effects of reduced PTPRD expression on cocaine self-administration supported PTPRD as a target for antiaddiction therapeutic agents. PTPRD heterozygous KO mice took longer to establish high rates of 1-mg/kg per infusion cocaine self-administration than WT littermate controls (Fig. 1). During the last three of nine successive exposures to 3-h sessions in which as many as 50 cocaine infusions were available on FR1 schedules, heterozygotes self-administered approximately half of the maximal amounts available, whereas WT littermates self-administered approximately 70% of the maximal available drug. Heterozygotes continued to self-administer at lower rates than WT mice on days 6–9. There were no differences in control responding on inactive levers during FR1 responding.
Fig. 1.
Numbers of 1-mg/kg cocaine infusions self-administered during 3-h sessions by WT (solid line, closed symbols) and heterozygous PTPRD-KO mice (dashed line, open circles) on experimental days shown. (Top) FR1 (1 mg/kg per infusion) and (Bottom) progressive-ratio schedules. Mice received 1-mg/kg cocaine infusions after completing 2, 4, 6, 9, and 12 lever presses on days 1–5, respectively (*P < 0.05, t test).
There is additional support from results of subsequent progressive ratio studies (Fig. 1). After several days’ experience with these progressive ratio schedules, PTPRD heterozygous KO mice displayed significantly lower break points. They therefore self-administered approximately half the number of infusions that were self-administered by WT littermate control mice. There were again no differences in control responding on inactive levers.
Motivated by the convergent effects of heterozygous PTPRD KO on conditioned place preference and self-administration testing and the prior findings for illudalic acid analogs in studies of PTPRF’s phosphatase and initial molecular modeling/docking studies, we selected a 7-BIA analog (i.e., 14, Fig. 2) for synthesis as a potential PTPRD phosphatase inhibitor. The synthetic path shown in Fig. 2 produced >2 g of 7-BIA with an overall 17% yield for this 13-step synthesis. The final product 14 was characterized by NMR spectroscopy, high-resolution mass spectroscopy, combustion analysis, and comparisons with previously reported values in the literature. Its purity was determined to be ≥98% based on analytical data. Mass spectrographic analyses identified some stability in 5% DMSO solution; approximately 23% remained intact after 2 mo storage at 4 °C. We retained amounts of purified compounds 11, 12, and 13 to use as comparison controls.
To establish an assay for PTPRD phosphatase inhibition and to compare results with those for the phosphatase from PTPRS, we produced recombinant human PTPRD and PTPRS D1 phosphatase domain fusion proteins. We produced BL21 cells transformed with pET-43.1 Ek/LIC constructs that were confirmed by sequencing to express phosphatase domain fusion proteins with verified human PTPRD and PTPRS phosphatases. Recombinant human PTPRD and PTPRS fusion proteins purified from these expressing cells converted p-nitrophenyl phosphate into paranitrophenolate, which provides strong 405-nM absorbance. Activity is dependent on the amount of enzyme and time. Activity is destroyed by boiling the purified recombinant fusion proteins.
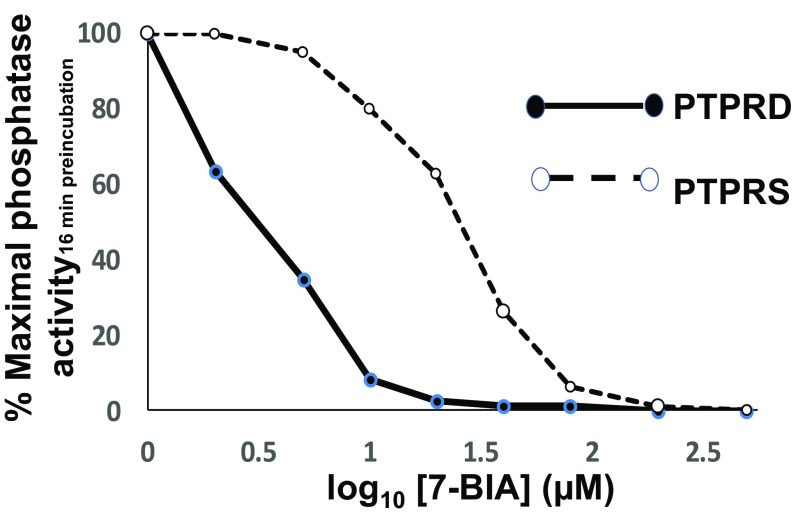
The phosphatase activities of recombinant human PTPRD and recombinant human PTPRS (Fig. 3 and SI Appendix, Fig. S1) fusion proteins were each inhibited by 7-BIA. Maximal inhibition developed over 16–18-min preincubations, consistent with a pseudoirreversible mechanism that has been postulated for interactions between illudalic acid analogs and PTPRF (26). Nonparallel Lineweaver–Burke plots (e.g., nominally noncompetitive) were compatible with a pseudoirreversible mechanism of action as postulated for 7-BIA interactions with PTPRF (SI Appendix, Fig. S2) (28). IC50 values, based on concentrations of 7-BIA that were preincubated with phosphatase, were approximately 1–3 µM (PTPRD) and 40 µM (PTPRS).
Fig. 3.
Concentration-dependent inhibition of the phosphatase activity of recombinant human PTPRD (solid line) and PTPRS (dashed line) D1 phosphatase domain fusion proteins by 16-min preincubation with 7-BIA at concentrations shown (log10 × 10−6 M; note that phosphatase assays diluted 7-BIA 10-fold; SI Appendix).
There is other in vitro evidence for specificity. No tested precursor (compounds 11–13; Fig. 2) significantly inhibited PTPRD or PTPRS phosphatase fusion protein activity. At concentrations as high as 10−5 M, 7-BIA failed to significantly influence binding or uptake at any of the 77 sites assessed by ligand binding or uptake assays.
We tested tolerability of 7-BIA vs. DMSO vehicle in mice using ascending-dose and repeated-dose i.p. administration. Treated mice failed to display obvious behavioral changes that were not seen in vehicle-injected animals. This was true of ascending doses as high as solubility limits (providing 60 mg/kg in solutions with some precipitate in maximally tolerated DMSO) and with 2-wk weekday 6 mg/kg dosing. There was no evidence for any drug-related gross pathologic processes. When sections of lung, liver, kidney, and gut were studied by board-certified veterinary pathologists, there was no evidence for histopathology related to acute ascending or 2-wk repeated administration.
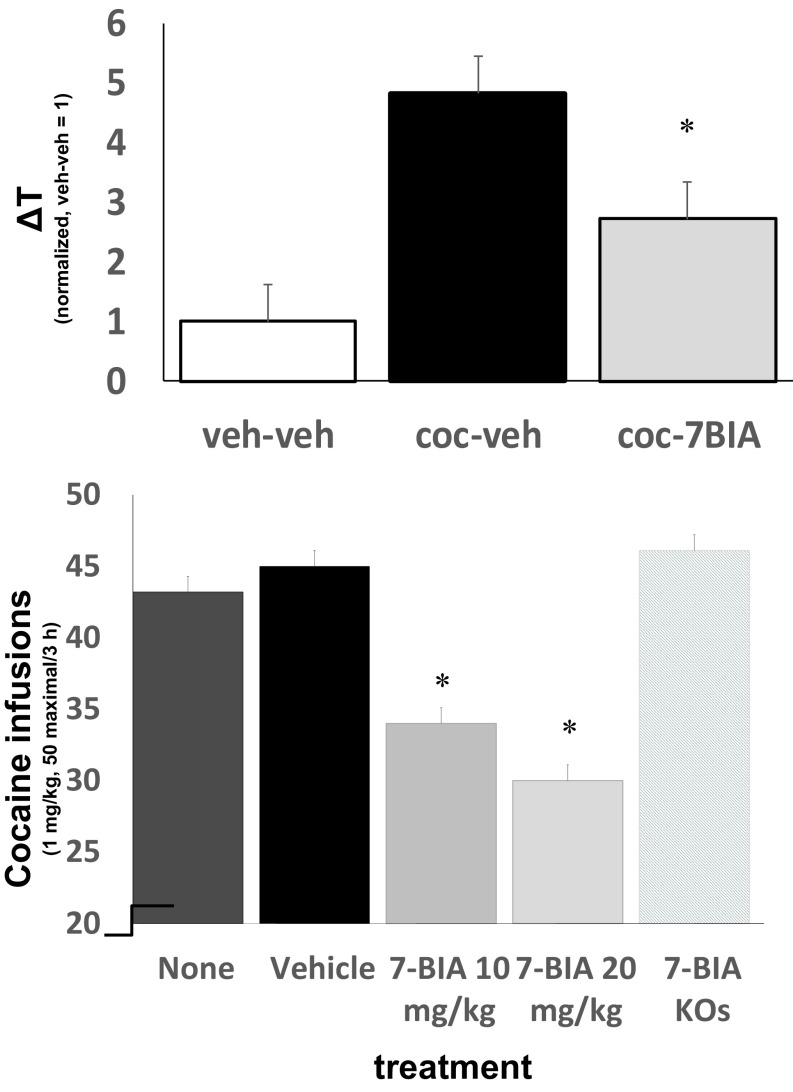
We then tested effects of pretreatment before each cocaine-conditioning session on preferences for places paired with 10 mg/kg cocaine (5). Pretreatments with 6 mg/kg i.p. 7-BIA 90 min before each conditioning session significantly reduced preference for cocaine-paired sides (Fig. 4). We confirmed significant levels of 7-BIA/metabolites in acetonitrile extracts of brains of 7-BIA–treated mice killed 90 min after 7-BIA injections by using mass spectrographic analyses. There was no significant difference in locomotor activity measured during the cocaine conditioning sessions preceded by vehicle vs. those preceded by 7-BIA injection. Pairing environments with 7-BIA itself provided no evidence for rewarding or aversive properties for this compound.
Fig. 4.
(Top) Cocaine-conditioned place preference in mice with vehicle (black) and 7-BIA (gray; >6 mg/kg) pretreatment before each conditioning session (*P = 0.03, t test; n = 12; Values normalized. 1 represents time (+/− SEM) in seconds spent (during postconditioning–preconditioning test sessions) in control mice which were pretreated prior to each conditioning session with saline vehicle for cocaine and DMSO vehicle for 7-BIA). (Bottom) Well-established cocaine self-administration is reduced significantly by 7-BIA pretreatments (90 min before session start) in WT but not in PTPRD heterozygous KO mice (n = 12–126 sessions; 13 WT and 6 heterozygous KO mice; *P < 0.05).
We next tested effects of 7-BIA administration before cocaine self-administration sessions. The 7-BIA pretreatments reduced cocaine self-administration in highly experienced WT mice that were lever-pressing to self-administer near-maximal available cocaine (50 1 mg/kg infusions on FR1 schedule) during ≥20 prior sessions. The 7-BIA pretreated mice failed to obtain at least 80% of available cocaine during 52% of sessions, compared with the 18–22% of sessions during which vehicle or nonpretreated mice failed to obtain at least this fraction of the maximal available cocaine (P = 10−4, t test). There were thus significant mean differences in self-administration (Fig. 4). Mice also failed to self-administer at least 80% of available cocaine during 31% of self-administration sessions that took place 1–2 d following single 7-BIA treatments, suggesting some persistence of the compound’s effects. There were again no significant effects on locomotor activity recorded when the mice were experiencing effects of cocaine with 7-BIA vs. vehicle pretreatments.
There is in vivo evidence for specificity. Most, if not all, of these 7-BIA effects appear to be attributable to the compound’s influences on PTPRD. Heterozygous PTPRD-KO mice with extensive histories of cocaine self-administration that were pretreated with 7-BIA failed to obtain at least 80% of the maximally available cocaine during only 9% of sessions.
Discussion
The present self-administration work in heterozygous PTPRD-KO mice adds to prior results to support PTPRD as a bona fide increasingly-“derisked” target for antiaddiction therapeutic agent development (29). Effects on fixed- and progressive-ratio responding combine to provide good evidence for reduced cocaine reward in heterozygous PTPRD-KO mice. Taken together with data from conditioned place-preference and human association studies, there is now substantial support for the idea that constitutive “trait” differences in levels of PTPRD expression result in differences in stimulant reward in humans and mouse models.
To test the idea that drug-induced, transient “state” differences in levels of PTPRD activity could also alter reward from stimulant administration, we needed to identify a compound that would alter PTPRD activities, was tolerable in vivo, and did not induce behavioral effects that would confound data from reward models. Our identification of 7-BIA as a PTPRD phosphatase inhibitor and our establishment of modest, if any, in vivo 7-BIA behavioral or pathological toxicities allowed us to use this compound in vivo for assays of cocaine reward. Cocaine provides robust place preference when paired with a specific environment during two conditioning sessions. 7-BIA treatment before each of these conditioning sessions reduces preference for cocaine-paired environments. These results support efficacy of transient PTPRD phosphatase modulation in reducing cocaine reward.
Cocaine maintains self-administration at high rates. In WT mice with well-established cocaine self-administration, 7-BIA pretreatments reduce self-administration. These 7-BIA effects are reversible and are not found in heterozygous PTPRD-KO mice. These self-administration data reinforce conditioned place preference results to support the idea that transient inhibition of PTPRD’s phosphatase can reduce cocaine reward. Elimination of 7-BIA’s effects in heterozygous KOs support the idea that much or all of 7-BIA’s in vivo action result from effects on PTPRD. Cocaine reward is reduced by transient reductions in PTPRD activity.
This work provides development and in vivo characterization of a lead compound that is specifically targeted to PTPRD, a cell-adhesion molecule/synaptic specifier relevant to addiction. Documentation of 7-BIA–induced changes in cocaine-conditioned place preference and self-administration solidifies 7-BIA as a lead compound for a novel antiaddiction therapeutic strategy. These pharmacological results, in turn, increase validation of PTPRD as a target for novel addiction therapeutic agents. Our development of improved chemical synthetic pathways that increase yields of 7-BIA, expression vectors for production of recombinant PTPRD and PTPRS phosphatase fusion proteins, purification of these PTPRD and PTPRS phosphatase fusion proteins, and development and validation of phosphatase assays using these fusion proteins provide background work that allowed in vitro characterization of 7-BIA as a PTPRD ligand and phosphatase inhibitor. We identified lack of gross toxicity in mice, a precondition to interpretable in vivo work. In vivo studies then identified 7-BIA’s ability to reduce cocaine reward in conditioned place preference and self-administration settings in WT mice.
There is evidence for substantial specificity of 7-BIA effects. In vitro support for specificity includes: (i) lack of PTPRD phosphatase inhibition from synthetic precursors, (ii) lack of 7-BIA effects on assays for 77 tested brain targets, (iii) >30-fold greater potency at PTPRD than at PTPRS phosphatases, and (iv) reported (22) >100-fold greater 7-BIA potency at PTPRS than at PTPRC, PTPN1, PTPN2, DUSP22, CDC25A, or CDC25B phosphatases. In vivo, ablation of 7-BIA effects on cocaine reward in heterozygous PTPRD-KO mice provides positive evidence for specificity, making it unlikely that the reduced cocaine reward reported is a result of 7-BIA’s abilities (22) to inhibit PTPRS or PTPRF’s phosphatases. We have identified no in vivo 7-BIA behavioral effect in WT mice; there is thus no behavioral evidence for behaviorally important nonspecific effects. Nevertheless, future studies that seek alterations in 7-BIA effects in mice with reduced expression of PTPRS or PTPRF can provide additional behavioral controls.
Binding of PTPRD by its extracellular ligands is likely to alter phosphatase activity of its key intracellular “D1” phosphatase domain (7). Interest in the phosphatase “business end” of PTPRD as a drug target was increased by nonconservative amino acid differences between PTPRD and its other subfamily members, PTPRF and PTPRS† (23).We have targeted the activity of PTPRD’s D1 domain in the present work. Prior studies have documented virtually perfect correlations between illudalic acid potencies in inhibiting phosphatase activities at PTPRF D1 vs. D1+D2 constructions (30). Our in vitro D1 domain results fit with our in vivo 7-BIA efficacy and selectivity results. Nevertheless, future studies of the effects of 7-BIA and related illudalic acid analogs on PTPRD’s D1+D2 domain proteins could add support for the mechanism of action of this compound.
There are limitations that may render 7-BIA a lead compound, but perhaps not a drug. 7-BIA displays limited solubility; mouse i.p. dosing greater than 20 mg/kg is thus problematic. The slow in vitro onset of PTPRD phosphatase inhibition is compatible with the pseudoirreversible mechanism of action that has been postulated for illudalic acid analog interactions with PTPRF, perhaps providing another limitation (21). We have documented levels of 7-BIA/metabolites in brains of mice killed at times when 7-BIA administration provides behavioral effects, but we have only limited information about 7-BIA’s cellular permeability. Although there is some selectivity for PTPRD phosphatase inhibition compared with PTPRS, even greater selectivity may be desirable. A motor-neuron phenotype reported in mice with homozygous KO of PTPRD and PTPRS (but not in heterozygotes) provides caution (31). We have identified preservation of approximately 23% of intact compound/salts in preliminary mass spectrographic analyses of 7-BIA stored in DMSO solution for 2 mo at 4 °C; improved stability would also be desirable. We have characterized in vitro time course, IC50 estimates, and Lineweaver–Burke analyses for 7-BIA, but have not performed full kinetic analyses of this inhibition. As we identify 7-BIA analogs that provide more selective PTPRD phosphatase inhibition and other desirable properties, fuller in vitro kinetic analyses as well as fuller in vivo pharmacodynamic, pharmacokinetic, and toxicologic analyses will be important.
Other cautions about PTPRD as a target for novel therapeutic agents come from associations between (i) total, lifelong human or mouse PTPRD KO and cognitive difficulties (5, 32) and (ii) findings that rare variation likely to dramatically alter PTPRD expression can promote or inhibit cancer formation or properties of cancer cells (33, 34). Neither humans nor mice with moderate differences in levels of PTPRD expression display any cognitive abnormalities. Neither we nor others have detected any tumors in studies of hundreds of mice with 50% reductions in PTPRD expression at ages up to 6 mo. There is no evidence that common human haplotypes that drive 70% individual differences in expression alter cancer propensities. We nevertheless need to be mindful of the possibilities that even intermittent pharmacological modulation of PTPRD activity might conceivably exert side effects on carcinogenesis, cognition, or other phenotypes.
Neuronal processes of PTPRD-expressing neurons grow when their PTPRD is allowed to make homomeric bonds with PTPRD expressed by adjacent cells (35). There are also heteromeric PTPRD ligands, including slit- and trk-like proteins, IL-1 receptor-like and accessory proteins, leucine-rich repeat and fibronectin type III domain-containing proteins, and netrin G ligand 3 (36–39). Ventral midbrain, striatal/accumbens, cerebral cortical, reticular thalamic, and other circuits that express PTPRD mRNA in likely dopamine, acetylcholine, glutamate, and GABA neurons (40) are thus likely to develop and adapt differently when they constitutively express PTPRD at differing levels. Lifelong differences in PTPRD activity, including those conferred by common human PTPRD intron 10 haplotypes or by heterozygous KOs, are thus likely to influence structures and functions of neuronal processes and synapses in brain regions that include those linked to rewarding effects of stimulants and other addictive substances. Lifelong differences in PTPRD activity that could function by providing differences in neuronal connectivities are now strongly linked to differences in vulnerability to stimulant effects by associations with mouse cocaine place preference (5), mouse cocaine self-administration, and human ratings of positive stimulant effects (19).
The current work provides evidence that acute and transient alterations in PTPRD activity influence stimulant reward. 7-BIA administration 90 min before cocaine conditioning or self-administration sessions produces substantial reductions in place preference and self-administration that are nearly as large as those provided by heterozygous KO (5). Data from self-administration supports partial reversal of these acute 7-BIA effects by the time of the next self-administration session. Such relatively rapid time courses focus our thinking on acute changes in phosphorylation status of proteins within PTPRD-expressing neurons. Studies of C. elegans KOs of its PTPRD homolog, ptp-3, elucidate candidate PTPRD-modulated phosphotyrosine homologs in 45 human proteins (8) including Cdk5 and the signal transducer and activator of transcription STAT3 (38). Cdk5 encodes a kinase that is regulated by its own phosphorylation and is strongly implicated in phosphorylation-induced changes in the properties of the MAPT microtubule-associated protein tau that is involved in neuroadaptive and maladaptive processes (41). PTPRD-mediated changes in the phosphorylation of STAT3 Y705 phosphotyrosine alter the ability of this transcription factor to homodimerize, move into the nucleus, and change transcription of other genes (36). Acute changes in tyrosine phosphorylation provide plausible mechanisms for influencing drug reward; both Cdk5 and STAT3 activities have been implicated in addictions (42, 43).
There is thus now a substantial rationale for optimizing 7-BIA–related structures and advancing improved PTPRD phosphatase inhibitors to clinical testing for substance use disorders. Success in this work will result in 7-BIA–related compounds that inhibit PTPRD phosphatase with increased solubility and stability; oral bioavailability; favorable biodistributions; increased specificities; lack of apparent behavioral, biochemical, or histopathological toxicities; lack of worrisome off-target effects; and beneficial effects on stimulant-conditioned place preference and self-administration. Success in these goals will also support studies of the use of PTPRD ligands in other phenotypes that have been associated with human PTPRD variation, including effects on vulnerability to develop restless leg syndrome (44), individual differences in densities of neurofibrillary neuropathology conditions in postmortem brains of individuals with Alzheimer’s disease (24), and mood instability (45). Success may also motivate identification of small molecules that can allow adult pharmacological modulation of other cell adhesion molecules [e.g., CDH13 (3)] that are candidates to play roles in addiction-related connectome signaling.
We initially focused on stimulant-use disorders in the present work because (i) combined human and mouse data are strongest for stimulants, (ii) focus on one drug class will focus human phase II clinical trials, and (iii) there are no FDA-approved medications for these disorders. Nevertheless, human genetic data support the effects of reduced PTPRD function on disorders of use of opiates, alcohol, and other addictive substances. It thus seems likely that PTPRD phosphatase inhibitors will ultimately display broad applicability in disorders of use of at least several addictive substances.
Supplementary Material
Acknowledgments
The authors thank Drs. A. Newman, D. White, H. Davis, J. Acri, I. Montoya, W. Wang, and T. Prisinzano for providing help and support. This work was supported by the Biomedical Research Institute of New Mexico (G.R.U., M.J.M.), Veterans’ Health Administration (G.R.U.), National Institutes of Health (J.D., A.S., K.C.R.), National Institute on Drug Abuse (A.S., I.M., K.C.R., G.R.U., Z.-X.X.), and National Institute on Alcohol Abuse and Alcoholism (A.S., I.M., K.C.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Jung J, Zhang H, Grant B, Chou P, American Society of Human Genetics 2017, October 17–21, 2017, Orlando, FL.
†Jung J, Zhang H, Grant B, Chou P, American Society of Human Genetics 2017, October 17–21, 2017, Orlando, FL.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720446115/-/DCSupplemental.
References
- 1.Substance Abuse and Mental Health Services Administration 2016. SAMHSA Results from the 2015 Survey of Drug Use in Households (Center for Behavioral Health Statistics and Quality, Rockville, MD)
- 2.Uhl GR, et al. Genome-wide association for methamphetamine dependence: Convergent results from 2 samples. Arch Gen Psychiatry. 2008;65:345–355. doi: 10.1001/archpsyc.65.3.345. [DOI] [PubMed] [Google Scholar]
- 3.Drgonova J, et al. Cadherin 13: Human cis-regulation and selectively-altered addiction phenotypes and cerebral cortical dopamine in knockout mice. Mol Med. 2016;22:537–547. doi: 10.2119/molmed.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drgonova J, et al. Altered CSMD1 expression alters cocaine-conditioned place preference: Mutual support for a complex locus from human and mouse models. PLoS One. 2015;10:e0120908. doi: 10.1371/journal.pone.0120908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drgonova J, et al. Mouse model for PTPRD associations with WED/RLS and addiction: Reduced expression alters locomotion, sleep behaviors and cocaine-conditioned place preference. Mol Med. 2015;21:717–725. doi: 10.2119/molmed.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Um JW, Ko J. LAR-RPTPs: Synaptic adhesion molecules that shape synapse development. Trends Cell Biol. 2013;23:465–475. doi: 10.1016/j.tcb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Craig AM. Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: Presynaptic hubs for synapse organization. Trends Neurosci. 2013;36:522–534. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell CJ, et al. Unbiased identification of substrates of protein tyrosine phosphatase ptp-3 in C. elegans. Mol Oncol. 2016;10:910–920. doi: 10.1016/j.molonc.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen LG, Rushlow WJ, Laviolette SR. Opiate exposure state controls dopamine D3 receptor and cdk5/calcineurin signaling in the basolateral amygdala during reward and withdrawal aversion memory formation. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:59–66. doi: 10.1016/j.pnpbp.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Ishiguro H, et al. NrCAM in addiction vulnerability: Positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31:572–584. doi: 10.1038/sj.npp.1300855. [DOI] [PubMed] [Google Scholar]
- 11.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson KJ, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu QR, et al. Pooled association genome scanning: Validation and use to identify addiction vulnerability loci in two samples. Proc Natl Acad Sci USA. 2005;102:11864–11869. doi: 10.1073/pnas.0500329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu QR, et al. Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:918–925. doi: 10.1002/ajmg.b.30436. [DOI] [PubMed] [Google Scholar]
- 15.Drgon T, et al. “Replicated” genome wide association for dependence on illegal substances: Genomic regions identified by overlapping clusters of nominally positive SNPs. Am J Med Genet B Neuropsychiatr Genet. 2011;156:125–138. doi: 10.1002/ajmg.b.31143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D, et al. Genome-wide association study of copy number variations (CNVs) with opioid dependence. Neuropsychopharmacology. 2015;40:1016–1026. doi: 10.1038/npp.2014.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhl GR, et al. Molecular genetics of nicotine dependence and abstinence: Whole genome association using 520,000 SNPs. BMC Genet. 2007;8:10. doi: 10.1186/1471-2156-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhl GR, et al. Molecular genetics of successful smoking cessation: Convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart AB, et al. Genome-wide association study of d-amphetamine response in healthy volunteers identifies putative associations, including cadherin 13 (CDH13) PLoS One. 2012;7:e42646. doi: 10.1371/journal.pone.0042646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joslyn G, Ravindranathan A, Brush G, Schuckit M, White RL. Human variation in alcohol response is influenced by variation in neuronal signaling genes. Alcohol Clin Exp Res. 2010;34:800–812. doi: 10.1111/j.1530-0277.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- 21.Ling Q, et al. Illudalic acid as a potential LAR inhibitor: Synthesis, SAR, and preliminary studies on the mechanism of action. Bioorg Med Chem. 2008;16:7399–7409. doi: 10.1016/j.bmc.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Ling Q, et al. Synthesis and LAR inhibition of 7-alkoxy analogues of illudalic acid. Yao Xue Xue Bao. 2010;45:1385–1397. [PubMed] [Google Scholar]
- 23.Hendriks WJ, Pulido R. Protein tyrosine phosphatase variants in human hereditary disorders and disease susceptibilities. Biochim Biophys Acta. 2013;1832:1673–1696. doi: 10.1016/j.bbadis.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Chibnik LB, et al. Susceptibility to neurofibrillary tangles: Role of the PTPRD locus and limited pleiotropy with other neuropathologies. Mol Psychiatry. 2017;23:1521–1529. doi: 10.1038/mp.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall FS, Sora I, Uhl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- 26.Moriya Y, et al. Sex differences in the effects of adolescent social deprivation on alcohol consumption in μ-opioid receptor knockout mice. Psychopharmacology (Berl) 2015;232:1471–1482. doi: 10.1007/s00213-014-3784-y. [DOI] [PubMed] [Google Scholar]
- 27. Tissue Expression of PTPRD. Summary. The Human Protein Atlas. Available at www.proteinatlas.org/ENSG00000153707-PTPRD/tissue.
- 28. Inhibition of Enzyme Activity. Available at www.csun.edu/∼hcchm001/5enzyme.pdf. Accessed November 14, 2017.
- 29.Uhl GR, Drgonova J. Cell adhesion molecules: Druggable targets for modulating the connectome and brain disorders? Neuropsychopharmacology. 2014;39:235. doi: 10.1038/npp.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, et al. Discovery of novel inhibitor of human leukocyte common antigen-related phosphatase. Biochim Biophys Acta. 2005;1726:34–41. doi: 10.1016/j.bbagen.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choucair N, et al. Evidence that homozygous PTPRD gene microdeletion causes trigonocephaly, hearing loss, and intellectual disability. Mol Cytogenet. 2015;8:39. doi: 10.1186/s13039-015-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, et al. Protein tyrosine phosphatase receptor-type δ acts as a negative regulator suppressing breast cancer. Oncotarget. 2017;8:98798–98811. doi: 10.18632/oncotarget.22000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodward EL, et al. Genomic complexity and targeted genes in anaplastic thyroid cancer cell lines. Endocr Relat Cancer. 2017;24:209–220. doi: 10.1530/ERC-16-0522. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Bixby JL. Receptor tyrosine phosphatase-delta is a homophilic, neurite-promoting cell adhesion molecular for CNS neurons. Mol Cell Neurosci. 1999;14:370–384. doi: 10.1006/mcne.1999.0789. [DOI] [PubMed] [Google Scholar]
- 36.Yamagata A, et al. Structure of Slitrk2-PTPδ complex reveals mechanisms for splicing-dependent trans-synaptic adhesion. Sci Rep. 2015;5:9686. doi: 10.1038/srep09686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida T, et al. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J Neurosci. 2012;32:2588–2600. doi: 10.1523/JNEUROSCI.4637-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J Biol Chem. 2010;285:13966–13978. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Z, Liu J, Ding H, Xu F, Liu H. Structural basis of SALM5-induced PTPδ dimerization for synaptic differentiation. Nat Commun. 2018;9:268. doi: 10.1038/s41467-017-02414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen brain atlas images 2018 Available at http://mouse.brain-map.org/. Accessed November 14, 2017.
- 41.Bhounsule AS, Bhatt LK, Prabhavalkar KS, Oza M. Cyclin dependent kinase 5: A novel avenue for Alzheimer’s disease. Brain Res Bull. 2017;132:28–38. doi: 10.1016/j.brainresbull.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi S, et al. Increased activity of cyclin-dependent kinase 5 leads to attenuation of cocaine-mediated dopamine signaling. Proc Natl Acad Sci USA. 2005;102:1737–1742. doi: 10.1073/pnas.0409456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen JX, et al. Activation of TLR4/STAT3 signaling in VTA contributes to the acquisition and maintenance of morphine-induced conditioned place preference. Behav Brain Res. 2017;335:151–157. doi: 10.1016/j.bbr.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 44.Earley CJ, Uhl GR, Clemens S, Ferré S. Connectome and molecular pharmacological differences in the dopaminergic system in restless legs syndrome (RLS): Plastic changes and neuroadaptations that may contribute to augmentation. Sleep Med. 2017;31:71–77. doi: 10.1016/j.sleep.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward J, et al. Genome-wide analysis in UK Biobank identifies four loci associated with mood instability and genetic correlation with major depressive disorder, anxiety disorder and schizophrenia. Transl Psychiatry. 2017;7:1264. doi: 10.1038/s41398-017-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.