Significance
Immune thrombocytopenia (ITP) patients with antiplatelet glycoprotein (GP) Ib-IX autoantibodies appear refractory to conventional treatments; however, the mechanism remains elusive. Here we show that the platelets undergo apoptosis in ITP patients with anti-GPIbα autoantibodies. We demonstrate that anti-GPIbα antibody binding activates Akt, which elicits platelet apoptosis through activation of phosphodiesterase (PDE3A) and PDE3A-mediated PKA inhibition. Phosphatidylserine (PS) exposure results in phagocytosis of anti-GPIbα antibody-bound platelets by macrophages in the liver. Notably, inhibition or genetic ablation of Akt or Akt-regulated apoptotic signaling or blockage of PS exposure rescues the platelets from clearance. Therefore, our findings reveal pathogenic mechanisms of ITP with anti-GPIbα autoantibodies and, more importantly, suggest therapeutic strategies for thrombocytopenia caused by autoantibodies or other pathogenic factors.
Keywords: immune thrombocytopenia, platelet, apoptosis, Akt, phosphatidylserine exposure
Abstract
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet count which can cause fatal hemorrhage. ITP patients with antiplatelet glycoprotein (GP) Ib-IX autoantibodies appear refractory to conventional treatments, and the mechanism remains elusive. Here we show that the platelets undergo apoptosis in ITP patients with anti-GPIbα autoantibodies. Consistent with these findings, the anti-GPIbα monoclonal antibodies AN51 and SZ2 induce platelet apoptosis in vitro. We demonstrate that anti-GPIbα antibody binding activates Akt, which elicits platelet apoptosis through activation of phosphodiesterase (PDE3A) and PDE3A-mediated PKA inhibition. Genetic ablation or chemical inhibition of Akt or blocking of Akt signaling abolishes anti-GPIbα antibody-induced platelet apoptosis. We further demonstrate that the antibody-bound platelets are removed in vivo through an apoptosis-dependent manner. Phosphatidylserine (PS) exposure on apoptotic platelets results in phagocytosis of platelets by macrophages in the liver. Notably, inhibition or genetic ablation of Akt or Akt-regulated apoptotic signaling or blockage of PS exposure protects the platelets from clearance. Therefore, our findings reveal pathogenic mechanisms of ITP with anti-GPIbα autoantibodies and, more importantly, suggest therapeutic strategies for thrombocytopenia caused by autoantibodies or other pathogenic factors.
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet count (1, 2) which is caused primarily by autoantibodies against two major receptors of platelets, the fibrinogen receptor glycoprotein (GP) IIb/IIIa and the von Willebrand factor (VWF) receptor GPIb-IX complex (3–5). The autoantibody-bound platelets are thought to be removed by Fc-dependent phagocytosis in the spleen (1, 2, 6). Therefore, the main therapeutic strategies for ITP are immune suppression, immune modulation, and splenectomy (1, 2, 7). However, ITP patients with anti–GPIb-IX autoantibodies present more severe decreases in platelet count (4) and are less responsive to conventional therapies such as steroid treatments (8), i.v. IgG (IVIG) (5, 9), and even splenectomy (10, 11), suggesting that a different pathogenic mechanism may be involved in anti–GPIb-IX autoantibody-induced platelet clearance.
Anti-GPIbα monoclonal antibodies were found to activate platelets in vitro (12–16) and induce platelet clearance in vivo (12, 17–20). More recent studies demonstrated that anti-GPIbα antibodies induced phagocytosis of platelets in the liver through an Fc-independent mechanism (12, 17, 20). Anti-GPIbα antibodies targeting the N terminus of the receptor cause it to cluster, resulting in phagocytosis of platelets by microphages in the liver (12). On the other hand, GPIbα desialylation was demonstrated to contribute to platelet clearance in an hepatocyte Ashwell–Morell receptor-dependent manner (20). Moreover, shear-induced unfolding of the GPIbα mechanosensory domain by anti-GPIbα monoclonal antibodies was found to trigger signaling, leading to platelet clearance (21). Therefore, while increasing evidence suggests that anti-GPIbα autoantibodies may induce platelet clearance via an Fc-independent manner, the mechanism for anti-GPIbα antibody-induced thrombocytopenia remains elusive.
GPIbα, the main subunit of the GPIb-IX complex, contains binding sites for several important ligands including VWF and thrombin at the N-terminal extracellular domain (16, 22, 23). The interaction of the VWF multimer with GPIbα induces translocation and cross-linking of GPIb-IX complexes in lipid rafts (24–27), triggering signaling cascades (28, 29) and leading to platelet activation and thrombus formation (30, 31). Interestingly, we found that the GPIbα–VWF interaction could also induce platelet apoptosis, but the mechanism remains unknown (32). We recently reported that protein kinase A (PKA)-mediated platelet apoptosis occurs extensively in pathophysiological conditions (33). Moreover, accumulating evidence suggests that various pathological stimuli lead to thrombocytopenia in many common diseases, such as infection, cancer, diabetes, and heart and circulation diseases (34–37). However, little is known about the pathogenesis leading to thrombocytopenia.
In this study, we find that anti-GPIbα monoclonal antibodies induce Akt activation and Akt-mediated platelet apoptosis. We demonstrate that platelets undergo apoptosis in ITP patients with anti-GPIbα autoantibodies. The apoptotic platelets are phagocytized by macrophages in the liver in a phosphatidylserine (PS) exposure-dependent manner. Inhibition or genetic ablation of Akt or Akt-regulated apoptotic signaling or blockage of PS exposure rescues the platelets from clearance. Therefore our findings reveal pathogenic mechanisms of ITP with anti-GPIbα autoantibodies and, more importantly, suggest therapeutic strategies for thrombocytopenia caused by autoantibodies or other pathogenic factors.
Results
Anti-GPIbα Antibodies Induce Platelet Apoptosis.
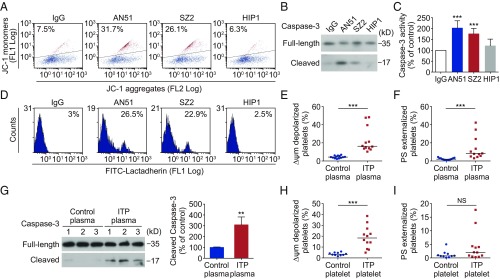
To investigate the pathogenesis of ITP with anti-GPIbα antibodies, we selected anti-GPIbα monoclonal antibodies (SI Appendix, Table S1) to examine their effects on platelets. We hypothesized that anti-GPIbα antibodies could induce platelet apoptosis. To test this, we incubated the anti-GPIbα antibodies AN51 and SZ2 with human platelets. We found that these antibodies induced marked mitochondrial transmembrane potential (ΔΨm) depolarization (Fig. 1A), which initiates mitochondria-mediated intrinsic programmed apoptosis in platelets (32–35). Moreover, AN51 and SZ2 significantly elevated caspase-3 activity in platelets, as indicated by the appearance of 17-kDa fragments (Fig. 1B), and total caspase-3 activity (Fig. 1C). Caspases combined with other apoptogenic enzymes disrupt plasma membrane integrity leading to PS externalization during apoptosis (32–35). We found that AN51 and SZ2 indeed induced PS exposure in the platelets (Fig. 1D). These data indicate that the anti-GPIbα antibodies induce platelet apoptosis. Moreover, as is consistent with previous reports (12–16), anti-GPIbα antibodies also induced platelet activation, as indicated by P-selectin exposure and PAC-1 binding (SI Appendix, Fig. S1). In contrast, the anti-GPIIb/IIIa antibodies SZ21 and D57 did not induce platelet apoptosis or activation (SI Appendix, Fig. S1). In addition, the anti-GPIbα antibody HIP1 also did not induce obvious platelet apoptosis (Fig. 1 A–D).
Fig. 1.
Anti-GPIbα antibodies induce platelet apoptosis. (A–D) Washed human platelets were incubated with 10 μg/mL normal mouse IgG or anti-GPIbα antibody AN51, SZ2, or HIP1 at 37 °C for 8 h. (A) Representative flow cytometric figures of platelet Δψm depolarization. The JC-1 monomers reflect the monomeric form of JC-1 that appeared in the cytosol after mitochondrial Δψm depolarization, and the JC-1 aggregates represent potential-dependent aggregation in the mitochondria. (B) Western blot analysis of caspase 3 with anti–caspase-3 antibody. (C) Analysis of caspase-3 activity with ELISA, n = 6. (D) Representative flow cytometric figures of platelet PS exposure. (E–G) Washed normal human platelets were incubated with plasma from healthy donors (control plasma) or from ITP patients with only anti-GPIbα autoantibodies (ITP plasma) at 37 °C for 8 h (1:1, vol/vol). Platelet Δψm depolarization (E) and PS exposure (F) were detected by flow cytometry. (G, Left) Caspase 3 was analyzed with anti–caspase-3 antibody by Western blot. (Right) Densitometry of immunoblot for cleaved caspase 3 from the Western blot data. (H and I) Platelet Δψm depolarization (H) and PS exposure (I) were detected by flow cytometry in PRP isolated from healthy donors (control platelets) and ITP patients with anti-GPIbα autoantibodies (ITP platelets). Data in C and G are expressed as mean ± SD. In E, F, H, and I horizontal lines indicate the median values, and each dot represents one patient. **P < 0.01, ***P < 0.001 compared with control by one-way ANOVA (C), Mann-Whitney U test (E, F, H, and I), or Student’s t test (G). NS, not significant.
Platelets Undergo Apoptosis in ITP Patients with Anti-GPIbα Autoantibodies.
To verify the observations with anti-GPIbα monoclonal antibodies in ITP patients, we identified 12 ITP patients with anti-GPIbα autoantibodies (SI Appendix, Table S2) using the flow cytometric immunobead array (38). After healthy human platelets were incubated with plasma from the ITP patients, we found that the plasma obviously induced ΔΨm depolarization (Fig. 1E), PS exposure (Fig. 1F), and caspase-3 activation in the platelets (Fig. 1G). These data suggest that the anti-GPIbα autoantibody plasma can induce platelet apoptosis in vitro. To rule out other factors, such as cytokines and growth factors, that might be present in ITP plasma and influence the results, we purified IgG fractions from the ITP plasmas containing anti-GPIbα autoantibodies. Compared with IgG fractions from normal plasmas, IgG fractions from ITP plasmas induced obvious ΔΨm depolarization, PS exposure, and caspase-3 activation in the platelets (SI Appendix, Fig. S2).
We also directly examined the platelets from the ITP patients for apoptotic events. ΔΨm depolarization was detected in the platelets from ITP patients with anti-GPIbα autoantibodies (Fig. 1H). Interestingly, unlike the results with plasma in vitro, there was no significant difference in PS exposure between platelets from ITP patients and platelets from healthy controls (Fig. 1I). The reason might be that PS-exposed platelets had been removed from the circulation in vivo. Moreover, P-selectin exposure was obviously elevated in healthy human platelets incubated with plasma containing anti-GPIbα autoantibodies (SI Appendix, Fig. S3A) and in platelets from the patients (SI Appendix, Fig. S3B), suggesting the platelets were activated. In addition, we found that, compared with the significant effects of the anti-GPIbα autoantibody plasma on platelet ΔΨm depolarization and P-selectin exposure, plasma from ITP patients with anti-GPIIb/IIIa autoantibodies induced only moderate ΔΨm depolarization and P-selectin exposure in healthy human platelets (SI Appendix, Fig. S4). Taken together, these data suggest that the platelets undergo apoptosis in ITP patients with anti-GPIbα autoantibodies.
Anti-GPIbα Antibody Induces Platelet Apoptosis by Inhibiting PKA Activity.
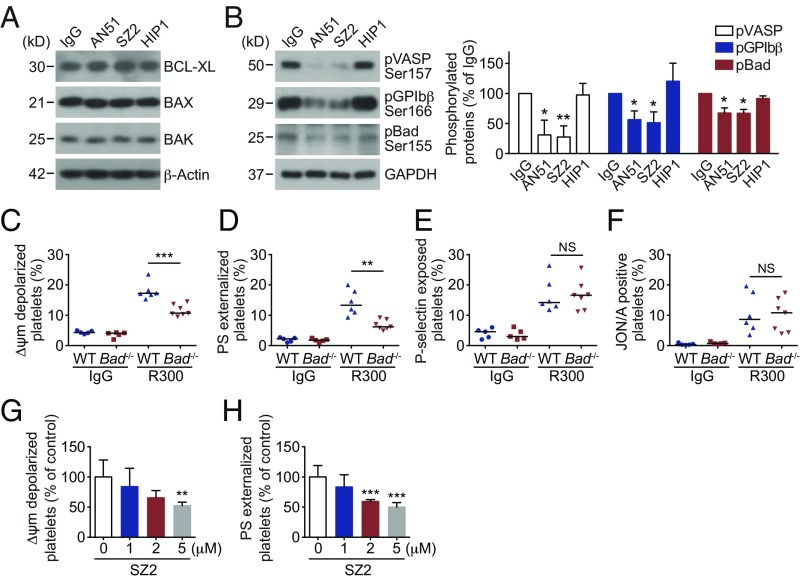
Next, we investigated the mechanism of anti-GPIbα antibody-induced platelet apoptosis. First we detected the amounts of apoptotic proteins in the platelets. Bcl-xL, Bak, and Bax did not vary in the antibody-treated (Fig. 2A) and anti-GPIbα autoantibody plasma-treated (SI Appendix, Fig. S5) platelets. We reported recently that PKA plays a key role in regulating platelet apoptosis (33). Reduction of PKA activity resulted in dephosphorylation of Bad at Ser155 leading to platelet apoptosis (33). Therefore, we detected PKA activity and found that PKA activity was reduced in AN51- and SZ2-treated platelets (Fig. 2B) but not in SZ21- or D57-treated platelets (SI Appendix, Fig. S6), as indicated by dephosphorylation of the PKA substrates GPIbβ Ser166 (39) and vasodilator-associated stimulated phosphoprotein (VASP) (Fig. 2B) (40). Phosphorylation of Bad at Ser155, which is mediated by PKA (41), was also reduced (Fig. 2B). Similarly, anti-GPIbα autoantibody plasma reduced PKA activity in platelets (SI Appendix, Fig. S7). We further verified the role of PKA in platelet apoptosis with Bad-deficient platelets, which lack PKA substrate, and found that anti-GPIbα antibody-induced apoptotic events were obviously reduced (Fig. 2 C and D) but that the activation events were not obviously different in the Bad-deficient platelets (Fig. 2 E and F). In contrast, activation of PKA with forskolin, as indicated by phosphorylation of VASP at Ser157 (SI Appendix, Fig. S8), markedly reduced anti-GPIbα antibody-induced apoptotic events (Fig. 2 G and H). These data indicate that anti-GPIbα antibodies induce platelet apoptosis through inhibition of PKA activity.
Fig. 2.
Anti-GPIbα antibody-induced platelet apoptosis through inhibition of PKA activity. (A and B, Left) Western blot analysis of protein levels in human platelets treated with10 μg/mL IgG, AN51, SZ2, or HIP1 at 37 °C for 8 h with the indicated primary antibodies. Data are representative of three separate experiments. (B, Right) Densitometry of phosphorylated proteins in the Western blots at Left; n = 3. (C–F) PRP from WT or Bad−/− mice was incubated with 5 μg/mL R300 (a mixture of purified rat monoclonal antibodies against mouse GPIbα) at 37 °C for 8 h. Platelet Δψm depolarization (C), PS exposure (D), P-selectin externalization (E), and JON/A binding (F) were analyzed by flow cytometry. (G and H) Washed human platelets were pretreated with the indicated concentrations of the PKA activator forskolin at RT for 5 min and then were incubated with 10 μg/mL SZ2 at 37 °C for 8 h. Platelet Δψm depolarization (G) and PS exposure (H) were detected by flow cytometry; n = 6. Data are expressed as mean ± SD in B, G, and H. Horizontal lines in C–F indicate the median values, and each dot represents one mouse. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control or WT mice with Student’s t test in B and C–F or one-way ANOVA followed by Dunnett’s post hoc test in G and H. NS, not significant.
Akt Plays Key Roles in Anti-GPIbα Antibody-Induced Platelet Apoptosis.
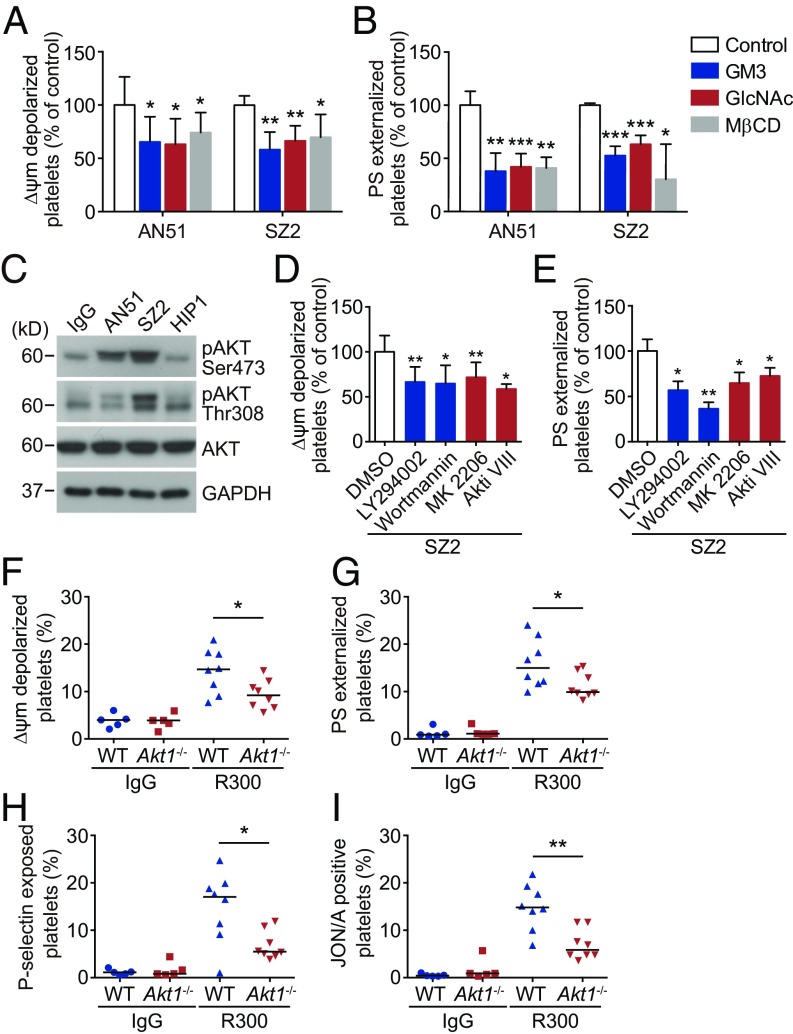
We further investigated why anti-GPIbα antibody binding induces apoptosis in PKA-mediated platelets. VWF-induced cross-linking of multiple GPIb-IX complexes in lipid rafts triggers signaling cascades leading to platelet activation (24–27). In light of these findings, we treated platelets with GM3 and GlcNAc, which inhibit GPIbα clustering (42, 43), and found that both these inhibitors significantly reduced AN51- and SZ2-induced ΔΨm depolarization and PS exposure (Fig. 3 A and B). Moreover, we used MβCD to disrupt lipid rafts (24) and found that MβCD obviously reduced AN51- and SZ2-induced apoptotic events (Fig. 3 A and B). In addition, consistent with the results of VWF binding (24–27), platelet activation was diminished by GM3, GlcNAc, and MβCD in AN51- and SZ2-treated platelets (SI Appendix, Fig. S9). These data suggest that anti-GPIbα antibodies may initiate platelet apoptosis by inducing GPIbα clustering in lipid rafts.
Fig. 3.
Akt plays key roles in anti-GPIbα antibody-induced platelet apoptosis. (A and B) Washed human platelets were pretreated with GM3 (100 μM), GlcNAc (100 mM), MβCD (1 mM), or vehicle control at 37 °C for 30 min and were further incubated with 10 μg/mL AN51 or SZ2 at 37 °C for 8 h. Platelet Δψm depolarization (A) and PS exposure (B) were detected by flow cytometry; n = 5. (C) Western blot analysis of Akt phosphorylation with anti-pAkt Ser-473 and Thr-308 antibodies. Data are representative of three separate experiments. (D and E) Washed human platelets were pretreated with the PI3K inhibitors LY294002 (20 μM) or wortmannin (100 μM), the Akt inhibitors MK2206 (6 μM) or Akti VIII (2 μM), or vehicle at RT for 5 min and were incubated with 10 μg/mL SZ2 at 37 °C for 8 h. Platelet Δψm depolarization (D) and PS exposure (E) were detected by flow cytometry; n = 7. (F–I) PRP from WT or Akt1−/− mice was incubated with 5 μg/mL R300 at 37 °C for 8 h. Δψm depolarization (F), PS exposure (G), P-selectin externalization (H), and JON/A binding (I) were analyzed by flow cytometry. Data in A, B, D, and E are expressed as mean ± SD. Horizontal lines in F–I indicate the median values, and each dot represents one mouse. *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle control or WT mice by Student’s t test.
Akt, a downstream effector of PI3K which interacts with the cytoplasmic domain of GPIbα (44), transduces VWF–GPIbα interaction signaling leading to platelet activation (28). Therefore, we hypothesized that Akt may be essential for anti-GPIbα antibody-induced apoptotic signaling. In support of this, we found that Akt was activated in AN51- and SZ2-treated (Fig. 3C) but not in SZ21- or D57-treated (SI Appendix, Fig. S6) platelets. Anti-GPIbα autoantibody plasma also markedly elevated Akt activity in platelets (SI Appendix, Fig. S10). Inhibitors of PI3K (LY294002 and wortmannin) or Akt (MK 2206 and Akti VIII) obviously reduced anti-GPIbα antibody-induced platelet apoptosis (Fig. 3 D and E).
There are three isoforms of Akt, all of which are expressed in both mouse and human platelets (28, 45–47). However, only Akt1 and Akt2 play roles in VWF-dependent signaling (28, 47). Therefore, we selected Akt1- and Akt2-knockout mice to investigate the role of Akt in antibody-induced signaling. We found that anti-GPIbα antibody-induced apoptotic events and activation were markedly reduced in both Akt1-deficient (Fig. 3 F–I) and Akt2-deficient (SI Appendix, Fig. S11) platelets. Taken together, these data demonstrate that Akt plays key roles in anti-GPIbα antibody-induced platelet apoptosis.
Akt Regulates PKA Activity Through Regulation of Phosphodiesterase.
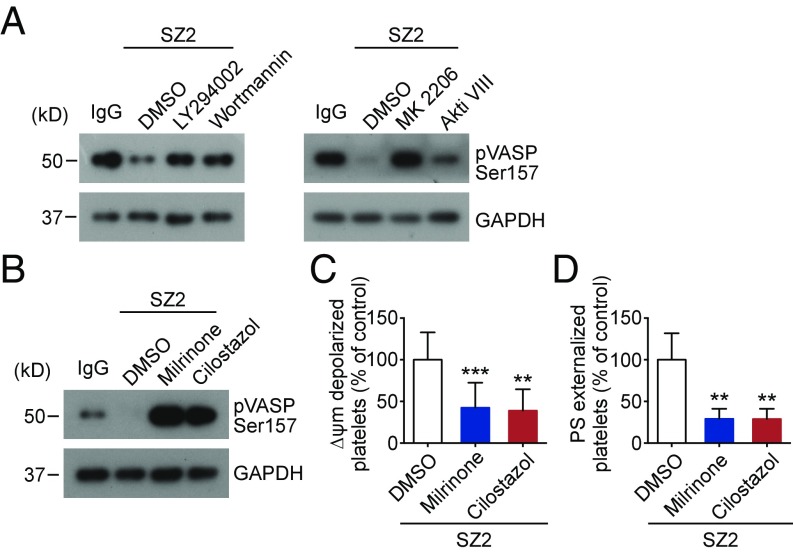
Akt was reported to directly activate phosphodiesterase (PDE3A) (48, 49). PDE3A hydrolyzes intracellular cAMP, leading to reduction of PKA activity (50). Therefore, it is possible that Akt regulates PKA-mediated platelet apoptosis through activation of PDE3A. To test this, platelets were treated with PI3K or Akt inhibitors. We found that inhibitors of PI3K (LY294002 and wortmannin) or Akt (MK 2206 and Akti VIII) markedly elevated PKA activity in SZ2-treated platelets (Fig. 4A). Correspondingly, the antibody-induced apoptotic events were markedly reduced by PI3K or Akt inhibitors (Fig. 3 D and E). Moreover, PDE3A inhibitors (milrinone and cilostazol) directly elevated PKA activity (Fig. 4B) and reduced apoptotic events in SZ2-treated platelets (Fig. 4 C and D). These data indicate that Akt reduces PKA activity and regulates PKA-mediated platelet apoptosis through activation of PDE3A.
Fig. 4.
Akt regulates PKA activity through regulation of PDE3A. (A) Washed human platelets were pretreated with PI3K inhibitors LY294002 (20 μM) and wortmannin (100 nM), Akt inhibitors MK2206 (6 μM) and Akti VIII (2 μM), or vehicle control at RT for 5 min and then were incubated with 10 μg/mL SZ2 at 37 °C for 8 h. VASP phosphorylation was analyzed with anti-pVASP Ser157 antibody by Western blot; data are representative of three separate experiments. (B–D) Washed human platelets were pretreated with PDE3A inhibitors milrinone (10 μM) and cilostazol (10 μM) or vehicle control at RT for 5 min and then were incubated with 10 μg/mL SZ2 at 37 °C for 8 h. VASP phosphorylation was analyzed with anti-pVASP Ser157 antibody by Western blot (B), and platelet Δψm depolarization (C) and PS exposure (D) were detected by flow cytometry; n = 7. Data are expressed as mean ± SD; **P < 0.01, ***P < 0.001, compared with vehicle control with Student’s t test.
Anti-GPIbα Antibody-Induced Platelet Apoptosis and Activation Occur Independently Through Two Separate Signaling Cascades.
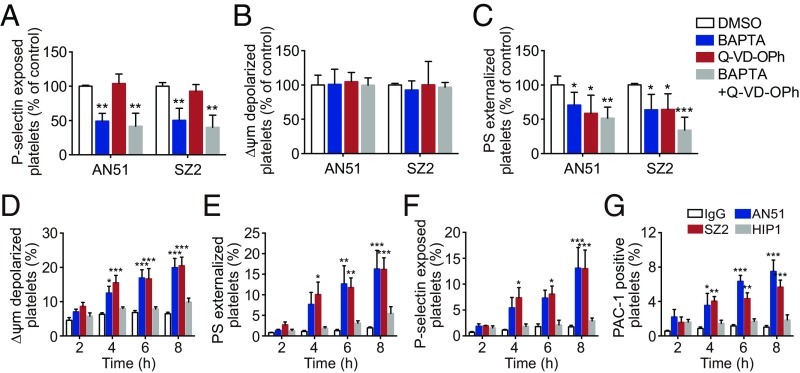
Deletion of Akt reduced anti-GPIbα antibody-induced apoptosis and activation (Fig. 3 F–I). However, Bad deficiency only impaired the antibody-induced apoptosis (Fig. 2 C–F), suggesting that the antibody-induced apoptosis and activation occur independently downstream of Akt. To further verify this, we used BAPTA [1,2-Bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetra acetic acid] to deplete intracellular calcium, which is essential for Akt-dependent platelet activation signaling (46, 51). BAPTA reduced P-selectin (Fig. 5A) but did not affect ΔΨm depolarization (Fig. 5B), suggesting that Ca2+ mobilization induced by anti-GPIbα antibodies only contributes to platelet activation. We incubated platelets with the caspase inhibitor Q-VD-OPh (quinoline-Val-Asp-difluorophenoxymethyl ketone) to block the apoptotic process. Q-VD-OPh only reduced PS externalization (Fig. 5C) but did not affect P-selectin exposure (Fig. 5A). Since ΔΨm depolarization is up-stream of caspase activation, we found that Q-VD-OPh did not affect ΔΨm depolarization (Fig. 5B). These findings indicate that anti-GPIbα antibody-induced platelet apoptosis and activation occur independently through two separate signaling cascades. Similar effects were observed in mouse platelets stimulated with anti-mouse GPIbα antibody R300 (SI Appendix, Fig. S12).
Fig. 5.
Anti-GPIbα antibody-induced platelet apoptosis and activation occur independently through two separate signaling cascades. (A–C) Washed human platelets were pretreated with the Ca2+ chelator BAPTA (10 μM), the pan-caspase inhibitor Q-VD-OPh (100 μM), or vehicle at RT for 5 min and were incubated with 10 μg/mL AN51 or SZ2 at 37 °C for 8 h. Platelet P-selectin externalization (A), Δψm depolarization (B), and PS exposure (C) were detected by flow cytometry; n = 7. (D–G) Time course of apoptosis and activation occurring in human platelets treated with 10 μg/mL IgG, AN51, SZ2, or HIP1. Data are expressed as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle control or normal IgG control by Student’s t test in A–C and by two-way ANOVA followed by Dunnett’s post hoc test in D–G.
Platelet apoptosis and activation alone may lead to platelet clearance in vivo. We therefore investigated the chronological order of anti-GPIbα antibody-induced apoptosis and activation. The results showed that apoptotic and activated events occurred almost simultaneously (Fig. 5 D–G). Similar effects of anti-mouse GPIbα polyclonal antibodies on mouse platelets were observed with R300 (SI Appendix, Fig. S13).
Anti-GPIbα Antibodies Induce Apoptosis-Dependent Platelet Clearance.
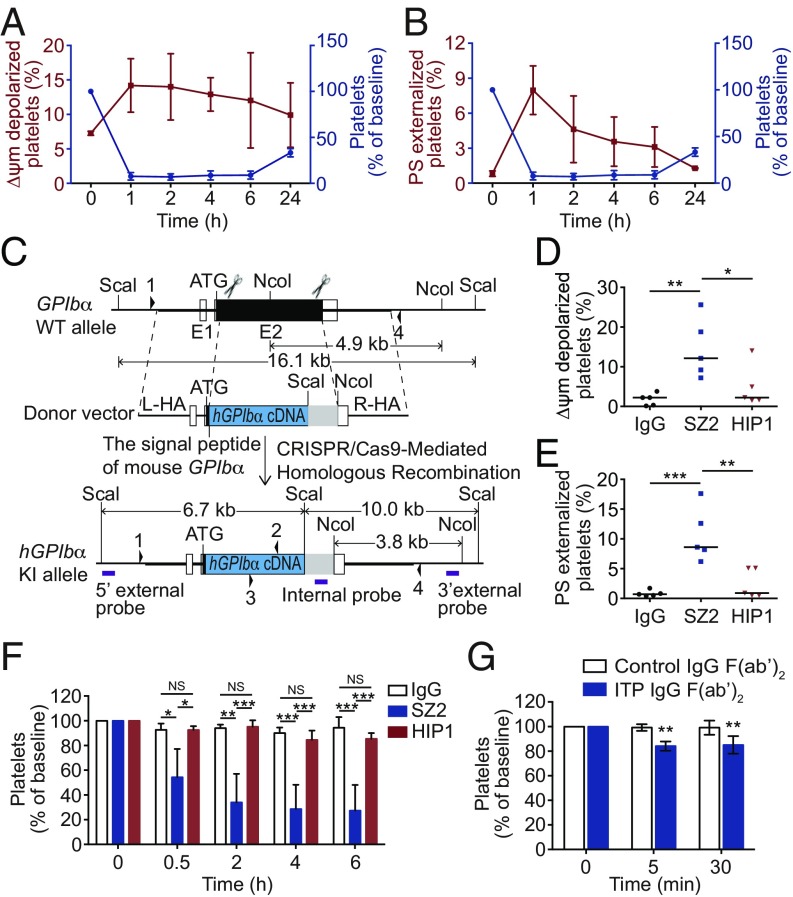
ITP patients with anti-GPIbα autoantibodies appear refractory to Fc-dependent therapy (5, 9–11). It has been known that anti-GPIbα antibodies induce thrombocytopenia in an Fc-independent manner (12, 17, 20). However, how the anti-GPIbα antibody-bound platelets are removed remained unclear. Therefore, we investigated whether platelet apoptosis contributed to platelet clearance in vivo. First, we demonstrated that both intact and F(ab′)2 fragments of anti-mouse GPIbα antibodies (R300) could induce platelet apoptosis in vitro (SI Appendix, Fig. S14 A and B). Second, we injected these antibodies into mice and found that, consistently with a previous report (17), both intact and F(ab′)2 fragments of R300 dose-dependently induced platelets clearance (SI Appendix, Fig. S14 C and D), further demonstrating that the Fc is not required for the anti-GPIbα antibody-induced platelet clearance. Third, apoptotic events were detected in the platelets from R300-treated mice. We found that the circulating platelet counts were negatively correlated with apoptotic events, Δψm depolarization, and PS exposure (Fig. 6 A and B), suggesting that platelet apoptosis may be essential for platelet clearance.
Fig. 6.
Anti-GPIbα antibodies induce apoptosis-dependent platelet clearance. (A and B) C57BL/6J mice were i.v. injected with 0.1 μg/g R300. Platelet count and Δψm depolarization (A) and platelet count and PS exposure (B) were measured at the indicated time points. n = 5 mice per group. (C) Schematic overview of the strategy to generate the hGPIbα-knockin allele. The humanized GPIbα coding sequence (hGPIbα cDNA) was fused to the signal peptide of the mouse GPIbα gene. The scissors indicate the Cas9 nuclease and its cutting sites at the GPIbα locus. The homologous arms of the donor vector are indicated as “L-HA” (1.7 kb) and “R-HA” (1.7 kb). Regions of homology between the WT locus and the donor vector are depicted by thick black lines, UTRs are shown as open bars, and the translated region is represented by solid bars. The restriction enzyme used for Southern blot analysis is shown. The purple boxes indicate the probe used for Southern blotting. The arrowheads indicate the locations of primers 1, 2, 3, and 4 used for genotyping and sequencing. (D and E) PRP from WT and hGPIbα mice was incubated with 10 μg/mL SZ2, HIP1, or normal mouse IgG at 37 °C for 8 h. Platelet Δψm depolarization (D) and PS exposure (E) were measured by flow cytometry. (F) hGPIbα mice were i.p. injected with 0.4 μg/g normal mouse IgG, SZ2, or HIP1. Platelet counts were determined at the indicated time points; n = 5 mice per group. (G) hGPIbα mice were i.v. injected with 200 μL IgG F(ab′)2 fragments from four healthy donors or from five ITP patients with only anti-GPIbα autoantibodies. Platelet counts were determined at the indicated time points. Baseline is defined as the platelet number before antibody injection. Data are expressed as mean ± SD in A, B, F, and G. Horizontal lines indicate the median values, and each dot represents one mouse in D and E. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA in D and E, by two-way ANOVA followed by Tukey’s post hoc test in F, and by two-way ANOVA followed by Bonferroni’s post hoc test in G. NS, not significant.
Therefore, we next tried to investigate the role of platelet apoptosis in anti-GPIbα antibody-induced platelet clearance. Fig. 1A shows that anti-GPIbα antibody SZ2, but not HIP1, induced platelet apoptosis. Because these monoclonal antibodies are all antibodies against human platelet GPIbα, we set up a humanized GPIbα murine model by using the human GPIbα gene to replace the mouse GPIbα gene (Fig. 6C and SI Appendix, Fig. S15). The expression of humanized GPIbα in the mouse platelets was verified by flow cytometry (SI Appendix, Fig. S16). The homozygous humanized GPIbα mice (hGPIbα) did not differ from WT mice in the numbers of platelets or red and white blood cells or in hemoglobin concentration (SI Appendix, Table S3). Consistent with the observation in human platelets, SZ2, but not HIP1, resulted in humanized GPIbα platelet apoptosis (Fig. 6 D and E). Then, we injected SZ2 and HIP1 into hGPIbα mice and found that SZ2, but not HIP1, induced platelet clearance (Fig. 6F). These data demonstrate that only the anti-GPIbα antibody, which induces platelet apoptosis, can initiate platelet clearance in vivo.
To further verify the apoptosis-dependent platelet clearance and evaluate the clinical relevance of these findings, we prepared F(ab′)2 fragments from the IgG fractions of ITP patients with only anti-GPIbα autoantibodies. The F(ab′)2 fragments induced the humanized GPIbα mouse platelet apoptosis in vitro (SI Appendix, Fig. S17). When injected into hGPIbα mice, the F(ab′)2 fragments indeed induced platelet clearance (Fig. 6G).
PS Exposure Is Responsible for Apoptotic Platelet Clearance.
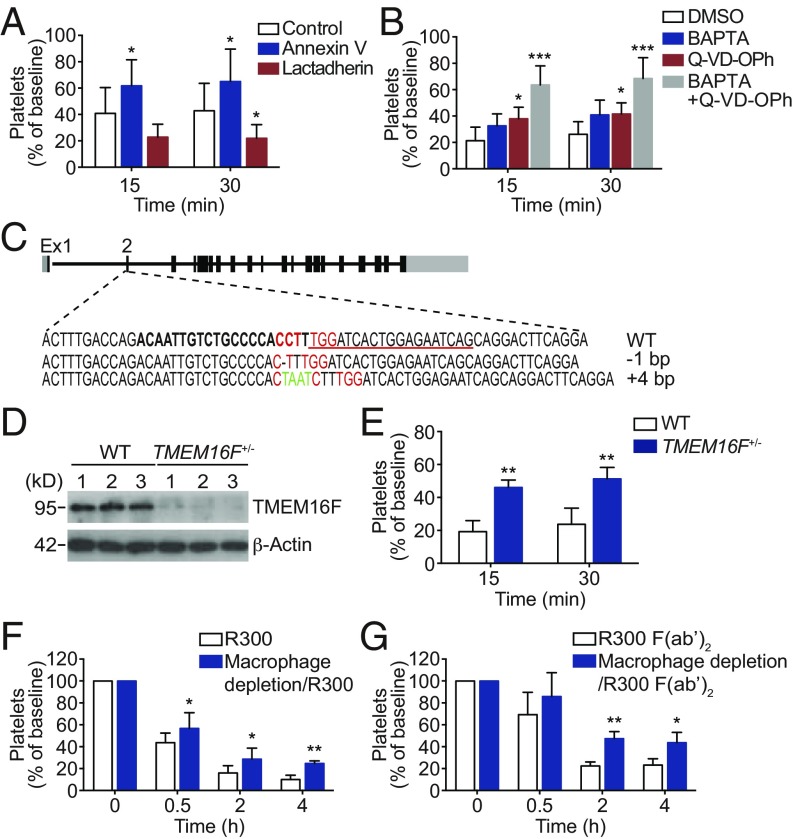
To explore the mechanism of apoptosis-dependent platelet clearance, we noticed that PS exposure is a late event of both platelet apoptosis and activation. As known, PS exposure is an “eat-me” signal leading to phagocytosis of apoptotic cells in vivo (52). Therefore, we investigated the role of PS in platelet phagocytosis. We found that the PS blocker annexin V effectively protected platelets from anti-GPIbα antibody (R300)-induced clearance (Fig. 7A). Moreover, annexin V rescued anti-human GPIbα antibody SZ2-induced platelet clearance in hGPIbα mice (SI Appendix, Fig. S18). In contrast, lactadherin, which enhances the interaction between PS and PS receptor (53), increased platelet clearance (Fig. 7A). These data suggest the role of PS exposure in anti-GPIbα antibody-induced platelet clearance.
Fig. 7.
PS exposure-dependent phagocytosis of platelets by macrophage. (A) C57BL/6J mice were i.v. preinjected with 0.5 μg/g annexin V, 0.025 μg/g lactadherin, or vehicle control and then were transfused with calcein-labeled R300-treated platelets. (B) Calcein-labeled mouse platelets pretreated with 10 μM BAPTA, 100 μM Q-VD-OPh, or vehicle control at RT for 5 min were incubated with R300 and then were transfused to C57BL/6J mice. The percentage of calcein-positive platelets remaining in circulation was assessed at the indicated time points by flow cytometry; n = 6–11 mice per group. Baseline is defined as the percentage of calcein-positive platelets within 1 min after platelet transfusion. (C) Generation of TMEM16F frameshift mutant mice using CRISPR/Cas9 genome editing. Exon 2 of the mouse TMEM16F gene was targeted with two CRISPR gRNAs indicated by bold letters and underlined, respectively (protospacer ajacent motif is shown in red). Founder alleles are depicted with deleted bases represented by dashes and inserted bases shown in green. (D) Western blot analysis of the TMEM16F level in WT and TMEM16F+/− mice. (E) C57BL/6J mice were i.v. transfused with calcein-labeled R300-treated platelets from TMEM16F+/− or WT littermates, and the percentage of calcein-positive platelets remaining in circulation was assessed at the indicated time points by flow cytometry; n = 5 mice per group. Baseline is defined as in A. (F and G) Effect of macrophage depletion on platelet clearance induced by R300 (F) and R300 F(ab′)2 (G) fragments. C57BL/6J mice were injected with control and clodronate liposomes 24 h before R300 (0.1 μg/g) or R300 F(ab′)2 fragments (0.3 μg/g) were injected i.p. into mice, and platelet number was determined at the indicated time points; n = 5 or 6 mice per group. Baseline is defined as the platelet number before anti-GPIbα antibody injection. Data are expressed as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001, by two-way ANOVA followed by Bonferroni’s post hoc test.
Since apoptosis and activation both contribute to PS exposure in anti-GPIbα antibody-treated platelets, both these two signaling pathways should contribute to anti-GPIbα antibody-induced platelet clearance in vivo as well. To investigate this, we injected BAPTA and Q-VD-OPh into mice. We found that, consistent with the in vitro data, BAPTA and Q-VD-OPh both obviously protected platelets from anti-GPIbα antibody-induced clearance. Moreover, the rescuing effects of Q-VD-OPh plus BAPTA were almost double those of Q-VD-OPh or BAPTA alone (Fig. 7B).
PS externalization during the activating process requires TMEM16F (54). To further confirm the role of PS exposure in platelet clearance, we generated TMEM16F-knockout mice (Fig. 7C). Because very few homozygotes of TMEM16F-knockout mice were born, we had to use heterozygotes of TMEM16F-knockout mice (TMEM16F+/−) for the experiment. The deficiency of TMEM16F in the TMEM16F+/− platelets was examined by Western blot (Fig. 7D). We found TMEM16F+/− and WT mice did not differ in the number of platelets and red and white blood cells or in hemoglobin concentration (SI Appendix, Table S4). Anti-GPIbα antibody-induced PS exposure was obviously reduced in the platelets from TMEM16F+/− mice (SI Appendix, Fig. S19). We i.v. transfused antibody-treated platelets from TMEM16F+/− mice and WT littermates into WT mice and found that the platelets from TMEM16F+/− mice were obviously less cleared than the platelets of WT littermates (Fig. 7E). These data demonstrate that PS exposure is essential for anti-GPIbα antibody-induced platelet clearance.
The Apoptotic Platelets Are Phagocytosed by Macrophages in the Liver.
Next, we investigated where the PS-exposed platelets are removed. Endothelial cells were reported to engulf bacterial-activated platelets (55); therefore, endothelial cells from anti-GPIbα antibody-treated mice were examined. No platelet was found to be colocalized with endothelial cells (SI Appendix, Fig. S20A). Neutrophils phagocytose activated platelets during acute myocardial infarction (56). However, we found that depletion of neutrophils could not rescue anti-GPIbα antibody-induced platelet clearance (SI Appendix, Fig. S20 B and C). These data suggest that endothelial cells or neutrophils may not contribute to anti-GPIbα antibody-induced platelet clearance. Then, we used Clophosome-N to deplete macrophages in vivo. After injection of R300 into the mice, we found that depletion of macrophages markedly rescued platelets from clearance (Fig. 7F). Macrophages contain Fc receptors, which can engulf platelets in an Fc-dependent manner. To investigate this possibility, we injected R300 F(ab′)2 into macrophage-depleted mice. Depletion of macrophages still protected platelets from clearance compared with results in undepleted mice (Fig. 7G), further indicating that Fc is not required for phagocytosis of antibody-bound platelets.
Macrophages present in the reticuloendothelial system throughout the whole body. We reported previously that anti-GPIbα antibody-bound platelets were removed by macrophages in the liver (12). To determine where the apoptotic platelets were removed, we labeled R300 and R300 F(ab′)2 with Alexa-Fluor 488 and injected the antibodies into mice. The fluorescence images indicated that the Alexa-Fluor 488 signal was primarily detected in the liver (SI Appendix, Fig. S21A). We further examined the liver (SI Appendix, Fig. S21B) and spleen (SI Appendix, Fig. S21C) by immunofluorescent staining and found that platelets were primarily colocalized with macrophages in the liver. Taken together, these data suggest that PS-exposed platelets are removed by macrophages in the liver.
Inhibition of Akt and Akt-Mediated Apoptosis Protects the Anti-GPIbα Antibody-Bound Platelets from Clearance in Vivo.
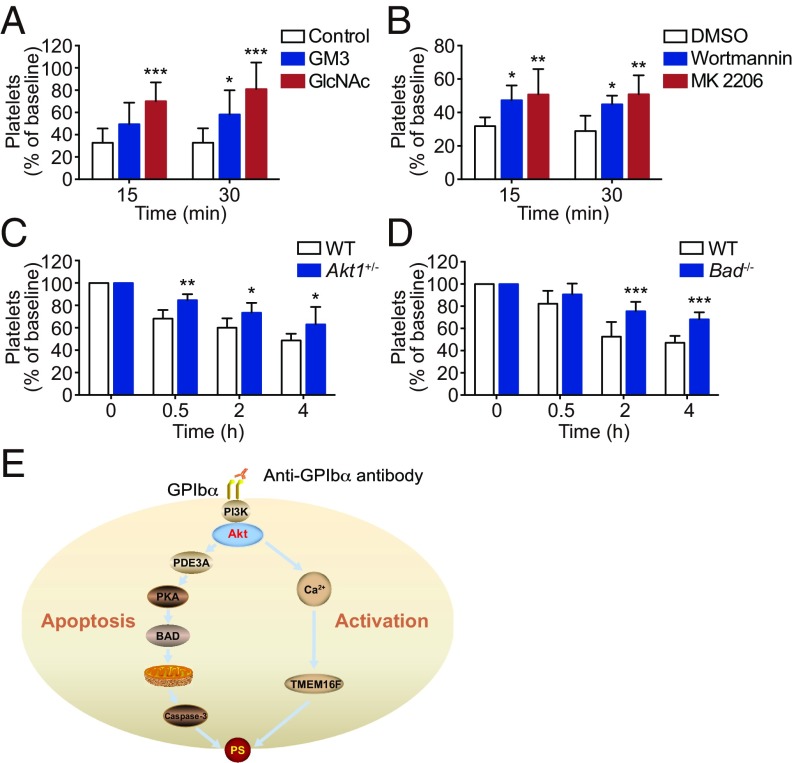
As shown in Fig. 3, GlcNAc and GM3 inhibited anti-GPIbα antibody-induced platelet apoptosis in vitro. Therefore, GlcNAc and GM3 should rescue the antibody-induced platelet clearance in vivo. To verify this, GlcNAc or GM3 was i.v. injected into mice, and then the mice were injected with calcein-labeled R300-treated platelets. As expected, GlcNAc and GM3 rescued anti-GPIbα antibody-induced platelet clearance (Fig. 8A). It was reported that GPIbα clustering might enhance the affinity of GPIbα with the CR3 receptor on macrophages, leading to platelet clearance (57). However, CR3 deficiency did not protect anti-GPIbα antibody-treated platelets from clearance (SI Appendix, Fig. S22). The reason may be that anti-GPIbα antibody binding has steric hindrance to block the interaction of GPIbα with CR3 receptors.
Fig. 8.
Inhibition of Akt activation and Akt-mediated apoptosis rescues anti-GPIbα antibody-induced platelet clearance in vivo. (A) C57BL/6J mice were i.v. preinjected with GM3 (6 μg/g), GlcNAc (110 μg/g), or vehicle control and then were transfused with calcein-labeled R300-treated platelets. (B) Mouse platelets were pretreated with 100 nM wortmannin, 6 μM MK2206, or vehicle control at RT for 5 min and then were transfused to C57BL/6J mice. The percentage of calcein-positive platelets remaining in circulation was assessed at the indicated time points by flow cytometry; n = 6–9 mice per group. Baseline is defined as the percentage of calcein-positive platelets within 1 min after platelet transfusion. (C and D) WT and Akt1+/− (C) and Bad−/− (D) mice were i.p. injected with R300 (0.1 μg/g). Platelet counts were determined at the indicated time points; n = 6–8 mice per group. Baseline is defined as the platelet number before anti-GPIbα antibody injection. Data are expressed as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001 compared by two-way ANOVA followed by Bonferroni post hoc test. (E) The dual roles of Akt in regulating activation and apoptosis in platelets. Akt, the downstream effector of PI3K, is activated after anti-GPIbα antibody binding. Akt activates platelets through Ca2+ elevation. Meanwhile, Akt activates PDE3A, leading to PKA inhibition and platelet apoptosis. The Akt-regulated activation and apoptosis signaling independently lead to PS externalization.
PI3K-Akt activation is essential for anti-GPIbα antibody-induced platelet apoptosis. Thus, it is conceivable that inhibition of PI3K-Akt activation should rescue anti-GPIbα antibody-induced platelet clearance in vivo. Indeed, Fig. 8B shows that PI3K and Akt inhibitors markedly reduced anti-GPIbα antibody-induced platelet clearance. We further verified the role of Akt in anti-GPIbα antibody-induced platelet clearance with Akt-deficient mice. Since only a very few Akt1−/− mice were generated, we had to use Akt1+/− mice to perform the experiment. After injection of anti-GPIbα antibodies into the Akt1+/− mice, platelet clearance was significantly prevented (Fig. 8C).
We further demonstrated the role of anti-GPIbα antibody-induced apoptosis in platelet clearance with Bad−/− mice. We found that after injection of R300 the platelets were less cleared in Bad−/− mice than in WT mice (Fig. 8D). Taken together, these data demonstrate that inhibition of Akt or Akt-mediated platelet apoptosis rescues anti-GPIbα antibody-induced platelet clearance in vivo.
Discussion
The data described in this study indicate that (i) anti-GPIbα antibodies induce Akt activation and Akt-mediated platelet apoptosis; (ii) Akt regulates platelet apoptosis, independent of activation signaling, through PDE3A-mediated PKA activity; (iii) PS exposure on apoptotic platelets rather than Fc results in phagocytosis of anti-GPIbα antibody-bound platelets in the liver; and (iv) agents that block Akt signaling or PS externalization protect the antibody-bound platelets from clearance and thus suggest therapeutic strategies for thrombocytopenia caused by autoantibodies or other pathogenic factors.
We demonstrate that anti-GPIbα antibodies induce Akt activation, which results in platelet apoptosis and activation. PI3K associated with the cytoplasmic domain of GPIbα transduces the VWF-binding signaling, leading to Akt activation (28, 45). We found that Akt, the downstream effector of PI3K, was activated after anti-GPIbα antibody binding. Inhibition of PI3K or Akt or genetic ablation of Akt disrupted the antibody-induced apoptosis and activation. Moreover, Akt activated platelets through Ca2+ elevation. These findings suggest that anti-GPIbα antibody activates platelets via signaling cascades similar to that of VWF binding (28). Distinct from the activation signaling, we found that Akt elicited platelet apoptosis via activation of PDE3A. We reported recently that inhibition of PKA caused intrinsically programmed platelet apoptosis (33). Consistent with this report, PKA activity was markedly reduced after anti-GPIbα antibody binding. We demonstrate that Akt activates PDE3A, which can reduce cAMP concentration, leading to PKA inhibition. In support of this finding, we reported previously that VWF-binding also induced platelet apoptosis (23). The current study provides a theoretical explanation for this finding. Therefore, our study identifies the dual roles of Akt in regulating both apoptosis and activation in platelets (Fig. 8E). Because Akt is abundant in platelets and eukaryotes and is involved in many functional signaling cascades (29, 46–48), the current findings may have general implications.
To explore the mechanism for anti-GPIbα antibody-induced Akt activation, we found that inhibitors of GPIbα clustering and the reagent that disrupts lipid rafts markedly reduced anti-GPIbα antibody-dependent platelet activation and apoptosis. These data suggest that anti-GPIbα antibodies may, like the VWF multimer (24–27), induce Akt activation by cross-linking GPIbα in lipid rafts. This possibility is supported by previous evidence. First, antibody-mediated cross-linking of target proteins has been demonstrated to associate with lipid rafts, rather than simple dimerization, to initiate signaling (58, 59). Second, two GPIbα molecules were in close proximity upon anti-GPIbα antibody binding, leading to platelet activation (12). Third, disrupting the association of GPIbα with lipid rafts abolished GPIbα-mediated platelet aggregation (24, 25). However, it is intriguing that, unlike AN51 and SZ2, the anti-GPIbα antibody HIP1 could not activate Akt and induce Akt-mediated platelet activation and apoptosis. Similar effects on platelets have been reported with other anti-GPIbα antibodies (12, 21). During the current submission, a report suggested that some anti-GPIbα antibodies, but not others, can exert a pulling force on GPIbα by cross-linking platelets under shear flow, which unfolds their mechanosensory domain, leading to platelet activation (21). This model provides an explanation for the different effects of anti-GPIbα antibodies on platelet activation under shear-flow conditions. However, in our current and previous (12) in vitro experiments and in similar experiments by others (24–26), the pretreated platelets were incubated under static conditions. There was no shear stress to pull the antibodies, but the antibodies still induced platelet activation. Interestingly, we find that the anti-GPIbα antibodies AK2 and HIP1 with epitopes located in the leucine-rich repeat region could not activate platelets (12, 21); on the other hand, epitopes of some of the reported anti-GPIbα antibodies which (except for VM16d) could activate platelets are located in the N- or C-terminal flanks or anionic sulfated sequence of GPIbα (12, 21). This finding suggests that epitope location or conformation-related affinity of the antibody may, as in VWF (60), play a role in the initiation of activation signaling. Future work is needed to solve this mystery.
We and others reported previously that Fc was not required for anti-GPIbα antibody-induced platelet clearance (12, 17, 20, 21, 61). Consistent with these findings, although binding to platelets, HIP1 did not induce platelet clearance in vivo. In contrast, SZ2, which induces platelet apoptosis, initiated platelet clearance. Blocking or genetic ablation of PS externalization, induced by platelet activation and apoptosis independently, prevented the platelets from clearance. We further demonstrated that the PS-exposed platelets were engulfed by macrophages in the liver. These findings demonstrate that the anti-GPIbα antibody-induced platelet clearance is through a PS-dependent rather than an Fc-dependent manner. Thus, our findings explain the long-standing puzzle that ITP patients with anti-GPIb-IX autoantibodies appear less responsive to conventional therapies, such as IVIG and even splenectomy (9–11).
Notably, GPIbα desialylation was demonstrated to contribute to anti-GPIbα antibody-induced platelet clearance (20). We verified that an inhibitor of sialidase rescued anti-GPIbα antibody-induced platelet clearance in our experimental condition (SI Appendix, Fig. S23). Anti-GPIbα antibody-induced platelet activation was found to be a prerequisite for GPIbα desialylation (20). The removal of sialic residues facilitates GPIbα clustering and accelerates platelet activation (20, 42). Therefore, it is likely that GPIbα desialylation contributes to platelet clearance by enhancing platelet activation and PS-dependent platelet phagocytosis. In support of this, platelets lacking sialylation of O-glycans were also primarily phagocytosed by macrophages in the liver (62). This explanation might also apply to GPIbα desialylation-mediated chilled platelet clearance (43, 63).
We demonstrate that anti-GPIbα antibodies induce platelet clearance through Akt activation and Akt-mediated platelet apoptosis and activation. Therefore, it is reasonable that inhibition of GPIbα clustering in lipid rafts, PI3K and Akt activation, or Akt-mediated activation and apoptosis signaling all significantly reduced anti-GPIbα antibody-induced platelet clearance. Thus, our findings provide various potential therapeutic strategies for the treatment of ITP with anti-GPIbα autoantibodies.
More importantly, we demonstrate that PS exposure is responsible for the phagocytosis of apoptotic platelets in the liver. PS externalization is a common and later-stage event for both apoptosis and activation (64). We and others reported that platelet apoptosis occurs extensively in physiologic conditions, blood bank platelets, and many common diseases (33–35). Platelet activation has long been demonstrated in many common diseases, such as infection, cancer, and heart diseases (36–38, 65). A large amount of evidence indicates that thrombocytopenia, which can result in fatal bleeding, occurs in many common diseases in which platelets are activated or undergo apoptosis (31–35). However, the means by which the apoptotic or activated platelets are removed from the circulation and result in thrombocytopenia remains elusive. Therefore, our findings provide a pathogenetic explanation for thrombocytopenia during various diseases in which the platelets are activated or undergo apoptosis. Inhibition of PS exposure-dependent platelet clearance represents a general therapeutic strategy for thrombocytopenia occurring in various common diseases.
In conclusion, we demonstrate that anti-GPIbα antibodies induce Akt activation and Akt-mediated platelet apoptosis. The apoptotic platelets are phagocytosed by macrophages in the liver in a PS exposure-dependent manner. Inhibition or genetic ablation of Akt or Akt-regulated apoptotic signaling or blocking of PS externalization protects the platelets from clearance. Therefore, our findings reveal the pathogenic mechanisms of anti-GPIbα autoantibodies in ITP and, more importantly, suggest therapeutic strategies for thrombocytopenia caused by autoantibodies or other pathogenic factors.
Materials and Methods
Mice.
Generation of human GPIbα-knockin mice.
For GPIbα targeting, exon 2 was targeted by two gRNAs designed to cut both ends of the exon to replace the mature mouse GPIbα chain-coding region with the human sequence (Fig. 6C). CRISPR single-guide RNAs (sgRNAs) were designed and screened for on-target activity. The T7 promoter sequence was added to the Cas9 and sgRNA templates by PCR amplification. T7-Cas9 and T7-sgRNA PCR products were purified and used as the template for in vitro transcription. Both the Cas9 mRNA and the sgRNA were purified. A circular donor vector was used to minimize random integrations. The donor plasmid containing human GPIbα cDNA and the WPRE-pA cassette was flanked by 1.7-kb and 1.7-kb homolog arms. C57BL/6 female mice and Kunming (KM) mouse strains were used as embryo donors and pseudopregnant foster mothers, respectively. Cas9 mRNA, sgRNAs, and donor vector were mixed at different concentrations and coinjected into the cytoplasm of fertilized zygotes at the one-cell stage. After injection, surviving zygotes were transferred into the oviducts of KM pseudopregnant females. Genomic DNA was extracted from the tail of the 7-d-old mice. The genotype for the human GPIbα-knockin allele was confirmed by PCR amplification, Southern blotting (SI Appendix, Fig. S15), and direct sequencing.
Generation of TMEM16F-knockout mice.
The TMEM16F-mutant mouse model was established by CRISPR/Cas9 genome editing technology to induce double-stranded breaks in exon 2 of TMEM16F. Cas9 mRNA was in vitro transcribed. Two independent guide RNAs targeting exon 2 of the TMEM16F gene were designed and transcribed in vitro using the MEGAshortscript Kit (Thermo Scientific). The sequences of sgRNAs were gRNA1 5’-ACAATTGTCTGCCCCACCTTTGG-3’ and gRNA2 5’-CTGATTCTCCAGTGATCCAAAGG-3’. The in vitro-transcribed Cas9 mRNA and sgRNAs were injected into the cytoplasm of C57BL/6J fertilized eggs, transferred to pseudopregnant recipients, and allowed to develop to term. Founders were screened for insertions/deletions (indels) by PCR amplification across the targeted region (F: 5′-TTTGACCTCTGGCTCATCTATTC-3′, R: 5′-CCTAGTCCTTCTGGGGTTGC-3′). PCR products from indel-carrying founders were Sanger sequenced to identify specific mutations. The founder mice were bred to WT C57BL/6J mice to generate heterozygous TMEM16F-mutant mice and then were intercrossed to generate homozygous TMEM16F-mutant mice.
Other mouse strains.
Bad−/− (66), Akt1−/− (67), and Akt2−/− (68) mice were generated as described previously. CR3−/− mice (003991) were purchased from The Jackson Laboratory. C57BL/6J WT mice were purchased from JOINN Laboratories. All mutations had been backcrossed onto the C57BL/6J background for at least 10 generations before this study. Mice were 6–12 wk old, and experiments included balanced groups of male and female mice unless otherwise stated. All animal experiments complied with the regulatory standards of and were approved by the Ethics Committee of The First Affiliated Hospital of Soochow University.
Patients and Healthy Volunteers.
Approval for obtaining whole-blood samples from healthy volunteers and patients was obtained from the Ethics Committee of The First Affiliated Hospital of Soochow University, and informed consent was obtained from all subjects according to the Declaration of Helsinki. Twelve ITP patients with anti-GPIbα autoantibodies and 30 ITP patients with anti-GPIIb/IIIa autoantibodies as characterized by flow cytometric immunobead array (38) were enrolled from The First Affiliated Hospital of Soochow University between May 8, 2016 and August 30, 2017, and age- and gender-matched healthy control subjects were recruited simultaneously for the studies.
Antibodies and Reagents.
Antibodies and reagents are provided in SI Appendix, Supplementary Materials.
Platelet Counts and Preparation.
Platelet and blood cell counts were performed with Sysmex XP-100 Hematologic Analyzer (Sysmex Corporation). The platelets from healthy volunteers were prepared as previously described (30–33). Briefly, whole blood was drawn from the inferior vena cava and anticoagulated with a 1:7 volume of acid–citrate–dextrose (ACD: 2.5% trisodium citrate, 2.0% d-glucose, 1.5% citric acid). Platelet-rich plasma (PRP) was collected from the whole blood by centrifugation at 200 × g for 11 min. Platelets were washed twice with CGS buffer (0.123 M NaCl, 0.033 M d-glucose, 0.013 M trisodium citrate, pH 6.5), resuspended in modified Tyrode’s buffer (MTB) (2.5 mM Hepes, 150 mM NaCl, 2.5 mM KCl, 12 mM NaHCO3, 5.5 mM d-glucose, 1 mM CaCl2, 1 mM MgCl2, pH 7.4) to a final concentration of 5 × 108/mL, and allowed to incubate at 22 °C for 1–2 h. For the preparation of murine platelets, whole blood from mice was collected from the postorbital vein using a 1:7 volume of ACD as anticoagulant. Platelets were washed with CGS buffer, resuspended in MTB to a concentration of 5 × 108/mL, and allowed to incubate at 22 °C for 1–2 h. For the preparation of platelets from patients, whole blood was drawn from the cubital vein and anticoagulated with ACD. For preparation of PRP, whole blood was anti-coagulated with a 1:9 volume of 3.8% trisodium citrate. Human and murine PRP was obtained by centrifugation at 200 × g and 100 × g, respectively.
In Vitro Platelet Antibody Assays.
Washed human platelets were treated with 10 μg/mL anti-human platelet monoclonal antibodies at 37 °C for 2–10 h. A relatively long time of incubation was selected to compensate for the no-shear-stress condition in vitro. Murine PRP was treated with 5 μg/mL anti-GPIbα polyclonal antibody R300 at 37 °C for 2–10 h. In inhibition experiments, human platelets or murine PRP were preincubated with various inhibitors and their corresponding vehicle controls at for 5 min at RT or at for 30 min at 37 °C and then were treated with AN51, SZ2, or R300 at 37 °C for 8 h.
Flow Cytometry.
P-selectin expression was detected with FITC-labeled anti-human (10 μg/mL) or anti-murine P-selectin antibody (1:5). GPIIb/IIIa activation was detected by FITC-labeled PAC-1 (25 μg/mL) binding to human platelets and PE-labeled JON/A (1:5) binding to murine platelets. Mitochondrial inner transmembrane potential (ΔΨm) depolarization in human or murine platelets was measured by JC-1 (2 μg/mL). PS exposure of human or murine platelets was detected by FITC-labeled lactadherin (10 μg/mL). Platelets were measured by a flow cytometer (FC 500; Beckman-Coulter).
Western Blot.
Washed human platelets (5 × 108/mL) were incubated with anti-GPIbα antibodies at 37 °C for 8 h or were preincubated with various inhibitors at RT for 5 min before anti-GPIbα antibody treatment and then were lysed with an equal volume of lysis buffer on ice for 30 min. For detection of the mouse TMEM16F level, washed murine platelets (5 × 108/mL) were lysed with an equal volume of lysis buffer on ice for 30 min. Proteins were separated and visualized by the ECL system. Quantification was performed with ImageJ software (NIH).
Caspase-3 Activity Assay.
Washed human platelets (5 × 108/mL) were incubated with 10 μg/mL AN51, SZ2, HIP1, or normal mouse IgG at 37 °C for 8 h and were lysed with an equal volume of lysis buffer on ice for 30 min. The caspase-3 activity assay was performed according to the manufacturer’s protocol. Briefly, 10 μL of platelet lysate per sample was mixed with 80 μL of reaction buffer and 10 μL of caspase-3 substrate (Ac-DEVD-pNA, 2 μM). Samples were further incubated at 37 °C for 4 h and activity was determined by an ELISA reader at an absorbance of 450 nm. The specific activity of caspase 3 was normalized for the total protein of sample.
Human IgG Purification.
EDTA-anticoagulated human plasma was diluted with PB (0.1 M Na2HPO4, 0.1 M NaH2PO4, pH 7.5) and applied to a protein G affinity column (GenScript). The column was washed thoroughly with PB, and the bound IgG was eluted with elution buffer (0.1 M glycine, pH 2.8). Purified IgG was dialyzed against PBS and concentrated to the original volume of the plasma samples.
IgG F(ab′)2 Fragmentation.
R300 F(ab′)2 fragments and human IgG F(ab′)2 fragments were generated using the Thermo Scientific IgG F(ab′)2 kit according to the manufacturer’s protocol. Briefly, 0.5 mg/mL of R300 or purified human IgG (<5 mg/mL) was desalted and digested with immobilized Pepsin in digestion buffer. Digest products were purified with Protein A beads and dialyzed. Human IgG F(ab′)2 fragments cleaved from 500 μL intact IgG were further concentrated to 300 μL. Purified F(ab′)2 was proved by SDS/PAGE and Coomassie blue staining.
Immunofluorescence Microscopy.
C57BL/6J mice were i.p. injected with normal rat IgG (0.1 μg/mL), R300 (0.1 μg/mL), or R300 F(ab′)2 fragments (0.4 μg/mL). Animals were killed after 4 h, and organs were immediately excised. Liver and spleen cryosections were fixed in ice-cold acetone and subsequently were blocked in 5% BSA/PBS at RT for 1 h. Mouse anti-mouse F4/80 (10 μg/mL) or rabbit anti-mouse CD31 (10 μg/mL) was incubated overnight at 4 °C, and Alexa-Fluor 488-conjugated goat anti-mouse (5 μg/mL) or Alexa-Fluor 488-conjugated goat anti-rabbit (5 μg/mL) antibody was incubated at RT for 1 h. Subsequently, R300 (10 μg/mL) and Alexa-Fluor 555-conjugated goat anti-rat antibody (5 μg/mL) were incubated at RT for 1 h. Cell nuclei were stained with 5 μg/mL DAPI.
Southern Blot Analysis.
Genomic DNA extracted from mouse tails was digested and electrophoresed in 1% agarose gel. The DNA in gel was transferred to a positively charged nylon membrane. The membrane was hybridized and detected using the DIG Luminescent Detection kit (Roche Group). For probe labeling, 3′ externally and internally DIG-labeled probes were prepared by PCR using Taq DNA polymerase and incorporating DIG-11-dUTP according to the manufacturer’s instructions.
Macrophage Depletion.
C57BL/6J mice (6- to 8-wk-old) were i.p. injected with control liposomes (700 μg per mouse) or clodronate liposomes (700 μg per mouse), and the liver was removed from mice after 24 h. Macrophage depletion was confirmed by immunofluorescence.
In Vivo Imaging Systems.
C57BL/6J mice (6- to 8-wk-old) were i.p. injected with rat IgG (0.1 μg/g), R300 (0.1 μg/g), or R300 F(ab′)2 fragments (0.4 μg/g) premixed with Alexa-Fluor 488-conjugated goat anti-rat antibody. The mice were killed 5 min after injection. The liver, spleen, lung, heart, and kidney were excised and imaged immediately using an in vivo imaging system (Caliper Life Sciences).
Platelet Clearance in Vivo.
C57BL/6J mice (6–8 wk old) i.p. received normal rat IgG, R300, or R300 F(ab′)2 in 100 μL of sterile PBS. Transgenic mice expressing human GPIbα (6- to 8-wk-old) were i.p. injected with normal mouse IgG, SZ2, or HIP1 or were i.v. injected with 200 μL IgG F(ab′)2 fragments from normal controls or ITP patients. The blood was collected from the post-glomus venous plexus and was anticoagulated with 3.8% trisodium citrate. Platelets were counted by a Sysmex XP-100 hematologic analyzer.
Posttransfusion Experiment.
Washed murine platelets labeled with 5 μg/mL calcein were incubated with 2 μg/mL R300 at RT for 1 h and were transfused to acceptor mice through the post-glomus venous plexus (1 × 108 platelets in 100 μL of MTB). Blood was collected from the post-glomus venous plexus at 1 min (baseline), 15 min, and 30 min, and total platelets were labeled with PE-conjugated anti-CD41 antibody. The percentage of calcein-labeled platelets remaining in circulation was assessed by flow cytometry. For blocking of PS and GPIbα clustering, mice were preinjected through the post-glomus venous plexus with annexin V (0.5 μg/g), lactadherin (0.025 μg/g), GM3 (6 μg/g), GlcNAc (110 μg/g), or vehicle control before platelet transfusion. For inhibition of calcium mobilization and caspase, PI3K, and Akt activity, calcein-labeled platelets were pretreated with BAPTA (10 μM), Q-VD-OPh (100 μM), wortmannin (100 nM), MK 2206 (6 μM), or vehicle control DMSO at RT for 5 min before incubation with 2 μg/mL R300.
Statistical Analysis.
All data are expressed as mean ± SD. Numeric data were analyzed using one-way (for a single variant) or two-way (for multiple variants) ANOVA. Two groups were compared by the two-tailed Student’s t test. The significance of data was assessed using GraphPad Prism 5 software. Differences were considered significant at P < 0.05. Different levels of significance are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001. All experiments requiring the use of animals were subject to randomization based on litter. No animals or samples were excluded from the study. Sample size was predetermined based on the variability observed in prior experiments and on preliminary data. Investigators were not blinded to outcome assessment.
Supplementary Material
Acknowledgments
We thank Zhongzhou Yang (Nanjing University) and Yulong He (Soochow University) for providing Akt1−/− and Akt2−/− mice. This work was supported by Key Program of the National Natural Science Foundation of China Grant 81130008 (to K.D.), National Natural Science Foundation of China Grants 81570102 and 81770117 (to K.D.), National Key Basic Research Program of China Grant 2012CB526600 (to K.D.), National Natural Science Foundation of China Grant 81770113 (to R.Y.), the Priority Academic Program Development of Jiangsu Higher Education Institutions, Jiangsu Provincial Special Program of Medical Science Grant BL2012005, Jiangsu Province Key Medical Center Grant ZX201102, and Jiangsu Province’s Outstanding Medical Academic Leader Program Award (to K.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808217115/-/DCSupplemental.
References
- 1.Neunert C, et al. American Society of Hematology The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 2.Stasi R, et al. Idiopathic thrombocytopenic purpura: Current concepts in pathophysiology and management. Thromb Haemost. 2008;99:4–13. doi: 10.1160/TH07-08-0513. [DOI] [PubMed] [Google Scholar]
- 3.McMillan R. Autoantibodies and autoantigens in chronic immune thrombocytopenic purpura. Semin Hematol. 2000;37:239–248. doi: 10.1016/s0037-1963(00)90102-1. [DOI] [PubMed] [Google Scholar]
- 4.Hou M, Stockelberg D, Kutti J, Wadenvik H. Antibodies against platelet GPIb/IX, GPIIb/IIIa, and other platelet antigens in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 1995;55:307–314. doi: 10.1111/j.1600-0609.1995.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 5.Go RS, Johnston KL, Bruden KC. The association between platelet autoantibody specificity and response to intravenous immunoglobulin G in the treatment of patients with immune thrombocytopenia. Haematologica. 2007;92:283–284. doi: 10.3324/haematol.10667. [DOI] [PubMed] [Google Scholar]
- 6.McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44(4 Suppl 5):S3–S11. doi: 10.1053/j.seminhematol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP) J Clin Med. 2017;6:E16. [Google Scholar]
- 8.Zeng Q, et al. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPIIbIIIa versus GPIbα antibodies. Am J Hematol. 2012;87:206–208. doi: 10.1002/ajh.22211. [DOI] [PubMed] [Google Scholar]
- 9.Peng J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: A multicenter cohort study. J Thromb Haemost. 2014;12:497–504. doi: 10.1111/jth.12524. [DOI] [PubMed] [Google Scholar]
- 10.McMillan R, Wang L, Tani P. Prospective evaluation of the immunobead assay for the diagnosis of adult chronic immune thrombocytopenic purpura (ITP) J Thromb Haemost. 2003;1:485–491. doi: 10.1046/j.1538-7836.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, et al. Platelet count evolution as a predictor of outcome after splenectomy for immune thrombocytopenic purpura. Int J Hematol. 2017;105:433–439. doi: 10.1007/s12185-016-2121-0. [DOI] [PubMed] [Google Scholar]
- 12.Yan R, et al. Glycoprotein Ibα clustering induces macrophage-mediated platelet clearance in the liver. Thromb Haemost. 2015;113:107–117. doi: 10.1160/TH14-03-0217. [DOI] [PubMed] [Google Scholar]
- 13.Yanabu M, et al. Tyrosine phosphorylation and p72syk activation by an anti-glycoprotein Ib monoclonal antibody. Blood. 1997;89:1590–1598. [PubMed] [Google Scholar]
- 14.Cauwenberghs N, et al. Fc-receptor dependent platelet aggregation induced by monoclonal antibodies against platelet glycoprotein Ib or von Willebrand factor. Thromb Haemost. 2001;85:679–685. [PubMed] [Google Scholar]
- 15.Bergmeier W, Rackebrandt K, Schröder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood. 2000;95:886–893. [PubMed] [Google Scholar]
- 16.Li C, et al. The maternal immune response to fetal platelet GPIbα causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti-FcRn therapies. J Clin Invest. 2011;121:4537–4547. doi: 10.1172/JCI57850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieswandt B, Bergmeier W, Rackebrandt K, Gessner JE, Zirngibl H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96:2520–2527. [PubMed] [Google Scholar]
- 18.Becker BH, Miller JL. Effects of an antiplatelet glycoprotein Ib antibody on hemostatic function in the guinea pig. Blood. 1989;74:690–694. [PubMed] [Google Scholar]
- 19.Cadroy Y, et al. Relative antithrombotic effects of monoclonal antibodies targeting different platelet glycoprotein-adhesive molecule interactions in nonhuman primates. Blood. 1994;83:3218–3224. [PubMed] [Google Scholar]
- 20.Li J, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quach ME, et al. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets. Blood. 2018;131:787–796. doi: 10.1182/blood-2017-05-784975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J Thromb Haemost. 2013;11:605–614. doi: 10.1111/jth.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrimpton CN, et al. Localization of the adhesion receptor glycoprotein Ib-IX-V complex to lipid rafts is required for platelet adhesion and activation. J Exp Med. 2002;196:1057–1066. doi: 10.1084/jem.20020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullam PM, et al. Physical proximity and functional interplay of the glycoprotein Ib-IX-V complex and the Fc receptor FcgammaRIIA on the platelet plasma membrane. J Biol Chem. 1998;273:5331–5336. doi: 10.1074/jbc.273.9.5331. [DOI] [PubMed] [Google Scholar]
- 26.Kasirer-Friede A, et al. Lateral clustering of platelet GP Ib-IX complexes leads to up-regulation of the adhesive function of integrin alpha IIbbeta 3. J Biol Chem. 2002;277:11949–11956. doi: 10.1074/jbc.M108727200. [DOI] [PubMed] [Google Scholar]
- 27.Gitz E, et al. Platelet interaction with von Willebrand factor is enhanced by shear-induced clustering of glycoprotein Ibα. Haematologica. 2013;98:1810–1818. doi: 10.3324/haematol.2013.087221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin H, Stojanovic A, Hay N, Du X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood. 2008;111:658–665. doi: 10.1182/blood-2007-04-085514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood. 2006;108:2596–2603. doi: 10.1182/blood-2006-01-011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Receptor-interacting protein kinase 3 promotes platelet activation and thrombosis. Proc Natl Acad Sci USA. 2017;114:2964–2969. doi: 10.1073/pnas.1610963114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai K, Bodnar R, Berndt MC, Du X. A critical role for 14-3-3zeta protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood. 2005;106:1975–1981. doi: 10.1182/blood-2005-01-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, et al. The glycoprotein Ibalpha-von Willebrand factor interaction induces platelet apoptosis. J Thromb Haemost. 2010;8:341–350. doi: 10.1111/j.1538-7836.2009.03653.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, et al. Protein kinase A determines platelet life span and survival by regulating apoptosis. J Clin Invest. 2017;127:4338–4351. doi: 10.1172/JCI95109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leytin V. Apoptosis in the anucleate platelet. Blood Rev. 2012;26:51–63. doi: 10.1016/j.blre.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Tang WH, et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation. 2014;129:1598–1609. doi: 10.1161/CIRCULATIONAHA.113.005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojha A, et al. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci Rep. 2017;7:41697. doi: 10.1038/srep41697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Es N, Sturk A, Middeldorp S, Nieuwland R. Effects of cancer on platelets. Semin Oncol. 2014;41:311–318. doi: 10.1053/j.seminoncol.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, et al. An improved flow cytometric immunobead array to detect autoantibodies in plasma from patients with immune thrombocytopenic purpura. Clin Chim Acta. 2015;438:396–400. doi: 10.1016/j.cca.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Wardell MR, Reynolds CC, Berndt MC, Wallace RW, Fox JE. Platelet glycoprotein Ib beta is phosphorylated on serine 166 by cyclic AMP-dependent protein kinase. J Biol Chem. 1989;264:15656–15661. [PubMed] [Google Scholar]
- 40.Smolenski A, et al. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 41.Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J. 2000;349:547–557. doi: 10.1042/0264-6021:3490547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Gitz E, et al. Improved platelet survival after cold storage by prevention of glycoprotein Ibα clustering in lipid rafts. Haematologica. 2012;97:1873–1881. doi: 10.3324/haematol.2012.066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmeister KM, et al. Glycosylation restores survival of chilled blood platelets. Science. 2003;301:1531–1534. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 44.Mu FT, et al. A functional 14-3-3zeta-independent association of PI3-kinase with glycoprotein Ib alpha, the major ligand-binding subunit of the platelet glycoprotein Ib-IX-V complex. Blood. 2008;111:4580–4587. doi: 10.1182/blood-2007-09-111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woulfe D, et al. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest. 2004;113:441–450. doi: 10.1172/JCI20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, et al. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004;104:1703–1710. doi: 10.1182/blood-2003-10-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Brien KA, Stojanovic-Terpo A, Hay N, Du X. An important role for Akt3 in platelet activation and thrombosis. Blood. 2011;118:4215–4223. doi: 10.1182/blood-2010-12-323204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Colman RW. Thrombin regulates intracellular cyclic AMP concentration in human platelets through phosphorylation/activation of phosphodiesterase 3A. Blood. 2007;110:1475–1482. doi: 10.1182/blood-2006-10-052522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han SJ, et al. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunter RW, Mackintosh C, Hers I. Protein kinase C-mediated phosphorylation and activation of PDE3A regulate cAMP levels in human platelets. J Biol Chem. 2009;284:12339–12348. doi: 10.1074/jbc.M807536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow TW, Hellums JD, Moake JL, Kroll MH. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation. Blood. 1992;80:113–120. [PubMed] [Google Scholar]
- 52.Savill J, Gregory C. Apoptotic PS to phagocyte TIM-4: Eat me. Immunity. 2007;27:830–832. doi: 10.1016/j.immuni.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 54.Fujii T, Sakata A, Nishimura S, Eto K, Nagata S. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc Natl Acad Sci USA. 2015;112:12800–12805. doi: 10.1073/pnas.1516594112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma R, et al. Phosphatidylserine-mediated platelet clearance by endothelium decreases platelet aggregates and procoagulant activity in sepsis. Sci Rep. 2017;7:4978. doi: 10.1038/s41598-017-04773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maugeri N, et al. Neutrophils phagocytose activated platelets in vivo: A phosphatidylserine, P-selectin, and beta2 integrin-dependent cell clearance program. Blood. 2009;113:5254–5265. doi: 10.1182/blood-2008-09-180794. [DOI] [PubMed] [Google Scholar]
- 57.Hoffmeister KM, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 58.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 60.Peng Y, Shrimpton CN, Dong JF, López JA. Gain of von Willebrand factor-binding function by mutagenesis of a species-conserved residue within the leucine-rich repeat region of platelet glycoprotein Ibalpha. Blood. 2005;106:1982–1987. doi: 10.1182/blood-2005-02-0514. [DOI] [PubMed] [Google Scholar]
- 61.Li J, et al. Fc-independent phagocytosis: Implications for IVIG and other therapies in immune-mediated thrombocytopenia. Cardiovasc Hematol Disord Drug Targets. 2013;13:50–58. doi: 10.2174/1871529x11313010006. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, et al. Sialylation on O-glycans protects platelets from clearance by liver Kupffer cells. Proc Natl Acad Sci USA. 2017;114:8360–8365. doi: 10.1073/pnas.1707662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rumjantseva V, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–1280. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoenwaelder SM, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114:663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 65.Linden MD, et al. Indices of platelet activation and the stability of coronary artery disease. J Thromb Haemost. 2007;5:761–765. doi: 10.1111/j.1538-7836.2007.02462.x. [DOI] [PubMed] [Google Scholar]
- 66.Ranger AM, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang ZZ, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 68.Dummler B, et al. Life with a single isoform of Akt: Mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.