Significance
The phosphorylation pattern of the Pol2 carboxy-terminal domain (CTD) Y1S2P3T4S5P6S7 repeats comprises an informational code coordinating transcription and RNA processing. We exploited fission yeast CTD phospho-site mutants and synthetic genetic arraying to illuminate opposing roles for Ser7 and Thr4 in transcription termination whereby: S7A elicits precocious termination via cleavage-polyadenylation factor (CPF) subunits and Rhn1; and T4A reduces termination and is lethal absent CPF subunits Ppn1 and Swd22. The findings that Y1F, S2A, and T4A are concordantly lethal with ppn1∆ and swd22∆ provide insights into a CTD vocabulary, implicating Tyr1-Ser2-Thr4 as a three-letter CTD word. This work underscores how the effects of mutating ostensibly inessential CTD marks are genetically buffered by other cellular factors that are functionally redundant to those marks.
Keywords: Pol2, CTD code, transcription termination, synthetic lethality
Abstract
The carboxy-terminal domain (CTD) code encrypted within the Y1S2P3T4S5P6S7 heptad repeats of RNA polymerase II (Pol2) is deeply rooted in eukaryal biology. Key steps to deciphering the code are identifying the events in gene expression that are governed by individual “letters” and then defining a vocabulary of multiletter “words” and their meaning. Thr4 and Ser7 exert opposite effects on the fission yeast pho1 gene, expression of which is repressed under phosphate-replete conditions by transcription of an upstream flanking long noncoding RNA (lncRNA). Here we attribute the derepression of pho1 by a CTD-S7A mutation to precocious termination of lncRNA synthesis, an effect that is erased by mutations of cleavage-polyadenylation factor (CPF) subunits Ctf1, Ssu72, Ppn1, Swd22, and Dis2 and termination factor Rhn1. By contrast, a CTD-T4A mutation hyperrepresses pho1, as do CPF subunit and Rhn1 mutations, implying that T4A reduces lncRNA termination. Moreover, CTD-T4A is synthetically lethal with ppn1∆ and swd22∆, signifying that Thr4 and the Ppn1•Swd22 module play important, functionally redundant roles in promoting Pol2 termination. We find that Ppn1 and Swd22 become essential for viability when the CTD array is curtailed and that S7A overcomes the need for Ppn1•Swd22 in the short CTD context. Mutational synergies highlight redundant essential functions of (i) Ppn1•Swd22 and Rhn1, (ii) Ppn1•Swd22 and Ctf1, and (iii) Ssu72 and Dis2 phosphatases. CTD alleles Y1F, S2A, and T4A have overlapping synthetic lethalities with ppn1∆ and swd22∆, suggesting that Tyr1-Ser2-Thr4 form a three-letter CTD word that abets termination, with Rhn1 being a likely “reader” of this word.
The carboxyl-terminal domain (CTD) of the Rpb1 subunit of RNA polymerase II (Pol2) consists of tandem repeated heptapeptides of consensus sequence Y1S2P3T4S5P6S7. The CTD is essential for cell viability because it recruits proteins that regulate transcription, modify chromatin structure, and catalyze or regulate RNA capping, splicing, and polyadenylation (1–4). The inherently plastic CTD structure is modulated dynamically by phosphorylation and dephosphorylation of the heptad Ser, Thr, and Tyr residues in rough synchrony with the steps of the transcription cycle (e.g., preinitiation, initiation, elongation, and termination). In turn, the primary structure of the CTD conveys informational cues about the transcription machinery—a CTD code—that is read by CTD-binding proteins (1–3). Insights into CTD coding principles have been gained by (i) probing biochemically and structurally how individual proteins recognize the CTD and (ii) genetically manipulating the composition and structure of the CTD and gauging effects on cell physiology.
The fission yeast Schizosaccharomyces pombe is an attractive model system for CTD structure–function analysis because the native heptad repeat array is relatively homogeneous vis-à-vis other taxa. The S. pombe CTD consists of 29 heptad repeats (SI Appendix, Fig. S1). The junction CTD segment to the body of Rpb1 consists of four repeats that deviate in size and/or sequence from the consensus heptad; this segment is referred to as the CTD “rump.” Distal to the rump is an array of 25 heptad repeats that adhere perfectly to the YSPTSPS consensus, with the single exception of an Ala in lieu of Pro3 in the fifth heptad downstream of the rump. The in vivo requirements for all seven amino acids of the Y1S2P3T4S5P6S7 repeat were gauged by introducing Ala and conservative substitutions in lieu of Tyr1, Ser2, Pro3, Thr4, Ser5, Pro6, and Ser7 of every heptad of the CTD array in the context of a fully functional Rpb1 subunit with a CTD composed of the rump plus 14 consensus heptads (5, 6). The salient findings were that (i) Tyr1, Pro3, Ser5, and Pro6 are essential for viability of fission yeast, by the criterion that Ala substitution is lethal, whereas Ser2, Thr4, and Ser7 are not, and (ii) Y1F, Y1F+S2A, Y1F+S7A, S2A+T4A, S2A+S7A, and T4A+S7A mutants are viable, signifying that Phe is functional in lieu of Tyr1 and that Ser5 is the only strictly essential phosphorylation site. The essentiality of Ser5-PO4 in fission yeast is linked to recruitment of the mRNA-capping apparatus to the Pol2 elongation complex (5). Indeed, the requirement for Ser5-PO4 (and for Pro6) can be bypassed by covalently fusing the capping enzyme to Pol2 (5, 7).
The ability of S. pombe to grow when the Tyr1, Ser2, Thr4, and Ser7 residues are uniformly replaced by a nonphosphorylatable side chain resonates with transcriptome analysis showing that only a small fraction of fission yeast mRNAs are dysregulated by CTD phospho-site mutations (8). How do we reconcile this scenario with the strong conservation of the consensus YSPTSPS heptad and the core assumption (to which we subscribe) that many essential steps in gene expression rely on phospho-CTD cues? To date, the four inessential CTD phospho-sites have not been correlated with specific events in fission yeast RNA biogenesis.
Our hypothesis is that the effects of mutating these phospho-sites are genetically buffered by other cellular factors that are functionally redundant to the phospho-mark or the side-chain hydroxyl. By identifying such functional redundancies and gauging their specificity for a particular phospho-site mutation, we expect to discern a pattern indicating which steps in gene expression rely on particular CTD coding letters. Here we address this via synthetic enhancement genetics, entailing the screening of a select collection of S. pombe genes for ones that, when deleted or mutated, are lethal in an rpb1-CTD mutant strain background.
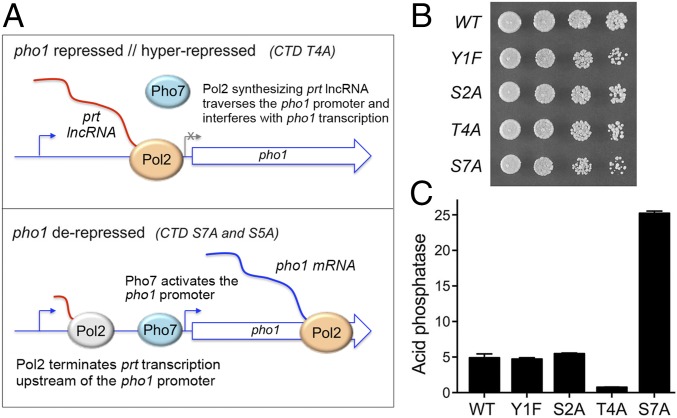
In this study we focus on genetic connections between the CTD and factors implicated in 3′-end formation and/or transcription termination. We do so in light of previous findings that CTD phospho-sites Thr4, Ser5, and Ser7 govern the expression of fission yeast genes involved in phosphate homeostasis (6, 8–11). The S. pombe phosphate regulon comprises three genes that specify, respectively, a cell surface acid phosphatase (Pho1), an inorganic phosphate transporter (Pho84), and a glycerophosphate transporter (Tgp1) (12). Expression of pho1, pho84, and tgp1 is actively repressed during growth in phosphate-rich medium by the transcription in cis of a long noncoding RNA (lncRNA) from the respective 5′ flanking genes prt, prt2, and nc-tgp1 (6, 9–11, 13–15). A model for the repressive arm of fission yeast phosphate homeostasis is that transcription of the upstream lncRNA interferes with the expression of the downstream mRNA genes by displacing the activating transcription factor Pho7 from its binding site(s) in the mRNA promoters that overlap the lncRNA transcription units (Fig. 1A) (9, 11, 16).
Fig. 1.
CTD control of phosphate homeostasis in phosphate-replete cells. (A) Models for the pho1 repressed/hyperrepressed (Upper) and derepressed (Lower) states of the prt–pho1 locus under phosphate-replete conditions. (B) Growth of S. pombe strains with the indicated full-length rpb1-CTD alleles (see SI Appendix, Fig. S1 for CTD amino acid sequences). Cells were inoculated in YES broth and grown at 30 °C. Exponentially growing cultures were adjusted to A600 of 0.1 and 3-µL aliquots of serial fivefold dilutions were spotted on YES agar and then incubated at 30 °C. (C) The indicated rpb1-CTD strains were grown to A600 of 0.5–0.9 in liquid culture in YES medium at 30 °C. Cells were harvested, washed with water, and assayed for Pho1 acid phosphatase activity by conversion of p-nitrophenylphosphate to p-nitrophenol. Activity is expressed as the ratio of A410 (p-nitrophenol production) to A600 (input cells). Each datum in the bar graph is the average of assays using cells from at least three independent cultures ± SEM.
Phosphate regulon expression under phosphate-replete conditions is governed by the phosphorylation state of the Pol2 CTD. For example, CTD mutations that prevent installation of the Ser7-PO4 or Ser5-PO4 marks derepress pho1 and pho84 in phosphate-replete cells. By contrast, prevention of the Thr4-PO4 mark hyperrepresses pho1 and pho84 under phosphate-rich conditions (6). Because such CTD mutations do not affect the activity of the lncRNA or mRNA gene promoters per se (9, 11), it is proposed that CTD status affects Pol2 termination, and thus the propensity to displace Pho7 from the downstream mRNA promoter, during lncRNA synthesis (Fig. 1A). Specifically, it is hypothesized that loss of the Ser7-PO4 or Ser5-PO4 marks leads to precocious termination of prt lncRNA transcription before the pho1 promoter and that loss of the Thr4-PO4 mark reduces prt termination and hence increases transcription across the pho1 promoter (Fig. 1A) (9).
This scenario begets several predictions. First, mutations of factors that normally promote cotranscriptional 3′ processing and transcription termination—these two events being functionally coupled (17)—might hyperrepress pho1 under phosphate-replete conditions. Second, synergies between CTD phospho-site mutants and 3′ processing/termination factor mutants might illuminate instances of overlapping or antagonistic functions. Third, genetic interactions among fission yeast proteins implicated in 3′ processing/termination and between these proteins and factors involved in sculpting the CTD phosphorylation array might cohere into a CTD interaction network. Here we put these ideas to the test.
Results
Opposite Effects of Thr4 and Ser7 Mutations on pho1 Expression.
For the present study, we employed a series of S. pombe rpb1 mutant strains in which the native CTD length was maintained as 29 heptads (4 rump and 25 consensus repeats) and Tyr1, Ser2, Thr4, or Ser7 in every heptad was replaced by Phe, Ala, Ala, and Ala, respectively (SI Appendix, Fig. S1). The full-length rpb1-CTD mutant strains thrived on YES (yeast extract, glucose, amino acid supplement) agar medium at 30 °C (Fig. 1B). Testing growth across a range of temperatures showed that Y1F, S2A, T4A, and S7A cells grew slowly at 20 °C and that the S2A mutant grew slowly at 37 °C (SI Appendix, Fig. S1). Western blotting of whole-cell extracts from midlog cultures grown at 30 °C with antibody against the Ser5-PO4 CTD mark, the only phospho-mark essential for fission yeast growth (5), showed that the levels of Ser5-phosphorylated Rpb1 were similar in WT, Y1F, S2A, T4A, and S7A cells (SI Appendix, Fig. S2). We surveyed the full-length rpb1-CTD mutants for effects on pho1 expression during exponential growth at 30 °C under phosphate-replete conditions. Acid phosphatase activity [a gauge of Pho1 enzyme level that correlates with pho1 mRNA level, as assayed by primer extension as well as RT-qPCR, RNA-sequencing (RNA-seq), and Northern blotting; refs. 6, 8–11, and 18] was quantified by incubating suspensions of serial dilutions of the phosphate-replete cells for 5 min with p-nitrophenylphosphate and assaying colorimetrically the formation of p-nitrophenol. The repressed basal Pho1 activity of WT rpb1-CTD cells was hyperrepressed by sevenfold in T4A cells and was derepressed by fivefold in S7A cells (Fig. 1C). By contrast, basal Pho1 expression was unaffected by the Y1F and S2A alleles (Fig. 1C).
Effects of Mutating Termination Factors and 3′-End–Formation Factors on pho1 Expression.
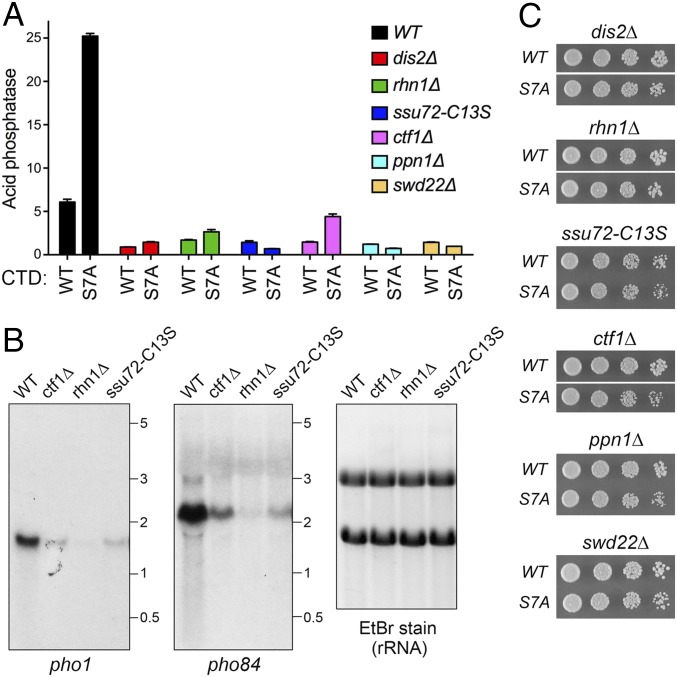
A model proposed for CTD control of pho1 expression invokes transcription termination during prt lncRNA synthesis as the tunable determinant of the activity of the flanking pho1 promoter controlled by the DNA-binding transcription factor Pho7 (Fig. 1A). A prediction of the model is that loss-of-function mutations in fission yeast proteins that promote cotranscriptional 3′ processing and transcription termination ought to hyperrepress Pho1 under phosphate-replete conditions by increasing the probability of Pol2 traversing the pho1 promoter. To test this idea, we monitored Pho1 expression in a series of fission yeast knockout strains lacking Dis2, Rhn1, Ctf1, Ppn1, or Swd22 as well as in a strain with a catalytically dead (C13S) version of Ssu72. The Ctf1, Ssu72, Ppn1, Swd22, and Dis2 proteins are constituents of the fission yeast cleavage polyadenylation factor (CPF) complex (19). Whereas Ctf1 and Ssu72 are considered CPF core subunits, Dis2, Ppn1, and Swd22 comprise a heteromeric subassembly (the DPS module) that associates with the core but is not necessary for core assembly (20). Ssu72 and Dis2 are protein phosphatase enzymes of the cysteinyl-phosphatase and binuclear metallophosphoesterase families, respectively. Dis2 is an ortholog of the budding yeast Glc7 phosphatase, which is implicated in coupling 3′ processing by CPF to transcription termination (21). A recent precision nuclear run-on sequencing (PRO-seq) analysis of the distribution of transcribing Pol2 in dis2+ and dis2∆ fission yeast cells showed that loss of Dis2 caused a global ∼500-bp 3′ extension of Pol2 distribution distal to the poly(A) site, indicative of reduced termination efficiency (22). Rhn1 is the fission yeast homolog of the budding yeast CTD-binding transcription termination factor Rtt103 (23). The dis2∆, rhn1∆, ctf1∆, ppn1∆, swd22∆, and ssu72-C13S strains all grew well on YES agar medium at 30 °C (Fig. 2C). [The ppn1∆ and swd22∆ cells had a cold-sensitive (cs) growth defect at 20 °C, and rhn1∆ cells displayed a temperature-sensitive (ts) growth defect at 37 °C (SI Appendix, Fig. S3A).]
Fig. 2.
Role of termination factor Rhn1 and 3′-end–formation factors in pho1 expression in rpb1-CTD WT and CTD-S7A cells. (A) S. pombe cells bearing the indicated rpb1-CTD alleles were grown in liquid culture at 30 °C and assayed for acid phosphatase activity as described in Fig. 1C. Each datum in the bar graph is the average of assays using cells from at least three independent cultures ± SEM. (B) Northern blot analysis of total RNA from exponentially growing phosphate-replete WT, ctf1∆, rhn1∆, and ssu72-C13S cells. The RNA was resolved by agarose gel electrophoresis and stained with ethidium bromide to visualize 28S and 18S rRNA (Right) before transfer to membrane and hybridization with 32P-labeled probes specific for pho1 (Left) or pho84 (Middle). Annealed probes were visualized by autoradiography. The positions and sizes (in kilobases) of RNA size standards are indicated. The Northern blots shown are representative of three biological replicates of the experiment, entailing isolation and analysis of RNA from three separate cultures of each of the fission yeast strains specified. (C) Growth of dis2∆, rhn1∆, ssu72-C13S, ctf1∆, ppn1∆, and swd22∆ strains with rpb1-CTD-WT or rpb1-CTD-S7A alleles. Serial fivefold dilutions of exponentially growing cells were spotted on YES agar and then incubated at 30 °C. The pairs of WT and S7A strains were spotted on the same agar plate in every case. In the three instances shown in which the WT and S7A spottings are separated by a white space, other rows of cell spottings on the plate separating WT and S7A strains were cropped out of the image.
We found that Pho1 expression in exponentially growing cells at 30 °C was uniformly hyperrepressed in dis2∆, rhn1∆, ctf1∆, ppn1∆, swd22∆, and ssu72-C13S cells under phosphate-replete conditions (Fig. 2A). In a previous analysis of the transcriptome of swd22∆ cells, it was noted that only 47 genes were underexpressed in the absence of Swd22 (20). From the perspective of the present study, it is noteworthy that the “swd22∆-down” gene set included pho1 and pho84, both of which are repressed by transcription of upstream flanking lncRNAs (11). Here we performed Northern analysis of pho1 and pho84 mRNA levels in WT, ctf1∆, rhn1∆, and ssu72-C13S cells and found that both genes were hyperrepressed in all three mutant strains (Fig. 2B). These results fortify the hypothesis that reducing the probability of Pol2 terminating during prt lncRNA synthesis leads to greater interference with the pho1 promoter.
To address whether the CPF mutations might affect aspects of pho1 mRNA biogenesis independently of prt lncRNA synthesis, we employed a plasmid-borne prt–pho1 reporter introduced into CPF-WT and mutant strains in which the chromosomal pho1 gene was deleted. In this experiment, we used a mutated version of the prt–pho1 reporter construct in which the prt promoter is inactivated by nucleotide changes in the HomolD and TATA box elements that drive prt lncRNA synthesis (SI Appendix, Fig. S3B) (9). This mutant reporter provides a readout of pho1 expression freed from interference by transcription of the flanking prt lncRNA. The Pho1 activity of the mutant plasmid in WT cells was high (i.e., derepressed) and was unaffected by the ppn1∆ and swd22∆ deletions (SI Appendix, Fig. S3B). Pho1 reporter expression was 17% less than the WT level in the dis2∆ and ssu72-C13S backgrounds and was 27% less than the WT level in ctf1∆ cells (SI Appendix, Fig. S3B); these modest effects on Pho1 expression uncoupled from prt lncRNA interference do not account for the fourfold to sevenfold decrements in Pho1 expression at the WT prt–pho1 locus in the CPF mutant strains (Fig. 2A). Thus, we surmise that the hyperrepressive effects of CPF subunit mutations on pho1 expression at the native prt–pho1 locus are not caused by inhibition of pho1 mRNA biogenesis per se.
Derepression of Pho1 Expression by CTD-S7A Depends on CPF Subunits and Rhn1.
The property of precocious termination during prt lncRNA synthesis proposed for the Pol2-S7A polymerase could reflect either (i) an inherent change in the Pol2-S7A elongation/termination balance in favor of termination (i.e., independent of the cleavage/polyadenylation and termination factors) or (ii) a change in the responsiveness of elongating Pol2-S7A to the action of cleavage/polyadenylation and termination factors. These two models beget distinct predictions concerning epistatic relationships between CTD-S7A and the CPF and Rhn1 mutants. To address this issue, we introduced the rpb1-CTD WT and rpb1-CTD-S7A alleles into the CPF subunit and Rhn1-mutant strains. The CTD-S7A allele did not affect their growth at 30 °C (Fig. 2C). We then assessed Pho1 expression under phosphate-replete conditions. The instructive findings were that dis2∆, rhn1∆, ssu72-C13S, ppn1∆, and swd22∆ eliminated the derepression of Pho1 elicited by CTD-S7A, and the effect of CTD-S7A was severely attenuated in ctf1∆ cells (Fig. 2A). Thus, the increase in Pho1 expression in S7A cells requires CPF subunits and Rhn1, consistent with model (ii) above.
In contrast to the CPF and Rhn1 mutants, the derepression of Pho1 expression by CTD-S7A was not attenuated in nab3∆ or sen1∆ cells (SI Appendix, Fig. S4), which lack the fission yeast homologs (24) of the Nab3 and Sen1 subunits of the budding yeast NNS complex implicated in noncoding RNA transcription termination (17), or in din1∆ cells that lack Din1 (SI Appendix, Fig. S4), an RNA pyrophosphohydrolase whose budding yeast homolog Rai1 interacts with the “torpedo” 5′–3′ exoribonuclease Rat1/Xrn1 (Dhp1 in fission yeast) that drives postcleavage transcription termination and with termination factor Rtt103 (25). Moreover, unlike the CPF and Rhn1 mutants, nab3∆ and sen1∆ mutations did not cause hyperrepression of Pho1 expression in CTD WT cells (SI Appendix, Fig. S4). The din1∆ mutation resulted in a modest 40% decrement in Pho1 activity in CTD WT cells. These results suggest that the proposed precocious prt lncRNA termination is not dependent on Nab3, Sen1, or Din1.
Distance Between prt and pho1 Start Sites Is a Tunable Governor of Pho1 Expression.
We hypothesize that the basal level of Pho1 expression in phosphate-replete cells is affected by whether Pol2 terminates prt transcription before reaching the pho1 promoter. The efficiency of this termination event is, in principle, governed by two parameters: (i) the probability that Pol2 will terminate over any interval segment of template DNA of the prt gene; and (ii) the total DNA length that Pol2 must travel to displace Pho7 from the pho1 promoter. The altered Pho1 expression caused by erasing Pol2 CTD phospho-sites or by mutations of CPF subunits and Rhn1 (Figs. 1 and 2) presumably reflects a reset in the first of these two parameters. To address the role of the second parameter, we altered the length of the prt transcription unit and assessed expression of the downstream pho1 gene. The experiments were performed using a plasmid-borne prt–pho1 reporter cassette introduced into otherwise WT fission yeast cells in which the chromosomal pho1 gene was deleted (11). This reporter faithfully reflects the homeostatic controls on the native pho1 locus (9). As depicted in SI Appendix, Fig. S5A, a 392-nt internal segment of the prt gene (from nucleotides +472 to +864 relative to the prt start site) was inverted (to maintain gene length but alter the sequence of the template and nascent RNA), deleted (to shorten the distance to the pho1 promoter), or tandemly duplicated and triplicated (to increase the distance to the pho1 promoter). Whereas segmental inversion had a modest effect on Pho1 activity (a 35% reduction) under phosphate-replete conditions, deletion of the segment resulted in 12-fold hyperrepression, and duplication and triplication of the segment elicited incremental threefold and fivefold derepression of Pho1 activity, respectively, compared with the WT prt–pho1 reporter (SI Appendix, Fig. S5B). These effects accord with the prediction that a longer transcription interval leads to a higher fraction of termination within that interval (and hence less interference with the pho1 promoter), and vice versa.
Mapping 3′ Polyadenylated Ends of prt lncRNA.
Previous characterization of the prt lncRNA included mapping of the transcription initiation site and detection in rrp6∆ cells (lacking a subunit of the nuclear exosome) of a prt–pho1 read-through transcript extending from the prt initiation site to the pho1 poly(A) site (6, 13, 14). A key prediction of our prt termination-centric model of the control of pho1 expression by CTD and CPF/Rhn1 is that the nascent prt transcript is polyadenylated and terminated at sites upstream of the pho1 promoter. However, to our knowledge, there has been no prior demonstration of a specific polyadenylated prt RNA other than the prt–pho1 read-through [notwithstanding that a ChIP-seq analysis found that the essential fission yeast termination factor Seb1 is present across the prt transcription unit and photoactivatable ribonucleoside-enhanced cross-linking and immunoprecipitation (PAR-CLIP) methods documented association of Seb1 with the 5′ segment of the prt RNA (26)].
Here, to query the existence of terminated prt transcripts, we mapped poly(A) sites in prt RNA by 3′-RACE using as template total RNA isolated from (i) rpb1-CTD-S7A cells expressing prt–pho1 at its natural chromosomal locus (four independent cDNA clones sequenced); (ii) rpb1-CTD-S7A pho1∆ cells bearing the prt–pho1 reporter plasmid (six independent cDNA clones sequenced); and (iii) rpb1-CTD-WT pho1∆ cells bearing the prt–pho1 reporter plasmid (eight independent cDNA clones sequenced). Fourteen of eighteen cDNAs (78%) had an identical junction to a poly(A) tail 351 nt downstream of the prt transcription start site (SI Appendix, Fig. S6A). The predominant prt poly(A) site is located 10 nt downstream of the first nucleotide of a fission yeast ATTTTT polyadenylation signal (PAS) (27). The ATTTTT hexanucleotide is distinctive among fission yeast PAS motifs in that it is situated closer to the cleavage site than the canonical AATAAA element (27). Four additional prt poly(A) junctions were each recovered once, at positions +282, +325, +333, and +338 of the prt transcript (SI Appendix, Fig. S6A).
To better interrogate the existence of the polyadenylated prt transcript suggested by 3′-RACE, we performed a Northern blot analysis of RNAs isolated from three independent cultures of rpb1-CTD-WT pho1∆ cells bearing the prt–pho1 reporter plasmid (SI Appendix, Fig. S7, WT lanes). Probing the blot with a 32P-labeled ssDNA complementary to the segment of the prt RNA from nucleotides +159 to +198 [i.e., upstream of the +351 poly(A) site identified by 3′-RACE] highlighted two classes of prt transcripts: an ∼2.5-kb RNA corresponding to the prt–pho1 read-through transcript and an ∼0.4-kb species (labeled “prt PAS” in SI Appendix, Fig. S7, Left) that we surmise corresponds to prt RNA that was cleaved and polyadenylated at the +351 site. Probing with a 32P-labeled ssDNA complementary to the segment of the pho1 mRNA from nucleotides +83 to +115 also highlighted two distinct RNA classes: a predominant ∼1.5-kb species corresponding to the pho1 mRNA and a minority species corresponding to the longer prt–pho1 read-through transcript (SI Appendix, Fig. S7, Right, WT lanes). No discrete pho1 RNAs smaller than the mature mRNA were detected by Northern blotting. Taken together, the 3′-RACE and Northern blot analyses affirm that the prt lncRNA is terminated at a site well upstream of the pho1 promoter, albeit clearly not with 100% efficiency, as indicated by the prt–pho1 read-through transcript.
Mutating prt poly(A) Signals Attenuates pho1 Derepression by CTD-S7A.
If S7A leads to precocious termination of prt lncRNA synthesis via a CPF-mediated pathway of prt polyadenylation at the +351 cleavage/polyadenylation site, then we might expect that mutation of the ATTTTT PAS immediately upstream of the +351 site would have an impact on the derepression of pho1 expression in phosphate-replete CTD-S7A cells and perhaps on basal pho1 expression in phosphate-replete CTD WT cells. To test this idea, we changed the PAS hexanucleotide to CTCGAG in the prt–pho1 reporter resident in rpb1-CTD-WT pho1∆ and rpb1-CTD-S7A pho1∆ cells and gauged acid phosphatase activity (SI Appendix, Fig. S6B). In CTD WT cells, the PAS mutation resulted in a 69% decrement in Pho1 expression. In CTD-S7A cells, the PAS mutation attenuated the derepression of Pho1, so that acid phosphatase activity was reduced by 56% compared with the WT PAS. These results implicate utilization of the +351 cleavage/polyadenylation site as a modulator of lncRNA repression of the flanking pho1 gene.
The finding that the PAS mutation did not entirely eliminate the derepressive effect of S7A (i.e., unlike the CPF subunit and Rhn1 mutations) suggested that there might be additional cleavage/polyadenylation sites at distal positions in the prt transcription unit. However, because such putative polyadenylated prt transcripts would be subject to rapid intranuclear decay triggered by a cluster of cis-acting determinants of selective removal (DSR) elements located at +399 to +438 of the prt transcript (9, 13, 28), they would not be readily detected by 3′-RACE. To address this point, we performed 3′-RACE using RNA isolated from a fission yeast strain that lacks Mmi1 (29), the RNA-binding protein that recognizes the DSR motifs and elicits RNA decay. Analysis of the PCR-amplified poly(A)+ prt cDNAs from mmi1∆ cells revealed a fragment longer than that detected in mmi1+ cells. Sequencing of 13 independent cDNA clones showed that 12 had a junction to a poly(A) tail 589 nt downstream of the prt transcription start site, whereas one clone had a junction 1 nt 5′ of the other 12 cDNAs (SI Appendix, Fig. S6C). These prt poly(A) sites are located 22 and 21 nt downstream of the first nucleotide of a canonical fission yeast AATAAA PAS (27), hereafter referred to as “prt PAS-2,” and 151 nt downstream of the last DSR motif of the prt DSR cluster (SI Appendix, Fig. S6C).
Additional evidence for 3′ processing and termination of prt at the +589 poly(A) site was obtained by Northern analysis of RNAs isolated from three independent cultures of rpb1-CTD-WT pho1∆ cells bearing a previously characterized prt–pho1 reporter plasmid in which the clustered DSR motifs in the prt gene were mutated to eliminate Mmi1 binding (SI Appendix, Fig. S7) (9). The DSR mutations result in 10-fold lower Pho1 acid phosphatase expression and a fivefold increase in prt RNA (as gauged by RT-qPCR) under phosphate-replete conditions, and they strongly attenuate the pho1 response to phosphate starvation (9). Here we found that the DSR mutations resulted in the appearance of an ∼0.6-kb prt transcript (referred to as “prt PAS2” in SI Appendix, Fig. S7) that we surmise corresponds to prt RNA that was cleaved and polyadenylated at the +589 poly(A) site. The DSR mutations also elicited an approximately fourfold increase in the abundance of the prt–pho1 read-through transcript (detected with either prt or pho1 probes) and a decrement in the level of the pho1 mRNA (SI Appendix, Fig. S7).
Mutating the prt PAS-2 AATAAA hexamer to GGCGGG resulted in a 50% reduction in Pho1 expression in CTD WT cells and a 29% decrement in Pho1 levels in CTD-S7A cells (SI Appendix, Fig. S6B). Combining the ATTTTT (PAS) and AATAAA (PAS-2) prt mutations additively reduced Pho1 expression by 84% in CTD WT cells and 78% in CTD-S7A cells (SI Appendix, Fig. S6B). Taken together, these genetic experiments and RNA analyses highlight 3′ processing and termination of prt lncRNA synthesis upstream of pho1 via the canonical poly(A) pathway as a tunable influence on pho1 expression.
Pho1 Derepression by Csk1 and Cdk9 Kinase Mutants and Epistasis with CPF Subunits and Rhn1.
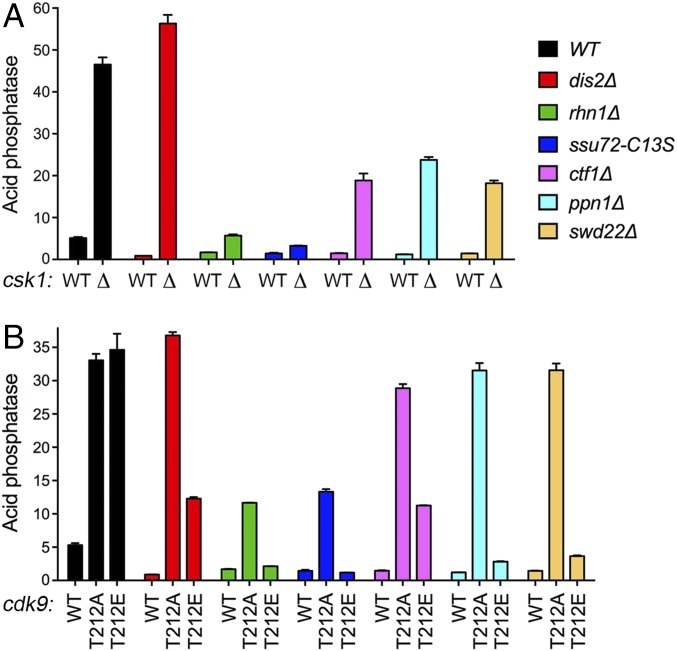
The protein kinase Csk1 is a negative regulator of the phosphate response, as judged by the fact that pho1 expression is constitutively turned on in csk1∆ cells under phosphate-replete conditions (Fig. 3A) (6, 12, 18). Csk1 is a cyclin-dependent kinase (CDK)-activating kinase with several physiological targets, including Cdk9 (30, 31). The essential Cdk9 kinase, in a complex with its cyclin partner Pch1, catalyzes Ser phosphorylation of the Pol2 CTD (31, 32). Csk1 stimulates the CTD kinase activity of Cdk9•Pch1 by phosphorylating Cdk9 on residue Thr212 of the activating “T-loop” segment. The activated Cdk9•Pch1 enzyme phosphorylates the Pol2 CTD at positions Ser2 and Ser5 of the CTD heptad (31). The nonphosphorylatable Cdk9-T212A mutant protein is refractory to activation by Csk1 in vitro. The phosphomimetic Cdk9 mutation T212E enhances the kinase activity of recombinant Cdk9•Pch1 by about threefold compared with the WT enzyme that had not been activated by Csk1 and also compared with the T212A mutant enzyme. Nonetheless, the kinase activity of Cdk9-T212E•Pch1 is threefold lower than that of WT Cdk9•Pch1 that had been activated by Csk1 (31). Thus, Cdk9-T212E is a hypomorphic mutation.
Fig. 3.
Pho1 derepression by Csk1 and Cdk9 kinase mutants and epistasis with CPF subunits and Rhn1. S. pombe strains bearing the indicated csk1 alleles (A) or cdk9 alleles (B) in combination with CPF subunit or Rhn1 mutations as specified were grown in liquid culture at 30 °C and assayed for acid phosphatase activity. Each datum in the bar graph is the average of assays using cells from at least three independent cultures ± SEM.
The fission yeast csk1∆, cdk9-T212A, and cdk9-T212E strains phenocopy each other with respect to derepression of Pho1 under phosphate-replete conditions (Fig. 3, black bars) (6). A key question is whether derepression by Csk1 kinase deletion or hypomorphic Cdk9 mutations entails effects on 3′ processing or termination. Epistatic relationships were illuminated by measuring the effect of csk1∆, cdk9-T212A, and cdk9-T212E on Pho1 expression in the various 3′ processing and termination factor-mutant backgrounds (Fig. 3).
The derepression by csk1∆ was erased in the rhn1∆ and ssu72-C13S strains, signifying that the protein phosphatase activity of Ssu72 and the presence of the CTD-binding termination factor Rhn1 are critical for the effects of Csk1 loss on phosphate homeostasis. By contrast, there was no decrement in csk1∆ derepression of Pho1 in the dis2∆ strain (Fig. 3A), implying that the protein phosphatase activity of Dis2 does not come into play for csk1∆ dysregulation of Pho1. [The derepression by csk1∆ is thereby distinguished from derepression by CTD-S7A, which is dependent on Dis2.] The derepressive effect of csk1∆ was maintained in the ctf1∆, ppn1∆ and swd22∆ strains with respect to the fold increase in Pho1 activity compared with low background in the equivalent csk1+ strains (13-fold for ctf1∆, 20-fold for ppn1∆, 13-fold for swd22∆) but was diminished by two- to 2.5-fold with respect to the absolute level of derepressed Pho1 in the csk1∆ single mutant (Fig. 3A).
The hierarchy of effects of processing/termination factor mutants on the derepression of Pho1 by cdk9-T212A was akin to that seen in csk1∆ cells, insofar as the cdk9-T212A phenotype was similarly unaffected by dis2∆ and the impacts of the other mutations clustered into two groups comprising (i) ctf1∆, ppn1∆, and swd22∆ and (ii) rhn1∆ and ssu72-C13S (Fig. 3B). The distinctions were that ctf1∆, ppn1∆, and swd22∆ did not antagonize derepression by cdk9-T212A, although they partially blunted the csk1∆ phenotype, and rhn1∆ and ssu72-C13S cells maintained derepression by cdk9-T212A, albeit to an intermediate level, whereas they virtually abolished derepression by csk1∆. These results suggest that the events that lead to Pho1 derepression in cdk9-T212A cells are less acutely dependent on the ensemble of processing/termination factors than the underlying events in csk1∆ cells.
A different set of epistatic effects was seen for cdk9-T212E than for cdk9-T212A. The derepression of Pho1 by cdk9-T212E was erased in rhn1∆, ssu72-C13S, ppn1∆, and swd22∆ cells and was partially blunted in dis2∆ and ctf1∆ cells (Fig. 3B). Of the three protein kinase alleles analyzed in Fig. 3, cdk9-T212E was most similar to CTD-S7A with respect to the effects of CPF subunit and Rhn1 mutations on dysregulation of Pho1 expression.
Synthetic Genetic Interactions of CTD Phospho-Site Mutants.
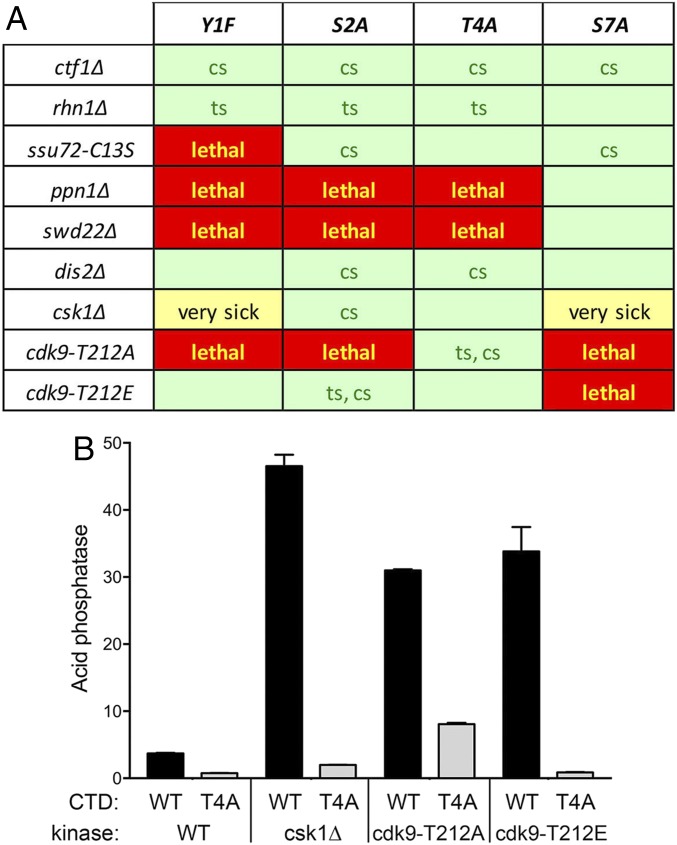
To query genetic interactions of CTD phospho-site mutations with cleavage/termination factors and kinases, haploid fission yeast strains with the full-length rpb1-CTD alleles Y1F, S2A, T4A, and S7A (marked with a 3′ flanking natMX gene) were mated to haploid strains with null or missense mutations in CPF subunits, Rhn1, and protein kinases (marked with 3′ flanking kanMX, hygMX, or ura4 genes). The resulting heterozygous diploids were sporulated and, for each allelic pair, a random collection of 500–1,000 viable haploid progeny was screened by serial replica-plating for the presence of the flanking markers. A failure to recover any viable haploids with both markers while recovering the three other haploid progeny (the unmarked and the two singly marked haploids) with the expected frequencies was taken as evidence of synthetic lethality between the CTD allele and the test allele. (The lethal allelic pairs are indicated by red boxes in the matrix shown in Fig. 4A.) The double-mutant haploids that passed selection were spotted on YES agar at 20–37 °C in parallel with the component single mutants. Two allelic pairs that grew very poorly at 30 and 34 °C and that failed to grow at 37 or 25 °C (classified as “very sick”) are denoted by yellow boxes in Fig. 4A. The double mutants that thrived at 30 °C are denoted by light green boxes in Fig. 4A. Double mutants that displayed an enhanced ts or cs growth defect vis-à-vis one of the single mutants are annotated as such in Fig. 4A.
Fig. 4.
Synthetic genetic interactions of CTD phospho-site mutants. (A) Synthetically lethal pairs of alleles are highlighted in red boxes. Viable double mutants without a synthetic defect are indicated by plain green boxes. Viable double mutants that displayed ts or cs defects are annotated as such in their respective green boxes. The yellow boxes indicate severe synthetic growth defect (very sick). (B) S. pombe strains bearing the indicated csk1 or cdk9 alleles in combination with CTD WT or CTD-T4A alleles as specified were grown in liquid culture at 30 °C and assayed for acid phosphatase activity. Each datum in the bar graph is the average of assays using cells from at least three independent cultures ± SEM.
The results of the synthetic genetic array highlight allele-specific interactions of CTD phospho-site mutations that provide insights into the function of the CTD-PO4 marks (or the amino acid side-chain hydroxyl groups) in fission yeast. To wit, we see that S7A displays few or no synthetic growth defects with the CPF subunit or Rhn1 mutants (Fig. 2C), consistent with our inferences from the phosphate homeostasis experiments that S7A elicits precocious prt transcription termination and that the CPF subunit and Rhn1 mutants diminish prt termination. By contrast, T4A, which is imputed to antagonize termination in the prt–pho1 system, is synthetically lethal in the absence of Ppn1 or Swd22 (Fig. 4A). Previous tiling-array studies of the swd22∆ transcriptome showed that the absence of Swd22 leads to a defect in termination of a limited subset of S. pombe genes (20). We surmise from the synthetic lethality observed here that Thr4-PO4 (or the Thr-OH) and the Ppn1/Swd22 subcomplex of CPF each play important but genetically redundant roles in directing essential transcription termination events in fission yeast. A salient outcome of the genetic array was that Y1F and S2A cluster with T4A with respect to their synthetic lethality with ppn1∆ and swd22∆ (and not with ctf1∆, dis2∆, or rhn1∆) (Fig. 4A), implying that Tyr1-PO4 (or the Tyr-OH) and Ser2-PO4 (or the Ser-OH) also function in cleavage/polyadenylation and/or termination in a manner that is redundant to the Ppn1/Swd22 component of CPF. The strong genetic interactions of CTD phospho-sites with CPF subunits contrasted with the lack of synergy between CTD mutations Y1F, S2A, T4A, or S7A and nab3∆, sen1∆, and din1∆.
In keeping with their opposing effects on phosphate homeostasis, S7A and T4A diverged in their genetic interactions with the Csk1 and Cdk9 kinases. A key finding was that S7A and the three kinase mutants, each of which elicited derepression of prt-regulated pho1 expression in a manner that relied wholly or in part on the subunits of the CPF complex and Rhn1, were synthetically lethal for the S7A cdk9-T212A and S7A cdk9-T212E allelic pairs or were synthetically very sick in the case of the S7A csk1∆ pair (Fig. 4A). Taken together, these results suggest that Ser7-PO4 (or the Ser-OH) and the Csk1 and Cdk9 kinases function redundantly in preventing precocious termination. By contrast, the csk1∆ and two cdk9 mutants were viable in combination with T4A (Fig. 4A). We took advantage of these viable double mutants to probe epistatic relations between the kinase alleles that derepress Pho1 and the CTD-T4A allele that hyperrepresses Pho1 (Fig. 4B). The salient findings were that T4A “wins out” against csk1∆ and cdk9-T212E, i.e., the derepression elicited in the csk1∆ and cdk9-T212E single mutants was erased in the csk1∆ T4A and cdk9-T212E T4A double mutants (Fig. 4B). T4A attenuated but did not eliminate the derepression of Pho1 expression by cdk9-T212A (Fig. 4B). Whereas csk1∆ and cdk9-T212E were viable when paired with Y1F and S2A (albeit with varying conditional or synthetic sick growth defects), the cdk9-T212A allele was synthetically lethal with Y1F and S2A (Fig. 4A). These results underscore the inferences from Fig. 3B that the Cdk9 T212A and T212E mutations are not equivalent in their impact on the various cellular events in which Cdk9 participates.
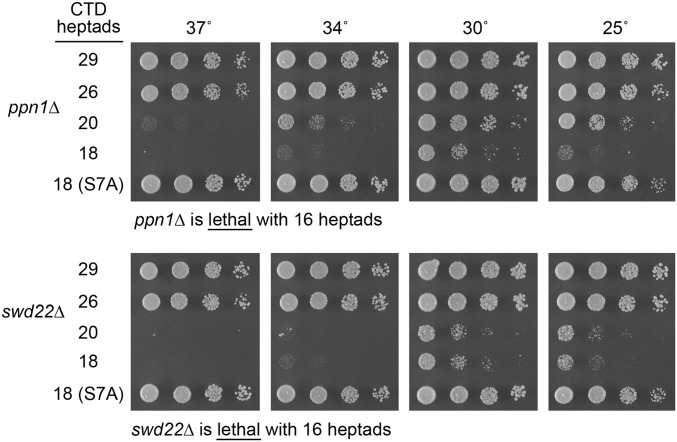
Ppn1 and Swd22 Are Essential for Viability When the CTD Array Is Shortened.
In light of previous findings that the phosphatase activity of Ssu72 becomes essential for fission yeast growth when CTD length is shortened to 16 repeats (rump plus 12 consensus heptads) (6), we tested for mutational synergies between the several CPF subunit and Rhn1 mutants and our collection of rpb1 alleles with serially truncated CTDs (33). In an otherwise WT background, a 16-repeat rpb1-CTD allele has no effect on fission yeast growth (33). Here we found that (i) shortening the CTD array to 20 or 18 repeats in the ppn1∆ and swd22∆ strains elicited a slow-growth defect at 25 and 30 °C and a failure to grow at 34 and 37 °C and (ii) ppn1∆ and swd22∆ were lethal when the CTD was truncated to 16 repeats (Fig. 5). By contrast, growth of rhn1∆ cells at 20–34 °C was unaffected by shortening the CTD to 16 repeats. The 16-heptad CTD allele also had no effect on the growth of dis2∆ cells at 20–37 °C. Growth of the ctf1∆ strain at 20–37 °C was unaffected by CTD shortening to 20 repeats; further shortening to 16 repeats had no effect on growth of ctf1∆ at 30–37 °C but did elicit a cs defect seen as failure to thrive at 20 °C. Thus, like Ssu72, the Ppn1 and Swd22 subunits of CPF are distinctive in that they become essential for vegetative growth when the CTD array is curtailed.
Fig. 5.
Ppn1 and Swd22 are essential for viability when the CTD array is shortened. Exponentially growing cultures of S. pombe ppn1∆ (Upper) and swd22∆ (Lower) strains bearing chromosomal rpb1-CTD alleles with 29, 26, 20, or 18 CTD heptads were adjusted to A600 of 0.1, and aliquots of serial fivefold dilutions were spotted to YES agar and incubated at the indicated temperatures. As noted below the panels, ppn1∆ and swd22∆ were lethal in the context of a 16-heptad CTD array. The slow-growth and ts defects of ppn1∆ and swd22∆ in the context of CTD shortened to 18 repeats were fully suppressed by mutating the Ser7 positions of all consensus heptads to Ala.
S7A Overcomes the Requirement for Ppn1 and Swd22 When the CTD Is Short.
A likely consequence of CTD truncation is that it promotes competition among the many cellular CTD-binding proteins and protein complexes for occupancy of the residual CTD heptads of the Pol2 elongation complex. Accordingly, the lethality or impaired growth of ppn1∆ and swd22∆ when the heptad array is reduced to 16 or 18 repeats indicates a competitive disadvantage in CPF recruitment to the shorter CTD when Ppn1 or Swd22 is absent. Given the results of our phosphate homeostasis experiments indicating that CTD-S7A exerts effects on prt termination opposite those of ppn1∆ and swd22∆, we queried whether replacing Ser7 with Ala might ameliorate the requirement for Ppn1 and Swd22 for growth when the CTD is truncated. The salient findings were that the slow-growth and ts defects of ppn1∆ and swd22∆ in the context of CTD shortened to 18 repeats (rump plus 14 consensus heptads) were fully suppressed by mutating the 14 Ser7 positions to Ala (Fig. 5). However, a shortened 18-heptad CTD in which the 14 Ser7 and 14 Thr4 positions were mutated to Ala (T4A+S7A; ref. 8) did not ameliorate the slow-growth and ts defects of ppn1∆ and swd22∆. Collectively, our results point to S7A as a gain-of-function mutation and to T4A as a loss-of-function mutation with respect to CTD interactions with components of the cleavage/polyadenylation/termination machinery.
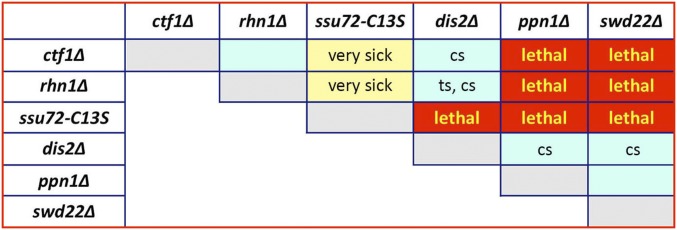
Mutational Synergies Among 3′ Processing Factors and Termination Factors.
Haploids with mutations in the inessential CPF subunits Ctf1, Ssu72, Dis2, Ppn1, and Swd22 and the termination factor Rhn1 were crossed in all pairwise combinations to test for synthetic genetic interactions. The results, compiled in Fig. 6, provide further insights into the organization of the fission yeast 3′ processing/termination machinery. Previous studies indicated that CPF comprises a core complex (of which Ctf1 and Ssu72 are subunits) plus a DPS module composed of Dis2, Ppn1, and Swd22 (20). A series of affinity-tag purification experiments in subunit-null strains showed that (i) the absence of Ppn1 resulted in failure of Dis2 and Swd22 to associate with one another and with the CPF core; (ii) the absence of Swd22 did not affect the association of Ppn1 with Dis2 but resulted in failure of Ppn1•Dis2 to associate with the CPF core; and (iii) the absence of Dis2 did not affect the assimilation of Ppn1 and Swd22 with the CPF core (20). Here, we observed no synthetic growth defects in ppn1∆ swd22∆, ppn1∆ dis2∆, and swd22∆ dis2∆ double mutants (Fig. 6). Taken together, the physical and genetic interactions indicate that the DPS module is dispensable en bloc for vegetative growth.
Fig. 6.
Mutational synergies among 3′ processing factors and termination factors. Synthetically lethal pairs of alleles are highlighted in red boxes. Viable double mutants without a synthetic defect are indicated by plain cyan boxes. Viable double mutants that displayed ts or cs defects are annotated as such in their respective cyan boxes. The yellow boxes indicate a severe synthetic growth defect (very sick).
The key findings in regard to the DPS module were that ppn1∆, swd22∆, and dis2∆ were each synthetically lethal with the ssu72-C13S allele that specifies a catalytically dead version of the Ssu72 protein phosphatase (Fig. 6). Because the three DPS mutants all result in a failure to assimilate the Dis2 protein phosphatase into the CPF complex, we surmise that the synthetic lethality of DPS mutants and ssu72-C13S signifies that (i) a CPF-associated protein phosphatase activity is essential for fission yeast viability; and (ii) either Dis2 or Ssu72 can fulfill this requirement. This conclusion resonates with an earlier observation that an ssu72∆-null allele is synthetically lethal with an allele of ppn1 that is defective for interaction with Dis2 (20).
We observed additional instructive synergies whereby ppn1∆ and swd22∆ were each synthetically lethal with ctf1∆ and rhn1∆ (Fig. 6). By contrast, dis2∆ was viable (albeit ts and cs) when paired with rhn1∆ and was viable (although cs) when paired with ctf1∆ (Fig. 6). Thus, the Ppn1•Swd22 portion of the module becomes essential for CPF function when Ctf1 is missing from the core, in a manner that does not rely on the protein phosphatase component of DPS. Genetic redundancy of Ppn1 and Swd22 with Rhn1 underscores a role for Ppn1•Swd22 in transcription termination. Note that there was no synthetic growth defect when ctf1∆ and rhn1∆ were paired (Fig. 6). The ctf1∆ and rhn1∆ alleles were each viable but were synthetically very sick when paired with ssu72-C13S (Fig. 6), a phenotype that was clearly more severe than the effect of dis2∆ in the ctf1∆ and rhn1∆ backgrounds. These results indicate that the Ssu72 and Dis2 phosphatases are not functionally equivalent, with Ssu72 being the more important for growth in the absence of Ctf1 or Rhn1. Finally, we found no synergies between CPF and Rhn1 mutants and nab3∆, sen1∆, or din1∆.
csk1∆ Suppresses the Synthetic Lethality of dis2∆ ssu72-C13S.
The lethality accompanying simultaneous inactivation of any of seven pairwise combinations of CPF subunits and/or Rhn1 (Fig. 6) is presumably the consequence of a severe termination defect impacting the expression of essential S. pombe genes. We considered the prospect that such a lethal termination defect in a double mutant might be ameliorated by a third mutation that exerts an opposite effect, e.g., the csk1∆ allele that we hypothesize promotes precocious termination in the prt transcription unit. To test this idea, we crossed viable double mutants of csk1∆ plus ctf1∆, rhn1∆, or ssu72-C13S (Fig. 3A) with differentially marked single mutants ppn1∆, swd22∆, and dis2∆ and then screened by random spore analysis for viable triple mutants. Only in the cross of csk1∆ ssu72-C13S with dis2∆ did we recover a viable csk1∆ dis2∆ ssu72-C13S triple mutant. The csk1∆ dis2∆ ssu72-C13S strain grew as well as the csk1∆ ssu72-C13S double mutant on YES agar at 30 °C and 34 °C (said growth being slower than that of WT and of the dis2∆ single mutant) but had a more severe ts and cs defect than did csk1∆ ssu72-C13S at 37 °C and 25 °C (SI Appendix, Fig. S8A). The finding that loss of the Csk1 kinase rescued the lethality incurred by simultaneous inactivation of the two protein phosphatase subunits of CPF fortifies the model that Csk1 exerts an effect on termination opposite that of Dis2 and Ssu72 and hints that the Dis2 and Ssu72 phosphatase might act on an essential phosphoprotein substrate that is generated (directly or indirectly) by Csk1. With respect to Pho1 expression, we saw that the csk1∆ dis2∆ ssu72-C13S triple mutant phenocopied the csk1∆ ssu72-C13S double mutant in erasing the derepression of Pho1 caused by csk1∆ rather than maintaining the derepression seen in the csk1∆ dis2∆ double mutant (SI Appendix, Fig. S8B).
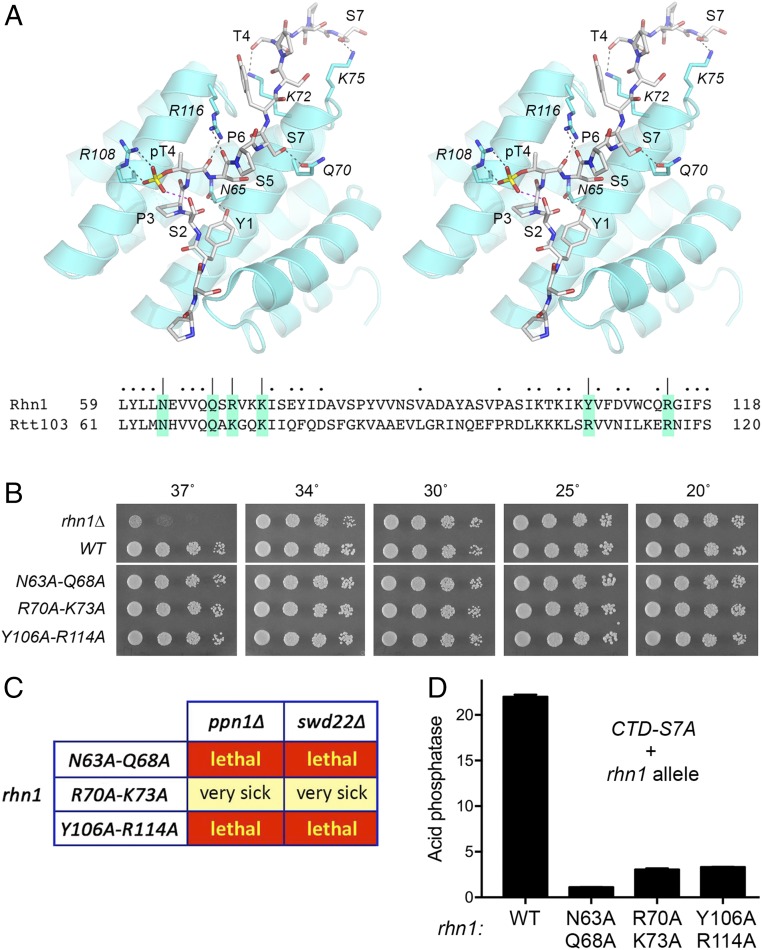
Effect of Mutating the CTD-Binding Site of Rhn1.
Fission yeast Rhn1 is a homolog of the budding yeast phospho-CTD–binding transcription termination factor Rtt103 (23). Rtt103 is a “reader” of the Thr4-PO4 and Ser2-PO4 letters of the CTD code. Structures of the Rtt103 CTD interaction domain in complex with CTD-Thr4-PO4 and CTD-Ser2-PO4 peptides have delineated the determinants of CTD recognition (34–37), as depicted in Fig. 7A for the Rtt103 complex with the CTD peptide PSY1S2P3(pT4)S5P6S7YSPTSPS containing a single Thr4-PO4 heptad and a downstream unmodified heptad (36). Alignment of the amino acid sequence of the segment of Rtt103 that comprises the CTD interface to the corresponding sequence of Rhn1 highlights the conservation of functional groups that engage the CTD (Fig. 7A). The CTD contacts in Rtt103 involve Asn65 (H-bond to the Tyr1-OH of the phosphorylated heptad), Gln70 (H-bond to the Ser7-OH of the phosphorylated heptad), Arg116 (bidentate H-bonds to the pThr4 and Ser5 main-chain carbonyls), Arg108 (bidentate contacts to the pThr4 phosphate oxygens), Lys72 (H-bond to the Thr4-OH of the downstream heptad), and Lys75 (H-bond to Ser7 of the downstream heptad) (Fig. 7A). The equivalent amino acids in Rhn1 are Asn63, Gln68, Arg114, Tyr106, Arg70, and Lys73. To assess the contributions of CTD binding to Rhn1 function in fission yeast, we replaced the chromosomal rhn1+ gene with a series of three rhn1 alleles, N63A-Q68A, R70A-K73A, and Y106A-R114A, in which pairs of CTD-interacting side chains were mutated to Ala. The mutated Rhn1 proteins evidently had biological activity, insofar as they restored WT growth at 37 °C vis-à-vis the ts growth defect of the rhn1∆ strain (Fig. 7B). However, in the ppn1∆ and swd22∆ genetic backgrounds, in which Rhn1 is essential for growth, the rhn1-N63A-Q68A and rhn1-Y106A-R114A alleles were both lethal (Fig. 7C), signifying that Rhn1 contacts to the phosphorylated CTD heptad are crucial for Rhn1 in vivo activity when the DPS module of CPF is compromised. The rhn1-R70A-K73A allele conferred a severe growth defect when paired with ppn1∆ or swd22∆, i.e., the double mutants formed pinpoint colonies at 25 and 30 °C and failed to grow at higher or lower temperatures. We surmise that Rhn1 contacts to the unmodified flanking CTD heptad are also important for Rhn1 function in vivo. The rhn1 N63A-Q68A, R70A-K73A, and Y106A-R114A mutations phenocopied rhn1∆ with respect to elimination of the derepression of Pho1 expression by CTD-S7A (Fig. 7D).
Fig. 7.
Effect of mutating the CTD-binding site of Rhn1. (A, Upper) Stereoview of the structure of the Rtt103 CTD-interaction domain in complex with a CTD peptide containing Thr4-PO4 (Protein Data Bank ID code 5LVF). The CTD peptide is depicted as a stick model with gray carbons; the CTD amino acids are labeled in plain font. Rtt103 side chains that contact the CTD are shown as stick models with cyan carbons and are labeled in italics. Hydrogen-bond interactions between Rtt103 and the CTD are indicated by black dashed lines; an intramolecular H-bond from Ser2-OH to the Thr4 phosphate is indicated by a magenta dashed line. (A, Lower) Alignment of the amino acid sequence of the CTD-interacting segment of Rtt103 to the equivalent segment of Rhn1. Amino acids targeted for Ala scanning in Rhn1 are shaded and denoted by |. Other positions of side-chain identity/similarity are indicated by dots above the Rhn1 sequence. (B) Serial dilutions of the indicated rhn1 strains were spot tested for growth on YES agar at the indicated temperatures. All rhn1 strains were spotted on the same agar plate in every case. The white space between the WT and N63A-Q68A spottings indicates that a single intervening row of cell spottings (of a strain not relevant to the experiment) was cropped out of the image. (C) Mutational synergies. Synthetically lethal pairs of alleles are highlighted in red boxes. The yellow boxes indicate a severe synthetic growth defect (very sick). (D) S. pombe strains bearing the rpb1-CTD-S7A allele in combination with Rhn1 mutants as specified were grown in liquid culture at 30 °C and were assayed for acid phosphatase activity. Each datum in the bar graph is the average of assays using cells from at least three independent cultures ± SEM.
Discussion
The present study highlights 3′ processing and termination as a control point in the lncRNA-mediated repression of 3′-flanking gene expression that underlies fission yeast phosphate homeostasis. In particular, the prt–pho1 locus is established as a sensitive readout of cellular influences on 3′ processing/termination via the degree of interference with Pho1 expression by prt lncRNA transcription. By exploiting this system to interrogate the effects of CTD phospho-site mutations and the genetic interactions of CTD mutations with 3′ processing and termination factors (summarized in SI Appendix, Fig. S9), we provide insights into the functions of Ser7, Thr4, and Tyr1, the “orphan” letters (4) of the CTD code.
S7A Elicits Precocious Termination.
The findings that derepression of pho1 expression at the prt-pho1 locus by S7A is expunged by loss-of-function mutations of CPF subunits Ctf1, Ssu72, Ppn1, Swd22, and Dis2 and termination factor Rhn1 implicate Ser7 status as a determinant of Pol2 termination; to wit, Pol2-S7A is prone to precocious termination during prt lncRNA synthesis. Our inference is that (i) Ser7-PO4 (or the Ser7-OH) normally exerts a negative influence on the interaction of the 3′ processing/termination machinery with the Pol2 elongation complex (SI Appendix, Fig. S9) and (ii) said interaction is enhanced when all Ser7 marks are changed to Ala. This idea is underscored by our demonstration that S7A rescues the severe growth defects caused by absence of Ppn1 or Swd22 (present study) or a catalytically inactivated Ssu72-C13S (6) in the context of a shortened CTD heptad array, i.e., S7A affords a gain of function for the core CPF complex that lacks the Ppn1•Swd22 module.
T4A Reduces Termination.
The CTD-T4A allele exerts a hyperrepressive effect on prt-pho1 opposite that of S7A. Indeed, hyperrepression of pho1 by T4A wins out in a CTD-(T4A+S7A) double mutant (8), consistent with the model that T4A reduces termination during prt lncRNA transcription either by diminishing interaction of the 3′ processing/termination machinery with the Pol2 elongation complex or rendering the elongation complex less responsive to the action of those factors. The concordant effects of T4A and processing/termination factor mutations in negating the derepressive impact of S7A (Fig. 2A and ref. 8), csk1∆, and cdk9-T212E (Figs. 3 and 4B) further support the notion that Thr4-PO4 (or the threonine-OH) acts as a positive effector of Pol2 termination (SI Appendix, Fig. S9). However, it is clear that the Thr4-PO4 mark is not essential per se for termination in fission yeast, based on the fairly benign effect of T4A on vegetative growth and the finding that T4A affects the expression of only seven protein-coding genes (8). A highly instructive outcome in the present study is that T4A is synthetically lethal in the absence of the otherwise inessential CPF subunits Ppn1 and Swd22. Thus, we propose that the Thr4-PO4 mark and Ppn1•Swd22 play critical but genetically redundant roles in termination in fission yeast.
Rhn1, the homolog of budding yeast Rtt103, is the likely reader of the Thr4-PO4 CTD coding letter with respect to termination. Rhn1 is not a subunit of the fission yeast CPF complex (20), and it is inessential for vegetative growth. We find here that rhn1∆ (like T4A) is synthetically lethal in the absence of Ppn1•Swd22 and that the activity of Rhn1 in sustaining cell growth in the ppn1∆ or swd22∆ backgrounds is abolished by mutations in its phospho-CTD–binding site. In the WT CPF complex, the CTD-T4A rhn1∆ double mutation (which simply lowers the restrictive temperature of the rhn1∆ allele to 34 °C) has little impact. These results would place Thr4-PO4 and Rhn1 in the same subpathway of termination in fission yeast. Our data are consistent with recent studies in budding yeast that establish a physical association of Thr4-phosphorylated CTD with Rtt103 (35, 36, 38), although in budding yeast the T4A mutation impacted termination specifically at small nucleolar RNA genes (35). A budding yeast T4V mutant strain exhibited a more general increase in Pol2 density distal to poly(A) sites of protein-coding genes (36).
Tyr1 and Termination.
The fission yeast CTD-Y1F mutation had no overt impact on pho1 expression under phosphate-replete conditions, and hence there was no sign that the absence of the Tyr1-PO4 mark impacts lncRNA-mediated transcriptional interference. Instead, a persuasive genetic connection between the Tyr1-PO4 mark or the Tyr hydroxyl and 3′ processing/termination was made via the findings that Y1F is synthetically lethal in the absence of Ppn1•Swd22 or when the Ssu72 phosphatase is catalytically dead. We would attribute these synthetic phenotypes directly to the Y1F mutation and not to a possible indirect impact on the adjacent Ser2-coding letter because (i) the spectrum of synthetic lethality of Y1F with three different CPF mutations is broader than that of the S2A allele and (ii) we showed previously that a Y1F allele had no effect on the overall level of the Ser2-PO4 mark, as probed with a phospho-specific antibody (7).
Our results point to either Tyr1-PO4 or the Tyr hydroxyl acting as a positive effector of Pol2 termination in fission yeast. This scenario is distinct from the negative role assigned to the Tyr1-PO4 mark in budding yeast Pol2 termination, whereby CTD Tyr phosphorylation impairs recruitment of the termination factors Rtt103 and Pcf11 (39) and the removal of the Tyr1-PO4 mark by the Glc7 protein phosphatase subunit of CPF as Pol2 traverses the poly(A) site leads to engagement of Rtt103 and Pcf11 that then leads to CPF-coupled termination (21). Whereas Glc7 is essential for viability in Saccharomyces cerevisiae, the orthologous CPF phosphatase subunit Dis2 is inessential in S. pombe. We show here that loss of Dis2 is genetically buffered by the Ssu72 phosphatase subunit of fission yeast CPF.
Our invocation of a positive termination function for the Tyr1-PO4 or the Tyr hydroxyl in fission yeast based on the Y1F genetics is in tune with a recent report that replacement of three-fourths of the Tyr residues in the mammalian CTD with Phe resulted in a generalized termination defect manifest as widespread read-through transcription over long distances 3′ of mammalian genes (40).
Tyr1-Ser2-Thr4 as a Three-Letter Word in the CTD Code.
Our best hope was that a synthetic genetic array of CTD phospho-site mutations in fission yeast would (i) identify or affirm the cellular transactions to which individual CTD coding letters contribute and (ii) shed light on the vocabulary of the code, i.e., if two different phospho-site mutations elicit highly overlapping mutational synergies, then the two CTD letters comprise a “word” that regulates certain cellular transactions. The array experiments conducted here show that Y1F, S2A, and T4A have overlapping synthetic lethalities with deletions of CPF subunits Ppn1 and Swd22 (SI Appendix, Fig. S9), suggesting that Tyr1-Ser2-Thr4 form a three-letter word in the CTD code that promotes Pol2 termination (and mutations of which impair termination in certain genetic backgrounds). There was already a strong suggestion that Tyr1-Ser2 comprise a two-letter CTD word in fission yeast, based on the observation that the majority of the protein-coding RNAs that were affected in Y1F cells were coordinately affected in S2A cells (8). With respect to termination, we hypothesize that the “spelling” of the three-letter word is likely to be Tyr1-Ser2-Thr4(PO4), based on how the Rtt103/Rhn1 termination factor recognizes the Thr4-phospho-CTD (Fig. 7A). The termination factor makes a hydrogen bond from a conserved Asn side chain to the CTD Tyr1 hydroxyl, and mutation of the Asn to Ala is detrimental to Rhn1 function. We envision that changing the CTD Tyr1 letter to Phe, thereby subtracting the hydroxyl group, has the same negative effect on Rhn1 activity. The Ser2 hydroxyl of the phospho-heptad makes no contacts with Rtt103, but it does donate a hydrogen bond to the phosphate group of Thr4-PO4 (Fig. 7A), and it is likely that this interaction aids in establishing or stabilizing a conformation of the phospho-CTD that is suitable for binding to the termination factor.
Methods
Detailed methods are provided in SI Appendix for (i) the construction of fission yeast strains with Rpb1 CTD mutations; (ii) deletions of the ctf1, rhn1, dis2, ppn1, swd22, nab3, sen1, and din1 genes; and (iii) allelic exchanges at the rhn1 locus. The strains used in this study and their relevant genotypes are listed in SI Appendix, Table S1. The methods for testing mutational synergies, assaying cell-associated acid phosphatase activity, prt-pho1 reporter plasmids and assays, and RNA analyses via primer extension, 3′-RACE, and Northern blotting are described in detail in SI Appendix.
Supplementary Material
Acknowledgments
We thank Robert Fisher for the dis2∆ strain. This work was supported by NIH Grants R01-GM52470 and R35-GM126945.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810711115/-/DCSupplemental.
References
- 1.Eick D, Geyer M. The RNA polymerase II carboxy-terminal domain (CTD) code. Chem Rev. 2013;113:8456–8490. doi: 10.1021/cr400071f. [DOI] [PubMed] [Google Scholar]
- 2.Corden JL. RNA polymerase II C-terminal domain: Tethering transcription to transcript and template. Chem Rev. 2013;113:8423–8455. doi: 10.1021/cr400158h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeronimo C, Bataille AR, Robert F. The writers, readers, and functions of the RNA polymerase II C-terminal domain code. Chem Rev. 2013;113:8491–8522. doi: 10.1021/cr4001397. [DOI] [PubMed] [Google Scholar]
- 4.Yurko NM, Manley JL. The RNA polymerase II CTD “orphan” residues: Emerging insights into the functions of Tyr-1, Thr-4, and Ser-7. Transcription. 2018;9:30–40. doi: 10.1080/21541264.2017.1338176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwer B, Shuman S. Deciphering the RNA polymerase II CTD code in fission yeast. Mol Cell. 2011;43:311–318. doi: 10.1016/j.molcel.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwer B, Sanchez AM, Shuman S. RNA polymerase II CTD phospho-sites Ser5 and Ser7 govern phosphate homeostasis in fission yeast. RNA. 2015;21:1770–1780. doi: 10.1261/rna.052555.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwer B, Sanchez AM, Shuman S. Punctuation and syntax of the RNA polymerase II CTD code in fission yeast. Proc Natl Acad Sci USA. 2012;109:18024–18029. doi: 10.1073/pnas.1208995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwer B, Bitton DA, Sanchez AM, Bähler J, Shuman S. Individual letters of the RNA polymerase II CTD code govern distinct gene expression programs in fission yeast. Proc Natl Acad Sci USA. 2014;111:4185–4190. doi: 10.1073/pnas.1321842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee D, Sanchez AM, Goldgur Y, Shuman S, Schwer B. Transcription of lncRNA prt, clustered prt RNA sites for Mmi1 binding, and RNA polymerase II CTD phospho-sites govern the repression of pho1 gene expression under phosphate-replete conditions in fission yeast. RNA. 2016;22:1011–1025. doi: 10.1261/rna.056515.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez AM, Shuman S, Schwer B. Poly(A) site choice and Pol2 CTD Serine-5 status govern lncRNA control of phosphate-responsive tgp1 gene expression in fission yeast. RNA. 2018;24:237–250. doi: 10.1261/rna.063966.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg A, Sanchez AM, Shuman S, Schwer B. A long noncoding (lnc)RNA governs expression of the phosphate transporter Pho84 in fission yeast and has cascading effects on the flanking prt lncRNA and pho1 genes. J Biol Chem. 2018;293:4456–4467. doi: 10.1074/jbc.RA117.001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter-O’Connell I, Peel MT, Wykoff DD, O’Shea EK. Genome-wide characterization of the phosphate starvation response in Schizosaccharomyces pombe. BMC Genomics. 2012;13:697. doi: 10.1186/1471-2164-13-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah S, Wittmann S, Kilchert C, Vasiljeva L. lncRNA recruits RNAi and the exosome to dynamically regulate pho1 expression in response to phosphate levels in fission yeast. Genes Dev. 2014;28:231–244. doi: 10.1101/gad.230177.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee NN, et al. Mtr4-like protein coordinates nuclear RNA processing for heterochromatin assembly and for telomere maintenance. Cell. 2013;155:1061–1074. doi: 10.1016/j.cell.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ard R, Tong P, Allshire RC. Long non-coding RNA-mediated transcriptional interference of a permease gene confers drug tolerance in fission yeast. Nat Commun. 2014;5:5576. doi: 10.1038/ncomms6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwer B, Sanchez AM, Garg A, Chatterjee D, Shuman S. Defining the DNA binding site recognized by the fission yeast Zn2Cys6 transcription factor Pho7 and its role in phosphate homeostasis. MBio. 2017;8:e01218–17. doi: 10.1128/mBio.01218-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porrua O, Libri D. Transcription termination and the control of the transcriptome: Why, where and how to stop. Nat Rev Mol Cell Biol. 2015;16:190–202. doi: 10.1038/nrm3943. [DOI] [PubMed] [Google Scholar]
- 18.Henry TC, et al. Systematic screen of Schizosaccharomyces pombe deletion collection uncovers parallel evolution of the phosphate signal transduction pathway in yeasts. Eukaryot Cell. 2011;10:198–206. doi: 10.1128/EC.00216-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roguev A, et al. A comparative analysis of an orthologous proteomic environment in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Mol Cell Proteomics. 2004;3:125–132. doi: 10.1074/mcp.M300081-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Vanoosthuyse V, et al. CPF-associated phosphatase activity opposes condensin-mediated chromosome condensation. PLoS Genet. 2014;10:e1004415. doi: 10.1371/journal.pgen.1004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreieck A, et al. RNA polymerase II termination involves C-terminal-domain tyrosine dephosphorylation by CPF subunit Glc7. Nat Struct Mol Biol. 2014;21:175–179. doi: 10.1038/nsmb.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parua PK, et al. A Cdk9-PP1 switch regulates the elongation-termination transition of RNA polymerase II. Nature. 2018;558:460–464. doi: 10.1038/s41586-018-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiyama T, Sugioka-Sugiyama R, Hada K, Niwa R. Rhn1, a nuclear protein, is required for suppression of meiotic mRNAs in mitotically dividing fission yeast. PLoS One. 2012;7:e42962. doi: 10.1371/journal.pone.0042962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemay JF, et al. The Nrd1-like protein Seb1 coordinates cotranscriptional 3′ end processing and polyadenylation site selection. Genes Dev. 2016;30:1558–1572. doi: 10.1101/gad.280222.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M, et al. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature. 2004;432:517–522. doi: 10.1038/nature03041. [DOI] [PubMed] [Google Scholar]
- 26.Wittmann S, et al. The conserved protein Seb1 drives transcription termination by binding RNA polymerase II and nascent RNA. Nat Commun. 2017;8:14861. doi: 10.1038/ncomms14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mata J. Genome-wide mapping of polyadenylation sites in fission yeast reveals widespread alternative polyadenylation. RNA Biol. 2013;10:1407–1414. doi: 10.4161/rna.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilchert C, et al. Regulation of mRNA levels by decay-promoting introns that recruit the exosome specificity factor Mmi1. Cell Rep. 2015;13:2504–2515. doi: 10.1016/j.celrep.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiyama T, Sugioka-Sugiyama R. Red1 promotes the elimination of meiosis-specific mRNAs in vegetatively growing fission yeast. EMBO J. 2011;30:1027–1039. doi: 10.1038/emboj.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiz JE, Fisher RP. A CDK-activating kinase network is required in cell cycle control and transcription in fission yeast. Curr Biol. 2002;12:1100–1105. doi: 10.1016/s0960-9822(02)00903-x. [DOI] [PubMed] [Google Scholar]
- 31.Pei Y, et al. Cyclin-dependent kinase 9 (Cdk9) of fission yeast is activated by the CDK-activating kinase Csk1, overlaps functionally with the TFIIH-associated kinase Mcs6, and associates with the mRNA cap methyltransferase Pcm1 in vivo. Mol Cell Biol. 2006;26:777–788. doi: 10.1128/MCB.26.3.777-788.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei Y, Shuman S. Characterization of the Schizosaccharomyces pombe Cdk9/Pch1 protein kinase: Spt5 phosphorylation, autophosphorylation, and mutational analysis. J Biol Chem. 2003;278:43346–43356. doi: 10.1074/jbc.M307319200. [DOI] [PubMed] [Google Scholar]
- 33.Schneider S, Pei Y, Shuman S, Schwer B. Separable functions of the fission yeast Spt5 carboxyl-terminal domain (CTD) in capping enzyme binding and transcription elongation overlap with those of the RNA polymerase II CTD. Mol Cell Biol. 2010;30:2353–2364. doi: 10.1128/MCB.00116-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lunde BM, et al. Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat Struct Mol Biol. 2010;17:1195–1201. doi: 10.1038/nsmb.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemec CM, et al. Different phosphoisoforms of RNA polymerase II engage the Rtt103 termination factor in a structurally analogous manner. Proc Natl Acad Sci USA. 2017;114:E3944–E3953. doi: 10.1073/pnas.1700128114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jasnovidova O, Krejcikova M, Kubicek K, Stefl R. Structural insight into recognition of phosphorylated threonine-4 of RNA polymerase II C-terminal domain by Rtt103p. EMBO Rep. 2017;18:906–913. doi: 10.15252/embr.201643723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasnovidova O, et al. Structure and dynamics of the RNAPII CTDsome with Rtt103. Proc Natl Acad Sci USA. 2017;114:11133–11138. doi: 10.1073/pnas.1712450114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harlen KM, et al. Comprehensive RNA polymerase II interactomes reveal distinct and varied roles for each phospho-CTD residue. Cell Rep. 2016;15:2147–2158. doi: 10.1016/j.celrep.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer A, et al. CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science. 2012;336:1723–1725. doi: 10.1126/science.1219651. [DOI] [PubMed] [Google Scholar]
- 40.Shah N, et al. Tyrosine-1 of RNA polymerase II CTD controls global termination of gene transcription in mammals. Mol Cell. 2018;69:48–61.e6. doi: 10.1016/j.molcel.2017.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.