Abstract
A perfect flower in a mid-Cretaceous (early Cenomanian) Myanmar amber is described as Lijinganthus revoluta gen. et sp. nov. The fossil flower is actinomorphic and pentamerous, including calyx, corolla, stamens, and gynoecium. The sepals are tiny, while the petals are large and revolute. The stamens are dorsifixed, filamentous, and each has a longitudinally dehiscing bisporangiate anther. The gynoecium is in the centre of the flower, composed of three fused carpels with a stout style. Lijinganthus revoluta gen. et sp. nov. demonstrates a great resemblance to the flowers of Pentapetalae (Eudicots), adding new information to the enigmatic early evolutionary history of Pentapetalae and Eudicots.
Introduction
An increasing number of insects and plants have been reported in a mid-Cretaceous Myanmar ambers1–23. Among them, fossil flowers have shed light on the diversification of angiosperms during this important radiating period for angiosperms2,18–21,24. Core Eudicots comprise a major portion of the species diversity in extant angiosperms, and they underwent a rapid increase in diversity and abundance at the transition between the Early and Late Cretaceous19,25–28. Hitherto, the earliest record of a flower with distinct sepals and petals is approximately 94 Ma (the Cenomanian)29,30 (However, according to the latest study (Manchester et al.27), some fruit specimens belonging to the same taxon may be dated back to 105 Ma (the Albian)). Various molecular clocks indicate that angiosperms and Eudicots have a significantly earlier origin than the earliest fossil record indicates31–33. The gap between these estimations and the fossil record makes many conclusions in angiosperm systematics tentative. Here, we describe a new flower, Lijinganthus revoluta gen. et sp. nov., from an earliest Cenomanian-latest Albian (98.79 Ma)34 amber, which was collected from Noije Bum 2001 Summit Site, Hukawng Valley, Kachin, Myanmar (26°20′N, 96°36′E) (Fig. 1). This bisexual, pentamerous, actinomorphic flower with distinct sepals and petals, filamentous bisporangiate stamens, and trimerous gynoecium with superior ovary and axile placentation is unique, demonstrating a great resemblance to Pentapetalae (Core Eudicots) and thus shedding a new light on the evolution of Core Eudicots. Lijinganthus, as a representative of Pentapetalae, is among the first Core Eudicots. Together with contemporary fossil finds19,25–28, Lijinganthus suggests a Core Eudicot Boom at the transition from the Early to Late Cretaceous and helps to narrow the gap between fossil record and molecular clock estimates. The presence of a nectary disk in Lijinganthus suggests the possibility of insect-mediated pollination.
Figure 1.
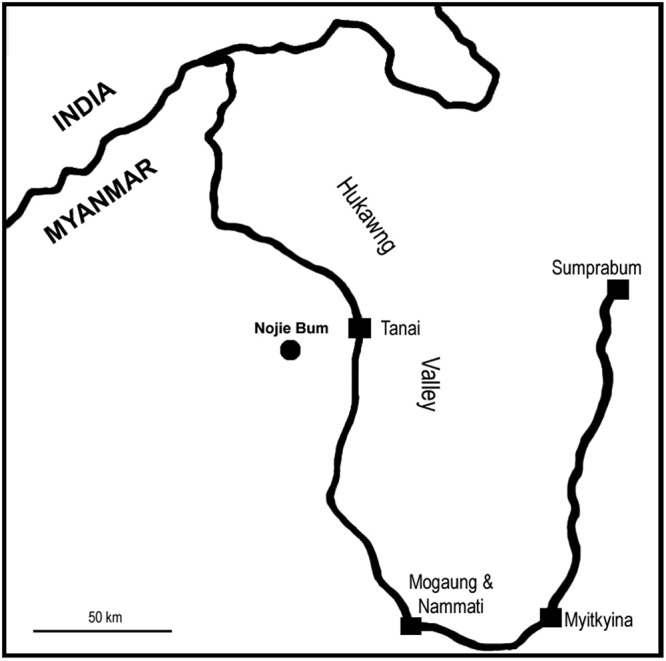
Nojie Bum, the fossil locality (round dot) of Lijinganthus revoluta gen. et sp. nov., in northern Myanmar. The major cities are shown as squares.
Results
Systematic palaeontology
Angiospermae
Eudicots
Pentapetalae
Family incertae sedis
Lijinganthus gen. nov.
Type species: Lijinganthus revoluta gen. et sp. nov.
Diagnosis: Flower actinomorphic, bisexual, with one whorl of five sepals, one whorl of five petals, androecium, and gynoecium. The calyx consisting of five distinct, small sepals with entire margins and rounded apices. The corolla consisting of five distinct, large, elongated oval, entire-margined revolute petals with rounded apices, alternating with the sepals. Androecium diplostemonous, filamentous. The anthers bisporangiate, introrse, dorsifixed, dehiscing by longitudinal slits. Nectary disk at the base of the gynoecium. The gynoecium tricarpous, lacking an obvious style and probably with axile placentation.
Lijinganthus revoluta gen. et sp. nov.
Figure 2.
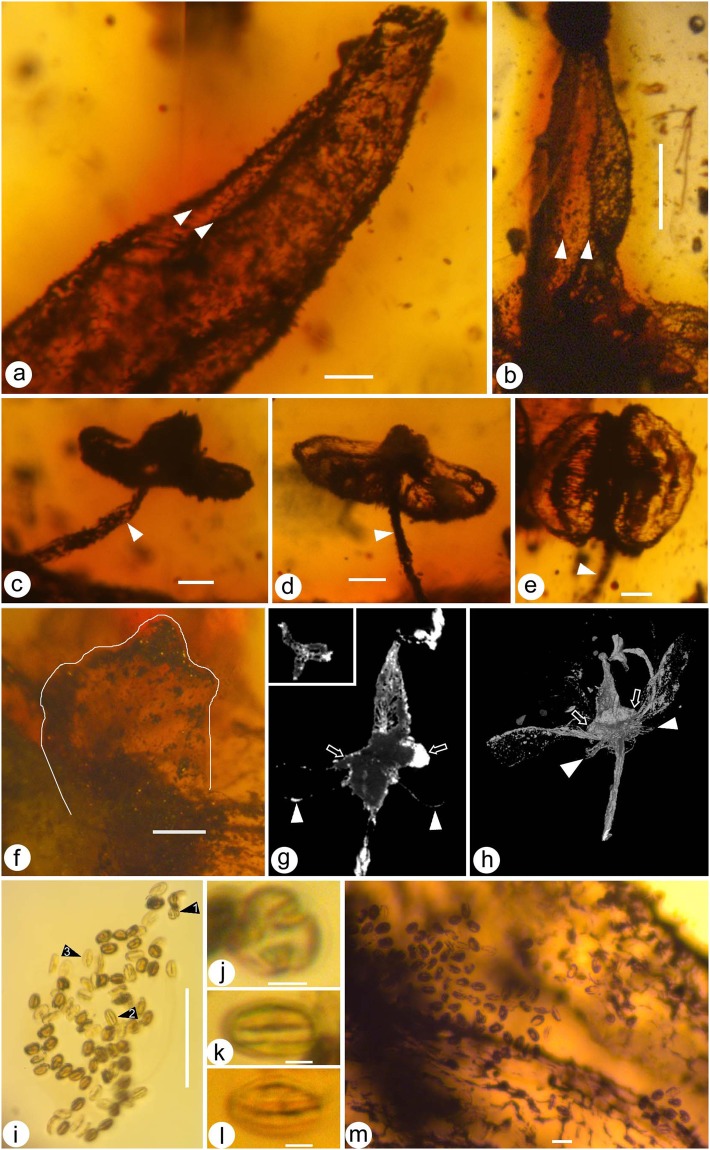
Lijinganthus revoluta gen. et sp. nov. embedded in a Myanmar amber. (a) Side view of the flower, showing physically connected pedicel (white triangle), petals (1–5), and stamens. Scale bar = 1 mm. (b) Top view of the flower, showing petals (1–5), anthers (white triangles), and ovary (double triangle). Note the relationship between stamens (a,b) and Petal 4. Scale bar = 1 mm. (c) Bottom view of the flower, showing revolute petals (1–5) and stamens. Scale bar = 1 mm. (d) Detailed view of the rectangular region in 1c, showing the relationship among sepal (white line), petal (4), and 2 filaments (white triangles). Scale bar = 0.1 mm.
Figure 3.
Detailed view of Lijinganthus revoluta gen. et sp. nov. embedded in a Myanmar amber. (a) Abaxial view of the distal portion of Petal 4, showing the revolute form and the margins (white triangles) of the petal. Scale bar = 0.1 mm. (b) Abaxial view of the distal portion of Petal 1, showing the margins (white triangles) of revolute petal. Scale bar = 0.5 mm. (c–e) Top (c,d) and adaxial (e) views of the dorsifixed bisporangiate anthers on the termini of slender filaments (white triangles). Scale bar = 0.1 mm. (f) Detailed view of a sepal (white line). Scale bar = 0.1 mm. (g) Micro-CT virtual section of the gynoecium, showing the conical profile of the ovary, nectary disk (arrows), inconspicuous style, and petals (white triangles). The inset shows the cross section of the ovary near the tip. (h) Micro-CT virtual view showing relationship among the pedicel, sepals (white triangles), petals, stamen, nectary disk (arrows), conical ovary, and inconspicuous style in the centre. (i) Approximately 70 tricolpate pollen grains closely associated with the flower. Scale bar = 0.1 mm. (j–l) Different views of tricolpate pollen grains, enlarged from those marked as 1–3, respectively, in (i). (j) polar view; (k,l) equatorial views. Scale bar = 5 μm. (m) Numerous pollen grains adjacent to one of the petals of the flower. Scale bar = 20 μm.
Figure 4.
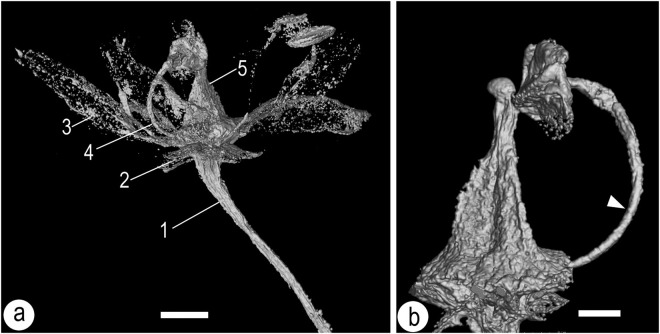
Detailed view of Lijinganthus revoluta gen. et sp. nov. embedded in a Myanmar amber. (a) Micro-CT visualization of the flower. 1, pedicel; 2, sepal; 3, petal; 4, stamen; 5, ovary. Scale bar = 0.5 mm. (b) Detailed view of a stamen and the ovary, showing the slender filament (white triangles) attached to the dorsal of the anther that opens along two adaxial longitudinal slits. Scale bar = 0.2 mm.
Figure 5.

Reconstruction of Lijinganthus revoluta gen. et sp. nov.
Description: The flower (including the pedicel) is 6.5 mm long and 4.8 mm wide. The flower includes a pedicel, sepals, petals, stamens, and a gynoecium (Figs 2a–c, 3h and 4a). The pedicle is 0.2 mm in diameter and 3.8 mm long, slightly tapering distally. There are five distinct sepals, each 0.4-0.5 mm long and 0.32-0.33 mm wide, with an obtuse or round tip (Figs 2d, 3f,h and 4a). Alternating with the sepals are five distinct petals, each approximately 1.8 mm long and 0.67 mm wide, revolute (Figs 2a–d, 3a,b and 4a). At least eight distinct stamens are seen inserted on the nectary disk at the base of the ovary, each including a slender filament and a dorsifixed bisporangiate anther (Figs 2a–d, 3c–e,h and 4a,b). The filament is approximately 43 μm wide, 1.3 mm long, tapering distally (Figs 2a–d, 3c–e and 4a,b). The anther dehisces longitudinally, 0.5 mm wide and 0.5 mm long, without in situ pollen grains (Figs 3c–e,h and 4b). Several clumps of a single type of pollen grains are present in the same amber block and closely associated with the flower (Figs 3i,m and S1a–q). Pollen grains are tricolpate, 14–21 μm long and 10–12 μm in diameter (Fig. 3i–l). Nectary surrounds the base of the ovary (Fig. 3g–h). The gynoecium is situated centrally, including an ovary that tapers distally with no obvious style (Figs 3g,h and 4a,b). The ovary appears to be composed of three fused carpels, probably with axile placentation (Fig. 3g). The ovary is approximately 0.77 mm in diameter, with its tip only 0.13 mm in diameter, and round triangular in cross view (Figs 2b,c, 3g,h, 4b and 5).
Etymology: The generic name, Lijinganthus, is dedicated to poetess Ms. Jing Li (1967–2015) for her talent and poetry, and “anthus” from the Latin “anthos” (flower). The specific epithet revoluta is for the revolute form of the petals.
Type species: Lijinganthus revoluta gen. et sp. nov.
Holotype: PB22841.
Deposition: The Nanjing Institute of Geology and Palaeontology, Chines Academy of Sciences.
Type locality: Noije Bum 2001 Summit Site, Hukawng, Kachin, Myanmar (26°21′33.41″N, 96°43′11.88″E).
Horizon: the earliest Cenomanian-latest Albian, Cretaceous (98.79 Ma).
Remarks: Although only eight stamens are preserved in the flower, the spatial relationship between stamens and petals (Fig. 2d) and the presence of five petals (Fig. 2a–c) suggest that the total number of original stamens in Lijinganthus should be ten. Further research need to be done to confirm this.
The character combination of the fossil does not allow us to assign it to any known fossil or extant genus of angiosperms, thereby justifying a new genus.
Discussion
Comparison with extant angiosperms
The occurrence of over 800 single type of tricolpate pollen grains closely associated with Lijinganthus revoluta gen. et sp. nov. (preserved in the same block, and their distances from the flower range from 2.5 to 0 mm (Figs 3i,m and S1a–q)) strongly suggests a eudicot affinity for Lijinganthus, as tricolpate pollen grains are a characteristic feature of Eudicots, which are frequently termed Tricolpates. Among Eudicots, the occurrence of distinct calyx and corolla in Lijinganthus distinguishes it from the basal eudicots (with undifferentiated perianth)30 and Gunnerales (lacking perianth)35, suggesting that Lijinganthus belongs to the Pentapetalae in Core Eudicots. Several features of Lijinganthus are also seen in Crassulaceae (Saxifragales), but the latter has a basifixed anther, >3 more or less free carpels, decurrent stigma, and parietal placentation36,37 and is thus distinct from Lijinganthus with dorsifixed anther, fused carpels, capitate stigma, and axile placentation. Although the presence of distinct sepals and petals suggest that Lijinganthus is most likely related to the Superrosids, and its tricarpous gynoecium suggests a possible affinity to Malpighiales, we think it is premature to assign Lijinganthus to any group within Pentapetalae, at least for the time being.
Comparison with the Early Flowers of Core Eudicots
Previously, the earliest record of a flower with distinct calyx and corolla was marked by a fossil flower named “Rose Creek flower” from the Albian-Cenomanian30, which was recognized by Basinger and Dilcher in 198429 and reworked on and renamed as Dakotanthus cordiformis by Manhcester et al. (2018) with additional specimens (especially of fruits) using CT technology27. Lijinganthus is similar to Dakotanthus cordiformis in distinct pentamerous symmetry (5 sepals and 5 petals) and bisexuality, but it differs from the latter in long (rather than ovate) petals, a slender (rather than stout) filament, a bisporangiate (rather than tetrasporangiate) anther, tricolpate (rather than tricolporate) pollen grains, a trimerous (rather than pentamerous) gynoecium, and 1 (rather than 5) style27,29. Dakotanthus cordiformis is initially interpreted as approximately 94 Ma old29, but recent study, with more specimens (especially of fruits) from strata other than the original locality (Rose Creek), suggests that the age of Dakotanthus cordiformis may be extended to 105 Ma27. Dakotanthus cordiformis was considered to be “the first fossils with unequivocal features of core eudicots” from the Albian-Cenomanian30. The age of 98.79 Ma of Lijinganthus places our flower near the Albian/Cenomanian boundary (97.2 Ma to100.5 Ma by various authors). The concentrated occurrence of various flowers of different lineages (Lachnociona terriae (Brunelliaceae/Cunoniaceae (Oxalidaels) + Rosids + Saxifragles), Tropidogyne pikei and T. pentaptera (Cunoniaceae), Eoëpigynia burmensis (Cornaceae), Dakotanthus cordiformis (Quillajaceae)) and Lijinganthus at about the same time19,25–28 seems to suggest the Core Eudicots underwent a rapid diversification (“Core Eudicot Boom”) at the very beginning of the Late Cretaceous (Table 1). It is intriguing to investigate whether there is a coupling between this important plant event and the Upper Albian OAE 1d event (including rapid CO2 concentration rising)38 as well as the decline of Gnetales and Bennettitales.
Table 1.
Comparison among the flowers of pioneer Core Eudicots in the mid-Cretaceous (Albian-Cenomanian).
| Symmetry | Gender | Sepal | Petal | Stamen | Filament | Pollen sac | Pollen grain | Floral cup | Ovary | Placentation | Carpel | Style | Preservation media | Locality | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lijinganthus revoluta | actinomorphic | bisexual | 5, free | 5, free, revolute | 8(10?), dorsifixed, introse | slender | 2 | tricolpate | None | superior | axile | 3, fused | amber | Burma | This study | |
| Dakotanthus cordiformis | actinomorphic | bisexual | 5, free | 5, free, spatulate | 10?, dorsifixed | stout | 4 | tricolporate | superior | axile | 5, fused | siltstone | USA | Manchester et al.27 | ||
| Lachnociona terriae | actinomorphic | unisexual | 5, free | none | 10 | slender | present | superi, half-inferior | 5, fused or not | connivent | Amber | Burma | Poinar et al.19 | |||
| Tropidogyne pentaptera | actinomorphic | bisexual | 5, slightly fused | 0 | 5? dorsifixed | short, slender | present | inferior | 2, fused | 2 | amber | Burma | Poinar et al.28 | |||
| Eoȅpigynia burmensis | actinomorphic | bisexual | 4, fused | 4, free, valvate | 4, dorsifixed, introse | slender | ? | tricolporate? | present | inferior | ? | fused | amber | Burma | Poinar et al.18 | |
| Caliciflora mauldinensis | actinomorphic | Bisexual | 5, free, revolute-valvate | 5, free, keeled-conduplic-ate | 8, dorsifixed | short or none | 4 | tricolporate | present | superior | marginal? | 3, free | siltstone | USA | Friis et al.26 |
The presence of nectary disk in Lijinganthus suggests that insects may have begun interacting with flowers by the Cenomanian. Whether such an interaction is a major driving force for the diversification of Core Eudicots is apparently a question deserving further investigation.
Earlier Origins
Various molecular clock studies have indicated that Eudicots and angiosperms originated much earlier than formerly assumed31–33. The discovery of Lijinganthus from the late Albian-Early Cenomanian (98.79 Ma) adds to the diversity and abundance of early Core Eudicots and helps to reconcile the conflicts between different schools and studies. Compatible with the molecular studies, the early age of Lijinganthus, together with recently found earlier-than-recorded fossils of Poaceae39 and Solanaceae40 as well as various contemporaneous Core Eudicots19,25–28 points to a cryptic and unexpectedly longer history of angiosperms and Eudicots. It is noteworthy that this conclusion is compatible with previously documented pre-Cretaceous traces of angiosperms41–48.
Conclusions
Lijinganthus revoluta gen. et sp. nov. and other contemporaneous fossil flowers suggest a Core Eudicot Boom at the very beginning of the Late Cretaceous. Increasing number of reports of early fossil flowers seem to converge earlier origins of various lineages that have been predicted by molecular clocks.
Methods
The specimen was collected from Noije Bum 2001 Summit Site, Hukawng Valley, Kachin, Myanmar (26°20′N, 96°36′E) (Fig. 1). Paleontological studies indicate that the specimen belongs to the earliest Cenomanian-latest Albian, Early Cretaceous (98.79 Ma)34, which is generally agreed on by Poinar et al.28 and Xing et al.49. Two parallel planes were made on the amber sample before observations. Observations and photographs were made with a Nikon SMZ1500 stereoscopic microscope at the Nanjing Institute of Geology and Palaeontology, Nanjing, China. Micro-CT was performed using a Zeiss Xradia 520 versa X-ray microscope at the Nanjing Institute of Geology and Palaeontology, Nanjing, China. The 3D reconstruction and virtual sections were generated using VGStudio MAX 3.0. All figures were organized for publication using Photoshop 7.0.
Electronic supplementary material
Acknowledgements
We appreciate Dr. Zongjun Yin and Ms. Suping Wu for help with Micro-CT, Mr. Yan Fang for help with the microscopy, and Ms. Lijun Chen for drawing the reconstruction. We appreciate Dr. Walter S. Judd for his suggestion on the possible affinity of this fossil taxon. This research is supported by the Strategic Priority Research Program (B) of Chinese Academy of Sciences (XDPB26000000), the National Natural Science Foundation of China (41688103, 91514302, 91114201) awarded to X.W. We thank the support from the State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology. We appreciate the constructive and detailed suggestions from two anonymous reviewers that made this paper much improved.
Author Contributions
D.H. and C.C. collected the specimen. X.W. initiated the study. Z.-J.L., X.W. drafted the manuscript. D.H., C.C., Z.-J.L., X.W. modified and finalized the manuscript.
Data Availability
The holotype (PB22841) is accessible in the palaeobotanical collection of the Nanjing Institute of Geology and Palaeontology, Chines Academy of Sciences, 39 Beijing Dong Road, Nanjing 210008, China.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35100-4.
References
- 1.Grimaldi DA, Engel MS, Nascimbene PC. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. American Museum Novitates. 2002;3361:1–77. doi: 10.1206/0003-0082(2002)361<0001:FCAFMB>2.0.CO;2. [DOI] [Google Scholar]
- 2.Poinar GO, Jr., Chambers KL. Palaeoanthella huangii gen. and sp. nov., an early Cretaceous flower (Angiospermae) in Burmese amber. Sida. 2005;21:2087–2092. [Google Scholar]
- 3.Cai C, Hsiao Y, Huang D. A new genus and species of polypore fungus beetle in Upper Cretaceous Burmese amber (Coleoptera, Tetratomidae, Eustrophinae) Cretaceous Research. 2016;60:275–280. doi: 10.1016/j.cretres.2015.12.010. [DOI] [Google Scholar]
- 4.Cai C, Huang D. A new genus of whip-scorpions in Upper Cretaceous Burmese amber: Earliest fossil record of the extant subfamily Thelyphoninae (Arachnida: Thelyphonida: Thelyphonidae) Cretaceous Research. 2017;69:100–105. doi: 10.1016/j.cretres.2016.09.004. [DOI] [Google Scholar]
- 5.Cai C, Huang D. The first Mesozoic palmetto beetle (Coleoptera: Smicripidae) in Upper Cretaceous Burmese amber. Cretaceous Research. 2016;64:45–49. doi: 10.1016/j.cretres.2016.04.001. [DOI] [Google Scholar]
- 6.Cai C, Huang D. Cretoleptochromus archaicus gen. et sp. nov., a new genus of ant-likestone beetles in Upper Cretaceous Burmese amber (Coleoptera, Staphylinidae, Scydmaeninae) Cretaceous Research. 2016;63:7–19. doi: 10.1016/j.cretres.2016.02.016. [DOI] [Google Scholar]
- 7.Huang D, et al. The first araripeneurine antlion in Burmese amber (Neuroptera: Myrmeleontidae) Cretaceous Research. 2016;63:1–6. doi: 10.1016/j.cretres.2016.02.010. [DOI] [Google Scholar]
- 8.Huang D, et al. A new genus of alderflies (Megaloptera: Sialidae) in Upper Cretaceous Burmese amber. Cretaceous Research. 2016;64:7–11. doi: 10.1016/j.cretres.2016.03.012. [DOI] [Google Scholar]
- 9.Azar D, Hakim M, Huang D. A new compsocid booklouse from the Cretaceous amber of Myanmar (Psocodea: Troctomorpha: Amphientometae: Compsocidae) Cretaceous Research. 2016;68:28–33. doi: 10.1016/j.cretres.2016.08.003. [DOI] [Google Scholar]
- 10.Engel MS, Huang D, Breitkreuz LCV, Cai C, Alvarado M. Two new species of mid-Cretaceous webspinners in amber from northern Myanmar (Embiodea: Clothodidae, Oligotomidae) Cretaceous Research. 2016;58:118–124. doi: 10.1016/j.cretres.2015.10.007. [DOI] [Google Scholar]
- 11.Engel MS, et al. A new twisted-wing parasitoid from mid-Cretaceous amber of Myanmar (Strepsiptera) Cretaceous Research. 2016;58:160–167. doi: 10.1016/j.cretres.2015.10.008. [DOI] [Google Scholar]
- 12.Engel, M. S. et al. An apterous scelionid wasp in mid-Cretaceous Burmese amber (Hymenoptera: Scelionidae). Comptes Rendus Palevol (2016c).
- 13.Engel MS, Huang D, Alqarni AS, Cai C. An unusual new lineage of sawflies (Hymenoptera) in Upper Cretaceous amber from northern Myanmar. Cretaceous Research. 2016;60:281–286. doi: 10.1016/j.cretres.2015.12.014. [DOI] [Google Scholar]
- 14.Engel MS, Huang D, Alqarni AS, Cai C. A remarkable evanioid wasp in mid-Cretaceous amber from northern Myanmar (Hymenoptera: Evanioidea) Cretaceous Research. 2016;60:121–127. doi: 10.1016/j.cretres.2015.11.012. [DOI] [Google Scholar]
- 15.Rasnitsyn AP, Poinar GO, Jr., Brown A. Bizarre wingless parasistic wasp from mid-Cretaceous Burmese amber (Hymenoptera, Ceraphrononidea, Aptenoperissidae fam. nov.) Cretaceous Research. 2016;69:113–118. doi: 10.1016/j.cretres.2016.09.003. [DOI] [Google Scholar]
- 16.Poinar G., Jr. A mid-Cretaceous Lauraceae flower, Cascolaurus burmitis gen. & sp. nov., in Myanmar amber. Cretaceous Research. 2017;71:96–101. doi: 10.1016/j.cretres.2016.11.015. [DOI] [Google Scholar]
- 17.Poinar G, Jr, Lambert JB, Wu Y. Araucarian source of fossiliferous Burmese amber: spectroscopic and anatomical evidence. Journal of the Botanical Research Institute of Texas. 2007;1:449–455. [Google Scholar]
- 18.Poinar GO, Jr., Chambers KL, Buckley R. Eoëpigynia burmensis gen. and sp. nov., an Early Cretaceous eudicot flower (Angiospermae) in Burmese amber. Journal of the Botanical Research Institute of Texas. 2007;1:91–96. [Google Scholar]
- 19.Poinar GO, Jr., Chambers K, Buckley R. An early Cretaceous angiosperm fossil of possible significance in rosid floral diversification. Journal of the Botanical Research Institute of Texas. 2008;2:1183–1192. [Google Scholar]
- 20.Poinar GO, Jr., Chambers KL, Wunderlich J. Micropetasos, a new genus of angiosperms from mid-Cretaceous Burmese amber. Journal of the Botanical Research Institute of Texas. 2013;7:745–750. [Google Scholar]
- 21.Poinar GO, Jr., Buckley R, Chen H. A primitive mid-Cretaceous angiosperm flower, Antiquifloris latifibris gen. & sp. nov., in Myanmar amber. Journal of the Botanical Research Institute of Texas. 2016;10:155–162. [Google Scholar]
- 22.Engel MS, Huang D. A new crown wasp in Cretaceous amber from Myanmar (Hymenoptera: Stephanidae) Cretaceous Research. 2017;69:56–61. doi: 10.1016/j.cretres.2016.08.014. [DOI] [Google Scholar]
- 23.Lü, L., Cai, C. & Huang, D. The earliest oxyteline rove beetle in amber and its systematic implications (Coleoptera: Staphylinidae: Oxytelinae). Cretaceous Research (2017).
- 24.Chambers KL, Poinar GO, Jr., Buckley R. Tropidogyne, a new genus of Early Cretaceous Eudicots (Angiospermae) from Burmese amber. Novon. 2010;20:23–29. doi: 10.3417/2008039. [DOI] [Google Scholar]
- 25.Poinar GO, Chambers KL, Buckley R. Eoëpigynia burmensis gen. et sp. nov., an Early Cretaceous eudicot flower (Angiospermae) in Burmese amber. Journal of Botanical Research Institute Texas. 2007;1:91–96. [Google Scholar]
- 26.Friis Else Marie, Pedersen Kaj Raunsgaard, Crane Peter R. The emergence of core eudicots: new floral evidence from the earliest Late Cretaceous. Proceedings of the Royal Society B: Biological Sciences. 2016;283(1845):20161325. doi: 10.1098/rspb.2016.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manchester SR, Dilcher DL, Judd WS, Corder B, Basinger JF. Early eudicot flower and fruit: Dakotanthus gen. nov. from the Cretaceous Dakotat Formation of Kansas and Nebraska, USA. Acta Palaeobotanica. 2018;58:27–40. doi: 10.2478/acpa-2018-0006. [DOI] [Google Scholar]
- 28.Poinar GO, Chambers KL. Tropidogyne pentaptera, sp. nov., a new mid-Cretaceous fossil angiosperm flower in Burmese amber. Palaeodiversity. 2017;10:135–140. doi: 10.18476/pale.v10.a10. [DOI] [Google Scholar]
- 29.Basinger JF, Dilcher DL. Ancient bisexual flowers. Science. 1984;224:511–513. doi: 10.1126/science.224.4648.511. [DOI] [PubMed] [Google Scholar]
- 30.Friis EM, Crane PR, Pedersen KR. The early flowers and angiosperm evolution. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 31.Foster CSP, et al. Evaluating the impact of genomic data and priors on Bayesian estimates of the angiosperm evolutionary timescale. Systematic Biology. 2016;66:338–351. doi: 10.1093/sysbio/syw086. [DOI] [PubMed] [Google Scholar]
- 32.Barba-Montoya, J., Reis, M. d., Schneider, H., Donoghue, P. C. J. & Yang, Z. Constraining uncertianty in the timescale of angiosperm evolution and veracity of a Cretaceous Terrestrial Revolution. New Phytologist (2018). [DOI] [PMC free article] [PubMed]
- 33.Zeng L, et al. Resolution of deep eudicot phylogeny and their temporal diversification using nuclear genes from transcriptomic and genomic datasets. New Phytologist. 2017;214:1338–1354. doi: 10.1111/nph.14503. [DOI] [PubMed] [Google Scholar]
- 34.Shi G, et al. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretaceous Research. 2012;37:155–163. doi: 10.1016/j.cretres.2012.03.014. [DOI] [Google Scholar]
- 35.APG. APG IV: An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society181, 1–20 (2016).
- 36.Xu Zhenghao, Deng Meihua. Identification and Control of Common Weeds: Volume 2. Dordrecht: Springer Netherlands; 2017. [Google Scholar]
- 37.Stevens, P. F. Angiosperm Phylogeny Website. Version 14, http://www.mobot.org/MOBOT/research/APweb/ (2018).
- 38.Scott RW, Formolo M, Rush N, Owens JD, Oboh-Ikuenobe F. Upper Albian OAE 1d event in the Chihuahua Trough, New Mexico, USA. Cretaceous Research. 2013;46:136–150. doi: 10.1016/j.cretres.2013.08.011. [DOI] [Google Scholar]
- 39.Prasad V, et al. Late Cretaceous origin of the rice tribe provides evidence for early diversification in Poaceae. Nature Communication. 2011;2:480. doi: 10.1038/ncomms1482. [DOI] [PubMed] [Google Scholar]
- 40.Wilf P, Carvalho MR, Gandolfo MA, Cúneo NR. Eocene lantern fruits from Gondwanan Patagonia and the early origins of Solanaceae. Science. 2017;355:71–75. doi: 10.1126/science.aag2737. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z-J, Wang X. Yuhania: A unique angiosperm from the Middle Jurassic of Inner Mongolia, China. Historical Biology. 2017;29:431–441. doi: 10.1080/08912963.2016.1178740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z-J, Wang X. A perfect flower from the Jurassic of China. Historical Biology. 2016;28:707–719. doi: 10.1080/08912963.2015.1020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han G, et al. A whole plant herbaceous angiosperm from the Middle Jurassic of China. Acta Geologica Sinica. 2016;90:19–29. doi: 10.1111/1755-6724.12983. [DOI] [Google Scholar]
- 44.Wang X, Wang S. Xingxueanthus: an enigmatic Jurassic seed plant and its implications for the origin of angiospermy. Acta Geologica Sinica. 2010;84:47–55. doi: 10.1111/j.1755-6724.2010.00169.x. [DOI] [Google Scholar]
- 45.Wang X. Schmeissneria: An angiosperm from the Early Jurassic. Journal of Systematics and Evolution. 2010;48:326–335. doi: 10.1111/j.1759-6831.2010.00090.x. [DOI] [Google Scholar]
- 46.Hochuli PA, Feist-Burkhardt S. Angiosperm-like pollen and Afropollis from the Middle Triassic (Anisian) of the Germanic Basin (Northern Switzerland) Frontiers in Plant Science. 2013;4:344. doi: 10.3389/fpls.2013.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hochuli PA, Feist-Burkhardt S. A boreal early cradle of angiosperms? angiosperm-like pollen from the Middle Triassic of the Barents Sea (Norway) Journal of Micropalaeontology. 2004;23:97–104. doi: 10.1144/jm.23.2.97. [DOI] [Google Scholar]
- 48.Cornet B, Habib D. Angiosperm-like pollen from the ammonite-dated Oxfordian (Upper Jurassic) of France. Review of Palaeobotany and Palynology. 1992;71:269–294. doi: 10.1016/0034-6667(92)90167-F. [DOI] [Google Scholar]
- 49.Xing, L. et al. A mid-Cretaceous embryonic-to-neonate snake in amber from Myanmar. Science Advances4 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The holotype (PB22841) is accessible in the palaeobotanical collection of the Nanjing Institute of Geology and Palaeontology, Chines Academy of Sciences, 39 Beijing Dong Road, Nanjing 210008, China.