Abstract
Despite reported evidence on the relationship between higher serum aluminum levels and poor outcomes in patients on chronic hemodialysis (CHD), the acceptable cutoff value of serum aluminum for mortality remains unclear. A retrospective observational cohort study with 636 Taiwanese patients on CHD was conducted to investigate the impact of serum aluminum levels on mortality. The predictors were bivariate serum aluminum level (<6 and ≥6 ng/mL) and the Outcomes were all-cause and cardiovascular (CV) mortality. During the mean follow-up of 5.3 ± 2.9 years, 253 all-cause and 173 CV deaths occurred. Crude analysis showed that a serum aluminum level of ≥6 ng/mL was a significant predictor of all-cause [hazard ratio (HR), 1.80; 95% confidence interval (CI), 1.40–2.23] and CV (HR, 1.84; 95% CI, 1.36–2.50) mortality. After multivariable adjustment, the serum aluminum level of ≥6 ng/mL remained a significant predictor of all-cause mortality (HR, 1.37, 95% CI, 1.05–1.81) but became insignificant for CV mortality (HR, 1.29; 95% CI, 0.92–1.81). Therefore, our study revealed that a serum aluminum level of ≥6 ng/mL was independently associated with all-cause death in patients on CHD, suggesting that early intervention for aluminum level in patients on CHD might be beneficial even in the absence of overt aluminum toxicity.
Introduction
Identifying risk factors for mortality may help in early intervention approaches to improve the survival of patients on chronic hemodialysis (CHD) who have a substantially reduced life expectancy1,2. Controlling aluminum levels is an important issue for patients with chronic kidney disease (CKD) because systemic aluminum toxicity is harmful3. Moreover, an elevated serum aluminum level can lead to dialysis dementia4, osteomalacia, a very low bone turnover rate with marked accumulation of unmineralized osteoid5, iron-resistant microcytic anemia6 and cardiomegaly7 in dialysis patients.
Currently, severe aluminum toxicity (serum aluminum level >200 ng/mL) in patients on CHD is uncommon8,9 due to the removal of aluminum from water used for dialysis by reverse osmosis and deionization as well as the use of widely available nonaluminum-containing phosphate binders. However, controlling serum aluminum levels remains an important issue for patients on CHD. Aluminum removal by dialysis is not efficient, and the possible source of aluminum accumulation in patients on CHD is oral (aluminum-containing phosphate binders and antacids) and injectable medications (calcitriol, vitamins B complex, iron and erythropoietin) that are commonly administered to dialysis patients10,11. Therefore, the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines12 recommend that the baseline serum aluminum level should be below 20 ng/mL and that aluminum levels and risk for aluminum toxicity should be assessed at least once per year.
Chazan et al. demonstrated that elevated aluminum levels are associated with mortality in a study on 10646 patients on maintenance CHD. The annual mortality rate was 18% higher for patients with serum aluminum levels between 40.9 and 59.8 ng/mL and progressively increased to 60% higher for those with aluminum levels above 199.7 ng/mL than for those with levels below 38.9 ng/mL13. One recent study reported that patients on CHD with serum aluminum levels more than 9 ng/mL had significantly poorer outcomes than those with levels below 6 ng/mL over a year of observation14, demonstrating that aluminum, even within an apparently acceptable range (i.e., <20 ng/mL), is also associated with increased mortality in patients on CHD. However, a significant difference between the groups with serum aluminum levels below 6 ng/mL and 6–9 ng/mL was not observed in that study14. Moreover, the evidence on the serum aluminum cutoff value to assess its clinical significance in association with mortality in patients on CHD remains unclear due to the insufficient observation time and insufficient adjustment for risk factors.
Therefore, we conducted a retrospective observational cohort study to test the effect of a serum aluminum cutoff value of 6 ng/mL (upper normal limit) on all-cause and cardiovascular mortality in patients on CHD.
Methods
Study design and patients
This retrospective observational cohort study was conducted at a single medical center, Shin Kong Wu Ho-Su Memorial Hospital, and used the medical records of patients undergoing hemodialysis from December 2006 to December 2012. Medical records of only those patients who were receiving regular hemodialysis for at least 3 months before data collection and those who were clinically stable without hospitalization during the 3 months preceding data collection were included. Initially, 805 patients were eligible for this study. Furthermore, the medical records of patients with unavailable serum aluminum levels were excluded. Finally, the records of 636 patients on CHD were selected. Patient outcomes were observed until December 2015. Patients who died at the hospital during follow-up were identified from the discharge diagnosis and death certificates in hospital charts, which the causes of death were classified into CV event, cerebrovascular event, gastrointestinal bleeding event, and unknown etiology by the attending physician of nephrologist. Patients who were transferred to other dialysis centers, switched to peritoneal dialysis, or received renal transplantation were censored.
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Shin Kong Wu Ho-Su Memorial Hospital. Informed consent was waived because the study was based on a medical chart review. Patient information was protected by anonymization and de-identification prior to analysis.
Demographic and laboratory data
Demographic and laboratory data were obtained from the medical records and included age; sex; hemodialysis vintage; cardiothoracic ratio (CTR); levels of aluminum, blood urea nitrogen, serum creatinine, albumin, uric acid, total cholesterol, triglycerides, hemoglobin, intact parathyroid hormone, ionized calcium, serum phosphate, and alkaline phosphatase; iron profile; urea kinetics; history of diabetes mellitus (DM), hypertension, coronary artery disease, or cerebrovascular disease; and prescription of renin–angiotensin system blockers, lipid-lowering agents, beta-blockers, and antiplatelet agents. Coronary artery disease was defined as a history of exertional angina, significant arterial occlusive disease disclosed by an angiogram, past myocardial infarction, coronary artery bypass surgery, or angioplasty. Cerebrovascular disease was defined as a history of cerebrovascular accidents, either hemorrhage or infarction. CVD was diagnosed based on a documented history of coronary artery or cerebrovascular disease. Blood samples were collected before, following an at least 8-h fast, and immediately after the dialysis session. The blood samples post dialysis were used to assess the urea kinetics. Biochemical analyses were conducted using standard commercially available assays and automated test machines (Beckman Coulter, Lane Cove, NSW, Australia). Intact parathyroid hormone levels were measured using the Roche Elecsys assay (Roche Diagnostics, Basel, Switzerland). Aluminum levels were measured by graphite furnace atomic absorption spectrometry using GBC 906AA (Braeside VIC, Australia).
Statistical analysis
Data are presented as mean ± standard deviation or median with interquartile range as appropriate for continuous data and number (%) for categorical data. Student’s t-test was used to compare the means of continuous variables, and the χ2 test was used for categorical variables. Continuous aluminum levels were natural-log transformed (ln) to approximate a normal distribution. Linear regression analyses were performed using ln(aluminum) as the dependent variable. Variables were chosen into the multivariable analysis by stepwise methods; P-values by F-statistic for entry and removal were <0.05 and >0.10, respectively. Moreover, serum aluminum levels were categorized into two groups based on the cutoff of 6 ng/mL. Survival curves were estimated using the Kaplan–Meier method and tested by the log-rank test. Cox proportional regression model was used to determine the risk of death. The assumption of proportionality was not violated by testing for the interaction between time and variables. Additionally, subgroup analyses were performed for the following variables: DM, age (≤60 and >60 years), sex, previous CVD, and ionized calcium level (≤4.5 and >4.5 mg/dL). A two-tailed P-value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS for Windows version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The mean age, length of follow-up, and mean HD vintage among the entire cohort of 636 patients on CHD were 62.8 ± 13.2 years, 5.3 ± 2.9 years, and 5.0 ± 4.7 years, respectively. Of the total, 47.8% were males and 38.5%, 39.9%, and 27.6% had DM, hypertension, and CVD, respectively (Table 1). Right-tail distribution of serum aluminum level was observed in the entire cohort (Fig. 1). Table 1 lists the demographic and clinical data of the participants stratified by the serum aluminum level (<6 and ≥6 ng/mL). Those with higher aluminum levels were older and predominantly females. Moreover, those with serum aluminum levels of ≥6 ng/mL had significantly higher alkaline phosphatase levels and CTR and lower creatinine, uric acid and albumin levels; the frequencies of antiplatelet and lipid-lowering agent prescriptions were also higher in this group.
Table 1.
Baseline characteristics of the study population.
| Characteristic | All (n = 636) | Al < 6 ng/mL (n = 322) | Al ≥ 6 ng/mL (n = 314) | P |
|---|---|---|---|---|
| Aluminum (ng/mL) | 7.7 ± 7.6 | 3.7 ± 1.3 | 11.7 ± 9.1 | NA |
| Age (years) | 62.8 ± 13.2 | 61.6 ± 14.1 | 64.0 ± 12.1 | 0.021 |
| Male sex | 304 (47.8) | 172 (53) | 132 (42) | 0.004 |
| Duration of dialysis (years) | 5.0 ± 4.7 | 4.6 ± 4.7 | 5.3 ± 4.8 | 0.078 |
| Diabetes mellitus | 244 (38.5) | 114 (35) | 130 (41) | 0.143 |
| Cardiovascular disease | 175 (27.6) | 87 (27) | 88 (28) | 0.832 |
| Hypertension | 254 (39.9) | 121 (37) | 133 (42) | 0.218 |
| Blood nitrogen (mg/dL) | 69 ± 18 | 68 ± 17 | 69 ± 19 | 0.576 |
| Creatinine (mg/dL) | 9.5 ± 2.3 | 9.8 ± 2.2 | 9.3 ± 2.3 | 0.001 |
| Uric acid (mg/dL) | 6.6 ± 2.3 | 6.9 ± 1.9 | 6.3 ± 2.6 | 0.001 |
| Albumin (g/dL) | 4.1 ± 0.4 | 4.2 ± 0.4 | 4.1 ± 0.4 | 0.031 |
| Triglyceride (mg/dL) | 163 ± 149 | 155 ± 141 | 171 ± 157 | 0.180 |
| Cholesterol (mg/dL) | 175 ± 44 | 173 ± 40 | 177 ± 47 | 0.252 |
| Kt/V | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.429 |
| Hemoglobin (g/dL) | 10.4 ± 1.4 | 10.4 ± 1.4 | 10.3 ± 1.5 | 0.601 |
| Transferrin saturation (%) | 35.3 ± 15.2 | 36.2 ± 16.1 | 34.3 ± 14.2 | 0.116 |
| Ionized calcium (mg/dL) | 4.6 ± 0.4 | 4.6 ± 0.4 | 4.6 ± 0.4 | 0.123 |
| Phosphate (mg/dL) | 5.2 ± 1.4 | 5.1 ± 1.4 | 5.2 ± 1.4 | 0.397 |
| Alkaline phosphatase (U/L) | 98 ± 70 | 90 ± 57 | 107 ± 80 | 0.002 |
| iPTH (pg/mL) | 163 ± 199 | 158 ± 221 | 168 ± 174 | 0.510 |
| Cardiothoracic ratio (%) | 50.6 ± 6.7 | 49.3 ± 7.0 | 51.9 ± 6.2 | <0.001 |
| Medications | ||||
| Antiplatelet agents | 220 (34.8) | 99 (31) | 121 (38) | 0.044 |
| RAS blockaders | 219 (31.8) | 119 (37) | 100 (31) | 0.149 |
| Beta-blocker | 116 (18.3) | 56 (17) | 60 (19) | 0.613 |
| Lipid-lowering agents | 122 (19.2) | 50 (15.6) | 72 (22.9) | 0.020 |
Values are expressed as number (%) of patients or as mean ± standard deviation Abbreviations: Al, aluminum; Kt/V, urea kinetics; iPTH, intact parathyroid hormone; NA, not available; RAS, renin–angiotensin system.
Figure 1.
The distribution of serum aluminum levels in patients on chronic hemodialysis.
Factors significantly associated with serum aluminum levels in patients on CHD
As summarized in Table 2, older age, female sex, longer HD vintage, higher ionized calcium and alkaline phosphatase levels, lower creatinine level, and higher CTR were significantly associated with higher ln(aluminum) levels in patients on CHD. Moreover, multiple linear regression analysis with a stepwise method for parameter selection demonstrated that ln(aluminum) levels exhibited a significantly positive association with HD duration, ionized calcium and alkaline phosphatase levels, and CTR.
Table 2.
Determinants of serum log (aluminum level) by simple linear regression analysis.
| Parameter | Crude | Multivariable | ||
|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | |
| Age (per year) | 0.005 (0.001, 0.009) | 0.029 | ||
| Male versus female | −0.182 (−0.29, −0.072) | 0.001 | ||
| Duration of dialysis (per year) | 0.019 (0.008, 0.031) | 0.001 | 0.012 (0.001, 0.025) | 0.045 |
| Diabetes mellitus | 0.047 (−0.067, 0.160) | 0.419 | ||
| Cardiovascular disease | 0.088 (−0.035, 0.211) | 0.160 | ||
| Hypertension | 0.069 (−0.044, 0.181) | 0.232 | ||
| Creatinine (mg/dL) | −0.030 (−0.054, 0.006) | 0.015 | ||
| Uric acid (mg/dL) | ||||
| Hemoglobin (per g/dL) | 0.006 (−0.032, 0.043) | 0.769 | ||
| Albumin (per g/dL) | −0.118 (−0.256, 0.020) | 0.094 | ||
| Kt/V (per unit) | 0.082 (−0.161, 0.325) | 0.508 | ||
| Ionized calcium (per mg/dL) | 0.186 (0.067, 0.305) | 0.002 | 0.135 (0.010, 0.261) | 0.035 |
| Phosphate (per mg/dL) | 0.023 (−0.015, 0.061) | 0.238 | ||
| ALK-P (per 10 U/L) | 0.016 (0.008, 0.024) | <0.001 | 0.015 (0.006, 0.023) | 0.001 |
| iPTH (per 10 pg/mL) | 0.002 (0.000, 0.005) | 0.087 | ||
| Cardiothoracic ratio (per 1%) | 0.021 (0.013, 0.029) | <0.001 | 0.019 (0.011, 0.027) | <0.001 |
Abbreviations: CI, confidence interval; Kt/V, urea kinetics; ALK-P, alkaline phosphatase; iPTH, intact parathyroid hormone.
All-cause mortality in patients on CHD
On follow-up, there were 253 (39.7%) deaths due to all causes, including 173 fatal CV events, 33 infectious diseases, 13 malignancies, 9 cerebrovascular events, 7 gastrointestinal bleeding events, and 18 deaths due to unknown etiology. Figure 2A shows the Kaplan–Meier survival curves of all-cause mortality according to the bivariate aluminum levels (<6 and ≥6 ng/mL). The difference in survival among the two groups was significant for all-cause mortality (χ2 = 20.4; P < 0.001). Results of the Cox proportional hazards regression analysis are shown in Table 2. The crude hazard ratio (HR) of the bivariate aluminum levels (<6 and ≥6 ng/mL) for all-cause mortality was 1.80 [95% confidence interval (CI), 1.40–2.32]. After multivariable adjustment, the bivariate aluminum levels (<6 and ≥6 ng/mL) remained a significant predictor of mortality (HR, 1.37; 95% CI, 1.05–1.81). Moreover, the continuous ln(aluminum) level was a significant predictor of all-cause mortality in both crude and multivariable analyses.
Figure 2.
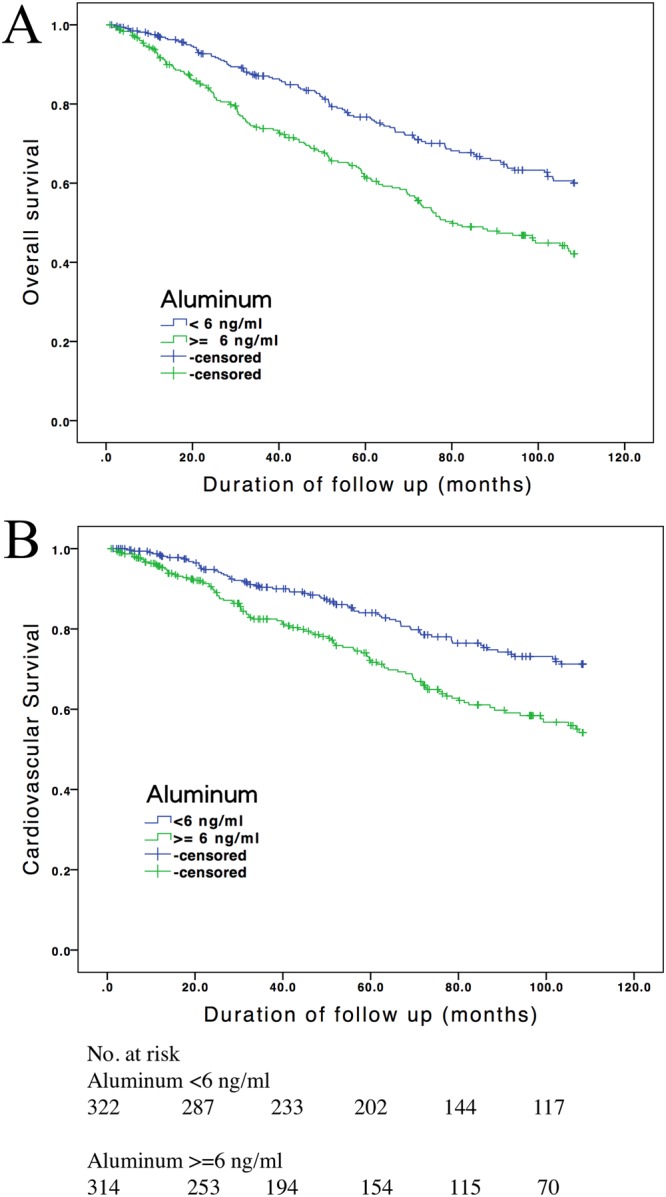
Probabilities of survival according to serum aluminum levels (<6 and ≥6 ng/mL) (A) in all-cause mortality with a log-rank test (χ2 = 20.4; P < 0.001) and (B) in cardiovascular mortality with a log-rank test (χ2 = 15.1; P < 0.001).
Cardiovascular mortality in patients on CHD
There were 173 fatal CV events during the observation period of this study. Figure 2B shows the Kaplan–Meier survival curves of CV mortality according to the bivariate aluminum levels (<6 and ≥6 ng/mL). The difference in survival among the two groups was significant for all-cause mortality (χ2 = 15.1; P < 0.001). As presented in Table 3, the crude HR of the bivariate aluminum levels (<6 and ≥6 ng/mL) for CV mortality was 1.84 (95% CI, 1.36–2.50). After multivariable adjustment, HR (1.29; 95% CI, 0.92–1.81) became an insignificant predictor of CV mortality. The predictability for CV mortality of continuous ln (aluminum) level followed the same pattern as bivariate aluminum levels. However, the HRs of the bivariate aluminum levels (1.50; 95% CI, 1.07–2.09) and continuous ln (aluminum) (1.28; 95% CI, 1.03–1.60) for CV mortality became significant in the full multivariable model without adjusting the parameter of CTR.
Table 3.
Multivariable Cox regression analysis of risk factor for mortality.
| Aluminum cutoff value of 6 ng/mL | Every 1 increment in ln(Aluminum) | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| All-cause mortality | ||||
| Crude | 1.80 (1.40–2.32) | <0.001 | 1.48 (1.25–1.74) | <0.001 |
| Mode 1 | 1.79 (1.39–2.31) | <0.001 | 1.45 (1.23–1.71) | <0.001 |
| Mode 2 | 1.55 (1.19–2.02) | 0.001 | 1.34 (1.12–1.59) | <0.001 |
| Mode 3 | 1.37 (1.05–1.81) | 0.023 | 1.25 (1.04–1.50) | 0.015 |
| Mode 4 | 1.56 (1.19–2.04) | 0.001 | 1.37 (1.15–1.64) | <0.001 |
| Cardiovascular mortality | ||||
| Crude | 1.84 (1.36–2.50) | <0.001 | 1.47 (1.21–1.79) | <0.001 |
| Mode 1 | 1.86 (1.36–2.53) | <0.001 | 1.46 (1.20–1.78) | <0.001 |
| Mode 2 | 1.60 (1.16–2.20) | 0.003 | 1.35 (1.10–1.67) | 0.003 |
| Mode 3 | 1.29 (0.92–1.81) | 0.127 | 1.18 (0.94–1.48) | 0.137 |
| Mode 4 | 1.50 (1.07–2.09) | 0.016 | 1.28 (1.03–1.60) | 0.023 |
Multivariate model 1 is adjusted for age, sex, and hemodialysis vintage. Multivariate model 2 comprises model 1 as well as adjustments for diabetes mellitus, cardiovascular disease, and CTR. Multivariate model 3 comprises model 2 as well as adjustments for levels of creatinine, albumin, hemoglobin, ionized calcium, phosphate, alkaline phosphatase, and transferrin saturation; KT/V; as well as prescriptions of antiplatelet medications, renin–angiotensin system blockers, beta-blockers, and lipid-lowering agents. Multivariate model 4 comprises model 3 without CTR Abbreviations: CI, confidence interval; CTR, cardiothoracic ratio; Kt/V, urea kinetics; ln, natural log transformation.
Subgroup analysis
We also analyzed the association of the bivariate aluminum levels (<6 and ≥6 ng/mL) with all-cause and CV mortality stratified by covariates, including a history of DM or CVD, age (≤60 and >60 years), sex, and ionized calcium level (≤4.5 and >4.5 mg/dL). As shown in Fig. 3, after multivariable adjustment, the bivariate aluminum levels (<6 and ≥6 ng/mL) were significantly predictive of both all-cause and CV mortality in those with a CVD history, ionized calcium level of ≤4.5 ng/dL, and no history of DM.
Figure 3.
Subgroup analysis of the effect of serum aluminum levels (<6 and ≥6 ng/mL) on (A) all-cause mortality and (B) cardiovascular mortality among patients on chronic hemodialysis. The full model comprised adjusted variables including age; sex; hemodialysis vintage; diabetes mellitus; cardiovascular disease; cardiothoracic ratio; levels of creatinine, albumin, hemoglobin, ionized calcium, phosphate, and alkaline phosphatase; transferrin saturation; urea kinetics; as well as prescription of antiplatelet agents, renin–angiotensin blockers, beta-blockers, and lipid-lowering agents.
Discussion
In this study on 636 patients on CHD with the longest follow-up being 9 years, we found that a higher serum aluminum level (≥6 ng/mL) was independently associated with higher all-cause mortality after adjusting for potential confounders. Moreover, we found a linear association of ln(aluminum) level with all-cause death in patients on CHD. Interestingly, serum aluminum level was not independently associated with CV mortality and it might mediate the CV outcomes though the CTR level in patients on CHD. These findings expand our understanding of the association of serum aluminum levels with adverse outcomes in patients on CHD and emphasize that clinicians should be vigilant of potential early events even in patients in whom serum aluminum levels are within the acceptable range (below 20 ng/mL), as suggested by the KDOQI, and adopt early intervention approaches to prevent further aluminum accumulation in the body.
Aside from acute toxicity, aluminum accumulation will lead to gradual cellular damage. Aluminum has been reported to accumulate in all tissues in animals, preferentially in the liver, heart, bone, and brain15. Previous studies have provided several potential possible mechanisms to explain the association of serum aluminum levels with mortality in patients on CHD. One such mechanism is cellular oxidative stress induced by aluminum16,17, which may cause anemia and atherosclerosis in humans18, consequently increasing mortality in dialysis patients19,20. Another potential mechanism is chronic inflammation, as demonstrated by enhanced proinflammatory and proapoptotic gene expression in human brain cells exposed to aluminum sulfate21 and by the association of inflammatory markers with plasma aluminum levels in individuals with asthma22. Consequently, inflammation was found to be associated with an increased risk of mortality in patients on CHD23,24. Another mechanism that may underlie aluminum-associated mortality is protein–energy wasting induced by aluminum25. Hypoalbuminemia is recognized as a strong predictor of mortality in the CKD population26. The final consideration is the hazardous effect of aluminum in cardiac remodeling7,27, which the cardiotoxicity of aluminum might be attributable to oxidative stress and dysregulation of the intracellular redox system18. Some reports have found a significant association between heart damage and aluminum levels28,29. Undoubtedly, CVD is the leading cause of death in patients on CHD30. Taken together, these mechanisms might explain the significant association of serum aluminum levels with mortality of patients on CHD found in the current study. However, concise elucidation of the underlying mechanisms requires further investigations.
Adjustment for potential confounders by multiple linear regression analysis in the current study revealed that ln(aluminum) level was positively associated with HD duration, ionized calcium and alkaline phosphatase levels, and CTR. Patients with longer dialysis duration are expected to have higher aluminum levels owing to challenges in the efficient removal of aluminum by dialysis, leading to progressive accumulation over time. Aluminum will block calcium entry into the bone, consequently inducing osteomalacia5. Thereafter, hyperparathyroidism might be a mechanism to protect against aluminum-induced osteomalacia, perhaps by increasing bone turnover with elevated alkaline phosphatase levels31. Moreover, studies found evidence for aluminum-induced cardiac injury28 and cardiac complications29; higher aluminum loading in CHD patients was associated with cardiomegaly7 and cardiothoracic ratio (>0.5)27; cardiotoxicity of aluminum might be attributable to oxidative stress and dysregulation of the intracellular redox system18. Such clinical findings in previous studies can support our data.
An interesting finding from our data was that the higher serum aluminum level was not significantly associated with CV death in patients on CHD after adjusting the confounders but the association became significant after removing the parameter of CTR. Therefore, one hypothesis was made that higher serum aluminum level may contribute to the CV death through the higher CTR level, which a well-documented risk factor for poor prognosis in chronic dialysis20,32–37.
In subgroup analysis, aluminum levels had a significant predictive power for all-cause and CV mortality in patients with ionized calcium levels of ≤4.5 mg/dL, history of DM, and history of CVD. DM is a strong predictor of mortality in patients on CHD, which might negate the impact of aluminum levels on outcomes. A previous in vitro study indicated that aluminum inhibits the regeneration of reduced glutathione, thereby leading to oxidative damage16, which may induce atherosclerosis in humans18. Moreover, aluminum is harmful to cardiomyocytes. Such evidence supports our finding that patients with no history of CVD and those with lower calcium levels are more vulnerable to aluminum toxicity than those without.
The findings of the current study highlight the need for the close monitoring of aluminum levels in patients on CHD, even in those with aluminum levels below 20 ng/mL, as recommended by the KDOQI. Certain products containing aluminum that are commonly administered to dialysis patients include aluminum-containing phosphate binders and antacids, iron- and calcium-containing medications, calcitriol, vitamin B complex, erythropoietin, and insulin10,11,38,39. The first step in the prevention of aluminum toxicity is minimizing exposure to these medications, particularly aluminum-containing phosphate binders. Moreover, intensive hemodialysis (six times per week for 4–6 weeks) with a high-flux artificial kidney can efficiently remove aluminum12,40. Finally, the efficacy of early deferoxamine therapy still requires further evaluations to ascertain its benefits and risks. The KDOQI guidelines suggest the deferoxamine stimulation test and initiation of therapy in patients with serum aluminum levels of more than 20 ng/mL12.
Our study has several limitations. First, this was a single-center retrospective study; therefore, these findings may not be generalizable to all patient populations on CHD. Further multicenter studies as well as those including subjects from different ethnicities are needed to confirm our findings. Second, we only assessed baseline covariates to predict mortality, which might have resulted in biased estimates for associated variables that were time-varying predictors. Third, unstimulated serum aluminum level was used as the predictor, which may only reflect recent, limited exposure to aluminum and cannot provide information on the actual aluminum burden41. However, it is an easy and noninvasive approach to assess aluminum loading in patients on CHD, aside from bone biopsy and the deferoxamine stimulation test. Finally, data on the history of aluminum-containing medication exposure were unavailable in this cohort. However, we used serum aluminum level as the predictor, as higher frequency of aluminum exposure would lead to higher serum aluminum levels. Despite these limitations, the current study has several strengths. First, the follow-up period was long enough to include enough patients who reached the primary outcome. Second, adjustment was possible for several well-established factors related to mortality in dialysis.
In conclusion, we identified an independent association of a high aluminum level (≥6 ng/mL) and all-cause mortality in patients on CHD. Moreover, our results suggest a dose-dependent effect of serum aluminum levels on mortality and implicate that serum aluminum levels should be maintained as low as possible in patients on CHD. However, further study is still needed to clarify the benefits and harms of lowering serum aluminum levels in CHD patients in whom serum aluminum levels are within the acceptable range (below 20 ng/mL).
Electronic supplementary material
Acknowledgements
Support: Shin Kong Wu Ho-Su Memorial Hospital (2018SKHADR004) sponsored this study.
Author Contributions
Conceived and designed the experiments: L.-B.S., T.-M.H.; performed the experiments: F.-Y.W., T.-M.H.; wrote the manuscript: L.-B.S., T.-M.H.; analyzed the data: all authors; contributed reagents/materials/analysis tools: all authors; approved the manuscript: all authors.
Data Availability
All data generated or analysed during this study are included in this published article (Supplementary Information files: S1 dataset).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34799-5.
References
- 1.Shinzato T, et al. Report of the annual statistical survey of the Japanese Society for Dialysis Therapy in 1996. Kidney Int. 1999;55:700–712. doi: 10.1046/j.1523-1755.1999.00297.x. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz A, et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- 3.Wills MR, Savory J. Aluminum and chronic renal failure: sources, absorption, transport, and toxicity. Crit Rev Clin Lab Sci. 1989;27:59–107. doi: 10.3109/10408368909106590. [DOI] [PubMed] [Google Scholar]
- 4.Rob PM, Niederstadt C, Reusche E. Dementia in patients undergoing long-term dialysis: aetiology, differential diagnoses, epidemiology and management. CNS Drugs. 2001;15:691–699. doi: 10.2165/00023210-200115090-00003. [DOI] [PubMed] [Google Scholar]
- 5.Andress DL, Maloney NA, Endres DB, Sherrard DJ. Aluminum-associated bone disease in chronic renal failure: high prevalence in a long-term dialysis population. J Bone Miner Res. 1986;1:391–398. doi: 10.1002/jbmr.5650010503. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal Anil K. Practical Approach to the Diagnosis and Treatment of Anemia Associated With CKD in Elderly. Journal of the American Medical Directors Association. 2006;7(9):S7–S12. doi: 10.1016/j.jamda.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.London GM, et al. Association between aluminum accumulation and cardiac hypertrophy in hemodialyzed patients. Am J Kidney Dis. 1989;13:75–83. doi: 10.1016/S0272-6386(89)80120-9. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe JA, Liftman C, Glickman JD. Frequency of elevated serum aluminum levels in adult dialysis patients. Am J Kidney Dis. 2005;46:316–319. doi: 10.1053/j.ajkd.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Gault PM, Allen KR, Newton KE. Plasma aluminium: a redundant test for patients on dialysis? Ann Clin Biochem. 2005;42:51–54. doi: 10.1258/0004563053026862. [DOI] [PubMed] [Google Scholar]
- 10.Bohrer D, et al. Drugs as a hidden source of aluminium for chronic renal patients. Nephrol Dial Transplant. 2007;22:605–611. doi: 10.1093/ndt/gfl569. [DOI] [PubMed] [Google Scholar]
- 11.Bohrer D, et al. Role of medication in the level of aluminium in the blood of chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24:1277–1281. doi: 10.1093/ndt/gfn631. [DOI] [PubMed] [Google Scholar]
- 12.National Kidney, F. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis42, S1–201 (2003). [PubMed]
- 13.Chazan JA, Lew NL, Lowrie EG. Increased serum aluminum. An independent risk factor for mortality in patients undergoing long-term hemodialysis. Arch Intern Med. 1991;151:319–322. doi: 10.1001/archinte.1991.00400020075016. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CW, et al. Association of low serum aluminum level with mortality in hemodialysis patients. Ther Clin Risk Manag. 2016;12:1417–1424. doi: 10.2147/TCRM.S113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez MA, Bernal CA, Mahieu S, Carrillo MC. The interactions between the chronic exposure to Aluminum and liver regeneration on bile flow and organic anion transport in rats. Biol Trace Elem Res. 2009;127:164–176. doi: 10.1007/s12011-008-8234-4. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K, Yoshino M. Aluminum decreases the glutathione regeneration by the inhibition of NADP-isocitrate dehydrogenase in mitochondria. J Cell Biochem. 2004;93:1267–1271. doi: 10.1002/jcb.20261. [DOI] [PubMed] [Google Scholar]
- 17.Exley C. The pro-oxidant activity of aluminum. Free Radic Biol Med. 2004;36:380–387. doi: 10.1016/j.freeradbiomed.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Morena M, Delbosc S, Dupuy AM, Canaud B, Cristol JP. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. Hemodial Int. 2005;9:37–46. doi: 10.1111/j.1492-7535.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 19.Stenvinkel P. The role of inflammation in the anaemia of end-stage renal disease. Nephrol Dial Transplant. 2001;16(Suppl 7):36–40. doi: 10.1093/ndt/16.suppl_7.36. [DOI] [PubMed] [Google Scholar]
- 20.Tsai MH, Liou HH, Leu JG, Yen MF, Chen HH. Sites of peripheral artery occlusive disease as a predictor for all-cause and cardiovascular mortality in chronic hemodialysis. PLoS One. 2015;10:e0128968. doi: 10.1371/journal.pone.0128968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukiw WJ, Percy ME, Kruck TP. Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J Inorg Biochem. 2005;99:1895–1898. doi: 10.1016/j.jinorgbio.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Guo CH, Chen PC, Hsia S, Hsu GS, Liu PJ. The relationship of plasma aluminum to oxidant-antioxidant and inflammation status in asthma patients. Environ Toxicol Pharmacol. 2013;35:30–38. doi: 10.1016/j.etap.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Elewa U, et al. Cardiovascular risk biomarkers in CKD: the inflammation link and the road less traveled. Int Urol Nephrol. 2012;44:1731–1744. doi: 10.1007/s11255-012-0271-4. [DOI] [PubMed] [Google Scholar]
- 25.Krewski D, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10(Suppl 1):1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TL, Fang YW, Leu JG, Tsai MH. Association between serum aluminum levels and cardiothoracic ratio in patients on chronic hemodialysis. PLoS One. 2017;12:e0190008. doi: 10.1371/journal.pone.0190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghorbel I, et al. Effects of dietary extra virgin olive oil and its fractions on antioxidant status and DNA damage in the heart of rats co-exposed to aluminum and acrylamide. Food Funct. 2015;6:3098–3108. doi: 10.1039/c5fo00342c. [DOI] [PubMed] [Google Scholar]
- 29.Neophytou AM, et al. Ischemic Heart Disease Incidence in Relation to Fine versus Total Particulate Matter Exposure in a US Aluminum Industry Cohort. PLoS One. 2016;11:e0156613. doi: 10.1371/journal.pone.0156613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saran R, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69:A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slatopolsky E. The interaction of parathyroid hormone and aluminum in renal osteodystrophy. Kidney Int. 1987;31:842–854. doi: 10.1038/ki.1987.75. [DOI] [PubMed] [Google Scholar]
- 32.Yen TH, Lin JL, Lin-Tan DT, Hsu KH. Cardiothoracic ratio, inflammation, malnutrition, and mortality in diabetes patients on maintenance hemodialysis. Am J Med Sci. 2009;337:421–428. doi: 10.1097/MAJ.0b013e31819bbec1. [DOI] [PubMed] [Google Scholar]
- 33.Chen KH, et al. Cardiothoracic ratio, malnutrition, inflammation, and two-year mortality in non-diabetic patients on maintenance hemodialysis. Kidney Blood Press Res. 2008;31:143–151. doi: 10.1159/000127388. [DOI] [PubMed] [Google Scholar]
- 34.Chen KH, et al. Cardiothoracic ratio association with mortality in patients on maintenance peritoneal dialysis. Ther Apher Dial. 2011;15:81–88. doi: 10.1111/j.1744-9987.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- 35.Yotsueda R, et al. Cardiothoracic Ratio and All-Cause Mortality and Cardiovascular Disease Events in Hemodialysis Patients: The Q-Cohort Study. Am J Kidney Dis. 2017;70:84–92. doi: 10.1053/j.ajkd.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Ogata H, et al. The cardiothoracic ratio and all-cause and cardiovascular disease mortality in patients undergoing maintenance hemodialysis: results of the MBD-5D study. Clin Exp Nephrol. 2017;21:797–806. doi: 10.1007/s10157-017-1380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okute Y, et al. Cardiothoracic Ratio as a Predictor of Cardiovascular Events in a Cohort of Hemodialysis Patients. J Atheroscler Thromb. 2017;24:412–421. doi: 10.5551/jat.36426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinke CM, Breitkreutz J, Leuenberger H. Aluminium in over-the-counter drugs: risks outweigh benefits? Drug Saf. 2003;26:1011–1025. doi: 10.2165/00002018-200326140-00003. [DOI] [PubMed] [Google Scholar]
- 39.Gura KM. Aluminum contamination in parenteral products. Curr Opin Clin Nutr Metab Care. 2014;17:551–557. doi: 10.1097/MCO.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 40.Molitoris BA, Alfrey AC, Alfrey PS, Miller NL. Rapid removal of DFO-chelated aluminum during hemodialysis using polysulfone dialyzers. Kidney Int. 1988;34:98–101. doi: 10.1038/ki.1988.150. [DOI] [PubMed] [Google Scholar]
- 41.D’Haese PC, et al. Value of serum aluminium monitoring in dialysis patients: a multicentre study. Nephrol Dial Transplant. 1990;5:45–53. doi: 10.1093/ndt/5.1.45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (Supplementary Information files: S1 dataset).




