Abstract
Gout is a chronic disease associated with deposition of monosodium urate crystals and accompanied by diabetes, hypertension, and dyslipidemia. Hypertriglyceridemia is common among patients with gout, and fenofibrate is usually used to reduce triglyceride levels. The aim of this study is to determine the effect of uric acid reduction by fenofibrate in patients with gout administered uric acid lowering agents (viz., the xanthine oxidase inhibitors allopurinol and febuxostat). Data from 863 patients with gout were collected from electronic medical records comprising information on underlying diseases, laboratory findings, and drug histories. Among all the patients, 70 (8.11%) took fenofibrate with allopurinol or febuxostat. Male and young patients took fenofibrate more frequently, and hypertension was less frequent in patients administered xanthine oxidase inhibitors and fenofibrate than in those administered only xanthine oxidase inhibitors. After the treatment, serum uric acid levels more significantly decreased (−1.81 ± 2.41 vs. −2.40 ± 2.28 mg/dL, p = 0.043) in patients with fenofibrate cotreatment, than in those administered allopurinol or febuxostat alone. The effect of uric acid reduction was larger (b = −1.098, p < 0.001) in patients taking glucocorticoids than in those administered other treatments. There was no difference in the levels of creatinine, blood urea nitrogen, and aminotransferases between patients treated with and without fenofibrate. Fenofibrate additionally reduced uric acid levels without showing any change in the results of renal or liver function tests, suggesting that the addition of fenofibrate is a reasonable option for treating gout in patients having high triglyceride levels.
Introduction
Gout is a chronic disease caused by inflammatory responses to the deposition of monosodium urate (MSU) crystals. Elevated serum urate concentration leads to the crystallization of MSU, and hyperuricemia is an important etiologic factor in the development of gout1,2. Uric acid is the final product of metabolism of endogenous and ingested purine in humans3. Increased production or inadequate renal excretion of uric acid is the main cause of hyperuricemia, while diet and comorbidities including obesity and renal impairment are regarded as the risk factors. The manifestation consists of long asymptomatic periods, acute flares with pain and swelling of involved joints, and chronic gouty arthritis with tophi4. Uric acid lowering strategy is essential to prevent acute flares and permanent joint damage in gout, and xanthine oxidase inhibitors—allopurinol and febuxostat—are commonly used5.
These agents block xanthine oxidases that catalyze the oxidation of hypoxanthine to xanthine and xanthine to uric acid, thereby effectively reducing uric acid synthesis6. These hypouricemic agents are used in increasing doses to achieve the target uric acid level of <6.0 mg/dL (360 µmol/L); the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) recommendations for the treatment of gout suggest lowering uric acid levels below 5.0 mg/dL (300 µmol/L) in more severe disease conditions with tophi, frequent flares, or chronic gouty arthritis7,8.
Gout is well known to be associated with other metabolic disorders such as diabetes, dyslipidemia, or obesity, and especially dyslipidemia with elevated low-density lipoprotein (LDL) cholesterol and hypertriglyceidemia9. Elevated serum uric acid contributes to high LDL cholesterol and hypertriglyceridemia, but allopurinol can reduce triglyceride accumulation by decreasing intracellular uric acid levels10. Hypertriglyceridemia is caused by alcohol consumption, obesity, and sedentary lifestyle, and has been found to contribute to the development of gout11,12. Fenofibrate is the treatment of choice in patients with moderate to severe triglyceridemia because it enhances the oxidation of fatty acids in the liver and muscle and reduces hepatic lipogenesis13,14. Fibrates are fibric acid derivatives that activate the nuclear transcription factor peroxisome proliferator-activated receptor (PPAR)-α, which controls fatty acid transport and β-oxidation and results in a reduction in triglyceride levels and an increase in high-density lipoprotein cholesterol levels15,16. Fenofibrate is widely used to modify the lipid profile of patients with hypertriglyceridemia or type 2 diabetes and has a preventive effect on the progression of atherosclerosis in those patients17,18.
In clinical practice, physicians have prescribed fenofibrate for the treatment of hypertriglyceridemia in patients with gout. Fenofibrate has been reported to reduce serum uric acid levels in other populations19. However, the effect on the uric acid level or the adverse effect of fenofibrate coadministered with xanthine oxidase inhibitors has rarely been investigated in patients with gout. Only a few studies have examined by how much the uric acid level could be reduced by adding fenofibrate to patients with gout who received standard therapy.
Therefore, we sought to determine the effect of fenofibrate on the serum uric acid level in patients with gout by comparing serial serum uric acid levels between patients receiving xanthine oxidase inhibitors and fenofibrate and those receiving only xanthine oxidase inhibitors. In addition, we investigated whether fenofibrate coadministration affects renal or hepatic function in these patients.
Results
Basic characteristics of patients with gout
Table 1 shows the comparison of characteristics between patients administered allopurinol or febuxostat and patients cotreated with fenofibrate. Of the 863 patients with gout, 70 (8.1%) and 793 (91.9%) had prescriptions for allopurinol plus fenofibrate or febuxostat plus fenofibrate and allopurinol or febuxostat, respectively. Among them, 789 patients (91.4%) were men; the mean age was 50.6 ± 14.9 years; and 394 (45.7%) and 127 (14.7%) patients had hypertension and diabetes, respectively. The patients with gout on allopurinol plus fenofibrate or febuxostat plus fenofibrate were younger (46.9 ± 12.0 vs. 50.9 ± 15.1 years, p = 0.012) and had less hypertension (22/70, 31.4 vs. 372/793, 46.9%, p = 0.013) than those with gout who were not on fenofibrate. The levels of serum triglyceride decreased from 418.4 ± 190.0 to 316.2 ± 185.2 mg/dL, p = 0.002), suggesting good fenofibrate treatment adherence of the patients.
Table 1.
Characteristics of study participants by treatment groups.
| Total | Allopurinol or febuxostat | Allopurinol plus fenofibrate or febuxostat plus fenofibrate | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| Total | 863 | 793 | 70 | ||||
| Sex | 0.026 | ||||||
| Male | 789 | 91.4 | 720 | 90.8 | 69 | 98.6 | |
| Female | 74 | 8.6 | 73 | 9.2 | 1 | 1.4 | |
| Age, years | 0.014 | ||||||
| –29 | 53 | 6.1 | 51 | 6.4 | 2 | 2.9 | |
| 30–39 | 171 | 19.8 | 148 | 18.7 | 23 | 32.9 | |
| 40–49 | 208 | 24.1 | 190 | 24.0 | 18 | 25.7 | |
| 50–59 | 194 | 22.5 | 180 | 22.7 | 14 | 20.0 | |
| 60–69 | 124 | 14.4 | 113 | 14.2 | 11 | 15.7 | |
| 70– | 113 | 13.1 | 111 | 14.0 | 2 | 2.9 | |
| Age (mean/SD) | 50.6 | 14.9 | 50.9 | 15.1 | 46.9 | 12.0 | 0.012 |
| Hypertension | 0.013 | ||||||
| No | 469 | 54.3 | 421 | 53.1 | 48 | 68.6 | |
| Yes | 394 | 45.7 | 372 | 46.9 | 22 | 31.4 | |
| Hyperlipidemia | <0.0001 | ||||||
| No | 563 | 65.2 | 554 | 69.9 | 9 | 12.9 | |
| Yes | 300 | 34.8 | 239 | 30.1 | 61 | 87.1 | |
| Diabetes mellitus | 0.806 | ||||||
| No | 736 | 85.3 | 677 | 85.4 | 59 | 84.3 | |
| Yes | 127 | 14.7 | 116 | 14.6 | 11 | 15.7 | |
| Colchicine | 0.683 | ||||||
| No | 661 | 76.6 | 606 | 76.4 | 55 | 78.6 | |
| Yes | 202 | 23.4 | 187 | 23.6 | 15 | 21.4 | |
| Glucocorticoids | 0.247 | ||||||
| No | 627 | 72.7 | 572 | 72.1 | 55 | 78.6 | |
| Yes | 236 | 27.3 | 221 | 27.9 | 15 | 21.4 | |
| NSAIDs | 0.156 | ||||||
| No | 628 | 72.8 | 572 | 72.1 | 56 | 80.0 | |
| Yes | 235 | 27.2 | 221 | 27.9 | 14 | 20.0 | |
| HMG-CoA reductase inhibitor | 0.780 | ||||||
| No | 820 | 95.0 | 753 | 95.0 | 67 | 95.7 | |
| Yes | 43 | 5.0 | 40 | 5.0 | 3 | 4.3 | |
| Angiotensin receptor | 0.424 | ||||||
| No | 812 | 94.1 | 744 | 93.8 | 68 | 97.1 | |
| Yes | 51 | 5.9 | 49 | 6.2 | 2 | 2.9 | |
| Year of diagnosis | 0.904 | ||||||
| 1995–2000 | 11 | 1.3 | 10 | 1.3 | 1 | 1.4 | |
| 2001–2005 | 124 | 14.4 | 114 | 14.4 | 10 | 14.3 | |
| 2006–2010 | 215 | 24.9 | 195 | 24.6 | 20 | 28.6 | |
| 2011–2015 | 343 | 39.7 | 315 | 39.7 | 28 | 40.0 | |
| 2016–2018 | 170 | 19.7 | 159 | 20.1 | 11 | 15.7 | |
SD: standard deviation; NSAIDs: nonsteroidal anti-inflammatory drugs; HMG-CoA: 3-hydroxy-3-methylglutaryl-CoA.
Comparison of uric acid reduction between patients treated with and without fenofibrate
Changes in serum uric acid levels with and without fenofibrate are shown in Fig. 1. Before the medication, the average uric acid level did not differ between the patients on allopurinol or febuxostat and those on allopurinol plus fenofibrate or febuxostat plus fenofibrate (8.89 ± 2.01 vs. 8.74 ± 1.66 mg/dL, p = 0.548). After treatment, both groups showed decreased serum uric acid levels, which more significantly decreased in patients cotreated with fenofibrate than in those on allopurinol or febuxostat alone (−1.81 ± 2.41 vs. −2.40 ± 2.28 mg/dL, p = 0.043).
Figure 1.
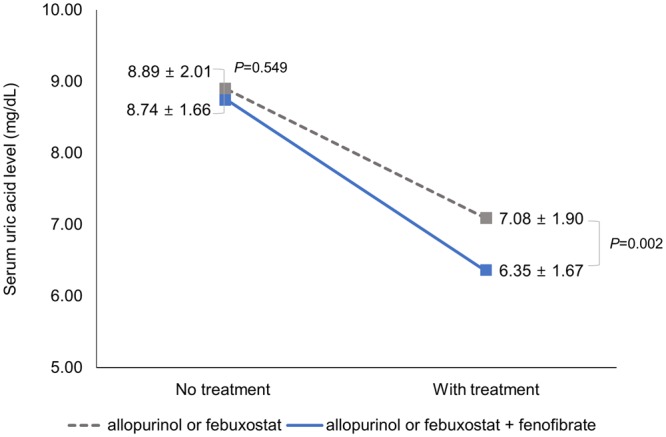
Change in serum uric acid levels according to treatment groups (group without fenofibrate vs. with fenofibrate). Gray dotted lines indicate the reduced levels of uric acid in patients administered allopurinol or febuxostat; blue line indicates the reduced levels of uric acid in the patients co-administered with fenofibrate. The interaction effect on the uric acid levels between treatment groups and whether with or without treatment was not statistically significant (p = 0.084).
Table 2 shows the results of the multiple linear regression, which assessed the association between the types of medication and change in serum uric acid levels. Even after adjusting for the confounders, we found that the serum uric acid level decreased more in patients on allopurinol plus fenofibrate or febuxostat plus fenofibrate than it did in patients with gout but not treated with fenofibrate (b = −0.879, p = 0.003). Furthermore, serum uric acid levels decreased more in patients with gout who were administered glucocorticoids than in patients who were not (b = −1.098, p < 0.001).
Table 2.
Association between treatment groups (with and without fenofibrate) and change in serum acid level.
| Variables | b | S.E. | 95% CI | p-value | |
|---|---|---|---|---|---|
| Treatment groups | |||||
| allopurinol or febuxostat | Ref. | ||||
| allopurinol or febuxostat + fenofibrate | −0.879 | 0.300 | −1.468 | −0.290 | 0.003 |
| Sex | |||||
| Men | Ref. | ||||
| Women | 0.456 | 0.285 | −0.103 | 1.015 | 0.110 |
| Age | |||||
| −29 | Ref. | ||||
| 30–39 | 0.353 | 0.353 | −0.338 | 1.044 | 0.317 |
| 40–49 | 0.213 | 0.345 | −0.464 | 0.890 | 0.538 |
| 50–59 | 0.052 | 0.352 | −0.637 | 0.741 | 0.883 |
| 60–69 | −0.105 | 0.374 | −0.837 | 0.628 | 0.779 |
| 70– | −0.364 | 0.387 | −1.122 | 0.394 | 0.346 |
| Hypertension | |||||
| No | Ref. | ||||
| Yes | −0.330 | 0.176 | −0.675 | 0.015 | 0.061 |
| Hyperlipidemia | |||||
| No | Ref. | ||||
| Yes | 0.024 | 0.180 | −0.329 | 0.376 | 0.896 |
| Diabetes mellitus | |||||
| No | Ref. | ||||
| Yes | −0.048 | 0.230 | −0.499 | 0.404 | 0.836 |
| Colchicine | |||||
| No | Ref. | ||||
| Yes | 0.158 | 0.188 | −0.211 | 0.527 | 0.400 |
| Steroid | |||||
| No | Ref. | ||||
| Yes | −1.098 | 0.182 | −1.454 | −0.742 | <0.0001 |
| NSAID | |||||
| No | Ref. | ||||
| Yes | 0.127 | 0.177 | −0.220 | 0.475 | 0.472 |
| HMG-CoA reductase inhibitor | |||||
| No | Ref. | ||||
| Yes | −0.405 | 0.388 | −1.167 | 0.356 | 0.297 |
| Angiotensin receptor | |||||
| No | Ref. | ||||
| Yes | 0.466 | 0.360 | −0.240 | 1.172 | 0.195 |
| Year of diagnosis | |||||
| 1995–2000 | Ref. | ||||
| 2001–2005 | 0.843 | 0.701 | −0.531 | 2.218 | 0.229 |
| 2006–2010 | 1.489 | 0.692 | 0.133 | 2.846 | 0.031 |
| 2011–2015 | 0.505 | 0.688 | −0.843 | 1.852 | 0.463 |
| 2016–2018 | 0.128 | 0.697 | −1.238 | 1.495 | 0.854 |
SD: standard deviation; NSAIDs: nonsteroidal anti-inflammatory drugs; HMG-CoA: 3-hydroxy-3-methylglutaryl-CoA. Outcome variable was change in serum uric acid level (‘after drug administration’ minus ‘before drug administration’). The negative estimation means decreased average serum uric acid level compared with the reference group.
The levels of serum uric acid at enrolment were compared to the levels before the index measure to exclude spontaneous changes of serum uric acid. Pre-enrollment levels of serum uric acid were collected at the closest measurement between 30 and 150 days prior to the time of enrolment. There was no difference in the levels of serum uric acid between before and at enrolment (Supplementary Table 1).
Comparison of the results of renal and liver function tests between patients treated with and without fenofibrate
Table 3 shows the results of the liver and renal function tests before and after the use of medication according to the treatment groups. Mean creatinine, blood urea nitrogen (BUN), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels before and after medication were not significantly different between patients on allopurinol or febuxostat and those on allopurinol plus fenofibrate or febuxostat plus fenofibrate.
Table 3.
Results of laboratory tests before and after medication use according to medication treatment groups.
| Total N | No treatment | With treatment | |||
|---|---|---|---|---|---|
| Mean/n | SD/Row/% | Mean/n | SD/Row% | ||
| Creatinine (mg/dL) | |||||
| allopurinol or febuxostat | 762 | 1.81 | 2.01 | 1.84 | 2.18 |
| allopurinol or febuxostat plus fenofibrate | 68 | 1.21 | 0.29 | 1.26 | 0.27 |
| BUN (mg/dL) | |||||
| allopurinol or febuxostat | 760 | 22.23 | 17.22 | 23.07 | 18.6 |
| allopurinol or febuxostat plus fenofibrate | 68 | 15.54 | 8.04 | 15.24 | 5.07 |
| AST (IU/L) | |||||
| allopurinol or febuxostat | 760 | 28.89 | 22.33 | 30.15 | 21.31 |
| allopurinol or febuxostat plus fenofibrate | 69 | 33.58 | 22.14 | 33.09 | 17.6 |
| ALT (U/L) | |||||
| allopurinol or febuxostat | 759 | 35.79 | 44.0 | 35.72 | 30.15 |
| allopurinol or febuxostat plus fenofibrate | 67 | 46.16 | 39.86 | 40.09 | 27.23 |
| AST* | |||||
| allopurinol or febuxostat | 698 | 11 | 1.6 | 16 | 2.3 |
| allopurinol or febuxostat plus fenofibrate | 67 | 3 | 4.5 | 1 | 1.5 |
| ALT* | |||||
| allopurinol or febuxostat | 698 | 26 | 3.7 | 31 | 4.4 |
| allopurinol or febuxostat plus fenofibrate | 67 | 6 | 9.0 | 3 | 4.5 |
| Bilirubin | |||||
| allopurinol or febuxostat | 755 | 0.73 | 0.37 | 0.76 | 0.41 |
| allopurinol or febuxostat plus fenofibrate | 68 | 0.71 | 0.35 | 0.68 | 0.25 |
| Total cholesterol | |||||
| allopurinol or febuxostat | 758 | 181.0 | 42.0 | 180.96 | 39.83 |
| allopurinol or febuxostat plus fenofibrate | 68 | 203.2 | 43.7 | 190.81 | 38.15 |
| HDL cholesterol | |||||
| allopurinol or febuxostat | 430 | 42.91 | 12.18 | 42.91 | 12.18 |
| allopurinol or febuxostat plus fenofibrate | 56 | 39.13 | 9.99 | 39.13 | 9.99 |
| LDL cholesterol | |||||
| allopurinol or febuxostat | 187 | 113.71 | 38.41 | 109.49 | 34.26 |
| allopurinol or febuxostat plus fenofibrate | 23 | 120.04 | 39.71 | 102.27 | 42.5 |
| Triglyceride | |||||
| allopurinol or febuxostat | 469 | 196.41 | 106.76 | 187.71 | 109.39 |
| allopurinol or febuxostat plus fenofibrate | 62 | 418.42 | 190.01 | 316.17 | 185.15 |
| Glucose | |||||
| allopurinol or febuxostat | 754 | 110.41 | 33.86 | 106.55 | 28.27 |
| allopurinol or febuxostat plus fenofibrate | 68 | 106.68 | 27.09 | 105.39 | 27.03 |
SD: standard deviation; BUN: blood urea nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; HDL: high density lipoprotein; LDL: low density lipoprotein.
*The number and proportion of patients who exceed three times of the maximum normal range before and after medication.
Discussion
In addition to gout, hyperuricemia also leads to hypertension, diabetes, and dyslipidemia, and has become a public health burden worldwide because of its high prevalence and clinical significance20. Although uric acid has a beneficial effect as an oxygen radical scavenger, hyperuricemia correlates closely with cardiovascular risk and all-cause mortality21,22. The aging society and decreased human physical activity caused by social development, have increased the incidence of gout with obesity, dyslipidemia, diabetes, hypertension, and hyperuricemia6.
In this study, we analyzed the clinical data of 863 patients with gout who were administered xanthine oxidase inhibitors, and fenofibrate decreased the uric acid level by approximately 0.73 mg/dL. Furthermore, cotreatment with fenofibrate and xanthine oxidase inhibitors did not affect the serum levels of the kidney and liver function markers.
Several studies have examined the effect of fenofibrate on uric acid levels in gout. The studies showed that uric acid levels decreased by 19 and 23% after fenofibrate treatment of patients with gout who were on urate-lowering agents23,24. However, although the reduction of urate levels was assessed in a small number (10 and 14) of patients with gout, this study collected sufficient clinical data to evaluate the general effect of fenofibrate. Furthermore, in the previous studies, patients treated with only allopurinol were enrolled, whereas our patients were prescribed allopurinol or febuxostat. Febuxostat is considered more effective than allopurinol in achieving target uric acid levels25,26.
The uric acid levels were reduced in patients taking xanthine oxidase inhibitors and fenofibrate compared with those taking only xanthine oxidase inhibitors (−1.81 vs. −2.4 mg/dL, p = 0.043). Fenofibrate decreases serum uric acid levels by increasing urinary excretion of uric acid by inhibiting the renal organic anion transporter urate transporter 1 (URAT1; solute carrier family 22 member 12, SLC22A12)19,27. Moreover, it was shown to raise urinary pH, and acidic urinary pH could be associated with the formation of uric acid stones, which is one of the comorbidities of gout and a common complication of uricosuric agents28. Therefore, the addition of fenofibrate is reasonable, considering that agents that increase the renal excretion of uric acid are likely to lead to the development of uric acid stones.
More studies on the effect of fenofibrate on uric acid reduction were carried out in patients with hypertriglyceridemia or diabetes19,29,30. In the randomized and controlled fenofibrate intervention and event lowering in diabetes (FIELD) study conducted to determine the effect of fenofibrate on type 2 diabetes, uric acid levels were decreased by 10.3% in 622 patients with diabetes who took allopurinol. In addition, the first gout event in 5 years was more frequent with placebo treatment than with fenofibrate among patients with diabetes31.
Treatment strategies for acute gout such as the use of anti-inflammatory drugs including colchicine, nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids are frequently maintained because of the need for prophylaxis to prevent further recurrent gout flares5,7. In this study, among the treatments with anti-inflammatory drugs, statins, and angiotensin receptor modulating agents, glucocorticoids were correlated significantly with uric acid reduction. This finding could be explained by the fact that glucocorticoids enhance urinary excretion of uric acid by directly acting on uric acid transport in the renal tubules30–32. Moreover, the effect of glucocorticoids on lowering uric acid was similar to that of allopurinol in previous reports32–34.
Since mild liver biochemical abnormalities are known to occur in 5–10% of patients on fenofibrate, baseline and regular periodic aminotransferase testing is recommended35. Fenofibrate-induced hepatotoxicity is hepatocellular, cholestatic, or of a mixed pattern, and ranges from an acute and self-limiting to chronic liver injury36. However, in this study, there was no differences in the levels of AST and ALT between patients treated with and without fenofibrate. Furthermore, previous studies showed the beneficial effects of fenofibrate on levels of liver enzymes in patients with fatty liver and its preventive effects on fatty liver disease in mice37,38. In several studies including a large-scale randomized controlled trial, 2% of the patients taking fenofibrate had more elevated creatinine levels when compared with the control group patients14,39. Fenofibrate-associated nephrotoxicity occurs in patients with pre-existing renal disease, and high dosage poses a higher risk than low dosage40. However, our patients with gout showed no change in levels of creatinine and BUN before and after treatment, and no difference occurred between patients treated with and without fenofibrate.
A limitation of this study is the lack of data on gout flares, which should ultimately be prevented in the treatment of gout. However, this is a novel study that investigated the effect of fenofibrate on the reduction of uric acid in a large population of patients with gout who were administered febuxostat and allopurinol. The status of these patients is significant because it is similar to that in the current clinical situation, indicating that it could be applied to the management of patients with gout and elevated triglycerides. Another limitation is that during the study period of more than 20 years, there have been changes in the use of the drug for lowering serum uric acid level because febuxostat was introduced in 2012 in the subject hospital. However, there was no significant difference in the ratio of the prescription count of allopurinol and febuxostat between two the treatment groups (group without fenofibrate vs. with fenofibrate) as shown in Supplementary Table 2. Finally, our analysis did not contain a confirmation for patient adherence to medication. It is a well-known and inevitable limitation of retrospective data. However, the level of adherence would be randomly distributed in both treatment groups, and there are no indications or factors that adherence was higher in a certain group as compared to that in the other group.
In conclusion, this data shows that the addition of fenofibrate reduced uric acid levels in patients with gout who were taking allopurinol or febuxostat, and they showed no significant change in renal or hepatic functions. Therefore, cotreatment of fenofibrate with xanthine oxidase inhibitors might be a favorable therapeutic strategy in patients with gout who have hypertriglyceridemia.
Methods
Data source and study participants
We used the data from the electronic medical record (EMR) system of the Ajou University Hospital, which has been operating as a tertiary teaching hospital in Korea since 1994. The EMR system collects information based on unique, de-identified numbers for patients combined with their age and sex, the diagnostic codes based on the International Classification of Diseases (ICD-10), admission and discharge dates, laboratory test results, and prescribed medications/treatments from about 2.9 million patients (including 543,617 inpatients). The information about diagnostic codes including combined diseases was obtained from the EMR and validated by manual chart review (H.-A.K. and J.-Y.J).
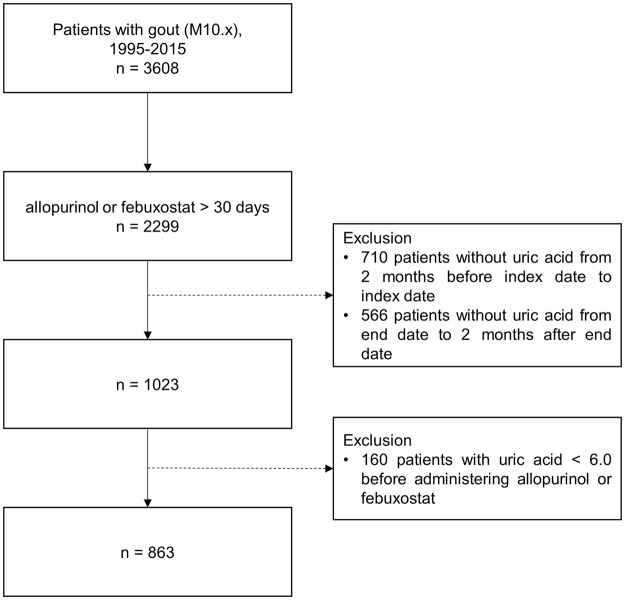
To investigate the effect of allopurinol or febuxostat with fenofibrate on uric acid levels, we selected 3,608 patients with gout (ICD-10 code: M10.x) between 1995 and 31 February 2018. Among the 3,608 patients, we first selected patients treated for at least 30 consecutive days with allopurinol or febuxostat (n = 2,299). We excluded those who did not have a uric acid test within 2 months before taking allopurinol or febuxostat (n = 710) and within 2 months after taking allopurinol or febuxostat for 30 days (n = 566).
We excluded 160 patients with uric acid levels <6.0 before medication use. Finally, this study included the 863 patients with gout. The flowchart for selecting the study participants is displayed in Fig. 2. In addition, we eliminated 98 patients who did not have renal and liver function tests within 2 months before taking allopurinol or febuxostat and within 2 months after taking allopurinol or febuxostat for 30 days, to examine whether patients taking additional fenofibrate had adverse drug effects on the liver or kidneys.
Figure 2.
Flowchart depicting the method of selection of the study participants. Data of 1,456 patients were excluded during the collection of laboratory results.
Outcomes
The primary outcome was serum uric acid (mg/dL), which was measured within 2 months before the date of prescription of hypouricemic agents and within 2 months after a consecutive 30-days of prescription of hypouricemic agents. Renal (creatinine and BUN) and liver (AST and ALT) function tests were performed within the same period.
Exposure
The patients were classified into two treatment groups using prescribed medication based on data extracted from the EMR of Ajou University: (1) the group administered allopurinol or febuxostat for 30 consecutive days, and (2) the group co-administered fenofibrate during 30-days of allopurinol or febuxostat treatment.
Confounders
Data on demographics, comorbidities, and use of other medication were included in this study. All confounders were assessed during the period of exposure. The demographic factors were sex and age. Comorbidities (hypertension, hyperlipidemia, and diabetes mellitus) were included by reviewing the patients’ prescriptions in the medical records. Medication for the treatment of patients with gout were included: NSAIDs, glucocorticoids (methylprednisolone, prednisolone, deflazacort, triamcinolone, and hydrocortisone), and colchicine. This study also included drugs that could affect serum uric acid level, viz., statin and angiotensin receptors.
Statistical analysis
We compared the characteristics of the allopurinol or febuxostat and allopurinol plus fenofibrate or febuxostat plus fenofibrate groups using chi-square and independent t-test. We used the independent t-test to compare the difference in serum uric acid levels between the two groups (allopurinol or febuxostat vs. allopurinol plus fenofibrate or febuxostat plus fenofibrate). Two-way ANOVA was used for testing the interaction effect between two the treatment groups and time. Multiple linear regression was conducted to determine the effect of fenofibrate on the difference (‘after drug administration’ minus ‘before drug administration’) in serum uric acid levels in patients with gout. To examine whether patients coadministered fenofibrate had adverse drug reactions, we calculated the average creatinine, BUN, AST, and ALT values before and after medication based on the groups. For the liver function test, we calculated the number and proportion of patients who exceed three times of the maximum normal range before and after medication. A p < 0.05 was considered statistically significant. Data were managed using the Microsoft SQL Server (Microsoft Corp), and all statistical analyses were conducted using the SAS software package (ver. 9.4; SAS Institute, Cary, NC, USA).
Ethics statement
This study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-MED-OBS-18-102), which waived the requirement for informed consent because the anonymized data were analyzed retrospectively.
Electronic supplementary material
Acknowledgements
This research was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant numbers: HI16C0982, HI17C0970, and HI16C0992).
Author Contributions
J.-Y.J., Y.C.: design, data collection and analysis, and manuscript writing. C.-H.S.: data collection and analysis and critical revision of the manuscript. D.Y., H.-A.K.: conception and design, data collection and analysis, and manuscript writing. All authors have read and approved the final manuscript.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to legal restrictions imposed by the government of South Korea in relation to the Personal Information Protection Act, but are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ju-Yang Jung and Young Choi contributed equally.
Contributor Information
Dukyong Yoon, Email: d.yoon.ajou@gmail.com.
Hyoun-Ah Kim, Email: nakhada@naver.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35175-z.
References
- 1.Neogi T, et al. Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases. 2015;74:1789–1798. doi: 10.1136/annrheumdis-2015-208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nature Reviews Rheumatology. 2017;13:639–647. doi: 10.1038/nrrheum.2017.155. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis and Rheumatism. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 4.Richette P, Bardin T. Gout. The Lancet. 2010;375:318–328. doi: 10.1016/s0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 5.Davies Kristen, Bukhari Marwan A S. Recent pharmacological advances in the management of gout. Rheumatology. 2017;57(6):951–958. doi: 10.1093/rheumatology/kex343. [DOI] [PubMed] [Google Scholar]
- 6.Pascart, T. & Liote, F. Gout: state of the art after a decade of developments. Rheumatology, 10.1093/rheumatology/key002 (2018). [DOI] [PubMed]
- 7.Richette P, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Annals of the Rheumatic Diseases. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 8.Khanna D, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care & Research. 2012;64:1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwabara M, et al. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: A five-year cohort study in Japan. International Journal of Cardiology. 2018;261:183–188. doi: 10.1016/j.ijcard.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Lanaspa MA, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PloS One. 2012;7:e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JH, et al. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: a prospective study. Arthritis Care & Research. 2013;65:133–140. doi: 10.1002/acr.21824. [DOI] [PubMed] [Google Scholar]
- 12.Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovascular Diabetology. 2014;13:159. doi: 10.1186/s12933-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopf T, et al. Influence of fenofibrate treatment on triacylglycerides, diacylglycerides and fatty acids in fructose fed rats. PloS One. 2014;9:e106849. doi: 10.1371/journal.pone.0106849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keech A, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. The Lancet. 2005;366:1849–1861. doi: 10.1016/s0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 15.Adkins JC, Faulds D. Micronised fenofibrate: a review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidaemia. Drugs. 1997;54:615–633. doi: 10.2165/00003495-199754040-00007. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ghani RA, et al. The influence of fenofibrate on lipid profile, endothelial dysfunction, and inflammatory markers in type 2 diabetes mellitus patients with typical and mixed dyslipidemia. Journal of Clinical Lipidology. 2013;7:446–453. doi: 10.1016/j.jacl.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Rajamani K, et al. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. The Lancet. 2009;373:1780–1788. doi: 10.1016/s0140-6736(09)60698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derosa G, Maffioli P, Sahebkar A. Plasma uric acid concentrations are reduced by fenofibrate: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacological Research. 2015;102:63–70. doi: 10.1016/j.phrs.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Borghi C, et al. Serum uric acid and the risk of cardiovascular and renal disease. Journal of Hypertension. 2015;33:1729–1741. doi: 10.1097/hjh.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 21.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho Sung Kweon, Chang Yoosoo, Kim Inah, Ryu Seungho. U-Shaped Association Between Serum Uric Acid Level and Risk of Mortality. Arthritis & Rheumatology. 2018;70(7):1122–1132. doi: 10.1002/art.40472. [DOI] [PubMed] [Google Scholar]
- 23.Feher MD, Hepburn AL, Hogarth MB, Ball SG, Kaye SA. Fenofibrate enhances urate reduction in men treated with allopurinol for hyperuricaemia and gout. Rheumatology. 2003;42:321–325. doi: 10.1093/rheumatology/keg103. [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Lee CH, Lee J. Effect of fenofibrate in combination with urate lowering agents in patients with gout. The Korean Journal of Internal Medicine. 2006;21:89–93. doi: 10.3904/kjim.2006.21.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, et al. Comparative efficacy and safety of urate-lowering therapy for the treatment of hyperuricemia: a systematic review and network meta-analysis. Scientific Reports. 2016;6:33082. doi: 10.1038/srep33082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, et al. An allopurinol-controlled, multicenter, randomized, double-blind, parallel between-group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. International Journal of Rheumatic Diseases. 2014;17:679–686. doi: 10.1111/1756-185x.12266. [DOI] [PubMed] [Google Scholar]
- 27.Uetake D, et al. Effect of fenofibrate on uric acid metabolism and urate transporter 1. Internal Medicine (Tokyo, Japan) 2010;49:89–94. doi: 10.2169/internalmedicine.49.2597. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi S, et al. Relationship between insulin resistance and low urinary pH in patients with gout, and effects of PPARalpha agonists on urine pH. Hormone and Metabolic Research. 2007;39:511–514. doi: 10.1055/s-2007-982517. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi Y, et al. Effect of fenofibrate on uric acid metabolism in Japanese hyperlipidemic patients. Journal of Atherosclerosis and Thrombosis. 2004;11:335–340. doi: 10.5551/jat.11.335. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Hada T. Effect of fenofibrate on plasma concentration and urinary excretion of purine bases and oxypurinol. The Journal of Rheumatology. 2001;28:2294–2297. [PubMed] [Google Scholar]
- 31.Waldman B, et al. Effect of fenofibrate on uric acid and gout in type 2 diabetes: a post-hoc analysis of the randomised, controlled FIELD study. The Lancet Diabetes & Endocrinology. 2018;6:310–318. doi: 10.1016/s2213-8587(18)30029-9. [DOI] [PubMed] [Google Scholar]
- 32.Shi L, Berkemeyer S, Buyken AE, Maser-Gluth C, Remer T. Glucocorticoids and body fat associated with renal uric acid and oxalate, but not calcium excretion, in healthy children. Metabolism: Clinical and Experimental. 2010;59:134–139. doi: 10.1016/j.metabol.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, et al. Prednisone in Uric Acid lowering in Symptomatic Heart Failure Patients With Hyperuricemia (PUSH-PATH) study. The Canadian Journal of Cardiology. 2013;29:1048–1054. doi: 10.1016/j.cjca.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Shibutani Y, Ueo T, Takahashi S, Moriwaki Y, Yamamoto T. Effect of ACTH on renal excretion of purine bases in a patient with isolated ACTH deficiency. Clinica Chimica Acta. 2000;294:185–192. doi: 10.1016/S0009-8981(99)00263-6. [DOI] [PubMed] [Google Scholar]
- 35.Dohmen K, et al. Fenofibrate-induced liver injury. World Journal of Gastroenterology. 2005;11:7702–7703. doi: 10.3748/wjg.v11.i48.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad J, et al. Identification and Characterization of Fenofibrate-Induced Liver Injury. Digestive Diseases and Sciences. 2017;62:3596–3604. doi: 10.1007/s10620-017-4812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaghoubi M, et al. Comparison of fenofibrate and pioglitazone effects on patients with nonalcoholic fatty liver disease. European Journal of Gastroenterology & Hepatology. 2017;29:1385–1388. doi: 10.1097/meg.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 38.van der Veen JN, et al. Fenofibrate, but not ezetimibe, prevents fatty liver disease in mice lacking phosphatidylethanolamine N-methyltransferase. Journal of Lipid Research. 2017;58:656–667. doi: 10.1194/jlr.M070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossner S, Oro L. Fenofibrate therapy of hyperlipoproteinaemia. A dose-response study and a comparison with clofibrate. Atherosclerosis. 1981;38:273–282. doi: 10.1016/0021-9150(81)90043-5. [DOI] [PubMed] [Google Scholar]
- 40.Attridge RL, Linn WD, Ryan L, Koeller J, Frei CR. Evaluation of the incidence and risk factors for development of fenofibrate-associated nephrotoxicity. Journal of Clinical Lipidology. 2012;6:19–26. doi: 10.1016/j.jacl.2011.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to legal restrictions imposed by the government of South Korea in relation to the Personal Information Protection Act, but are available from the corresponding author on reasonable request.