Abstract
OBJECTIVE.
To determine incidence of and risk factors for readmissions with multidrug-resistant organism (MDRO) infections among patients with previous MDRO infection.
DESIGN.
Retrospective cohort of patients admitted between January 1, 2006, and October 1, 2015.
SETTING.
Barnes-Jewish Hospital, a 1,250-bed academic tertiary referral center in St Louis, Missouri.
METHODS.
We identified patients with MDROs obtained from the bloodstream, bronchoalveolar lavage (BAL)/bronchial wash, or other sterile sites. Centers for Disease Control and prevention (CDC) and European CDC definitions of MDROs were utilized. All readmissions ≤1 year from discharge from the index MDRO hospitalization were evaluated for bloodstream, BAL/bronchial wash, or other sterile site cultures positive for the same or different MDROs.
RESULTS.
In total, 4,429 unique patients had a positive culture for an MDRO; 3,453 of these (78.0%) survived the index hospitalization. Moreover, 2,127 patients (61.6%) were readmitted ≥1 time within a year, for a total of 5,849 readmissions. Furthermore, 512 patients (24.1%) had the same or a different MDRO isolated from blood, BAL/bronchial wash, or another sterile site during a readmission. Bone marrow transplant, end-stage renal disease, lymphoma, methicillin-resistant Staphylococcus aureus, or carbapenem-resistant Pseudomonas aeruginosa during index hospitalization were factors associated with increased risk of having an MDRO isolated during a readmission. MDROs isolated during readmissions were in the same class of MDRO as the index hospitalization 9%–78% of the time, with variation by index pathogen.
CONCLUSIONS.
Readmissions among patients with MDRO infections are frequent. Various patient and organism factors predispose to readmission. When readmitted patients had an MDRO, it was often a pathogen in the same class as that isolated during the index admission, with the exception of Acinetobacter (~9%).
Hospital readmissions are associated with reduced quality of life and increased mortality.1–4 An improved understanding of risk factors for readmission is critical to develop targeted interventions to prevent readmissions. One major cause of readmissions is infection, which accounted for as many as 30% of 30-day readmissions.5 In addition, infections during hospitalization, particularly sepsis, are associated with frequent 30-day readmissions, with estimated rates ranging from 7% to 43%.6–16
Although multidrug-resistant organism (MDRO) infections are known to increase the length of stay and healthcare costs,17 their role as a risk factor for readmission has not been extensively studied. Data suggest that patients with MDRO infections are at higher risk of readmission, though causes of readmission in these cohorts are not well defined.18–25 Furthermore, infection as a reason for readmission in sepsis patients (with or without MDROs) is poorly characterized due to data limitations and heterogeneity among study designs, with estimates of readmission rates due to infection ranging from 20% to 70%.7,10,12,13
Data concerning the rate of readmissions in patients who had an MDRO infection and the rate of MDRO infections during readmission in patients with a prior MDRO infection are lacking. We studied hospitalized patients with MDRO infections, confirmed by positive cultures from the bloodstream, bronchoalveolar lavage (BAL)/bronchial wash, or other sterile site to determine readmission rates and whether the same or a different MDRO was isolated in sterile site or BAL/bronchial wash cultures during a readmission. We did not use a comparison group of patients with non-MDRO infections because this population of patients tends to be different from patients with MDRO infections.26,27 Understanding the rate of readmission with MDROs in patients with prior MDRO infections will help guide clinical decision making by improving the use of targeted empiric antimicrobials in readmitted patients, by facilitating readmission reduction interventions targeted toward patients at high readmission risk, and by improving infection prevention efforts by alerting personnel when patients at high-risk for MDRO infection are admitted so that proper isolation precautions can be initiated.
MATERIALS AND METHODS
Study Location and Patient Population
This study was conducted at Barnes-Jewish Hospital (BJH), a 1,250-bed academic medical center located in St Louis, Missouri. The study period was January 1, 2006 to October 1, 2015. Hospitalized patients with a positive sterile site or BAL/bronchial wash culture for Enterobacteriaceae, Enterococcus spp, Staphylococcus aureus, Pseudomonas aeruginosa, or Acinetobacter spp were analyzed for eligibility. Antimicrobial susceptibilities for all pathogens were determined using disc diffusion methodology. Sterile sites were defined as bloodstream; pleural, intra-abdominal, pericardial, cerebrospinal, and synovial fluids; bone marrow; and surgical specimens collected from lymph nodes; the central nervous system (CNS), liver, spleen, kidney, pancreas, ovary, or vascular tissue. The Washington University School of Medicine Institutional Review Board approved this study.
Study Design and Data Collection
Utilizing a retrospective cohort study design, the first hospitalization between January 2006 and October 2015 of all patients aged ≥18 years with multidrug-resistant Enterobacteriaceae,Enterococcus spp, S. aureus, P. aeruginosa, or Acinetobacter spp isolated from bloodstream, other sterile sites, or BAL/bronchial wash culture were identified. Patients with >1 of these MDROs were considered to have a polymicrobial infection. Other potentially drug-resistant organisms were not included in the analysis. The primary end points were any readmission and readmission during which an MDRO infection occurred. Baseline characteristics, including age, gender, race, Acute Physiology and Chronic Health Evaluation (APACHE) II22 scores (calculated based on clinical data present during the 24 hours after positive blood cultures were drawn), Charlson comorbidity index, and medical comorbidities (based on International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis codes) were obtained. Patients who died during the index hospitalization or were discharged on hospice were considered to expire at the time of hospital discharge and were excluded from analyses related to readmissions.
Definitions
We assessed 30-day mortality using the BJC Healthcare Informatics database. Barnes-Jewish Hospital is the main adult teaching institution for BJC Healthcare, a large integrated healthcare system of both inpatient and outpatient care. The system includes 13 hospitals in a compact geographic region surrounding and including St Louis, Missouri. Barnes-Jewish Hospital has >50,000 admissions annually, and the BJC system has >140,000 admissions annually. All index hospitalizations used to define the cohort occurred at BJH. Readmissions to BJH or any other BJC acute-care facility were captured. All data were derived from the medical informatics database maintained by the Center for Clinical Excellence, BJC HealthCare. To be categorized as having an MDRO during a readmission, blood, BAL/bronchial wash, or sterile-site cultures positive for an MDRO could be obtained any time during the readmission. Patient death dates are included in the informatics database. For patients with <1 year of follow-up care at BJC HealthCare after index hospitalization, the Social Security Death Index (SSDI) was used to identify patient deaths. Patients without follow-up in the BJC system and who were not in the SSDI were considered lost to follow-up on their last date of care in a BJC facility. Index MDRO admission survivors were considered censored at their date of death or loss to follow-up.
Defining MDROs
We utilized multiple definitions of drug resistance as outlined by the Centers for Disease Control and Prevention (CDC) and European CDC (Supplemental Table 1).28–30 Any Enterobacteriaceae was presumed to be an extended-spectrum β-lactamase (ESBL) producer if ceftriaxone or ceftazidime was intermediate or resistant. Patients were considered to have a vancomycin intermediate S. aureus (VISA) infection if S. aureus was isolated in culture and was determined to have a vancomycin minimum inhibitory concentration (MIC) of 4 or 8 μg/mL, in accordance with Clinical and Laboratory Standards Institute recommendations.31
Statistical Analysis
Comparisons between index MDRO admission survivors and nonsurvivors were performed using the χ2 or the Fisher exact test for categorical values and using the Student t test or Mann-Whitney U test for continuous variables. Readmissions by index hospitalization pathogen group were analyzed using Kaplan-Meier curves. Continuous variables were reported as means with standard deviations or medians and interquartile ranges (IQRs). Categorical data were expressed as frequencies. In bivariate analysis, the relative risk of in-hospital mortality or discharge on hospice from index MDRO hospitalization was calculated using drug-resistant S. aureus as the reference group because it was the largest group and had the lowest mortality. For bivariate analysis of variables associated with readmissions or readmissions with an MDRO, a Cox proportional hazards model was used. The proportional hazards assumption was checked graphically using a log-log survival plot. A P value of <.05 was considered significant in all analyses. Multivariate Cox proportional hazards models were used to determine risk factors for readmission overall and risk factors for a readmission during which an MDRO was isolated. Factors associated with mortality in bivariate analysis (P < .20) were entered into a Cox proportional hazards model to determine hazard ratios (HRs) for readmission. Nonsignificant predictors were retained in final models if their P value was <.20 in bivariate analysis. All variables entered into the model were assessed for collinearity, and clinically plausible interaction terms were tested in the model (Supplemental Table 2). Model diagnostics included plotting of DFBETAs to detect influential cases and martingale residuals for a residuals pattern. Linearity of the Charlson comorbidity index for the outcome was confirmed prior to model entry. All analyses were conducted with SPSS version 24 software (IBM, Armonk, NY).
RESULTS
A total of 4,429 patients with MDROs from sterile sites or BAL/bronchial wash cultures were identified, and 976 patients (22.0%) died during the index hospitalization or were discharged to hospice. Nonsurvivors were older and had more underlying comorbidities and higher APACHE-II scores than survivors (Supplemental Table 3). In bivariate analysis, nonsurvivors were more likely to have any drug-resistant Enterococcus, Acinetobacter, or P. aeruginosa than survivors and less likely to have a drug-resistant S. aureus infection (Supplemental Table 3). Patients with any drug-resistant Acinetobacter had the highest index hospitalization mortality (n = 44, 41.1%), with a relative risk (RR) of death of 2.45 compared to patients with drug-resistant S. aureus (P < .0001) (Supplemental Table 4). There were significant differences in the APACHE-II scores among patients in the different index pathogen groups (P < .001) (Supplemental Table 5). Additional differences in patient characteristics among index pathogen groups are shown in Supplemental Table 5. Comorbidities, age, gender, ICU utilization, length of hospital stay, and the site from which an MDRO was isolated were all significantly different between groups (Supplemental Table 5).
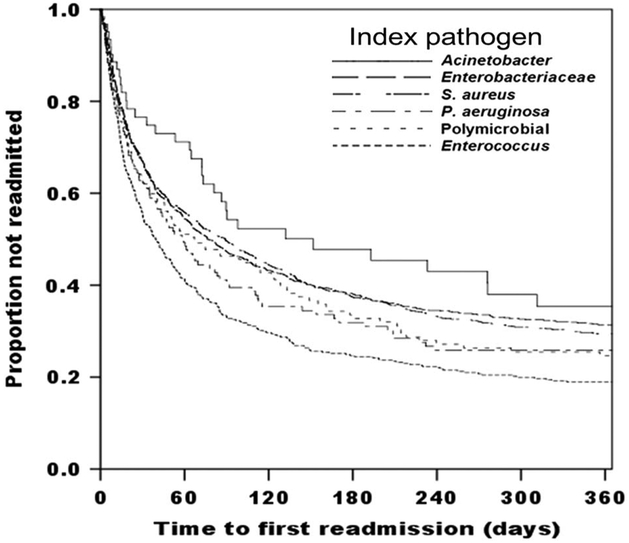
Among the 3,453 index MDRO hospitalization survivors, 1,121 (32.5%) were readmitted at least once within 30 days after discharge from the index hospitalization, and 2,127 (61.6%) were readmitted within 1 year. These 2,127 patients had a total of 5,849 readmissions within 1 year, with a median of 2 readmissions per patient (IQR, 1–4; range, 1–28). The median time to first readmission was 26.9 days (IQR, 9.9–76.9). Survivors infected with drug-resistant Acinetobacter were least likely to be readmitted, and those infected with drug-resistant Enterococcus most likely to be readmitted (Figure 1) (P < .001). Differences in baseline characteristics between survivors readmitted within 1 year and those who were not are shown in Table 1.
FIGURE 1.
Time to first readmission by index multidrug-resistant organism (MDRO) hospitalization pathogen type. Patients censored at death or loss to follow-up. Log-rank, 44.340; P < .001; 5° of freedom. All index pathogens are drug resistant.
TABLE 1.
Comparison of Survivors of the Index MDRO Hospitalization Readmitted Within 1 Year to Those Who Were Not Readmitted Within 1 Year After Discharge From the Index MDRO Hospitalization
| Index Admission Characteristics | No. (%) of Survivors Not Readmitted (N = 1,326)a |
No. (%) of Survivors Any Readmission (N = 2,127)a |
P Valueb |
|---|---|---|---|
| Age, y (± SD) | 57.8 ±17.0 | 56.7 ± 15.8 | .152 |
| Gender, male | 771 (58.1) | 1230 (57.8) | .653 |
| Ethnicity, white | 919 (69.3) | 1348 (63.4) | .078 |
| Any ICU stay | 734 (55.4) | 966 (45.4) | .002 |
| Bone marrow transplant | 19 (1.4) | 74 (3.5) | <.001 |
| Solid organ transplant | 56 (4.2) | 126 (5.9) | .314 |
| Cardiovascular disease | 773 (58.3) | 1209 (56.8) | .230 |
| Congestive heart failure | 311 (23.5) | 488 (22.9) | .006 |
| Chronic respiratory failure | 394 (29.7) | 561 (26.4) | .030 |
| Diabetes mellitus | 453 (34.2) | 758 (35.6) | .293 |
| Chronic kidney disease | 263 (19.8) | 506 (23.8) | <.001 |
| End-stage renal disease | 126 (9.5) | 274 (12.9) | .003 |
| Solid organ malignancy | 270 (20.4) | 471 (22.1) | .014 |
| Leukemia | 83 (6.3) | 274 (12.9) | <.001 |
| Lymphoma | 59 (4.4) | 120 (5.6) | .091 |
| Cirrhosis | 78 (5.9) | 149 (7.0) | .002 |
| Charlson comorbidity score (IQR) | 3 (1–5) | 4 (2–6) | <.001 |
| APACHE-II score (± SD) | 11.4±5.2 | 11.2±5.0 | .535 |
| Culture source | |||
| Blood | 908 (68.5) | 1565 (73.6) | .001 |
| Bronchial wash/BAL | 246 (18.6) | 282 (13.3) | <.001 |
| Other sterile site | 270 (20.4) | 422 (19.8) | .709 |
| > 1 site | 97 (7.3) | 138 (6.5) | .348 |
| Index drug-resistant organism c | |||
| Staphylococcus aureus | 588 (44.3) | 873 (41.0) | .056 |
| Enterococcus | 181 (13.7) | 402 (18.9) | <.001 |
| Enterobacteriaceae | 403 (30.4) | 605 (28.4) | .221 |
| Acinetobacter | 29 (2.2) | 34 (1.6) | .209 |
| Pseudomonas aeruginosa | 55 (4.1) | 95 (4.5) | .655 |
| Polymicrobial | 70 (5.3) | 118 (5.5) | .735 |
| Index discharge disposition | |||
| Left hospital against medical advice | 14 (1.1) | 9 (0.4) | .079 |
| Home | 682 (51.4) | 1301 (61.2) | .486 |
| SNF/LTACH/Rehab/NH | 586 (44.2) | 783 (36.8) | .959 |
| Other hospital | 44 (3.3) | 34 (1.6) | .235 |
| Index LOS, d (IQR) | 15.1 (7.8–29.8) | 13.9 (7.0–30.1) | .147 |
NOTE. MDRO, multidrug-resistant organism; APACHE-II, Acute Physiology and Chronic Health Evaluation; BAL, bronchoalveolar lavage; ICU, intensive care unit; IQR, interquartile range; LTACH, long-term acute-care hospital; LOS, length of stay; NH, nursing home; SNF, skilled nursing facility; SD, standard deviation.
Unless otherwise specified.
P values were obtained using univariate Cox regression.
Drug resistance was defined according to the definitions in Supplemental Table 1.
Of the survivors who were not readmitted within 1 year, 500 (14.5%) were lost to follow-up. In bivariate analysis, patients lost to follow-up were significantly (P < .05) more likely to have cardiovascular disease, chronic respiratory failure, be older, have had an ICU stay during index hospitalization, and be discharged to a skilled nursing facility (SNF), a long-term acute-care hospital (LTACH), or a nursing home (NH) (data not shown).
In a multivariate Cox proportional hazards model of survivors, any ICU stay during the index hospitalization was associated with a reduced HR for readmission (HR, 0.88) as was having a positive bronchoscopy culture (HR, 0.83) or infection with a drug-resistant Acinetobacter (HR, 0.71). Factors associated with increased risk of readmission were cirrhosis (HR, 1.24), leukemia (HR, 1.44), Charlson comorbidity index (HR, 1.06 per unit increase), VRE (HR, 1.15), and carbapenem-resistant P. aeruginosa during index hospitalization (HR, 1.40) (Table 2).
TABLE 2.
Index MDRO Hospitalization Factors Associated With Any Readmission Within 1 Year in a Multivariable Cox Proportional Hazards Modela
| Factors From Index MDRO Hospitalizationb |
HR (95% CI) | P Value |
|---|---|---|
| Leukemia | 1.44 (1.24–1.68) | <.001 |
| Carbapenem-resistant Pseudomonas | 1.39 (1.12–1.72) | .002 |
| Cirrhosis | 1.23 (1.04–1.47) | .018 |
| Vancomycin-resistant Enterococci | 1.15 (1.01–1.32) | .041 |
| Charlson comorbidity index | 1.06 (1.04–1.08) | <.001 |
| ICU stay | 0.88 (0.80–0.97) | .011 |
| Positive bronchoscopy culture | 0.84 (0.70–0.99) | .031 |
| Drug-resistant Acinetobacter | 0.70 (0.52–0.95) | .027 |
NOTE. MDRO, multidrug-resistant organism; CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; ESBL, extended-spectrum β-lactamase; MRSA, methicillin-resistant Staphylococcus aureus.
Population = survivors of index hospitalization.
Other variables included in the model that were not significant: age, ethnicity, receipt of a bone marrow transplant, congestive heart failure, cirrhosis, chronic kidney disease, chronic respiratory failure, end-stage renal disease, lymphoma, solid organ malignancy, blood culture with an MDRO, ESBL Enterobacteriaceae, MRSA, and length of stay.
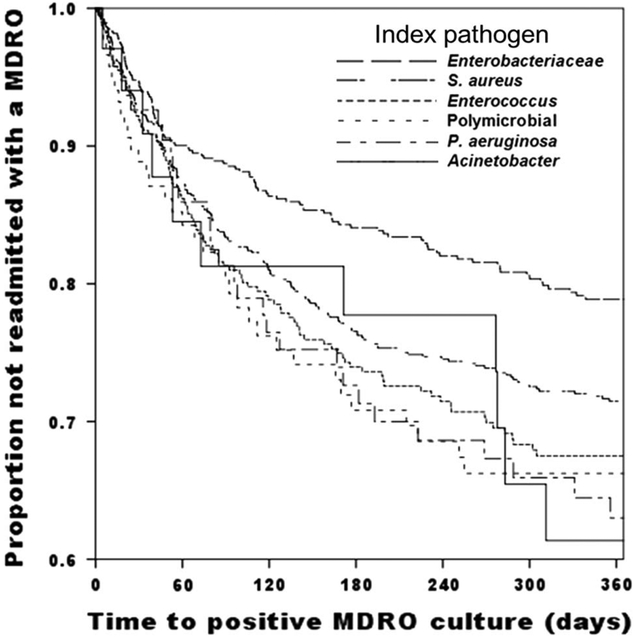
Of the 2,127 patients with at least 1 readmission within 1 year, 512 (24.1%) had an MDRO isolated during at least 1 readmission. The median time to a new positive MDRO culture after discharge from the index hospitalization was 61.9 days (IQR, 24.3–140.6). Patients with index drug-resistant Enterobacteriaceae were the least likely and patients with index drug-resistant Acinetobacter were the most likely to be readmitted with another MDRO infection (P = .001) (Figure 2). Differences in baseline characteristics between readmitted patients with and without another MDRO during readmission are shown in Supplemental Table 6. Patients with readmissions that did not end prior to 365 days after index hospitalization discharge could have had MDROs isolated after 365 days that were not captured by this study, though this involved only 14 patients (0.7%).
FIGURE 2.
Time to first positive multidrug-resistant organism (MDRO) culture within 1 year of index MDRO hospitalization by index hospitalization pathogen. All index pathogens are drug resistant. Log-rank, 21.220; 5° of freedom; P= .001. Note y-axis scale from 0.6 to 1.0.
We performed a multivariate Cox proportional hazards model comparing patients with an MDRO during a readmission to patients without any MDROs during readmission. Bone marrow transplant (HR, 1.79), end-stage renal disease (HR, 1.43), lymphoma (HR, 1.49), methicillin-resistant S. aureus (MRSA) during index hospitalization (HR, 1.29), and carbapenem-resistant P. aeruginosa during index hospitalization (HR, 1.99) were all associated with a significantly increased risk of having an MDRO isolated during a readmission (Table 3).
TABLE 3.
Index MDRO Hospitalization Factors Associated With Readmission (Within 1 Year) in Which an MDRO Was Isolated Compared to Readmitted Patients Without MDROs a
| Factor From Index MDRO Hospitalizationb |
HR (95% CI) | P Value |
|---|---|---|
| Carbapenem-resistant Pseudomonas | 1.98 (1.35–2.90) | <.001 |
| MRSA | 1.31 (1.03–1.68) | .047 |
| Bone marrow transplant | 1.80 (1.14–2.84) | .012 |
| Lymphoma | 1.48 (1.05–2.09) | .022 |
| End-stage renal disease | 1.42 (1.05–1.92) | .020 |
NOTE. MDRO, multidrug-resistant organism; HR, hazard ratio; CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus.
Multivariable Cox proportional hazards model.
Other variables included in the model that were not significant: ethnicity, sex, cardiovascular disease, congestive heart failure, chronic kidney disease, diabetes, leukemia, solid organ malignancy, Charlson comorbidity index, positive blood culture MDRO, positive other sterile site MDRO culture, VRE, drug-resistant Acinetobacter, discharge disposition, and index hospital length of stay.
In patients who had an MDRO isolated during readmission, the readmission MDRO was in the same pathogen class as the index hospitalization MDRO 68%–80% of the time, with 1 exception. For patients with drug-resistant Acinetobacter during index hospitalization, only 9.1% had drug-resistant Acinetobacter isolated during a readmission (Table 4), though numbers were small in this group. Discordant results between index and readmission cultures are shown in Supplemental Figure 1. To investigate why Acinetobacter was an outlier, we analyzed baseline characteristics of the cohort stratified by index hospitalization pathogen group. The proportion of patients who died or were discharged on hospice was significantly different between index pathogens, with Acinetobacter patients having the highest rate of death or discharge on hospice (P < .001) (Supplemental Table 5). Drug-resistant Acinetobacter patients were equally likely to have no bacterial cultures collected during readmissions (17.6%) and to be lost to follow-up (14.3%) as patients from other pathogen groups (data not shown).
TABLE 4.
Patient Survival, Readmission, and MDRO Recurrence Characteristics by Index Hospitalization Pathogen Group
| Index Drug-Resistant Pathogen |
No. (%) Surviving Index Hospitalization |
No. (%) Readmitted Within 1 Year |
No. (%) Readmitted With an MDRO |
No. (%) Readmitted (With Any MDRO) With an Isolate From the Same Class as the Index |
|---|---|---|---|---|
| Staphylococcus aureus | 1,461 (83.3) | 873 (59.8) | 216 (24.7) | 169 (78.2) |
| Enterococcus | 583 (68.6) | 402 (69.0) | 102 (27.1) | 73 (71.6) |
| Enterobacteriaceae | 1,008 (82.4) | 605 (60.0) | 110 (18.2) | 78 (70.9) |
| Acinetobacter | 63 (58.9) | 34 (54.0) | 11 (32.4) | 1 (9.1) |
| Pseudomonas | 150 (75.0) | 95 (63.3) | 31 (32.6) | 21 (67.7) |
| Polymicrobial | 188 (66.3) | 118 (62.8) | 35 (29.7) |
NOTE. MDRO, multidrug-resistant organism.
DISCUSSION
All-cause readmissions within 1 year after index MDRO hospitalization were frequent in this study. The 30-day and 1-year all-cause readmission rates were higher than in previously published studies examining readmissions after systemic infections, suggesting a higher readmission rate in patients with MDRO infections.6–10,12–15,20 Although data are limited, other studies suggest rates of readmission for MDROs similar to those in our study (32%).32 In the present study, the rate of readmission differed by type of MDRO isolated during the index hospitalization. Surviving patients with drug-resistant Acinetobacter were least likely to be readmitted within 1 year, and patients with drug-resistant Enterococcus were the most likely to be readmitted within 1 year. Independent risk factors for readmission within 1 year included certain comorbidities and certain pathogens (Table 2). These results suggest that patient and pathogen factors both contribute to all-cause readmission risk. Risk factors for mortality during the index MDRO hospitalization were less common as risk factors for readmission, which may explain why certain factors such as an ICU stay, which are associated with an increased risk of death, were thus associated with a reduced risk of readmission.
Almost 25% of patients readmitted within 1 year after an MDRO infection had an MDRO isolated during a readmission. The rate of readmissions during which an MDRO was isolated differed by the MDRO isolated at the index hospitalization. Few studies are available for comparison, but it appears that our readmission rate for MDRO patients may be similar to those of other cohorts.32,33 Whether drug resistance itself is a risk factor for readmission is not clear and is not addressed by this study. Previous work has shown higher rates of readmission for antibiotic-resistant (vs antibiotic-susceptible) pathogens.18,32 A history of MRSA, VRE, or an ESBL gram-negative organism was associated with 1.6-fold increased odds for readmission in one cohort.34 Another study showed no difference in readmission rates between methicillin-sensitive and MRSA when adjusting for other comorbidities and severity of illness.35 In the context of our study, it is important to recognize that our patients were likely at high risk of readmission due to their high baseline severity of illness and their infections. Because we did not include a group of patients with non-MDRO infections as a comparator, the impact of drug resistance on readmission rates cannot be deduced from the present study. Multicenter studies will likely be required to address the impact of drug resistance on readmission rates and will need to consider factors such as the adequacy of source control and appropriateness of therapy in calculation of readmission risk.
In the present study, independent risk factors for MDRO isolation during a readmission included comorbidities (receipt of BMT, ESRD, lymphoma) and certain pathogens during the index hospitalization (VRE, MRSA, and carbapenem-resistant P. aeruginosa) compared with patients who were readmitted but had no MDROs isolated. These results suggest that patient and pathogen factors both contribute to the risk of readmission with an MDRO. In addition, we found that the MDRO isolated during a readmission is in the same class as the index hospitalization MDRO 67%–78% of the time, except for Acinetobacter (~9%), though numbers were small in the Acinetobacter group. Recognizing the heterogeneity of MDROs that occur during readmissions will help to better define patients in need of broad-spectrum empiric antimicrobial therapy at readmission.
Our study has several limitations. The retrospective design of the study makes it difficult to elucidate possible confounders that could have biased the outcome measures. This was a single-center study, and results may not be generalizable to other centers. However, the high rates of readmission and readmissions during which MDROs were isolated should be applicable to other tertiary-care referral centers with similar patient case mixes. We relied on clinical cultures to define MDRO recurrence, which likely underestimates the actual rate of readmissions with MDROs because we did not capture surveillance cultures that might identify colonization. It is possible that patients received antibiotics prior to having cultures collected during readmissions, which would also lower the apparent MDRO recurrence rate. As diagnostic microbiology methods continue to improve, the incidence of culture-negative sepsis will likely decrease, making it easier to more accurately assess populations at highest risk of readmissions with an MDRO recurrence.
We were limited by a lack of data on non-MDRO pathogens with which to compare readmission rates, but previous work has shown that patients acquiring MDRO infections are fundamentally different than patients with non-MDRO infections.26,27 Therefore, it was more important to compare patients with different types of MDROs rather than compare patients with non-MDRO infections to those with drug-resistant infections due to the same organism.
Another limitation of our study is loss to follow-up. Some patients may have gone to a facility outside of the BJC Healthcare network where we were unable to capture their MDRO recurrence status. Patients censored at loss to follow-up were more likely to be discharged to a SNF, LTACH, or NH, where the local physicians could have diagnosed and treated MDRO infections without our knowledge. In addition, patients lost to follow-up were more likely to have had an ICU stay during their index MDRO hospitalization, which could explain why having an ICU stay was associated with a reduced hazard ratio for readmission. The study was also limited by a lack of treatment data to assess the appropriateness of antibiotic therapy as a potential reason for readmissions in this cohort.
In conclusion, all-cause readmissions and readmissions during which an MDRO was isolated are common among patients with MDRO infections. Patient characteristics and pathogen type both contribute to the risk of readmissions overall and readmissions during which an MDRO was isolated. By analyzing readmissions with MDROs, we aimed to elucidate risk factors for readmission in this population, to provide opportunities for targeted interventions to potentially prevent readmissions, and to help define the risk of MDRO infection during readmission so that infection prevention programs can initiate the proper isolation precautions and reduce the spread of MDROs within hospitals. In addition, our research gives providers guidance about which patients are most likely to need broad-spectrum antimicrobials when they are readmitted, based on the knowledge that MDRO recurrence is high in this population.
Supplementary Material
ACKNOWLEDGMENTS
Financial support: This work was supported by the Washington University Institute of Clinical and Translational Sciences (grant no. UL1TR000448) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) (to J.P.B.). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. This work was also supported by the Barnes-Jewish Hospital Foundation (to M.H.K.) and by the Washington University Institute of Clinical and Translational Sciences grant (no. UL1TR000448, subaward no. KL2TR000450) from the National Center for Advancing Translational Sciences (NCATS) ofthe NIH (to J.H.K.).
Footnotes
Potential conflicts of interest: All authors report no further conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.254.
REFERENCES
- 1.Pearson S, Stewart S, Rubenach S. Is health-related quality of life among older, chronically ill patients associated with unplanned readmission to hospital? Aust N Z J Med 1999;29:701–706. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med 2005;165:1274–1279. [DOI] [PubMed] [Google Scholar]
- 3.Hu Y, McMurry TL, Stukenborg GJ, Kozower BD. Readmission predicts 90-day mortality after esophagectomy: analysis of Surveillance, Epidemiology, and End Results Registry linked to Medicare outcomes. J Thorac Cardiovasc Surg 2015;150: 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hicks CW, Tosoian JJ, Craig-Schapiro R, et al. Early hospital readmission for gastrointestinal-related complications predicts long-term mortality after pancreatectomy. Am J Surg 2015; 210:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gohil SK, Datta R, Cao C, et al. Impact of hospital population case-mix, including poverty, on hospital all-cause and infection-related 30-day readmission rates. Clin Infect Dis 2015;61:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 2015;313:1055–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun A, Netzer G, Small DS, et al. Association between index hospitalization and hospital readmission in sepsis survivors. Crit Care Med 2016;44:478–487. [DOI] [PubMed] [Google Scholar]
- 8.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med 2014;190:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu V, Lei X, Prescott HC, Kipnis P, Iwashyna TJ, Escobar GJ. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med 2014;9:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortego A, Gaieski DF, Fuchs BD, et al. Hospital-based acute care use in survivors of septic shock. Crit Care Med 2015;43:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med 2014;29:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly JP, Hohmann SF, Wang HE. Unplanned readmissions after hospitalization for severe sepsis at academic medical center-affiliated hospitals. Crit Care Med 2015;43:1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med 2015;43:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TK, Fuchs BD, Small DS, et al. Post-acute care use and hospital readmission after sepsis. Ann Am Thorac Soc 2015;12:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang DW, Tseng CH, Shapiro MF. Rehospitalizations following sepsis: common and costly. Crit Care Med 2015;43:2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S. Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA 2017;317:530–531. [DOI] [PubMed] [Google Scholar]
- 17.Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2014;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andruska A, Micek ST, Shindo Y, et al. Pneumonia pathogen characterization Is an independent determinant of hospital readmission. Chest 2015;148:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta R, Brown S, Nguyen VQ, et al. Quantifying the exposure to antibiotic-resistant pathogens among patients discharged from a single hospital across all California healthcare facilities. Infect Control Hosp Epidemiol 2015;36:1275–1282. [DOI] [PubMed] [Google Scholar]
- 20.Emerson CB, Eyzaguirre LM, Albrecht JS, Comer AC, Harris AD, Furuno JP. Healthcare-associated infection and hospital readmission. Infect Control Hosp Epidemiol 2012;33: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micek ST, Heard KM, Gowan M, Kollef MH. Identifying critically ill patients at risk for inappropriate antibiotic therapy: a pilot study of a point-of-care decision support alert. Crit Care Med 2014;42:1832–1838. [DOI] [PubMed] [Google Scholar]
- 22.Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011;69:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quezada Joaquin NM, Diekema DJ, Perencevich EN, Bailey G, Winokur PL, Schweizer ML. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra T, Neelakanta A, Dombecki C, et al. Burden of Clostridium difficile infection on hospital readmissions and its potential impact under the Hospital Readmission Reduction Program. Am J Infect Control 2015;43:314–317. [DOI] [PubMed] [Google Scholar]
- 25.Duggal A, Barsoum W, Schmitt SK. Patients with prosthetic joint infection on IV antibiotics are at high risk for readmission. Clin Orthop Relat Res 2009;467:1727–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unusual Susceptibility Profiles Alert. Centers for Disease Control and Prevention website. http://www.cdc.gov/nhsn/pdfs/gen-support/usp-alert-current.pdf. Accessed July 19, 2016.
- 27.Multidrug-Resistant Organism and Clostridium difficile Infection (MDRO/CDI) Module. Centers for Disease Control and Prevention website. http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDA Dcurrent.pdf. Published 2016. Accessed July 19, 2016.
- 28.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–281. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement CLSI document M100-S25. Vol. 25 Wayne, PA: CLSI; 2015. [Google Scholar]
- 30.Messina JA, Cober E, Richter SS, et al. Hospital readmissions in patients with Carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2016;37:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison GM, Muldoon EG, Kent DM, et al. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014;58:812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaw LK, Robinson JO, Ho KM. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. Lancet Infect Dis 2014;14:967–975. [DOI] [PubMed] [Google Scholar]
- 33.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002;136:834–844. [DOI] [PubMed] [Google Scholar]
- 34.Butler AM, Olsen MA, Merz LR, et al. Attributable costs of enterococcal bloodstream infections in a nonsurgical hospital cohort. Infect Control Hosp Epidemiol 2010;31:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palacios-Baena ZR, Gutierrez-Gutierrez B, De Cueto M, et al. Development and validation of the INCREMENT-ESBL predictive score for mortality in patients with bloodstream infections due to extended-spectrum-beta-lactamase-producing Enter-obacteriaceae. J Antimicrob Chemother 2017;72:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.