Abstract
One in ten infants born in the United States is born preterm, or prior to 37 weeks gestation. Exposure to elevated levels of metals, such as lead and arsenic, has been linked to higher risk of preterm birth (PTB), but consequences of lower levels of exposure and less studied metals are unclear. We examined the associations between 17 urinary trace metals individually and in mixtures in relation to PTB. The LIFECODES birth cohort enrolled pregnant women at <15 weeks gestation at Brigham and Women’s Hospital in Boston. We selected cases of PTB (n=99) and unmatched controls (n=291) and analyzed urine samples for a panel of trace metals (median: 26 weeks gestation). We used logistic regression models to calculate the odds ratio (OR) for PTB and subtypes of PTB based on presentation at delivery. Subtypes included spontaneous and placental PTB. We used elastic net (ENET) regularization to identify individual metals or pairwise interactions that had the strongest associations with PTB, and principal components analysis (PCA) to identify classes of exposures associated with the outcome. We observed increased odds of PTB (OR: 1.41, 95% Confidence Interval [CI]: 1.12, 1.78) in association with an interquartile range difference in urinary copper (Cu). We also observed an increased OR for selenium (OR: 1.33, 95% CI:0.98, 1.81). ENET selected Cu as the most important trace metal associated with PTB. PCA identified 3 principal components (PCs) that roughly reflected exposure to toxic metals, essential metals, and metals with seafood as a common source of exposure. PCs reflecting essential metals were associated with an increased odds of overall and spontaneous PTB. Maternal urinary copper in the third trimester was associated with increased risk of PTB, and statistical analyses for mixtures indicated that after accounting for correlation this metal was the most important statistical predictor of the outcome.
Keywords: metals, preterm birth, mixtures analysis
1. Introduction
Preterm birth (PTB), defined as birth prior to 37 weeks gestation, is one of the most important causes of neonatal morbidity and mortality and is associated with adverse health outcomes later in life (CDC 2017). The Centers for Disease Control and Prevention (CDC) estimated that in 2016, one in ten infants in the United States was born preterm (CDC 2017). Risk factors for PTB include, but are not limited to, maternal age, race, low socioeconomic status, illicit drug use, history of PTB, multiple pregnancy, pregnancy complications, and other medical disorders (Frey and Klebanoff 2016; Goldenberg et al. 2008). PTB can be further classified into subtypes based on presentation at birth, and risk factors as well as mechanisms may differ between these subtypes (McElrath et al. 2008).
A growing body of evidence suggests associations between exposure to chemicals during pregnancy and an increased risk of PTB (Ferguson et al. 2013; Ferguson and Chin 2017; Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes 2007; Simmons et al. 2010). For metals specifically, the evidence for the association between lead (Pb) and PTB is well-established (Cantonwine et al. 2010; Cheng et al. 2017; Jelliffe-Pawlowski et al. 2006; Perkins et al. 2014; Torres-Sanchez et al. 1999). Arsenic (As) and cadmium (Cd) exposure are also associated with increased risk of PTB (Ferguson and Chin 2017). Most of these studies have examined populations with historically high exposure to metals, such as As in Bangladesh and Pb and Cd in China; as such, the findings may not predict effects in populations with lower levels of exposure(Myers et al. 2010; Wang et al. 2016; Yang etal. 2016).
Furthermore, most studies that have assessed the relationships between multiple metals and PTB have focused on single-pollutant models only (Shirai et al. 2010; Tsuji et al. 2018; Wai et al. 2017). Since exposures in humans never occur in isolation, it is an important next step to investigate multiple metals simultaneously (Braun et al. 2016; Claus Henn et al. 2014; Taylor et al. 2016). However, there is a small but growing number of analyses using advanced statistical methodologies to analyze metal mixtures and health outcomes to better understand aggregate effects or to identify “bad actors” within a correlated set of exposures (Bobb et al. 2015; Deyssenroth et al. 2018; Horton et al. 2018; Sanders et al. 2015; Valeri et al. 2017). These previous studies have used methods, such as Bayesian kernel machine regression (BKMR) and weight quantile sums (WQS) to explore metal mixtures in relation to health outcomes, however they have not been utilized in the study of PTB.
In the present study we analyzed a panel of 17 trace metals measured in urine samples from the 3rd trimester of pregnancy and estimated associations with PTB. In addition, because this study has the largest number of trace metal analytes to date to address this research question, we investigated the effects of mixtures using two approaches. First, we used elastic net (ENET) regularization to identify the individual metals and pairwise interactions from the mixture that are most strongly associated with PTB. Second, we used principal components analysis (PCA) to examine associations with correlated groupings.
2. Methods
The LIFECODES birth cohort, an on-going prospective cohort study originally designed to identify risk factors for preeclampsia, recruits pregnant women who are planning to deliver at Brigham and Women’s Hospital in Boston, Massachusetts, USA. Women are enrolled and consented at <15 weeks gestation and participate in four study visits (Cantonwine et al. 2016). At the first visit, participants complete a questionnaire providing demographic information, tobacco and alcohol use, and pregnancy as well as medical history. Urine samples are collected at up to four timepoints for each participant (median 10, 18, 26, and 35 weeks of gestation) and stored at −80°C. The present analysis utilizes participants from a nested case-control study of PTB that includes women who were recruited from 2006-2008 (Ferguson et al. 2014). There were approximately 150 singleton preterm births and 1100 singleton term births recruited into the LIFECODES birth cohort during that period, and demographic characteristics of the overall cohort were very similar to those of the participants who were included in the case-control study (Cantonwine et al. 2016). The nested case-control study selected nearly all the cases of preterm birth as well as unmatched controls in a 3:1 ratio. Individuals from the case-control study were included in the present analysis if they had available urine samples from the third study visit.
Gestational age to determine preterm birth was calculated using the last menstrual period verified by ultrasound and two maternal-fetal medicine specialists. Preterm birth was defined as delivery before 37 weeks completed gestation. In addition, previous research suggests that cases of PTB differ by presentation at delivery. In a previous study of newborns born before 28 weeks gestation, deliveries with a spontaneous presentation (i.e., with preterm premature rupture of the membranes [PPROM] or spontaneous preterm labor) had similar placental characteristics indicative of inflammation (McElrath et al. 2008). Alternatively, PTB that occurred with presentation of intrauterine growth restriction or preeclampsia had evidence of poor placentation. Thus, in our study population, cases of PTB were further classified into spontaneous PTB (n = 46) and placental PTB (n = 22) for analysis based on these presentations at delivery. Cases that did not fall into either category were not examined separately, as we had no prior hypothesis about a unifying mechanism. The study was approved by the Institutional Review Board at Brigham and Women’s Hospital and was deemed exempt by the University of Michigan and the National Institute of Environmental Health Sciences (NIEHS).
2.1. Urinary trace metals analysis
Urine samples from the visit at median 26 weeks gestation were analyzed at NSF International (Ann Arbor, MI, USA) as part of a pilot study for the Children’s Health and Exposure Assessment Resource (CHEAR), a program designed to expand resources for analyzing environmental exposures in NIH-funded studies on children’s health (Balshaw et al. 2017; NIEHS 2018). Seventeen trace metals were analyzed on a Thermo Fisher (Waltham, MA, USA) ICAPRQ inductively coupled plasma mass spectrometry (ICPMS) and CETAC ASX-520 autosampler, including As, barium (Ba), beryllium (Be), Cd, copper (Cu), chromium (Cr), mercury (Hg), manganese (Mn), molybdenum (Mo), nickel (Ni), Pb, selenium (Se), tin (Sn), thallium (Tl), uranium (U), tungsten (W), and zinc (Zn). A PFA-ST nebulizer, quartz spray chamber, quartz torch, quartz injector tube, and nickel sample and skimmer cones were utilized in the analysis. An in-house method was developed based on the CDC Laboratory Procedure Manual for Urine Multi-Element ICP-DRC-MS (method no. 3018.3 and 3018A.2; revised 2012 March 19). The LOD was determined by calculating three times the standard deviation of the background level of the blanks. Additional methods on the analysis of trace metals are provided as Appendix 1 in the supplemental material.
Some metal concentrations below the limit of detection (LOD) were reported by the ICP-MS. These machine-reported values were kept as is and concentrations not reported by the ICP-MS were replaced with the LOD divided by the square root of two (Hornung and Reed 1990). If a metal had >70% of the participants below the LOD (i.e., either machine-reported or imputed), it was treated as detect versus non-detect in subsequent analyses. To account for urine dilution, we corrected for urinary specific gravity using the formula MSG = M [(1.015-1) / (SG-1)], where MSG is the specific gravity-corrected metal concentration, M is the measured metal concentration in urine, 1.015 is the median specific gravity of all participants, and SG is the specific gravity for the individual sample (Ferguson et al. 2014).
2.2. Statistical analysis
We examined population characteristics and pregnancy-related variables of the cases and controls individually and overall, including maternal age, race/ethnicity, education, health insurance provider, prepregnancy body mass index (BMI), self-reported tobacco use during pregnancy, self-reported alcohol use during pregnancy, parity, use of assisted reproductive technology (ART), self-reported multivitamin use during pregnancy, and sex of the neonate.
We analyzed trace metal distributions by calculating geometric mean (GM) concentrations as well as 25 th, 50th, 75 th, and 95 th percentiles. In addition, we calculated the median, 25 th, and 75 th percentile of the specific gravity-corrected metals concentrations in cases and controls separately. For those treated as detect versus non-detect, we calculated the number and percentage detected within each group. In addition to looking at the distribution of metals by case status, we calculated the GM by levels of demographic covariates and tested for differences between the groups using general linear models.
In our primary analysis, we used logistic regression models to calculate odds ratios (OR) and 95% Confidence Intervals (CI) for overall PTB in association with each urinary trace metal concentration. To account for specific gravity, we compared associations across four models: 1) metals uncorrected and models unadjusted; 2) metals specific gravity-corrected and models unadjusted; 3) metals uncorrected and models adjusted for specific gravity; and 4) metals specific gravity-corrected and models adjusted for specific gravity (O'Brien et al. 2016). Because results were consistent across models and because approach 4) removes the potential collider between covariates and specific gravity (O'Brien et al. 2016), all final models were presented with specific gravity-corrected trace metal concentrations with specific gravity additionally included as a covariate.
We selected other covariates for the adjusted models using a forward stepwise selection procedure, examining the effect of including covariates that were associated with urinary trace metal concentrations and keeping those variables that changed effect estimates by >10%. All final models were adjusted for maternal age (continuous), race (white, black, and other), education (≤ high school graduate, technical school, junior college/some college, ≥ college graduate), pre-pregnancy BMI (continuous), and gestational age at time of sample collection (continuous). History of PTB, smoking during pregnancy, health insurance provider, sex, parity, use of ART, and multi-vitamin use in pregnancy were also considered as covariates, but had no significant effect on the estimates and were not included in the final models. As a secondary analysis, we recreated the same statistical models but examined spontaneous and placental PTB separately, with other types of PTB excluded from each model. Additionally, we examined associations with tertiles of exposure to examine deviations from linearity. Since many of these trace elements are also essential nutrients (Cu, Mn, Mo, Ni, Se, Zn), we set the second tertile as the reference level for those metals (Bauer et al. 2017). For those that are non-essential (As, Ba, Cd, Hg, Pb, Sn, Tl), we set the reference level at the first tertile. These analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
In addition to modeling single chemicals, we examined the exposures as a mixture using two methods: ENET regularization and PCA. ENET was used to determine which element(s) of the mixture were driving the association with PTB and its subtypes while adjusting for covariates (Zou and Hastie 2005). ENET is a regularization and variable selection method, which can handle high dimensional data. It is a combination of the least absolute shrinkage and selection operator (LASSO), which drives the coefficients of irrelevant variables to zero, and ridge regression, which penalizes the square of the regression coefficients and shrinks the coefficients of highly correlated variables proportionally toward but never to zero (Friedman et al. 2010). Thus, ENET selects a group of variables by shrinking some effect estimates and allowing others to be shrunk to zero. A timing parameter α controls the balance between the LASSO and ridge penalties. We used cross validation to optimize the α We performed a second ENET in which we allowed for inclusion of all possible pairwise interactions in the model, and we did not mandate that main effects be included for those interaction terms. After the ENET procedures, we refit generalized linear models (GLMs) to produce ORs and 95% CIs adjusting for covariates. For these analyses, we used the “glmnet” package in R version 3.4.1.
PCA is a variable reduction method that produces components, called principal components (PCs), without considering the outcome of interest (O'Rourke and Hatcher 2013). These PCs are linear combinations of observed variables that are weighted by loadings corresponding to each variable. Loadings are produced via an eigenequation to satisfy the principle of least squares, and a higher loading indicates that the variable is represented more in the given component. The number of PCs is equal to the number of variables, however only the first few will be meaningful. There are several approaches to determine the cutoff for the number of components. We used a combination of: 1) the “Eigenvalue-one” method where only components with an eigenvalue ≥1 are selected; 2) the Scree test, which uses a plot of the eigenvalues; and 3) keeping the components that account for a specified proportion of the variance (O'Rourke and Hatcher 2013). We used a varimax rotation, which is an orthogonal rotation used to maximize the variance in the factor pattern matrix. In the first estimation of the PCs, we included the 14 metals with >80% values over the LOD, excluding the metals that were treated as detect/non-detect in single-pollutant models. Ba and Tl loaded onto multiple components, indicating that they were complex items (O'Rourke and Hatcher 2013). Thus, we re-created PCs without Ba and Tl. We then fit the PCs into a logistic regression model adjusting for covariates. We used SAS 9.4 for PCA analysis.
3. Results
Table 1 displays the distribution of demographic characteristics for all participants and by case status. Participants were mostly over the age of 30 years, with a median of 32.7 years in the full population. Most participants were White (60.0%) and had private health insurance providers (83.2%), with only minor differences noted between cases and controls. The distribution of education levels was similar between groups, with 39% of all participants reporting they were college graduates or higher. Slightly more than half of the participants had a normal BMI prior to pregnancy (58.0%) with both groups having similar distributions. There was little self-reported tobacco use (6.4%) or alcohol (3.8%) consumption during pregnancy in our population. Approximately 9.0% of participants reported using ART with no difference between groups. Most participants took multivitamins during pregnancy (70.8%) and there was no significant difference in multivitamin use between case and control participants.
Table 1.
Distribution of demographic characteristics overall and by case status: median (25th, 75th percentiles) or n (%).
| Demographic Characteristic | Overall (n = 390) |
Cases (n = 99) |
Controls (n = 291) |
P1 |
|---|---|---|---|---|
| Maternal age, years | 32.2 (29.2, 35.7) | 33.0 (30.2, 36.3) | 31.9 (28.9,35.6) | 0.14 |
| 24 years or younger | 41 (10.5) | 7(7.1) | 34(11.7) | |
| 25-29 years | 76 (19.5) | 17 (17.2) | 59 (20.3) | |
| 30-34 years | 159 (40.8) | 43 (43.4) | 116(39.9) | |
| 35+ years | 114 (29.2) | 32 (32.3) | 82 (28.2) | |
| Race/ethnicity | 0.74 | |||
| White | 234 (60.0) | 61 (61.6) | 173 (59.5) | |
| African American | 58 (14.9) | 14 (14.2) | 44 (15.1) | |
| Other | 98 (25.1) | 24 (24.2) | 74 (25.4) | |
| Education | 0.20 | |||
| High school degree or less | 53 (13.6) | 17 (17.2) | 36(12.4) | |
| Technical college | 59 (15.1) | 17 (17.2) | 42 (14.4) | |
| Junior college or some college | 116 (29.7) | 28 (28.3) | 88 (30.2) | |
| ≥ College graduate | 152 (39.0) | 36 (36.3) | 116 (39.9) | |
| Missing | 10 (2.6) | 1 (1.0) | 9(3.1) | |
| Health insurance | 0.44 | |||
| Private/HMO/self-pay | 317 (81.3) | 84 (84.9) | 233 (80.1) | |
| Public | 64 (16.4) | 14 (14.1) | 50 (17.2) | |
| Missing | 9 (2.3) | 1 (1.0) | 8 (2.7) | |
| Pre-pregnancy BMI | 0.40 | |||
| <25 kg/m2 | 226 (58.0) | 56 (56.6) | 170 (58.4) | |
| 25-30 kg/m2 | 94 (24.1) | 21 (21.2) | 73 (25.1) | |
| >30 kg/m2 | 70 (17.9) | 22 (22.2) | 48 (16.5) | |
| Tobacco use | 0.22 | |||
| None in pregnancy | 360 (92.3) | 90 (90.9) | 270 (92.8) | |
| Some in pregnancy | 25 (6.4) | 9 (9.1) | 16 (5.5) | |
| Missing | 5(1.3) | 0(0) | 5(1.7) | |
| Alcohol use | ||||
| None in pregnancy | 366 (93.9) | 96 (100.0) | 270 (92.8) | |
| Some in pregnancy | 15 (3.8) | 0 (0) | 15 (5.1) | |
| Missing | 9 (2.3) | 3 (3.0) | 6 (2.1) | |
| Parity | 0.74 | |||
| Nulliparous | 171 (43.9) | 42 (42.4) | 129 (44.3) | |
| Parous | 219 (56.1) | 57 (57.6) | 162 (55.7) | |
| History of PTB | ||||
| No | 350 (89.7) | 77 (77.8) | 273 (93.8) | <0.001 |
| Yes | 40 (10.3) | 22 (22.2) | 18 (6.2) | |
| Use of ART | 0.44 | |||
| No | 355 (91.0) | 92 (92.9) | 263 (90.4) | |
| Yes | 35 (9.0) | 7 (7.1) | 28 (9.6) | |
| Multivitamin use | ||||
| No | 109 (28.0) | 31 (31.3) | 78 (26.8) | 0.44 |
| Yes | 276 (70.8) | 68 (68.7) | 208 (71.5) | |
| Missing | 5 (1.2) | 0 (0) | 5 (1.7) | |
| Sex of neonate | 0.09 | |||
| Male | 170 (43.6) | 36 (36.4) | 134 (46.1) | |
| Female | 220 (56.4) | 63 (63.6) | 157 (53.9) |
p-value denotes differences between cases and controls by t-test.
Abbreviations: BMI, body mass index; PTB, preterm birth; ART, assisted reproductive technology.
Most analytes were detected in a high proportion of urine samples (Table 2). The percentage below the LOD was greater than 80% for Be, Cr, U, W. These metals were subsequently treated as detect versus non-detect in all the statistical models. In comparison to women from the 2007–2008 National Health and Nutrition Examination Survey (NHANES), some urinary trace metals in this study population were higher while others were lower (CDC 2018). The most notable differences were the higher concentrations of As (GM 18.5 ppb) and Mn (GM 0.85 ppb) in our population compared to the NHANES population (GM 7.14 ppb and 0.13 ppb, respectively; Table 2). Urinary Cu, Ni, Se, Zn, and Cr were not measured by NHANES; however, all but Cr were measured by the Canadian Health Measures Survey Cycle 1 (2007-2009). The women in our study had higher concentrations of Cu, Ni, and Zn compared to the women from the Canadian survey. In that study population, females aged 20-39 years had a GM of 7.82 ppb for urinary Cu which was slightly lower compared to what was observed in our study population (9.65 ppb) (Health Canada 2010).
Table 2.
Distribution of trace metals from third trimester urine samples (ppb) in all women from the present study population and from women in the National Health and Nutrition Examination Survey (NHANES) from 2007-2008.1
| Metals | N | LOD | n (%) <LOD |
GM | 25% | 50% | 75% | 95% | NHANES GM |
|---|---|---|---|---|---|---|---|---|---|
| As | 390 | 0.30 | 0 (0) | 18.5 | 9.66 | 18.1 | 32.5 | 95.6 | 7.14 |
| Ba | 390 | 0.10 | 4 (1.03) | 1.74 | 1.01 | 1.95 | 3.46 | 6.33 | 1.51 |
| Cd | 390 | 0.06 | 218 (55.9) | 0.08 | 0.04 | 0.08 | 0.14 | 0.42 | 0.19 |
| Cu | 390 | 2.50 | 32 (8.20) | 9.65 | 7.08 | 9.22 | 12.2 | 19.8 | 7.821 |
| Hg | 390 | 0.05 | 32 (8.20) | 0.47 | 0.27 | 0.51 | 0.97 | 2.15 | 0.43 |
| Mn | 390 | 0.08 | 6 (l.50) | 0.85 | 0.51 | 0.72 | 1.15 | 5.05 | 0.132 |
| Mo | 390 | 0.30 | 0 (0) | 51.7 | 37.1 | 51.3 | 69.8 | 132.3 | 40.5 |
| Ni | 390 | 0.80 | 54 (13.9) | 2.60 | 1.87 | 2.84 | 3.98 | 6.83 | 1.001 |
| Pb | 390 | 0.10 | 92 (23.6) | 0.27 | 0.17 | 0.35 | 0.62 | 1.32 | 0.44 |
| Se | 390 | 5.00 | 3 (0.80) | 37.7 | 30.0 | 37.5 | 46.3 | 67.4 | 42.41 |
| Sn | 390 | 0.10 | 24 (6.20) | 0.71 | 0.36 | 0.61 | 1.22 | 4.54 | 0.622 |
| Tl | 390 | 0.02 | 61 (15.6) | 0.10 | 0.08 | 0.13 | 0.18 | 0.28 | 0.17 |
| Zn | 390 | 2.00 | 0 (0) | 227 | 149 | 251 | 379 | 688 | 1621 |
| Be | 390 | 0.04 | 356 (91.3) | 0.02 | 0.01 | 0.02 | 0.04 | 0.11 | <LOD |
| Cr | 390 | 0.40 | 330 (84.6) | 0.20 | 0.10 | 0.22 | 0.41 | 1.06 | NR |
| U | 390 | 0.01 | 342 (87.7) | 0.006 | 0.004 | 0.007 | 0.01 | 0.04 | 0.006 |
| W | 390 | 0.20 | 309 (79.2) | 0.13 | 0.09 | 0.14 | 0.24 | 0.53 | 0.09 |
Abbreviations: Arsenic (As), Barium (Ba), Cadmium (Cd), Copper (Cu), Mercury (Hg), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), Lead (Pb), Selenium (Se), Tin (Sn), Thallium (Tl), Zinc (Zn), Beryllium (Be), Chromium (Cr), Uranium (U), Tungsten (W), Limit of Detection (LOD), Geometric Mean (GM), Not Reported (NR)
Where NHANES measurements were unavailable, we present urinary concentrations from the Canadian Health Measures Survey Cycle 1, 2007-2009, which includes females ages 20-39 years.
Mn and Sn levels measured in 2011-2012.
Shading denotes metals with >70% of samples below the limit of detection.
In crude comparisons of specific-gravity corrected trace metal concentrations (Table A), we observed higher levels of urinary Cu in cases (median 10.0 ppb) compared to controls (median 8.99 ppb). Also among cases, we observed lower levels of Ba (median 1.88 ppb vs. 1.95 ppb in controls) and higher levels of Se (median: 39.8 ppb vs 38.8 ppb in controls). Detection of Cr was higher in cases (21.2%) compared to controls (13.4%) as well.
We noted several differences in GM urinary metal concentrations by demographic characteristics (Table B). Some notable differences included higher concentrations of Se and Zn in African-American women compared to White women. Those who graduated from college had much lower concentrations of Zn compared to women with a high school degree or less. Cu and Zn were higher for women with BMI ≥25 kg/m2 compared to women with BMI <25 kg/2. Women who smoked during pregnancy also had higher Cu and Zn in their urine compared to women who did not smoke during pregnancy, along with higher Cd and Pb; this is expected as Cd, Cu, Pb, and Zn are some of the metals found in cigarette smoke (Bernhard et al. 2005; Pappas 2011).
Adjusted ORs for PTB in association with 3rd trimester urinary trace metals were mostly elevated (Table 3). We observed an elevated OR of 1.41 (95% CI 1.12, 1.78) in association with an interquartile range (IQR) increase in Cu. Ba (OR 1.27, 95% CI 0.90, 1.77) and Se (OR 1.33, 95% CI 0.98, 1.81) had elevated ORs that were suggestive as well. Sn (OR 0.89, 95% CI 0.65, 1.22) and Be detection (OR 0.48, 95% CI 0.18, 1.29) were protective for PTB but had wide confidence intervals. When we analyzed by the phenotypes of PTB, i.e., spontaneous and placental, the association for Cu appeared for both spontaneous (OR 1.52, 95% CI 1.14, 2.03) and placental (OR 1.50, 95% CI 0.99, 2.27) PTB. The association we observed for Se appeared to be driven by an association with spontaneous (OR 1.69, 95% CI 1.11, 2.57) rather than placental PTB (OR 0.83, 95% CI 0.53, 1.29). Associations between Ba and PTB were similar across subtypes. We also observed several additional associations among subtype analyses that were not apparent when examining overall PTB. Detection of Cr in urine samples was associated with an increased OR for placental PTB (OR 4.19, 95% CI 1.16, 15.1). We also observed positive associations between spontaneous PTB and U (OR 2.05, 95% CI 0.88, 4.80) as well as W (OR 1.94, 95% CI 0.95, 3.97).
Table 3.
Adjusted1 odds ratio (95% Confidence Interval) of overall, spontaneous, and placental preterm birth (PTB) in association with an interquartile range difference in urinary trace metal concentration and by principal components (PC).
| Overall PTB | Spontaneous PTB | Placental PTB | |
| Metal | (n cases = 99) (n controls = 291) |
(n cases = 46) (n controls = 291) |
(n cases = 22) (n controls = 291) |
| As | 0.98 (0.72, 1.33) | 0.89 (0.58, 1.38) | 1.07 (0.49, 2.35) |
| Ba | 1.27 (0.90, 1.77) | 1.27 (0.84, 1.93) | 1.51 (0.75, 3.04) |
| Cd | 1.03 (0.78, 1.37) | 1.13 (0.79, 1.63) | 1.73 (0.64, 4.66) |
| Cu | 1.41 (1.12, 1.78) | 1.52 (1.14, 2.03) | 1.50 (0.99, 2.27) |
| Hg | 1.13 (0.85, 1.51) | 1.28 (0.82, 1.98) | 1.02 (0.70, 1.50) |
| Mn | 1.08 (0.85, 1.37) | 1.14 (0.77, 1.69) | 0.97 (0.58, 1.61) |
| Mo | 1.05 (0.80, 1.37) | 1.14 (0.71, 1.82) | 0.97 (0.60, 1.58) |
| Ni | 0.96 (0.75, 1.22) | 1.19 (0.77, 1.85) | 0.99 (0.71, 1.39) |
| Pb | 1.19 (0.91, 1.55) | 1.15 (0.82, 1.60) | 1.45 (0.91, 2.31) |
| Se | 1.33 (0.98, 1.81) | 1.69 (1.11, 2.57) | 0.83 (0.53, 1.29) |
| Sn | 0.89 (0.65, 1.22) | 0.99 (0.68, 1.43) | 0.83 (0.53, 1.29) |
| Tl | 0.96 (0.79, 1.16) | 0.95 (0.64, 1.41) | 0.96 (0.66, 1.42) |
| Zn | 1.15 (0.85, 1.57) | 1.21 (0.78, 1.90) | 1.04 (0.50, 2.14) |
| Be | 0.48 (0.18, 1.29) | 0.37 (0.09, 1.64) | 0.93 (0.17, 4.98) |
| Cr | 1.83 (0.93, 3.61) | 1.39 (0.57, 3.37) | 4.19 (1.16, 15.1) |
| U | 1.61 (0.79, 3.27) | 2.05 (0.88, 4.80) | 1.58 (0.36, 6.87) |
| W | 1.33 (0.73, 2.42) | 1.94 (0.95, 3.97) | 0.71 (0.16, 3.09) |
| Principal Components | |||
| Toxic | 1.03 (0.79, 1.34) | 1.02 (0.72, 1.44) | 1.37 (0.80, 2.33) |
| Essential | 1.36 (1.05, 1.76) | 1.58 (1.14, 2.20) | 1.16 (0.64, 2.10) |
| Fish-related | 1.05 (0.83, 1.35) | 1.09 (0.79, 1.51) | 0.93 (0.59, 1.46) |
Adjusted for specific gravity, maternal age, race, education, pre-pregnancy BMI, gestational age at time of sample collection. Shading denotes metals with >70% of samples below the limit of detection modeled as detect vs. non-detect.
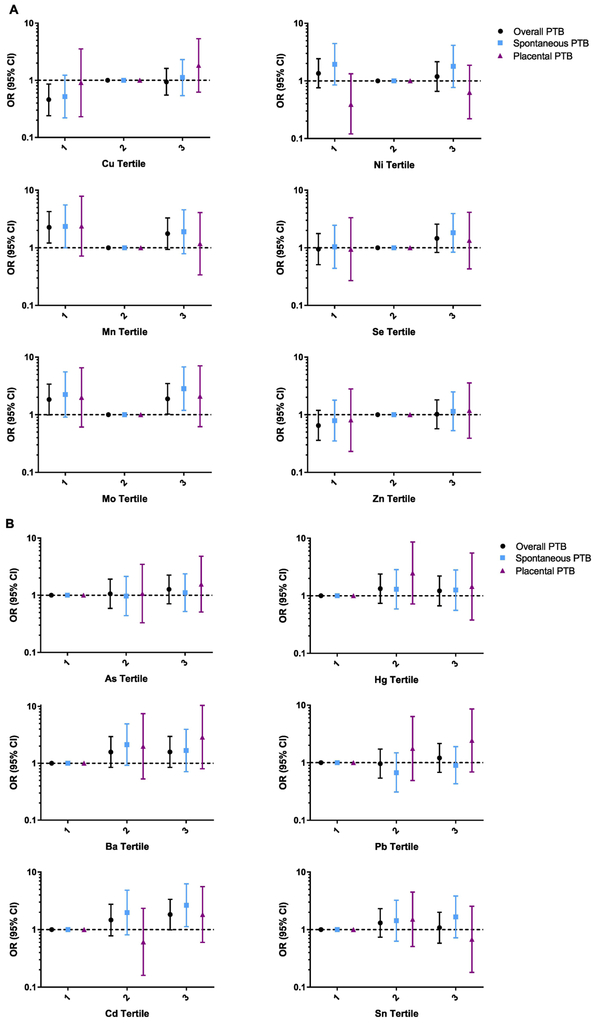
Figure 1 highlights the results from the tertile analysis, separated by essential vs. non-essential trace metals. Among essential metals, we observed the anticipated U-shaped response curve for Mn and Mo; however, with Cu, associations appeared to be linear. For non-essential metals, we observed positive trends between Cd and spontaneous PTB (ptrend = 0.03) and a non-significant trend between Pb and placental PTB (ptrend = 0.16).
Figure 1.
A. Adjusted1 odds ratios (95% confidence intervals) of all and subtypes of preterm birth (PTB) by tertiles of exposure. Referent levels were set at tertile 2 for essential metals (Cu, Mn, Mo, Ni, Se, Zn).
B. Adjusted1 odds ratios (95% confidence intervals) of all and subtypes of preterm birth (PTB) by tertiles of exposure. Referent levels were set at tertile 1 for non-essential metals (As, Ba, Cd, Hg, Pb, Sn).
1 Models adjusted for specific gravity, maternal age, race, education, pre-pregnancy BMI, gestational age at time of sample collection
Urinary trace metals displayed low to moderate correlations with one another (Figure A). In ENET analyses of individual urinary trace metals, Cu was the only metal selected, and effect estimates for all other trace metals were shrunk to zero. Note, the optimal tuning parameter, selected via crossvalidation, was 0.9 which favors shrinkage to zero rather than including multiple parameters in the model. When we included all pairwise interaction terms, the tuning parameter was more relaxed (alpha=0.1). In this model ENET selected Cu only for main effects, as well as several interaction terms (e.g., Cu*Cd). OR and 95% CIs for interaction terms are displayed in Table 4; however, refitted effect estimates should be interpreted with caution because effect estimate can be inflated and precision overestimated (Pavlou et al. 2016). We did not perform ENET among subtypes of PTB due to limitations in sample size.
Table 4.
Adjusted1 ORs (95% CI) for overall preterm birth in association with third trimester urinary metals refitting selected exposures from elastic net (ENET) regularization methods into generalized linear models
| Main Effects Model | Main Effects and Interaction Model |
|
|---|---|---|
| Metal | Overall Preterm Birth | |
| Ba | ||
| Cu | 1.90 (1.28, 3.02) | 1.09 (0.23, 4.71) |
| Hg | ||
| Mo | ||
| Se | ||
| Be | ||
| U | ||
| W | ||
| As:Be2 | 0.65 (0.40, 0.94) | |
| Cd:Cu | 0.92 (0.83, 1.02) | |
| Cu:Cr | ||
| Cu:Mo | 1.09 (0.93, 1.29) | |
| Cu:Se | 1.06 (0.78, 1.49) | |
| Cu:Zn | 1.01 (0.86, 1.20) | |
| Cu:W | ||
| Ni:Cr2 | 2.12 (1.18, 3.84) | |
| Tl:Cr2 | ||
Adjusted for specific gravity, maternal age, race, education, pre-pregnancy BMI, and gestational age at time of collection
Be and Cr have >70% of samples below the limit of detection and are modeled as detect vs. non-detect
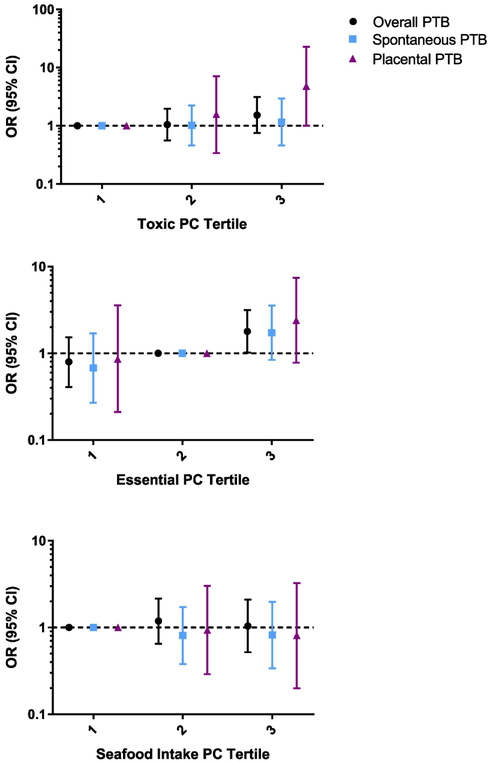
We identified three meaningful PCs in the analysis. PC1 was characterized by high loading from primarily toxic metals, Cd, Mn, and Pb (Table D). PC2 was characterized by high loading from essential metals, Cu, Se, and Zn. PC3 had high loading from metals that have previously been linked to seafood intake, As, Hg, and Sn (ATSDR 1999, 2005a, 2007b). PC1 was not associated with overall (OR 1.03, 95% CI 0.79, 1.34) or either subtype of PTB (Table 3). The PC for essential metals was associated with an increased risk of overall (OR 1.36, 95% CI 1.05, 1.76) and spontaneous (OR 1.58, 95% CI 1.14, 2.20) PTB. Finally, the associations between PC3 and PTB were null. In addition, we analyzed the PC as tertiles with the reference level at tertile 1 for PC1 and 3 and tertile 2 for PC2 (Figure 2). We observed mostly null associations across all types of PTB for each PC. We did observe an increased OR in the third tertile for PC2.
Figure 2.
Adjusted1 odds ratios (95% confidence intervals) of all and subtypes of preterm birth (PTB) by tertiles of principal components. Referent levels were set at tertile 2 for essential PC (PC2) and tertile 1 for toxic and seafood-related (PC1 and 3).
4. Discussion
Our analysis of 17 individual metals from 3rd trimester urine samples in pregnant women revealed positive associations between Cu and an increased risk of PTB, which was consistently elevated among subtypes of PTB and was the only metal selected from correlated exposures in ENET analysis. Additionally, the PC where Cu loaded onto a component with other essential metals, Se and Zn, was associated with an increased odds of overall and particularly spontaneous PTB. In contrast, we observed null associations between individual toxic metals and PTB in single-pollutant as well as mixtures models.
Our findings for Cu contrast with other studies where Cu deficiency was associated with increased risk of PTB (Algerwie and Khatri 1998; Galinier et al. 2005; Tsuzuki et al. 2013). The studies measured Cu from maternal serum or plasma or cord blood samples taken at birth or shortly thereafter, whereas we measured third trimester urinary Cu. Cu can be reliably measured in several biological matrices, including but not limited to blood, urine, hair, and nails (ATSDR 2004). A systematic review assessed the variety of methods used to determine Cu status in humans and concluded with the limited data available that serum Cu seems to be a more useful biomarker because it is better at detecting both Cu overload and deficiency than other biomarkers (Harvey et al. 2009).
Previous studies in pregnant women observed similar urinary concentrations to levels measured in our study population. A study from Australia found a median of 8.08 μg/L urinary Cu in pregnant women (n=173) for samples collected two weeks before delivery, which is similar to levels in our population (median: 9.22 ppb) (Callan et al. 2013). In a cohort of women in Spain, researchers also observed similar or slightly higher concentrations of urinary Cu compared with our study population with a median of 15 μg/g creatinine at the third trimester (Fort et al. 2014). Finally, as shown in Table 2, urinary concentrations in women participating in the Canadian Health Survey were also similar to what we observed in pregnant women from the Boston area (Health Canada 2010).
Cu is an essential nutrient that plays a role in several reduction and oxidation processes (Danzeisen et al. 2007; Uriu-Adams et al. 2010). The Institute of Medicine recommends a dietary intake of 900 μg/L per day (ATSDR 2004). Animal studies have shown that Cu deficiency in pregnancy is associated with many teratogenic effects, such as impaired development of the heart, blood vessels, and brain (Keen et al. 1998). However, too much Cu can lead to accumulation in the liver and brain and lead to oxidative stress (Danzeisen et al. 2007). The US Environmental Protection Agency and the Occupational Safety and Health Administration have set upper limit regulatory levels for Cu in drinking water and air in occupational settings, respectively (ATSDR 2004). Cu overload is rare because of the mechanisms in place designed to keep homeostatic balance, but case studies of women with Wilson’s disease, where those mechanisms are disrupted, have shown that it may be associated with intrauterine growth restriction and preeclampsia during pregnancy (Walker et al. 2011). Animal studies also show various developmental effects, such as decrease in average litter size and fetal body weight when mother rats are fed high doses of Cu in their diet (ATSDR 2004). Se, another essential trace metal, was also associated with increased OR of preterm birth in our study population. Similar to Cu, lower levels of Se in pregnancy have been associated with PTB, however in our population Se concentrations in urine are higher than other cohorts who have measured urinary concentrations during pregnancy (Fort et al. 2014; Health Canada 2010; Rayman et al. 2011; Zachara 2018). Recently, a study out of China observed similar patters with high concentrations of Cu and Se in cord serum and decreased birth weight, birth length, and head circumference (Tang et al. 2016).
In contrast with essential metals, we only observed slight or moderate associations with well-documented toxic metals like Pb and Cd, as well as Mn which is essential but can be toxic at high doses (Aschner and Aschner 2005; Williams et al. 2012). Additionally, we observed that our toxic metal component in PCA analysis was not associated with PTB. These compounds have been associated in several studies with PTB (Cheng et al. 2017; Li et al. 2017; Perkins et al. 2014; Wang et al. 2016; Yang et al. 2016). Notably, however, our study captures urinary levels of Cd and Pb that are equivalent to or lower than those observed in the general US population.
The last PC included As, Hg, and Sn, which have each been associated with seafood consumption (ATSDR 1999, 2005a, 2007b), and was not associated with PTB. Prenatal exposure to As in drinking water has been associated with increased risk of PTB in previous studies (Almberg et al. 2017; Rahman et al. 2018). However, both studies assessed exposure by measuring community water systems and not biological samples. We expected to observe associations between As and PTB in this study, especially because urinary concentrations were higher in this population compared to what was observed in NHANES in the same time period. Our inability to detect associations may be due to the fact that we did not measure the elemental, inorganic, and organic forms separately for these metals nor for individual As species. Most of the As found in seafood is the organic form, which is less harmful than the inorganic form which can be found in water, soil, and air (ATSDR 2007b). Because we were not able to distinguish these sources, true associations with species of As may have been obscured. Sn and Hg exposure have also been linked at adverse reproductive or birth outcomes (ATSDR 2005a; Xue et al. 2007); however, these compounds are also found in seafood which may have protective effects outweighing adverse consequences.
Between 2006-2008, Boston had a population of approximately 610,000, placing it among the top 25 most populated cities in the U.S. As a large metropolitan city, residents in and around Boston are exposed to a host of environmental chemicals, including metals, from traffic pollution, nearby industries, and the built environment. Other exposure sources for these metals include diet and water. Cu is commonly found in the environment and humans are exposed via ingestion, inhalation, and dermal contact (ATSDR 2004). Most drinking water sources in Massachusetts do not have high levels of Cu, so most exposure from drinking water is likely from Cu pipes and brass fittings (Massachusetts Department of Public Health 2016). According to ATSDR, MA also has a number of industrial facilities that release Cu into the air and water, in addition to other sources of environmental Cu, including waste water practices and combustion, which could be potential sources of exposure and explain the elevated levels observed in our study population (ATSDR 2004).
This study had several limitations. Although we measured a large panel of metals, urine may not be the ideal biomarker for all of them. Pb, W, and Zn are more appropriately analyzed in blood, although urine biomarkers can indicate more recent exposure to metals and are highly correlated with concentrations earlier in pregnancy (ATSDR 2005b, c, 2007a; Fort et al. 2014). We were also only able to measure metals from a single urine sample taken at the third visit (~26 weeks gestation). Measuring these metals at multiple time points would give a better overall picture of exposure during pregnancy. Also, despite having a larger number of cases than previous studies, when we consider the subtypes of PTB we had more limited power. Finally, this study may be limited in its generalizability because of its assessment of associations within a population from Brigham and Women’s Hospital, a tertiary care center, where more high-risk pregnancies are delivered compared to other hospitals. Nevertheless, the trace metal concentrations observed in this study population are more similar to what is observed in the rest of the US population than in previous studies that focus on populations with extremely high exposure levels.
We analyzed the metals as a mixture using two different methods, ENET and PCA. However, these methods have their own limitations as well. ENET is a prediction model, and therefore the selected chemicals and interactions, while the most statistically relevant for predicting PTB, may not be biologically meaningful. PCA also selects PCs mathematically and therefore the clusters may not be biologically meaningful, which can limit the interpretability. PCA can identify a group of toxic metals that are associated with PTB, but we do not know which metal within that group is important. Currently, there is no gold standard among biostatisticians and epidemiologist with respect to the best methods for analyzing complicated mixtures (Braun et al. 2016; Taylor et al. 2016). We selected ENET and PCA as two promising approaches for 1) assessing the most important predictor within a correlated set of exposures, and 2) identifying the effect of combined rather than individual exposures, respectively. While the results from ENET in this example provided little additional information beyond what we learned from single pollutant models, the results from PCA may have identified groups of metals with possible shared exposure sources. Thus, these findings may be useful for targeted public health measures in populations exposed to elevated levels of multiple metals simultaneously.
5. Conclusions
In summary, we found that several essential urinary trace metal concentrations were associated with overall as well as subtypes of PTB. Mixtures analyses confirmed findings in single-pollutant models but did not provide notable additional information in our study. Further research is necessary to confirm these findings and to understand the mechanisms behind the risk of essential metals and PTB.
Supplementary Material
Highlights.
We analyzed 17 urinary trace metals in collaboration with the Children’s Health and Exposure Assessment Resource (CHEAR).
Urinary Cu concentrations from third trimester were associated with increased odds of preterm birth in single pollutant model
Elevated concentrations of other essential trace metals, Se and Zn, also has a positive association with increased odds of preterm birth
ENET and PCA results confirmed findings from the single pollutant models.
Acknowledgments
Funding:
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (Z1AES103321). Additional funding was provided by NIEHS (R01ES018872). Analysis was provided by the Children’s Health Exposure Analysis Research (CHEAR) Program (U2CES026555).
Abbreviations:
- ART
assisted reproductive technology
- BMI
body mass index
- CHEAR
Children’s Health and Exposure Assessment Resource
- ENET
elastic net
- GLM
generalized linear model
- PCA
principal component analysis
- PC
principal component
- PTB
preterm birth
- SG
specific gravity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algerwie MH, Khatri PC 1998. Serum copper in newborns and their mothers. Indian J Pediatr 65:899–903. [DOI] [PubMed] [Google Scholar]
- Almberg KS, Turyk ME, Jones RM, Rankin K, Freels S, Graber JM, et al. 2017. Arsenic in drinking water and adverse birth outcomes in ohio. Environ Res 157:52–59. [DOI] [PubMed] [Google Scholar]
- Aschner, Aschner M 2005. Nutritional aspects of manganese homeostasis. Mol Aspects Med 26:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. 1999. Toxicological profile for mercury. Atlanta, GA. [Google Scholar]
- ATSDR. 2004. Toxicological profile for copper. Atlanta, GA. [Google Scholar]
- ATSDR. 2005a. Toxicological profile for tin. Atlanta, GA. [Google Scholar]
- ATSDR. 2005b. Toxicological profile for zinc. Atlanta, GA. [PubMed] [Google Scholar]
- ATSDR. 2005c. Toxicological profile for tungsten. Atlanta, GA. [PubMed] [Google Scholar]
- ATSDR. 2007a. Toxicological profile for lead. Atlanta, GA. [Google Scholar]
- ATSDR. 2007b. Toxicological profile for arsenic. Atlanta, GA. [PubMed] [Google Scholar]
- Balshaw DM, Collman GW, Gray KA, Thompson CL. 2017. The children's health exposure analysis resource: Enabling research into the environmental influences on children's health outcomes. Curr Opin Pediatr 29:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JA, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna G, et al. 2017. Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility. Environ Int 108:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard D, Rossmann A, Wick G. 2005. Metals in cigarette smoke. IUBMB Life 57:805–809. [DOI] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF. 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect 124:A6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan AC, Hinwood AL, Ramalingam M, Boyce M, Heyworth J, McCafferty P, et al. 2013. Maternal exposure to metals--concentrations and predictors of exposure. Environ Res 126:111–117. [DOI] [PubMed] [Google Scholar]
- Cantonwine D, Hu H, Sanchez BN, Lamadrid-Figueroa H, Smith D, Ettinger AS, et al. 2010. Critical windows of fetal lead exposure: Adverse impacts on length of gestation and risk of premature delivery. J Occup Environ Med 52:1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Ferguson KK, Mukherjee B, Chen YH, Smith NA, Robinson JN, et al. 2016. Utilizing longitudinal measures of fetal growth to create a standard method to assess the impacts of maternal disease and environmental exposure. PLoS One 11:e0146532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2017. Preterm birth. Available: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm [accessed February 2, 2018 2018].
- CDC. 2018. Fourth report on human exposure to environmental chemicals, updated tables, (march 2018). Available: https://www.cdc.gov/exposurereport/ [accessed 04/01/2018.
- Cheng L, Zhang B, Huo W, Cao Z, Liu W, Liao J, et al. 2017. Fetal exposure to lead during pregnancy and the risk of preterm and early-term deliveries. Int J Hyg Environ Health 220:984–989. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Coull BA, Wright RO. 2014. Chemical mixtures and children's health. Curr Opin Pediatr 26:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzeisen R, Araya M, Harrison B, Keen C, Solioz M, Thiele D, et al. 2007. How reliable and robust are current biomarkers for copper status? Brit J Nutr 98:676–683. [DOI] [PubMed] [Google Scholar]
- Deyssenroth MA, Gennings C, Liu SH, Peng S, Hao K, Lambertini L, et al. 2018. Intrauterine multimetal exposure is associated with reduced fetal growth through modulation of the placental gene network. Environment international 120:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, O'Neill MS, Meeker JD. 2013. Environmental contaminant exposures and preterm birth: A comprehensive review. J Toxicol Environ Health B Crit Rev 16:69–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. 2014. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Chin HB. 2017. Environmental chemicals and preterm birth: Biological mechanisms and the state of the science. Curr Epidemiol Rep 4:56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort M, Cosin-Tomas M, Grimalt JO, Querol X, Casas M, Sunyer J. 2014. Assessment of exposure to trace metals in a cohort of pregnant women from an urban center by urine analysis in the first and third trimesters of pregnancy. Environ Sci Pollut Res Int 21:9234–9241. [DOI] [PubMed] [Google Scholar]
- Frey HA, Klebanoff MA. 2016. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med 21:68–73. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. 2010. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Galinier A, Periquet B, Lambert W, Garcia J, Assouline C, Rolland M, et al. 2005. Reference range for micronutrients and nutritional marker proteins in cord blood of neonates appropriated for gestational ages. Early Hum Dev 81:583–593. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. 2008. Epidemiology and causes of preterm birth. Lancet 371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LJ, Ashton K, Hooper L, Casgrain A, Fairweather-Tait SJ. 2009. Methods of assessment of copper status in humans: A systematic review. Am J Clin Nutr 89:2009S–2024S. [DOI] [PubMed] [Google Scholar]
- Health Canada. 2010. Report on human biomonitoring of environmental chemicals in canada: Results of the canadian health measures survey cycle 1 (2007-2009). Ottawa, Canada. [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene 5:46–51. [Google Scholar]
- Horton MK, Hsu L, Henn BC, Margolis A, Austin C, Svensson K, et al. 2018. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environment international 121:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. 2007. The role of environmental toxicants in preterm birth In: Preterm birth: Causes, consequences, and prevention, (Behrman R, Butler A, eds). Washington (DC):National Academies Press (US). [PubMed] [Google Scholar]
- Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V. 2006. Effect of magnitude and timing of maternal pregnancy blood lead (pb) levels on birth outcomes. J Perinatol 26:154–162. [DOI] [PubMed] [Google Scholar]
- Keen CL, Uriu-Hare JY, Hawk SN, Jankowski MA, Daston GP, Kwik-Uribe CL, et al. 1998. Effect of copper deficiency on prenatal development and pregnancy outcome. American Journal of Clinical Nutrition 67:1003s–1011s. [DOI] [PubMed] [Google Scholar]
- Li J, Wang H, Hao JH, Chen YH, Liu L, Yu Z, et al. 2017. Maternal serum lead level during pregnancy is positively correlated with risk of preterm birth in a chinese population. Environ Pollut 227:484–489. [DOI] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health. 2016. Copper and your health Available: https://www.mass.gov/service-details/copper-and-your-health [accessed August 9 2018].
- McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. 2008. Pregnancy disorders that lead to delivery before the 28th week of gestation: An epidemiologic approach to classification. Am J Epidemiol 168:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, et al. 2010. Maternal drinking water arsenic exposure and perinatal outcomes in inner mongolia, china. J Epidemiol Community Health 64:325–329. [DOI] [PubMed] [Google Scholar]
- NIEHS. 2018. Children's health exposure and analysis resource. Available: https://chearprogram.org/ [accessed April 26, 2018 2018]. [Google Scholar]
- O'Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect 124:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke N, Hatcher L. 2013. A step-by-step approach for using sas for factor analysis and structural equation modeling, second edition. Cary, North Carolina:SAS Institute Inc. . [Google Scholar]
- Pappas RS. 2011. Toxic elements in tobacco and in cigarette smoke: Inflammation and sensitization. Metallomics 3:1181–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou M, Ambler G, Seaman S, De Iorio M, Omar RZ. 2016. Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat Med 35:1159–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E. 2014. Very low maternal lead level in pregnancy and birth outcomes in an eastern massachusetts population. Ann Epidemiol 24:915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ML, Kile ML, Rodrigues EG, Valeri L, Raj A, Mazumdar M, et al. 2018. Prenatal arsenic exposure, child marriage, and pregnancy weight gain: Associations with preterm birth in bangladesh. Environ Int 112:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP, Wijnen H, Vader H, Kooistra L, Pop V. 2011. Maternal selenium status during early gestation and risk for preterm birth. CMAJ 183:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, Claus Henn B, Wright RO. 2015. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: A review of recent literature. Curr Environ Health Rep 2:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. 2010. Maternal exposure to low-level heavy metals during pregnancy and birth size. J Environ Sci Health A Tox Hazard Subst Environ Eng 45:1468–1474. [DOI] [PubMed] [Google Scholar]
- Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. 2010. Preventing preterm birth and neonatal mortality: Exploring the epidemiology, causes, and interventions. Semin Perinatol 34:408–415. [DOI] [PubMed] [Google Scholar]
- Tang M, Xu C, Lin N, Yin S, Zhang Y, Yu X, et al. 2016. Toxic effects of trace elements on newborns and their birth outcomes. The Science of the total environment 550:73–79. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Joubert BR, Braun JM, Dilworth C, Gennings C, Hauser R, et al. 2016. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: Lessons from an innovative workshop. Environ Health Perspect 124:A227–A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sanchez LE, Berkowitz G, Lopez-Carrillo L, Torres-Arreola L, Rios C, Lopez-Cervantes M. 1999. Intrauterine lead exposure and preterm birth. Environ Res 81:297–301. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Shibata E, Morokuma S, Tanaka R, Senju A, Araki S, et al. 2018. The association between whole blood concentrations of heavy metals in pregnant women and premature births: The japan environment and children's study (jecs). Environ Res 166:562–569. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Morimoto N, Hosokawa S, Matsushita T. 2013. Associations of maternal and neonatal serum trace element concentrations with neonatal birth weight. PLoS One 8:e75627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriu-Adams JY, Scherr RE, Lanoue L, Keen CL. 2010. Influence of copper on early development: Prenatal and postnatal considerations. Biofactors 36:136–152. [DOI] [PubMed] [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, et al. 2017. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20-40 months of age: Evidence from rural bangladesh. Environmental health perspectives 125:067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai KM, Mar O, Kosaka S, Umemura M, Watanabe C. 2017. Prenatal heavy metal exposure and adverse birth outcomes in myanmar: A birth-cohort study. Int J Environ Res Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LR, Rattigan M, Canterino J. 2011. A case of isolated elevated copper levels during pregnancy. J Pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu L, Hu YF, Hao JH, Chen YH, Su PY, et al. 2016. Association of maternal serum cadmium level during pregnancy with risk of preterm birth in a chinese population. Environ Pollut 216:851–857. [DOI] [PubMed] [Google Scholar]
- Williams M, Todd GD, Roney N, Crawford J, Coles C, McClure PR, et al. 2012. Agency for toxic substances and disease registry (atsdr) toxicological profiles In: Toxicological profile for manganese. Atlanta (GA):Agency for Toxic Substances and Disease Registry (US). [PubMed] [Google Scholar]
- Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L. 2007. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environmental health perspectives 115:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huo W, Zhang B, Zheng T, Li Y, Pan X, et al. 2016. Maternal urinary cadmium concentrations in relation to preterm birth in the healthy baby cohort study in china. Environ Int 94:300–306. [DOI] [PubMed] [Google Scholar]
- Zachara BA. 2018. Selenium in complicated pregnancy. A review. Adv Clin Chem 86:157–178. [DOI] [PubMed] [Google Scholar]
- Zou H, Hastie T 2005. Regularization and variable selection via the elastic net. J Roy Stat Soc B 67:301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.