Abstract
α-Vinylation of phosphonates, phosphine oxides, sulfones, sulfonamides, and sulfoxides has been achieved by selective C–H zincation and copper-catalyzed C(sp3)–C(sp2) cross-coupling reaction using vinylphenyliodonium salts. The vinylation transformation proceeds in high efficiency and stereospecificity under mild reaction conditions. This zincative cross-coupling reaction represents a general alkenylation strategy, which is also applicable for α-alkenylation of esters, amides, and nitriles in the synthesis of β, γ-unsaturated carbonyl compounds.
Keywords: copper catalysis, iodonium salt, C(sp3)–H functionalization, vinylation
Graphical Abstract
C(sp3)–H-FunktionalisierungIodoniumsalzeKupferkatalyseVinylierungGeneral vinylation strategy: α-Vinylation of phosphonates, phosphine oxides, sulfones, sulfonamides, sulfoxides, and carbonyl derivatives has been achieved effectively by a one-pot C–H zincation and copper-catalyzed C(sp3)–C(sp2) cross-coupling reaction using vinylphenyliodonium salts under mild conditions. ■■ picture had to be simplified ■■
Universelle Vinylierungsstrategie: Die α-Vinylierung von Phosphonaten, Phosphinoxiden, Sulfonen, Sulfonamiden, Sulfoxiden und Carbonylderivaten gelingt durch eine Ein-Topf-Reaktion aus C-H-Zinkierung und Kupfer-katalyiserter C-(sp3)-C(sp2)-Kreuzkupplung unter Verwendung von Vinylphenyliodoniumsalzen unter milden Bedingungen.
Alkene-containing molecules are ubiquitously desirable targets. Olefins are one of the most important functional groups and have rich and robust chemistry that is integral to organic synthesis and chemical science.[1] Therefore, the development of effective methods for incorporating this functional group is an important goal. Toward this, great attention has been focused on developing C(sp3)–C(sp2) bond-formation reactions, such as the reactions of alkyl halides,[2] alkyl organometallic compounds,[3] carbonyl and imines,[4] and carboxylic acids.[5] On the other hand, vinylation of C(sp3)–H bonds represents an appealing direct approach. For example, palladium-catalyzed β-C–H olefination of amides has been achieved by a sophisticated directing-group assisted strategy (Scheme 1a).[6] An elegant copper-catalyzed α-alkenylation of aldehydes has been reported via the synergy with amine catalysis (Scheme 1b).[7,8] Alternatively, a deprotonative/transition metal-catalyzed vinylation approach has been elegantly developed via in situ formed carbanion nucleophiles. Examples include α-vinylation of ketones,[9] nitromethane,[10] and pyridylmethyl ethers[11] (Scheme 1c). This deprotonative strategy is attractive without the requirement of directing groups, yet has received success only for relatively acidic C(sp3)–H bonds aforementioned. Nevertheless, in comparison to related arylation reactions, vinylation reactions of C(sp3)–H bonds are underexplored.[12] With olefins as one of the most useful functional groups, a general and effective alkenylation strategy is greatly desired and valuable.
Scheme 1.
Transition-metal-catalyzed alkenylation of C(sp3)–H bonds.
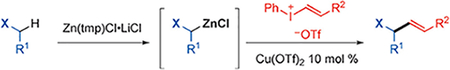
Here, we report an efficient α-vinylation method for phosphonate, sulfone, sulfoxide, and carbonyl derivatives by a modular C–H zincative/copper-catalyzed C(sp3)–C(sp2) cross-coupling reaction using vinylphenyliodonium salts (Scheme 1d). This approach utilizes Zn(tmp)Cl-mediated selective zincation of various C(sp3)–H bonds, from which the resulting nucleophilic organozinc intermediates could undergo a copper-catalyzed C(sp3)–C(sp2) cross-coupling reaction with vinylphenyliodonium salts. Vinyliodonium salts are an attractive electrophilic vinylation reagent[7b,8,13] due to their low toxicity, air- and moisture-stability, and high reactivity, despite being less explored than analogous diary-liodonium salts.[14] In this work, we demonstrate that such a zincative vinylation approach is modular and remarkably effective for a wide scope of C(sp3)–H bonds under mild conditions. The use of chiral zinc base has been explored toward the development of asymmetric vinylation reactions.
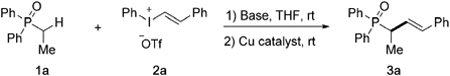
We initially chose ethyldiphenylphosphine oxide (1a) and trans-strenylphenyl iodonium salt (2a) as model substrates to attempt the vinylation of C(sp3)–H bond (Table 1). First, several commonly used bases were tested to promote deprotonative/copper-catalyzed vinylation with CuOTf·1/2PhMe catalyst in THF at room temperature, in all of which 1a was recovered with no vinylation product formed (Table 1, entries 1–5). A series of tetramethylpiperidine (tmp)-derived bases[15,16] were next examined, including Mg(tmp)Cl·LiCl, Zn(tmp)2·(LiCl)2 and Zn(tmp)Cl·LiCl (entries 6–8). Encouragingly, all afforded desired product 3a, with Zn(tmp)Cl·LiCl most effective (entry 8). Among different copper catalysts (entries 9–13), Cu(OTf)2 proved to be most effective to form 3a in 86% isolated yield (entry 13). Furthermore, the formation of 3a was improved by increasing the reaction temperature to 50°C (entry 14), which was chosen as the standard conditions for subsequent studies. Note that only trace amounts of desired product 3a were observed in the absence of a copper catalyst (entry 15).[17]
Table 1:
Condition optimization for α-vinylation of 1a.[a]
| Entry | Base | Catalyst | 3aa, Yield [%][b] |
|---|---|---|---|
| 1 | KOH | CuOTf·1/2 PhMe | 0 |
| 2 | KOtBu | CuOTf·1/2 PhMe | 0 |
| 3 | NaOMe | CuOTf·1/2 PhMe | 0 |
| 4 | KHMDS | CuOTf·l/2 PhMe | 0 |
| 5 | n-BuLi | CuOTf·1/2 PhMe | 0 |
| 6 | Mg(tmp)Cl·LiCl | CuOTf·1/2 PhMe | 47 |
| 7 | Zn(tmp)2·(LiCl)2 | CuOTf·1/2 PhMe | 29 |
| 8 | Zn(tmp)Cl·LiCl | CuOTf·1/2 PhMe | 83 |
| 9 | Zn(tmp)Cl·LiCl | CuCl | 80 |
| 10 | Zn(tmp)Cl·LiCl | CuBr | 81 |
| 11 | Zn(tmp)Cl·LiCl | Cu[CH3CN]4PF6 | 68 |
| 12 | Zn(tmp)Cl·LiCl | Cu(OAc)2 | 66 |
| 13 | Zn(tmp)Cl·LiCl | Cu(OTf)2 | 92 (86)[c] |
| 14[d] | Zn(tmp)Cl·LiCl | Cu(OTf)2 | (87)[c] |
| 15 | Zn(tmp)Cl·LiCl | - | trace |
Conditions: 1a (0.2 mmol), base (0.3 mmol), 2a (0.24 mmol), catalyst (10 mol%), THF (2 mL), RT.
Yields determined by 1H-NMR with CH2Br2 as an internal standard.
Yields of isolated products.
Reaction run at 50°C.
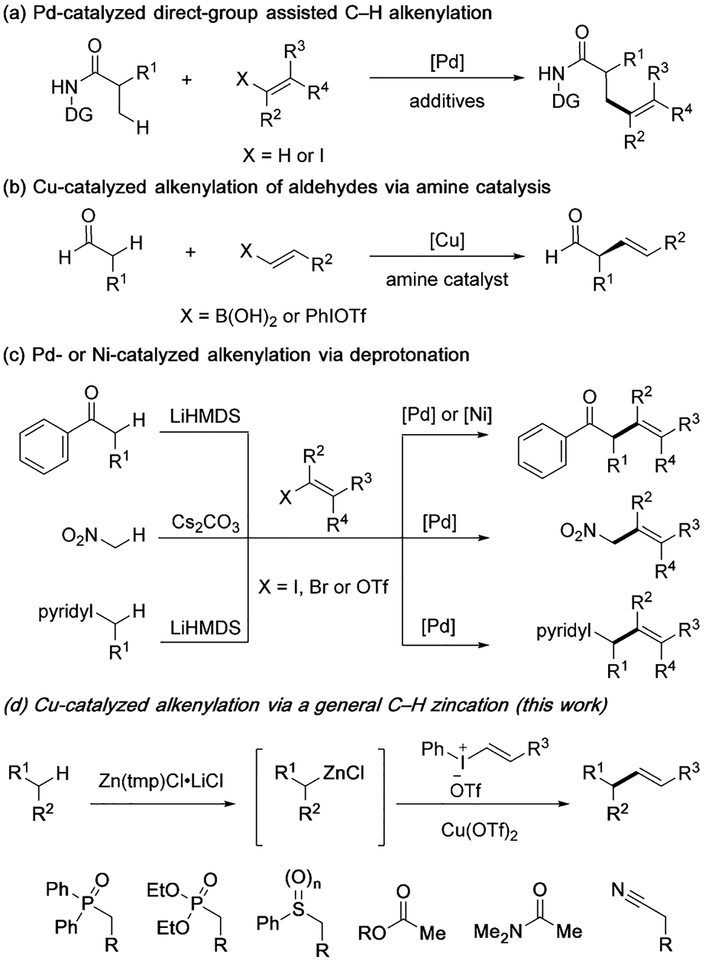
With standard conditions established, the α-vinylation reactions of phosphine oxide 1 was examined using different vinylphenyliodonium salts (Scheme 2). Note that diphenylphosphine oxides bearing one or no α-substituent group were found compatible to afford vinylation products 3a and 3b in excellent yields, while α-disubstituted phosphine oxide failed to undergo zincation. When the reactions of 1a were explored with vinyliodonium salts bearing different substituents on the phenyl group, such as 4-position electron-withdrawing chloride and electron-donating methoxy group, both readily formed the desired products 3c and 3d. Alkyl substituted vinyliodonium salts were well tolerated, such as benzyl, methyl and propyl groups that were demonstrated in the formation of 3e, 3f, and 3g, respectively. It is worth noting that halogens are tolerated to give vinylation products 3h and 3i, thus allowing further functionalization by transition-metal catalyzed cross-couplings.
Scheme 2.
α-Vinylation reactions of phosphine oxides with different vinylphenyliodonium salts 2. Conditions: 1 (0.2 mmol), Zn(tmp)Cl·LiCl(0.3 mmol), 2 (0.24 mmol), Cu(OTf)2 (0.02 mmol), THF (2 mL). Yields of isolated products.
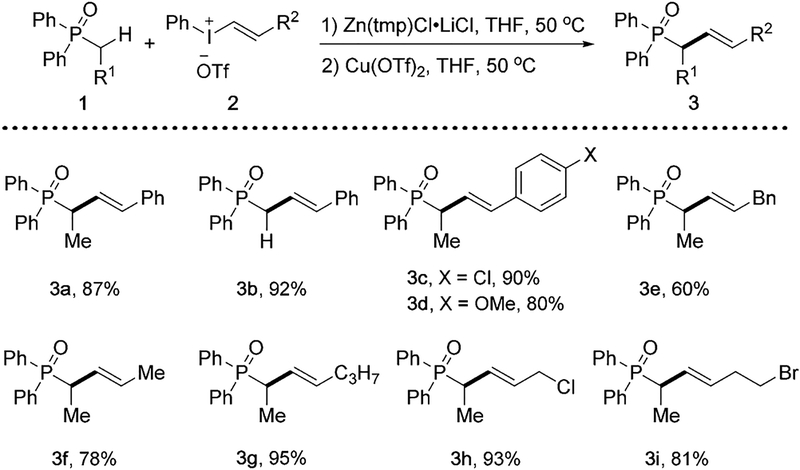
We also examined the vinylation reactions of phosphonates, a related class of important compounds having wide applications from agriculture to medicine[18] (Scheme 3). In the reactions with vinylphenyliodonium salt 2a, diethyl phosphonates 4a–4d all formed 5a–5d efficiently. Particularly, the reaction with cyclopropyl-substituted phosphonate 4d did not result in the formation of any ring-opening byproducts, indicating the vinylation step does not involve a radical intermediate. On the other hand, different alkenylphenyliodonium salts are also viable under standard conditions, providing desired phosphonates 5e–5i bearing different alkenyl groups at the α-position.
Scheme 3.
α-Vinylation reactions of phosphonates. Conditions: 4 (0.2 mmol), Zn(tmp)Cl·LiCl (0.3 mmol), 2 (0.24 mmol), Cu(OTf)2(0.02 mmol), THF (2 mL). Yields of isolated products.
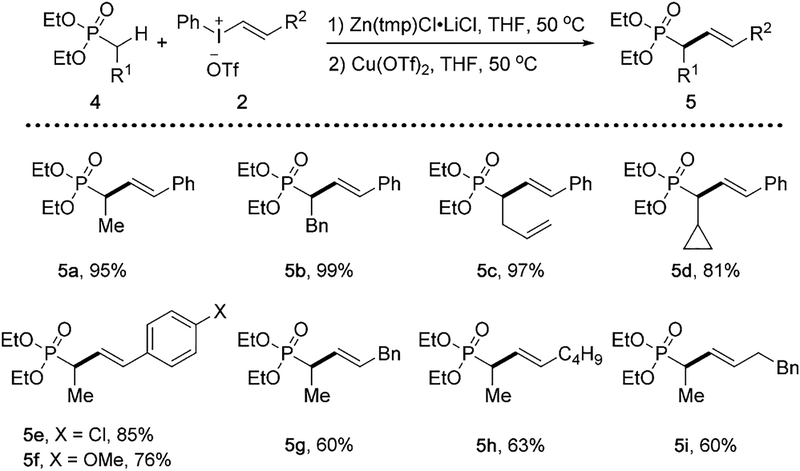
Using this zincative/cross-coupling approach, we next looked into α-vinylation of sulfone derivatives as a direct entry to allylic sulfones, which are highly valuable with its utilities as versatile building blocks and known biological activities[19] (Scheme 4). Upon further optimization, CuBr was chosen as the catalyst for the vinylation reactions of sulfones and sulfonamides at room temperature.[20] The vinylation reaction is applicable to a variety of sulfones, such as those bearing α-methyl, fluoro, or bromo group in the formation of 7a–7d. Sulfonamides also participated in the vinylation smoothly to give the desired products 7e–7f. Different vinylphenyliodonium salt partners are compatible for the successful formation benzyl- and propanyl-substituted vinyl sulfones 7g and 7h, respectively.
Scheme 4.
α-Vinylation of sulfones and sulfonamides. Conditions: 6 (0.2 mmol), Zn(tmp)Cl·LiCl (0.3 mmol), 2 (0.3 mmol), CuBr (0.02 mmol), THF (2 mL). Yields of isolated products.
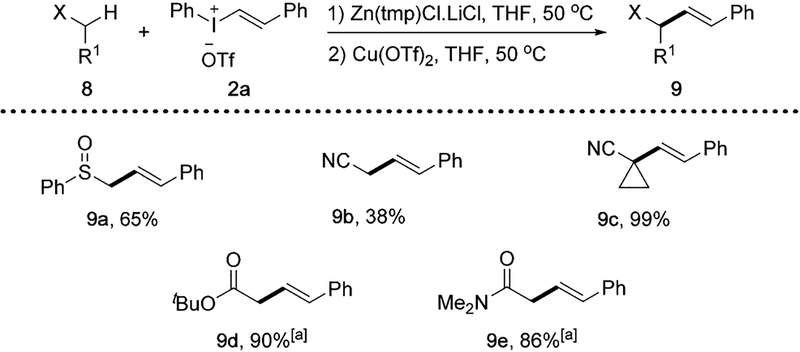
The applicability of this zincative/vinylation strategy was investigated among other types of C(sp3)–H bonds (Scheme 5). Under standard conditions, sulfoxide 8a provided the α-vinylation product 9a in 65% yield. α -Vinylation of nitrile derivatives were successful for the formation of 9b and 9c. α-Vinylation of tert-butyl acetate and N,N-dimethylacetamide were also effective, forming ester 9d and amide 9e in good yields. Note α-vinylation of ketones were ineffective under standard conditions. Overall, the generality of this copper-catalyzed vinylation demonstrated on a variety of C(sp3)–H bonds suggests its potential in constructing a wide range of vinyl–alkyl bonds.
Scheme 5.
α-Vinylation reactions of sulfoxide, nitriles, ester and amide. Conditions: 8 (0.2 mmol), Zn(tmp)Cl·LiCl (0.3 mmol), 2a (0.24 mmol), Cu(OTf)2 (0.02 mmol), THF (2 mL). Yields of isolated products. [a] Reaction run at RT.
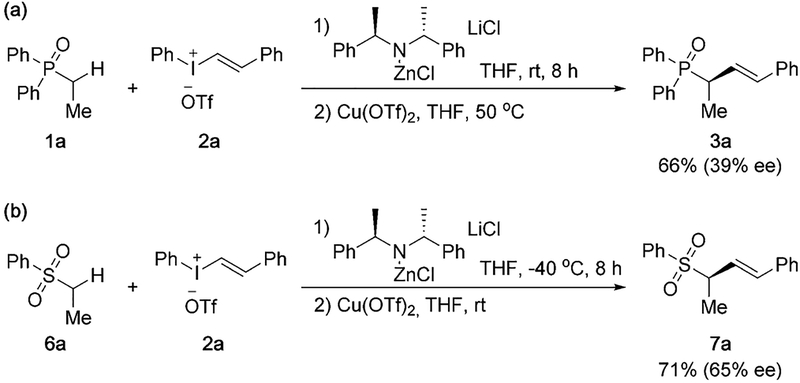
In our attempt to use chiral ligands to explore asymmetric versions of the vinylation reaction, our initial investigations remained unsuccessful. Alternatively, we examined the use of chiral zinc bases for stereoselective control of vinylation in the vinylation reaction of phosphine oxide 1a and sulfone 6a (Scheme 6). Moderate stereoinduction was observed with commercially available (R)-bis((R)-1-phenylethyl)amine-derived zinc base. Although the stereoselectivity at the current stage remains non-optimal, these initial results demonstrated the potential of developing an asymmetric vinylation reaction for the synthesis of optically active olefin-containing compounds.[20] Further investigations of asymmetrical versions of the vinylation reaction and the synthesis of optically active alkenyl-containing molecules will be reported in the future.
Scheme 6.
Synthesis of optically active α-vinylated phosphine oxide and sulfone. Yields of isolated products. The ee % values were determined by chiral HPLC analysis.
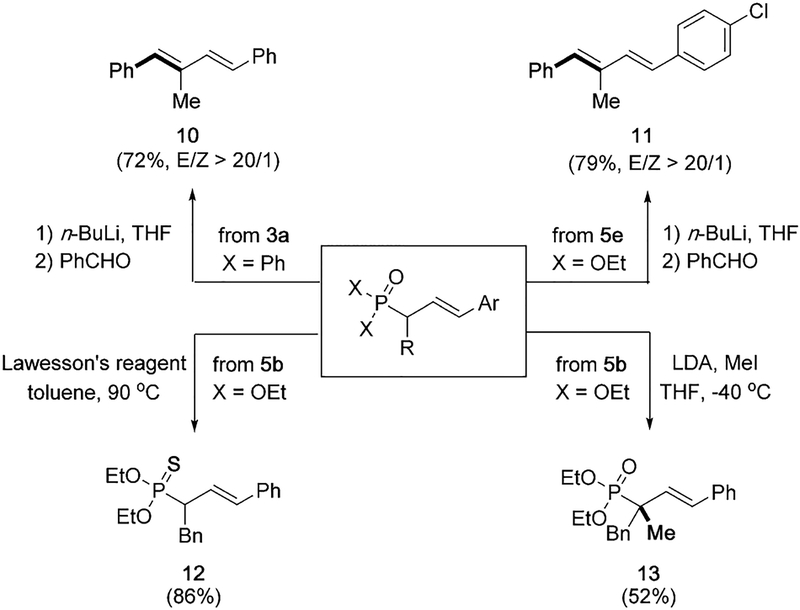
To further demonstrate synthetic utility of this general vinylation strategy, representative vinylated compounds were transformed into a broader range of functional molecules (Scheme 7). For example, phosphine oxide 3a and phosphonate 5e underwent Horner–Wadsworth–Emmons reactions to form trans-diene products 10 and 11, respectively. The treatment of phosphonate 5b with Lawesson’s reagent afforded thiophosphonate 12 in 86% yield, another useful class of molecules.[21] Furthermore, subsequent α -alkylation of phosphonate 5b led to the formation of phosphonate 13 containing fully substituted α-carbon atom, which offers a solution to the challenging α-disubstituted phosphonates under this zincative/vinylation transformation.
Scheme 7.
Representative transformations of vinylation phosphonates into diverse skeletons.
In summary, we have developed an efficient vinylation method for various C(sp3)–H bonds. This transformation was achieved by a one-pot C–H zincation and copper-catalyzed C(sp3)–C(sp2) bond formation using electrophilic vinyliodonium salts. The developed transformation is not only valuable and useful for a rapid entry to alkene-containing compounds of importance in pharmaceuticals and functional materials, but also presents a general tactic for introducing diverse functional groups onto similar skeletons.
Acknowledgements
We acknowledge financial support from Duke University. Q.W. is a fellow of the Alfred P. Sloan Foundation and a Camille Dreyfus Teacher-Scholar.
Footnotes
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/anie.201713278.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Selected reviews: Chemler SR, Trauner D, Danishefsky SJ, Angew. Chem. Int. Ed 2001, 40, 4544–4568;Chemler SR, Trauner D, Danishefsky SJ, Angew. Chem 2001, 113, 4676–4701;Denmark SE, Butler CR, Chem. Commun 2009, 20–33;Seechurn CCCJ, Kitching MO,Colacot TJ, Snieckus V, Angew. Chem. Int. Ed 2012, 51, 5062–5085;Seechurn CCCJ, Kitching MO,Colacot TJ, Snieckus V, Angew. Chem 2012, 124, 5150–5174.
- [2].For representative examples, see: Rudolph A, Lautens M, Angew. Chem. Int. Ed 2009, 48, 2656–2670;Rudolph A, Lautens M, Angew. Chem 2009, 121, 2694–2708;Dai X, Strotman NA, Fu GC, J. Am. Chem. Soc 2008, 130, 3302–3303;Krasovskiy A, Duplais C, Lipshutz BH, Org. Lett 2010, 12, 4742–4744;Lou S, Fu GC, J. Am. Chem. Soc 2010, 132, 5010–5011;Molander GA, Barcellos T, Traister KM, Org. Lett 2013, 15, 3342–3345;Cherney AH, Reisman SE, J. Am. Chem. Soc 2014, 136, 14365–14368;Choi J, Martin-Gago P, Fu GC, J. Am. Chem. Soc 2014, 136, 12161–12165;Johnson KA, Biswas S,Weix DJ, Chem. Eur. J 2016, 22, 7399–7402;Qiu C, Yao K,Zhang X, Gong H, Org. Biomol. Chem 2016, 14, 11332–11335;Cai Y, Benischke AD, Knochel P, Gosmini C, Chem. Eur. J 2017, 23, 250–253;Choi J, Fu GC, J. Am. Chem. Soc 2012, 134, 9102–9105.
- [3].For selected reviews, see: Jana R, Pathak TP, Sigman MS, Chem. Rev 2011, 111, 1417–1492;Zhou J, Fu GC, J. Am. Chem. Soc 2003, 125, 12527–12530.
- [4].For selected reviews, see: Montgomery J, Angew. Chem. Int. Ed 2004, 43, 3890–3908;Montgomery J, Angew. Chem 2004, 116, 3980–3998;Skucas E, Ngai MY, Komanduri V, Krische MJ, Acc. Chem. Res 2007, 40, 1394–1401;; for representative examples, see: Huddleston RR, Jang H-Y, Krische MJ, J. Am. Chem. Soc 2003, 125, 11488–11489;Patel SJ, Jamison TF, Angew. Chem. Int. Ed 2004, 43, 3941–3944;Patel SJ, Jamison TF,Angew. Chem 2004, 116, 4031–4034;Zhou C-Y, Zhu S-F, Wang L-X, Zhou Q-L, J. Am. Chem. Soc 2010, 132, 10955–10957;Wei C-H, Mannathan S, Cheng C-H, J. Am. Chem. Soc 2011, 133, 6942–6944;Nakai K, Yoshida Y, Kurahashi T, Matsubara S,J. Am. Chem. Soc 2014, 136, 7797–7800;Jackson EP, Montgomery J, J. Am. Chem. Soc 2015, 137, 958–963;Huang Y, Huang R-Z, Zhao Y, J. Am. Chem. Soc 2016, 138, 6571–6576.
- [5].Edwards JT, Merchant RR, McClymont KS, Knouse KW,Qin T, Malins LR, Vokits B, Shaw SA, Bao DH, Wei FL,Zhou T, Eastgate MD, Baran PS, Nature 2017, 545, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Wasa M, Engle KM, Yu J-Q, J. Am. Chem. Soc 2010, 132, 3680–3681; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stowers KJ, Fortner KC, Sanford MS, J. Am. Chem. Soc 2011, 133, 6541–6544; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li S, Chen G, Feng C-G,Gong W, Yu J-Q, J. Am. Chem. Soc 2014, 136, 5267–5270; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang B, Lu C, Zhang S-Y, He G, Nack WA, Chen G, Org. Lett 2014, 16, 6260–6263. [DOI] [PubMed] [Google Scholar]
- [7].a) Stevens JM, MacMillan DWC, J. Am. Chem. Soc 2013, 135, 11756–11759; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Skucas E, MacMillan DWC, J. Am. Chem. Soc 2012, 134, 9090–9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guo J, Lin L, Liu Y, Li X, Liu X, Feng X, Org. Lett 2016, 18, 5540–5543. [DOI] [PubMed] [Google Scholar]
- [9].For selected examples, see: Huang J, Bunel E, Faul MM, Org. Lett 2007, 9, 4343–4346;Jiang L, Weist S, Jansat S, Org. Lett 2009, 11, 1543–1546;Ankner T, Cosner CC, Helquist P, Chem. Eur. J 2013, 19, 1858–1871;Grigalunas M,Ankner T, Norrby P-O, Wiest O, Helquist P, J. Am. Chem. Soc 2015, 137, 7019–7022;Grigalunas M, Norrby P-O, Wiest O,Helquist P, Angew. Chem. Int. Ed 2015, 54, 11822–11825;Grigalunas M, Norrby P-O, Wiest O,Helquist P, Angew. Chem 2015, 127, 11988–11991.
- [10].Padilla-Salinas R, Walvoord RR, Tcyrulnikov S, Kozlowski MC, Org. Lett 2013, 15, 3966–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang X, Kim B-S, Li M, Walsh PJ, Org. Lett 2016, 18, 2371–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Radical vinylation reactions were also reported. see: Li J, Zhang J, Tan H, Wang DZ, Org. Lett 2015, 17, 2522–2525;Liu X, Sun S, Meng Z, Lou H, Liu L, Org. Lett 2015, 17, 2396–2399.
- [13].a) Cahard E, Bremeyer N, Gaunt MJ, Angew. Chem. Int. Ed 2013, 52, 9284–9288; [DOI] [PubMed] [Google Scholar]; Cahard E, Bremeyer N, Gaunt MJ, Angew. Chem 2013, 125, 9454–9458; [DOI] [PubMed] [Google Scholar]; b) Holt D, Gaunt MJ, Angew. Chem. Int. Ed 2015, 54, 7857–7861; [DOI] [PubMed] [Google Scholar]; Holt D, Gaunt MJ, Angew. Chem 2015, 127, 7968–7972; [Google Scholar]; c) Ivanova MV, Bayle A, Besset T, Poisson T, Pannecoucke X, Angew. Chem. Int. Ed 2015, 54, 13406–13410; [DOI] [PubMed] [Google Scholar]; Ivanova MV, Bayle A, Besset T, Poisson T, Pannecoucke X, Angew. Chem 2015, 127, 13604–13608. [DOI] [PubMed] [Google Scholar]
- [14].For selected reviews, see: Bielawski M, Olofsson B, Chem. Commun 2007, 2521–2523;Bielawski M, Olofsson B, Org. Synth 2009, 86, 308;Merritt EA, Olofsson B, Angew. Chem. Int. Ed 2009, 48, 9052–9070;Merritt EA, Olofsson B, Angew. Chem 2009, 121, 9214–9234;; for selected examples, see: Phipps RJ, Grimster NP,Gaunt MJ, J. Am. Chem. Soc 2008, 130, 8172–8174;Liu C,Zhang W, Dai L-X, You S-L, Org. Lett 2012, 14, 4525–4527;Zhu S, MacMillan DWC, J. Am. Chem. Soc 2012, 134, 10815–10818;Baralle A, Fensterbank L, Goddard JP, Ollivier C, Chem. Eur. J 2013, 19, 10809–10813;Farid U, Malmedy F, Claveau R, Albers L, Wirth T, Angew. Chem. Int. Ed 2013, 52, 7018–7022;Farid U, Malmedy F, Claveau R, Albers L, Wirth T, Angew. Chem 2013, 125, 7156–7160;Huang Z, Sam QP, Dong G, Chem. Sci 2015, 6, 5491–5498.
- [15].For selected references, see: Hlavinka ML, Greco JF, Hagadorn JR, Chem. Commun 2005, 5304–5306;Uchiyama M,Matsumoto Y, Nobuto D, Furuyama T, Yamaguchi K, Morokuma K, J. Am. Chem. Soc 2006, 128, 8748–8750;Hlavinka ML, Hagadorn JR, Organometallics 2007, 26, 4105–4108;Wunderlich SH, Knochel P, Angew. Chem. Int. Ed 2007, 46, 7685–7688;Wunderlich SH, Knochel P, Angew. Chem 2007, 119, 7829–7832;Wunderlich S, Knochel P, Chem. Commun 2008, 6387–6389;Unsinn A, Ford MJ, Knochel P, Org. Lett 2013, 15, 1128–1131;Duez S, Steib AK, Knochel P, Org. Lett 2012, 14, 1951–1953.
- [16].a) McDonald SL, Wang Q, Angew. Chem. Int. Ed 2014, 53, 1867–1871; [DOI] [PubMed] [Google Scholar]; McDonald SL, Wang Q, Angew. Chem 2014, 126, 1898–1902; [Google Scholar]; b) McDonald SL, Wang Q, Chem. Commun 2014, 50, 2535–2538; [DOI] [PubMed] [Google Scholar]; c) Liu C, Wang Q, Org. Lett 2016, 18, 5118–5121. [DOI] [PubMed] [Google Scholar]
- [17].See the Supporting Information for the proposed mechanism and related control experiments.
- [18].a) Pankiewicz KW, Malinowski K, Jayaram HN, Lesiak-Watanabe K, Watanabe KA, Curr. Med. Chem 1999, 6, 629–634; [PubMed] [Google Scholar]; b) Khandazhinskaya A, Yasko M, Shirokova E, Curr. Med. Chem 2006, 13, 2953–2980; [DOI] [PubMed] [Google Scholar]; c) Watkins WJ, Chen JM, Cho A, Chong L, Collins N, Fardis M, Huang W, Hung M, Kirschberg T, Lee WA, Liu X, Thomas W, Xu J, Zeynalzadegan A, Zhang J, Bioorg. Med. Chem. Lett 2006, 16, 3479–3483; [DOI] [PubMed] [Google Scholar]; d) Demmer CS, Krogsgaard-Larsen N, Bunch L, Chem. Rev 2011, 111, 7981–8006; [DOI] [PubMed] [Google Scholar]; e) Queffélec C, Petit M, Janvier P, Knight DA, Bujoli B, Chem. Rev 2012, 112, 3777–3807; [DOI] [PubMed] [Google Scholar]; f) Montchamp JL, Acc. Chem. Res 2014, 47, 77–87. [DOI] [PubMed] [Google Scholar]
- [19].For examples, see: Yamada M, Ichikawa T, Ii M, Itoh K, Tamura N, Kitazaki T, Bioorg. Med. Chem 2008, 16, 3941–3958;Wilden JD, J. Chem. Res 2010, 34, 541–548;Chen X, Hussain S, Parveen S, Zhang S, Yang Y, Zhu C, Curr. Med. Chem 2012, 19, 3578–3604;Shoaib Ahmad Shah S, Rivera G, Ashfaq M, Mini-Rev. Med. Chem 2013, 13, 70–86;Reck F, Zhou F, Girardot M, Kern G, Eyermann CJ, Hales NJ, Ramsay RR, Gravestock MB, J. Med. Chem 2005, 48, 499–506;El-Awa A, Noshi MN, du Jourdin XM, Fuchs PL, Chem. Rev 2009, 109, 2315–2349.
- [20].See the Supporting Information for addtional experimental data.
- [21].a) Rushing SD, Hammer RP, J. Am. Chem. Soc 2001, 123, 4861–4862; [DOI] [PubMed] [Google Scholar]; b) Maier L, Synth. React. Inorg. Met.-Org. Chem 1976, 6, 133–155. [Google Scholar]