Abstract
Notch signaling research dates back to more than one hundred years, beginning with the identification of the Notch mutant in the fruit fly Drosophila melanogaster. Since then, research on Notch and related genes in flies has laid the foundation of what we now know as the Notch signaling pathway. In the 1990s, basic biological and biochemical studies of Notch signaling components in mammalian systems, as well as identification of rare mutations in Notch signaling pathway genes in human patients with rare Mendelian diseases or cancer, increased the significance of this pathway in human biology and medicine. In the 21st century, Drosophila and other genetic model organisms continue to play a leading role in understanding basic Notch biology. Furthermore, these model organisms can be used in a translational manner to study underlying mechanisms of Notch-related human diseases and to investigate the function of novel disease associated genes and variants. In this chapter, we first briefly review the major contributions of Drosophila to Notch signaling research, discussing the similarities and differences between the fly and human pathways. Next, we introduce several biological contexts in Drosophila in which Notch signaling has been extensively characterized. Finally, we discuss a number of genetic diseases caused by mutations in genes in the Notch signaling pathway in humans and we expand on how Drosophila can be used to study rare genetic variants associated with these and novel disorders. By combining modern genomics and state-of-the art technologies, Drosophila research is continuing to reveal exciting biology that sheds light onto mechanisms of disease.
Keywords: Notch signaling, Drosophila, Mendelian Diseases, Functional Genomics, Translational Research
1. Discovery and expansion of the Notch signaling pathway in Drosophila
The first fly Notch (gene symbol: N) mutant was discovered in the laboratory of Thomas Hunt Morgan in 1913, and is named so based on its dominant wing notching phenotype [1,2]. In addition to notched wings, Notch null mutant flies exhibit additional dominant wing vein and mechanosensory bristle density abnormalities, as well as recessive embryonic lethality [3]. This lethality is primarily caused by the conversion of epidermal cells into neurons due to defects in lateral inhibition during neurogenesis, a developmental defect known as the “neurogenic” phenotype [4–7]. The Notch gene was cloned and sequenced in the mid-1980s and was found to encode a large transmembrane receptor-like protein [8,9]. Additional genes that exhibit similar phenotypes when mutated such as Delta (Dl)[10], Serrate (Ser) [11,12], neuralized (neur) [13], mastermind (mam) [14], Hairless (H)[15] and deltex (dx) [16,17] were cloned and characterized around the same time. Additional core genes of the pathway, such as Suppressor o f Hairless [Su(H))][18,19], and genes in the Enhancer o f split-Complex [E(spl)-C, spl is a hypomorphic allele of Notch] [20], were identified through genetic interaction screens with other genes in the pathway and were also cloned in the 1990s[21–26]. Interestingly, epistatic analyses laid the basic outline of the pathway prior to the molecular cloning of many of these genes [27,1], demonstrating the power of pure genetic studies.
Technological advances allowed investigators to look for additional regulators of the pathway that were missed by previous genetic screens. One drawback of classic mutagenesis screens using X-rays and chemical mutagens such as EMS (Ethyl methanesulfonate) is that it is often challenging and labor intensive to map and clone the affected gene and to identify the molecular lesions. Development of transposon (e.g. P-elements, PiggyBac)-based techniques provided a new tool to perform random mutagenesis screens to quickly identify new mutants that exhibit Notch signaling related phenotypes or to molecularly clone previously identified mutants that were left unmapped [28–31].
In Drosophila, embryonic developmental defects can be masked if maternal mRNAs and/or proteins that are deposited into the oocyte by the mother during oogenesis are sufficient for the animals to progress through embryogenesis. Hence, for genes that are abundantly expressed in oocytes, null mutants do not exhibit classical embryomic neurogenic phenotypes but typically die at a later developmental stage and were therefore missed in classic embryonic screens. Such maternal effect genes can be uncovered by generating oocytes that are homozygous for the mutation by combining a FLP (FLiPpase) /FRT (Flippase Recognition Target) system-based site directed mitotic recombination technique [32] with a germline-specific dominant female sterile (DFS) mutation[33,34]. Using this FLP-DFS technique, several novel maternal effect genes were identified [35–37]. In addition, development of reverse genetic strategies based on knowledge of the molecular map of the fly genome allowed to generate mutations in genes that have been implicated in Notch signaling in other systems but have not been studied in Drosophila such as Presenilin (Pns)[38–40]. Finally, additional modifier screens[41–53], somatic mutagenesis screens [54–69], genome-wide or targeted transgenic RNAi (RNA interference) based screens [70–76] and UAS (Upstream Activation Sequence)/GAL4 system [77]-mediated over-expression screens [78–82] have increased our knowledge of genes that regulate Notch signaling in vivo. These genetic screens, along with cell culture based assays[83–86] and systems biology driven approaches including transcriptomics [87–96] and proteomics[97–100] have allowed fly researchers to continue to discover new genes that regulate Notch signaling in diverse contexts. Because diagrams that illustrate Notch signaling now look more like a complicated intertwined web[101] rather than a simple linear pathway[27], the pathway is now occasionally referred to as the “Notch Signaling Network[102,1]” or the “Notch Signaling System[103]” to emphasize the complexity and dynamic nature of the pathway.
2. The Drosophila Notch signaling pathway and its relationship to the mammalian pathway.
Studies of the Notch signaling pathway in Drosophila have provided the framework for subsequent studies in other model organisms, including human[1]. One key advantage of studying Notch signaling in fruit flies is the genetic simplicity of the pathway compared to other organisms. Most core Notch pathway components are encoded by single genes in the fly genome while the structure and function of these factors remain largely conserved between flies and mammals. For example, the Drosophila genome contains one gene (Notch) that encodes for the Notch receptor, whereas the human genome contains four (NOTCH1–4; Table 1)[104]. Even the simple C. elegans genome encodes two Notch receptors [lin-12 (cell LINeage defective-12) and glp-1 (abnormal Germ Line Proliferation-1)][105,106], giving Drosophila an advantage when trying to determine whether certain biological phenomena depend on Notch signaling or when performing structure-function studies of Notch in an in vivo setting [107,108]. In this section, we provide an outline of the Notch signaling pathway as currently understood in Drosophila melanogaster (Fig. 1), while pointing out some key differences found between the fly and mammalian pathways.
Table 1: List of Drosophila genes discussed in this chapter along with their human homologs, disease association and functions.
Human genes in bold have been linked diseases based on OMIM (https://www.omim.org/). The fly genes that are homologous to these genes are also shown in bold. Fly genes with “?” have mammalian homologs that have been shown to be involved in the Notch pathway but their role in Notch signaling in Drosophila have not been studied or are not clear. See [440–449] to find the full list of genes involved in Arp2/3-WASp, ESCRT, AP-3, HOPS and V-ATPase complexes in Drosophila and human.
| Drosophllo gene (symbol) | Human homolog(s) (OMIM disease #) | Protein Functions |
|---|---|---|
| Notch (N) | NOTCH1 (#109730, #616028), NOTCH2 (#102500, #610205), NOTCH3 (#125310, #130720, #615293), N0TCH4 | Receptor |
| Delta (Dl) | DLL1, DLL3 (#277300), DLL4 (#616589) | Ligand |
| Serrate (Ser) | JAG1 (#118450, #187500), JAG2 | |
| rumi | POGLUT1 (#615696,617232) | Receptor glycosylation |
| O-fucosyltransferase 1 (O-fut1) | POFUT1 (#615327) | |
| Shams | GXYLT1, GXYLT2 | |
| fringe | LFNG (#609813), MFNG, RFNG | |
| EGF-domain O-GlcNAc transferase (Eogt) | EOGT (#615297) | |
| neurolized (neur) | NEURL1, NEURL1B | E3 ligase for ligand |
| mind bomb 1(mib1) | MIB1 (#615092) | |
| Furin1 (Fur1)?, Furin2(Fur2)? | FUR | S1 cleavage |
| kuzbanian (kuz) | ADAM10 (#615537) | S2 cleavage |
| Presenilin (Psn) | PSEN1 (#172700, #600274, #607822, #613694, #613737), PSEN2 (#606889, #613697) | S3 cleavage |
| aph-1 | APH1A, APH1B | |
| nicastrin (nct) | NCSTN (#142690) | |
| pen-2 | PSENEN (#613736) | |
| shibire(shi) | DNM1 (#616346), DNM2 (#160150, #606482, #615368), DNM3 | Receptor and ligand endocytosis |
| Sec15 | EXOCS, EXOC6B | Ligand trafficking |
| Rab11 | RAB11A, RAB11B | |
| Arp2/3 Complex: 8 genes (e.g. Arp2, Arp3) [Ref.440] | Arp2/3Complex: 9genes [Ref.445] | |
| WASp | WAS (#301000), WASL | |
| Ehbp1 | EHBP1 (#611868), EHBP1L | |
| temp | PTAR1 | |
| Numb | NUMB, NUMBL | Receptor trafficking |
| Sanpodo (Spdo) | - | |
| deltex (dx) | DTX1, DTX2, DTX3, DTX3L, DTX4 | |
| supressor of deltex (su(dx)) | ITCH (#613385), WWP1, WWP2 | |
| lethal(2)giant discs 1 (l(2)gd1) | CC2D1A (#608443), CC2D1B | |
| ESCRT complex: 20 genes (e.g. shrub, Vps25) [Ref.441] | ESCRT complex: 30 genes (#114480, #600795, #605387, #614898, #614696) [Ref.446] | |
| AP-3 complex: 4 genes (e.g. carmine (cm), ruby(rb))[Ref.442] | AP-3 complex: 7 genes (#608233, #617050, #617276)) [Ref.447] | |
| HOPS complex: 7 genes (e.g. carnation (car), deep orange (dor)) [Ref .443] | HOPS complex: 8 genes (#208085, #616683, #617303) [Ref.448] | |
| V-ATPase complex: 33 genes (e.g. VhaAC39–1, Vha68–2) [Ref.444] | V-ATPase complex: 23 genes (#124480, #219200, #259700, #267300, #278250, #259700, #616455, #617402, #617403) [Ref .449] | Vesicle acidification |
| Supressor of Hairless (Su(H)) | RBPJ (#614814) | Transcription factor |
| Hairless (H) | - | Corepressor |
| - | SPEN (SHARP/Mint) | |
| - | FHL1 (KyoT2) (#300695, #300696, #300717, #300718) | |
| groucho(gro) | TLE1, TLE2, TLE3, TLE4, TIE5, TLE6 (#616814) | |
| C-terminal Binding Protein (CtBP) | CTBP1, CTBP2 | |
| mastermind (mam) | MAML1, MAML2, MAML3 | Coactivator |
| nejire(nej) | EP300 (#114500, #613684), CREBBP (#180849) | |
| Enhancer of split complex [E(spl)-C): 7 bHLH repressor genes: e.g. E(spl)-m8) | HES1, HES2, HES3, HES4, HES5, HES6, HES7 (#613686) | Target Genes |
| sage? | MESP2 (#608681) | |
| Doc?1, Doc2?, Doc3? | TBX6 (#122600) | |
| Cdk8? | CDK8 | NICD degradation |
| archipelago (ago)? | FBXW7 |
Figure 1: Schematic diagram of the Drosophila Notch signaling pathway.
Canonical Notch signaling takes place between two juxtaposed cells (left: signal sending cell, right: signal receiving cell). Different steps of signal activation and functions of molecules depicted here are described in detail in the main text. Note that some players depicted here only regulate Notch signaling in specific contexts. Abbreviations for proteins shown are based on FlyBase gene symbol nomenclature (also see Table 1).
2.1. Biosynthesis and trafficking of the Notch receptor
For Notch signaling to be activated in a canonical fashion, two cells, one signal receiving and one signal sending, need to be juxtaposed (juxtacrine signaling). The Notch receptor is synthesized in the signal receiving cell and undergoes a number of post-translational modifications (PTMs) in both the endoplasmic reticulum (ER) and the Golgi apparatus[109]. In the ER, the extracellular domain of Notch becomes heavily O-glucosylated by Rumi (protein O-glucosyltransferase)[54] and O-fucosylated by O-fut1 (protein O-fucosyltransferase)[110,35]. The monosaccharides added by these enzymes to selective EGF (epidermal growth factor)-like repeats of Notch can further be elongated by Shams (glucoside xylosyltransferase)[111,112] and Fringe (Fng, O-Fucosylpeptide β3-N-acetylglucosaminyltransferase)[113–115,29] in the ER or Golgi apparatus. Experiments using enzymatically inactive mutants and transgenic over-expression strains have revealed that glycosylation of Notch is critical for ligand selectivity as well as for proper signal activation upon ligand-receptor interaction[116,117]. Both Drosophila and mammalian Notch receptors have also been shown to undergo additional glycosylation by Eogt [EGF-domain O-GlcNAc (N-Acetylglucosamine) transferase] in the ER[118,119]. Eogt mutant flies do not exhibit obvious Notch signaling defects but genetically interact with other members of the pathway, indicating that this gene plays a modulatory role.
In addition, Notch undergoes the first (S1) proteolytic cleavage mediated by an unknown (potentially a furin-like) protease, in the Golgi. It has been reported that in Drosophila cells the majority of the Notch receptor found at the cell membrane consists of the ~300 kDa full-length protein[120,121] while in mammals, most Notch at the cell surface has undergone S1 cleavage [122,123]. Although there has been some controversy in the Drosophila literature [124], S1 cleavage is not absolutely required for signal activation but rather it seems to facilitate the transport of the receptor to the cell surface contributing to signal strength[125], similar to the effect of S1 cleavage on mammalian Notch receptors [126].
2.2. Biosynthesis and trafficking of the ligands and ligand-receptor interaction
Notch receptors that have undergone proper PTMs in the ER and Golgi are exported to the plasma membrane where they can physically interact with ligands presented by the neighboring signal-sending cells. While the Notch receptor is expressed relatively broadly[127,128], the ligands Delta[129–133] and Serrate[11,12,134] exhibit unique and dynamic patterns of expressions during development. Together with the selective expression of Fng[29], which facilitates Notch-Delta interactions while suppressing Notch-Serrate interactions[135,114], the spatial and temporal pattern of ligand expression plays a critical role in determining where Notch signaling becomes activated. In mammals, three orthologs of Delta [Delta-like (DLL)1,3,4] and two orthologs of Serrate [Jagged (JAG) 1,2] are present. The existence of multiple DLL and JAG ligands, together with the presence of three fng orthologs (Lunatic (LFNG), Manic (MFNG) and Radical (RFNG) Fringe), increases the complexity of Notch signaling in mammals compared to Drosophila[104]. Multiple ligands can be expressed in the same tissue and can bind/activate the four Notch receptors with varying affinities. Furthermore, DLL3, the most divergent of the DLL paralogs, functions as a decoy ligand due to the lack of monoubiquitination sites in the cytoplasmic domain required for receptor activation[136]. Hence, this protein has been proposed to inhibit rather than activate Notch signaling in a cell autonomous manner by binding to the Notch receptors in cis (cis-inhibition) and preventing them from binding to ligands presented in trans[137]. Interestingly, three mammalian orthologs of fng have recently been shown to modify the same Notch receptor in different manners; some modifications can inhibit certain ligand-receptor interactions, others can potentiate them[138]. Hence, four receptors × five (four primarily activating and one inhibiting) ligands × 3 Fng enzymes generates a much more complicated scenario in mammals, compared to the one receptor × two (primarily activating) ligands × one Fng enzyme system in Drosophila.
Ligand-receptor interaction is necessary but not sufficient for canonical Notch signaling activation. After ligand and receptor bind to each another, the signal sending cell endocytoses the ligand, generating a physical force that unravels a second (S2) cleavage site embedded in the negative regulatory region (NRR) of the Notch receptor. Without the pulling force, three LNR (Lin-12/Notch Repeat) domains within the NRR limit the access of ADAM (A Disintegrin and Metalloprotease) proteases and prevent them from cleaving the S2 site[139,140]. Upon the conformational change mediated by ligand-endocytosis and force generation, Kuzbanian (Kuz, ADAM10 in human) cleaves the S2 site, shedding the majority of the extracellular domain and leaving behind a membrane-tethered portion of the Notch receptor referred to as the NEXT (Notch extracellular truncation)[141]. In order to endocytose the ligands and generate the pulling force, cytoplasmic domains of Delta or Serrate must be mono-ubiquitinated by E3 ubiquitin ligases Neuralized (Neur) or Mindbomb 1 (Mib1)[142,143]. neur and mib1 are differentially expressed and function in different Notch dependent biological processes during Drosophila development[144–149].
Although mind bomb 2 (mib2, MIB2 in human) is present in the fly genome[150], its in vivo role in Notch signaling is not clear [151]. The human genome contains two neur orthologs (NEURL1, NEURL1B) and one mib1 ortholog (MIB1). Although studies based on cultured cells indicate that these genes can all regulate Notch activity[152–154], only Mib1 has been reported to exhibit a strong Notch signaling defect in vivo when mutated in mice [155]. Hence, the dependence of the Notch pathway on ligand mono-ubiquitination by Neur and Mib family proteins seems to have diverged and/or acquired a high degree of redundancy during evolution.
2.3. Proteolytic cleavages of the Notch receptor and transcriptional regulation
After S2 cleavage of Notch, NEXT is further processed by the γ-secretase complex, an intramembrane protease composed of Presenilin (Psn), Nicastrin (Nct), Anterior pharynx defective 1 (Aph-1), and Presenilin enhancer-2 (Pen-2). Two Psn orthologs (PSEN1 and PSEN2) and two aph-1 orthologs (APH1A and APH1B) together with single orthologs for Nct (NCSTN) and pen-2 (PSENEN) exist in the human genome. γ-secretase performs the S3 cleavage of NEXT to release the Notch intracellular domain (NICD) from the membrane [156]. It remains unclear whether γ-secretase primarily processes NEXT at the cell membrane, within endocytic vesicles or both. The requirement of the genes that primarily function in Clathrin-dependent endocytosis [e.g. by Dynamin encoded by the shi (shibire) gene] for Notch activation in signal-receiving cells in certain contexts supports that S3 cleavage takes place in endocytic vesicles[157,158]. However, other studies argue that S3 cleavage primarily occurs at the plasma membrane and that endocytosis is not required[159], suggesting that this may be a context-specific issue. Indeed, proteins and molecular machineries that regulate endocytic trafficking and degradation of Notch receptors such as Dx (E3 ligase)[160,161,53], Suppressor of dx [Su(dx), E3 ligase] [162,163], ESCRT (Endosomal Sorting Complexes Required for Transport) complex (multivesicular body formation) [62,164], Lethal (2) giant discs 1 [L(2)gd1, adaptor protein?][165,166], AP-3 (Adaptor Protein-3) complex (late endosomal trafficking) and HOPS (HOmotypic fusion and Protein Sorting, endosome-lysosome fusion) complexes[167,168] and Vacuolar-ATPase (V-ATPase, vesicle acidification) complex [65,169] can fine-tune Notch activity, likely by regulating the efficiency of Notch cleavage in different subcellular compartments and/or modulating the balance between ligand-dependent and -independent signaling activities[170,171].
After being released from the membrane, NICD trafficks to the nucleus and forms a transcriptional activation complex with Su(H) [RBPJ (Recombination signal Binding Protein for immunoglobulin kappa J region) in human] [172,173,18] and Mam [MAML (Mastermind-like)1–3 in human] [174,175]. In the absence of NICD, Su(H) is bound to the co-repressor Hairless (H)[176,15,177] which in turn recruits additional co-repressors such as Groucho [TLE (Transducin-like enhancer protein) 1–6 in human, TLE5 is also referred to as AES (Amino-terminal Enhancer Of Split)] and CtBP (C-terminal Binding Protein, CTBP1–2 in human) to silence target genes [178–182]. Once NICD enters the nucleus and binds to Su(H), H is no longer able to bind to Su(H)[183]. The active NICD-Su(H)-Mam complex further recruits transcriptional co-activators such as the histone acetyltransferase CBP [CREB (cAMP response element binding protein)-binding protein]/p300 [nejire (nej) in Drosophila, EP300 (E1A binding protein P300) and CREBBP (CREB-binding protein) in human] to initiate transcription of downstream target genes [71,184]. While most genes that are involved in the transcriptional activation complex are conserved between flies and mammals, no direct homolog of Hairless exists in mammalian genomes. Instead, two structurally unrelated co-repressors, KyoT2 (encoded by the FHL1 gene in human)[185] and SHARP [SMRT/HDAC1 Associated Repressor Protein, encoded by the SPEN (SPlit ENds family transcriptional repressor) gene in human, also called Mint] [186,187] play the same function, binding to RBPJ and further recruiting additional corepressors to silence transcription[188]. Interestingly, Hairless and KyoT2/SHARP bind to RBPJ through different molecular mechanisms[183,189,190], suggesting that these genes were integrated into the Notch pathway independently through convergent evolution.
A number of Notch target genes have been identified to date[191], but the best characterized target genes are found in the E(spl)-C[177,192–194]. E(spl)-C encodes seven basic helix-loop-helix (bHLH) proteins that function as transcriptional repressors (E(spl)-m3, m5, m7, m8, mβ, mγ, mδ)[195–198]. In addition, the gene that encodes Gro as well as four Bearded family proteins (E(spl)-m2, m4, m6, mα), a group of small proteins that inhibit Neur function, are also found at this locus[199–202]. bHLH E(spl) proteins antagonize the activity of proneural bHLH proteins such as Achaete and Scute during neurogenesis and this relationship is generally conserved in mammals [203–206]. Homologs of bHLH E(spl) genes are known as HES (Hairy and Enhancer o f Split) genes in mammals (HES1–7 human)[207,208]. Together with the structurally and evolutionarily related HEY (Hairy/Enhancer-of-split related with YRPW motif protein) genes (HEY1, HEY2, HEYL in human) which are also under the control of Notch signaling in many contexts [209,210], these factors play critical roles in developmental events that involve proneural transcription factors as well as in a number of other Notch-dependent contexts [211,212].
Finally, signal termination of Notch is mediated by ubiquitin-proteasomal degradation of the NICD. Based on experiments in mammalian systems, the PEST [proline (P), glutamic acid (E), serine (S) and threonine (T)-rich] domain near the C-terminus of NICD is required for phosphorylation by CDK8 (Cyclin-dependent kinase 8) and subsequent poly-ubiquitination by the ubiquitin E3 ligase FBXW7 (F-BoX and WD repeat domain containing) 7. However, whether Cdk8 and archipelago (ago, FBXW7 homolog) also play similar roles in the Drosophila Notch signaling pathway in vivo waits further confirmation.
2.4. Non-canonical activation of the pathway and additional facts to note
In addition to the canonical signaling pathway described above, a number of studies have revealed non-canonical ways by which Notch signal can be activated (e.g. non-canonical ligands, Su(H)-independent signaling, signal crosstalk). Due to space limitations, we will not discuss these alternative pathways here and refer the readers to the following review articles[213–217].
As we have seen, there are a number of similarities between the Drosophila and human (mammalian) Notch signaling pathways but there are a number of differences we referred to that one should keep in mind. As we have already discussed, duplication (JAG1/2), triplication (DLL1/3/4 and LFNG/MFNG/RFNG) and quadruplication (NOTCH1–4) of core genes in the pathway during mammalian evolution have increased the complexity of the pathway compared to Drosophila. Some genes maintained redundancy while others acquired novel functions or became subfunctionalized to fine-tune the pathway in mammals. In addition, there have been new genes that have been incorporated into the pathway, some of which do not have an obvious ortholog in Drosophila (e.g. KyoT2, SHARP). In addition, one should also keep in mind that there are other key biological differences (e.g. minimal role of CpG DNA methylation in gene regulation in Drosophila[218,219], lack of primary cilia in most somatic cells[220,221]) that are known to exist between insects and mammals and that may impact the translation of some findings from Drosophila to human.
3. Notch signaling in Drosophila development
Since the identification of Notch and other members of the canonical signaling pathway as fundamental genes involved in the embryonic and post-embryonic development of Drosophila, numerous studies have focused on elucidating the role of Notch signaling during the development of diverse organs in the fly[222,223]. Over the years, several tissues that exhibit characteristic morphological defects when Notch signaling activity is altered have been used as models to understand how the pathway works and to further identify novel pathway members. These include the embryonic central nervous system (brain and ventral nerve cord), the adult peripheral nervous system (mechanosensory bristles, chordotonal organs and eyes), adult appendages (wings and legs), hematopoietic organs (lymph gland) and reproductive organs (ovary and testis). In addition, studies on post-developmental functions of Notch signaling, such as its role in synaptic plasiticity [224,225] and stem cell maintenance/differentiation[226–228], are also being explored. Here, we will focus on the role of Notch signaling during the development of the adult mechanosensory organs (bristles) and the wing margin during Drosophila development. These two tissues are well established model systems to study three conceptually distinct modes of Notch signaling that are reiteratively used during morphogenesis and organogenesis across evolution: lateral inhibition, lineage decisions and inductive signaling[223,229–231].
3.1. Notch signal-mediated lateral inhibition during early development of mechanosensory bristles
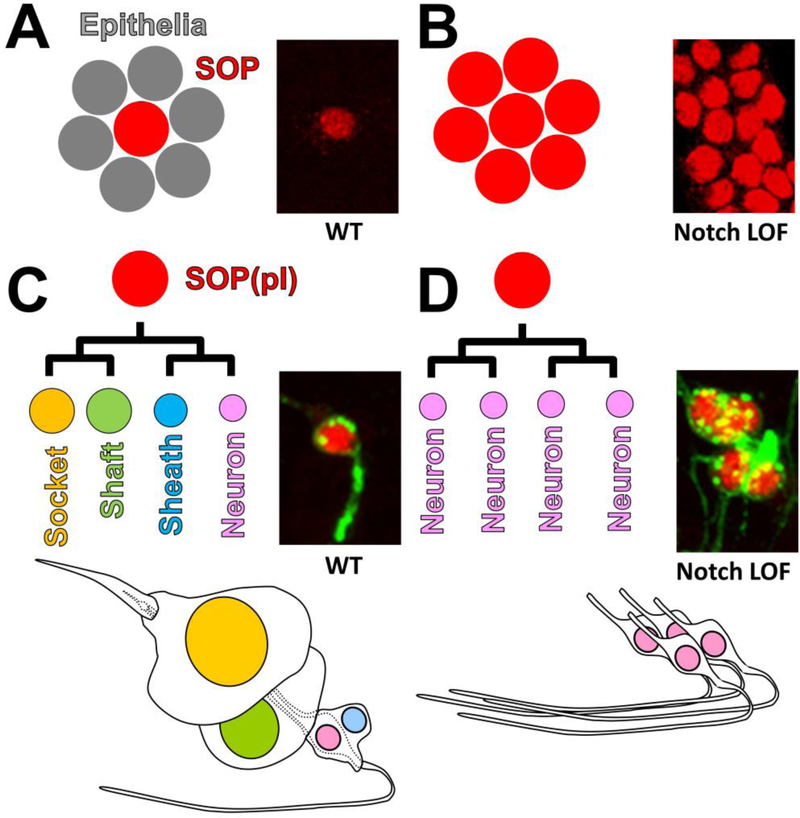
Mechanosensory bristles are part of the peripheral nervous system that allow the fly to sense mechanical forces and provide proprioception for coordinated movement and behavior (Fig. 2A–B)[232,233]. The bristles in the notum (dorsal thorax) are formed in a highly reproducible and stereotypical fashion[229] and their development can be easily traced using fixed or live imaging strategies [234]. Bristle precursor cells, which are called sensory organ precursor (SOP) cells, are selected out from a group of cells referred to as the proneural cluster (Fig. 2C). Proneural clusters are specific groups of ectodermal cells that begin to express proneural bHLH transcription factors of the Achaete-Scute Complex (AS-C, ASCL1–5 in human[235]). A single SOP is selected from a proneural cluster through Notch-mediated lateral inhibition [230]. Lateral inhibition is achieved through a genetic circuitry that works through a feedback loop that involves inductive and repressive transcriptional relationships between Notch signaling components and several transcription factors (Fig. 2D)[109,236,204]. In a proneural cluster, all cells initially express both Notch and Delta and have equal potential to either become an SOP or an epithelial cell. Delta activates Notch in neighboring cells, which leads to expression of downstream target genes in the E(spl)-C. E(spl) proteins function as transcriptional repressors and down-regulate the expression of AS-C, which are positive regulators of Delta transcription. Thus, decrease in AS-C expression due to upregulation of E(spl) leads to the reduction of Delta expression in the signal-receiving cell.
Figure 2: Notch signaling is required for lateral inhibition and lineage decisions during mechanosensory organ development.
A) Photograph of the fly notum. Large (macrochaetae) and small (microchaetae) are organized in a stereotypical fashion. B) Schematic diagram of a single mechanosensory organ (bristle). C) Schematic diagram representing the development of a single bristle. “N” indicates cells that activate Notch signaling. D-D’) Schematic diagrams of lateral inhibition during the selection of a sensory organ precursor (SOP) cell. In the beginning both cells have the potential to become an SOP. As development progresses, two cells acquire distinct fates through amplification of small differences through transcriptional feedback loops built into the stem. Cells that become the net signal sending cell becomes the SOP (labeled in red), and the net signal receiving cell(s) takes the epithelial cell fate. Panels B and C were adapted and mofidied from [108].
In addition to inducing the expression of Delta, AS-C bHLH transcription factors positively regulate the expression of a zinc finger nuclear protein called Senseless (Sens) [GFI1 (Growth Factor Independent 1 transcriptional repressor) in human] [237–239]. Sens participates in this genetic circuitry by promoting the transcription of AS-C target genes by working as a transcriptional coactivator through physical interactions with AS-C bHLH proteins. In addition, at low expression levels Sens functions as a transcriptional repressor through direct binding to DNA, thus acting as a binary switch to further amplify the feedback loop that is established by AS-C, Delta and E(spl)[238]. Within the young proneural cluster, the expression level of AS-C, Sens and Notch signaling components are similar among the cells. However, at some time point during development, the equilibrium of Notch-Delta signaling becomes disrupted which is thought to be through a stochastic event[240–242]. When one cell receives less Notch signal, expression of E(spl) within this cell is reduced and Delta expression becomes derepressed. Thus, cells receiving less Notch signal begin to express higher levels of Delta, which in turn can send stronger Delta mediated Notch signals to neighboring cells. Through this feed-forward loop, one cell that continues to send the signal to neighboring cell eventually becomes selected out as the SOP (low Notch activity), while the other cells remain in the epithelial cell fate [high Notch activity, (Fig. 2C–D)]. This mechanism allows the bristles on the fly notum to be formed in an evenly spaced manner. Loss of Notch signaling during this process, which occurs between 0 to 14 hours after puparium formation (hAPF), leads to generation of more SOPs at the expense of epithelial cells (Fig. 3A–B)[230]. During lateral inhibition of the SOPs, only Notch-Delta signaling is essential and Notch-Serrate signaling does not seem to be required[108,243]. Thus, the loss of Serrate does not show any defect in bristle spacing, whereas the loss of Delta in mutant clones exhibits bristle tufting in the adult notum[244].
Figure 3: Phenotypic consequences of Notch signaling loss during mechanosensory organ development.
A) Notch signaling mediates the lateral inhibition to specify an SOP from a proneural cluster. Cells that receive high Notch signaling becomes epidermal cells. B) Upon loss of Notch signaling during lateral inhibition, all cells takes the SOP cell fate. Photographs show SOPs marked by Senseless expression (Red). C) Reiterative Notch signaling is required to specify the four cell fates of the mechanosensory organ. The cells that receives the highest amount of Notch signaling becomes the Socket cells while the cells that receive the least becomes the neuron. D) Upon loss of Notch signaling during lineage decisions all cells take on the neuronal fate. Photographs show neuronal nuclei and membrane, labeled by antibodies against Elav (Red) and Hrp (Green). Panels A and B were adapted and mofidied from [108].
3.2. Notch signaling-mediated lineage/cell fate decisions upon asymmetric cell division of sensory organ precursor cells
Each bristle is composed of four cells: a socket, a shaft, a sheath and a mechanosensory neuron. These four cells are generated by a series of asymmetric cell divisions of the SOP and subsequent lineage specification through Notch signaling (Fig. 2B–C) [245,246]. Socket and shaft cells are located externally and provide the mechanical apparatus for mechanosensation. The neuron and the sheath cell, which is thought to function as a glial cell, are located internally and cannot be observed by simple visual examination of the fly notum. The dendrite of the mechanosensory neuron is located at the base of the bristle and is thought to contain mechanosensitive ion channels that open to depolarize the neuron upon deflection of the shaft cell [247]. The axon of the neuron targets the central nervous system to transmit the signal to higher nervous system centers [248].
The SOP, also referred to as the pI cell in this context, first divides along the anterior-posterior axis of the body to give rise to the posterior pIIa cell, the precursor cell of the external cells, and the anterior pIIb cell which gives rise to the internal cells (Fig. 2C). When the SOP divides, the cell fate determinants Neur and Numb are segregated into the pIIb cell but not into the pIIa cell [249,250]. This unequal inheritance of cell fate determinants, mediated by the Par3 (encoded by the bazooka gene in Drosophila)-Par6-aPKC (atypical Protein Kinase C) complex, determines the subsequent direction of Notch signaling between the pIIa and pIIb cells in order to specify distinct fates. Both pIIa and pIIb express comparable levels of Notch, Delta and Serrate but Neur, essential for ligand activity by promoting their mono-ubiquitination and endocytosis, is apportioned to the pIIb cell. Hence, ligands in the pIIb cell have the ability to signal, whereas ligands in the pIIa cell do not[249]. In addition, Numb (NUMB and NUMBL in human), an endocytic adaptor protein, acts in the pIIb cell to block signal reception by promoting the endocytosis of Notch and Sanpodo (Spdo)[251,67,252]. Spdo encodes a transmembrane protein required for cell fate specification at the cell surface by further modulating the trafficking of the Notch receptor [253]. Unlike Numb, Spdo has no obvious human homolog. Furthermore, proteins that regulate the proper trafficking of the ligands to the apical signaling interface, such as Sec15 (Secretory 15, component of the Exocyst complex)[55], Rab11 (small GTPase involved in vesicle recycling and exocytosis)[254], EHBP-1 [EH (Eps15 Homology) domain Binding Protein-1, adaptor protein that binds to Sec15 and Rab11) [60], Tempura (geranylgeranyltransferase for certain Rabs including Rab11)[58] and the Arp (Actin-related protein) 2/3-WASp (Wiskott-Aldrich Syndrome protein) complex (regulator of cytoskeleton and vesicle trafficking through Actin polymerization) [56,255] are also critical for proper communication between the two cells. Together, these mechanisms create a bias so that the pIIb cell becomes the signal-sending cell while the pIIa cell becomes the signal-receiving cell.
The pIIa and pIIb cells further undergo several rounds of asymmetric cell divisions and signaling to specify the four distinct cell types. An additional glial cell is formed through asymmetric division of the pIIb cell but this cell undergoes apoptosis and does not contribute to the mechanosensory organ in the adult notum [256,257]. A complete loss of Notch signaling during this process, which occurs between 16 to 24 hAPF, leads to a neurogenic phenotype (Fig. 3C–D)[230]. As a result, external socket and shaft cells, as well as the internal sheath cells, are lost leading to a balding phenotype on the notum. In contrast, gain of Notch signaling during this process leads to generation of more external cells at the expense of internal cells, thus exhibiting a multiple socket cell phenotype in the most extreme case [258]. The two ligands, Delta and Serrate, act redundantly during linage decisions to form the bristle. The loss of function of either ligand alone does not show lineage specification defects but cells mutant for both ligands exhibit a strong balding defect similar to loss of Notch[243]. In summary, Notch is used for both lateral inhibition and cell fate specification during the development of the mechanosensory organ, and is regulated by a number of distinct factors.
3.3. Notch mediated inductive signaling during the formation of the wing margin
The wing of a fly is a bilayered structure composed of dorsal and ventral wing blades that are bound together via integrin mediated attachment (Fig. 4A–B)[259]. The two surfaces of the wing blade meet at the wing margin to form the rim of the wing. Mechanosensory and chemosensory bristles are located along the anterior wing margin, whereas noninnervated bristles align the posterior wing margin. The wing margin is formed during the larval stage within the wing imaginal disc, which gives rise to the future wing and notum tissue of the adult fly. The dorsal domain of the wing imaginal disc expresses the selector gene apterous, which encodes a homeodomain transcription factor with two LIM (Lin11, Isl-1 and Mec-3) domains[260]. Apterous turns on the expression of Serrate and Fng specifically in the dorsal domain, whereas Notch and Delta are expressed in both compartments[261–263]. Serrate can signal to the ventral compartment but cannot signal within the dorsal compartment due to differences in Fng modification of Notch in the two compartments [264,265,114,266,267]. Conversely, Delta can signal to the dorsal cells but cannot signal to the ventral compartment[114,266,268]. This bidirectional signaling through Delta and Serrate along the dorsal-ventral boundary leads to the activation of Notch, which in turn activates genes specific for the wing margin such as wingless (wg) and cut (Fig. 3C)[269–271,262,272]. Wg (WNT1 in human), a Wnt signaling ligand, acts as a morphogen to pattern the wing along the dorsal-ventral axis[273,272,274] whereas Cut is a homeodomain transcription factor that is involved in maintaining the expression of Wg as well as repressing the expression of Delta and Serrate within the future wing margin tissue [275,276,270]. At later stages in wing margin development, the cells that coexpress Wg and Cut down-regulate the expression of Notch ligands, whereas cells flanking the wing margin cells express high levels of Delta and Serrate via high Wnt signaling activation. Thus, Delta and Serrate from the flanking cells continue to signal to the wing margin cells to maintain the expression of Wg and Cut, reinforcing the establishment of a solid compartmental boundary through a positive feedback loop[277,276]. Loss of Notch signaling during this induction leads to the loss of wing margin tissue (Figure 4D–E). Unlike decisions in the bristle lineage where Delta and Serrate act redundantly, both ligands are necessary for wing margin specification. Hence, loss of either Delta or Serrate alone leads to a reduction in Wg and Cut expression, resulting in the notching of the wing[267]. Mild loss of wing margin tissue at the distal tip of the wing can even be seen in flies that are heterozygous for a null mutation of Notch [3]. This haploinsufficiency phenotype of Notch, which originally gave the name “Notch” to the gene and the pathway, emphasizes the strict dosage sensitivity of inductive signaling during wing margin formation.
Figure 4: Notch signaling is required for inductive signaling during wing margin development.
A) Photograph of a DAPI-stained wing imaginal disc that is pseudocolored for the dorsal domain (green) and the future wing margin (red). B) Schematic diagram of a transverse section of a part of the fly thorax. The dorsal wing imaginal disc (green) gives rise to the notum (dorsal thorax) and the dorsal wing blade. The ventral wing imaginal disc forms the ventral wing blade. The boundary between the dorsal and ventral compartment becomes the wing margin (red). C) Notch mediated inductive signaling specifies the future wing margin during imaginal disc development. Serrate (Ser) signals from the dorsal to the ventral compartment (red arrows) whereas Delta (Dl) signals from the ventral to the dorsal compartment (yellow). D) Upon Notch signaling activation at the dorsal-ventral boundary, genes such as Wingless (red) and Cut (not shown) are expressed, specifying the wing margin. E) Upon loss of Notch signaling during inductive signaling, these genes fail to be expressed and the wings exhibit a “notching” phenotype. Abbreviation of axes in panels A-B: D (Dorsal), V (Ventral), A (Anterior), P(Posterior), M (Medial), L (Lateral). Panel C was adapted and mofidied from [108].
4). Human diseases caused by rare mutations in Notch pathway genes
In parallel to efforts to reveal the genes and mechanisms that coordinate the Notch signaling pathway using model organisms and cultured cell lines, medical research has uncovered a strong link between Notch and many human diseases[278–283]. To date, inherited or de novo mutations in human genes that encode core components of the pathway such as the receptors, ligands, transcription factors and downstream target genes have been shown to cause diverse Mendelian disorders[284,285]. By studying these rare diseases and patients from a clinical perspective, physicians and scientists made discoveries that had major impacts on basic Notch research. In addition, there is growing evidence that misregulation of Notch signaling may participate in more common disorders, ranging from neuropsychiatric to metabolic disorders [286–289]. Furthermore, somatic mutations in genes in the pathway and/or misregulation of Notch signaling activity has also been linked to oncogenesis and tumor progression in different cancer types[290,141,291]. Here, we will provide an overview of Mendelian disorders caused by mutations in genes that encode core Notch signaling components in human, most of which are catalogued in Online Mendelian Inheritance in Man (OMIM)[292], an online database of human genotypes and phenotypes. The role of Notch signaling in cancer will be further discussed in other chapters of this book (e.g. Chapters 9, 15 and 18).
4.1. Adams-Oliver syndrome
Adams-Oliver syndrome (AOS) is a developmental disorder characterized by aplasia cutis congenital (a congenital skin defect, typically of the scalp) and transverse limb defects (typically digital amputations) [293,294]. In addition, some AOS patients exhibit nervous system and cardiac/vascular abnormalities. Dominant mutations in NOTCH1 (OMIM #616028), DLL4 (OMIM #616589), RBPJ (OMIM #614814) and recessive mutations in EOGT (OMIM #615297) are known to cause this condition. Additional mutations in DOCK6 [Dedicator O f CytoKinesis 6, guanine nucleotide exchange factor (GEF) for Rho-GTPases, OMIM #614219] and ARHGAP31 [RHo GTPase Activating Protein 31, GTPase-activating protein (GAP) for Rho-GTPases, OMIM #100300] have also been linked to AOS but the relationship between these genes and Notch signaling is currently unknown. A number of missense, nonsense and frameshift mutations in NOTCH1 [295,296] and DLL4[297] have been found in patients with this condition, suggesting that haploinsufficiency is the underlying mechanism of the dominant inheritance for these genes. AOS-linked mutations identified in RBPJ have been shown to impair the DNA binding capacity of the encoded protein[298].
4.2. Alagille syndrome and Hajdu-Cheney syndrome
Alagille Syndrome is a developmental disorder that affects a number of organ systems including the liver (paucity of intrahepatic bile ducts), cardiovascular system (stenosis of the pulmonic valve), kidney (renal dysplasia), skeleton (abnormal “butterfly” vertebrae), eye (posterior embryotoxon, a characteristic defect in the layers of the eye called the ring of Schwalbe) and dysmorphic facial features [299]. The main manifestation of disease is seen in the liver where bile duct formation is defective, resulting in chronic cholestasis [300]. Dominant nonsense, frameshift and missense mutations in JAG1 (~90% of cases, OMIM #118450)[301,302] or NOTCH2 (a few % of cases, OMIM #610205)[303] cause this condition, suggesting that haploinsufficiency of these genes is the underlying genetic mechanism. Dominant mutations in NOTCH2 are also associated with a different congenital disease called Hajdu-Cheney syndrome that primarily manifests as a skeletal disease (OMIM #102500) [304–306]. Mutations identified in this disease are late truncating mutations that remove the C-terminal PEST domain of NOTCH2, which likely acts as gain-of-function alleles by increasing its stability. Hence, both loss- and gain-of-function mutations in NOTCH2 cause rare genetic disorders that are phenotypically and mechanistically distinct from each other.
4.3. Aortic Valve Diseases and Tetralogy of Fallot
Notch signaling plays a key role during the development of the cardiovascular system[280]. Notch is used reiteratively in cardiac development: during cardiomyocyte specification and differentiation, atrioventricular canal development, cardiac valve development, ventricular trabeculation, and outflow tract development. Cardiac defects are often seen in patients with AOS, Alagille Syndrome and Hajdu-Cheney syndrome with a number of different cardiac lesions. Dominant mutations in NOTCH1 and JAG1 have also been linked to primary congenital heart diseases such as Aortic Valve Disease 1 (OMIM #109730)[307,308] and Tetralogy of Fallot (OMIM #187500)[309], respectively. NOTCH1 mutations linked to Aortic Valve Disease are nonsense and frameshift mutations, suggesting a haploinsufficient mechanism [310]. Why some patients with loss-of-function mutations in NOTCH1 exhibit Adams-Oliver syndrome while others only present cardiac symptoms is unclear. To date, all mutations in JAG1 that are linked to Tetralogy of Fallot are missense alleles[309,311–313], which may have different consequences from the Alagille syndrome-linked mutations in this gene. In addition, dominant mutations (one nonsense and one missense, respectively) in MIB1 (OMIM #615092)[314] and MIB2 (OMIM #N/A)[315] have been linked to Left Ventricular Noncompaction (LVNC), a form of cardiomyopathy. Patients with a mutation in MIB2 also exhibit gastrointestinal phenotypes and have been classified as Ménétrier disease [315]. In sum, cardiac defects are often associated with mutations affecting Notch signaling, which is likely due to the fact that Notch signaling plays a number of critical roles during cardiovascular development in vertebrates [316]. These phenotypes can be presented together with defects in other organ systems, reflecting the highly pleiotropic nature of this pathway.
4.4. Spondylocostal Dysostosis
Notch signaling also affects skeletal development, and mutations in several core signaling components and downstream target genes have been associated with rare skeletal disorders[317,318]. Spondylocostal Dysostosis (SCDO) is primarily an autosomal recessive disorder, presenting with abnormal vertebra formation and patterning defects. Five of the six types of SCDO identified to date are caused by recessive mutations in core Notch signaling pathway components and downstream target genes: SCDO1; DLL3 (OMIM #277300)[319], SCDO2; MESP2 (Mesoderm posterior bHLH transcription factor 2, Notch target gene, OMIM #608681)[320], SCDO3; LFNG (OMIM #609813)[321], SCDO4; HES7 (Notch target gene, OMIM #613686)[322], and SCDO5; TBX6 (T-box 6, Notch target gene [323,324], OMIM #122600, autosomal dominant forms have also been reported)[325,326]. The sixth SCDO gene, RIPPLY2 (Ripply transcriptional repressor 2, OMIM #616566), lies downstream of the pathway and regulates the expression and/or function ofMESP2 and TBX6[327]. In mice, many of these genes have been shown to play a critical role in somitogenesis [328,329], indicating that SCDO is caused by misregulation of an evolutionarily conserved transcriptional pathway that regulates somite (precursor of vertebra and other tissues) formation[330,331].
4.5. CADASIL and NOTCH3-related disorders
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is the most common heritable cause of stroke and vascular dementia, characterized by five main symptoms: migraine with aura, subcortical ischemia, mood disorders, apathy, and cognitive decline generally found in families with an autosomal dominant pattern of inheritance [332]. Accumulation of granular osmophilic material (GOM), which accompanies vascular smooth muscle degeneration and arteriopathy in postmortem CADASIL patient brain tissue, is a characteristic pathological feature of the disease[333]. Over 90% of CADASIL patients carry a dominant mutation in NOTCH3 (OMIM #125310) and over 170 mutations have been identified to date[334,335]. Interestingly, the majority of the mutations involve loss or gain of cysteine residues in one of the 34 EGF repeats in the extracellular domain of NOTCH3[336]. The odd numbers of cysteines (5 or 7) per EGF repeat caused by CADASIL NOTCH3 mutations are thought to disrupt the formation of proper intra-molecular disulfide bonds. Although no logical explanation has been proposed, it is interesting to note that the majority of the mutations are clustered between EGFr-1–5 and the distribution of CADASIL associated missense mutations along the extracellular domain of NOTCH3 is uneven[335].
Whether CADASIL is caused by loss or gain of function of NOTCH3 has been under extensive debate [337]. Some CADASIL mutations behave as loss-of-function alleles of NOTCH3 based on ligand-receptor binding and signaling assays performed in cultured cells and in mouse models [338–342]. However, considering that heterozygous deletions of the NOTCH3 locus have not been associated with CADASIL in human patients, and that Notch3 knockout mice do not exhibit pathological phenotypes that are characteristic for the disease [343], the degree to which defects in Notch signaling contributes to the pathogenesis of this disorder remains unclear. Others propose that the pathogenesis of CADASIL is due to a toxic-gain-of-function (neomorphic effect) of NOTCH3 and that non-physiological intermolecular disulfide bonds formed between the free cysteine residues of NOTCH3 and other transmembrane and/or secreted proteins is the primary cause of disease [344–346]. The extracellular portion of NOTCH3 has been found to be associated with or included in the GOM[347–349], which also consists of numerous proteins including Clusterin and Collagen18α1/Endostatin[350]. However, it remains to be determined whether extracellular accumulation/aggregation of secreted and cell surface proteins in the GOM is due to direct interaction of these factors with mutant NOTCH3 protein. In addition, whether there is a causal connection between GOM formation and clinical symptoms found in CADASIL patients still needs to be investigated and clarified. Furthermore, since most studies have been performed only on a small subset of pathogenic mutations in NOTCH3, further studies on a spectrum of mutations are needed to reveal the full molecular pathology of the disease.
Mutations in NOTCH3 have also been found in patients with lateral meningocele syndrome (LMNS, OMIM #130720)[351] where de novo NOTCH3 variants are identified, and in a single family with an autosomal dominantly inherited infantile myofibromatosis 2 (IMF2, OMIM #615293)[352]. The former disease is characterized by distinct facial characteristics, hypotonia, hyperextensibility and meningocele-related neurologic phenotypes such as bladder dysfunction, while the latter disorder is characterized by formation of benign tumors in connective tissues that arise due to excessive mescenchymal cell proliferation. Other reported cases of infantile myofibromatosis have been linked to the PDGFRB (Platelet Derived Growth Factor Receptor Beta) gene (OMIM #228550), and the role Notch signaling in the pathogenesis of this disease is unknown. Both disorders have been proposed to be caused through gain-of-function mechanisms (late truncating mutations that delete the PEST domain for LMNS[351]; missense mutation in the NRR domain for IMF2[352]), but further functional studies and additional patient identification are necessary to reveal a clear genotype-phenotype relationship.
4.6. Other Mendelian diseases caused by mutations in Notch signaling pathway genes: γ-secretase complex related disorders as an example
In addition to the diseases described above, there are a number of Mendelian diseases that are caused by mutations in homologs of Drosophila genes that are known to be critical for Notch signaling. However, since many genes are pleiotropic and have functions outside of the Notch signaling pathway, it is not clear which aspect, if any, of the patient’s phenotypes can be explained by defects in Notch signaling.
For example, dominant missense mutations in PSEN1 (OMIM #607822, 600274, 172700) and PSEN2 (OMIM #606889), that encode catalytic subunits of the γ-secretase complex, cause rare early onset familial forms of Alzheimer’s disease (AD) and other forms of dementia. Although several studies have implicated the role of Notch signaling in AD pathogenesis [353], the primary mechanism by which mutations in PSENs cause AD seems to be through altered proteolytic processing of Amyloid Precursor Protein (APP), another well characterized substrate of the γ-secretase complex [354]. Additional dominant missense mutations in PSEN1 (OMIM #613694) and PSEN2 (OMIM #613697) have also been found in patients with dilated cardiomyopathy[355–357]. The functional consequences of these missense mutations are unclear and whether defects in Notch signaling may be contributing to this condition has not been investigated. Furthermore, loss-of-function mutations in PSEN1 and other components of the γ-secretase complex cause another type of disease known as familial acne inversa. This condition, also known as hidradenitis suppurativa, is a chronic relapsing skin inflammatory disease that has been linked to haploinsufficiency of PSEN1 (OMIM #613737), NCSTN (Nicastrin, OMIM #142690) and PSENEN (Presenilin enhancer gamma-secretase subunit, OMIM #613736). Since Notch signaling plays multiple key roles in the development and maintenance of the skin[358] and immune system[359], it has been proposed that defects in Notch signaling contributes to the pathogenesis [360], but additional experimental evidence is needed to strengthen this model.
Similarly, dominant mutations in POFUT1 (Protein O-fucosyltransferase 1, OMIM #615327)[361], POGLUT1 [Protein O-glucosyltransferase 1, OMIM #615696, this gene is also linked to muscular dystrophy (OMIM #617232))[362] and ADAM10 (OMIM #615537) [363] cause skin disorders that results in pigmentation defects (Dowling-Degos disease or reticulate acropigmentation of Kitamura). Considering that Notch regulates multiple aspects of melanocyte development[364], it is likely that defects in Notch signaling contribute to the pathogenesis of these diseases[365,366]. However, direct experimental evidence is necessary to test this hypothesis. Likewise, mutations in a number of genes encoding general cellular machineries that affect Notch receptor trafficking and activation (e.g. Clathrin-Dynamin mediated endocytosis, ESCRT, AP-3, HOPS, V-ATPase complexes) are also linked to diverse diseases but additional work is required to determine the degree by which Notch signaling defects contribute to the pathology of these disorders.
In summary, genes that have been well established to function in Notch signaling are linked to a number of Mendelian diseases. The fact that the Notch pathway is pleiotropic likeky contributes to the broad range of human phenotypes affecting a wide range of organ systems. In addition, the strict dosage dependence of the pathway may explain the involvement of both gain- and loss-of-function mechanisms and both recessive and dominant modes of inheritance leading to disease. Further investigations that focus on of the functional impacts of each pathogenic mutations will likely provide better mechanistic understandings of how specific phenotypes associated with these disorders may be explained by defects in Notch signaling.
5. Using Drosophila to study rare functional variants in genes linked to Notch signaling pathway and beyond
Advances in sequencing technologies are accelerating the pace of disease gene discovery [367]. Currently (February 2017), of 149 genes that have been linked to Notch signaling in Drosophila melanogaster, 130 are conserved in human (87%) and 48 (37%) have human homologs that are linked to Mendelian diseases based on FlyBase[368], a manually curated database for Drosophila genetics and biology, and OMIM[292]. Identification of some of these disease genes was made possible through whole-exome sequencing [315,306,304,305,363,362]. As more and more exomes and genomes are sequenced in research and clinical diagnostic laboratories using high-throughput sequencing technologies[369–371], new human diseases that are caused by mutations in genes that have been previously linked to Notch signaling in flies are likely to be identified. In addition, the list of novel rare variants of unknown significance (VUS) in known Notch-related disease genes will also likely to continue to expand. Proper interpretation of the functional consequences of these VUS will become critical for researchers to identify the underlying causes of disease and for clinicians to make medical decisions for patients in the era of personalized medicine.
For Notch-related disorders, a number of in vitro and in vivo assays in mammalian systems have been established to assess the function of disease-associated variants. For example, several Alagille syndrome linked mutations in JAG1 (p.L37S, p.P163L and p.R184H) were shown to affect subcellular localization, glycosylation, and signaling capability of JAG1 using skeletal muscle derived cell lines, leading to the proposal of a haploinsufficient (loss-of-function) model of disease pathogenesis[372,373]. In another study, however, two Alagille syndrome linked mutations in JAG1 (p.C284F and p.E1003X) were reported to exhibit a dominant-negative effect on Notch signaling when overexpressed in NIH3T3 cells[374].
Conflicting results obtained through in vitro experiments are typically resolved using in vivo model systems. To date, most in vivo studies that attempt to understand the functional consequences of disease-associated variants in Notch related diseases have been performed in the mouse (Mus musculus). One key advantage of mouse models is that one can screen for phenotypic similarities between the mutant mice and disease patients. For example, heterozygous inactivation of Rbpj in muce causes cardiac phenotypes that are often seen in human diseases[375]. Similarly, cardiac phenotypes seen in LVNC patients that carry mutations in MIB1 were successfully phenocopied in heart specific Mib1 knockout mice[314]. Importantly, reduced Notch1 signaling in the developing heart was observed in these animals suggesting that loss-of-function of MIB1 and subsequent reduction in Notch activation is likely to be associated with LVNC. Some studies in mice have used gene knock-in strategies to introduce analogous mutations into the mouse ortholog of the human gene to understand the function of specific disease-linked mutations. For example, one study modeled two CADASIL-linked mutations (p.C455R and p.R1031C) in mice and showed that these mutations behave as hypomorphic alleles[350]. Furthermore, Clusterin and Collagen18α1/Endostatin, materials found in GOM in CADASIL patient brain vessels, accumulated in brain blood vessels of the mice, proving a phenotypic link between the human patients and the mouse models. Although important insights into the role of disease associated NOTCH3 variants in vascular biology can be obtained by these types of studies, a potential confound of these mouse mutants is that they do not exhibit key features of CADASIL such as development of spontaneous stroke [350,376]. Similar arguments have been made for mouse knock-in models for AD-linked mutations in PSEN1 [377–382]. Nevertheless, these models provide valuable information about the role of the genes and variants in a physiological setting, a complex systemic environment that cannot be easily mimicked in cell or tissue culture based studies.
One large drawback for gene modification based experiments in mice is the cost and time that is required to generate reagents and to complete the analysis of a given variant. When hundreds of novel VUS are identified from large sequencing efforts, it is unrealistic and uneconomical to use the mice to study all of these variants in vivo. In vitro experiments can be used as a first line of screening prior to the generation of mouse models, but slight defects that may be amplified through intercellular feedback loops in vivo (e.g. during lateral inhibition and inductive signaling) may be missed through simple cell based assays. Furthermore, if the disease-linked variants affect animo acids that are not conserved between human and mouse, a knock-in strategy cannot be applied. Based on the deep biological knowledge of Notch signaling and rich genetic toolkits the community has generated to characterize this pathway[383–385], Drosophila can be a powerful tool to bridge this gap. Here, we will discuss several strategies to functionally characterize disease-associated variants using Drosophila, starting with the identification of the potential fly ortholog of a gene of interest. We will close this section by providing examples of such Drosophila studies that have been performed to study disease-associated genes involved in Notch signaling.
5.1. Using bioinformatics to aggregate existing knowledge and resources
The first step in disease-linked variant functional studies using Drosophila is to perform bioinformatics analyses to identify the strongest Drosophila ortholog candidate for the human gene of interest. There are a number of orthology prediction programs that use different algorithms and criteria to predict the most likely ortholog candidate [386]. User-friendly online tools such as DIOPT (Drosophila RNAi Screening Center Integrative Ortholog Prediction Tool)[387] and HCOP (Human Genome Organization Gene Nomenclature Committee Comparison of Orthology Predictions Search)[388] integrates a number of these programs to provide the users with an arbitrary score. The higher the DIOPT or HCOP scores are for a given gene combination, more likely the two genes are to be true orthologs. Due to the two rounds of whole genome duplication evens that likely to have occurred in ancestral vertebrates [389] (although there is still some debate [390]), there are many cases in which multiple human genes are orthologous to a single fly gene as seen for Notch (NOTCH1–4), Delta (DLL1,3,4) and Serrate (JAG1,2) (Table 1). Once a fly gene of interest is identified, one can determine whether the gene has been linked to Notch signaling in Drosophila by using PubMed[391] or FlyBase[368]. Information such as known gene function, expression patterns, physical interactors, available reagents and publication records can be obtained through these websites. A newly developed integrative online resource called MARRVEL (Model organism Aggregated Resources for Rare Variant ExpLoration) [392,393] integrates DIOPT [387], Flybase[368] as well as additional human genomics [292,394–398] and model organisms databases [399–404] to help the users to perform a wide survey of the gene and variant of interest. These searches are important to confirm that the gene/variant of interest is worth investigating in depth prior to initiating the any experiments in model organisms.
For genes that have been linked to Notch signaling in Drosophila, it is important to determine the context in which this link has been made and to find out the tools and experimental strategies that were used to make the conclusion. One gene may have been functionally studied using a clean null allele in the context of embryonic central nervous system development, while another gene may have been studied using tissue-specific RNAi expression in the developing wing margin without proper control experiments. By obtaining information about the biological context and experimental strategy that was used in previous studies, one can determine how to design a set of experiments to test the function of the variant of interest. It is also important to determine whether the reagents used in the previous studies are available through stock centers or individual laboratories. If the mutant or transgenic stocks are available from public stock centers such as BDSC (Bloomington Drosophila Stock Center) [383], DGGR (Drosophila Genomics and Genetic Resources)[405], VDRC (Vienna Drosophila Resource Center)[406] or from individual labs upon request, this will save time and resources. Additional genetic tools such as Notch signaling reporters[407] and classical alleles of core Notch signaling pathway genes that can be useful for signaling and genetic interaction studies are also available from some of these stock centers. Many monoclonal antibodies (e.g. anti-Notch, anti-Delta) and constructs/plasmids that are useful for Notch signaling studies in Drosophila [e.g. transgenic vectors, cDNA clones and BACs (Bacterial Artificial Chromosomes)] are also available from DSHB (Developmental Studies Hybridoma Bank) [385] and DGRC (Drosophila Genomics Resource Center) [384], respectively. In summary, by performing a thorough search of the existing knowledge and resources using online tools and databases, one can obtain sufficient information to design a set of experiments to test the functional significance of a variant of interest in Drosophila.
5.2. Selecting the best strategy to study the variant of interest in flies
One important consideration when studying a human missense variant in Drosophila is whether the mutated/altered amino acid is conserved or not. While there are some exceptional cases (see the TM2D3 case discussed below in Section 5.3.2), conserved amino acids tends to be functionally more important[408]. In addition, the conservation of the residue allows one to test the function of the variant in the context of the fly gene/protein. By introducing the analogous variant in a fly cDNA or genomic rescue construct and expressing them in the mutant background in Drosophila, one can test if the variant behaves differently from the wild-type/reference fly gene. Also, if the variant of interest is conserved, site-directed mutagenesis using CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat)-Cas9 System can be employed to edit the endogenous fly gene to create a clean knock-in allele via homology directed repair (HDR)[409].
If the amino acid is not conserved, one needs to somehow “humanize” the fly gene to be able to test the impact of the variant. There are a number of strategies to achieve this, and one powerful strategy that our laboratory has been using recently is based on the T2AGAL4 system (Fig. 5A)[410]. This method allows one to generate a convenient “2-in-1” strain that can dramatically facilitate gene humanization experiments in Drosophila[411]. The first step is to introduce an artificial exon consisting of a splice acceptor (SA), ribosomal skipping T2A sequence, GAL4 [Yeast transcription factor that activates UAS (Upstream Activating Sequence)] coding sequence and a transcriptional termination (polyA) signal into a coding intron (introns flanked by two coding exons) of a gene of interest. Introduction of this cassette can be performed via CRISPR/HDR or through recombinase mediated cassette exchange (RMCE) of MiMIC (Minos-Mediated Integration Cassette) insertions[412,413]. MiMIC is an engineered versatile transposable element that has been extensively mobilized in the fly genome and can be used as an entry point to manipulate genes in many sophisticated ways [414–416]. By flanking the T2A-GAL4 cassette with inverted attP sites, one can further convert this line into a GFP-tagged protein trap line via RMCE to enable a number of sophisticated biochemical, cell biological and genetic experiments [411]. If the gene lacks an intron, the GAL4-polyA cassette can be knocked into the first coding exon via the CRISPR/HDR (Fig. 5B). The T2A-GAL4 and GAL4 knock-in strains function as loss-of-function alleles due to the polyA signal. At the same time, these cassettes produce a GAL4 protein that is expressed in the same temporal and spatial manner as the host gene. The T2A ribosomal skipping peptide ensures that the protein is physically separated from the host protein so that GAL4 can enter the nucleus [410,417]. Upon nuclear entry, GAL4 will drive the expression of a cDNA of interest under the control of UAS elements[77]. Hence, by combining the T2AGAL4/GAL4 knock-in lines with a UAS-human cDNA transgenic line, one can replace the fly protein with a human protein to determine whether the two are interchangeable. Easily scorable phenotypes such as lethality or sterility can be used as crude assays to determine whether the human protein can function in the fly body. If one observes a complete or partial rescue with a reference (wild-type) human cDNA, one can use this as a reference point to compare how well the variant cDNA functions [418,419]. Further rescue experiments of Notch related phenotypes (e.g. neurogenic, wing notching) or signaling defects (e.g. activation of Notch reporters or target genes) will provide information on whether the variant impacts Notch signaling in vivo.
Figure 5: Strategies to “humanize” Drosophila genes in vivo.
A) For genes that have coding introns (introns flanking two coding exons), one can insert a T2A-GAL4 cassette via CRISPR and HDR (homology directed repair). When the gene of interest is translated, the splice acceptor (SA) forces the splicing machinery to include the T2A-GAL4 cassette. The transcriptional termination site (polyA) stops the transcription, preventing the rest of the gene to be transcribed. When the transcript (mRNA) is translated, N-terminal of the fly protein is made but is prematurely truncated due to the T2A (2A) ribosomal skipping sequence, leading to generation of nonfunctional proteins in most cases. T2A sequence further allows the GAL4 protein to be translated, which in turn translocates to the nucleus to activate the expression of human cDNAs (wild-type/reference or mutant/variant) under the control of UAS elements. B) For genes that do not have a coding intron, one can knock-in a GAL4 in the fly gene of interest. GAL4 will be transcribed and translated in the same temporal and special manner as the fly gene, allowing one to express the human cDNA in a mutant background. Grey boxes: 5’ and 3’ untranslated regions. Orange box: Fly coding sequence (CDS).
In addition to this T2A-GAL4 strategy, one can also make use of mutant fly strains that have been previously characterized and try to rescue the mutant phenotypes using UAS-human cDNA transgenic lines and ubiquitous- or tissue-specific GAL4 drivers. To date, we have rescued a number of fly mutants by ubiquitous expression of human cDNAs [420–422,58,423–425], suggesting that many human genes have shared molecular functions and can replace the fly orthologs in vivo. A more rudimentary humanization experiment can be performed by co-expressing an RNAi against a fly gene together with a human cDNA. Furthermore, in addition to rescue/replacement based functional studies, one can perform over-expression based experiments in a wild-type background using the GAL4/UAS system to determine whether there are any differences observed when reference and variant forms are compared. This could be especially useful for cases in which a hypermorphic (gain-of-function), antimorphic (dominant negative), or neomorphic scenarios are suspected. However, ectopic over-expression based phenotypes observed through these studies need to be assessed with caution since they may not reflect the physiological function of the variant. Similarly, negative data may simply be due to the lack of sensitivity of the phenotype or assay that is being performed. Hence, positive data are strongly indicative that the variant has a functional impact in human, but one cannot rule out a candidate gene/variant due to negative data obtained from Drosophila studies.
5.3. Functional studies of disease associated variants in Notch signaling genes in Drosophila
Although functional studies of human disease associated variants in genes linked to Notch signaling have not been performed extensively in Drosophila, the following two examples related to Alzheimer’s disease illustrate the value of assessing Notch signaling related phenotypes in flies to elucidate the possible impact of disease-associated variants.
5.3.1. Early onset familial Alzheimer’s disease and PSEN1
Autosomal dominant mutations in PSEN1 are found in a number of families that exhibit rare early-onset forms of familial Alzheimer’s disease (FAD)[426]. There are >240 missense variants that have been identified to date [427] but potential impacts on PSEN1 function have not been experimentally defined for most of them. The age of onset of FAD varies from 24–65 years, suggesting that some alleles maybe more detrimental than others. By introducing 14 representative PSEN1 mutations found in conserved amino acids into the fly Psn homolog cDNA driven by an endogenous promoter, Seidner and colleagues performed a series of rescue experiments to determine whether there is any genotypephenotype correlation that they can identify that parallel the severity in human patients [428]. By assessing rescue of lethality, examining morphological defects in the wing margin, bristle and eye, and performing in vivo reporter assays in a Psn null mutant background, they were able to group the FAD-linked variants into three distinct classes, which correlated well with the severity of disease presentation in human patients. It is interesting to note that the authors also attempted rescue of the fly Psn null mutant with human PSEN1 or PSEN2 transgenes but they failed, suggesting that human PSEN1 and PSEN2 cannot function in the context of the fly γ-secretase complex. Humanization of the entire γ-secretase complex (Psn, nct, pen-2, aph-1) may circumvent the problem but this needs to be experimentally tested.
5.3.2. Late onset sporadic Alzheimer’s disease and TM2D3
Compared to FAD, the genetic causes of Late-onset Alzheimer’s disease (LOAD) remain to be defined. Since LOAD is much more common than FAD and found sporadically, a number of Genome-Wide Association Studies (GWAS), using common variants have been performed and a number of loci that increase the risk of LOAD have been identified [429,430]. Other than the well-established coding variants (p.C112R and p.R158C) in APOE (APOlipoprotein E, OMIM #104310)[431–433], however, the significance of most of these variants awaits to be experimentally verified. Through a recent exome-wide association study, the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) consortium[434,435] identified a rare coding variant in a previously uncharacterized gene that has a strong effect size on LOAD[436]. A P155L variant in TM2D3 (TM2 domain containing 3) was associated with a ~7.5 fold chance of developing LOAD and a 10-year earlier age of onset in an Icelandic population. However, the study was not able to replicate this finding in other populations since this allele was 10 times less frequent in non-Icelanders. TM2D3 encodes a transmembrane protein whose function has never been studied in vertebrate species. Furthermore, the p.P155L variant was predicted to be benign using multiple mutation prediction programs, leading to skepticism that this variant has functional consequences. The fly TM2D3 homolog almondex (amx) was one of the earliest genes together with Notch, Delta, neur, mam, E(spl) and big brain (bib, endocing an Aquaporin-family protein), to be linked to Notch signaling based on its strong embryonic neurogenic phenotype when maternally mutated [4,5]. amx mutants undergo normal development, likely due to a large maternal contribution, but all embryos that are laid by homozygous or hemizygous (mutant over a deficiency of the locus) amx mutant females exhibit a strong neurogenic phenotype and die as embryos [437,5]. Although the molecular function of Amx is still unknown, genetic epistasis experiments have suggested that Amx likely functions at the S3 cleavage step of Notch activation [438]. Considering that PSEN1 and PSEN2, two genes that cause FAD also act at the same step in the Notch pathway, and that maternal-zygotic Psn null mutant embryos phenocopy the maternal amx mutant phenotype in Drosophila[38,39], the p.P155L in TM2D3 was an excellent candidate variant that may increase the risk of LOAD through regulation of the γ-secretase complex. Since the variant amino acid (p.P155) is not conserved between human and Drosophila, we humanized the fly amx gene by generating a genomic rescue construct in which the fly amx coding region has been replaced by the human sequence. Interestingly, the reference TM2D3 was able to partially rescue the neurogenic phenotype and lethality of the maternal amx mutant embryos, whereas TM2D3 with the p.P155L variant was not able to do so[436]. Hence, p.P155L associated with LOAD was shown to be a functional variant based on Notch-signaling related phenotypic assay performed in vivo, and further functional studies are ongoing to determine the precise molecular function of TM2D3/Amx in vivo.
In summary, genetic tools and phenotypic assays in Drosophila provide valuable information to assess the functional consequences of disease-linked variants in vivo. Even for conditions such as AD for which the pathogenic involvement of Notch signaling is still obscure, Notch signaling related phenotypes in Drosophila tissues such as the wing, bristle, and embryonic nervous system can be used as robust and reproducible phenotypic readouts to determine the functionality of disease-associated human variants of interest. Similar strategies can be employed to determine the functionalities of many VUS that are identified through massive sequencing efforts.
6. Conclusions
Notch signaling is a unique pathway that regulates diverse biological processes through a relatively simple and straightforward signal transduction mechanism. Studies in Drosophila have played a pioneering role in assembling the Notch signaling pathway, elucidating numerous factors that fine-tune it in diverse contexts. By combining a number of genetic tools and resources, fly researchers have uncovered a number of biological functions of this pathway in a variety of developmental and post-developmental settings.
Although Notch signaling in Drosophila has been extensively studied over the last century, exciting new discoveries continue to be made in the fly field that impacts the larger biomedical community. Large-scale screens using newer technologies will likely continue to expand the list of genes that regulate Notch signaling in vivo in Drosophila, which could subsequently be used as a starting point when studying the function of orthologous genes in vertebrates. Through efforts of clinicians and human geneticists, a number of human diseases that are caused by mutations in genes linked to Notch signaling have been discovered, increasing the significance of the pathway in human health. We foresee that such gene/disease lists will continue to expand through efforts in the human genomics field. Understanding the functional consequences of VUS is critical for these studies, and experiments in Drosophila can accelerate such efforts.
Moving forward, close communications and collaborations among Drosophila researchers, human geneticists and clinicians will provide a synergistic effect to quickly identify novel human disease linked genes, study the function of variants of interest, and begin to understand the biological function of these genes in vivo[439]. The rich knowledge regarding various biological contexts that are regulated by Notch signaling and the extensive genetic tools that are available to Drosophila researchers provide a unique advantage when studying novel human disease genes in the context of Notch signaling. Through further information exchange and collaborations with vertebrate biologists, biochemists, molecular biologists, structural biologists and bioinformaticians, such knowledge can further be translated to develop potential therapies for patients. Drosophila will continue to be at the forefront of the Notch signaling field, and discoveries made using the fly will provide valuable information to understand human pathology and possibly tame this pathway when necessary.
7. Acknowledgements
We apologize to our colleagues for not being able to cite their works. We thank Drs. Andrew K. Groves, Hamed Jafar-Nejad, Hillary K. Graves and Michael F. Wangler for constructive comments and helpful suggestions. S.Y. is supported by the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital (NRI Fellowship), the Naman Family Fund for Basic Research and the Caroline Wiess Law Fund for Research in Molecular Medicine (BCM Junior Faculty Seed Funding Program), Alzheimer’s Association (NIRH-15–364099), Simons Foundation Autism Research Initiative (Award#368479), and the National Institutes of Health (NIH, U54 NS093793). J.L.S. received support from the NIH (GMR2556929).
Abbreviations:
- AD
Alzheimer’s disease
- ADAM10
A Disintegrin and Metalloprotease 10
- AES
Amino-terminal Enhancer of Split
- ago
archipelago
- amx
almondex
- AOS
Adams-Oliver Syndrome
- AP-3
Adaptor Protein-3
- Aph
Anterior pharynx defective
- APOE
APOlipoprotein E
- APP
Amyloid Precursor Protein
- aPKC
atypical Protein Kinase C
- ARHGAP31
Rho GTPase-activating protein 31
- Arp2/3
Actin-related protein 2/3
- AS-C
Achaete-Scute Complex
- BAC
Bacterial Artificial Chromosome
- bib
big brain
- BDSC
Bloomington Drosophila Stock Center
- bHLH
basic Helix-Loop-Helix
- C. elegans
Caenorhabditis elegans
- CADASIL
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts andLeukoencephalopathy
- CBP/CREBBP
CREB Binding Protein
- CDK8
Cyclin-Dependent Kinase 8
- CREB
cAMP response element binding protein
- cDNA
complementary DeoxyriboNucleic Acid
- CHARGE
Cohorts for Heart and Aging Research in Genomic Epidemiology
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- CtBP
C-terminal Binding Protein
- DFS
Dominant Female Sterile
- DGGR
Drosophila Genomics and Genetic Resources
- DGRC
Drosophila Genomics Resource Center
- DIOPT
Drosophila RNAi Screening Center Integrative Ortholog Prediction Tool
- Dl
Delta
- DLL
DeLta-Like
- DOCK6
Dedicator Of Cytokinesis 6
- DSHB
Developmental Studies Hybridoma Bank
- dx
deltex
- E(spl)-C
Enhancer of split-Complex
- EGF
Epidermal Growth Factor
- EHBP-1
EH (Eps15 Homology) domain Binding Protein-1
- elav
embryonic lethal abnormal vision
- EMS
Ethyl MethaneSulfonate
- Eogt
EGF-domain O-GlcNAc transferase
- EP300
E1A binding protein P300
- ER
Endoplasmic Reticulum
- ESCRT
Endosomal Sorting Complexes Required for Transport
- FAD
Familial Alzheimer’s Disease
- FBXW7
F-BoX and WD repeat domain containing 7
- FHL1
Four and a H alf LIM domains 1
- FLP
FLiPpase
- Fng
Fringe
- FRT
Flippase Recognition Target
- GAP
GTPase-Activating Protein
- GEF
Guanine nucleotide Exchange Factor
- GFI1
Growth Factor Independent 1 transcriptional repressor
- glcNAc
N-Acetylglucosamine
- glp-1
Germ Line Proliferation defective-1
- GOM
granular osmophilic material
- Gro
Groucho
- GWAS
Genome-Wide Association Studies
- H
Hairless
- hAPF
hours After Puparium Formation
- HCOP
Human genome organization gene nomenclature committee Comparison of Orthology Predictions search
- HDR
Homology Directed Repair
- HES
Hairy and Enhancer of Split
- HEY
Hairy/Enhancer-of-split related with YRPW motif protein
- HOPS
HOmotypic fusion and Protein Sorting
- IMF
Infantile MyoFibromatosis
- JAG
JAGged
- kuz
kuzbanian
- l(2)gd1
lethal (2) giant discs 1
- LFNG
Lunatic FiNGe
- LIM
Lin11, Isl-1 and Mec-3
- lin-12
cell LINeage defective-12
- LMNS
Lateral MeNingocele Syndrome
- LNR
Lin-12/Notch Repeat
- LOAD
Late-Onset Alzheimer’s Disease
- LVNC
Left Ventricular NonCompaction
- mam
mastermind
- MAML
Mastermind-like
- MARRVEL
Model organism Aggregated Resources for Rare Variant ExpLoration
- MESP2
Mesoderm posterior bHLH transcription factor 2
- MFNG
Manic FriNGe
- mib
mindbomb
- MiMIC
Minos-Mediated Integration Cassette
- mRNA
messenger RiboNucleic Acid
- Nct/NCSTN
Nicastrin
- nej
nejire
- neur/NEURL
neutralized
- NEXT
Notch extracellular truncation
- NICD
Notch intracellular domain
- NRR
Negative Regulatory Region
- O-fut1
O-fucosyltransferase-1
- OMIM
Online Mendelian Inheritance in Man
- PDGFRB
Platelet Derived Growth Factor Receptor Beta
- pen/PSENEN
presenilin enhancer
- PEST
proline (P), glutamic acid (E), serine (S) and threonine (T)-rich
- POFUT1
Protein O-fucosyltransferase 1
- POGLUT1
Protein O-glucosyltransferase 1
- PTM
post-translational modification
- Psn/PSEN
Presenilin
- RBPJ
Recombination signal Binding Protein for immunoglobulin kappa J region
- RFNG
Radical FriNGe
- RIPPLY2
RIPPLY transcriptional repressor 2
- RMCE
Recombinase Mediated Cassette Exchange
- RNAi
RNA interference
- SA
Splice Acceptor
- SCDO
SpondyloCostal DysOstosis
- Sec15
Secretory 15
- Ser
Serrate
- SHARP
SMRT/HDAC1 Associated Repressor Protein
- shi
shibire
- SOP
Sensory Organ Precursor
- spdo
sanpodo
- SPEN
Split ENds family transcriptional repressor
- spl
split
- Su(dx)
Suppressor of deltex
- Su(H)
Suppressor of Hairless
- TBX6
T-box 6
- Temp
Tempura
- TLE
Transducin Like Enhancer protein
- TM2D3
TM2 domain containing 3
- UAS
Upstream Activation Sequence
- V-ATPase
Vacuolar-ATPase
- VDRC
Vienna Drosophila Resource Center
- VUS
Variant of Unknown Significance
- WASp
Wiskott-Aldrich Syndrome protein
- wg
wingless
References
- 1.Artavanis-Tsakonas S, Muskavitch MA (2010) Notch: the past, the present, and the future. Curr Top Dev Biol 92:1–29 [DOI] [PubMed] [Google Scholar]
- 2.Dexter JS (1914) The analysis of a case of continuous variation in Drosophila by a study of its linkage relations. American Naturalist 48:712–758 [Google Scholar]
- 3.Lindsley DL, Zimm GG (1992) The Genome of Drosophila melanogaster. Academic Press, [Google Scholar]
- 4.Lehmann R, Dietrich U, Jiménez F, Campos-Ortega JA (1981) Mutations of early neurogenesis in Drosophila. Wilehm Roux Arch Dev Biol (Dev Genes Evo) 190 (4):226–229 [DOI] [PubMed] [Google Scholar]
- 5.Lehmann R, Jimenez F, Dietrich U, Campos-Ortega JA (1983) On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Wilehm Roux Arch Dev Biol (Dev Genes Evo) 192 (2): 62–74 [DOI] [PubMed] [Google Scholar]
- 6.Poulson DF (1936) Embryology of Drosophila. Records of the Genetics Society of America 5 [Google Scholar]
- 7.Poulson DF (1937) Chromosomal Deficiencies and the Embryonic Development of Drosophila Melanogaster. Proceedings of the National Academy of Sciences of the United States of America 23 (3): 133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S (1985) Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 43 (3 Pt 2):567–581 [DOI] [PubMed] [Google Scholar]
- 9.Kidd S, Kelley MR, Young MW (1986) Sequence of the notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol 6 (9):3094–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopczynski CC, Alton AK, Fechtel K, Kooh PJ, Muskavitch MA (1988) Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes & Development 2 (12b):1723–1735 [DOI] [PubMed] [Google Scholar]
- 11.Thomas U, Speicher SA, Knust E (1991) The Drosophila gene Serrate encodes an EGF-like transmembrane protein with a complex expression pattern in embryos and wing discs. Development 111 (3):749–761 [DOI] [PubMed] [Google Scholar]
- 12.Fleming RJ, Scottgale TN, Diederich RJ, Artavanis-Tsakonas S (1990) The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev 4 (12A):2188–2201 [DOI] [PubMed] [Google Scholar]
- 13.Boulianne GL, de la Concha A, Campos-Ortega JA, Jan LY, Jan YN (1991) The Drosophila neurogenic gene neuralized encodes a novel protein and is expressed in precursors of larval and adult neurons. The EMBO journal 10 (10):2975–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smoller D, Friedel C, Schmid A, Bettler D, Lam L, Yedvobnick B (1990) The Drosophila neurogenic locus mastermind encodes a nuclear protein unusually rich in amino acid homopolymers. Genes Dev 4 (10):1688–1700 [DOI] [PubMed] [Google Scholar]
- 15.Maier D, Stumm G, Kuhn K, Preiss A (1992) Hairless, a Drosophila gene involved in neural development, encodes a novel, serine rich protein. Mech Dev 38 (2):143–156 [DOI] [PubMed] [Google Scholar]
- 16.Xu T, Artavanis-Tsakonas S (1990) deltex, a locus interacting with the neurogenic genes, Notch, Delta and mastermind in Drosophila melanogaster. Genetics 126 (3):665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan TH, Sturtevant AH, Bridges CB (1922). Year Book Carnegie Inst Wash 22:283–287 [Google Scholar]
- 18.Fortini ME, Artavanis-Tsakonas S (1994) The suppressor of hairless protein participates in notch receptor signaling. Cell 79 (2):273–282 [DOI] [PubMed] [Google Scholar]
- 19.Plunkett CR (1926) The interaction of genetic and environmental factors in development. Journal of Experimental Zoology 46:181–244 [Google Scholar]
- 20.Welshons WJ (1956) Dosage experiments with split mutants in the presence of an enhancer of split. Drosophila Inf Serv 30:157–158 [Google Scholar]
- 21.Busseau I, Diederich RJ, Xu T, Artavanis-Tsakonas S (1994) A member of the Notch group of interacting loci, deltex encodes a cytoplasmic basic protein. Genetics 136 (2):585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go MJ, Artavanis-Tsakonas S (1998) A genetic screen for novel components of the notch signaling pathway during Drosophila bristle development. Genetics 150 (1):211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furukawa T, Kawaichi M, Matsunami N, Ryo H, Nishida Y, Honjo T (1991) The Drosophila RBP-J kappa gene encodes the binding protein for the immunoglobulin J kappa recombination signal sequence. J Biol Chem 266 (34):23334–23340 [PubMed] [Google Scholar]
- 24.Schweisguth F, Posakony JW (1992) Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell 69:11991212 [DOI] [PubMed] [Google Scholar]
- 25.Delidakis C, Artavanis-Tsakonas S (1992) The Enhancer of split [E(spl)] locus of Drosophila encodes seven independent helix-loop-helix proteins. Proceedings of the National Academy of Sciences of the United States of America 89 (18): 8731–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrons H, Knust E, Campos-Ortega JA (1992) The Enhancer of split complex and adjacent genes in the 96F region of Drosophila melanogaster are required for segregation of neural and epidermal progenitor cells. Genetics 132 (2):481–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artavanis-Tsakonas S, Matsuno K, Fortini ME (1995) Notch signaling. Science 268 (5208):225–232 [DOI] [PubMed] [Google Scholar]
- 28.Goto S, Taniguchi M, Muraoka M, Toyoda H, Sado Y, Kawakita M, Hayashi S (2001) UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat Cell Biol 3 (9):816–822 [DOI] [PubMed] [Google Scholar]
- 29.Irvine KD, Wieschaus E (1994) fringe, a Boundary-specific signaling molecule, mediates interactions between dorsal and ventral cells during Drosophila wing development. Cell 79 (4):595–606 [DOI] [PubMed] [Google Scholar]
- 30.Periz G, Fortini ME (1999) Ca(2+)-ATPase function is required for intracellular trafficking of the Notch receptor in Drosophila. The EMBO journal 18 (21):5983–5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian X, Hansen D, Schedl T, Skeath JB (2004) Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development 131 (23):5807–5815 [DOI] [PubMed] [Google Scholar]
- 32.Xu T, Rubin GM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 (4):1223–1237 [DOI] [PubMed] [Google Scholar]
- 33.Chou TB, Perrimon N (1992) Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics 131 (3):643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrimon N, Lanjuin A, Arnold C, Noll E (1996) Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics 144 (4): 1681–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasamura T, Sasaki N, Miyashita F, Nakao S, Ishikawa HO, Ito M, Kitagawa M, Harigaya K, Spana E, Bilder D, Perrimon N, Matsuno K (2003) neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development 130 (20):4785–4795 [DOI] [PubMed] [Google Scholar]
- 36.Selva EM, Hong K, Baeg GH, Beverley SM, Turco SJ, Perrimon N, Hacker U (2001) Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events. Nat Cell Biol 3 (9):809–815 [DOI] [PubMed] [Google Scholar]
- 37.Goode S, Melnick M, Chou TB, Perrimon N (1996) The neurogenic genes egghead and brainiac define a novel signaling pathway essential for epithelial morphogenesis during Drosophila oogenesis. Development 122 (12):3863–3879 [DOI] [PubMed] [Google Scholar]
- 38.Struhl G, Greenwald I (1999) Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398 (6727):522–525 [DOI] [PubMed] [Google Scholar]
- 39.Ye Y, Lukinova N, Fortini ME (1999) Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature 398 (6727):525–529 [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Livne-Bar I, Zhou L, Boulianne GL (1999) Drosophila presenilin is required for neuronal differentiation and affects notch subcellular localization and signaling. J Neurosci 19 (19):8435–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahoney MB, Parks AL, Ruddy DA, Tiong SY, Esengil H, Phan AC, Philandrinos P, Winter CG, Chatterjee R, Huppert K, Fisher WW, L’Archeveque L, Mapa FA, Woo W, Ellis MC, Curtis D (2006) Presenilin-based genetic screens in Drosophila melanogaster identify novel notch pathway modifiers. Genetics 172 (4):2309–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eun SH, Lea K, Overstreet E, Stevens S, Lee JH, Fischer JA (2007) Identification of genes that interact with Drosophila liquid facets. Genetics 175 (3): 1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hing HK, Bangalore L, Sun X, Artavanis-Tsakonas S (1999) Mutations in the heatshock cognate 70 protein (hsc4) modulate Notch signaling. Eur J Cell Biol 78 (10):690–697 [DOI] [PubMed] [Google Scholar]
- 44.Rottgen G, Wagner T, Hinz U (1998) A genetic screen for elements of the network that regulates neurogenesis in Drosophila. Mol Gen Genet 257 (4):442–451 [DOI] [PubMed] [Google Scholar]
- 45.Royet J, Bouwmeester T, Cohen SM (1998) Notchless encodes a novel WD40-repeat-containing protein that modulates Notch signaling activity. The EMBO journal 17 (24):7351–7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiber SL, Preiss A, Nagel AC, Wech I, Maier D (2002) Genetic screen for modifiers of the rough eye phenotype resulting from overexpression of the Notch antagonist hairless in Drosophila. Genesis 33 (3):141–152 [DOI] [PubMed] [Google Scholar]
- 47.Shalaby NA, Parks AL, Morreale EJ, Osswalt MC, Pfau KM, Pierce EL, Muskavitch MA (2009) A screen for modifiers of notch signaling uncovers Amun, a protein with a critical role in sensory organ development. Genetics 182 (4): 1061–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Hoef DL, Hughes J, Livne-Bar I, Garza D, Konsolaki M, Boulianne GL (2009) Identifying genes that interact with Drosophila presenilin and amyloid precursor protein. Genesis 47 (4):246–260 [DOI] [PubMed] [Google Scholar]
- 49.Verheyen EM, Purcell KJ, Fortini ME, Artavanis-Tsakonas S (1996) Analysis of dominant enhancers and suppressors of activated Notch in Drosophila. Genetics 144 (3): 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yedvobnick B, Helms W, Barrett B (2001) Identification of chromosomal deficiencies that modify Drosophila mastermind mutant phenotypes. Genesis 30 (4):250–258 [DOI] [PubMed] [Google Scholar]
- 51.Mishra AK, Sachan N, Mutsuddi M, Mukherjee A (2015) Kinase active Misshapen regulates Notch signaling in Drosophila melanogaster. Exp Cell Res 339 (1):51–60 [DOI] [PubMed] [Google Scholar]
- 52.Bray S, Musisi H, Bienz M (2005) Bre1 is required for Notch signaling and histone modification. Dev Cell 8 (2):279–286 [DOI] [PubMed] [Google Scholar]
- 53.Hori K, Sen A, Kirchhausen T, Artavanis-Tsakonas S (2011) Synergy between the ESCRT-III complex and Deltex defines a ligand-independent Notch signal. J Cell Biol 195 (6): 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acar M, Jafar-Nejad H, Takeuchi H, Rajan A, Ibrani D, Rana NA, Pan H, Haltiwanger RS, Bellen HJ (2008) Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell 132 (2):247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ (2005) Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell 9 (3):351–363 [DOI] [PubMed] [Google Scholar]
- 56.Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ (2009) The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol 11 (7):815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tien AC, Rajan A, Schulze KL, Ryoo HD, Acar M, Steller H, Bellen HJ (2008) Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster. J Cell Biol 182 (6): 1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charng WL, Yamamoto S, Jaiswal M, Bayat V, Xiong B, Zhang K, Sandoval H, David G, Gibbs S, Lu HC, Chen K, Giagtzoglou N, Bellen HJ (2014) Drosophila Tempura, a novel protein prenyltransferase alpha subunit, regulates notch signaling via Rab1 and Rab11. PLoS Biol 12(1):e1001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giagtzoglou N, Li T, Yamamoto S, Bellen HJ (2013) Drosophila EHBP1 regulates Scabrous secretion during Notch-mediated lateral inhibition. J Cell Sci 126 (Pt 16):3686–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giagtzoglou N, Yamamoto S, Zitserman D, Graves HK, Schulze KL, Wang H, Klein H, Roegiers F, Bellen HJ (2012) dEHBP1 controls exocytosis and recycling of Delta during asymmetric divisions. J Cell Biol 196 (1):65–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaccari T, Bilder D (2005) The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 9 (5):687–698 [DOI] [PubMed] [Google Scholar]
- 62.Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, Stenmark H, Bilder D (2009) Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J Cell Sci 122 (Pt 14):2413–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Domanitskaya E, Schupbach T (2012) CoREST acts as a positive regulator of Notch signaling in the follicle cells of Drosophila melanogaster. J Cell Sci 125 (Pt 2):399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Y, Yan Y, Denef N, Schupbach T (2011) Regulation of somatic myosin activity by protein phosphatase 1beta controls Drosophila oocyte polarization. Development 138 (10): 1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan Y, Denef N, Schupbach T (2009) The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell 17 (3):387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA (2002) The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell 3 (2):221–231 [DOI] [PubMed] [Google Scholar]
- 67.Hutterer A, Knoblich JA (2005) Numb and alpha-Adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs. EMBO Rep 6 (9):836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallagher CM, Knoblich JA (2006) The conserved c2 domain protein lethal (2) giant discs regulates protein trafficking in Drosophila. Dev Cell 11 (5): 641–653 [DOI] [PubMed] [Google Scholar]
- 69.Haberman AS, Akbar MA, Ray S, Kramer H (2010) Drosophila acinus encodes a novel regulator of endocytic and autophagic trafficking. Development 137 (13):2157–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dornier E, Coumailleau F, Ottavi JF, Moretti J, Boucheix C, Mauduit P, Schweisguth F, Rubinstein E (2012) TspanC8 tetraspanins regulate ADAM10/Kuzbanian trafficking and promote Notch activation in flies and mammals. J Cell Biol 199 (3):481–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia D, Soylemez M, Calvin G, Bornmann R, Bryant J, Hanna C, Huang YC, Deng WM (2015) A large-scale in vivo RNAi screen to identify genes involved in Notch-mediated follicle cell differentiation and cell cycle switches. Sci Rep 5:12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berns N, Woichansky I, Friedrichsen S, Kraft N, Riechmann V (2014) A genome-scale in vivo RNAi analysis of epithelial development in Drosophila identifies new proliferation domains outside of the stem cell niche. J Cell Sci 127 (Pt 12):2736–2748 [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Liu M, Su Y, Du J, Zhu AJ (2012) A targeted in vivo RNAi screen reveals deubiquitinases as new regulators of Notch signaling. G3 (Bethesda) 2 (12):1563–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saj A, Arziman Z, Stempfle D, van Belle W, Sauder U, Horn T, Durrenberger M, Paro R, Boutros M, Merdes G (2010) A combined ex vivo and in vivo RNAi screen for notch regulators in Drosophila reveals an extensive notch interaction network. Dev Cell 18 (5):862–876 [DOI] [PubMed] [Google Scholar]
- 75.Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, Dietzl G, Dickson BJ, Knoblich JA (2009) Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature 458 (7241):987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez-Lamarca MJ, Snowdon LA, Seib E, Klein T, Bray SJ (2015) Rme-8 depletion perturbs Notch recycling and predisposes to pathogenic signaling. J Cell Biol 210 (2):303–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 (2):401–415 [DOI] [PubMed] [Google Scholar]
- 78.Vallejo DM, Caparros E, Dominguez M (2011) Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. The EMBO journal 30 (4):756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hall LE, Alexander SJ, Chang M, Woodling NS, Yedvobnick B (2004) An EP overexpression screen for genetic modifiers of Notch pathway function in Drosophila melanogaster. Genet Res 83 (2):71–82 [DOI] [PubMed] [Google Scholar]
- 80.Da Ros VG, Gutierrez-Perez I, Ferres-Marco D, Dominguez M (2013) Dampening the signals transduced through hedgehog via microRNA miR-7 facilitates notch-induced tumourigenesis. PLoS Biol 11 (5):e1001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pi H, Huang YC, Chen IC, Lin CD, Yeh HF, Pai LM (2011) Identification of 11-amino acid peptides that disrupt Notch-mediated processes in Drosophila. J Biomed Sci 18:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adam JC, Montell DJ (2004) A role for extra macrochaetae downstream of Notch in follicle cell differentiation. Development 131 (23):5971–5980 [DOI] [PubMed] [Google Scholar]
- 83.Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D (2002) aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell 3 (1):85–97 [DOI] [PubMed] [Google Scholar]
- 84.Mourikis P, Lake RJ, Firnhaber CB, DeDecker BS (2010) Modifiers of notch transcriptional activity identified by genome-wide RNAi. BMC Dev Biol 10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodfellow H, Krejci A, Moshkin Y, Verrijzer CP, Karch F, Bray SJ (2007) Gene-specific targeting of the histone chaperone asf1 to mediate silencing. Dev Cell 13 (4):593–600 [DOI] [PubMed] [Google Scholar]
- 86.Li J, Housden BE, Bray SJ (2014) Notch signaling assays in Drosophila cultured cell lines. Methods Mol Biol 1187:131–141 [DOI] [PubMed] [Google Scholar]
- 87.Bernard F, Krejci A, Housden B, Adryan B, Bray SJ (2010) Specificity of Notch pathway activation: twist controls the transcriptional output in adult muscle progenitors. Development 137 (16):2633–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Slaninova V, Krafcikova M, Perez-Gomez R, Steffal P, Trantirek L, Bray SJ, Krejci A (2016) Notch stimulates growth by direct regulation of genes involved in the control of glycolysis and the tricarboxylic acid cycle. Open Biol 6 (2): 150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pezeron G, Millen K, Boukhatmi H, Bray S (2014) Notch directly regulates the cell morphogenesis genes Reck, talin and trio in adult muscle progenitors. J Cell Sci 127 (Pt 21):4634–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Babaoglan AB, Housden BE, Furriols M, Bray SJ (2013) Deadpan contributes to the robustness of the notch response. PLoS One 8 (9):e75632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Housden BE, Fu AQ, Krejci A, Bernard F, Fischer B, Tavare S, Russell S, Bray SJ (2013) Transcriptional dynamics elicited by a short pulse of notch activation involves feed-forward regulation by E(spl)/Hes genes. PLoS Genet 9 (1):e1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Djiane A, Krejci A, Bernard F, Fexova S, Millen K, Bray SJ (2013) Dissecting the mechanisms of Notch induced hyperplasia. The EMBO journal 32 (1):60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krejci A, Bernard F, Housden BE, Collins S, Bray SJ (2009) Direct response to Notch activation: signaling crosstalk and incoherent logic. Sci Signal 2 (55):ra1. [DOI] [PubMed] [Google Scholar]
- 94.Pines MK, Housden BE, Bernard F, Bray SJ, Roper K (2010) The cytolinker Pigs is a direct target and a negative regulator of Notch signalling. Development 137 (6):913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Terriente-Felix A, Li J, Collins S, Mulligan A, Reekie I, Bernard F, Krejci A, Bray S (2013) Notch cooperates with Lozenge/Runx to lock haemocytes into a differentiation programme. Development 140 (4):926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zacharioudaki E, Housden BE, Garinis G, Stojnic R, Delidakis C, Bray SJ (2016) Genes implicated in stem cell identity and temporal programme are directly targeted by Notch in neuroblast tumours. Development 143 (2):219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S (2005) Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol 7 (12): 1191–1201 [DOI] [PubMed] [Google Scholar]
- 98.Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Vaidya N, Beekman C, Wong C, Rhee DY, Cenaj O, McKillip E, Shah S, Stapleton M, Wan KH, Yu C, Parsa B, Carlson JW, Chen X, Kapadia B, Vijayraghavan K, Gygi SP, Celniker SE, Obar RA, Artavanis-Tsakonas S (2011) A Protein Complex Network of Drosophila melanogaster. Cell 147 (3):690–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guruharsha KG, Hori K, Obar RA, Artavanis-Tsakonas S (2014) Proteomic analysis of the Notch interactome. Methods Mol Biol 1187:181–192 [DOI] [PubMed] [Google Scholar]
- 100.Moshkin YM, Kan TW, Goodfellow H, Bezstarosti K, Maeda RK, Pilyugin M, Karch F, Bray SJ, Demmers JA, Verrijzer CP (2009) Histone chaperones ASF1 and NAP1 differentially modulate removal of active histone marks by LID-RPD3 complexes during NOTCH silencing. Mol Cell 35(6):782–793 [DOI] [PubMed] [Google Scholar]
- 101.Ilagan MX, Kopan R (2007) SnapShot: notch signaling pathway. Cell 128 (6):1246. [DOI] [PubMed] [Google Scholar]
- 102.Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S (2007) Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol 19 (2):166–175 [DOI] [PubMed] [Google Scholar]
- 103.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S (2012) The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 13 (9):654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137 (2):216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yochem J, Weston K, Greenwald I (1988) The Caenorhabditis elegans lin-12 gene encodes a transmembrane protein with overall similarity to Drosophila Notch. Nature 335 (6190):547–550 [DOI] [PubMed] [Google Scholar]
- 106.Yochem J, Greenwald I (1989) glp-1 and lin-12, genes implicated in distinct cell-cell interactions in C. elegans, encode similar transmembrane proteins. Cell 58 (3):553–563 [DOI] [PubMed] [Google Scholar]
- 107.Leonardi J, Fernandez-Valdivia R, Li YD, Simcox AA, Jafar-Nejad H (2011) Multiple O-glucosylation sites on Notch function as a buffer against temperature-dependent loss of signaling. Development 138 (16):3569–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamamoto S, Charng WL, Rana NA, Kakuda S, Jaiswal M, Bayat V, Xiong B, Zhang K, Sandoval H, David G, Wang H, Haltiwanger RS, Bellen HJ (2012) A mutation in EGF repeat-8 of Notch discriminates between Serrate/Jagged and Delta family ligands. Science 338 (6111): 12291232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fortini ME (2009) Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16 (5):633–647 [DOI] [PubMed] [Google Scholar]
- 110.Okajima T, Irvine KD (2002) Regulation of notch signaling by o-linked fucose. Cell 111(6):893–904 [DOI] [PubMed] [Google Scholar]
- 111.Sethi MK, Buettner FF, Krylov VB, Takeuchi H, Nifantiev NE, Haltiwanger RS, Gerardy-Schahn R, Bakker H (2010) Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats. J Biol Chem 285 (3): 1582–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee TV, Sethi MK, Leonardi J, Rana NA, Buettner FF, Haltiwanger RS, Bakker H, Jafar-Nejad H (2013) Negative regulation of notch signaling by xylose. PLoS Genet 9 (6):e1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruckner K, Perez L, Clausen H, Cohen S (2000) Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 406 (6794):411–415 [DOI] [PubMed] [Google Scholar]
- 114.Panin VM, Papayannopoulos V, Wilson R, Irvine KD (1997) Fringe modulates Notch-ligand interactions. Nature 387 (6636):908–912 [DOI] [PubMed] [Google Scholar]
- 115.Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, Wilson R, Wang Y, Stanley P, Irvine KD, Haltiwanger RS, Vogt TF (2000) Fringe is a glycosyltransferase that modifies Notch. Nature 406 (6794):369–375 [DOI] [PubMed] [Google Scholar]
- 116.Jafar-Nejad H, Leonardi J, Fernandez-Valdivia R (2010) Role of glycans and glycosyltransferases in the regulation of Notch signaling. Glycobiology 20 (8):931–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rana NA, Haltiwanger RS (2011) Fringe benefits: functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr Opin Struct Biol 21 (5):583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muller R, Jenny A, Stanley P (2013) The EGF repeat-specific O-GlcNAc-transferase Eogt interacts with notch signaling and pyrimidine metabolism pathways in Drosophila. PLoS One 8(5):e62835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakaidani Y, Ichiyanagi N, Saito C, Nomura T, Ito M, Nishio Y, Nadano D, Matsuda T, Furukawa K, Okajima T (2012) O-linked-N-acetylglucosamine modification of mammalian Notch receptors by an atypical O-GlcNAc transferase Eogt1. Biochem Biophys Res Commun 419 (1): 1419 [DOI] [PubMed] [Google Scholar]
- 120.Johansen KM, Fehon RG, Artavanis-Tsakonas S (1989) The notch gene product is a glycoprotein expressed on the cell surface of both epidermal and neuronal precursor cells during Drosophila development. J Cell Biol 109 (5):2427–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kidd S, Baylies MK, Gasic GP, Young MW (1989) Structure and distribution of the Notch protein in developing Drosophila. Genes Dev 3 (8):1113–1129 [DOI] [PubMed] [Google Scholar]
- 122.Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S (1997) Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 90 (2):281–291 [DOI] [PubMed] [Google Scholar]
- 123.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A (1998) The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proceedings of the National Academy of Sciences of the United States of America 95 (14):8108–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kidd S, Lieber T (2002) Furin cleavage is not a requirement for Drosophila Notch function. Mech Dev 115 (1–2):41–51 [DOI] [PubMed] [Google Scholar]
- 125.Lake RJ, Grimm LM, Veraksa A, Banos A, Artavanis-Tsakonas S (2009) In vivo analysis of the Notch receptor S1 cleavage. PLoS One 4 (8):e6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gordon WR, Vardar-Ulu D, L’Heureux S, Ashworth T, Malecki MJ, Sanchez-Irizarry C, McArthur DG, Histen G, Mitchell JL, Aster JC, Blacklow SC (2009) Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One 4(8):e6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fehon RG, Johansen K, Rebay I, Artavanis-Tsakonas S (1991) Complex cellular and subcellular regulation of notch expression during embryonic and imaginal development of Drosophila: implications for notch function. J Cell Biol 113 (3):657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hartley DA, Xu TA, Artavanis-Tsakonas S (1987) The embryonic expression of the Notch locus of Drosophila melanogaster and the implications of point mutations in the extracellular EGF-like domain of the predicted protein. The EMBO journal 6 (11):3407–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vassin H, Bremer KA, Knust E, Campos-Ortega JA (1987) The neurogenic gene Delta of Drosophila melanogaster is expressed in neurogenic territories and encodes a putative transmembrane protein with EGF-like repeats. The EMBO journal 6 (11):3431–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bender LB, Kooh PJ, Muskavitch MA (1993) Complex function and expression of Delta during Drosophila oogenesis. Genetics 133 (4):967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kopczynski CC, Muskavitch MA (1989) Complex spatio-temporal accumulation of alternative transcripts from the neurogenic gene Delta during Drosophila embryogenesis. Development 107 (3):623–636 [DOI] [PubMed] [Google Scholar]
- 132.Parks AL, Turner FR, Muskavitch MA (1995) Relationships between complex Delta expression and the specification of retinal cell fates during Drosophila eye development. Mech Dev 50 (2–3):201–216 [DOI] [PubMed] [Google Scholar]
- 133.Kooh PJ, Fehon RG, Muskavitch MA (1993) Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophila development. Development 117 (2):493–507 [DOI] [PubMed] [Google Scholar]
- 134.Bachmann A, Knust E (1998) Dissection of cis-regulatory elements of the Drosophila gene Serrate. Dev Genes Evol 208 (6):346–351 [DOI] [PubMed] [Google Scholar]
- 135.Xu A, Haines N, Dlugosz M, Rana NA, Takeuchi H, Haltiwanger RS, Irvine KD (2007) In vitro reconstitution of the modulation of Drosophila Notch-ligand binding by Fringe. J Biol Chem 282 (48):35153–35162 [DOI] [PubMed] [Google Scholar]
- 136.Heuss SF, Ndiaye-Lobry D, Six EM, Israel A, Logeat F (2008) The intracellular region of Notch ligands Dll1 and Dll3 regulates their trafficking and signaling activity. Proceedings of the National Academy of Sciences of the United States of America 105 (32): 11212–11217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ladi E, Nichols JT, Ge W, Miyamoto A, Yao C, Yang LT, Boulter J, Sun YE, Kintner C, Weinmaster G (2005) The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol 170 (6):983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kakuda S, Haltiwanger RS (2017) Deciphering the Fringe-Mediated Notch Code: Identification of Activating and Inhibiting Sites Allowing Discrimination between Ligands. Dev Cell 40 (2): 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC (2007) Structural basis for autoinhibition of Notch. Nat Struct Mol Biol 14 (4):295–300 [DOI] [PubMed] [Google Scholar]
- 140.Gordon WR, Roy M, Vardar-Ulu D, Garfinkel M, Mansour MR, Aster JC, Blacklow SC (2009) Structure of the Notch1-negative regulatory region: implications for normal activation and pathogenic signaling in T-ALL. Blood 113 (18):4381–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kovall RA, Blacklow SC (2010) Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol 92:31–71 [DOI] [PubMed] [Google Scholar]
- 142.Le Bras S, Loyer N, Le Borgne R (2011) The multiple facets of ubiquitination in the regulation of notch signaling pathway. Traffic 12 (2): 149–161 [DOI] [PubMed] [Google Scholar]
- 143.Weinmaster G, Fischer JA (2011) Notch ligand ubiquitylation: what is it good for? Dev Cell 21 (1):134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang W, Struhl G (2005) Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development 132 (12):2883–2894 [DOI] [PubMed] [Google Scholar]
- 145.Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM (2005) The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development 132 (10):2319–2332 [DOI] [PubMed] [Google Scholar]
- 146.Le Borgne R, Remaud S, Hamel S, Schweisguth F (2005) Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol 3 (4):e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pitsouli C, Delidakis C (2005) The interplay between DSL proteins and ubiquitin ligases in Notch signaling. Development 132 (18):4041–4050 [DOI] [PubMed] [Google Scholar]
- 148.Lai EC, Deblandre GA, Kintner C, Rubin GM (2001) Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell 1 (6):783–794 [DOI] [PubMed] [Google Scholar]
- 149.Pavlopoulos E, Pitsouli C, Klueg KM, Muskavitch MA, Moschonas NK, Delidakis C (2001) neuralized Encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev Cell 1 (6):807–816 [DOI] [PubMed] [Google Scholar]
- 150.Koo BK, Yoon KJ, Yoo KW, Lim HS, Song R, So JH, Kim CH, Kong YY (2005) Mind bomb-2 is an E3 ligase for Notch ligand. J Biol Chem 280 (23):22335–22342 [DOI] [PubMed] [Google Scholar]
- 151.Nguyen HT, Voza F, Ezzeddine N, Frasch M (2007) Drosophila mind bomb2 is required for maintaining muscle integrity and survival. J Cell Biol 179 (2):219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rullinkov G, Tamme R, Sarapuu A, Lauren J, Sepp M, Palm K, Timmusk T (2009) Neuralized-2: expression in human and rodents and interaction with Delta-like ligands. Biochem Biophys Res Commun 389 (3):420–425 [DOI] [PubMed] [Google Scholar]
- 153.Koutelou E, Sato S, Tomomori-Sato C, Florens L, Swanson SK, Washburn MP, Kokkinaki M, Conaway RC, Conaway JW, Moschonas NK (2008) Neuralized-like 1 (Neurl1) targeted to the plasma membrane by N-myristoylation regulates the Notch ligand Jagged1. J Biol Chem 283 (7):3846–3853 [DOI] [PubMed] [Google Scholar]
- 154.Teider N, Scott DK, Neiss A, Weeraratne SD, Amani VM, Wang Y, Marquez VE, Cho YJ, Pomeroy SL (2010) Neuralized1 causes apoptosis and downregulates Notch target genes in medulloblastoma. Neuro Oncol 12 (12):1244–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koo BK, Yoon MJ, Yoon KJ, Im SK, Kim YY, Kim CH, Suh PG, Jan YN, Kong YY (2007) An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS One 2 (11):e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tagami S, Okochi M, Yanagida K, Ikuta A, Fukumori A, Matsumoto N, Ishizuka-Katsura Y, Nakayama T, Itoh N, Jiang J, Nishitomi K, Kamino K, Morihara T, Hashimoto R, Tanaka T, Kudo T, Chiba S, Takeda M (2008) Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol Cell Biol 28 (1):165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D (2008) Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol 180 (4):755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seugnet L, Simpson P, Haenlin M (1997) Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol 192 (2):585–598 [DOI] [PubMed] [Google Scholar]
- 159.Struhl G, Adachi A (2000) Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell 6 (3):625–636 [DOI] [PubMed] [Google Scholar]
- 160.Yamada K, Fuwa TJ, Ayukawa T, Tanaka T, Nakamura A, Wilkin MB, Baron M, Matsuno K (2011) Roles of Drosophila deltex in Notch receptor endocytic trafficking and activation. Genes Cells 16 (3):261–272 [DOI] [PubMed] [Google Scholar]
- 161.Fuwa TJ, Hori K, Sasamura T, Higgs J, Baron M, Matsuno K (2006) The first deltex null mutant indicates tissue-specific deltex-dependent Notch signaling in Drosophila. Mol Genet Genomics 275 (3):251–263 [DOI] [PubMed] [Google Scholar]
- 162.Mazaleyrat SL, Fostier M, Wilkin MB, Aslam H, Evans DA, Cornell M, Baron M (2003) Down-regulation of Notch target gene expression by Suppressor of deltex. Dev Biol 255 (2):363–372 [DOI] [PubMed] [Google Scholar]
- 163.Cornell M, Evans DA, Mann R, Fostier M, Flasza M, Monthatong M, Artavanis-Tsakonas S, Baron M (1999) The Drosophila melanogaster Suppressor of deltex gene, a regulator of the Notch receptor signaling pathway, is an E3 class ubiquitin ligase. Genetics 152 (2):567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Herz HM, Woodfield SE, Chen Z, Bolduc C, Bergmann A (2009) Common and distinct genetic properties of ESCRT-II components in Drosophila. PLoS One 4 (1):e4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schneider M, Troost T, Grawe F, Martinez-Arias A, Klein T (2013) Activation of Notch in lgd mutant cells requires the fusion of late endosomes with the lysosome. J Cell Sci 126 (Pt 2):645–656 [DOI] [PubMed] [Google Scholar]
- 166.Troost T, Jaeckel S, Ohlenhard N, Klein T (2012) The tumour suppressor Lethal (2) giant discs is required for the function of the ESCRT-III component Shrub/CHMP4. J Cell Sci 125 (Pt3):763–776 [DOI] [PubMed] [Google Scholar]
- 167.Wilkin M, Tongngok P, Gensch N, Clemence S, Motoki M, Yamada K, Hori K, Taniguchi-Kanai M, Franklin E, Matsuno K, Baron M (2008) Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev Cell 15 (5):762–772 [DOI] [PubMed] [Google Scholar]
- 168.Takats S, Pircs K, Nagy P, Varga A, Karpati M, Hegedus K, Kramer H, Kovacs AL, Sass M, Juhasz G (2014) Interaction of the HOPS complex with Syntaxin 17 mediates autophagosome clearance in Drosophila. Mol Biol Cell 25 (8): 1338–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D (2010) The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 137(11):1825–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Baron M (2012) Endocytic routes to Notch activation. Semin Cell Dev Biol 23 (4):437–442 [DOI] [PubMed] [Google Scholar]
- 171.Shimizu H, Woodcock SA, Wilkin MB, Trubenova B, Monk NA, Baron M (2014) Compensatory flux changes within an endocytic trafficking network maintain thermal robustness of Notch signaling. Cell 157 (5):1160–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Furukawa T, Maruyama S, Kawaichi M, Honjo T (1992) The Drosophila homolog of the immunoglobulin recombination signal-binding protein regulates peripheral nervous system development. Cell 69 (7):1191–1197 [DOI] [PubMed] [Google Scholar]
- 173.Schweisguth F, Posakony JW (1992) Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell 69(7): 1199–1212 [DOI] [PubMed] [Google Scholar]
- 174.Wu L, Sun T, Kobayashi K, Gao P, Griffin JD (2002) Identification of a family of mastermindlike transcriptional coactivators for mammalian notch receptors. Mol Cell Biol 22 (21):7688–7700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kitagawa M, Oyama T, Kawashima T, Yedvobnick B, Kumar A, Matsuno K, Harigaya K (2001) A human protein with sequence similarity to Drosophila mastermind coordinates the nuclear form of notch and a CSL protein to build a transcriptional activator complex on target promoters. Mol Cell Biol 21 (13):4337–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bang AG, Posakony JW (1992) The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev 6 (9):1752–1769 [DOI] [PubMed] [Google Scholar]
- 177.Bailey AM, Posakony JW (1995) Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev 9 (21):2609–2622 [DOI] [PubMed] [Google Scholar]
- 178.Barolo S, Stone T, Bang AG, Posakony JW (2002) Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev 16 (15):1964–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nagel AC, Krejci A, Tenin G, Bravo-Patino A, Bray S, Maier D, Preiss A (2005) Hairless-mediated repression of notch target genes requires the combined activity of Groucho and CtBP corepressors. Mol Cell Biol 25 (23):10433–10441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Paroush Z, Finley RL Jr., Kidd T, Wainwright SM, Ingham PW, Brent R, Ish-Horowicz D (1994) Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell 79 (5):805–815 [DOI] [PubMed] [Google Scholar]
- 181.Stifani S, Blaumueller CM, Redhead NJ, Hill RE, Artavanis-Tsakonas S (1992) Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat Genet 2 (2):119–127 [DOI] [PubMed] [Google Scholar]
- 182.Morel V, Lecourtois M, Massiani O, Maier D, Preiss A, Schweisguth F (2001) Transcriptional repression by suppressor of hairless involves the binding of a hairless-dCtBP complex in Drosophila. Curr Biol 11 (10):789–792 [DOI] [PubMed] [Google Scholar]
- 183.Yuan Z, Praxenthaler H, Tabaja N, Torella R, Preiss A, Maier D, Kovall RA (2016) Structure and Function of the Su(H)-Hairless Repressor Complex, the Major Antagonist of Notch Signaling in Drosophila melanogaster. PLoS Biol 14 (7):e1002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Skalska L, Stojnic R, Li J, Fischer B, Cerda-Moya G, Sakai H, Tajbakhsh S, Russell S, Adryan B, Bray SJ (2015) Chromatin signatures at Notch-regulated enhancers reveal large-scale changes in H3K56ac upon activation. The EMBO journal 34 (14):1889–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Taniguchi Y, Furukawa T, Tun T, Han H, Honjo T (1998) LIM protein KyoT2 negatively regulates transcription by association with the RBP-J DNA-binding protein. Mol Cell Biol 18(1):644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oswald F, Kostezka U, Astrahantseff K, Bourteele S, Dillinger K, Zechner U, Ludwig L, Wilda M, Hameister H, Knochel W, Liptay S, Schmid RM (2002) SHARP is a novel component of the Notch/RBP-Jkappa signalling pathway. The EMBO journal 21 (20):5417–5426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuroda K, Han H, Tani S, Tanigaki K, Tun T, Furukawa T, Taniguchi Y, Kurooka H, Hamada Y, Toyokuni S, Honjo T (2003) Regulation of marginal zone B cell development by MINT, a suppressor of Notch/RBP-J signaling pathway. Immunity 18 (2):301–312 [DOI] [PubMed] [Google Scholar]
- 188.Borggrefe T, Oswald F (2014) Keeping notch target genes off: a CSL corepressor caught in the act. Structure 22 (1):3–5 [DOI] [PubMed] [Google Scholar]
- 189.Collins KJ, Yuan Z, Kovall RA (2014) Structure and function of the CSL-KyoT2 corepressor complex: a negative regulator of Notch signaling. Structure 22 (1):70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Borggrefe T, Oswald F (2016) Setting the Stage for Notch: The Drosophila Su(H)-Hairless Repressor Complex. PLoS Biol 14 (7):e1002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bray S, Bernard F (2010) Notch targets and their regulation. Curr Top Dev Biol 92:253–275 [DOI] [PubMed] [Google Scholar]
- 192.Jennings B, Preiss A, Delidakis C, Bray S (1994) The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development 120 (12):3537–3548 [DOI] [PubMed] [Google Scholar]
- 193.Lecourtois M, Schweisguth F (1995) The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev 9 (21):2598–2608 [DOI] [PubMed] [Google Scholar]
- 194.de Celis JF, de Celis J, Ligoxygakis P, Preiss A, Delidakis C, Bray S (1996) Functional relationships between Notch, Su(H) and the bHLH genes of the E(spl) complex: the E(spl) genes mediate only a subset of Notch activities during imaginal development. Development 122 (9):2719–2728 [DOI] [PubMed] [Google Scholar]
- 195.Knust E, Schrons H, Grawe F, Campos-Ortega JA (1992) Seven genes of the Enhancer of split complex of Drosophila melanogaster encode helix-loop-helix proteins. Genetics 132 (2):505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Delidakis C, Preiss A, Hartley DA, Artavanis-Tsakonas S (1991) Two genetically and molecularly distinct functions involved in early neurogenesis reside within the Enhancer of split locus of Drosophila melanogaster. Genetics 129 (3):803–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Klambt C, Knust E, Tietze K, Campos-Ortega JA (1989) Closely related transcripts encoded by the neurogenic gene complex enhancer of split of Drosophila melanogaster. The EMBO journal 8 (1):203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Delidakis C, Monastirioti M, Magadi SS (2014) E(spl): genetic, developmental, and evolutionary aspects of a group of invertebrate Hes proteins with close ties to Notch signaling. Curr Top Dev Biol 110:217–262 [DOI] [PubMed] [Google Scholar]
- 199.Hartley DA, Preiss A, Artavanis-Tsakonas S (1988) A deduced gene product from the Drosophila neurogenic locus, enhancer of split, shows homology to mammalian G-protein beta subunit. Cell 55 (5):785–795 [DOI] [PubMed] [Google Scholar]
- 200.Lai EC, Bodner R, Posakony JW (2000) The enhancer of split complex of Drosophila includes four Notch-regulated members of the bearded gene family. Development 127 (16):3441–3455 [DOI] [PubMed] [Google Scholar]
- 201.Ziemer A, Tietze K, Knust E, Campos-Ortega JA (1988) Genetic analysis of enhancer of split, a locus involved in neurogenesis in Drosophila melanogaster. Genetics 119 (1):63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wurmbach E, Wech I, Preiss A (1999) The Enhancer of split complex of Drosophila melanogaster harbors three classes of Notch responsive genes. Mech Dev 80 (2): 171–180 [DOI] [PubMed] [Google Scholar]
- 203.Oellers N, Dehio M, Knust E (1994) bHLH proteins encoded by the Enhancer of split complex of Drosophila negatively interfere with transcriptional activation mediated by proneural genes. Mol Gen Genet 244 (5):465–473 [DOI] [PubMed] [Google Scholar]
- 204.Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P (1996) Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Development 122 (1):161–171 [DOI] [PubMed] [Google Scholar]
- 205.Gigliani F, Longo F, Gaddini L, Battaglia PA (1996) Interactions among the bHLH domains of the proteins encoded by the Enhancer of split and achaete-scute gene complexes of Drosophila. Mol Gen Genet 251 (6):628–634 [DOI] [PubMed] [Google Scholar]
- 206.Nakao K, Campos-Ortega JA (1996) Persistent expression of genes of the enhancer of split complex suppresses neural development in Drosophila. Neuron 16 (2):275–286 [DOI] [PubMed] [Google Scholar]
- 207.Akazawa C, Sasai Y, Nakanishi S, Kageyama R (1992) Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem 267 (30):21879–21885 [PubMed] [Google Scholar]
- 208.Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S (1992) Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev 6 (12B):2620–2634 [DOI] [PubMed] [Google Scholar]
- 209.Kokubo H, Lun Y, Johnson RL (1999) Identification and expression of a novel family of bHLH cDNAs related to Drosophila hairy and enhancer of split. Biochem Biophys Res Commun 260 (2):459–465 [DOI] [PubMed] [Google Scholar]
- 210.Leimeister C, Externbrink A, Klamt B, Gessler M (1999) Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev 85 (1–2):173–177 [DOI] [PubMed] [Google Scholar]
- 211.Weber D, Wiese C, Gessler M (2014) Hey bHLH transcription factors. Curr Top Dev Biol 110:285–315 [DOI] [PubMed] [Google Scholar]
- 212.Kobayashi T, Kageyama R (2014) Expression dynamics and functions of Hes factors in development and diseases. Curr Top Dev Biol 110:263–283 [DOI] [PubMed] [Google Scholar]
- 213.Yamamoto S, Charng WL, Bellen HJ (2010) Endocytosis and intracellular trafficking of Notch and its ligands. Curr Top Dev Biol 92:165–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Palmer WH, Deng WM (2015) Ligand-Independent Mechanisms of Notch Activity. Trends Cell Biol 25 (11):697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Heitzler P (2010) Biodiversity and noncanonical Notch signaling. Curr Top Dev Biol 92:457–481 [DOI] [PubMed] [Google Scholar]
- 216.D’Souza B, Meloty-Kapella L, Weinmaster G (2010) Canonical and non-canonical Notch ligands. Curr Top Dev Biol 92:73–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Johnson EA (2011) HIF takes it up a notch. Sci Signal 4 (181):pe33. [DOI] [PubMed] [Google Scholar]
- 218.Schwanbeck R (2015) The role of epigenetic mechanisms in Notch signaling during development. J Cell Physiol 230 (5):969–981 [DOI] [PubMed] [Google Scholar]
- 219.Takayama S, Dhahbi J, Roberts A, Mao G, Heo SJ, Pachter L, Martin DI, Boffelli D (2014) Genome methylation in D. melanogaster is found at specific short motifs and is independent of DNMT2 activity. Genome Res 24 (5):821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jana SC, Bettencourt-Dias M, Durand B, Megraw TL (2016) Drosophila melanogaster as a model for basal body research. Cilia 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E (2011) A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145(7): 1129–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bray S (1998) Notch signalling in Drosophila: three ways to use a pathway. Semin Cell Dev Biol 9 (6):591–597 [DOI] [PubMed] [Google Scholar]
- 223.Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7 (9):678–689 [DOI] [PubMed] [Google Scholar]
- 224.Kidd S, Struhl G, Lieber T (2015) Notch is required in adult Drosophila sensory neurons for morphological and functional plasticity of the olfactory circuit. PLoS Genet 11 (5):e1005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lieber T, Kidd S, Struhl G (2011) DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron 69 (3):468–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Koch U, Lehal R, Radtke F (2013) Stem cells living with a Notch. Development 140 (4):689–704 [DOI] [PubMed] [Google Scholar]
- 227.Udolph G (2012) Notch signaling and the generation of cell diversity in Drosophila neuroblast lineages. Adv Exp Med Biol 727:47–60 [DOI] [PubMed] [Google Scholar]
- 228.Xie T, Song X, Jin Z, Pan L, Weng C, Chen S, Zhang N (2008) Interactions between stem cells and their niche in the Drosophila ovary. Cold Spring Harb Symp Quant Biol 73:39–47 [DOI] [PubMed] [Google Scholar]
- 229.Hartenstein V, Posakony JW (1989) Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107 (2):389–405 [DOI] [PubMed] [Google Scholar]
- 230.Hartenstein V, Posakony JW (1990) A dual function of the Notch gene in Drosophila sensillum development. Dev Biol 142 (1): 13–30 [DOI] [PubMed] [Google Scholar]
- 231.Lai EC, Orgogozo V (2004) A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol 269 (1): 1–17 [DOI] [PubMed] [Google Scholar]
- 232.Garcia-Bellido A, Santamaria P (1978) Developmental Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics 88 (3):469–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Campuzano S, Modolell J (1992) Patterning of the Drosophila nervous system: the achaetescute gene complex. Trends Genet 8 (6):202–208 [DOI] [PubMed] [Google Scholar]
- 234.Couturier L, Schweisguth F (2014) Antibody uptake assay and in vivo imaging to study intracellular trafficking of Notch and Delta in Drosophila. Methods Mol Biol 1187:79–86 [DOI] [PubMed] [Google Scholar]
- 235.Huang C, Chan JA, Schuurmans C (2014) Proneural bHLH genes in development and disease. Curr Top Dev Biol 110:75–127 [DOI] [PubMed] [Google Scholar]
- 236.Furman DP, Bukharina TA (2008) How Drosophila melanogaster Forms its Mechanoreceptors. Curr Genomics 9 (5):312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nolo R, Abbott LA, Bellen HJ (2000) Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102 (3):349–362 [DOI] [PubMed] [Google Scholar]
- 238.Acar M, Jafar-Nejad H, Giagtzoglou N, Yallampalli S, David G, He Y, Delidakis C, Bellen HJ (2006) Senseless physically interacts with proneural proteins and functions as a transcriptional co-activator. Development 133 (10):1979–1989 [DOI] [PubMed] [Google Scholar]
- 239.Jafar-Nejad H, Acar M, Nolo R, Lacin H, Pan H, Parkhurst SM, Bellen HJ (2003) Senseless acts as a binary switch during sensory organ precursor selection. Genes Dev 17 (23):2966–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Barad O, Hornstein E, Barkai N (2011) Robust selection of sensory organ precursors by the Notch-Delta pathway. Curr Opin Cell Biol 23 (6):663–667 [DOI] [PubMed] [Google Scholar]
- 241.Corson F, Couturier L, Rouault H, Mazouni K, Schweisguth F (2017) Self-organized Notch dynamics generate stereotyped sensory organ patterns in Drosophila. Science 356 (6337). doi: 10.1126/science.aai7407 [DOI] [PubMed] [Google Scholar]
- 242.Troost T, Schneider M, Klein T (2015) A re-examination of the selection of the sensory organ precursor of the bristle sensilla of Drosophila melanogaster. PLoS Genet 11 (1):e1004911. doi: 10.1371/journal.pgen.1004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zeng C, Younger-Shepherd S, Jan LY, Jan YN (1998) Delta and Serrate are redundant Notch ligands required for asymmetric cell divisions within the Drosophila sensory organ lineage. Genes Dev 12 (8):1086–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Parks AL, Muskavitch MA (1993) Delta function is required for bristle organ determination and morphogenesis in Drosophila. Dev Biol 157 (2):484–496 [DOI] [PubMed] [Google Scholar]
- 245.Jan YN, Jan LY (2001) Asymmetric cell division in the Drosophila nervous system. Nat Rev Neurosci 2 (11):772–779 [DOI] [PubMed] [Google Scholar]
- 246.Schweisguth F (2015) Asymmetric cell division in the Drosophila bristle lineage: from the polarization of sensory organ precursor cells to Notch-mediated binary fate decision. Wiley Interdiscip Rev Dev Biol 4 (3):299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW (2000) A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell 103 (6):957–969 [DOI] [PubMed] [Google Scholar]
- 248.Chen BE, Kondo M, Garnier A, Watson FL, Puettmann-Holgado R, Lamar DR, Schmucker D (2006) The molecular diversity of Dscam is functionally required for neuronal wiring specificity in Drosophila. Cell 125 (3):607–620 [DOI] [PubMed] [Google Scholar]
- 249.Le Borgne R, Schweisguth F (2003) Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev Cell 5 (1):139–148 [DOI] [PubMed] [Google Scholar]
- 250.Rhyu MS, Jan LY, Jan YN (1994) Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76 (3):477–491 [DOI] [PubMed] [Google Scholar]
- 251.Roegiers F, Jan LY, Jan YN (2005) Regulation of membrane localization of Sanpodo by lethal giant larvae and neuralized in asymmetrically dividing cells of Drosophila sensory organs. Mol Biol Cell 16 (8):3480–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Langevin J, Le Borgne R, Rosenfeld F, Gho M, Schweisguth F, Bellaiche Y (2005) Lethal giant larvae controls the localization of notch-signaling regulators numb, neuralized, and Sanpodo in Drosophila sensory-organ precursor cells. Curr Biol 15 (10):955–962 [DOI] [PubMed] [Google Scholar]
- 253.O’Connor-Giles KM, Skeath JB (2003) Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev Cell 5(2):231–243 [DOI] [PubMed] [Google Scholar]
- 254.Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA (2005) Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122 (5):763–773 [DOI] [PubMed] [Google Scholar]
- 255.Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED (2001) Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. The Journal of cell biology 152 (1): 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Reddy GV, Rodrigues V (1999) A glial cell arises from an additional division within the mechanosensory lineage during development of the microchaete on the Drosophila notum. Development 126 (20):4617–4622 [DOI] [PubMed] [Google Scholar]
- 257.Gho M, Bellaiche Y, Schweisguth F (1999) Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development 126 (16):3573–3584 [DOI] [PubMed] [Google Scholar]
- 258.Guo M, Jan LY, Jan YN (1996) Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron 17 (1):27–41 [DOI] [PubMed] [Google Scholar]
- 259.Blair SS (2007) Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol 23:293–319 [DOI] [PubMed] [Google Scholar]
- 260.Bourgouin C, Lundgren SE, Thomas JB (1992) Apterous is a Drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron 9 (3):549–561 [DOI] [PubMed] [Google Scholar]
- 261.Klein T, Couso JP, Martinez Arias A (1998) Wing development and specification of dorsal cell fates in the absence of apterous in Drosophila. Curr Biol 8 (7):417–420 [DOI] [PubMed] [Google Scholar]
- 262.Kim J, Irvine KD, Carroll SB (1995) Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell 82 (5):795–802 [DOI] [PubMed] [Google Scholar]
- 263.Bachmann A, Knust E (1998) Positive and negative control of Serrate expression during early development of the Drosophila wing. Mech Dev 76 (1–2):67–78 [DOI] [PubMed] [Google Scholar]
- 264.Fleming RJ, Gu Y, Hukriede NA (1997) Serrate-mediated activation of Notch is specifically blocked by the product of the gene fringe in the dorsal compartment of the Drosophila wing imaginal disc. Development 124 (15):2973–2981 [DOI] [PubMed] [Google Scholar]
- 265.Rauskolb C, Correia T, Irvine KD (1999) Fringe-dependent separation of dorsal and ventral cells in the Drosophila wing. Nature 401 (6752):476–480 [DOI] [PubMed] [Google Scholar]
- 266.Klein T, Arias AM (1998) Interactions among Delta, Serrate and Fringe modulate Notch activity during Drosophila wing development. Development 125 (15):2951–2962 [DOI] [PubMed] [Google Scholar]
- 267.de Celis JF, Garcia-Bellido A, Bray SJ (1996) Activation and function of Notch at the dorsalventral boundary of the wing imaginal disc. Development 122 (1):359–369 [DOI] [PubMed] [Google Scholar]
- 268.Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN (1996) Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev 10 (4):421–434 [DOI] [PubMed] [Google Scholar]
- 269.Jack J, DeLotto Y (1992) Effect of wing scalloping mutations on cut expression and sense organ differentiation in the Drosophila wing margin. Genetics 131 (2):353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Micchelli CA, Rulifson EJ, Blair SS (1997) The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124 (8):1485–1495 [DOI] [PubMed] [Google Scholar]
- 271.Rulifson EJ, Blair SS (1995) Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 121 (9):2813–2824 [DOI] [PubMed] [Google Scholar]
- 272.Neumann CJ, Cohen SM (1997) Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124 (4):871–880 [DOI] [PubMed] [Google Scholar]
- 273.Zecca M, Basler K, Struhl G (1996) Direct and long-range action of a wingless morphogen gradient. Cell 87 (5):833–844 [DOI] [PubMed] [Google Scholar]
- 274.Alexandre C, Baena-Lopez A, Vincent JP (2014) Patterning and growth control by membrane-tethered Wingless. Nature 505 (7482):180–185. doi: 10.1038/nature12879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Blochlinger K, Bodmer R, Jack J, Jan LY, Jan YN (1988) Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature 333 (6174):629–635 [DOI] [PubMed] [Google Scholar]
- 276.de Celis JF, Bray S (1997) Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124 (17): 3241–3251 [DOI] [PubMed] [Google Scholar]
- 277.Michel M, Aliee M, Rudolf K, Bialas L, Julicher F, Dahmann C (2016) The Selector Gene apterous and Notch Are Required to Locally Increase Mechanical Cell Bond Tension at the Drosophila Dorsoventral Compartment Boundary. PLoS One 11 (8):e0161668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Stanley P, Okajima T (2010) Roles of glycosylation in Notch signaling. Curr Top Dev Biol 92:131–164 [DOI] [PubMed] [Google Scholar]
- 279.Gridley T (2010) Notch signaling in the vasculature. Curr Top Dev Biol 92:277–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.MacGrogan D, Nus M, de la Pompa JL (2010) Notch signaling in cardiac development and disease. Curr Top Dev Biol 92:333–365 [DOI] [PubMed] [Google Scholar]
- 281.Liu J, Sato C, Cerletti M, Wagers A (2010) Notch signaling in the regulation of stem cell selfrenewal and differentiation. Curr Top Dev Biol 92:367–409 [DOI] [PubMed] [Google Scholar]
- 282.Gridley T (2003) Notch signaling and inherited disease syndromes. Hum Mol Genet 12 Spec No 1:R9–13 [DOI] [PubMed] [Google Scholar]
- 283.Gridley T (1997) Notch signaling in vertebrate development and disease. Mol Cell Neurosci 9 (2): 103–108 [DOI] [PubMed] [Google Scholar]
- 284.Louvi A, Artavanis-Tsakonas S (2012) Notch and disease: a growing field. Semin Cell Dev Biol 23 (4):473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Penton AL, Leonard LD, Spinner NB (2012) Notch signaling in human development and disease. Semin Cell Dev Biol 23 (4):450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ables JL, Breunig JJ, Eisch AJ, Rakic P (2011) Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci 12 (5):269–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pierfelice T, Alberi L, Gaiano N (2011) Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69 (5):840–855 [DOI] [PubMed] [Google Scholar]
- 288.Bi P, Kuang S (2015) Notch signaling as a novel regulator of metabolism. Trends Endocrinol Metab 26 (5):248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Geisler F, Strazzabosco M (2015) Emerging roles of Notch signaling in liver disease. Hepatology 61 (1):382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Koch U, Radtke F (2010) Notch signaling in solid tumors. Curr Top Dev Biol 92:411–455 [DOI] [PubMed] [Google Scholar]
- 291.Ranganathan P, Weaver KL, Capobianco AJ (2011) Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 11 (5):338–351 [DOI] [PubMed] [Google Scholar]
- 292.Online Mendelian Inheritance in Man, https://www.omim.org/ (2017).
- 293.Hassed S, Li S, Mulvihill J, Aston C, Palmer S (2017) Adams-Oliver syndrome review of the literature: Refining the diagnostic phenotype. Am J Med Genet A 173 (3):790–800 [DOI] [PubMed] [Google Scholar]
- 294.Adams FH, Oliver CP (1945) Hereditary deformities in man due to arrested development. J Hered 36:3–7 [Google Scholar]
- 295.Stittrich AB, Lehman A, Bodian DL, Ashworth J, Zong Z, Li H, Lam P, Khromykh A, Iyer RK, Vockley JG, Baveja R, Silva ES, Dixon J, Leon EL, Solomon BD, Glusman G, Niederhuber JE, Roach JC, Patel MS (2014) Mutations in NOTCH1 cause Adams-Oliver syndrome. Am J Hum Genet 95 (3):275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Southgate L, Sukalo M, Karountzos AS, Taylor EJ, Collinson CS, Ruddy D, Snape KM, Dallapiccola B, Tolmie JL, Joss S, Brancati F, Digilio MC, Graul-Neumann LM, Salviati L, Coerdt W, Jacquemin E, Wuyts W, Zenker M, Machado RD, Trembath RC (2015) Haploinsufficiency of the NOTCH1 Receptor as a Cause of Adams-Oliver Syndrome With Variable Cardiac Anomalies. Circ Cardiovasc Genet 8 (4):572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Meester JA, Southgate L, Stittrich AB, Venselaar H, Beekmans SJ, den Hollander N, Bijlsma EK, Helderman-van den Enden A, Verheij JB, Glusman G, Roach JC, Lehman A, Patel MS, de Vries BB, Ruivenkamp C, Itin P, Prescott K, Clarke S, Trembath R, Zenker M, Sukalo M, Van Laer L, Loeys B, Wuyts W (2015) Heterozygous Loss-of-Function Mutations in DLL4 Cause Adams-Oliver Syndrome. Am J Hum Genet 97 (3):475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hassed SJ, Wiley GB, Wang S, Lee JY, Li S, Xu W, Zhao ZJ, Mulvihill JJ, Robertson J, Warner J, Gaffney PM (2012) RBPJ mutations identified in two families affected by Adams-Oliver syndrome. Am J Hum Genet 91 (2):391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Saleh M, Kamath BM, Chitayat D (2016) Alagille syndrome: clinical perspectives. Appl Clin Genet 9:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Alagille D, Estrada A, Hadchouel M, Gautier M, Odievre M, Dommergues JP (1987) Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr 110 (2):195–200 [DOI] [PubMed] [Google Scholar]
- 301.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC (1997) Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 16 (3):235–242 [DOI] [PubMed] [Google Scholar]
- 302.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB (1997) Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16 (3):243–251 [DOI] [PubMed] [Google Scholar]
- 303.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB (2006) NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet 79 (1):169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Majewski J, Schwartzentruber JA, Caqueret A, Patry L, Marcadier J, Fryns JP, Boycott KM, Ste-Marie LG, McKiernan FE, Marik I, Van Esch H, Michaud JL, Samuels ME (2011) Mutations in NOTCH2 in families with Hajdu-Cheney syndrome. Hum Mutat 32 (10): 1114–1117 [DOI] [PubMed] [Google Scholar]
- 305.Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, Mansour S, Holder SE, Brain CE, Burton BK, Kim KH, Pauli RM, Aftimos S, Stewart H, Kim CA, Holder-Espinasse M, Robertson SP, Drake WM, Trembath RC (2011) Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet 43 (4):303–305 [DOI] [PubMed] [Google Scholar]
- 306.Isidor B, Lindenbaum P, Pichon O, Bezieau S, Dina C, Jacquemont S, Martin-Coignard D, Thauvin-Robinet C, Le Merrer M, Mandel JL, David A, Faivre L, Cormier-Daire V, Redon R, Le Caignec C (2011) Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat Genet 43 (4):306–308 [DOI] [PubMed] [Google Scholar]
- 307.McBride KL, Riley MF, Zender GA, Fitzgerald-Butt SM, Towbin JA, Belmont JW, Cole SE (2008) NOTCH1 mutations in individuals with left ventricular outflow tract malformations reduce ligand-induced signaling. Hum Mol Genet 17 (18):2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D (2005) Mutations in NOTCH1 cause aortic valve disease. Nature 437 (7056):270–274 [DOI] [PubMed] [Google Scholar]
- 309.Krantz ID, Smith R, Colliton RP, Tinkel H, Zackai EH, Piccoli DA, Goldmuntz E, Spinner NB (1999) Jagged1 mutations in patients ascertained with isolated congenital heart defects. Am J Med Genet 84 (1):56–60 [PubMed] [Google Scholar]
- 310.Garg V (2006) Molecular genetics of aortic valve disease. Curr Opin Cardiol 21 (3): 180–184 [DOI] [PubMed] [Google Scholar]
- 311.Kola S, Koneti NR, Golla JP, Akka J, Gundimeda SD, Mundluru HP (2011) Mutational analysis of JAG1 gene in non-syndromic tetralogy of Fallot children. Clin Chim Acta 412 (23–24):2232–2236 [DOI] [PubMed] [Google Scholar]
- 312.Digilio MC, Luca AD, Lepri F, Guida V, Ferese R, Dentici ML, Angioni A, Marino B, Dallapiccola B (2013) JAG1 mutation in a patient with deletion 22q11.2 syndrome and tetralogy of Fallot. Am J Med Genet A 161A (12):3133–3136 [DOI] [PubMed] [Google Scholar]
- 313.Guida V, Chiappe F, Ferese R, Usala G, Maestrale G, Iannascoli C, Bellacchio E, Mingarelli R, Digilio MC, Marino B, Uda M, De Luca A, Dallapiccola B (2011) Novel and recurrent JAG1 mutations in patients with tetralogy of Fallot. Clin Genet 80 (6):591–594 [DOI] [PubMed] [Google Scholar]
- 314.Luxan G, Casanova JC, Martinez-Poveda B, Prados B, D’Amato G, MacGrogan D, Gonzalez-Rajal A, Dobarro D, Torroja C, Martinez F, Izquierdo-Garcia JL, Fernandez-Friera L, Sabater-Molina M, Kong YY, Pizarro G, Ibanez B, Medrano C, Garcia-Pavia P, Gimeno JR, Monserrat L, Jimenez-Borreguero LJ, de la Pompa JL (2013) Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med 19 (2): 193–201 [DOI] [PubMed] [Google Scholar]
- 315.Piccolo P, Attanasio S, Secco I, Sangermano R, Strisciuglio C, Limongelli G, Miele E, Mutarelli M, Banfi S, Nigro V, Pons T, Valencia A, Zentilin L, Campione S, Nardone G, Lynnes TC, Celestino-Soper PB, Spoonamore KG, D’Armiento FP, Giacca M, Staiano A, Vatta M, Collesi C, Brunetti-Pierri N (2016) MIB2 variants altering NOTCH signalling result in left ventricle hypertrabeculation/non-compaction and are associated with Menetrier-like gastropathy. Hum Mol Genet [DOI] [PubMed] [Google Scholar]
- 316.D’Amato G, Luxan G, de la Pompa JL (2016) Notch signalling in ventricular chamber development and cardiomyopathy. Febs J 283 (23):4223–4237 [DOI] [PubMed] [Google Scholar]
- 317.Tao J, Chen S, Lee B (2010) Alteration of Notch signaling in skeletal development and disease. Ann N Y Acad Sci 1192:257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zanotti S, Canalis E (2010) Notch and the skeleton. Mol Cell Biol 30 (4):886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD (2000) Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet 24 (4):438–441 [DOI] [PubMed] [Google Scholar]
- 320.Whittock NV, Sparrow DB, Wouters MA, Sillence D, Ellard S, Dunwoodie SL, Turnpenny PD (2004) Mutated MESP2 causes spondylocostal dysostosis in humans. Am J Hum Genet 74(6):1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sparrow DB, Chapman G, Wouters MA, Whittock NV, Ellard S, Fatkin D, Turnpenny PD, Kusumi K, Sillence D, Dunwoodie SL (2006) Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am J Hum Genet 78 (1):28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Sparrow DB, Guillen-Navarro E, Fatkin D, Dunwoodie SL (2008) Mutation of Hairy-and-Enhancer-of-Split-7 in humans causes spondylocostal dysostosis. Hum Mol Genet 17 (23):3761–3766 [DOI] [PubMed] [Google Scholar]
- 323.White PH, Farkas DR, Chapman DL (2005) Regulation of Tbx6 expression by Notch signaling. Genesis 42 (2):61–70 [DOI] [PubMed] [Google Scholar]
- 324.Yasuhiko Y, Haraguchi S, Kitajima S, Takahashi Y, Kanno J, Saga Y (2006) Tbx6-mediated Notch signaling controls somite-specific Mesp2 expression. Proceedings of the National Academy of Sciences of the United States of America 103 (10):3651–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wu N, Ming X, Xiao J, Wu Z, Chen X, Shinawi M, Shen Y, Yu G, Liu J, Xie H, Gucev ZS, Liu S, Yang N, Al-Kateb H, Chen J, Zhang J, Hauser N, Zhang T, Tasic V, Liu P, Su X, Pan X, Liu C, Wang L, Shen J, Shen J, Chen Y, Zhang T, Zhang J, Choy KW, Wang J, Wang Q, Li S, Zhou W, Guo J, Wang Y, Zhang C, Zhao H, An Y, Zhao Y, Wang J, Liu Z, Zuo Y, Tian Y, Weng X, Sutton VR, Wang H, Ming Y, Kulkarni S, Zhong TP, Giampietro PF, Dunwoodie SL, Cheung SW, Zhang X, Jin L, Lupski JR, Qiu G, Zhang F (2015) TBX6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med 372 (4):341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sparrow DB, McInerney-Leo A, Gucev ZS, Gardiner B, Marshall M, Leo PJ, Chapman DL, Tasic V, Shishko A, Brown MA, Duncan EL, Dunwoodie SL (2013) Autosomal dominant spondylocostal dysostosis is caused by mutation in TBX6. Hum Mol Genet 22 (8):1625–1631 [DOI] [PubMed] [Google Scholar]
- 327.Karaca E, Yuregir OO, Bozdogan ST, Aslan H, Pehlivan D, Jhangiani SN, Akdemir ZC, Gambin T, Bayram Y, Atik MM, Erdin S, Muzny D, Gibbs RA, Lupski JR (2015) Rare variants in the notch signaling pathway describe a novel type of autosomal recessive Klippel-Feil syndrome. Am J Med Genet A 167A (11):2795–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sparrow DB, Chapman G, Dunwoodie SL (2011) The mouse notches up another success: understanding the causes of human vertebral malformation. Mamm Genome 22 (7–8):362–376 [DOI] [PubMed] [Google Scholar]
- 329.Wahi K, Bochter MS, Cole SE (2016) The many roles of Notch signaling during vertebrate somitogenesis. Semin Cell Dev Biol 49:68–75 [DOI] [PubMed] [Google Scholar]
- 330.Pourquie O, Kusumi K (2001) When body segmentation goes wrong. Clin Genet 60 (6):409–416 [DOI] [PubMed] [Google Scholar]
- 331.Kageyama R, Niwa Y, Shimojo H, Kobayashi T, Ohtsuka T (2010) Ultradian oscillations in Notch signaling regulate dynamic biological events. Curr Top Dev Biol 92:311–331 [DOI] [PubMed] [Google Scholar]
- 332.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG (2009) Cadasil. Lancet Neurol 8 (7):643–653 [DOI] [PubMed] [Google Scholar]
- 333.Bergmann M, Ebke M, Yuan Y, Bruck W, Mugler M, Schwendemann G (1996) Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL): a morphological study of a German family. Acta Neuropathol 92 (4):341–350 [DOI] [PubMed] [Google Scholar]
- 334.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E (1996) Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383 (6602):707–710 [DOI] [PubMed] [Google Scholar]
- 335.Monet-Lepretre M, Bardot B, Lemaire B, Domenga V, Godin O, Dichgans M, Tournier-Lasserve E, Cohen-Tannoudji M, Chabriat H, Joutel A (2009) Distinct phenotypic and functional features of CADASIL mutations in the Notch3 ligand binding domain. Brain 132 (Pt 6): 1601–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, Cruaud C, Maciazek J, Weissenbach J, Bousser MG, Bach JF, Tournier-Lasserve E (1997) Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet 350 (9090): 1511–1515 [DOI] [PubMed] [Google Scholar]
- 337.Rutten JW, Haan J, Terwindt GM, van Duinen SG, Boon EM, Lesnik Oberstein SA (2014) Interpretation of NOTCH3 mutations in the diagnosis of CADASIL. Expert Rev Mol Diagn 14 (5):593–603 [DOI] [PubMed] [Google Scholar]
- 338.Peters N, Opherk C, Zacherle S, Capell A, Gempel P, Dichgans M (2004) CADASIL-associated Notch3 mutations have differential effects both on ligand binding and ligand-induced Notch3 receptor signaling through RBP-Jk. Exp Cell Res 299 (2):454–464 [DOI] [PubMed] [Google Scholar]
- 339.Joutel A (2011) Pathogenesis of CADASIL: transgenic and knock-out mice to probe function and dysfunction of the mutated gene, Notch3, in the cerebrovasculature. Bioessays 33 (1):73–80 [DOI] [PubMed] [Google Scholar]
- 340.Ayata C (2010) CADASIL: experimental insights from animal models. Stroke 41 (10 Suppl):S129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Haritunians T, Chow T, De Lange RP, Nichols JT, Ghavimi D, Dorrani N, St Clair DM, Weinmaster G, Schanen C (2005) Functional analysis of a recurrent missense mutation in Notch3 in CADASIL. J Neurol Neurosurg Psychiatry 76 (9):1242–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Joutel A, Monet M, Domenga V, Riant F, Tournier-Lasserve E (2004) Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect Jagged1 binding and Notch3 activity via the RBP/JK signaling Pathway. Am J Hum Genet 74 (2):338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A (2004) Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18 (22):2730–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Dichgans M, Ludwig H, Muller-Hocker J, Messerschmidt A, Gasser T (2000) Small in-frame deletions and missense mutations in CADASIL: 3D models predict misfolding of Notch3 EGF-like repeat domains. Eur J Hum Genet 8 (4):280–285 [DOI] [PubMed] [Google Scholar]
- 345.Donahue CP, Kosik KS (2004) Distribution pattern of Notch3 mutations suggests a gain-of-function mechanism for CADASIL. Genomics 83 (1):59–65 [DOI] [PubMed] [Google Scholar]
- 346.Opherk C, Duering M, Peters N, Karpinska A, Rosner S, Schneider E, Bader B, Giese A, Dichgans M (2009) CADASIL mutations enhance spontaneous multimerization of NOTCH3. Hum Mol Genet 18 (15):2761–2767 [DOI] [PubMed] [Google Scholar]
- 347.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E (2000) The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 105 (5):597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Joutel A, Favrole P, Labauge P, Chabriat H, Lescoat C, Andreux F, Domenga V, Cecillon M, Vahedi K, Ducros A, Cave-Riant F, Bousser MG, Tournier-Lasserve E (2001) Skin biopsy immunostaining with a Notch3 monoclonal antibody for CADASIL diagnosis. Lancet 358 (9298):2049–2051 [DOI] [PubMed] [Google Scholar]
- 349.Ishiko A, Shimizu A, Nagata E, Takahashi K, Tabira T, Suzuki N (2006) Notch3 ectodomain is a major component of granular osmiophilic material (GOM) in CADASIL. Acta Neuropathol 112 (3):333–339 [DOI] [PubMed] [Google Scholar]
- 350.Arboleda-Velasquez JF, Manent J, Lee JH, Tikka S, Ospina C, Vanderburg CR, Frosch MP, Rodriguez-Falcon M, Villen J, Gygi S, Lopera F, Kalimo H, Moskowitz MA, Ayata C, Louvi A, Artavanis-Tsakonas S (2011) Hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proceedings of the National Academy of Sciences of the United States of America 108 (21):E128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Gripp KW, Robbins KM, Sobreira NL, Witmer PD, Bird LM, Avela K, Makitie O, Alves D, Hogue JS, Zackai EH, Doheny KF, Stabley DL, Sol-Church K (2015) Truncating mutations in the last exon of NOTCH3 cause lateral meningocele syndrome. Am J Med Genet A 167A (2):271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Martignetti JA, Tian L, Li D, Ramirez MC, Camacho-Vanegas O, Camacho SC, Guo Y, Zand DJ, Bernstein AM, Masur SK, Kim CE, Otieno FG, Hou C, Abdel-Magid N, Tweddale B, Metry D, Fournet JC, Papp E, McPherson EW, Zabel C, Vaksmann G, Morisot C, Keating B, Sleiman PM, Cleveland JA, Everman DB, Zackai E, Hakonarson H (2013) Mutations in PDGFRB cause autosomal-dominant infantile myofibromatosis. Am J Hum Genet 92 (6): 1001–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Woo HN, Park JS, Gwon AR, Arumugam TV, Jo DG (2009) Alzheimer’s disease and Notch signaling. Biochem Biophys Res Commun 390 (4):1093–1097 [DOI] [PubMed] [Google Scholar]
- 354.Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8 (6):595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Li D, Parks SB, Kushner JD, Nauman D, Burgess D, Ludwigsen S, Partain J, Nixon RR, Allen CN, Irwin RP, Jakobs PM, Litt M, Hershberger RE (2006) Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am J Hum Genet 79 (6):1030–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, Rota M, Gwathmey JK, Dec GW, Aretz T, Leri A, Semigran MJ, Anversa P, Macgillivray TE, Tanzi RE, del Monte F (2010) Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation 121 (10): 1216–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, Rota M, Gwathmey JK, Dec GW, Aretz T, Leri A, Semigran MJ, Anversa P, Macgillivray TE, Tanzi RE, del Monte F (2011) Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation 121 (10): 1216–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Nowell C, Radtke F (2013) Cutaneous Notch signaling in health and disease. Cold Spring Harb Perspect Med 3 (12):a017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Yuan JS, Kousis PC, Suliman S, Visan I, Guidos CJ (2011) Functions of notch signaling in the immune system: consensus and controversies. Annu Rev Immunol 28:343–365 [DOI] [PubMed] [Google Scholar]
- 360.Pink AE, Simpson MA, Desai N, Trembath RC, Barker JN (2013) gamma-Secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J Invest Dermatol 133 (3):601–607 [DOI] [PubMed] [Google Scholar]
- 361.Li M, Cheng R, Liang J, Yan H, Zhang H, Yang L, Li C, Jiao Q, Lu Z, He J, Ji J, Shen Z, Li C, Hao F, Yu H, Yao Z (2013) Mutations in POFUT1, encoding protein O-fucosyltransferase 1, cause generalized Dowling-Degos disease. Am J Hum Genet 92 (6):895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Basmanav FB, Oprisoreanu AM, Pasternack SM, Thiele H, Fritz G, Wenzel J, Grosser L, Wehner M, Wolf S, Fagerberg C, Bygum A, Altmuller J, Rutten A, Parmentier L, El Shabrawi-Caelen L, Hafner C, Nurnberg P, Kruse R, Schoch S, Hanneken S, Betz RC (2014) Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomal-dominant Dowling-Degos disease. Am J Hum Genet 94 (1): 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Kono M, Sugiura K, Suganuma M, Hayashi M, Takama H, Suzuki T, Matsunaga K, Tomita Y, Akiyama M (2013) Whole-exome sequencing identifies ADAM10 mutations as a cause of reticulate acropigmentation of Kitamura, a clinical entity distinct from Dowling-Degos disease. Hum Mol Genet 22 (17):3524–3533 [DOI] [PubMed] [Google Scholar]
- 364.Liu J, Fukunaga-Kalabis M, Li L, Herlyn M (2015) Developmental pathways activated in melanocytes and melanoma. Arch Biochem Biophys 563:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yu H, Takeuchi H, Takeuchi M, Liu Q, Kantharia J, Haltiwanger RS, Li H (2016) Structural analysis of Notch-regulating Rumi reveals basis for pathogenic mutations. Nat Chem Biol 12(9):735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.McMillan BJ, Zimmerman B, Egan ED, Lofgren M, Xu X, Hesser A, Blacklow SC (2017) Structure of human POFUT1, its requirement in ligand-independent oncogenic Notch signaling, and functional effects of Dowling-Degos mutations. Glycobiology:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Gonzaga-Jauregui C, Lupski JR, Gibbs RA (2012) Human genome sequencing in health and disease. Annu Rev Med 63:35–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.http://flybase.org/ (2017).
- 369.Ramoni RB, Mulvihill JJ, Adams DR, Allard P, Ashley EA, Bernstein JA, Gahl WA, Hamid R, Loscalzo J, McCray AT, Shashi V, Tifft CJ, Wise AL (2017) The Undiagnosed Diseases Network: Accelerating Discovery about Health and Disease. Am J Hum Genet 100 (2): 185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Chong JX, Buckingham KJ, Jhangiani SN, Boehm C, Sobreira N, Smith JD, Harrell TM, McMillin MJ, Wiszniewski W, Gambin T, Coban Akdemir ZH, Doheny K, Scott AF, Avramopoulos D, Chakravarti A, Hoover-Fong J, Mathews D, Witmer PD, Ling H, Hetrick K, Watkins L, Patterson KE, Reinier F, Blue E, Muzny D, Kircher M, Bilguvar K, Lopez-Giraldez F, Sutton VR, Tabor HK, Leal SM, Gunel M, Mane S, Gibbs RA, Boerwinkle E, Hamosh A, Shendure J, Lupski JR, Lifton RP, Valle D, Nickerson DA, Bamshad MJ (2015) The Genetic Basis of Mendelian Phenotypes: Discoveries, Challenges, and Opportunities. Am J Hum Genet 97 (2):199–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Green RC, Goddard KA, Jarvik GP, Amendola LM, Appelbaum PS, Berg JS, Bernhardt BA, Biesecker LG, Biswas S, Blout CL, Bowling KM, Brothers KB, Burke W, Caga-Anan CF, Chinnaiyan AM, Chung WK, Clayton EW, Cooper GM, East K, Evans JP, Fullerton SM, Garraway LA, Garrett JR, Gray SW, Henderson GE, Hindorff LA, Holm IA, Lewis MH, Hutter CM, Janne PA, Joffe S, Kaufman D, Knoppers BM, Koenig BA, Krantz ID, Manolio TA, McCullough L, McEwen J, McGuire A, Muzny D, Myers RM, Nickerson DA, Ou J, Parsons DW, Petersen GM, Plon SE, Rehm HL, Roberts JS, Robinson D, Salama JS, Scollon S, Sharp RR, Shirts B, Spinner NB, Tabor HK, Tarczy-Hornoch P, Veenstra DL, Wagle N, Weck K, Wilfond BS, Wilhelmsen K, Wolf SM, Wynn J, Yu JH (2016) Clinical Sequencing Exploratory Research Consortium: Accelerating Evidence-Based Practice of Genomic Medicine. Am J Hum Genet 98 (6): 1051–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Morrissette JD, Colliton RP, Spinner NB (2001) Defective intracellular transport and processing of JAG1 missense mutations in Alagille syndrome. Hum Mol Genet 10 (4):405–413 [DOI] [PubMed] [Google Scholar]
- 373.Tada M, Itoh S, Ishii-Watabe A, Suzuki T, Kawasaki N (2012) Functional analysis of the Notch ligand Jagged1 missense mutant proteins underlying Alagille syndrome. Febs J 279(12):2096–2107 [DOI] [PubMed] [Google Scholar]
- 374.Boyer-Di Ponio J, Wright-Crosnier C, Groyer-Picard MT, Driancourt C, Beau I, Hadchouel M, Meunier-Rotival M (2007) Biological function of mutant forms of JAGGED1 proteins in Alagille syndrome: inhibitory effect on Notch signaling. Hum Mol Genet 16 (22):2683–2692 [DOI] [PubMed] [Google Scholar]
- 375.Nus M, MacGrogan D, Martinez-Poveda B, Benito Y, Casanova JC, Fernandez-Aviles F, Bermejo J, de la Pompa JL (2011) Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arterioscler Thromb Vasc Biol 31 (7): 1580–1588 [DOI] [PubMed] [Google Scholar]
- 376.Lundkvist J, Zhu S, Hansson EM, Schweinhardt P, Miao Q, Beatus P, Dannaeus K, Karlstrom H, Johansson CB, Viitanen M, Rozell B, Spenger C, Mohammed A, Kalimo H, Lendahl U (2005) Mice carrying a R142C Notch 3 knock-in mutation do not develop a CADASIL-like phenotype. Genesis 41 (1):13–22 [DOI] [PubMed] [Google Scholar]
- 377.Xia D, Watanabe H, Wu B, Lee SH, Li Y, Tsvetkov E, Bolshakov VY, Shen J, Kelleher RJ 3rd (2015) Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron 85 (5):967–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Xia D, Kelleher RJ 3rd, Shen J (2016) Loss of Abeta43 Production Caused by Presenilin-1 Mutations in the Knockin Mouse Brain. Neuron 90 (2):417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Veugelen S, Saito T, Saido TC, Chavez-Gutierrez L, De Strooper B (2016) Familial Alzheimer’s Disease Mutations in Presenilin Generate Amyloidogenic Abeta Peptide Seeds. Neuron 90 (2):410–416 [DOI] [PubMed] [Google Scholar]
- 380.Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP (1999) Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med 5 (1):101–106 [DOI] [PubMed] [Google Scholar]
- 381.Siman R, Reaume AG, Savage MJ, Trusko S, Lin YG, Scott RW, Flood DG (2000) Presenilin-1 P264L knock-in mutation: differential effects on abeta production, amyloid deposition, and neuronal vulnerability. J Neurosci 20 (23):8717–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Flood DG, Reaume AG, Dorfman KS, Lin YG, Lang DM, Trusko SP, Savage MJ, Annaert WG, De Strooper B, Siman R, Scott RW (2002) FAD mutant PS-1 gene-targeted mice: increased A beta 42 and A beta deposition without APP overproduction. Neurobiol Aging 23 (3):335–348 [DOI] [PubMed] [Google Scholar]
- 383.Bloomington Drosophila Stock Center, http://flystocks.bio.indiana.edu/ (2017).
- 384.Drosophila Genomics Resource Center, https://dgrc.bio.indiana.edu/ (2017).
- 385.Developmental Studies Hybridoma Bank, http://dshb.biology.uiowa.edu/ (2017).
- 386.Altenhoff AM, Dessimoz C (2012) Inferring orthology and paralogy. Methods Mol Biol 855:259–279 [DOI] [PubMed] [Google Scholar]
- 387.Drosophila RNAi Screening Center Integrative Ortholog Prediction Tool, http://www.flyrnai.org/cgi-bin/DRSCorthologs.pl (2017).
- 388.http://www.genenames.org/cgi-bin/hcop (2017).
- 389.Dehal P, Boore JL (2005) Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3 (10):e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kasahara M (2007) The 2R hypothesis: an update. Curr Opin Immunol 19 (5):547–552 [DOI] [PubMed] [Google Scholar]
- 391.PubMed (2017) https://www.ncbi.nlm.nih.gov/pubmed/.
- 392.Wang J, Al-Ouran R, Hu Y, Kim SY, Wan YW, Wangler MF, Yamamoto S, Chao HT, Comjean A, Mohr SE, Udn, Perrimon N, Liu Z, Bellen HJ (2017) MARRVEL: Integration of Human and Model Organism Genetic Resources to Facilitate Functional Annotation of the Human Genome. Am J Hum Genet 100 (6):843–853. doi: 10.1016/j.ajhg.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.http://marrvel.org/ (2017).
- 394.Database of Genomic Variants, http://dgv.tcag.ca/dgv/app/home/ (2017).
- 395.Exome Aggregation Consortium Browser, http://exac.broadinstitute.org/ (2017).
- 396.Geno2MP (2017) Genotype to Mendelian Phenotype Browser, http://geno2mp.gs.washington.edu/Geno2MP/.
- 397.DatabasE of genomiC varIation and Phenotype in Humans using Ensembl Resources, https://decipher.sanger.ac.uk/ (2017).
- 398.https://www.ncbi.nlm.nih.gov/clinvar/ (2017).
- 399.Saccharomyces Genome Database, http://www.yeastgenome.org/ (2017).
- 400.https://www.pombase.org/ (2017).
- 401.http://www.wormbase.org/ (2017).
- 402.Zebrafish Information Network, https://zfin.org/ (2017).
- 403.Mouse Genome Informatics, http://www.informatics.jax.org/ (2017).
- 404.Rat Genome Database, http://rgd.mcw.edu/ (2017).
- 405.Drosophila Genomics and Genetic Resources, http://www.dgrc.kit.ac.jp/ (2017).
- 406.Vienna Drosophila Resource Center, http://stockcenter.vdrc.at/ (2017). [Google Scholar]
- 407.Housden BE, Li J, Bray SJ (2014) Visualizing Notch signaling in vivo in Drosophila tissues. Methods Mol Biol 1187:101–113 [DOI] [PubMed] [Google Scholar]
- 408.Katsonis P, Koire A, Wilson SJ, Hsu TK, Lua RC, Wilkins AD, Lichtarge O (2014) Single nucleotide variations: biological impact and theoretical interpretation. Protein Sci 23 (12): 1650–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Gratz SJ, Rubinstein CD, Harrison MM, Wildonger J, O’Connor-Giles KM (2015) CRISPRCas9 Genome Editing in Drosophila. Curr Protoc Mol Biol 111:31 32, 31–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Diao F, White BH (2012) A novel approach for directing transgene expression in Drosophila: T2A-Gal4 in-frame fusion. Genetics 190 (3): 1139–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Bellen HJ, Yamamoto S (2015) Morgan’s legacy: fruit flies and the functional annotation of conserved genes. Cell 163 (1): 12–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Diao F, Ironfield H, Luan H, Diao F, Shropshire WC, Ewer J, Marr E, Potter CJ, Landgraf M, White BH (2015) Plug-and-play genetic access to drosophila cell types using exchangeable exon cassettes. Cell Rep 10 (8): 1410–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Gnerer JP, Venken KJ, Dierick HA (2015) Gene-specific cell labeling using MiMIC transposons. Nucleic Acids Res 43 (8):e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Nagarkar-Jaiswal S, DeLuca SZ, Lee PT, Lin WW, Pan H, Zuo Z, Lv J, Spradling AC, Bellen HJ (2015) A genetic toolkit for tagging intronic MiMIC containing genes. Elife 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Nagarkar-Jaiswal S, Lee PT, Campbell ME, Chen K, Anguiano-Zarate S, Gutierrez MC, Busby T, Lin WW, He Y, Schulze KL, Booth BW, Evans-Holm M, Venken KJ, Levis RW, Spradling AC, Hoskins RA, Bellen HJ (2015) A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. Elife 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Venken KJ, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, Carlson JW, Levis RW, Spradling AC, Hoskins RA, Bellen HJ (2011) MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods 8 (9):737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Daniels RW, Rossano AJ, Macleod GT, Ganetzky B (2014) Expression of multiple transgenes from a single construct using viral 2A peptides in Drosophila. PLoS One 9 (6):e100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Chao HT, Davids M, Burke E, Pappas JG, Rosenfeld JA, McCarty AJ, Davis T, Wolfe L, Toro C, Tifft C, Xia F, Stong N, Johnson TK, Warr CG, Yamamoto S, Adams DR, Markello TC, Gahl WA, Bellen HJ, Wangler MF, Malicdan MC (2017) A Syndromic Neurodevelopmental Disorder Caused by De Novo Variants in EBF3. Am J Hum Genet 100 (1): 128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Yoon WH, Sandoval H, Nagarkar-Jaiswal S, Jaiswal M, Yamamoto S, Haelterman NA, Putluri N, Putluri V, Sreekumar A, Tos T, Aksoy A, Donti T, Graham BH, Ohno M, Nishi E, Hunter J, Muzny DM, Carmichael J, Shen J, Arboleda VA, Nelson SF, Wangler MF, Karaca E, Lupski JR, Bellen HJ (2017) Loss of Nardilysin, a Mitochondrial Co-chaperone for alpha-Ketoglutarate Dehydrogenase, Promotes mTORC1 Activation and Neurodegeneration. Neuron 93 (1):115–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Sandoval H, Yao CK, Chen K, Jaiswal M, Donti T, Lin YQ, Bayat V, Xiong B, Zhang K, David G, Charng WL, Yamamoto S, Duraine L, Graham BH, Bellen HJ (2014) Mitochondrial fusion but not fission regulates larval growth and synaptic development through steroid hormone production. Elife 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yamamoto S, Jaiswal M, Charng WL, Gambin T, Karaca E, Mirzaa G, Wiszniewski W, Sandoval H, Haelterman NA, Xiong B, Zhang K, Bayat V, David G, Li T, Chen K, Gala U, Harel T, Pehlivan D, Penney S, Vissers LE, de Ligt J, Jhangiani SN, Xie Y, Tsang SH, Parman Y, Sivaci M, Battaloglu E, Muzny D, Wan YW, Liu Z, Lin-Moore AT, Clark RD, Curry CJ, Link N, Schulze KL, Boerwinkle E, Dobyns WB, Allikmets R, Gibbs RA, Chen R, Lupski JR, Wangler MF, Bellen HJ (2014) A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell 159 (1):200–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Wang S, Tan KL, Agosto MA, Xiong B, Yamamoto S, Sandoval H, Jaiswal M, Bayat V, Zhang K, Charng WL, David G, Duraine L, Venkatachalam K, Wensel TG, Bellen HJ (2015) The retromer complex is required for rhodopsin recycling and its loss leads to photoreceptor degeneration. PLoS Biol 12 (4):e1001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.David-Morrison G, Xu Z, Rui YN, Charng WL, Jaiswal M, Yamamoto S, Xiong B, Zhang K, Sandoval H, Duraine L, Zuo Z, Zhang S, Bellen HJ (2016) WAC Regulates mTOR Activity by Acting as an Adaptor for the TTT and Pontin/Reptin Complexes. Dev Cell 36 (2): 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Zhang K, Li Z, Jaiswal M, Bayat V, Xiong B, Sandoval H, Charng WL, David G, Haueter C, Yamamoto S, Graham BH, Bellen HJ (2013) The C8ORF38 homologue Sicily is a cytosolic chaperone for a mitochondrial complex I subunit. J Cell Biol 200 (6):807–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Xiong B, Bayat V, Jaiswal M, Zhang K, Sandoval H, Charng WL, Li T, David G, Duraine L, Lin YQ, Neely GG, Yamamoto S, Bellen HJ (2012) Crag is a GEF for Rab11 required for rhodopsin trafficking and maintenance of adult photoreceptor cells. PLoS Biol 10 (12):e1001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375 (6534):754–760 [DOI] [PubMed] [Google Scholar]
- 427.http://www.alzforum.org/mutations (2017).
- 428.Seidner GA, Ye Y, Faraday MM, Alvord WG, Fortini ME (2006) Modeling clinically heterogeneous presenilin mutations with transgenic Drosophila. Curr Biol 16 (10):1026–1033 [DOI] [PubMed] [Google Scholar]
- 429.Cuyvers E, Sleegers K (2016) Genetic variations underlying Alzheimer’s disease: evidence from genome-wide association studies and beyond. Lancet Neurol 15 (8):857–868 [DOI] [PubMed] [Google Scholar]
- 430.Wangler MF, Hu Y, Shulman JM (2017) Drosophila and genome-wide association studies: a review and resource for the functional dissection of human complex traits. Dis Model Mech 10(2):77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S (1993) Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet 342 (8873):697–699 [DOI] [PubMed] [Google Scholar]
- 432.Mahoney-Sanchez L, Belaidi AA, Bush AI, Ayton S (2016) The Complex Role of Apolipoprotein E in Alzheimer’s Disease: an Overview and Update. J Mol Neurosci 60 (3):325–335 [DOI] [PubMed] [Google Scholar]
- 433.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 (5123):921–923 [DOI] [PubMed] [Google Scholar]
- 434.Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium, http://www.chargeconsortium.com/ (2017).
- 435.Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, Uitterlinden AG, Harris TB, Witteman JC, Boerwinkle E (2009) Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet 2 (1):73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Jakobsdottir J, van der Lee SJ, Bis JC, Chouraki V, Li-Kroeger D, Yamamoto S, Grove ML, Naj A, Vronskaya M, Salazar JL, DeStefano AL, Brody JA, Smith AV, Amin N, Sims R, Ibrahim-Verbaas CA, Choi SH, Satizabal CL, Lopez OL, Beiser A, Ikram MA, Garcia ME, Hayward C, Varga TV, Ripatti S, Franks PW, Hallmans G, Rolandsson O, Jansson JH, Porteous DJ, Salomaa V, Eiriksdottir G, Rice KM, Bellen HJ, Levy D, Uitterlinden AG, Emilsson V, Rotter JI, Aspelund T, O’Donnell CJ, Fitzpatrick AL, Launer LJ, Hofman A, Wang LS, Williams J, Schellenberg GD, Boerwinkle E, Psaty BM, Seshadri S, Shulman JM, Gudnason V, van Duijn CM (2016) Rare Functional Variant in TM2D3 is Associated with Late-Onset Alzheimer’s Disease. PLoS Genet 12(10):e1006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Shannon MP (1972) Characterization of the female-sterile mutant almondex of Drosophila melanogaster. Genetica 43 (2):244–256 [DOI] [PubMed] [Google Scholar]
- 438.Michellod MA, Randsholt NB (2008) Implication of the Drosophila beta-amyloid peptide binding-like protein AMX in Notch signaling during early neurogenesis. Brain Res Bull 75 (2–4):305–309 [DOI] [PubMed] [Google Scholar]
- 439.Wangler MF, Yamamoto S, Bellen HJ (2015) Fruit flies in biomedical research. Genetics 199(3):639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.http://flybase.org/reports/FBgg0000158.html (2017).
- 441.http://flybase.org/reports/FBgg0000215.html (2017).
- 442.Flybase-AP3 (2017) http://flybase.org/reports/FBgg0000136.html.
- 443.Flybase-HOPS (2017) http://flybase.org/reports/FBgg0000106.html.
- 444.Flybase-VATPase (2017) http://flybase.org/reports/FBgg0000111.html.
- 445.HGNC-Arp2/3 (2017) http://www.genenames.org/cgi-bin/genefamilies/set/39.
- 446.HGNC-ESCRT (2017) http://www.genenames.org/cgi-bin/genefamilies/set/1111.
- 447.Dell’Angelica EC (2009) AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol 21 (4):552–559 [DOI] [PubMed] [Google Scholar]
- 448.Solinger JA, Spang A (2013) Tethering complexes in the endocytic pathway: CORVET and HOPS. Febs J 280 (12):2743–2757 [DOI] [PubMed] [Google Scholar]
- 449.HGNC-VATPase (2017) http://www.genenames.org/cgi-bin/genefamilies/set/415.