Abstract
Differences of EEG synchronization between normal old and young people during a working memory (WM) task were investigated. The synchronization likelihood (SL) is a novel method to assessed synchronization in multivariate time series for non-stationary systems. To evaluate this method to study the mechanisms of WM, we calculated the SL values in brain electrical activity for both resting state and task state. EEG signals were recorded from 14 young adults and 12 old adults during two different states, respectively. SL was used to measure EEG synchronization between 19 electrodes in delta, theta, alpha1, alpha2 and beta frequency bands. Bad task performance and significantly decreased EEG synchronization were found in old group compared to young group in alpha1, alpha2 and beta frequency bands during the WM task. Moreover, significantly decreased EEG synchronization in beta band in the elder was also detected during the resting state. The findings suggested that reduced EEG synchronization may be one of causes for WM capacity decline along with healthy aging.
Keywords: EEG, Synchronization, Working memory, Aging
Introduction
Advances in technology and medicine have made the proportion of people over 65 years old continuous increase. Aging is defined as progressive and widespread impairment of function, leading to an increased vulnerability to the changes of environment (Vysata et al. 2014). As a major risk factor, aging is also related to a series of physiological systems deterioration, which can result in many human diseases (López-Otín et al. 2013). For example, brain aging could lead to neurodegenerative diseases, such as dementia. Moreover, there is no definite time limit and unified diagnostic criteria for brain aging. Therefore, the elderly population is a heavy financial, pension and care burden to family as well as society now.
Over the past decade, many noninvasive neuroimaging technologies have been widely applied to acquisition of various neurophysiological signals for research in the field of cognitive neuroscience such as functional magnetic resonance imaging (fMRI; Draganski et al. 2013; Chew et al. 2016), electroencephalography (EEG; Mateos et al. 2017; Bernarding et al. 2017; Duru and Assem 2017), magnetoencephalography (MEG; Wilson et al. 2016), etc. In these techniques, EEG can be collected from the scalp of human by placing suitable electrodes with convenient acquisition. Furthermore, the high temporal resolution of EEG is a particularly suitable tool for assessing the changes of synchronization underlying brain activity signals. In recent years, EEG has been widely used to find abnormal changes in cognitive functions caused by diseases or healthy aging (John et al. 2018; Protopapa et al. 2016; Mazza and Brignani 2016).
Brain aging is a progressive process accompanied with gradual cognitive degeneration, as well as structural and neurophysiological changes within brain (Salat 2011; Grady 2012), especially decrease in memory capacity (Pinal et al. 2015; Rönnlund et al. 2015). Working memory (WM) is widely viewed as a prominent part in age-related cognitive decline (Reuter-Lorenz 2013), and age-related decrease of WM storage and central executive function has been observed in temporal tasks (Baudouin et al. 2006). Compared with young adults, old adults generally showed reduced WM performance, which is more obvious at high WM load (Nyberg et al. 2012; Heinzel et al. 2014). With behavioral differences between young and old people in WM tasks, age-related changes in activation of brain cortex have been reported (Grady 2008).
WM refers to the ability to temporarily maintain and manipulate information from the external environment in mind (Baddeley 2003), which is a complex cognitive process supported by integration as well as activation of local brain regions (Hampson et al. 2006). Meanwhile, synchronous neural oscillations have been thought of as an important mechanism related to the integration of specialized functional brain regions in cognitive processes (Ward 2003). There have been various measures proposed to estimate synchronization of neurophysiological systems by using time series of neurophysiological signals (Mumtaz et al. 2018; Vinck et al. 2011; Olbrich et al. 2014). Early traditional measures of synchronization mainly adopted linear analysis methods in time and frequency domain, such as correlation (Gevins and Schaffer 1980) and coherence (Zaveri et al. 1999), respectively. But linear analysis methods are not suitable for nonlinear signal. EEG is non-stationary signal, and it has shown nonlinearity for multichannel EEG (Rombouts et al. 1995), which indicates interactions between some brain regions may contain nonlinear components (Stam et al. 2003). Therefore, these linear approaches should not be suitable for investigating synchronization between different brain regions based on EEG signal (Stam et al. 2003). Considering the nonlinearity of neurophysiological systems, some nonlinear analysis methods have been applied to assess the synchronization of neurophysiological signals (Pereda et al. 2005). Synchronization likelihood (SL) is a measure of the statistical dependence between one time series and another or more other time series, which can detect linear and non-linear coupling between non-stationary systems (Stam and van Dijk 2002). Unlike others methods, the measure of SL isn’t sensitive to signals with different magnitudes or different degrees of freedom. In addition, SL also has other advantages. For example, the SL approach can detect coupling changes between dynamical systems with a high temporal resolution, and it has good robustness even if data contains considerable noise. So the SL method is very appropriate for detecting the interaction between EEG signals. For the past few years, SL has been widely used in EEG data to characterize the changes in synchronization of neurophysiological systems (Pijnenburg et al. 2004; Betzel et al. 2012).
In the present study, to investigate the age-related differences in brain functional synchronization, SL method was used to calculate EEG synchronization of young and old adults during a visual WM task.
Methods
Subjects
A total of twenty-six healthy subjects including fourteen young adults (age range 21–25 years; mean age, 22.6 ± 1.4 years) and twelve old adults (age range 57–83 years; mean age, 72.2 ± 6.7 years) were recruited from Xi’an Jiaotong University and voluntarily accepted to participate in this study. All subjects were right-handed males. All of them accomplished the mini-mental state examination (MMSE; Folstein et al. 1975) with scores above 28. Before the experiment session, written informed consent was signed. The study was approved by the local ethics committee.
Task description
In this study, each subject was required to participate in a visual WM task. This WM task was first proposed by Stam to study brain dynamics during WM condition (Stam 2000), and then was used by Pijnenburg et al. (2004) to investigate changes of cerebral functional activity in Alzheimer’s disease and mild cognitive impairment under WM condition.
The task processes consist of three steps, and are summarized as follows: (1) figures presentation. An A4 size paper with 12 stick figures (Fig. 1) was shown to the subject by the experimenter for 10 s. (2) Memory retention. The subject was asked to close his eyes for 1 min and try to memorize the figures in the A4 paper as many as possible. (3) Figure name recall. The subject was asked to open his eyes and required to say the names of those figures they could remember.
Fig. 1.
Paper with 12 stick figures used in the working memory task
For each subject, the number of figures remembered correctly was recorded as his scores of this task, which could evaluate the level of memory ability of subject. That is, the more scores one obtained, the high memory capacity one has. Before the experiment, the procedure and scoring rule of this task had been explained to the subject so that he could understand the task and concentrate on it.
EEG recording and preprocessing
The whole experiments were carried out in an electrically and acoustically shielded room. The scalp EEG signals were collected from 19 electrodes (Ag/AgCl)–Fp1, Fp2, F7, F3, Fz, F4, F8, C3, Cz, C4, P7, P3, Pz, P4, P8, O1, O2, T7, T8 using the Neuroscan EEG acquisition system (Neuroscan Inc., USA). The locations of all electrodes conformed to the International 10–20 system. Reference electrode and ground electrode were placed on the left mastoid and the forehead, respectively. A pair of electrodes placed above and below the left eye was used to record vertical electrooculogram (VEOG), and horizontal electrooculogram (HEOG) was recorded from two electrodes on the outer canthi of both eyes. Impedance of all sites was kept lower than 5 kΩ. Continuous EEG data was acquired with a sampling rate of 1000 Hz and a bandpass of 0.1–100 Hz under two conditions: (1) resting state with eyes closed; (2) period of memory retention during the task. During recording, each subject was seated in a comfortable chair and was asked to keep relaxed and minimize all body movement.
EEG data was preprocessed with Scan software (NeuroScan Inc., Herndon, VA, USA). First, ocular artifacts were corrected by using a regression analysis algorithm (NeuroScan Inc.). Next, an average reference montage including all of the recording electrodes was used. Then zero-phase-shift digital off-line filtering was performed on EEG data in the following five frequency bands: delta (0.5–3.5 Hz), theta (4–7 Hz), alpha1 (8–10 Hz), alpha2 (10–13 Hz) and beta (14–30 Hz). Then, two artifact-free epochs of 10 s duration for each subject were selected from EEG data. One epoch was chosen from resting condition. The other one was selected from recall phase with 1 min eyes closed after the presentation of the pictures. Finally, signals were down sampled to 500 Hz for further analysis.
Synchronization likelihood
The Synchronization likelihood (SL) method was proposed by Stam and van Dijk (2002), and it is an effective measure of generalized synchronization between two or more time series, which can be used to detect both linear and nonlinear coupling (Stam and van Dijk 2002). It measures the statistical likelihood that if a system is in the same state at two different times i and j, then the coupled system will be also in the same state at time i and j.
Supposed K simultaneously recorded EEG series xk,i, where k denotes EEG channel (k = 1, …, K) and i denotes discrete time (i = 1, …, N). The state of each EEG series at time i is defined as a embedded vector, reconstructed in the state space by using the method of time-delay embedding:
| 1 |
where l is the lag and m is the embedding dimension in the state space.
If the distance between vector Xk,i and Xk,j is smaller than a critical cutoff distance, channel k will be considered to be in the same state at the two time points. For each channel k at time i and j, the probability that the channel is in the same state is defined as:
| 2 |
here |·| denotes the Euclidean distance and θ is the Heaviside step function, θ(x) = 1 if x > 0 and otherwise θ(x) = 0. w1 and w2 are two windowing parameters, where w1 is the Theiler correction to avoid autocorrelation effects and w2 is a window used to improve the time resolution of the synchronization measure, which are chosen such that w1 ≪ w2 ≪ N. εk,i is the critical distance and is determined on the condition that , where 0 < Pref ≪ 1.
Then SL between one channel k and other K − 1 channels at time i is defined as follow:
| 3 |
SL describes the degree of synchronization between channel k and all the other K − 1 channels at time point i, which takes on values between Pref and 1. In the following pages, SLk is used to denote mean SLk,i over time points between channel k and other K − 1 channels, and SL denotes mean SLk over all electrodes. In particular, SL = Pref indicates no synchronization, while SL = 1 means maximal synchronization of all channels.
In this study, the Pref was set to 0.05. The time-delay embedding parameter m and l were set to 10 and 10, respectively. Two widowing parameters w1 and w2 were set to 100 and 400 samples, respectively.
Statistical analysis
The statistical analysis was performed using Statistical Product and Service Solutions (SPSS v20.0; SPSS, Chicago, IL, USA). Synchronization likelihood values of each frequency band were analyzed using a two-way repeated-measures analysis of variance (ANOVA) for WM task and resting state, respectively. There was a between-subject factor and a within-subject factor; between-subject factor was ‘group’ (two levels: old/young group) and within-subject factor was ‘electrode’ (19 levels: 19 electrodes). Greenhouse–Geisser correction was used to correct p values when Mauchly’s test of Sphericity was invalid. The value of p less than 0.05 was considered statistically significant.
Results
WM performance
The mean scores in this visual WM task were 4.67 (SD: 1.23) for the old group and 7.50 (SD: 1.51) for the young group. It indicated that the scores of the old group were significantly lower than that of the young group (Mann–Whitney U test, p < 0.001), which illustrated that the old subjects had bad performance in this task compared to the young participants.
EEG synchronization under WM condition
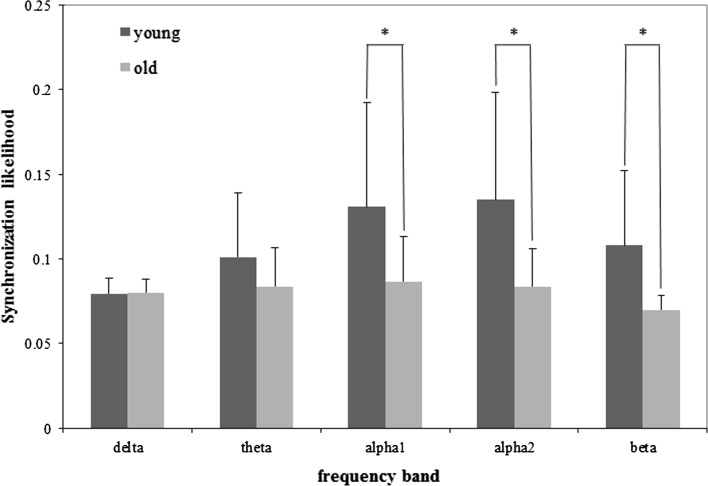
In both delta and theta bands, there was no significant difference between EEG synchronization of the young and that of old group under WM condition. The results showed that the effect of electrodes was significant (delta: F18, 432 = 4.705, p < 0.001; theta: F18, 432 = 10.086, p < 0.001). However, the interaction between ‘group’ and ‘electrode’ had no significance (p > 0.05).
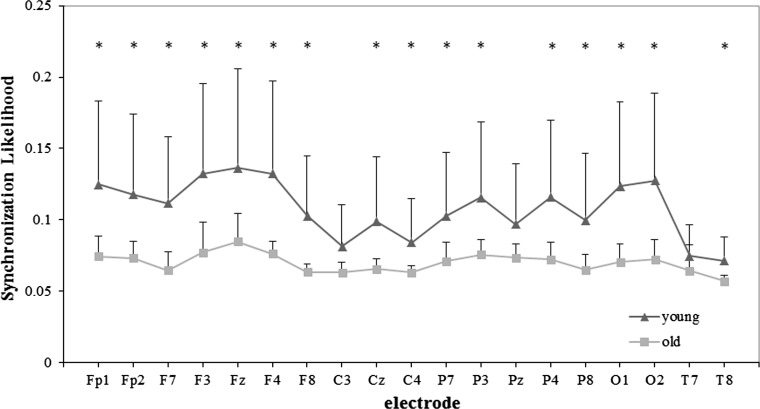
The EEG synchronization results showed that the young group was significantly higher than the old group in alpha1 (F1,24 = 4.902, p = 0.037), alpha2 (F1,24 = 6.529, p = 0.017) and beta (F1,24 = 7.898, p = 0.010) frequency bands during this task. In all these three frequency bands, the electrode effect was also notable (alpha1: F18, 432 = 12.632, p < 0.001; alpha2: F18,432 = 15.141, p < 0.001; beta: F18,432 = 12.539, p < 0.001). The group × electrode interaction was significant in alpha2 (F18,432 = 3.871, p = 0.018) and beta (F18,432 = 4.094, p = 0.011) frequency bands, but not significant difference in alpha1 frequency band (p > 0.05).
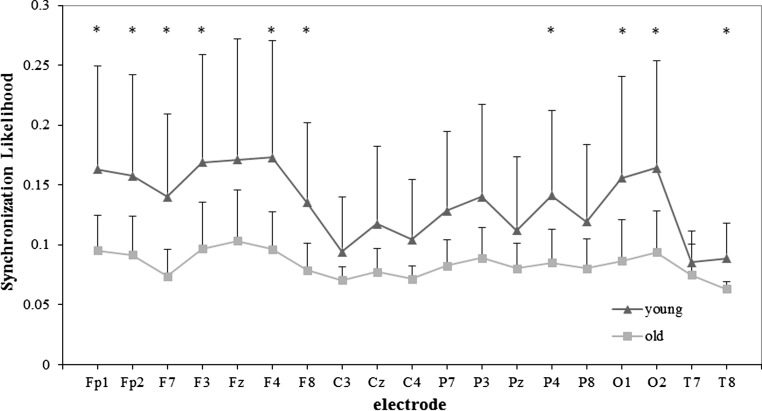
The mean SL values of old and young group in five frequency bands are all shown in Fig. 2, and detailed results for alpha2 and beta frequency bands are shown in Figs. 3 and 4, respectively.
Fig. 2.
Mean SL (+ SD) values of the old and young group for WM task in five frequency bands. Significance was tested by the independent t test (*p < 0.05)
Fig. 3.
SLk values of the young and old group for each electrode in alpha2 band for WM task. Significance was tested by the independent t test, and multiple comparisons correction was performed by the false discovery rate (FDR) procedure (*p < 0.05)
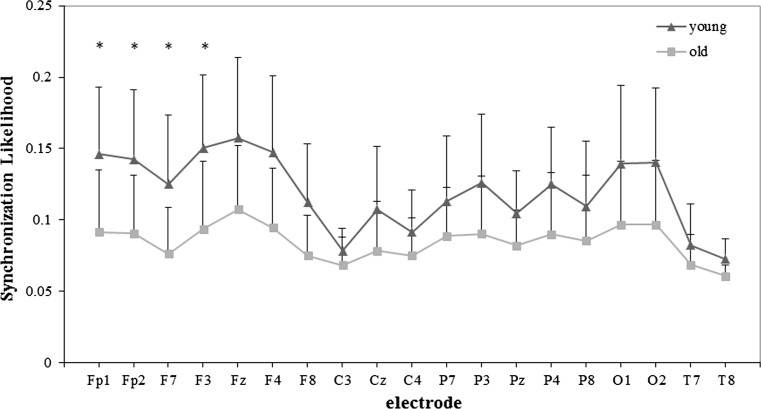
Fig. 4.
SLk values of the young and old group for each 19 electrodes in beta band for WM task. Significance was tested by the independent t test, and multiple comparisons correction was performed by the false discovery rate (FDR) procedure (*p < 0.05)
EEG synchronization under resting condition
In delta, theta, alpha1 and alpha2 bands, there was no significant difference between EEG synchronization of the young and that of old group under resting condition. The effect of electrodes was significant (delta: F18, 432 = 4.312, p = 0.002; theta: F18, 432 = 18.224, p < 0.001; alpha1: F18, 432 = 24.433, p < 0.001; alpha2: F18, 432 = 27.616, p < 0.001). But the interaction between ‘group’ and ‘electrode’ was non-significant (p > 0.05).
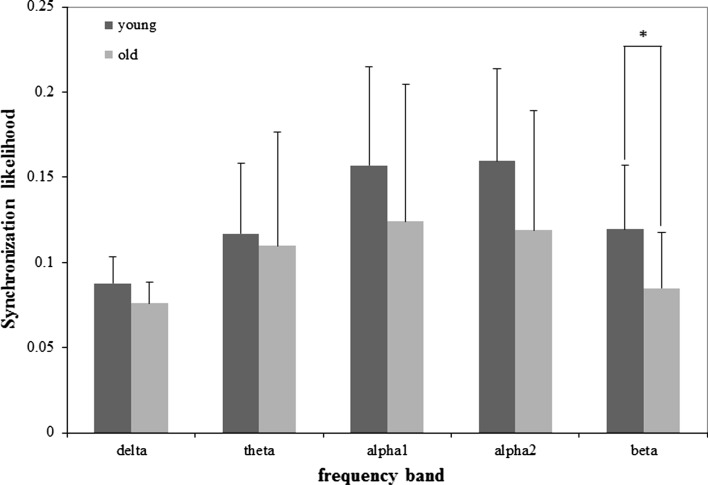
In beta band, EEG synchronization of the old group was lower than that of the young group, and it had a significant difference (F1, 24 = 5.714, p = 0.025). Meanwhile the electrodes effect was also significant (F18, 432 = 20.930, p < 0.001). Compared to the result under task condition, the group × electrode interaction was significant in this frequency band (F18, 432 = 3.742, p = 0.012).
The mean SL values of old and young group under resting condition in five frequency bands are shown in Fig. 5, and details of results for beta frequency band are shown in Fig. 6.
Fig. 5.
Mean SL (+ SD) values of the old and young group for resting state in five frequency bands. Significance was tested by the independent t test (*p < 0.05)
Fig. 6.
SLk values of the young and old group for each electrode in beta band for resting state. Significance was tested by the independent t test, and multiple comparisons correction was performed by the false discovery rate (FDR) procedure (*p < 0.05)
Discussion
In this visual WM task, the results revealed that young adults had higher mean scores than old adults. The bad behavioral performance of old adults reflected the degeneration of working-memory capacity with aging (Schneider-Garces et al. 2010).
Compared with young adults, old adults showed decreased EEG synchronization in both alpha1 and alpha2 bands during this WM task, while differences between two groups were not significant in resting state. In addition, EEG synchronization differences between two groups in beta band existed in more electrodes during WM task than resting state. These differences indicated that decrease of EEG synchronization for old people could reflect decline of cognitive function in WM task with aging.
With the decrease of EEG synchronous activities for old adults in alpha1 band during WM condition, no significant differences of group × electrode interaction were observed, which might imply that age-related decrease of EEG synchronization in this band showed a global effect over the whole brain cortex. As we know, alpha1 oscillation reflected global attentional arousal and processing (Klimesch 1997). Therefore, the result showed there might be an attention deficit in old people during WM tasks.
Previous studies have suggested that alpha2 oscillation takes an important part in selective stimulus encoding and is related to specific mode of the neural systems governing the processing and management of semantic information (Klimesch 1997, 1999). In this task, subjects were finally required to say the names of those figures they could remember, so semantic information processing was likely to be involved during the period of memory retention. In alpha2 band, we found a significant difference in group × electrode interaction. EEG synchronization differences between young and old adults in WM state mainly occurred in frontal and occipital lobe. Frontal lobe has been proved to be one of the functional areas in semantic cognition (Jefferies 2013). Recent fMRI and transcranial direct current stimulus (tDCS) studies have shown that the visual cortex located in the occipital lobe is important in retaining and consolidating visual WM information (Makovski and Lavidor 2014). We suggested that the reduced EEG synchronization of old adults in frontal and occipital lobe for alpha2 band might result in functional decline in semantic processing and memory consolidation.
Gola et al. (2013) proposed that beta oscillation played an important role in attentional processes. Under the task state, participants were required to focus their attention on memorizing figures, and memory retention was also necessary. In our behavioral results, it clearly showed that old adults acquired a relative low scores. On the one hand, old group paid less attention on the figures compared to young group; on the other hand, EEG synchronization of the elders in beta band was significant less than the young adults, to some extent, beta wave was closely linked to our attention, which was in line with precious study (Gola et al. 2013). Furthermore, research about neural oscillatory activity during episodic memory revealed that beta-band activity in posterior region reflected the integration and maintenance of the recent past events (Morton and Polyn 2016). The decreased EEG synchronization in beta band of old adults might indicate the functional degeneration in integrating and maintaining information with aging, which was one of reasons for the bad performance of old people in the task.
Besides, differences of EEG synchronization between old and young adults for WM task were also observed in frontal, central and parietal region, while the difference for resting state was only significant at frontal electrodes. By comparison with resting state, it required more brain regions closely connected to accomplish cognitive task during information processing.
In order to finish a WM task, both attention-related and memory-related processes were necessary during WM retention period (Michels et al. 2008). In this WM task, the decreased EEG synchronization of old adults in alpha1, alpha2 and beta bands reflected decline and deficits in attention and memory function along with healthy aging, which caused bad WM performance of old subjects.
Considering that older people were not familiar with using computer, figures were shown to subjects in a paper in the experiment. Next, computer would be used to present stimulus, it could make experiment more accurate and reliable.
Conclusion
Compared to young adults, healthy aging adults had bad behavioral performance in a visual WM task. Meanwhile, lower EEG synchronization in alpha1, alpha2 and beta frequency bands were observed in old adults. These findings showed age-related degeneration of attention and memory function. Besides, it was also proved that SL could be used as an effective measure to investigate function decline of WM with aging.
Acknowledgements
This study was funded by the National Key Research and Development Program of China (No. 2017YFB1300303), the National Natural Science Foundation of China (Grant No. 31271061), and the Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2015JM6289).
References
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Pouthas V, et al. Age-related changes in duration reproduction: involvement of working memory processes. Brain Cogn. 2006;62(1):17–23. doi: 10.1016/j.bandc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bernarding C, Strauss DJ, Hannemann R, et al. Neurodynamic evaluation of hearing aid features using EEG correlates of listening effort. Cogn Neurodyn. 2017;11(3):203–215. doi: 10.1007/s11571-017-9425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Erickson MA, Malene A, et al. Synchronization dynamics and evidence for a repertoire of network states in resting EEG. Front Comput Neurosci. 2012;6:1–13. doi: 10.3389/fncom.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew LH, Teo J, Mountstephens J. Aesthetic preference recognition of 3D shapes using EEG. Cogn Neurodyn. 2016;10(2):165–173. doi: 10.1007/s11571-015-9363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Lutti A, Kherif F. Impact of brain aging and neurodegeneration on cognition: evidence from MRI. Curr Opin Neurol. 2013;26(6):640–645. doi: 10.1097/WCO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- Duru AD, Assem M. Investigating neural efficiency of elite karate athletes during a mental arithmetic task using EEG. Cogn Neurodyn. 2017;12(1):95–102. doi: 10.1007/s11571-017-9464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Schaffer RE. A critical review of electroencephalographic (EEG) correlates of higher cortical functions. Crit Rev Bioeng. 1980;4(2):113–164. [PubMed] [Google Scholar]
- Gola M, Magnuski M, Szumska I, et al. EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly subjects. Int J Psychophysiol. 2013;89(3):334–341. doi: 10.1016/j.ijpsycho.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, et al. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Lorenz RC, Brockhaus WR, et al. Working memory load-dependent brain response predicts behavioral training gains in older adults. J Neurosci. 2014;34(4):1224–1233. doi: 10.1523/JNEUROSCI.2463-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex. 2013;49(3):611–625. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- John TN, Puthankattil SD, Menon R. Analysis of long range dependence in the EEG signals of Alzheimer patients. Cogn Neurodyn. 2018;6:1–17. doi: 10.1007/s11571-017-9467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol. 1997;26(1–3):319–340. doi: 10.1016/S0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29(2–3):169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovski T, Lavidor M. Stimulating occipital cortex enhances visual working memory consolidation. Behav Brain Res. 2014;275:84–87. doi: 10.1016/j.bbr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Mateos DM, Erra RG, Wennberg R, et al. Measures of entropy and complexity in altered states of consciousness. Cogn Neurodyn. 2017;1:1–12. doi: 10.1007/s11571-017-9459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza V, Brignani D. Electrophysiological advances on multiple object processing in aging. Front Aging Neurosci. 2016;8:1–7. doi: 10.3389/fnagi.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L, Moazami-Goudarzi M, Jeanmonod D, et al. EEG alpha distinguishes between cuneal and precuneal activation in working memory. Neuroimage. 2008;40(3):1296–1310. doi: 10.1016/j.neuroimage.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Morton NW, Polyn SM. Beta-band activity represents the recent past during episodic encoding. Neuroimage. 2016;147:692–702. doi: 10.1016/j.neuroimage.2016.12.049. [DOI] [PubMed] [Google Scholar]
- Mumtaz W, Vuong PL, Malik AS, et al. A review on EEG-based methods for screening and diagnosing alcohol use disorder. Cogn Neurodyn. 2018;12(2):141–156. doi: 10.1007/s11571-017-9465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, et al. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Olbrich S, Tränkner A, Chittka T, et al. Functional connectivity in major depression: increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res Neuroimaging. 2014;222(1–2):91–99. doi: 10.1016/j.pscychresns.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Pereda E, Quiroga RQ, Bhattacharya J. Nonlinear multivariate analysis of neurophysiological signals. Prog Neurobiol. 2005;77(1–2):1–37. doi: 10.1016/j.pneurobio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Pijnenburg YAL, Made YV, van Walsum AMV, et al. EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin Neurophysiol. 2004;115(6):1332–1336. doi: 10.1016/j.clinph.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Pinal D, Zurrón M, Díaz F. An event related potentials study of the effects of age, load and maintenance duration on working memory recognition. PLoS ONE. 2015;10(11):e0143117. doi: 10.1371/journal.pone.0143117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopapa F, Siettos CI, Myatchin I, et al. Children with well controlled epilepsy possess different spatio-temporal patterns of causal network connectivity during a visual working memory task. Cogn Neurodyn. 2016;10(2):1–13. doi: 10.1007/s11571-015-9373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. Aging and cognitive neuroimaging: a fertile union. Perspect Psychol Sci. 2013;8(1):68–71. doi: 10.1177/1745691612469023. [DOI] [PubMed] [Google Scholar]
- Rombouts SARB, Keunen RWM, Stam CJ. Investigation of nonlinear structure in multichannel EEG. Phys Lett A. 1995;202(5–6):352–358. doi: 10.1016/0375-9601(95)00335-Z. [DOI] [Google Scholar]
- Rönnlund M, Sundström A, Nilsson LG. Interindividual differences in general cognitive ability from age 18 to age 65 years are extremely stable and strongly associated with working memory capacity. Intelligence. 2015;53:59–64. doi: 10.1016/j.intell.2015.08.011. [DOI] [Google Scholar]
- Salat DH. The declining infrastructure of the aging brain. Brain Connect. 2011;1(4):279–293. doi: 10.1089/brain.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, et al. Span, Crunch, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 2010;22(4):655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ. Brain dynamics in theta and alpha frequency bands and working memory performance in humans. Neurosci Lett. 2000;286(2):115–118. doi: 10.1016/S0304-3940(00)01109-5. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van Dijk BW. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Phys D Nonlinear Phenomena. 2002;163(3–4):236–251. doi: 10.1016/S0167-2789(01)00386-4. [DOI] [Google Scholar]
- Stam CJ, Made YV, Pijnenburg YAL, et al. EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2003;108(2):90–96. doi: 10.1034/j.1600-0404.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- Vinck M, Oostenveld R, Van WM, et al. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011;55(4):1548–1565. doi: 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Vysata O, Kukal J, Prochazka A, et al. Age-related changes in EEG coherence. Neurol Neurochir Pol. 2014;48(1):35–38. doi: 10.1016/j.pjnns.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7(12):553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Proskovec AL, et al. Neuroimaging with magnetoencephalography: a dynamic view of brain pathophysiology. Transl Res. 2016;175:17–36. doi: 10.1016/j.trsl.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri HP, Williams WJ, Sackellares JC, et al. Measuring the coherence of intracranial electroencephalograms. Clin Neurophysiol. 1999;110(10):1717–1725. doi: 10.1016/S1388-2457(99)00136-4. [DOI] [PubMed] [Google Scholar]