Abstract
This review covers aspects of cofilin and profilin regulations and their influence on actin polymerisation responsible for cell motility and metastasis. The regulation of their activity by phosphorylation and nitration, miRs, PI(4,5)P2 binding, pH, oxidative stress and post-translational modification is described. In this review, we have highlighted selected similarities, complementarities and differences between the two proteins and how their interplay affects actin filament dynamics.
Keywords: Cell motility, Metastasis, Regulation, Polymerisation, Actin
The spreading of cancer cells or metastasis is a highly complex mechanism that involves a cascade of events: namely (1) detachment of tumour cells from the primary tumour, (2) epithelial-mesenchymal transition, (3) anchorage-independent survival, (4) intravasation and dissemination into blood stream or lymphatic vessels, (5) extravasation and finally (6) migration/invasion in the secondary site and outgrowth (Yilmaz and Christofori 2009). An essential cellular event in all of these steps is cytoskeletal reorganisation that ultimately leads to the formation of supramolecular structures such as filopodia (sensory protrusions), invadopodia (invasive protrusions in motile carcinoma cells) and lamellipodia (locomotory protrusions in motile epithelial cells), which are all essential path-finding structures in chemotaxis, cell migration and invasion (Yilmaz and Christofori 2009). Numerous upstream signalling pathways and proteins spatiotemporally regulate the construction and destruction of these structures (Pollard and Borisy 2003; Olson and Sahai 2009; Oser et al. 2010; Nurnberg et al. 2011). Consequently, it is not surprising that targeting cytoskeletal reorganisation is an emerging field towards the development of antimetastatic therapies.
Cell motility is dependent of actin polymerisation
The cytoskeleton is an adaptive and dynamic cellular network composed of three distinct species of filamentous structures: microfilaments, intermediate filaments and microtubules. Although all three structures contribute to cell motility, microfilaments or the actin cytoskeleton plays the central role. Cell migration is a multistep process that occurs in response to an external signal detected by receptor proteins located on the cell membrane. The response key event is the dynamic transition of the cellular actin between its monomeric (G-actin) and filamentous (F-actin) form as well as the reorganisation of F-actin to provide the protrusion force for extension of the leading edge establishing polarised cell migration. Indeed, as movement begins, new focal adhesions, resulting from actin polymerisation, are formed at the leading edge that anchor the cell to the substratum. Subsequently, traction force on the substratum followed by actomyosin filament contraction pulls the cell body towards the leading edge and releases the cell rear adhesion (Li et al. 2005). In this process, the concentration in G-actin is of particular importance because association of G-actin at the barbed end near the plasma membrane of the lamellipodium is faster that its dissociation from the pointed end at the rear, resulting therefore in a net polymerisation. In vivo, regulation of this dynamic is dependent of various kinds of actin-binding proteins (ABPs) to regulate local sequestration of G-actin and F-actin depolymerisation. Among the numerous actin-binding proteins (ABPs) that regulate this process, some are of particular interest as they demonstrated tumour suppressor properties; cofilin and profilin are such ABPs and will be the focus of this review.
Cofilin and profilin basic functions
Cofilin belongs to a family of related proteins with similar biochemical activities called the actin depolymerizing factor (ADF)/cofilin family, comprising cofilin 1, cofilin 2 and ADF (also known as destrin) in mammals. Both cofilin 1 and ADF are expressed in non-muscle tissues, with cofilin 1 being the most abundant isoform and ADF accounting for about 5 to 10% of cofilin 1. Therefore, for this review, ‘cofilin’ will refer to cofilin 1. Binding both G-actin and F-actin, cofilin influences actin dynamics in a biphasic manner (Oser and Condeelis 2009): (1) depolymerises or severe F-actin near the pointed ends promoting disassembly and increasing the cellular concentration of G-actin leading consequently to an increase in F-actin turnover and (2) severs F-actin to create free barbed ends that can be used subsequently for actin polymerisation. These two functions are not mutually exclusive and which function predominates depends on the supply of G-actin monomers available for actin polymerisation and therefore will differ among cell types and subcellular compartments.
The supply of G-actin and creation on free barbed ends by cofilin allows F-actin polymerisation by profilin. Profilins are a large family of proteins present in all eukaryotes enabling actin cytoskeletal reorganisation to occur in an ordered and coordinated manner (Pandey and Chaudhary 2017). Profilins are small 15 kDa, structurally conserved proteins consisting of seven beta strands and four alpha helices even though the separate genes for different isoforms share little homology (Fedorov et al. 1994; Fedorov et al. 1997; Krishnan and Moens 2009). All profilin isoforms and splice variants bind to three ligands, actin, proline repeat motifs (PRMs) containing proteins and polyphosphatidylinositol (PPI) lipids with only very rare and tissue-specific exceptions. Since the different profilins have different affinities for the ligands, the function they play in modulating cellular functions in different tissue types also varies (Huang et al. 1996; Lambrechts et al. 1997; Obermann et al. 2005; Polet et al. 2007; Ding and Roy 2013). For simplicity, this review will be limited to discussing profilin 1, referred to as profilin.
One of profilin’s major functions is to maintain a pool of polymerisation-competent ATP-G-actin monomers (Witke 2004; Ding et al. 2012). It does this by accelerating by 1000-fold the nucleotide exchange of ADP to ATP (Goldschmidt-Clermont et al. 1992; Pollard and Borisy 2003; Paavilainen et al. 2004). Profilin has other important regulatory roles in a vast range of other cellular functions through its interaction with PPI lipids (Bae et al. 2010) and polyproline ligands (Ding et al. 2009). It also has a role in regulating the availability of free barbed ends and G-actin concentration giving it additional control of actin polymerisation rates (Rotty et al. 2015; Pernier et al. 2016). How profilin interacts with these ligands will influence the type of F-actin networks formed and thus the development and persistence of one type of protrusive structure over another (e.g. lamellipodia, filopodia or invadopodia), thus influencing cell motility (Lorente et al. 2014).
In malignant cells, the expression of these two actin-binding proteins is altered. Cofilin mRNA and protein levels are increased in various malignant cells (tumour cell lines and tissues from adenocarcinomas, osteosarcoma, lymphoid tissue neoplasms, astrocytoma, glioma and neuroblastoma) in comparison to control cells (for review, see Shishkin et al. 2016). Additionally, recent literature evidence suggests a correlation between dephosphorylated cofilin expression and poor prognosis in breast cancer patients (Maimaiti et al. 2016). Taken together, these results suggest that cofilin is involved in the formation of the malignant phenotype and that this is likely due to an overall misregulation of the cofilin pathway.
In contrast, profilin overexpression in a number of malignancies (breast, hepatic and pancreatic) results in a reduction in cell motility. Conversely, for these cells, reduction in profilin levels results in an increase in metastatic ability (Janke et al. 2000; Roy and Jacobson 2004; Wang et al. 2004; Schoppmeyer et al. 2017). The role of profilin in cancer is elusive and complex as high mRNA expression of profilin in other cancers such as clear cell renal cell carcinoma (Karamchandani et al. 2015) and gastric cancer (Cheng et al. 2015) is correlated with disease progression and poor patient outcomes. Different types of non-tumour tissues may also vary in responses to changes in profilin expression. Reducing profilin expression in human mammary epithelial cells (HMECs) results in enhanced motility (Zou et al. 2007) in contrast to other cell types such as human umbilical vein epithelial cells (HUVECs) that become less motile with lowered profilin expression (Ding et al. 2006). Profilin has a key role in multiple pathways involved in cell fate as changes in profilin expression can up or downregulate the level of expression of proteins involved in motility, proliferation and apoptosis (Coumans et al. 2014) giving it functions beyond its role as a resource for actin polymerisation.
Regulation of cofilin and profilin activity
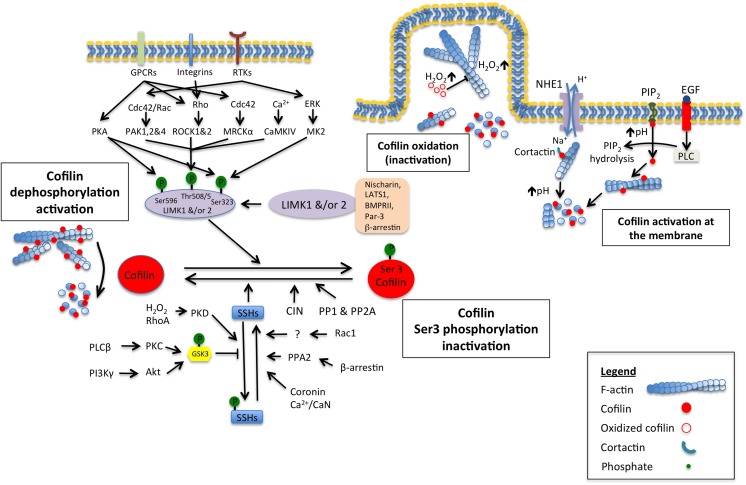
The regulation of cofilin activity is known to occur through multiple mechanisms which can be grouped into two broad categories: (1) phosphorylation/dephosphorylation of its serine 3 residue and (2) binding to phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) (Yonezawa et al. 1990; van Rheenen et al. 2007; Schulte et al. 2013) and cortactin (Oser et al. 2009). Apart from these, other mechanisms have been shown to contribute to the regulation of cofilin activity such as modification of the intracellular pH (Pope et al. 2004) and interaction with proteins such as cyclase-associated protein (CAP) (Zhang et al. 2013), Aip1 (Kueh et al. 2008), β-arrestin (Zoudilova et al. 2007), memo (Meira et al. 2009) and coronin (Cai et al. 2007; Marshall et al. 2009) that predominantly fine tune the primary mechanisms (Fig. 1).
Fig. 1.
Summary of the activation/inactivation mechanisms of cofilin
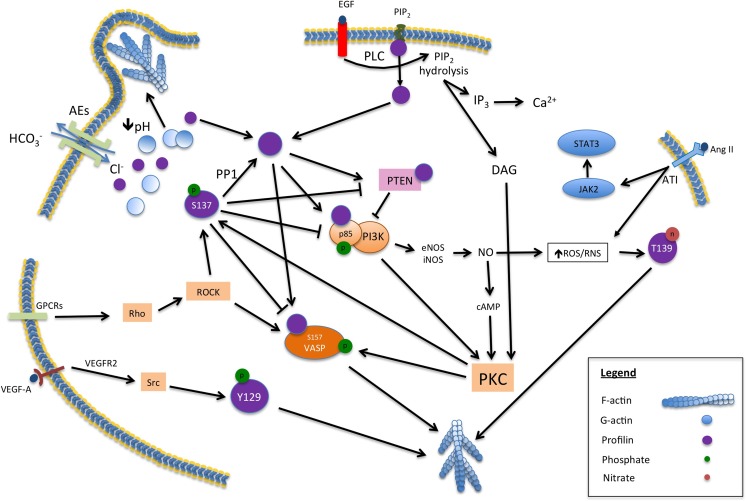
Multiple mechanisms also exist for regulating profilin activity including phosphorylation and binding to PI(4,5)P2, summarised in Fig. 2. Profilin phosphorylation is possible by both serine-threonine or tyrosine kinases and many theoretical or experimental sites have been identified (Hornbeck et al. 2012). While some of these serine-threonine or tyrosine residues are phosphorylated by single pathways, others such as S91, Y128 and S137 are potentially the targets of multiple kinases (summarised in the supplemental material in Gau et al. (2016)). Phosphorylation sites that have been associated with specific diseases or known to modify cellular processes include S137 in cancer (Diamond et al. 2015) and Huntington disease (Shao et al. 2008), Y129 in endothelial cell migration and angiogenesis (Fan et al. 2012) and a potential link in apoptosis for Y139 (Pecar Fonovic and Kos 2015). Phosphorylation of other residues such as T89 that have been shown to occur in vivo may also have functional significance (Gau et al. 2016). The observations that profilin activity in actin polymerisation is regulated by its interactions with PIP2 were made decades ago (Lassing and Lindberg 1985; Lassing and Lindberg 1988). Profilin has an increased affinity for PIP2 in higher density clusters and in this way, PIP2 concentration regulates profilin activity (Senju et al. 2017). Similar to cofilin, changes to intracellular pH alter profilin activity (McLachlan et al. 2007). As profilin binds to polyproline repeat motifs (PRMs) on a plethora of proteins involved in membrane trafficking, focal adhesions, Rac/rho and cdc42 signalling (Witke 2004) multiple mechanisms are involved in fine tuning profilin activity.
Fig. 2.
Summary of the mechanisms altering profilin function
Regulation of cofilin activity by Ser3 phosphorylation
As summarised in Fig. 1, cofilin activity is dependent of the phosphorylation of its serine 3 residue. Therefore, not surprisingly, there is a difference in the cellular distribution of the active/non-phosphorylated and the inactive/phosphorylated forms of cofilin. The active form is mainly found in lamellipodia and invadopodia, whereas the inactive form is more uniformly distributed throughout the cytoplasm, except at the leading edge (Song et al. 2006; Delorme et al. 2007; Bravo-Cordero et al. 2014). These observations are in agreement with the fact that inhibition of cofilin activity leads to defects in cell protrusion, cell polarity and chemotaxis (Mouneimne et al. 2006; Sidani et al. 2007). In mammals, inactivation of cofilin by phosphorylation is dependent of LIM-kinases 1 and 2 (LIMK) or the related testis-specific kinase (TESK) 1 and 2 while its activation by dephosphorylation is mediated by the phosphatase slingshot (SSH) or chronophin (CIN) phosphatase and by the protein phosphatases 1 and 2A (PP1 and PP2A) which promotes actin binding at the G-site (for review, see Bamburg and Wiggan 2002). Consequently, modifying the balance between these enzymes results in alteration of the cofilin activity cycle and a decrease in cell migration.
Role of the kinases
The role of LIMK1 (Bernard 2007; Manetti 2012) and SSH1 (Mizuno 2013) in migration has been extensively studied and reviews describing their structure, regulation and functions have been recently published; therefore, we will only briefly outlined their regulation.
Phosphorylation of the LIMKs threonine residue (Thr-508 in LIMK1 or Thr-505 in LIMK2) located in the activation loop of their kinase domains is thought to regulate their activity. Responsible for this phosphorylation are the downstream kinases ROCK, PAK1, PAK2 and PAK4 of the Rho family small GTPases, RhoA, Rac1, Cdc42 and the Ca2+/calmodulin-dependent protein kinase IV (CaMKIV). Additional mechanisms leading to LIMK1 activation have been identified and included: (1) Ser-323 phosphorylation that is mediated by activation of the p38 MAPK/MK2/LIMK1 pathway by VEGF-A (Kobayashi et al. 2006), (2) Ser-323 and Ser-596 phosphorylation by the cAMP-dependent protein kinase (also termed as protein kinase A, PKA) (Howe and Juliano 2000; Nadella et al. 2009). Negative regulators of LIMK activity have also been identified and include (1) caspase-3 that produces an inactive form of LIMK by proteolysis at the cleavage site (Asp-240) in response to apoptotic stimuli (Nagata et al. 1999; Tomiyoshi et al. 2004), (2) the bone morphogenic protein receptor II (BMPRII) that binds directly to the LIM domain of both LIMK (Lee-Hoeflich et al. 2004), (3) the direct interaction between the tumour suppressor LATS1 and the LIM domains of LIMK1 (Hirota et al. 2000; Yang et al. 2004) as well as with β-arrestin (Zoudilova et al. 2007), (4) the selective binding of the polarity protein Par-3 to LIMK2 (Chen and Macara 2006), (5) the inhibition of PAK1 activation by nischarin protein as well as with their direct association with LIMK (Ding et al. 2008), and by (6) SSH1-mediated dephosphorylation of Thr-508 and autophosphorylated residues (Soosairajah et al. 2005). Additional positive and negative regulators of LIMK activity have been identified and this is nicely reviewed in Manetti (2012).
Role of the phosphatases
The cofilin–phosphatase activity of SSH1 is highly regulated by F-actin binding (Kurita et al. 2008). This observation supports the idea that a high F-actin level in cells increases cofilin activity maintaining therefore the G- and F-actin ratio and contributing to cofilin-mediated actin filament turnover in protrusion structures. In contrast, it has been reported that SSH1 has also F-actin-stabilising and F-actin-bundling activities independently of cofilin–phosphatase activity (Kurita et al. 2007).
F-actin-mediated activation of the cofilin–phosphatase activity of SSH1 is regulated by interactions with 14-3-3 proteins. A model of SSH1 inactivation by phosphorylation of Ser-937 and Ser-978 localised within its C-terminal S domain has been proposed. Upon SSH1 phosphorylation, interaction with 14-3-3 proteins occurs sterically hindering SSH1 binding to F-actin and therefore activation of cofilin (Kurita et al. 2008). Regulation of Ser-937/Ser-978 phosphorylation and dephosphorylation is therefore important in the control of SSH1 activity and members of the PKD family were recently identified as the kinases responsible for these Ser-937/Ser-978 phosphorylations (Eiseler et al. 2009; Peterburs et al. 2009). Moreover, PKD also phosphorylates the conserved Ser-402 of SSH1 within the phosphatase domain providing an additional regulatory mechanism of SSH1 activity (Barisic et al. 2011).
The mechanism of Ser-937/Ser-978 dephosphorylation is unclear; however, β-arrestin has been shown to associate with SSH1, cofilin and PP2A, and may function as a scaffold to promote PP2A-mediated SSH1 dephosphorylation and SSH1-mediated cofilin activation (Xiao et al. 2010). Additionally, inhibition of Rac1 increases the binding of SSHs to 14-3-3 proteins and cofilin phosphorylation, indicating another possible regulatory mechanism of SSH1 dephosphorylation (Kligys et al. 2007). Another mechanism of SSH1 activity regulation involved the phosphatidylinositol 3-kinase (PI3K) pathways which in insulin-stimulated cells promoted accumulation of phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) followed by activation of the downstream effector AKT leading to SSH1 activation and cofilin dephosphorylation (Nishita et al. 2004). Additionally, the insulin receptor substrate-4 (IRS4) has been suggested to play a role in the localised activation of cofilin in membrane protrusions through a direct interaction with SSH1 and activation of PI3K (Homma et al. 2014). In neutrophils stimulated by chemoattractants, it has been shown that cofilin dephosphorylation is SSH2-dependent. Glycogen synthase kinase 3 β (GSK3β) phosphorylates the N-terminal region of SSH2 at Ser-21, Ser-25, Ser-32 and Ser-35 in the N-terminal A subdomain inhibiting its activity. In the presence of chemoattractants, GSK3β is phosphorylated by the PLCβ-PKC and PI3Kb-Akt signalling pathway, which suppresses their kinase activity leading to dephosphorylation and activation of SSH2 (Tang et al. 2011). Additional mechanisms of SSH activation have been identified and involve coronin isoforms (Cai et al. 2007; Marshall et al. 2009) and a channel-Ca2+-calcineurin (CaN) pathway (Wang et al. 2005).
Besides SSH, chronophin (CIN), a haloacid dehalogenase (HAD) phosphatase, has been identified as a specific phosphatase for cofilin (Gohla et al. 2005). Overexpression of CIN is associated with a decrease in cellular F-actin content while catalytically inactive CIN enhanced cellular phospho-cofilin pools and accumulation of polymerised actin. As opposed to SSH, CIN function is independent of a significant activity towards LIMK (Huang et al. 2006).
Regulation of profilin by phosphorylation
Profilin was described as far back as 1988 as a phosphorylation target of protein kinase C (PKC) (Hansson et al. 1988). The S137 residue was the first to be identified (Singh et al. 1996) and is located in the C-terminal α-helix close to the proline repeat recognition domain. Profilin S137 phosphorylation involves pathways downstream of PI3K mediated by PKC (Fig. 2) (Sathish et al. 2004). Although profilin can be phosphorylated by PKC in the presence of PIP2, in the absence of conventional agonists, including DAG, the isoenzyme and lipid specificity of PKC has an impact on the level of profilin phosphorylation. Phosphorylation was found to be maximal with PKCζ2 with a stoichiometry that suggested that only one residue of profilin was phosphorylated by PKC (Vemuri and Singh 2001). This fitted the observation that a single serine residue at the C-terminus of profilin, S137 was specifically phosphorylated by the PKC pathway (Singh et al. 1996). Profilin can interact with regulatory p85α. This interaction results in a feedback loop where the profilin:p85 complex increases PI3Kinase activity (Fig. 2), activating PKC that in turn phosphorylates profilin (Rizwani et al. 2014). This results in the inactivation of profilin functions that depend on PRM binding (Diamond et al. 2015).
Profilin can also be phosphorylated at S137 by the activation of Rho-associated kinase 1 (ROCK1) downstream of Rho-GTPase in an alternate pathway to PKC (Shao et al. 2008) (Fig. 2). The biological significance of profilin S137 phosphorylation by ROCK1 has been demonstrated in conditions such as Huntington disease (HD) and spinobulbar muscular dystrophy (SBMA) (Shao et al. 2008) where profilin phosphorylation lead to agglutination and inhibition of the huntingtin protein (Htt) and androgen receptor (AR), respectively (Diamond et al. 2015).
The balance between phosphorylated and dephosphorylated profilin at S137 is maintained by protein phosphatase-1 (PP1) that specifically targets this residue (Fig. 2) (Shao and Diamond 2012). In breast cancer, the switch between phosphorylated and dephosphorylated states regulates proliferation and survival through interactions with proteins with PRMs (Rizwani et al. 2014; Diamond et al. 2015). The regulation on profilin interactions with PRMs by phosphorylation has different outcomes for the cell depending on subcellular location. In the nucleus of MDA-MB-231 cells, phosphorylated profilin blocks apoptotic pathways leading to tumour promotion but in the cytoplasm, it is unphosphorylated profilin with uncompromised PRM and actin binding activity that is involved in pathways promoting proliferation and survival (Diamond et al. 2015).
Oligomerisation of profilin alters its susceptibility to phosphorylation; however, the in vivo function of this oligomerisation remains elusive. Profilin dimers are resistant to phosphorylation by PKC as the monomers interface with each other through their carboxy termini, effectively hiding the S137 residue. (Korupolu et al. 2009). In profilin tetramers, the S137 residues are exposed and while the tetramers have reduced PLP binding, they are preferentially phosphorylated over monomers (Korupolu et al. 2009). Tetramers and dimers have been reported in cell extracts (Babich et al. 1996; Skare et al. 2003) and tetramers in solution (Rennella et al. 2017) but the studies to determine if oligomerisation is biologically relevant in altering S137 phosphorylation are still to be carried out. Oxidising conditions are known to promote profilin oligomerisation (Mittermann et al. 1998) and profilin is upregulated by PKC/NF-κB pathways in response to reactive oxygen species (ROS) (Li et al. 2013). Profilin is linked to the oxidative metabolism of whole organisms (Pae and Romeo 2014) and individual cells (Li et al. 2013; Yao et al. 2014) so it is conceivable that under oxidative conditions that favour oligomerisation, profilin phosphorylation could be altered.
Profilin functions are also regulated by tyrosine phosphorylation at the Y129 residue (Fig. 2). This residue is part of the actin binding site and Y129 phosphorylation accelerates actin polymerisation rates by increasing the rate of nucleotide exchange and the affinity of profilin for actin (Fan et al. 2012). In endothelial cells, Y129 phosphorylation occurs after stimulation of vascular endothelial growth factor receptor kinase 2 (VEGFR2) by the growth factor VEGF-A at the leading edge of the cytoplasm. Both VEGFR2 and its downstream target kinase, Src, phosphorylate profilin at Y129 in response to VEGF-A. VEGF/Src phosphorylated profilin is important in endothelial tissue repair by promoting rapid actin polymerisation. This is an example of a very specific upregulation of profilin activity as this pathway is important in adults as a repair mechanism but is not involved in developmental angiogenesis (Fan et al. 2012). In the brain tumour, glioblastoma multiforme VEGF/Src phosphorylation of profilin at Y129 allows the formation of a complex between profilin and von Hippel-Linau (VHL) protein, preventing VHL degrading the transcription factor hypoxia-induced factor 1 alpha (HIF-1α). HIF-1α upregulates angiogenic factors including VEGF, resulting in further phosphorylation of profilin. Profilin phosphorylation is part of the feed forward mechanism in which the tumour drives aberrant vascularisation by the induction of HIF-1α. (Fan et al. 2014).
Regulation of cofilin and profilin by miRs
Cellular localisation of cofilin, profilin and other actin binding proteins is also regulated by miRs such as miR-92. The miR-17-92 cluster is highly expressed in metastatic tumours. By altering its expression with docosahexaenoic acid (DHA) treatment, the lung cancer cell line A549 changed the expression and cellular location of profilin 1 and cofilin 1 and VASP in a manner that mirrors the changes in distribution that occur in a solid tumour (Ali et al. 2016). In normal tissue adjacent to the tumour, profilin was predominantly cytoplasmic but the cells at the border of the lesion undergoing rapid actin turnover and migration into the interstitial area increased profilin accumulation both at the plasma membrane and nucleus. In the densely packed mid tumour section, profilin was concentrated at the nucleus. In A549 cells, DHA treatment decreased miR-17-92 expression that resulted in reduced phosphorylation of VASPS157 by PKC downstream of cAMP signalling (Ali et al. 2016). cAMP is elevated by NO (Wentworth et al. 2006). VASPS157 is pro-metastatic and anti-apoptotic and associates with profilin (Fig. 2). DHA also promoted S239 phosphorylation of VASP by PKG. VASPS239 is associated with cofilin and promotes cytoskeletal stability and apoptosis. By attenuating the expression of miR-17-92, DHA altered the cellular localisation of profilin/VASPS157 with a shift from the cytoplasm to the nucleus, while it increased the distribution of cofilin/VASPS239 at the cell boundary edge from the nucleus. The result of the downregulation of miR-17-92 was a decreased F-actin content of the cells and increased apoptosis. (Ali et al. 2016). The effects of increasing the nuclear concentration of profilin are unknown. Profilin has specific functions in the nucleus including pre-mRNA splicing (Skare et al. 2003), acting as an adaptor between actin and the exportin mechanism (Stuven et al. 2003) and is involved in transcription (Lederer et al. 2005). The role of the differential phosphorylation of VASP on nuclear profilin accumulation will be interesting to follow up. Alternate pathways to regulate cofilin and profilin exist as VASPS157 is phosphorylated by ROCK and VASPS239 by PKC (Wentworth et al. 2006).
Regulation by PI(4,5)P2 binding
Phosphoinositides, and principally PI(4,5)P2, have been recognised as essential players in the regulation of the mechanical properties of the cell membrane because they form the interface between the cytoskeleton and the plasma membrane. Biochemical studies have demonstrated that activities of numerous actin-binding proteins, including profilin and cofilin, can be regulated by direct interaction with PI(4,5)P2 (Tsujita and Itoh 2014; Senju et al. 2017). The plasma membrane is not homogenous and its PPIs exist as freely diffusing single molecules, small transient clusters, and also can form larger more stable aggregates (Levental et al. 2009; Kwiatkowska 2010; Wang and Richards 2012; Delage et al. 2013). Separate pools of PPIs are maintained by synthesis by inositide kinases and degradation by phosphatases that allows rapid but localised changes of PI(4,5)P2 concentration to spatially and temporally coordinate cellular responses (Krauss and Haucke 2007; Sun et al. 2013). Cofilin and profilin increased but still relatively low affinity for higher PI(4,5)P2 densities allows it to be recruited to and concentrated at sites of high actin polymerisation in a way that allows for rapid dynamics (Senju et al. 2017).
Binding of PI(4,5)P2 to cofilin leads to its reversible inactivation at the membrane. In vivo evidence using FRET- and FLIP-based experiments on mammary carcinoma cells revealed that EGF induces the release of cofilin from the membrane via phospholipase C (PLC)-mediated PI(4,5)P2 hydrolysis. Upon release, cofilin binds to and severs F-actin, creating free barbed ends for actin polymerisation (van Rheenen et al. 2007) (Fig. 1). Recently, the scaffolding protein memo was shown to amplify this mechanism (Meira et al. 2009).
The importance of profilin:PI(4,5)P2 interactions were made clear with the observation that the actin polymerisation activity of profilin is regulated by binding to PI(4,5)P2 (Lassing and Lindberg 1985; Lassing and Lindberg 1988). Membrane and cytoskeletal proteins are affected by alterations of membrane phosphoinositide concentration through activation of phospholipase C (PLC) and class I PI3Ks (Viaud et al. 2016). PI(4,5)P2 hydrolysis by PLC generates the second messengers inositol 1,4,5-triphosphate (IP3) and diacetyl glycerol (DAG) (Fig. 2). DAG activates protein kinase C (PKC) cascades and IP3 induces Ca2+ intracellular fluxes and so PI(4,5)P2 hydrolysis generates signals down two parallel pathways (Janmey and Lindberg 2004). Profilin bound to PI(4,5)P2 prevents its hydrolysis by unphosphorylated PLCγ but when EGF stimulates its receptor tyrosine kinase the resulting phosphorylation of PLC overcomes this inhibition (Goldschmidt-Clermont et al. 1991). As PLC enzymes are critical for maintaining intracellular calcium stores as well as the overall PI(4,5)P2/PI(3,4,5)P3 balance, profilin has emerged as a key regulator of cellular function (Jockusch et al. 2007). Phosphorylation of PI(4,5)P2 by PI3K class 1 enzymes produces PI(3,4,5)P3 that in turn is the source of PI(3,4)P2. Both these PPIs control different branches of PI3K signalling as well as interacting with profilin and other regulatory proteins (Li and Marshall 2015; Viaud et al. 2016).
Another important profilin interacting enzyme in phosphoinositide metabolism is phosphatase and tensin homologue 10 (PTEN) that converts PI(3,4,5)P3 back to PI(4,5)P2 (Fig. 2) (Das et al. 2009; Zaidi and Manna 2016). PTEN is an antagonist of PI3K enzymes and acts as a tumour suppressor agent by limiting PI(3,4)P2 availability (Gericke et al. 2013). Profilin binds to a PRM on PTEN preventing PTEN degradation by ubiquitination (Zaidi and Manna 2016). As the PRM binding site on profilin is itself sensitive to phosphorylation (Shao et al. 2008; Shao and Diamond 2012; Diamond et al. 2015), the complexity of relationship between the different profilin ligand binding sites emerges as inactivation of the PRM binding site has the potential to alter phosphoinositide membrane composition.
Profilin has two regions that bind PPIs electrostatically that overlap with both the actin and PRM binding domains (Lambrechts et al. 2002; Skare and Karlsson 2002) and profilin can potentially bind up to five inositol headgroups (Ostrander et al. 1995; Richer et al. 2008). Both actin (Lassing and Lindberg 1985; Lassing and Lindberg 1988) and PRMs (Lambrechts et al. 1997) compete with PPIs for binding to profilin and therefore regulate its activities. The PPI binding sites form part of a broad surface of exposed hydrophobic residues so changes in pH and fluxes in local ion concentrations have a role in regulating profilin:PPI interaction (for reviews, see Witke (2004) and Krishnan and Moens (2009)). In vitro profilin has a higher affinity for micellar PI(3,4)P2 (KD = 1.1 μM) and PI(3,4,5)P3 (KD = 5.7 μM) than for PI(4,5)P2 (KD = 11 μM) (Lu et al. 1996) or for submicellar PI(4,5)P2 (KD = 985 μM) concentrations (Moens and Bagatolli 2007). Despite its higher affinity for the rarer PPIs, the relative abundance of PI(4,5)P2 is the reason it has been proposed as the predominant membrane ligand of profilin (Ding et al. 2012).
Regulation through pH
Cofilin activities are affected by modification of the intracellular pH and this is highly dependent of the recruitment and activation of the sodium hydrogen exchanger NHE1. With respect to its actin severing and filament depolymerisation, it has been shown that cofilin is more potent at pH 8 than pH 6.5 (Yeoh et al. 2002). Secondly, the interaction between cofilin and PI(4,5)P2 is sensitive to pH as a local increase in pH at the cytoplasmic side of the plasma membrane facilitate PLC hydrolysis of PI(4,5) P2 leading to a decrease of clustering interaction between cofilin and PI(4,5)P2 (Zhao et al. 2010). Finally, local pH variations influence the binding of cofilin to cortactin. In invadopodia, binding of cortactin to cofilin negatively regulates cofilin activity. A key step in this interaction is tyrosine phosphorylation of cortactin after EGF stimulation (Oser and Condeelis 2009; Oser et al. 2009; Mader et al. 2011). The mechanism linking cortactin phosphorylation to cofilin activity has been described recently and also involved the activation in invadopodia of the pH-dependent key regulator NHE1. Therefore, cortactin phosphorylation induces a NHE1-dependent pH increase that disrupts cortactin binding to cofilin promoting its severing activities and resulting in the creation of free barbed ends that can be used for filament elongation (Magalhaes et al. 2011) (Fig. 1).
Profilin undergoes a partial unfolding at pH 6.4 that leads to a decrease in binding to both actin and PRMs, increasing the concentration of G-actin. (McLachlan et al. 2007). While a potential increase in the supply of G-actin molecules would favour ARP2/3 nucleation of branched actin filaments (Rotty et al. 2015; Pernier et al. 2016) (Fig. 2), the biological relevance of a low pH mechanism in regulating profilin/actin dynamics is not fully established. In motile cells, the pH gradients formed by the asymmetric distribution of NHE1 along the axis of movement of cells change from pH 7.6 to 6.9 from leading to trailing edges (Martin et al. 2011; Angelova et al. 2018) and so are still too high. The cytosol of apoptotic cells decreases to pH 5.7 due to H+ ions released from lysosomes (Nilsson et al. 2006). This pH is low enough to decrease profilin binding (McLachlan et al. 2007). Intracellular pH may be lowered by specific mechanisms such as Cl-/HCO3− anion exchangers (AEs) or through metabolic activity (Casey et al. 2010). Cytosol acidification is driven in part by the negatively charged inner leaflet of the plasma membrane attracting positively charged protons and causing the efflux of bases. As protons diffuse slowly due to the high number of interactions with fixed or slowly motile macromolecules, pH gradients may form (Casey et al. 2010). In the environment immediately adjacent to the inner leaflet of the plasma membrane, the pH may drop by up to 2 pH units so the alterations in profilin binding may be very localised (McLachlan et al. 2007).
Additional regulatory mechanisms
Oxidative stress
Recently, it has been shown that the higher H2O2 concentration at membranes and cell protrusions of migrating cells induces oxidation of cofilin cysteines 139 (C139) and 147 (C147) resulting in an inhibition of cofilin activity consequently enhancing either or both F-actin stability and net actin polymerisation at the leading edge and consequently affecting cell motility (Cameron et al. 2015) (Fig. 1).
Finally, the efficiency of cofilin actin binding and severing activity has been reported to be dependent of the mechanical forces that affect the structure and dynamics of the actin cytoskeleton (Hayakawa et al. 2011; Tojkander et al. 2015). Indeed, it appears that cofilin bind preferentially to less-tensile F-actin mediating their degradation, whereas F-actin filaments under tension are protected from effective severing.
One of the markers of oxidative damage in cells is the appearance of tyrosine nitrated proteins (Starr et al. 2011) and under oxidative stress profilin can become nitrated at the C-terminal T139 by peroxynitrite (Fig. 2) (Kasina et al. 2005). In vitro, the concentration of nitro-profilin increased with the level of peroxynitrite (Kasina et al. 2005) and in platelets, inducible nitric oxide synthase (iNOS) downstream of PI3K also increased profilin nitration (Kasina et al. 2006). Nitrated profilin increased its affinity to PRMs 20-fold and also decreased the critical concentration of actin from 250 to 150 nM but had no effect on the affinity to PI(4,5)P2 so the effect on profilin-PRM interactions and actin dynamics is profound (Kasina et al. 2005). In mice with systemic inflammation induced by lipopolysaccharide (LPS), the peroxynitrite produced by the reaction of superoxide released by neutrophils with NO breaks down to toxic ROS and reactive nitrogen species (RNS) (Starr et al. 2011). Susceptibility to oxidative/nitrosative damage increased with age and older mice expressed less of the protective superoxide dismutase (SOD) but more iNOS than young ones. Therefore, the aged mice had higher levels of nitrated profilin and other proteins in the lung tissue examined. This suggested that the loss of function to profilin and several other proteins involved in actin cytoskeleton regulation was due to nitration and contributed to the pathology (Starr et al. 2011).
Since the observation that overexpressing profilin increased vascular hypertrophy and hypertension (Moustafa-Bayoumi et al. 2007) much work has been done in understanding the link between oxidative stress and profilin. Activation of angiotensin (Ang) II type 1 receptor (AT1) by Ang II leads to the increased expression of profilin (Fig. 2), reduction of angiotensin converting enzyme 2 (ACE2), increased profilin/ERK and PI3K signalling cascades leading to the production of peroxynitrite and endothelial nitrous oxide synthase (eNOS) (Jin et al. 2012). In spontaneously hypertensive rats (SHR), the vascular hypertrophy was linked to an overexpression of profilin modulated by AT1 (Zhong et al. 2011). This phenotype was also associated with a reduction of ACE2 and elevated ERK1/2 and JNK phosphorylation. Similar results were obtained in human umbilical artery smooth muscle cells where profilin expression was induced by Ang II in a dose-dependent fashion. Profilin expression mediated by Ang II was also blocked when the JAK2/STAT3 pathway was inhibited (Fig. 2) (Cheng et al. 2011). The relationship of profilin, Ang II and ACE2 in mouse aortic tissue is interesting. Ang II treatment of the mice led to the decrease in ACE2 activity and increased profilin expression and oxidative damage. Treating the mice with Ang II and depleting ACE2 even further increased profilin expression, signalling through PI3K and ERK pathways to increase eNOS expression and phosphorylation leading to peroxynitrite formation (Jin et al. 2012).
Post-translational regulation of profilin
Changes in cellular concentration of profilin may also be the result of post-translation events. In Huntington’s disease, lower profilin levels in vivo corresponded to a more severe diagnosis with increased aggregation of the huntingtin protein (htt). Although mRNA transcripts of profilin 1 in mutant htt PC12 cells was unaltered from controls, and profilin 2a even increased, htt accumulation stimulated profilin clearance by the ubiquitin proteasome system with a reduction of F/G-actin ratio (Burnett et al. 2008). This is also relevant to breast cancer as for MDA-MB-231 cells, ubiquitination of profilin by the C-terminus of Hsc-70 interacting protein (CHIP) regulated profilin levels by targeting a proportion of ubiquitinated profilin to the proteasome. MDA-MB-231 cells depleted of CHIP had increased profilin levels and decreased motility (Choi et al. 2014) consistent with earlier studies (Bae et al. 2010). In contrast in ovarian cancer mutations in breast cancer susceptibility gene 1 (BRAC1) alters profilin expression (and cofilin) possibly by disruption of the ubiquitination pathways (Gau et al. 2015). Similarly, in pulmonary hypertension hypoxia upregulated profilin expression by the decreased targeting of profilin by the proteasome. This resulted in increased actin polymerisation and hence increased vascular hypertrophy (Zhao et al. 2017).
Conclusion
Cofilin and profilin have individually received continued interest in the last two decades due in part to their potential roles in cancer aggressiveness. However, to our knowledge, no studies have been investigating the dynamics between these two proteins and how these affect cell motility and cancer metastasis.
It is interesting to note that activation of the Rho/Rock pathway leads to phosphorylation of both cofilin and profilin. This results in seemingly opposite effects with a decreased actin polymerisation due to profilin and an inactivation of cofilin, which would favour filament persistence. Both cofilin and profilin compete for binding with PPI at the plasma membrane and both can be dissociated from the membrane by PLC mediated hydrolysis of PIP2 following activation by EGF. The dynamics of these interactions are currently unknown. The release of cofilin could increase the severing of the actin filaments while profilin would have the opposite effect possibly increasing the treadmill assembly/disassembly of actin filaments. Modulation of these effects could depend on pH gradient difference between cell regions. On the one hand, a lower pH at the membrane could dissociate profilin from G-actin increasing actin nucleation and filament formations. Free profilin could then interact through its PRM binding domains also promoting F-actin formation. On the other hand, low pH at the membrane would maintain cofilin inhibition by cortactin while further away, this inhibition could decrease as the pH increases resulting in filament severing. Finally, oxidative stress has a synergistic effect on both cofilin and profilin with the inhibition of cofilin following oxidation and the activation of profilin by nitration resulting in the promotion of filament formations. It is clear that much is still to be learned about the interplay between these two actors and how these proteins control cell motility in vivo and their deregulations in cancer and other diseases.
Conflict of interest
Joelle VF Coumans declares that she has no conflicts of interest. Rhonda J Davey declares that she has no conflicts of interest. Pierre DJ Moens declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Joelle V. F. Coumans and Rhonda J. Davey have participated equally in the writing of this review.
References
- Ali M, Heyob K, Jacob NK, Rogers LK. Alterative expression and localization of profilin 1/VASPpS157 and Cofilin 1/VASPpS239 regulates metastatic growth and is modified by DHA supplementation. Mol Cancer Ther. 2016;15(9):2220–2231. doi: 10.1158/1535-7163.MCT-16-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova Miglena I., Bitbol Anne-Florence, Seigneuret Michel, Staneva Galya, Kodama Atsuji, Sakuma Yuka, Kawakatsu Toshihiro, Imai Masayuki, Puff Nicolas. pH sensing by lipids in membranes: The fundamentals of pH-driven migration, polarization and deformations of lipid bilayer assemblies. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2018;1860(10):2042–2063. doi: 10.1016/j.bbamem.2018.02.026. [DOI] [PubMed] [Google Scholar]
- Babich M, Foti LR, Sykaluk LL, Clark CR. Profilin forms tetramers that bind to G-actin. Biochem Biophys Res Commun. 1996;218(1):125–131. doi: 10.1006/bbrc.1996.0022. [DOI] [PubMed] [Google Scholar]
- Bae YH, Ding Z, Das T, Wells A, Gertler F, Roy P. Profilin1 regulates PI(3,4)P2 and lamellipodin accumulation at the leading edge thus influencing motility of MDA-MB-231 cells. Proc Natl Acad Sci U S A. 2010;107(50):21547–21552. doi: 10.1073/pnas.1002309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, Wiggan OP. ADF/cofilin and actin dynamics in disease. Trends Cell Biol. 2002;12(12):598–605. doi: 10.1016/s0962-8924(02)02404-2. [DOI] [PubMed] [Google Scholar]
- Barisic S, Nagel AC, Franz-Wachtel M, Macek B, Preiss A, Link G, Maier D, Hausser A. Phosphorylation of Ser 402 impedes phosphatase activity of slingshot 1. EMBO Rep. 2011;12(6):527–533. doi: 10.1038/embor.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39(6):1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Bravo-Cordero Jose Javier, Hodgson Louis, Condeelis John S. Spatial regulation of tumor cell protrusions by RhoC. Cell Adhesion & Migration. 2014;8(3):263–267. doi: 10.4161/cam.28405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett BG, Andrews J, Ranganathan S, Fischbeck KH, Di Prospero NA. Expression of expanded polyglutamine targets profilin for degradation and alters actin dynamics. Neurobiol Dis. 2008;30(3):365–374. doi: 10.1016/j.nbd.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Marshall TW, Uetrecht AC, Schafer DA, Bear JE. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell. 2007;128(5):915–929. doi: 10.1016/j.cell.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JM, Gabrielsen M, Chim YH, Munro J, McGhee EJ, Sumpton D, Eaton P, Anderson KI, Yin H, Olson MF. Polarized cell motility induces hydrogen peroxide to inhibit cofilin via cysteine oxidation. Curr Biol. 2015;25(11):1520–1525. doi: 10.1016/j.cub.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11(1):50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J Cell Biol. 2006;172(5):671–678. doi: 10.1083/jcb.200510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JF, Ni GH, Chen MF, Li YJ, Wang YJ, Wang CL, Yuan Q, Shi RZ, Hu CP, Yang TL. Involvement of profilin-1 in angiotensin II-induced vascular smooth muscle cell proliferation. Vasc Pharmacol. 2011;55(1–3):34–41. doi: 10.1016/j.vph.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Zhu ZX, Zhou JS, Hu ZQ, Zhang JP, Cai QP, Wang LH. Silencing profilin-1 inhibits gastric cancer progression via integrin beta1/focal adhesion kinase pathway modulation. World J Gastroenterol. 2015;21(8):2323–2335. doi: 10.3748/wjg.v21.i8.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YN, Lee SK, Seo TW, Lee JS, Yoo SJ. C-terminus of Hsc70-interacting protein regulates profilin1 and breast cancer cell migration. Biochem Biophys Res Commun. 2014;446(4):1060–1066. doi: 10.1016/j.bbrc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Gau D, Poljak A, Wasinger V, Roy P, Moens PD. Profilin-1 overexpression in MDA-MB-231 breast cancer cells is associated with alterations in proteomics biomarkers of cell proliferation, survival, and motility as revealed by global proteomics analyses. OMICS. 2014;18(12):778–791. doi: 10.1089/omi.2014.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Bae YH, Wells A, Roy P. Profilin-1 overexpression upregulates PTEN and suppresses AKT activation in breast cancer cells. J Cell Physiol. 2009;218(2):436–443. doi: 10.1002/jcp.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage E, Puyaubert J, Zachowski A, Ruelland E. Signal transduction pathways involving phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: convergences and divergences among eukaryotic kingdoms. Prog Lipid Res. 2013;52(1):1–14. doi: 10.1016/j.plipres.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, Bokoch GM. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell. 2007;13(5):646–662. doi: 10.1016/j.devcel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MI, Cai S, Boudreau A, Carey CJ, Jr, Lyle N, Pappu RV, Swamidass SJ, Bissell M, Piwnica-Worms H, Shao J. Subcellular localization and Ser-137 phosphorylation regulate tumor-suppressive activity of profilin-1. J Biol Chem. 2015;290(14):9075–9086. doi: 10.1074/jbc.M114.619874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Roy P (2013) Profilin-1 versus profilin-2: two faces of the same coin? Breast Cancer Res 15(3):311. 10.1186/bcr3433 [DOI] [PMC free article] [PubMed]
- Ding Z, Lambrechts A, Parepally M, Roy P. Silencing profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J Cell Sci. 2006;119(Pt 19):4127–4137. doi: 10.1242/jcs.03178. [DOI] [PubMed] [Google Scholar]
- Ding Y, Milosavljevic T, Alahari SK. Nischarin inhibits LIM kinase to regulate cofilin phosphorylation and cell invasion. Mol Cell Biol. 2008;28(11):3742–3756. doi: 10.1128/MCB.01832-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Gau D, Deasy B, Wells A, Roy P. Both actin and polyproline interactions of profilin-1 are required for migration, invasion and capillary morphogenesis of vascular endothelial cells. Exp Cell Res. 2009;315(17):2963–2973. doi: 10.1016/j.yexcr.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Bae YH, Roy P. Molecular insights on context-specific role of profilin-1 in cell migration. Cell Adhes Migr. 2012;6(5):442–449. doi: 10.4161/cam.21832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseler T, Doppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009;11(5):545–556. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Arif A, Gong Y, Jia J, Eswarappa SM, Willard B, Horowitz A, Graham LM, Penn MS, Fox PL. Stimulus-dependent phosphorylation of profilin-1 in angiogenesis. Nat Cell Biol. 2012;14(10):1046–1056. doi: 10.1038/ncb2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Potdar AA, Gong Y, Eswarappa SM, Donnola S, Lathia JD, Hambardzumyan D, Rich JN, Fox PL. Profilin-1 phosphorylation directs angiocrine expression and glioblastoma progression through HIF-1alpha accumulation. Nat Cell Biol. 2014;16(5):445–456. doi: 10.1038/ncb2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov AA, Pollard TD, Almo SC. Purification, characterization and crystallization of human platelet profilin expressed in Escherichia coli. J Mol Biol. 1994;241(3):480–482. doi: 10.1006/jmbi.1994.1522. [DOI] [PubMed] [Google Scholar]
- Fedorov AA, Ball T, Mahoney NM, Valenta R, Almo SC. The molecular basis for allergen cross-reactivity: crystal structure and IgE-epitope mapping of birch pollen profilin. Structure. 1997;5(1):33–45. doi: 10.1016/s0969-2126(97)00164-0. [DOI] [PubMed] [Google Scholar]
- Gau DM, Lesnock JL, Hood BL, Bhargava R, Sun M, Darcy K, Luthra S, Chandran U, Conrads TP, Edwards RP, Kelley JL, Krivak TC, Roy P. BRCA1 deficiency in ovarian cancer is associated with alteration in expression of several key regulators of cell motility - a proteomics study. Cell Cycle. 2015;14(12):1884–1892. doi: 10.1080/15384101.2015.1036203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau David, Veon William, Zeng Xuemei, Yates Nathan, Shroff Sanjeev G., Koes David R., Roy Partha. Threonine 89 Is an Important Residue of Profilin-1 That Is Phosphorylatable by Protein Kinase A. PLOS ONE. 2016;11(5):e0156313. doi: 10.1371/journal.pone.0156313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, Leslie NR, Losche M, Ross AH. PtdIns(4,5)P2-mediated cell signaling: emerging principles and PTEN as a paradigm for regulatory mechanism. Adv Exp Med Biol. 2013;991:85–104. doi: 10.1007/978-94-007-6331-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohla A, Birkenfeld J, Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7(1):21–29. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont PJ, Kim JW, Machesky LM, Rhee SG, Pollard TD. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont PJ, Furman MI, Wachsstock D, Safer D, Nachmias VT, Pollard TD. The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 1992;3(9):1015–1024. doi: 10.1091/mbc.3.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A, Skoglund G, Lassing I, Lindberg U, Ingelman-Sundberg M. Protein kinase C-dependent phosphorylation of profilin is specifically stimulated by phosphatidylinositol bisphosphate (PIP2) Biochem Biophys Res Commun. 1988;150(2):526–531. doi: 10.1016/0006-291x(88)90425-1. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J Cell Biol. 2011;195(5):721–727. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J Cell Biol. 2000;149(5):1073–1086. doi: 10.1083/jcb.149.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma Y, Kanno S, Sasaki K, Nishita M, Yasui A, Asano T, Ohashi K, Mizuno K. Insulin receptor substrate-4 binds to Slingshot-1 phosphatase and promotes cofilin dephosphorylation. J Biol Chem. 2014;289(38):26302–26313. doi: 10.1074/jbc.M114.565945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P. V., Kornhauser J. M., Tkachev S., Zhang B., Skrzypek E., Murray B., Latham V., Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Research. 2011;40(D1):D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AK, Juliano RL. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol. 2000;2(9):593–600. doi: 10.1038/35023536. [DOI] [PubMed] [Google Scholar]
- Huang S, McDowell JM, Weise MJ, Meagher RB. The Arabidopsis profilin gene family. Evidence for an ancient split between constitutive and pollen-specific profilin genes. Plant Physiol. 1996;111(1):115–126. doi: 10.1104/pp.111.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18(1):26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Janke J, Schluter K, Jandrig B, Theile M, Kolble K, Arnold W, Grinstein E, Schwartz A, Estevez-Schwarz L, Schlag PM, Jockusch BM, Scherneck S. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191(10):1675–1686. doi: 10.1084/jem.191.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Lindberg U. Cytoskeletal regulation: rich in lipids. Nat Rev Mol Cell Biol. 2004;5(8):658–666. doi: 10.1038/nrm1434. [DOI] [PubMed] [Google Scholar]
- Jin Hai-Yan, Song Bei, Oudit Gavin Y., Davidge Sandra T., Yu Hui-Min, Jiang Yan-Yan, Gao Ping-Jin, Zhu Ding-Liang, Ning Guang, Kassiri Zamaneh, Penninger Josef M., Zhong Jiu-Chang. ACE2 Deficiency Enhances Angiotensin II-Mediated Aortic Profilin-1 Expression, Inflammation and Peroxynitrite Production. PLoS ONE. 2012;7(6):e38502. doi: 10.1371/journal.pone.0038502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch BM, Murk K, Rothkegel M. The profile of profilins. Rev Physiol Biochem Pharmacol. 2007;159:131–149. doi: 10.1007/112_2007_704. [DOI] [PubMed] [Google Scholar]
- Karamchandani JR, Gabril MY, Ibrahim R, Scorilas A, Filter E, Finelli A, Lee JY, Ordon M, Pasic M, Romaschin AD, Yousef GM. Profilin-1 expression is associated with high grade and stage and decreased disease-free survival in renal cell carcinoma. Hum Pathol. 2015;46(5):673–680. doi: 10.1016/j.humpath.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Kasina S, Rizwani W, Radhika KV, Singh SS. Nitration of profilin effects its interaction with poly (L-proline) and actin. J Biochem. 2005;138(6):687–695. doi: 10.1093/jb/mvi163. [DOI] [PubMed] [Google Scholar]
- Kasina S, Wasia R, Fasim A, Radhika KV, Singh SS. Phorbol ester mediated activation of inducible nitric oxide synthase results in platelet profilin nitration. Nitric Oxide. 2006;14(1):65–71. doi: 10.1016/j.niox.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Kligys K, Claiborne JN, DeBiase PJ, Hopkinson SB, Wu Y, Mizuno K, Jones JC. The slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization, and motility behavior of keratinocytes. J Biol Chem. 2007;282(44):32520–32528. doi: 10.1074/jbc.M707041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25(4):713–726. doi: 10.1038/sj.emboj.7600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korupolu RV, Achary MS, Aneesa F, Sathish K, Wasia R, Sairam M, Nagarajaram HA, Singh SS. Profilin oligomerization and its effect on poly (L-proline) binding and phosphorylation. Int J Biol Macromol. 2009;45(3):265–273. doi: 10.1016/j.ijbiomac.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Krauss M, Haucke V. Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO Rep. 2007;8(3):241–246. doi: 10.1038/sj.embor.7400919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Moens PDJ. Structure and functions of profilins. Biophys Rev. 2009;1(2):71–81. doi: 10.1007/s12551-009-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh HY, Charras GT, Mitchison TJ, Brieher WM. Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers. J Cell Biol. 2008;182(2):341–353. doi: 10.1083/jcb.200801027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita S, Gunji E, Ohashi K, Mizuno K. Actin filaments-stabilizing and -bundling activities of cofilin-phosphatase Slingshot-1. Genes Cells. 2007;12(5):663–676. doi: 10.1111/j.1365-2443.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- Kurita S, Watanabe Y, Gunji E, Ohashi K, Mizuno K. Molecular dissection of the mechanisms of substrate recognition and F-actin-mediated activation of cofilin-phosphatase Slingshot-1. J Biol Chem. 2008;283(47):32542–32552. doi: 10.1074/jbc.M804627200. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska K. One lipid, multiple functions: how various pools of PI(4,5)P(2) are created in the plasma membrane. Cell Mol Life Sci. 2010;67(23):3927–3946. doi: 10.1007/s00018-010-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts A, Verschelde JL, Jonckheere V, Goethals M, Vandekerckhove J, Ampe C. The mammalian profilin isoforms display complementary affinities for PIP2 and proline-rich sequences. EMBO J. 1997;16(3):484–494. doi: 10.1093/emboj/16.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts Anja, Jonckheere Veronique, Dewitte Daisy, Vandekerckhove Joel, Ampe Christophe. BMC Biochemistry. 2002;3(1):12. doi: 10.1186/1471-2091-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I, Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Lassing I, Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Lederer M, Jockusch BM, Rothkegel M. Profilin regulates the activity of p42POP, a novel Myb-related transcription factor. J Cell Sci. 2005;118(Pt 2):331–341. doi: 10.1242/jcs.01618. [DOI] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J. 2004;23(24):4792–4801. doi: 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental I, Christian DA, Wang YH, Madara JJ, Discher DE, Janmey PA. Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry. 2009;48(34):8241–8248. doi: 10.1021/bi9007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Marshall AJ. Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: a distinct branch of PI3K signaling. Cell Signal. 2015;27(9):1789–1798. doi: 10.1016/j.cellsig.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Li S, Guan JL, Chien S. Biochemistry and biomechanics of cell motility. Annu Rev Biomed Eng. 2005;7:105–150. doi: 10.1146/annurev.bioeng.7.060804.100340. [DOI] [PubMed] [Google Scholar]
- Li Zhenyu, Zhong Qiaoqing, Yang Tianlun, Xie Xiumei, Chen Meifang. The role of profilin-1 in endothelial cell injury induced by advanced glycation end products (AGEs) Cardiovascular Diabetology. 2013;12(1):141. doi: 10.1186/1475-2840-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente Gisela, Syriani Emilio, Morales Miguel. Actin Filaments at the Leading Edge of Cancer Cells Are Characterized by a High Mobile Fraction and Turnover Regulation by Profilin I. PLoS ONE. 2014;9(1):e85817. doi: 10.1371/journal.pone.0085817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PJ, Shieh WR, Rhee SG, Yin HL, Chen CS. Lipid products of phosphoinositide 3-kinase bind human profilin with high affinity. Biochemistry. 1996;35(44):14027–14034. doi: 10.1021/bi961878z. [DOI] [PubMed] [Google Scholar]
- Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71(5):1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes MA, Larson DR, Mader CC, Bravo-Cordero JJ, Gil-Henn H, Oser M, Chen X, Koleske AJ, Condeelis J. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 2011;195(5):903–920. doi: 10.1083/jcb.201103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaiti Y, Liu Z, Tan J, Abudureyimu K, Huang B, Liu C, Guo Y, Wang C, Nie X, Zhou J, Huang T. Dephosphorylated cofilin expression is associated with poor prognosis in cases of human breast cancer: a tissue microarray analysis. Onco Targets Ther. 2016;9:6461–6466. doi: 10.2147/OTT.S107321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti F. LIM kinases are attractive targets with many macromolecular partners and only a few small molecule regulators. Med Res Rev. 2012;32(5):968–998. doi: 10.1002/med.20230. [DOI] [PubMed] [Google Scholar]
- Marshall TW, Aloor HL, Bear JE. Coronin 2A regulates a subset of focal-adhesion-turnover events through the cofilin pathway. J Cell Sci. 2009;122(Pt 17):3061–3069. doi: 10.1242/jcs.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Pedersen SF, Schwab A, Stock C. Intracellular pH gradients in migrating cells. Am J Physiol Cell Physiol. 2011;300(3):C490–C495. doi: 10.1152/ajpcell.00280.2010. [DOI] [PubMed] [Google Scholar]
- McLachlan GD, Cahill SM, Girvin ME, Almo SC. Acid-induced equilibrium folding intermediate of human platelet profilin. Biochemistry. 2007;46(23):6931–6943. doi: 10.1021/bi0602359. [DOI] [PubMed] [Google Scholar]
- Meira M, Masson R, Stagljar I, Lienhard S, Maurer F, Boulay A, Hynes NE. Memo is a cofilin-interacting protein that influences PLCgamma1 and cofilin activities, and is essential for maintaining directionality during ErbB2-induced tumor-cell migration. J Cell Sci. 2009;122(Pt 6):787–797. doi: 10.1242/jcs.032094. [DOI] [PubMed] [Google Scholar]
- Mittermann I, Fetrow JS, Schaak DL, Almo SC, Kraft D, Herberle-Bors E, Valenta R. Oligomerization of profilins from birch, man and yeast. Profilin, a ligand for itself? Sex Plant Reprod. 1998;11:183–191. [Google Scholar]
- Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013;25(2):457–469. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Moens PD, Bagatolli LA. Profilin binding to sub-micellar concentrations of phosphatidylinositol (4,5) bisphosphate and phosphatidylinositol (3,4,5) trisphosphate. Biochim Biophys Acta. 2007;1768(3):439–449. doi: 10.1016/j.bbamem.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Mouneimne G, DesMarais V, Sidani M, Scemes E, Wang W, Song X, Eddy R, Condeelis J. Spatial and temporal control of cofilin activity is required for directional sensing during chemotaxis. Curr Biol. 2006;16(22):2193–2205. doi: 10.1016/j.cub.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Moustafa-Bayoumi M, Alhaj MA, El-Sayed O, Wisel S, Chotani MA, Abouelnaga ZA, Hassona MD, Rigatto K, Morris M, Nuovo G, Zweier JL, Goldschmidt-Clermont P, Hassanain H. Vascular hypertrophy and hypertension caused by transgenic overexpression of profilin 1. J Biol Chem. 2007;282(52):37632–37639. doi: 10.1074/jbc.M703227200. [DOI] [PubMed] [Google Scholar]
- Nadella KS, Saji M, Jacob NK, Pavel E, Ringel MD, Kirschner LS. Regulation of actin function by protein kinase A-mediated phosphorylation of Limk1. EMBO Rep. 2009;10(6):599–605. doi: 10.1038/embor.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Yang N, Mizuno K. The N-terminal LIM domain negatively regulates the kinase activity of LIM-kinase 1. Biochem J. 1999;343(Pt 1):99–105. [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Johansson U, Johansson AC, Kagedal K, Ollinger K. Cytosolic acidification and lysosomal alkalinization during TNF-alpha induced apoptosis in U937 cells. Apoptosis. 2006;11(7):1149–1159. doi: 10.1007/s10495-006-7108-5. [DOI] [PubMed] [Google Scholar]
- Nishita M, Wang Y, Tomizawa C, Suzuki A, Niwa R, Uemura T, Mizuno K. Phosphoinositide 3-kinase-mediated activation of cofilin phosphatase slingshot and its role for insulin-induced membrane protrusion. J Biol Chem. 2004;279(8):7193–7198. doi: 10.1074/jbc.M312591200. [DOI] [PubMed] [Google Scholar]
- Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat Rev Cancer. 2011;11(3):177–187. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- Obermann H, Raabe I, Balvers M, Brunswig B, Schulze W, Kirchhoff C. Novel testis-expressed profilin IV associated with acrosome biogenesis and spermatid elongation. Mol Hum Reprod. 2005;11(1):53–64. doi: 10.1093/molehr/gah132. [DOI] [PubMed] [Google Scholar]
- Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26(4):273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108(6):1252–1262. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186(4):571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Eddy R, Condeelis J. Actin-based motile processes in tumor cell invasion. In: Carlier M-F, editor. Actin-based motility. Dordrecht: Springer Netherlands; 2010. pp. 125–164. [Google Scholar]
- Ostrander DB, Gorman JA, Carman GM. Regulation of profilin localization in Saccharomyces cerevisiae by phosphoinositide metabolism. J Biol Chem. 1995;270(45):27045–27050. doi: 10.1074/jbc.270.45.27045. [DOI] [PubMed] [Google Scholar]
- Paavilainen VO, Bertling E, Falck S, Lappalainen P. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol. 2004;14(7):386–394. doi: 10.1016/j.tcb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Pae M, Romeo GR. The multifaceted role of profilin-1 in adipose tissue inflammation and glucose homeostasis. Adipocyte. 2014;3(1):69–74. doi: 10.4161/adip.26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey DK, Chaudhary B. Evolutionary expansion and structural functionalism of the ancient family of profilin proteins. Gene. 2017;626:70–86. doi: 10.1016/j.gene.2017.05.024. [DOI] [PubMed] [Google Scholar]
- Pečar Fonović Urša, Kos Janko. Cathepsin X Cleaves Profilin 1 C-Terminal Tyr139 and Influences Clathrin-Mediated Endocytosis. PLOS ONE. 2015;10(9):e0137217. doi: 10.1371/journal.pone.0137217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernier J, Shekhar S, Jegou A, Guichard B, Carlier MF. Profilin interaction with actin filament barbed end controls dynamic instability, capping, branching, and motility. Dev Cell. 2016;36(2):201–214. doi: 10.1016/j.devcel.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs P, Heering J, Link G, Pfizenmaier K, Olayioye MA, Hausser A. Protein kinase D regulates cell migration by direct phosphorylation of the cofilin phosphatase slingshot 1 like. Cancer Res. 2009;69(14):5634–5638. doi: 10.1158/0008-5472.CAN-09-0718. [DOI] [PubMed] [Google Scholar]
- Polet D, Lambrechts A, Vandepoele K, Vandekerckhove J, Ampe C. On the origin and evolution of vertebrate and viral profilins. FEBS Lett. 2007;581(2):211–217. doi: 10.1016/j.febslet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pope BJ, Zierler-Gould KM, Kuhne R, Weeds AG, Ball LJ. Solution structure of human cofilin: actin binding, pH sensitivity, and relationship to actin-depolymerizing factor. J Biol Chem. 2004;279(6):4840–4848. doi: 10.1074/jbc.M310148200. [DOI] [PubMed] [Google Scholar]
- Rennella Enrico, Sekhar Ashok, Kay Lewis E. Self-Assembly of Human Profilin-1 Detected by Carr–Purcell–Meiboom–Gill Nuclear Magnetic Resonance (CPMG NMR) Spectroscopy. Biochemistry. 2017;56(5):692–703. doi: 10.1021/acs.biochem.6b01263. [DOI] [PubMed] [Google Scholar]
- Richer SM, Stewart NK, Tomaszewski JW, Stone MJ, Oakley MG. NMR investigation of the binding between human profilin I and inositol 1,4,5-triphosphate, the soluble headgroup of phosphatidylinositol 4,5-bisphosphate. Biochemistry. 2008;47(51):13455–13462. doi: 10.1021/bi801535f. [DOI] [PubMed] [Google Scholar]
- Rizwani Wasia, Fasim Aneesa, Sharma Deepshikha, Reddy Divya J., Bin Omar Nabil A. M., Singh Surya S. S137 Phosphorylation of Profilin 1 Is an Important Signaling Event in Breast Cancer Progression. PLoS ONE. 2014;9(8):e103868. doi: 10.1371/journal.pone.0103868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotty JD, Wu C, Haynes EM, Suarez C, Winkelman JD, Johnson HE, Haugh JM, Kovar DR, Bear JE. Profilin-1 serves as a gatekeeper for actin assembly by Arp2/3-dependent and -independent pathways. Dev Cell. 2015;32(1):54–67. doi: 10.1016/j.devcel.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Jacobson K. Overexpression of profilin reduces the migration of invasive breast cancer cells. Cell Motil Cytoskeleton. 2004;57(2):84–95. doi: 10.1002/cm.10160. [DOI] [PubMed] [Google Scholar]
- Sathish K, Padma B, Munugalavadla V, Bhargavi V, Radhika KV, Wasia R, Sairam M, Singh SS. Phosphorylation of profilin regulates its interaction with actin and poly (L-proline) Cell Signal. 2004;16(5):589–596. doi: 10.1016/j.cellsig.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Schoppmeyer R, Zhao R, Cheng H, Hamed M, Liu C, Zhou X, Schwarz EC, Zhou Y, Knorck A, Schwar G, Ji S, Liu L, Long J, Helms V, Hoth M, Yu X, Qu B. Human profilin 1 is a negative regulator of CTL mediated cell-killing and migration. Eur J Immunol. 2017;47(9):1562–1572. doi: 10.1002/eji.201747124. [DOI] [PubMed] [Google Scholar]
- Schulte B, John I, Simon B, Brockmann C, Oelmeier SA, Jahraus B, Kirchgessner H, Riplinger S, Carlomagno T, Wabnitz GH, Samstag Y. A reducing milieu renders cofilin insensitive to phosphatidylinositol 4,5-bisphosphate (PIP2) inhibition. J Biol Chem. 2013;288(41):29430–29439. doi: 10.1074/jbc.M113.479766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju Yosuke, Kalimeri Maria, Koskela Essi V., Somerharju Pentti, Zhao Hongxia, Vattulainen Ilpo, Lappalainen Pekka. Mechanistic principles underlying regulation of the actin cytoskeleton by phosphoinositides. Proceedings of the National Academy of Sciences. 2017;114(43):E8977–E8986. doi: 10.1073/pnas.1705032114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Jieya, Diamond Marc I. Protein Phosphatase 1 Dephosphorylates Profilin-1 at Ser-137. PLoS ONE. 2012;7(3):e32802. doi: 10.1371/journal.pone.0032802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J., Welch W. J., DiProspero N. A., Diamond M. I. Phosphorylation of Profilin by ROCK1 Regulates Polyglutamine Aggregation. Molecular and Cellular Biology. 2008;28(17):5196–5208. doi: 10.1128/MCB.00079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkin Sergey, Eremina Lidia, Pashintseva Natalya, Kovalev Leonid, Kovaleva Marina. Cofilin-1 and Other ADF/Cofilin Superfamily Members in Human Malignant Cells. International Journal of Molecular Sciences. 2016;18(1):10. doi: 10.3390/ijms18010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidani M, Wessels D, Mouneimne G, Ghosh M, Goswami S, Sarmiento C, Wang W, Kuhl S, El-Sibai M, Backer JM, Eddy R, Soll D, Condeelis J. Cofilin determines the migration behavior and turning frequency of metastatic cancer cells. J Cell Biol. 2007;179(4):777–791. doi: 10.1083/jcb.200707009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SS, Chauhan A, Murakami N, Styles J, Elzinga M, Chauhan VP. Phosphoinositide-dependent in vitro phosphorylation of profilin by protein kinase C. Phospholipid specificity and localization of the phosphorylation site. Recept Signal Transduct. 1996;6(2):77–86. [PubMed] [Google Scholar]
- Skare P, Karlsson R. Evidence for two interaction regions for phosphatidylinositol(4,5)-bisphosphate on mammalian profilin I. FEBS Lett. 2002;522(1–3):119–124. doi: 10.1016/s0014-5793(02)02913-7. [DOI] [PubMed] [Google Scholar]
- Skare P, Kreivi JP, Bergstrom A, Karlsson R. Profilin I colocalizes with speckles and Cajal bodies: a possible role in pre-mRNA splicing. Exp Cell Res. 2003;286(1):12–21. doi: 10.1016/s0014-4827(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Song X, Chen X, Yamaguchi H, Mouneimne G, Condeelis JS, Eddy RJ. Initiation of cofilin activity in response to EGF is uncoupled from cofilin phosphorylation and dephosphorylation in carcinoma cells. J Cell Sci. 2006;119(Pt 14):2871–2881. doi: 10.1242/jcs.03017. [DOI] [PubMed] [Google Scholar]
- Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, Sampath R, Bamburg JR, Bernard O. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24(3):473–486. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free Radic Biol Med. 2011;50(2):371–380. doi: 10.1016/j.freeradbiomed.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuven T, Hartmann E, Gorlich D. Exportin 6: a novel nuclear export receptor that is specific for profilin.actin complexes. EMBO J. 2003;22(21):5928–5940. doi: 10.1093/emboj/cdg565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Thapa N, Hedman AC, Anderson RA. Phosphatidylinositol 4,5-bisphosphate: targeted production and signaling. Bioessays. 2013;35(6):513–522. doi: 10.1002/bies.201200171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Xu W, Harden TK, Sondek J, Sun L, Li L, Wu D. A PLCbeta/PI3Kgamma-GSK3 signaling pathway regulates cofilin phosphatase slingshot2 and neutrophil polarization and chemotaxis. Dev Cell. 2011;21(6):1038–1050. doi: 10.1016/j.devcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojkander S, Gateva G, Husain A, Krishnan R, Lappalainen P (2015) Generation of contractile actomyosin bundles depends on mechanosensitive actin filament assembly and disassembly. Elife 4:e06126. 10.7554/eLife.06126 [DOI] [PMC free article] [PubMed]
- Tomiyoshi G, Horita Y, Nishita M, Ohashi K, Mizuno K. Caspase-mediated cleavage and activation of LIM-kinase 1 and its role in apoptotic membrane blebbing. Genes Cells. 2004;9(6):591–600. doi: 10.1111/j.1356-9597.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Tsujita Kazuya, Itoh Toshiki. Phosphoinositides in the regulation of actin cortex and cell migration. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2015;1851(6):824–831. doi: 10.1016/j.bbalip.2014.10.011. [DOI] [PubMed] [Google Scholar]
- van Rheenen J, Song X, van Roosmalen W, Cammer M, Chen X, Desmarais V, Yip SC, Backer JM, Eddy RJ, Condeelis JS. EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells. J Cell Biol. 2007;179(6):1247–1259. doi: 10.1083/jcb.200706206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri B, Singh SS. Protein kinase C isozyme-specific phosphorylation of profilin. Cell Signal. 2001;13(6):433–439. doi: 10.1016/s0898-6568(01)00164-4. [DOI] [PubMed] [Google Scholar]
- Viaud J, Mansour R, Antkowiak A, Mujalli A, Valet C, Chicanne G, Xuereb JM, Terrisse AD, Severin S, Gratacap MP, Gaits-Iacovoni F, Payrastre B. Phosphoinositides: important lipids in the coordination of cell dynamics. Biochimie. 2016;125:250–258. doi: 10.1016/j.biochi.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Wang J, Richards DA. Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biol Open. 2012;1(9):857–862. doi: 10.1242/bio.20122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64(23):8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shibasaki F, Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J Biol Chem. 2005;280(13):12683–12689. doi: 10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- Wentworth JK, Pula G, Poole AW. Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated on Ser157 by protein kinase C-dependent and -independent mechanisms in thrombin-stimulated human platelets. Biochem J. 2006;393(Pt 2):555–564. doi: 10.1042/BJ20050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14(8):461–469. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, Haas W, Kovacs JJ, Shukla AK, Hara MR, Hernandez M, Lachmann A, Zhao S, Lin Y, Cheng Y, Mizuno K, Ma'ayan A, Gygi SP, Lefkowitz RJ. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci U S A. 2010;107(34):15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yu K, Hao Y, Li DM, Stewart R, Insogna KL, Xu T. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat Cell Biol. 2004;6(7):609–617. doi: 10.1038/ncb1140. [DOI] [PubMed] [Google Scholar]
- Yao Wantong, Ji Shunrong, Qin Yi, Yang Jingxuan, Xu Jin, Zhang Bo, Xu Wenyan, Liu Jiang, Shi Si, Liu Liang, Liu Chen, Long Jiang, Ni Quanxing, Li Min, Yu Xianjun. Profilin-1 suppresses tumorigenicity in pancreatic cancer through regulation of the SIRT3-HIF1α axis. Molecular Cancer. 2014;13(1):187. doi: 10.1186/1476-4598-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh S, Pope B, Mannherz HG, Weeds A. Determining the differences in actin binding by human ADF and cofilin. J Mol Biol. 2002;315(4):911–925. doi: 10.1006/jmbi.2001.5280. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- Yonezawa N, Nishida E, Iida K, Yahara I, Sakai H. Inhibition of the interactions of cofilin, destrin, and deoxyribonuclease I with actin by phosphoinositides. J Biol Chem. 1990;265(15):8382–8386. [PubMed] [Google Scholar]
- Zaidi AH, Manna SK. Profilin-PTEN interaction suppresses NF-kappaB activation via inhibition of IKK phosphorylation. Biochem J. 2016;473(7):859–872. doi: 10.1042/BJ20150624. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ghai P, Wu H, Wang C, Field J, Zhou GL. Mammalian adenylyl cyclase-associated protein 1 (CAP1) regulates cofilin function, the actin cytoskeleton, and cell adhesion. J Biol Chem. 2013;288(29):20966–20977. doi: 10.1074/jbc.M113.484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Hakala M, Lappalainen P. ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP(2)-density sensor. Biophys J. 2010;98(10):2327–2336. doi: 10.1016/j.bpj.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wade B, Ma J, Hart CM, Sutliff RL. Hypoxia alters ubiquitination of proteins associated with cellular proliferation in mouse lung. FASEB J. 2017;31(1 Supplement):1016.1018–1016.1018. [Google Scholar]
- Zhong JC, Ye JY, Jin HY, Yu X, Yu HM, Zhu DL, Gao PJ, Huang DY, Shuster M, Loibner H, Guo JM, Yu XY, Xiao BX, Gong ZH, Penninger JM, Oudit GY. Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. Regul Pept. 2011;166(1–3):90–97. doi: 10.1016/j.regpep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Zou L, Jaramillo M, Whaley D, Wells A, Panchapakesa V, Das T, Roy P. Profilin-1 is a negative regulator of mammary carcinoma aggressiveness. Br J Cancer. 2007;97(10):1361–1371. doi: 10.1038/sj.bjc.6604038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282(28):20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]