Abstract
Considerable debate surrounds the question of whether or not quantum mechanics plays a significant, non-trivial role in photosynthetic light harvesting. Many have proposed that quantum superpositions and/or quantum transport phenomena may be responsible for the efficiency and robustness of energy transport present in biological systems. The critical experimental observations comprise the observation of coherent oscillations or “quantum beats” via femtosecond laser spectroscopy, which have been observed in many different light harvesting systems. Part Two of this review aims to provide an overview of experimental observations of energy transfer in the most studied light harvesting systems. Length scales, derived from crystallographic studies, are combined with energy and time scales of the beats observed via spectroscopy. A consensus is emerging that most long-lived (hundreds of femtoseconds) coherent phenomena are of vibrational or vibronic origin, where the latter may result in coherent excitation transport within a protein complex. In contrast, energy transport between proteins is likely to be incoherent in nature. The question of whether evolution has selected for these non-trivial quantum phenomena may be an unanswerable question, as dense packings of chromophores will lead to strong coupling and hence non-trivial quantum phenomena. As such, one cannot discern whether evolution has optimised light harvesting systems for high chromophore density or for the ensuing quantum effects as these are inextricably linked and cannot be switched off.
Keywords: Photosynthesis, Quantum coherence, Light harvesting, Quantum biology, Protein
Introduction
Part One of this review details the quantum framework within which light harvesting processes in biological systems can be understood. Now that some formalism has been introduced, we can look deeper into real systems that have been observed to harbour quantum coherent mechanisms. Quantum beats are observed to be a near ubiquitous feature of photosynthetic systems; however, understanding their origins is a difficult task and remains a matter of current debate. Part Two of this review aims to put forward analyses that attempt to tease apart the mechanisms that may produce the observed quantum beats and uncover their consequences with regard to light harvesting efficiency and robustness. A multitude of mechanisms have been suggested and most are documented here. Some may find the theoretical aspects somewhat nebulous and at this point, reference to some of the definitions in Part One may be of use.
Many of the mechanisms underpinning coherent dynamics in photosynthesis appear to be vibronic in nature, that is, the transfer of excitation energy between excited electronic states harbours coherent, coupled states comprising a mixture of both electronic and vibrational degrees of freedom. There appear to be many reasons for this with regard to efficiency and robustness of light harvesting, which would provide a key evolutionary advantage for organisms living in low light where every photon is precious. It has been proposed that quantum mechanisms play a non-trivial role in light harvesting as coherences between states are stable over a time scale that is commensurate with the relevant energy transfer times. It is unclear whether or not coherent quantum effects directly increase the efficiency of light harvesting or if the energy transport process in more akin to Förster resonance energy transfer (FRET). Regardless, their presence is curious as it opens up many questions for fundamental physics as well as biophysics. Namely, how can non-trivial quantum effects propagate in hot, wet cellular environments and what makes these quantum states so robust?
Observations of quantum behaviour in light harvesting systems
The following discussion will be mainly concerned with understanding the time, length and energy scales involved in potentially coherent light harvesting processes and the implications for efficiency and robustness. Many of the specifics such as coupling energies, exciton energies, and vibrational wavelengths will largely be left out and the reader can be directed to the references given in-text.
The oscillatory patterns observed in spectroscopic measurements have been termed “quantum beats”. They are evidence of some form of coherent interference phenomena underlying the spectra. The spectral data and associated quantum beats must be interpreted by building a theoretical model that supports the observations; however, multiple models can often be invoked to reproduce and fit the data. Hence, we are faced with a difficult task of trying to understand the fundamental source of coherence. To that end, interpretations of the results of spectroscopic studies have ebbed and flowed and thus, for the purpose of this text, an emphasis will be placed on global observations and a few key interpretations of the results. Exacerbating these difficulties, there is often an inconsistency in units between papers. Quantum mechanically speaking, wavenumber, frequency, period, wavelength and energy are all proportional (or inversely so) up to a constant and, hence, are used interchangeably.
Most photosynthetic organisms share common features, namely a light harvesting antenna and a reaction centre; organisms that use rhodopsins to generate a proton gradient are, however, an exception. It has been posited that all photosynthetic organisms that make use of a reaction centre for charge separation derive from a single ancestral bacterial reaction centre that evolved in different niches for either oxygen/hydrogen or sulphur oxidation (Cardona 2014). Six bacterial clades (delineated by their constituent light harvesting chromophores) appeared, the most notable three being purple bacteria, green sulphur bacteria and cyanobacteria. Through a series of endosymbiotic events involving cyanobacteria, the eukaryotic organisms—red algae, green algae and plants, and protists—evolved taking the cyanobacterial light harvesting system and altering it in innumerable ways (Neilson and Durnford 2010). Interestingly, no photosynthetic eukaryotes appear to have evolved from the endosymbiosis of other photosynthetic bacteria while cyanobacteria appear to have been involved in at least two distinct endosymbiotic events (the generation of a proto-red alga and, more recently, Paulinella sp. (Delaye et al. 2016)). Quantum beats of various forms have been observed in almost all of these light harvesting systems.
Quantum processes in antennae
The antenna is usually the first point of excitation for light harvesting systems and responsible for the efficient and robust transport of excitation energy, in the form of an exciton, to the reaction centre. Organisms that live in environments with very low photon flux have large and elaborate light harvesting antennae as every captured photon counts. Furthermore, the energy from each one of those photons must be efficiently and robustly carried to the reaction centre to prevent energy loss via fluorescence emission (the time scale of which is roughly in nanoseconds). This highlights a key evolutionary trade-off for these extremophiles: they must have a large surface with a high density of chromophores from which they can capture photons but they also must be able to transport the excitations to the closest reaction centre on a nanosecond timescale. A further element of this trade-off is energy input in making antennae and reaction centres. If an organism makes a plethora of reaction centres, then energy is being wasted as many will not be used. Hence, there is a need for increases in efficiency of transport in the antennae themselves. Yet another complexity comes from the environment where perturbations can cause decay and decoherence, potentially leading to reduced efficiency. There is hence a need for robustness against these effects which many organisms seem to exhibit in their transfer processes.
Green bacteria
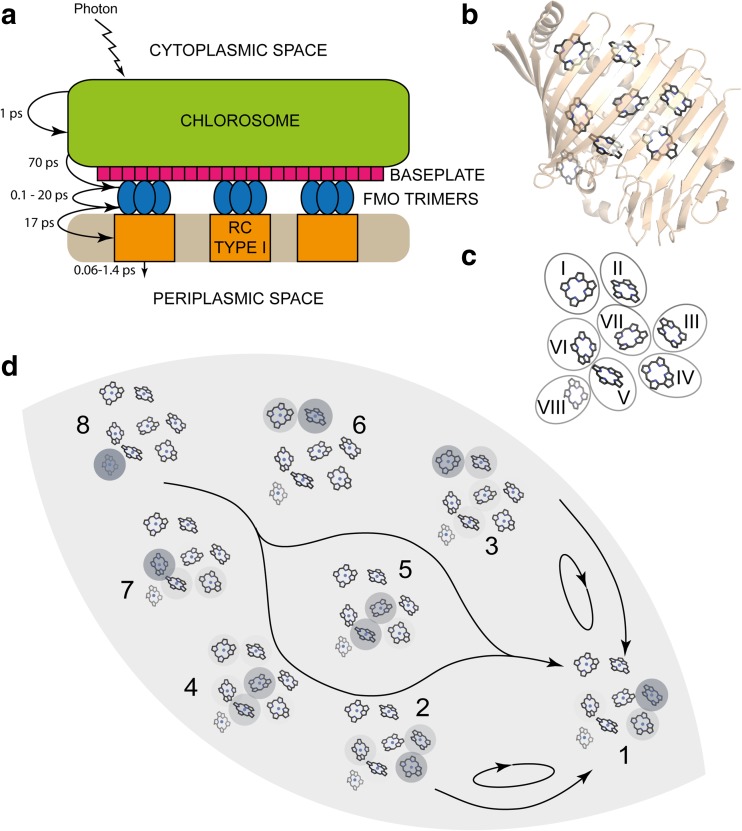
The predominant antenna of green bacteria is the chlorosome—a large, highly ordered aggregate of bacteriochlorophyll (BChl) molecules enclosed in a lipid/protein membrane. In green non-sulphur bacteria, the chlorosome is coupled directly to the reaction centre via the bacteriochlorophyll/carotenoid-containing protein baseplate. In green sulphur bacteria, an additional coupling protein is added between the baseplate and the reaction centre known as the Fenna-Matthews-Olson (FMO) complex. The FMO complex is a trimeric protein structure almost entirely composed of β-sheet secondary structural elements that encapsulate seven closely packed bacteriochlorophyll molecules in addition to one peripheral bacteriochlorophyll molecule that appears to weakly couple to the other seven (Fig. 1).
Fig. 1.
a Morphology of the green sulphur bacteria light harvesting apparatus. In green non-sulphur bacteria, the RC type I is replaced with RC type II and the FMO complex is missing. Transfer and charge separation times are given. b Structure of the FMO monomer (PDB 3eni). c Chlorophyll arrangement and chromophore labels for the FMO monomer. d Exciton states and energy flow downhill between them from state 8 to state 1. Acutely studied transfer rates and coherence times are labelled as arrows and ellipses respectively. Exciton states were calculated by finding eigenvectors of the FMO Hamiltonian (Adolphs and Renger 2006) in Mathematica. Exciton states are calculated from the Hamiltonian given by. Chlorophyll tails are removed for clarity. Structures were rendered in PyMol
One of the more recent and comprehensive studies into light harvesting energy transfer time scales (Dostál et al. 2016) outlines specific steps in energy transfer of the green sulphur bacterium Chlorobium tepidum. The chlorosome has a very large absorption cross section and produces a strong and wide absorption feature in the spectra; hence, the laser excitation spectrum only partially covers the chlorosome absorption spectrum so as to not obscure other energy transfer features.
The details of time scales and morphology are documented in Fig. 1a. Firstly, relaxation within the chlorosome to the red edge of its absorption band occurs with a time constant of 940 fs (a lower limit given only partial excitation) followed by a further slower decay component of 10 ps due to excitations that appear to be lost via annihilation (when two excitons interact to destroy themselves and emit their energy—a process that takes place when a high density of excitons is present). Cross peaks between the chlorosome and FMO bands indicate a transfer between these states with a time constant of 70 ps. States resident on the baseplate are obscured in the spectra. All internal transfer processes within the FMO are completed within 100 fs to 20 ps with 25% of trapped lowest energy FMO populations decaying with a time constant of 200 ps. Energy transfer between the FMO and the reaction centre occurs with a time constant of 17 ps and reaction centre populations decay with a time constant of 60 fs and 1.4 ps corresponding to energy relaxation and charge separation, respectively. Many of these time scales are corroborated by earlier transient absorption studies (below) and provide the perfect platform to discuss the separability/non-separability of time scales of coherence relevant to energy transfer.
Chlorosome
In an evolutionary search for an efficient antenna, the chlorosome is an excellent example. Its construction is such that chromophores have large interaction energies merely by the BChl molecules being in spatial contact with each other within highly ordered domains. These domains take the shape of concentric rods, sheets and spirals. Tight packing gives rise to large interaction energies between chromophores and hence the possibility of a large degree of exciton delocalisation. Estimates of exciton delocalisation from spectroscopic studies, however, vary wildly. The regions over which the exciton is delocalised are often referred to as the coherent domains. Because of weak long range interactions, static crystal defects and the influence of the vibrational environment pervading the chlorosome structure, excitons are not extended across the entire chlorosome but are partially localised into fluctuating coherent (exciton) domains. The coherent domains will then undergo fluorescence energy transfer (FRET) between one another incoherently.
There is another interesting feature of the gross chlorosome structure which ties partially into the broader question of why plants are not black. The design of the chlorosome is such that the capture cross section is maximised while not allowing excitations to fluoresce. The absorption probability of a photon is small, and hence the thickness of the chlorosome needs to be maximised. Excitons have a characteristic diffusion length given the speed with which they can propagate and a finite lifetime before their re-emission. As such, there is an upper limit on the size of the chlorosome before capture becomes inefficient (i.e. the time needed to reach the reaction centre is such that the exciton will not decay by fluorescence before reaching the reaction centre).
Early estimates based on dipole couplings (possibly an erroneous approximation given the close proximity of chlorophylls) place the size of the delocalised exciton state as 2–3 chromophores in Chlorobium and 10–12 chromophores in Chloroflexus (Prokhorenko et al. 2000). Later work, however, suggests that the size of the coherent domains will be largely indiscernible as small differences in the phase of coherent oscillations within different coherent domains will greatly reduce the dephasing time of electronic coherence upon averaging (Jun et al. 2014). It is further suggested that coherent domains will lie on single rods/lamellae over 20 nm due to the ordering of the transition moments in the structure and energy will (to a first approximation) be transferred incoherently between these coherent domains. The exciton states of the chlorosome (without the influence of the environment) are highly extended. As such, environmental perturbations happening on distant regions of the exciton will destroy the superposition. During energy transfer in the chlorosome, it is likely that only randomised instances of exciton states are apparent, given fluctuations from the environment, making them hard to observe. Furthermore, the chlorosome is packed with chlorophyll molecules meaning that the number of excitons will be enormous. Hence, we are unable to observe the different energy levels in the chlorosome as they form a broad continuum making observations of quantum beats in 2DES almost impossible.
However, a number of quantum beats have been observed in spectroscopic measurements of the chlorosome. A set of long-lived modes appear with low frequency which dephase over 1 ps in Chloroflexus and 1.5–2 ps in Chlorobium (Savikhin et al. 1994, 1995a, 1995b). These modes have been ascribed to either interfering ground state vibrations following resonant impulsive Raman scattering or intra/inter-molecular vibronic modes (Ma et al. 1999; Prokhorenko et al. 2000). These coherent beatings are independent of the spectral line shape evolution and different parts of the emission spectrum show different vibrational modes and phase flips (Savikhin et al. 1994). Hence, the diffusion of excitons through the chlorosome probably does not produce electronic coherences; however, electronic coherences may be enhanced by intensity borrowing from vibronic coupling (Prokhorenko et al. 2000; Dostál et al. 2014). Vibrational coherences dephase too quickly to aid in transferring coherence between coherent domains given that the time scale of the random energy diffusion process between coherent domains is 4–5 ps between rods; however, they may aid in intra-domain relaxation (Prokhorenko et al. 2000; Dostál et al. 2014). By observing changes in the lineshapes, the energy transfer in the single coherent domains takes place in 20–30 fs. Electronic or vibronic coherence is potentially manifest as a high-frequency mode which dephases over 60 fs and the coherent domain can potentially complete a sampling of the possible energy transfer pathways under the high-frequency coherences.
FMO complex
The FMO complex has been used as both a theoretical and experimental prototype for studies into quantum effects in biology. Its closely packed arrangement of chromophores gives rise to large interaction energies and the possibility for coherence providing efficient funnelling of excitations downhill in energy to the reaction centre. The efficiency and coherence dynamics of FMO appear to be robust to alterations of the system’s vibrational reservoir and site energies (chromophore excitation energies) (Hayes et al. 2011; Maiuri et al. 2018). It should be noted that the site energies in many theoretical calculations are only estimates. The reason for this is that they are largely unobservable and that which we can observe when probing the energy is the eigenvalues of the energy matrix (the Hamiltonian) corresponding to the exciton energies. In Fig. 1, the FMO structure (Fig. 1b), the chromophore sites (Fig. 1c) and the calculated exciton states are shown via shading over the chromophore arrangement and energy flow (Fig. 1d).
Due to the delocalisation of excitonic states across the seven main chromophores in the FMO complex, energy is not simply transferred down a chromophore ladder but funnelled along pathways between the delocalised exciton states (Fig. 1d). Protein electrostatics induce differences in site energies across chromophores which are coupled to one another, producing delocalised excitons and resulting in a very different absorption spectrum to that of bacteriochlorophyll in solution. Site energies, exciton energies and their delocalisations are still a matter of debate. The spectrum of the FMO trimer contains features for only seven excitons and not the full 24 expected if all eight chromophores were strongly coupled within the FMO trimer (Thyrhaug et al. 2016). Hence, inter-monomer quantum coherent effects can be ruled out with coherence confined to single FMO monomers. In 2DES spectra, all major diagonal peaks are connected by cross peaks indicating transfer between excitons. There are many possible pathways for energy transfer from initially excited states to the lowest energy state, although specific processes are preferred, as shown in Fig. 1d. The fastest and thus most prevalent energy transfer steps tend to involve transfer between exciton states with some spatial overlap. This is evident in the putative composition of chromophore sites in each exciton (Fig. 1d) and hence the overlap between each exciton participating in energy transfer. Coherence between excitons can thus produce path coherence in the energy transfer processes throughout the complex down to the trap site which funnels to the reaction centre.
Since the initial discovery of quantum beats in the FMO complex, there has been considerable debate surrounding the origin of coherences. Two dominant coherent oscillations are observed on the cross peaks between excitons one and two (160 cm−1) and excitons one and three (200 cm−1) with coherence times ranging from 100 fs to 1.1 ps (around 300 fs at room temperature) depending on the method and author (Engel et al. 2007; Hayes et al. 2010; Panitchayangkoon et al. 2010, 2011; Thyrhaug et al. 2016, 2018; Duan et al. 2017). Oscillatory features are observed in peak widths and quantum beats are seen to grow in amplitude at early times indicating possible transfer of coherence from higher energy excitonic states relaxing to lower lying ones. Coherence transfer could then support the sampling of multiple relaxation pathways to the sink exciton. The transfer of coherence via quantum transport is also supported by the changes in the oscillatory phase between populations and coherences (π phase flip). However, it has remained unclear whether coherence arises from vibrations or electronic superpositions. Indeed, growing evidence suggests that it may not be possible to separate vibrational and electronic dynamics of the system. In many cases, the gaps in exciton energy and the energies of vibrations resident on chlorophylls from Raman studies are resonant, and hence, could result in strong coherent coupling and vibronic states with long coherence times.
Many have attempted to tackle the problem of biological relevance and robustness of coherences to perturbations in the FMO system. Firstly, the presence of coherence may not be the result of evolutionary optimisation of system-environment couplings but merely the result of optimising the excitonic structure of the FMO complex (Hayes et al. 2011). Lifetimes of the strongest coherences between excitons in 2DES remain unchanged (1.1–1.4 ps) in FMO complexes that have: a random incorporation of different chlorophyll species with increased rigidity of their tails; 30% deuteration; or complete incorporation of carbon-13. The quantum beating frequencies between specific excitons are also unchanged upon these perturbations. Thus, at least, some of the observed coherences do not arise from nuclear motions alone. This is especially apparent in partially deuterated samples, which suffer inhomogeneous broadening and dephasing of nuclear motions as different chromophores now have different constituent vibrational modes. Coherences are also robust to changes in the excitonic structure, not just the thermal reservoir (Maiuri et al. 2018). Alterations to the excitonic Hamiltonian of the system can be made by site-directed mutagenesis thereby altering the site energies of specific chromophores by interaction with altered neighbouring amino acid residues. The changes in the exciton structure and hence excitation delocalisation can be observed via dramatic changes in the absorption spectra; however, the crystal structures of the mutants are unpublished. Interestingly, the strongest quantum beats that have been observed are resilient to alterations in the excitonic structure showing similar frequencies and dephasing times. Both observations of robustness to changes in the vibrational environment and exciton structure suggest that quantum beats may arise from vibronic coherence or at least suggest that the strongest vibrational and electronic coherences are energetically resonant.
It has been postulated that correlated motions in the protein environment around the chromophores may also play a critical role in supporting long-lived coherences. Correlated nuclear environments and non-Markovian vibrational perturbations may be generated by either synchronised and uncoupled nuclear motions for individual chromophores or the response of the chromophores which are embedded in a same protein matrix. If the nature of the environment was critical, dephasing rates would increase as protein modes become more populated with increasing temperature. Hence, a correlated, fluctuating electrostatic environment provided by the protein may protect quantum coherences if they exist on the appropriate time scales enabling oscillatory population dynamics (Panitchayangkoon et al. 2010). If system-environment coupling is strong enough, fluctuations may cause entanglement with the environment in a non-trivial way and energy/information may be exchanged between system and environment on a femtosecond time scale (Panitchayangkoon et al. 2011).
By looking at the movement of peaks in the spectra (spectral motions) of each exciton, excitons appear to experience correlated changes in different regions of the spectrum with frequencies agreeing with that of excited state vibrations (Rolczynski et al. 2018). This correlation lasts for much of the first picosecond of the spectral evolution. Correlations may be explained by the residence of vibrations on individual chlorophylls that comprise each different exciton; local motions on one chromophore affect multiple excitons and the global exciton energy. Excitons may possibly be inheriting vibrational phase information from each other which enhances quantum beating lifetimes. Coupling of vibrational modes in different chromophores at distant ends of the protein cannot be a mechanical effect as the speed of sound in a protein is too slow. Thus, electromagnetic interactions must correlate vibrations in distinct chromophores separated by protein. Synchronised oscillations may stem from embedding multiple identical chromophores with the same internal vibrational modes in close proximity within an organised protein matrix and the response of the chromophores ensures that the spectral motions in the excited state will be correlated.
Lessons from studying green bacteria
The close packing of large numbers of BChl molecules in the chlorosome results in dynamic, delocalised exciton states. Thus, the chlorosome increases the photon capture cross section and the width of the absorption spectrum using a single type of chromophore. The size of the chlorosome is limited by the fluorescence lifetime of the excitons and the time for the energy to reach the reaction centre via exciton diffusion. Understanding coherence in these systems is quite difficult, given that the excitons are not exactly in energy eigenstates.
The arrangement of chromophores in the FMO protein again produces a series of exciton states. Robust quantum beats are observed in spectra. Many of these beats have a resonance between the energies of the constituent vibrations and exciton energy gaps. Specific coherences that have a resonance between vibrational energies and exciton energy gaps are robust to changes in the site energies and constituent vibrational modes. The robustness of these coherences may be by design to form vibronic states or merely just the coincidence of energies possibly leading to vibration-assisted transitions.
Purple bacteria
The two predominant light harvesting antennae of purple bacteria are comprised of integral membrane proteins containing chlorophyll and carotenoid molecules ordered in symmetric ring structures (Fig. 2a). Light harvesting complex 2 (LH2) is a peripheral antenna that funnels excitations to light harvesting complex 1 (LH1) which surrounds the reaction centre. The number of subunits in each ring structure varies from species to species, and even within a single organism. There are also variations on this symmetric ring structure including a lemniscate (“figure eight”) structure that occurs upon interaction with an accessory protein. Some species have additional light harvesting antennae which have subtle differences in the energies of their constituent chromophores (Chmeliov et al. 2013). It should be noted that LH1 and LH2 are in no way related to LHCI and LHCII of higher plants despite a naming similarity.
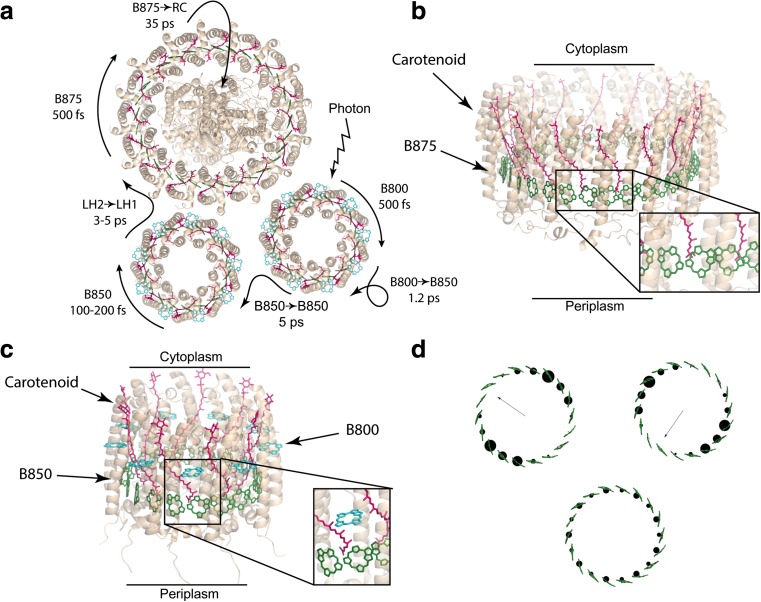
Fig. 2.
a Representative relaxation and energy transfer times between different states and rings and morphology of proteins embedded in the chromatophore membrane. b Crystal structure of LH I (PDB: 3wmm) with the chlorophyll ring labelled. The inset highlights the alternating chlorophyll orientations. c Crystal structure of LH II (PDB: 2fkw) with the chlorophyll rings labelled. The inset highlights the alternating chlorophyll orientations of B850. d A selection of important exciton states of the B850 ring; two isoenergetic first excited states at the top (with their dipole moments) and the ground state at the bottom. Exciton states were calculated by finding eigenvectors of the B850 Hamiltonian (Strümpfer and Schulten 2009) in Mathematica. Chlorophyll tails have been removed for clarity and structures were rendered in PyMol
The structural symmetry of the LH1 and LH2 complexes permits some interesting pathways for energy transfer, super-transfer and localisation effects which arise due to superpositions of electronic states interfering to generate delocalised excitons and drastically altering the dipole moments of the system. The symmetry of the assembled protein complex leads to sets of energy eigenstates (exciton states) in rings that form a ladder of pairs of degenerate (isoenergetic) states along with a ground and highest excited state that are non-degenerate. Two states from each set (the lowest two excited states) have a large dipole moment; the rest have dipole moments that are an order of magnitude smaller including the ground state (which is almost optically dark). The inclusion of disorder disrupts this excitonic structure and leads to different, slightly randomised delocalisation patterns of excitons. The randomised excitons after environmental interactions often have larger dipole moments; however, these dipole moments are still negligible compared with the lowest excited exciton states. The two lowest excited states and the ground state are shown in Fig. 2d for B850 LH2. A similar pattern is found for LH1.
LH2 is comprised of two sets of concentric bacteriochlorophyll rings (B800 and another which is species dependent called B850; Fig. 2a, c). The B800 ring is comprised of monomeric bacteriochlorophyll molecules with their transition dipoles roughly in the plane and tangential to the ring with the same orientation (handedness) around. The B850 ring is comprised of dimeric bacteriochlorophyll molecules with their transition dipoles roughly tangent and in the plane of the ring but alternating in direction within the pair. LH1 (Fig. 2b) is comprised of a single B875 ring formed by pairs of bacteriochlorophylls in alternating orientation as in the B850 ring of LH2. However, there is often a break in the ring disrupting the symmetry. Carotenoids in both LH1 and LH2 are from here on largely ignored in the analysis. Within LH2, the relaxation of the B800 ring to its lowest excited state is on the order of 500 fs (Cohen Stuart et al. 2011) whereas the relaxation time of the B850 ring is 100–200 fs (Book et al. 2000). Energy is transferred between the two LH2 rings on a time scale of 1.2 ps and then on to LH1 over 3–5 ps (Fig. 2a). Lastly, energy relaxes on the LH1 over 500 fs and is transferred to the reaction centre over 35 ps. Structural arrangements and transfer rates are shown in Fig. 2.
LH2
Many estimates of the average extent of delocalisation of the excitation on LH2 have been made ranging between 2 and 13 chromophore molecules on the B850 ring (Chachisvilis and Sundström 1996; Chachisvilis et al. 1997; Book et al. 2000; Dahlberg et al. 2015). This is due to dephasing from the environment contracting the superposition with further variability depending on the method and analysis used. Dephasing from the environment also destroys coherence between the more randomised exciton states that are now produced; this is similar to that of the chlorosome but to a smaller extent. Given environmental dephasing, it is apparent that the extent of delocalisation on the different rings is time dependent and relaxes to a smaller number of chromophores (possibly only 2) over 50–100 fs. At room temperature, it is observed that B800 experiences a larger degree of dephasing than the B850 ring and hence the members of the B850 ring are more strongly coupled to each other (and less strongly coupled to the environment) than the B800 ring. Differences in coupling are also evident in the redshift of B850 relative to B800 (50 nm); more strongly coupled chromophores have a broader absorption spectrum due to exciton splitting and hence the lowest excited state will be redder with more strongly coupled chromophores. Some vibrational coherences are observed in B800 that are not observed in B850 suggesting their suppression in the latter (Book et al. 2000).
It is unlikely that most exciton levels in each ring will manifest themselves during energy transfer given their negligible dipole moments (thereby reducing the transition probability) and, thus, only a few coherences between excitons are dominant in this system. As such, B800 only decays to two states of B850 with any great probability. The two optically active exciton states of B850 are seen to have coupled phases and persistent oscillations that last for 30–500 fs while dephasing on the B800 ring follows a 177-fs time scale (Cohen Stuart et al. 2011; Harel and Engel 2012; Fidler et al. 2013). These two optically active B850 states are also observed to have persistent oscillations coupled to the lowest states of the B800 ring. B850 relaxation pathways carry a π/2 phase change between the spectral regions corresponding to B800 and B850 states. This could be indicative of a complicated mechanism that avoids or enhances destructive interference at an optically dark state through multiple pathways carrying different phases. These oscillations between B850 and B800 have a delicate interplay and it has been suggested the coherent states that generate the oscillations have a 90% probability of being localised on either B800 or B850 at any given instant. Specific quantum beats are observed for different polarisation schemes in 2DES with different lifetimes suggesting some specific quantum beats do not arise from purely electronic or vibrational coherences. It should be noted that many of the quantum beats observed do indeed correspond to vibrational states and not all fall into the mixed electronic/vibrational category. Hence, it has been suggested that the B850 ring harbours coupled dark vibrational states and higher excited states (Singh et al. 2015). Furthermore, quantum beats can still be observed between states of the B800 and B850 ring in organisms grown in 30% D2O with a phase change across the spectrum similar to that of the native scenario (Dahlberg et al. 2015). Vibronic coherence between states of LH2, while lasting less than 100 fs, has a similar lifetime to that of the energy transfer between them, however, its biological relevance is unclear assuming the upper state plays an intermediate role.
Using single-molecule tools on LH2, a deeper look into the distribution of mechanisms can be foraged for, as single instances of energy transfer can be observed (not just an ensemble average) (Hildner et al. 2013). Quantum beats are observed and persist for at least 400 fs across all LH2 complexes rendering energy transfer robust to static disorder which randomises the exciton states in the ensemble. The modes observed have a broad distribution which is reflective of the distribution of instances of randomised exciton states. It is suggested that the predominant oscillation corresponds to coherence between excitons in B800 and B850 to an optically dark state of B850. A π phase jump is also noted in the first 10 fs which may result from a change in accumulated phase during relaxation. This phase jump may imply a modification of the energy transfer pathways toward a different low-energy optically accessible state of B850 highlighting that long-lived coherences may play a crucial role in allowing flexibility in transfer pathways. Coherence thus survives long enough to average over local inhomogeneities of the excited state and allow for funnelling to the lowest energy state.
LH1
A number of low-frequency quantum beats are observed in B877 and B820 LH1 variants which last between 300 fs and 8 ps and are generally ascribed to vibrational coherences in both the ground and excited states given their frequency dependence and phase shifts (Chachisvilis et al. 1994; Chachisvilis and Sundström 1996; Ma et al. 2017). Upon dissociation of the ring into protein subunits in detergent, a dramatic change in frequency content of quantum beats occurs where low-frequency modes are no longer observed. This is possibly due to a loss of contact between B820 macrocycles from which inter-molecular modes are derived. Furthermore, observed anisotropy in pump-probe population decay is characteristic of changes in the dipole moment and suggests selection of an exciton from a superposition of exciton states (Diffey et al. 1998). Just as with states of LH2, vibronic resonances are observed in B820 which decay over 600 fs (Ferretti et al. 2014). Hence, vibronic coherences are potentially able to sustain and regenerate electronic coherence on energy transfer time scales. In the LH1 of Thermochromatium tepidum, calcium and barium metal ions bind to the chlorophylls which alter the excitonic structure of the rings raising or lowering the absorption energy. A difference in the energy level structure that is produced by these variants does not appear to yield appreciable difference in population transfer or dephasing rates (estimated to be 57 fs via changes in the lineshape) (Ma et al. 2017). Phase changes are seen in the spectra of calcium and barium substitutes highlighting two different pathways of energy transfer to the reaction centre. Oscillations are apparent for 1 ps and have two dominant modes, one of which is resonant with the energy difference of the first two excited states and the ground state. Relaxation thus potentially takes place in a vibronic manner which is accomplished by linking higher and lower states with vibrational coherences which is irrespective of the energy difference between exciton states and independent of temperature.
Lessons from studying purple bacteria
Exciton dynamics in these ring structures is highly complex, so how can we interpret energy transfer in light of these data? As the transition dipole moment scales with the square root of the number of chromophore molecules and only certain superpositions will be optically active due to the summation of dipole moments, we can posit that energy transfer is coherent among chromophores within the rings, relaxing to a low-lying state. Following that, the enhanced dipole moment derived from the superposition generates super-transfer to neighbouring rings improving the spectral overlap of long distance transfer which can be viewed as conventional FRET. Hence, evolution in this system may have enhanced bright states for super-transfer between LH1 and LH2 and not possess coherence between them (Strümpfer et al. 2012; Kassal et al. 2013; Baghbanzadeh and Kassal 2016). It is also clear given results from purple bacteria and green bacteria that many observed quantum beats curiously have a resonance in the energies of the constituent vibrations and exciton energy gaps. This may lead to vibration-assisted transitions between excitons and in some cases vibronic states.
Antennae derived from cyanobacteria
Cyanobacteria are the progenitors of all eukaryotic photosynthetic life which is a complex story of theft and piracy. Hence, there are many shared features in chromophore composition and antenna structures. One of the key light harvesting antennae of cyanobacteria is the phycobilisome which is a large assembly of rings of proteins including the chromophore containing phycobiliproteins and linker proteins. Upon the primary endosymbiotic event driving the evolution of early eukaryotic photosynthetic life, a cyanobacterium was harnessed and its descendants remain today as the modern plastids. The first endosymbiotic event created what would become modern red algae. Within the chloroplast of modern red algae, the phycobilisome structure still exists with minimal alteration. At a later evolutionary time, a primitive red alga was engulfed to produce many of today’s photosynthetic and non-photosynthetic protists which include cryptophytes, heterokonts and alveolates. Within this supergroup of organisms, all have abandoned the phycobilisome, pilfering many of the proteins involved to produce derivative light harvesting proteins. Lastly, a group of dinoflagellates are purportedly tertiary endosymbionts and hence have captured the light harvesting apparatus of a protist and have evolved new light harvesting proteins.
At this point, we need to recognise that limited research has been done to understand the nature of coherent energy transport in the antennae of the greater light harvesting complexes (LHCI and LHCII) in cyanobacteria and its descendants that surround the reaction centres (Schlau-Cohen et al. 2012; Müh and Renger 2012; Wells et al. 2014; Duan et al. 2015; Lewis et al. 2016; Ramanan et al. 2017). Some research has provided numbers for bulk dephasing rates but less on specific vibrational and excitonic coherences; however, some specific oscillations are observed in 2DES. The reason for this is that the LHCs are very energetically complex, with large numbers of chromophores and it is difficult to tease apart specific coherences and energy transfer steps. Hence, while many of the processes we are discussing here are likely to be present in these systems, analysis of smaller systems provides greater insight.
Cyanobacteria, red algae and glaucophytes: the phycobilisome
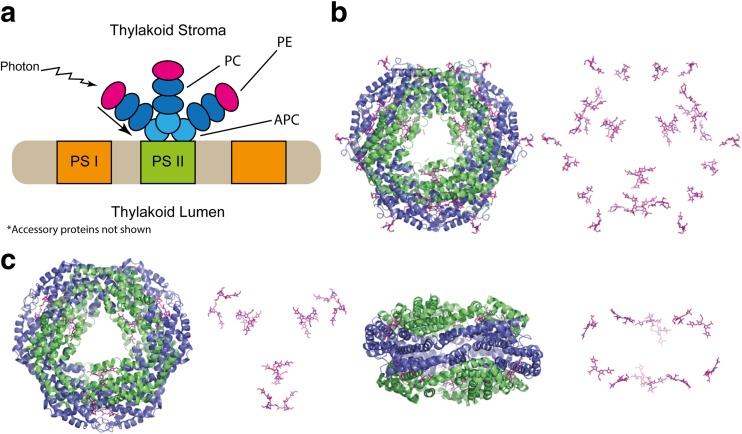
The phycobilisome (PBS) is a large protein complex comprising around 600 polypeptides arranged in large rod-like stacks directed outward asteriform from photosystem II in the thylakoid of cyanobacteria, red algae and glaucophytes. The main chromophorylated proteins are the phycobiliproteins which are αβ heterodimers arranged into trimer rings (αβ)3 and/or hexamer double rings ((αβ)3)2 with covalently attached linear tetrapyrrole chromophores as the main light harvesting pigment. The phycobiliproteins that comprise each stack have been sorted into three main categories. At the end, distal to the photosystem are the bluest absorbing complexes—the phycoerythrins. Further down the stack, the absorption and emission becomes redder through phycocyanin and allophycocyanin, respectively, until the photosystem is reached (Fig. 3a) (Zhang et al. 2017). A certain degree of ingenuity should be noted in this complex, whereby having a synchronised energetic and spatial gradient, excitations are efficiently funnelled by virtue of the uni-directionality of the system. In other systems, such as the chlorosome, the energetic gradient through space is non-existent and despite the lack of funnelling, efficiency is maintained and must be derived from other means.
Fig. 3.
a Morphology of the phycobilisome light harvesting apparatus. Also highlighted is the flow of energy down the phycobilisome. Note that the relative amounts of each PE/PC are light dependent. b Crystal structure of R-PE (PDB: 1b8d) with and without protein. c Crystal structure of APC in two orientations (PDB: 1kn1) with and without protein. Structures rendered in PyMol
There is not a great deal of experimental evidence suggesting the existence of large-scale quantum effects in the PBS light harvesting system. This may be because the spatially ordered energy funnel functions efficiently via FRET without the need for other phenomena. Additionally, the density of chromophores in PBS is lower than that in other light harvesting systems, with no chromophores in physical contact with each other. The most significant quantum effect in the PBS is that some individual pairs of chromophores are strongly coupled to form delocalised excitonic states (coherence between the individual chromophores) resulting in energy splitting, and hence, broader spectral absorption and overlap between the chromophores of different phycobiliproteins. To date, no studies have analysed energy transfer through this structure as a whole using coherent techniques and very little data is available on the dynamics of energy flow through the system tracking individual pigments. Broadly, in cyanobacteria, energy flows from C-phycocyanin to allophycocyanin to the photosystem with fluorescence rise times of 60 ps and 120 ps while in red algae, energy flows from B-phycoerythrin to C-phycocyanin to allophycocyanin to the photosystem with fluorescence rise times 30, 57 and 150 ps (Yamazaki et al. 1984).
In the allophycocyanin (APC) trimer, delocalised excitonic effects have been observed by circular dichroism spectroscopy where three electronic transitions exist as opposed to one electronic transition in individual subunits (Zhang et al. 2001). Furthermore, two-colour pump-probe polarisation anisotropy highlights the existence of delocalised excitonic states from large initial anisotropy which decays over around 550 fs (Womick and Moran 2009). Splitting generates a dramatic change in the absorption spectrum and pushes the fluorescence maximum toward the red (lower energy), increasing spectral overlap with the reaction centre absorption (Karpulevich et al. 2016). Quantum beats are observed in APC and appear to decay within 35 fs and correspond to a Raman active frequency that is also resonant with the exciton energy gap. Vibronic mixing is also reported in APC, which increases the rate of internal conversion and subsequent relaxation to the lowest excited exciton state (Womick and Moran 2009).
The initial anisotropy of individual C-phycocyanin (C-PC) monomers is minimal, suggesting exciton effects are not present until they are arranged into a quaternary structure (Edington et al. 1995). In C-phycocyanin, it has been revealed that the predominant coherences observed originate from vibrations in the excited state and are damped over 330 fs; however, these do not correspond to the energies of the exciton gaps (Womick et al. 2012). It is possible that a vibrational mode may become delocalised over the excitons. This delocalisation may be derived from oscillations in the dipole moment stimulated by the vibration which affects the neighbouring chromophores or may be a symptom of the excitons being delocalised over the same sites.
In R-phycoerythrin (R-PE), phycourobilin chromophores act as primary acceptors and their excitations are localised whereas the innermost pair of phycoerythrobilin (PEB) chromophores is strongly coupled with the excitons possibly delocalised across them. Quantum beats have been observed in this system (Womick et al. 2011); however, no strong case has been made for whether this is excitonic or vibrational in origin.
Thus, to date, it does not appear as though extended coherent quantum effects are present in the phycobilisome. That said, splitting due to strong coupling and vibronic effects via coupling to vibrational modes may play an important role in both widening the absorption spectra (and hence the spectral overlap between donor and acceptor states) and allowing for efficient down conversion of excitation energy, respectively.
Lessons from studying the phycobilisome
The phycobilisome appears to be unique, in that unlike other light harvesting complexes discussed, it does not appear to produce a slew of quantum beats in ultrafast spectroscopy. The only evidence for non-trivial quantum phenomena is the observation of exciton splitting resulting from the juxtaposition of chromophores. Yet, it is an efficient antenna system: relying on the coincidence of spatial and energetic gradients from the tips of the rod structures to the central APC core that leads to the photosystem reaction centre. The lesson appears to be that one does not need to utilise quantum transport to make a photon efficient photosynthetic complex.
Cryptophytes
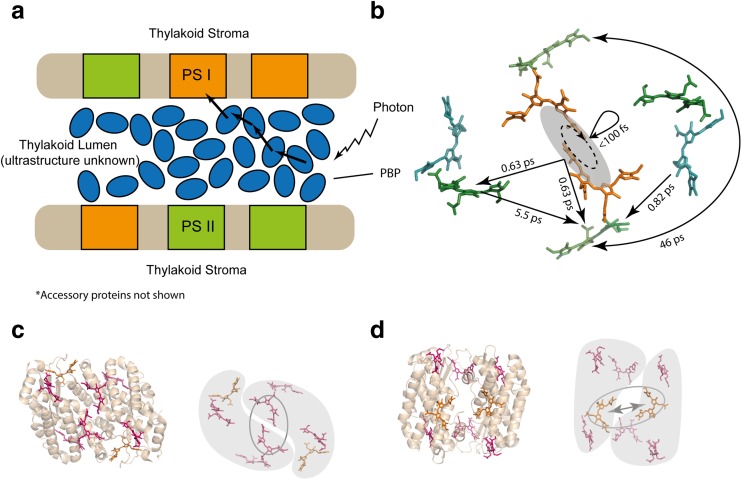
The light harvesting antennae of the cryptophytes are derived from the phycobilisome of an ancestral red alga, a product of secondary endosymbiosis. The soluble cryptophyte phycobiliproteins comprise an asymmetric heterodimer of dimers (usually (α1β)(α2β)), where the β subunit has evolved from the red algal β subunit, while the usually different α subunits (α1 and α2) are novel peptides. Each α subunit carries one covalently linked linear tetrapyrrole chromophore while each β subunit carries three covalently linked linear tetrapyrrole chromophores. These protein assemblies are transported across the thylakoid membrane and reside within the lumen (Fig. 4a) as opposed to the stroma for the phycobilisome (Gould et al. 2007). The β subunit is encoded by a single plastid gene while the genome of the cryptophyte Guillardia theta shows multiple, nuclear-encoded α subunit genes (Curtis et al. 2012). It is unknown if these cryptophyte phycobiliproteins are arranged in an ordered assembly within the thylakoid lumen or reside as a concentrated solution (or glass) of proteins; however, electron dense material is observed in the lumen (Fig. 4a) (Wehrmeyer 1970).
Fig. 4.
a Morphology of the cryptophyte light harvesting apparatus and suggested energy transfer pathway. b PC645 energy transfer rates as illustrative of other PBPs. Energy transfer and relaxation are shown on one half of the dimer to avoid crowding the figure. Coherences are shown as dashed ellipses (PDB: 4lms). c Crystal structure of the closed form PE545 with and without protein. The two dimer halves are shaded and the central pair is circled (PDB: 1xg0). d Crystal structure of the closed form PE555 with and without protein. The two dimer halves are shaded with the central pair circled and arrows highlighting their increased separation. (PDB: 4lmx). Phycocyanin, green; mesobiliverdin, blue; dihydrobiliverdin, orange; and phycoerythrin, red. Structures rendered in PyMol
Crystallographic studies have shown that two distinct quaternary forms exist within the cryptophyte phycobiliprotein family that have been called the closed and open forms (Fig. 6c, d, respectively) (Harrop et al. 2014). The closed form exhibits a central pair of chromophores that are in van der Waals contact across the dimer interface, while the open form has a central, water-filled channel (Harrop et al. 2014; Wilk et al. 1999). The open form quaternary structure has been generated by a single residue insertion into the cryptophyte-derived α subunit which results in the separation of the central chromophore pair having dramatic effects on exciton states, energy structure and observed quantum beats. This open state appears to be confined to the Hemiselmis genus while the closed state appears to be the ancestral form. The time scales of energy transfer between proteins have not been worked out, nor has the time scale for transfer to the photosystem reaction centre. However, within each protein, time scales have been found for transfer events between chromophores (or excitons), relaxation between exciton states and the fluorescence lifetime of 2.5 ns (Doust et al. 2004). Both open and closed states show some coherent dynamics, most of which are vibronic in nature (Arpin et al. 2015).
Fig. 6.
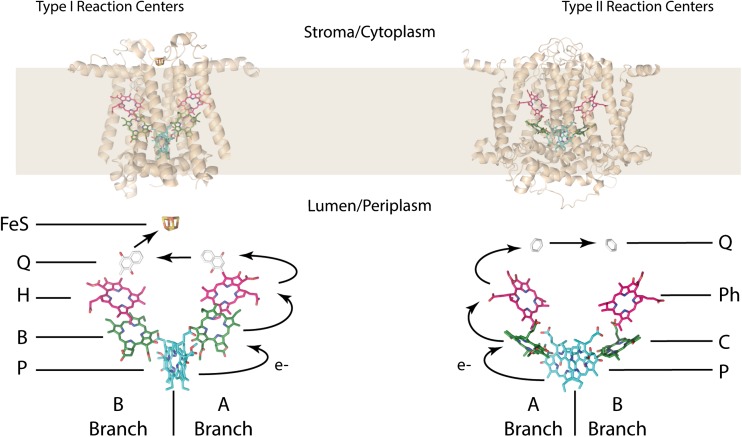
Structure of the reaction centres (types I and II) with labelled chromophores and electron transport between them. Reaction centres have been stripped of accessory peptide chains and other electron transport chain proteins (PDB: 3ZKI - type II and PDB: 1JB0 - type I). Structures rendered in PyMol
Closed form cryptophyte phycobiliproteins
Two closed form phycobiliproteins have been extensively characterised by laser spectroscopy: PC645 from the cryptophyte Chroomonas sp. CCMP270 and PE545 from Rhodomonas sp. CS24. In PC645, there is a noted presence of energy level splitting due to the close proximity of the central chromophores generating delocalisation and coherence firstly between chromophores, generating excitons, and secondly, coherence between the excitons (MacColl et al. 1999). Excitations are funnelled from dihydroviliberdin (DBV) chromophores in the central dimer to any of the phycocyanobilin (PCB) chromophores of the complex in 0.63 ps which show concerted dynamics and decay over 0.6 and 5.5 ps, respectively (Fig. 4b) (Marin et al. 2011). Excitation energy transfer is concluded by an energy transfer step to the lowest energy PCB and decays within 44–46 ps. Energy can also be transferred from a mesobiliverdin (MBV) to PCB on a time scale of 0.82 ps (Marin et al. 2011).
Within the central dimer, internal conversion processes producing a decay between exciton states are evidenced by a rotation in the dipole moment (Marin et al. 2011). Oscillatory features are evidenced between the DBV dimer states and also separately with the excited MBV state over 400 fs (Collini et al. 2010). The oscillations of the upper DBV state with MBV have a frequency confluent with the energy difference between the states indicative of electronic coherence; however, these observations may also be vibrational in origin, as reported (Turner et al. 2011). Initial studies using two-colour laser spectroscopy to excite specific coherences assigned a ladder of coherences to separable vibrational and electronic states to the DBV dimer, decohering at 900 fs and 300 fs, respectively (Richards et al. 2014). However, a follow-up study was able to discern the vibronic nature of these states by examining the two-colour spectra as a function of laser intensity (Novelli et al. 2015). With increasing intensity, one particular mode shows saturation (as opposed to a cubic increase as expected and observed for other modes). The only models that can accurately predict this behaviour are vibronic where the central DBV dimer is coupled to vibrational modes with this vibronic coherence decaying over 280 fs. In the DBV-PCB cross peaks, a quantum beat is observed which endures for 350 fs, showing indications of electronic coherence while being close in energy to vibrational states (Dean et al. 2016). Coherence between these exciton states would be remarkable given the large detuning away from energetic resonance with each other. This quantum beat may either be due to intensity borrowing from vibrations on each chromophore or, more likely given the energy gap involved, due to vibronic states resident across multiple chromophores from vibrations distorting the electronic state. For energy transfer purposes, it is proposed (Dean et al. 2016) that 3–4% of the excitation is transferred from the DBV pair to PCB chromophores in the vibronic state each vibrational period. This effect acts as a mechanism of directing energy flow across very large gaps in energy out of resonance and is 3.5 times the rate predicted by FRET.
Due to the residence of the phycobiliproteins in the thylakoid lumen, energy transfer must be robust to alterations in pH values (or if not, must provide some alteration to energy transfer to avoid oxidative stress). For pH values between 5.7 and 7.4, oscillatory dynamics appears to be robust for PC645 (Turner et al. 2012). At all pH values, modes decay on time scales of 170–300 fs. Hence, the protein appears to protect quantum beats against pH quenching while fluorescence is pH dependent, possibly due to the build-up of excitation at the red-most chromophore at the periphery of the protein.
In the closed form PE545 from Rhodomonas sp. CS24, the excitation is funnelled from four peripheral PEB chromophores to a central dimeric pair of PEBs within 1 ps, then transferred to lower energy DBV chromophores at the periphery over 4 ps, and finally relaxes to the red-most chromophore (one of the DBVs) in 22 ps (Doust et al. 2004). In PE545, exciton splitting in the central dimeric pair of PEBs is observed as with PC645 (MacColl et al. 1999). Quantum beat oscillations are noted in transient absorption kinetics which are in agreement with vibrational frequencies (Doust et al. 2004). With delocalisation of the excited state across the dimer pair, it is suggested that quantum coherence would aid in the eventual selection of the trap state at the red-most PEB (Doust et al. 2004). Similar to PC645, an oscillation between the central pair and peripheral PEBs is noted and dephases over 130 fs which is possibly due to vibronic resonance (Collini et al. 2010). In contrast, a control PBS protein with a single PEB attached does not show such quantum beats (Wong et al. 2012). The phase and amplitude of the cross peaks in PE545 show electronic (or at least vibronic) coherence signatures and the absorption energies are very well correlated with the emission energies damped over 100 fs.
Open form cryptophyte phycobiliproteins
Open form phycobiliproteins comprise the other type of cryptophyte light harvesting protein and they appear to be confined to the Hemiselmis clade, namely PC577 from Hemiselmis pacifica and PC612 from Hemiselmis virescens—which have a similar chromophore composition to PC645, and PE555 from Hemiselmis andersenii—that has the same chromophore composition as the closed form PE545 (Harrop et al. 2014). Despite the water-filled hole present in PE555 (suggesting little exciton splitting and hence no coherence between these states), quantum beats are still observed and persist over 100 fs (Collini 2012; Harrop et al. 2014). This was determined by examining shape and amplitude correlations of 2DES peaks and is, hence, indirect and requires further study. Quantum beats are also observed directly on cross peaks in 2DES; however, these are weak and vibrational in character (Harrop et al. 2014).
PC612 does not show signs of exciton splitting nor does it produce quantum beat signals due to the presence of the large water-filled hole between the central pair of DBV chromophores, decreasing their coupling (MacColl et al. 1999; Harrop et al. 2014). In PC612, excitation is funnelled from DBV to PCB with constants of 0.6 and 15 ps with slower transfer between PCBs compared to PC645 (McClure et al. 2014). In PC577, coherent oscillatory dynamics are evident in the first 1.5 ps (with a dephasing rate of 0.4 ps), possibly due to vibrational coherence, given phase changes across the spectrum. Furthermore, in PC577 (McClure et al. 2014), oscillatory dynamics decaying over 62 fs and 46 fs is observed which may correspond to reorganisation of the solvent in the water-filled hole. This generates a “solvent quake” which is observed as a redshift in emission energy. This mechanism may be involved in the functioning of the central pair of open form PBPs that is now separated and their interaction mediated by the solvent. However, the organisation of the solvation shell may be irrelevant to energy transfer.
Lessons from studying cryptophyte phycobiliproteins
A general theme begins to emerge from these results when taken as a whole. Firstly, in the closed form, exciton splitting from the strong coupling of the central chromophore pair is an important feature as it broadens the absorption spectra and increases the magnitude of the dipole moment of the excitation (depending on the orientation of the constituent dipoles). In contrast, for the open form, the introduction of a water-filled hole between the central pair appears to reduce the strength of quantum beats. Secondly, some of the quantum beats observed seem to be derived from vibronic coherences (in the closed form) or at least vibrational and excitonic coherences that are energetically resonant. Thirdly, the elucidation of the origin of quantum beats is a hard problem. It is not fully understood if quantum coherence helps to guide the selection of a trap site or whether this is a classical phenomenon.
From all the light harvesting systems discussed up to this point, it appears that electronic coherence alone cannot produce quantum beats with lifetime longer than 100 fs. Instead, consensus suggests that vibronic states are required to generate robustness against environmental dephasing. Furthermore, it is not understood how all these proteins fit together as elements of a greater light harvesting system within the thylakoid lumen, nor is it understood whether coherence is maintained between proteins and how a trap site is selected globally. To conclude, an important question can be asked: as efficient funnelling to the lowest energy state within a protein is observed, how is it that the excitation can then hop to the reaction centre which may be many proteins away?
Dinoflagellates
The photosynthetic dinoflagellates are the product of yet another series of endosymbiotic events deriving their chloroplast from different sources. The dinoflagellates that utilise peridinin as their main light harvesting carotenoid form a group of organisms. They contain a membrane-bound system similar to the LHC of plants and a water-soluble protein containing peridinin and chlorophyll a (peridinin chlorophyll-binding protein—PCP). PCP resides in the lumen and each PCP contains eight peridinins and two chlorophylls in a quasi-2-fold symmetric arrangement that is encapsulated in an α-helix-rich protein shell (Fig. 5) (Hofmann et al. 1996). Each chlorophyll is surrounded by four peridinin chromophores closely packed within the centre of the peripheral protein cage. Excitonic coherence across the entire quasi-2-fold symmetric chromophore arrangement has been suggested on theoretical and experimental grounds to explain strong circular dichroism signals that are observed (Bricker and Lo 2015). The energy transfer mechanism of PCP is unique. The closely packed nature of PCP chromophores gives rise to an interesting quantum behaviour. Strong coupling increases the delocalisation of excitation while strained conformations of the molecules allow controlled dynamic localisation of the state. The localised state results in a longer lived higher excited states due to mechanically unavailable relaxation pathways (Roscioli et al. 2017).
Fig. 5.
a Morphology of the dinoflagellate light harvesting apparatus with putative energy transfer path. b Crystal structure of PCP monomer (PDB: 1ppr) in two orientations and with protein and lipid stripped away highlighting chlorophyll (in green) surrounded by 4 peridinins each (in magenta). Structures rendered in PyMol
Energy transfer processes are initially mediated by a mixed chlorophyll-peridinin exciton state upon excitation of peridinins and electronic coherence effects are confined to 50 fs in Amphidinium carterae. Off-diagonal peaks in 2DES measurements between peridinins and between chlorophyll and peridinin decay rapidly (dephasing time of 20 fs) due to the excited state’s vibrational motions distorting the peridinins by torsional modes thereby transforming the structure of the ordered chromophore cluster and localising the excitation on one of the chlorophylls. The rate of energy transfer to the lowest energy state is estimated to be around 2 orders of magnitude faster than what is predicted by Förster transfer. The initial quantum coherent process may account for 50–70% of the very high efficiency (90%) for energy transfer from peridinin to chlorophyll.
Lessons from studying dinoflagellates
Initial energy transfer is very fast (~ 20 fs) and thus cannot be FRET. Instead, initial dynamics proceeds through a very complex energy transfer mechanism using delocalisation and rapid decay after a specific molecular motion. The PCP complex has not been analysed in depth for quantum coherence. This is true for many other peripheral light harvesting proteins (such as water-soluble chlorophyll-binding proteins) which may also harbour curious quantum behaviours owing to carotenoid/chlorophyll mixtures. Part of reason for the lack of research on some of these proteins is their congested spectra, limiting the ability to discern specific origins for spectral features. As a crystallographic assembly, PCP forms a trimer. It is unclear whether or not coherent energy transfer occurs between each monomer and perhaps may function in a similar manner as the FMO complex. Finally, the ultrastructural arrangement of the PCP trimer within the thylakoid lumen is not known; hence, there is no clear model as to how the excitation moves from PCP to the target photosystem reaction centre.
Reaction centres
The role of the reaction centre (RC) is to develop a usable charge separation (voltage) that can ultimately be used to produce a free H+ gradient across the thylakoid membrane which then drives the ATPase to transform ADP to ATP, thus storing chemical energy. Typically, a charge separation event is initiated by the excitation of two central chromophores (the special pair) which results in an electron being passed along a chain of other chromophores until it reaches a quinone. However, this is not always the case as some other pathways are present where charge separation is initiated on chromophores other than the special pair. In any case, the special pair acts as the terminal electron donor. There are variations on this basic theme and studies either analyse the reaction centre from purple sulphur bacteria or the reaction centre of photosystem II (PSII) from cyanobacteria. The reaction centres across the entire cyanobacterial lineage are genetically related with minor tweaks in chromophore composition and, therefore, some recent data in green plants will be included. Reaction centres show a distinct quasi-symmetry in the chromophoric arrangement with only slight asymmetry in the special pair of chromophores that generate charge separation and direct the excitation down one branch of the quasi-symmetric dimer (Fig. 6).
Quantum coherences in this system are a little more intricate due to the charge transfer (CT) states. Because electron-deficient and electron-abundant chromophores are now possible states of the system, we must consider them as valid states in our formalism and not just consider electronic excitations in the form of excitons. Because the exciton states are coupled to the CT states, we must consider the possibility of a coherent superposition of them. A superposition of exciton and CT states may aid in robustness, allowing the system to sample two different charge transfer pathways simultaneously before the CT state has even been selected and localised. We must consider what sorts of coherence would help charge transfer—charge transfer relies on the overlap of the donor and acceptor (initial and final) electronic orbital states which is a very short-range effect and hence molecular motions in the form of vibrations may potentially help guide selection of a CT state and the subsequent pathway robustly.
Some of the earliest signatures of coherence in the reaction centre were from electron paramagnetic resonance (EPR) studies where the appearance of nuclear quantum beats was found. These studies look at the response of an unpaired electron to a magnetic field over time and have found a coherent resonance pattern (quantum beats between spins) (Link et al. 2001). These quantum beats were ascribed to coherent nuclear motions but more modern work focuses on optical studies into these systems.
Type II reaction centres
The excitation and charge transfer kinetics of type II reaction centres (those of purple bacteria and the cyanobacterial photosystem II) follow from the photoexcitation of the special pair (the two delocalised excited exciton states denoted as P (lower) and P* (upper)) either from an antenna, from chlorophylls in the greater LHCII (for cyanobacteria) or from (bacterio)chlorophylls in the RC itself (BA or BB shown in Fig. 6). The dynamics of the exciton generated on the special pair and the surrounding electrostatic environment generates a charge separation where an electron from the special pair is transferred directly to a bacteriopheophytin on the A branch of the quasi-2-fold dimeric structure (denoted HA shown in Fig. 6) in 3 ps or via an intermediate bacteriochlorophyll on that same branch (BA) in 3 ps and then 1 ps. Charge separated states are denoted as PA+HA− or PA+BA−, the minus sign stating the position of the electron. The electron is then transferred to a quinone (QA) in 200 ps and onto the opposing quinone (QB) in 100 μs and moved along the electron transport chain which ultimately generates a proton gradient. Note that the A/B branch notation changes for either eukaryotes or prokaryotes but will be used as standard throughout.
In reaction centres with an oxidised special pair (which disallows charge separation), quantum beats are observed to persist for 400 fs on H and B (Lee et al. 2007). Generally, spectroscopic methods cannot distinguish features from either the A or B branches in the reaction centre due to their isoenergetic nature. Quantum beats present between H and B match their energy difference and hence may be due to electronic coherence (Westenhoff et al. 2012). This is accompanied by many other quantum beats possibly due to vibrational and electronic states. Further beats are possibly derived from the electrostatic response of the protein. Models including a mode that is localised to H semi-adequately describe the data; however, vibrational modes coupled to H and B produce almost identical changes to the spectra immediately after excitation. These data, however, also suggest that the coherence between H and B outlives populations of excited states upon them which presents a problem. This could be due to correlations in nuclear motions modulating the energy levels of B and H excitations. This modulation produces correlated fluctuations of the exciton states of P and B enabling the transfer of quantum coherences induced by the environment. Another proposal is that vibrational coherences, although derived from the excited state of a chromophore, continue to oscillate in the ground state, which assumes the ground and excited states have similar vibrational structures (Paleček et al. 2017). Excitation generates a coherence that is purely vibrational on B or has a mixed vibronic character over B and H and, upon energy transfer to P, this quantum beat is shifted to the ground state of B or H. This would result in a π phase shift which has been observed during the characteristic time of transfer to P and hence it may not be necessary to invoke wavelike transfer in the reaction centre (Paleček et al. 2017). Thus, the accessory chromophore B may act as a sink of energy accelerating transfer by retaining vibrational energy following exciton transfer.
In non-oxidised reaction centres, slow quantum beats are observed between the excited states of the special pair across a number of frequencies and do not fully dephase after charge separation. Further quantum beats are seen in the bacteriochlorophyll region of the spectra concurrent with a redshift of the absorption peak upon charge separation (Vos et al. 1991, 1994). Oscillatory dynamics are observed over the first 2 ps of spectral evolution but become highly irregular after a few hundred femtoseconds—possibly indicating coherence is lost on a hundred femtosecond time scale. There are a few quantum beats of interest. Firstly, ultra-low frequency quantum beats are perhaps related to inter-chromophore or local concerted protein dynamics. These dynamics facilitate the energetic movement of the state to a position where the energy difference to the charge separated state is zero. The low frequency modes are not thermally relaxed on the time scale of charge separation (over 500 fs). These low frequency modes on the special pair exist along the charge transfer reaction coordinate and are associated with the formation of the charge separated state. Secondly, a higher frequency mode is observed which abruptly dephases between 200 and 800 fs. This higher frequency quantum beat is possibly an anharmonic vibrational motion coupled to and arising from the special pair exciton states. The vibrational motion on the special pair exciton state alters the charge distribution, oscillating over the pigment complex leading to a distribution where charge separation can occur (and thus the P+B− state) (Spörlein et al. 1998). This oscillation is also observed with many of the same features in reaction centres lacking a bacteriochlorophyll band, hence justifying the oscillations origin as the special pair (Streltsov et al. 1998). Furthermore, in mutant reaction centres with alterations in the local protein environment, a reduced amplitude of this mode is observed and correlates to a reduced charge transport rate. All these quantum beats appear to be activated by the P to P* transition and not thermal motions (Vos et al. 1998).
Coherences in the signal assigned to the electrochromic shift (a colour change due to local electric fields and redox reactions—namely charge separation) of the bacteriochlorophyll absorption band suggest the possibility of coherent electron transfer to the P+HA− state. One of the ultra-low-frequency quantum beats perhaps modulates the electrochromic shift (not just the P to P* transition) and given spectral dependence of this low frequency mode is coupled to the formation of the BA− state. There is also the possibility of another channel via which charge transfer can occur, initiated by the formation of a B* state which decays to a P+B− state with a time scale of 0.2–0.5 ps (Ma et al. 2018). The coherence lifetime of these dynamics is 54 fs, highlighted by the anti-diagonal broadening in 2DES. The frequencies observed in the dynamics correspond to mainly vibrational modes and are resonant with the energy difference giving rise to vibronic resonance between the pathways.
Type I reaction centres
The excitation and charge transfer kinetics of type I reaction centres (that of photosystem I and the analogous green bacterial RC) are similar to those of purple bacteria and PSII. The photoexcitation of the special pair (again denoted as P and P*) either from an antenna or from a chlorophyll/pheophytin (CA/B or PhA/B shown in Fig. 6) initiates the reaction. For simplicity, much of the nomenclature concerning the branches from purple bacteria will be used here. Two main pathways are noted in charge separation kinetics. The first of which follows charge separation on the special pair transferring to the accessory chlorophyll (P+C−) then on to the pheophytin state (P+Ph−). The second pathway is the direct charge separation of the chlorophyll and pheophytin (C+Ph−) followed by the charge separation across the special pair and the pheophytin (P+Ph−). The excitation is then transferred to quinones and on to the metal cluster.
The type I reaction centre component of cyanobacteria through higher plants is strongly coupled to low-frequency chromophore and protein modes as in type II reaction centres (Sarkisov et al. 2006). A large complement of quantum beats are observed with a broad range of decay times (< 2.5 ps). Many of the quantum beats are resonant with the energy gap involving special pair exciton states with similar frequency to that of type II reaction centres (Fuller et al. 2014). Simulations of a vibronic model are in good agreement with the data. This resonance appears to speed up energy relaxation, energy transport and charge separation, which comes in bursts of population (probability) increase of the charge separated state in simulations.
Of particular interest is the possibility of process coherence across multiple transfer pathways: the system may sample multiple pathways at once in a quantum superposition. Excited states of the chlorophylls are isoenergetic, so multiple chromophores can act as the primary electron donor. The two routes are via the special pair-chlorophyll charge-separated state coupled to the special pair exciton state (pathway I), or via an exciton/charge separated chlorophyll-pheophytin state (pathway II) (Romero et al. 2014, 2017). The three states initiating the two transfer pathways have varying degrees of delocalisation. Two separate quantum beats have been observed that suggest vibronic coupling firstly between the states of pathway I and secondly between all three states. Vibronic states again emerge due to the confluence of the exciton energy gap and the energy of one vibrational quantum. Coherences present across the primary transfer states initiating separation suggest a mechanism of coherent charge transfer showing long-lived coherences over 500 ps. Considering these states initiate both pathways, it is possible that this allows sampling of the energy landscape before selecting the most viable path given the present state of the environment.
Lessons from studying reaction centres
Along with harbouring exciton states, reaction centres are also capable of producing coherent charge separated states. As appears throughout light harvesting systems, these states are vibronic in nature. Coherence seems to play an important role in selecting the reaction path from an initial superposition of possible electron transport pathways. This is potentially a means of decreasing the back reaction of charge transport and also a means of increasing robustness to the environment by selecting a more efficient transfer path. The vibronic nature of these exciton-charge transfer states appears to be an efficient mechanism by which the electron state moves along the reaction path by emitting a vibration. Vibrations appear to also remain coherent after the excited state has transferred away from a particular molecule and the net effect of this on efficiency (if any) is unknown. Lastly, little is known about coherence and energy transfer in the greater membrane-bound light harvesting complexes, of which the reaction centre forms a central part, in cyanobacteria and its descendants. This is possibly due to the energetic disorder in the system obfuscating analysis.
Bacteriorhodopsin
Many photosynthetic archaea and some halophilic bacteria generate a useable proton gradient directly without the need of an oxidation step. In the archaeon Halobacterium salinarum, this mechanism of energy production is switched on when oxygen levels decrease below the level needed for effective respiration. The proteins responsible for this are rhodopsins (or more specifically bacteriorhodopsin). Bacteriorhodopsin is composed of a homo-trimer of seven transmembrane helix proteins each with a single retinal chromophore embedded in the centre. The photo-process that drives this proton pump is as follows: all-trans retinal is excited to a higher electronic orbital upon excitation from a photon, and it then relaxes to its ground state with some of the excitation energy moving the retinal into a different 13-cis conformational state. The new local dielectric environment and pH value around the retinal coaxes the deprotonation of the Schiff base of the retinal which releases a proton into the extracellular space. The retinal then rotationally relaxes and is reprotonated from the cytoplasmic space. There is no antenna here to capture photons, just a single absorption event and conformational change.
Vibrational and rotational states and their coherence are of importance in both the motion between retinal isomers and the dynamics in the isomerised state for efficient and robust deprotonation. However, just as vibrational coherence can affect the nature of electronic coherence, the converse is also true. The electronic and vibrational states are not necessarily separable and the process of isomerisation may sojourn through a vibronically coherent path. The process of retinal isomerisation takes approximately 1 ps to complete (Kraack et al. 2011). Following isomerisation, a complex and concerted series of conformational changes in the protein along with solvent reorganisation is generated to drive the charge transfer event and to offer alternating access between the cytoplasmic and extracellular space (Nango et al. 2016). These protein structural changes limit the reverse reaction and direct proton flow. Of interest to coherence is the isomerisation event and subsequent relaxation of the retinal and influences on deprotonation and later conformational changes. No evidence is present for coherence between the excited state of retinal and the charge separated state of retinal (i.e. the state of the electron after separation is not kept track of by the state of the retinal). In bacteriorhodopsin, only one charge transfer pathway exists and hence coherence would only influence the efficiency in deprotonation, if at all.
In bacteriorhodopsin, all non-trivial coherences are predominantly vibrational owing to the presence of a single chromophore; however, determining the ground or excited state origin of coherent dynamics is paramount to understanding the relevance of these motions to charge separation. By tuning the excitation pulse to either be resonant or detuned to the red or blue in relation to the absorption maximum, details about the origin of coherent motions can be ascertained. Red detuned excitation (lower energy than the absorbance maximum) highlights ground state vibrational coherences which lie in the high frequency regime whereas blue detuned excitation (higher energy than the absorbance maximum) provides information about the excited state coherences which are of lower frequency in retinal (Kraack et al. 2011). This assignment is supported by wavelength dependence of quantum beat oscillations. When excited by high energy light, energy is redistributed by vibrational relaxation and deposited into low frequency vibrational modes promoting key nuclear motions. The generation of these modes is likely due to impulsive Raman scattering or the reorganisation of higher frequency modes from the ground state. Vibrational coherence of low frequency modes in the excited state decohere over 300 fs—one third of the time scale of retinal isomerisation reported.
Under incoherent illumination, the efficiency of retinal charge separation (photon in—proton out which is seen as a shift in the absorption spectrum of the retinal) is approximately 65%. However, using laser pulse shaping, the efficiency can be optimised or anti-optimised by 24% (Prokhorenko et al. 2006). Optimal illumination consists of periodic electric field amplitude modulations that drive a specific vibrational mode whereas anti-optimal pulse shapes are highly aperiodic and no specific vibrational modes are driven. The mode that is driven by optimal illumination is just slightly off resonant with a torsional mode in the retinal, driving isomerisation in the excited state. This creation of a specific vibrational superposition drives efficient isomerisation and charge transfer by the displacement of the state along the reaction coordinate. This is in contrast to the anti-optimal pulse whose excitation does not itself decrease the excited state population but whose essentially random phase destroys any coherent vibrational motions.
Lessons from studying bacteriorhodopsin
There is a fundamental difference in the mechanism by which bacteriorhodopsin creates a charge separation compared to the photosystems and reaction centres. Bacteriorhodopsin utilises a structural change in retinal that includes the relocation of nuclei, whereas the reaction centres relocate electrons via charge transfer states. Thus, the coherent states associated with the bacteriorhodopsin reaction are primarily vibrational rather than vibronic.
Summary and conclusions
Structural studies, particularly x-ray crystallography, have shown that light harvesting proteins organise and arrange light-active chromophore molecules in order to capture light energy, funnel it to a target, and, in the case of reaction centres, to facilitate the creation of a charge separated state to power photosynthesis. In many of these proteins, chromophores are arranged so that they are at high density, tightly packed and in physical contact. Indeed, the green photosynthetic bacterial chlorosome has jettisoned the protein and stacked chromophores into organised structures within a lipid compartment, gaining maximal chromophore density.
The tight packing of chromophores is usually associated with strong coupling. This coupling means that the chromophores do not act independently when interacting with electromagnetic fields, instead they form delocalised excitons. In the multi-chromophore exciton, the energy levels are split, producing transitions with higher and lower energy when compared to isolated chromophores. Thus, the tight packing of chromophores has two consequences for light harvesting: it increases the physical capture area of the antenna; and it broadens the absorption spectrum. Each of these consequences could be seen as an enhancement of the properties of the antenna complex and hence may be selected for by evolution. In the extreme cases, chromophore packing is extended around circular arrays (LH1/2) or linear arrays (chlorosome). In these cases, the exciton structure is dynamically delocalised. The exciton states can migrate through the system via diffusion. The challenge in these systems is understanding how the excitation can leave the system, ultimately progressing to the photosystem or reaction centre with high probability.
The advent of ultrafast, femtosecond laser technology has facilitated the development of spectroscopic techniques that probe energy transfer pathways through light harvesting and photosynthetic systems and proteins. These measurements have observed quantum beats in most biological light harvesting systems/proteins, with beat lifetimes ranging from tens of femtoseconds through to picoseconds. The presence of beats is a signature of underlying coherent phenomena. The difficulty is determining the nature of these coherences and whether they play a role in photosynthesis and/or light harvesting. When chromophore complexes form excitons delocalised across the chromophore array, beats often arise from superpositions of exciton states. Although interpretation is still mired in controversy, the beat frequencies and decoherence times are usually commensurate with that of vibrational and vibronic states. Purely vibrational coherences are not likely to play key roles in light harvesting; however, we note that they may be important in the function of reaction centers and bacteriorhodopsin. It is also apparent that time scales of coherence and time scales of energy transfer are not separable and nor are the energy scales of couplings and environmental effects in most light harvesting systems. As such, energy transfer lies in an intermediate region that is not strictly classical nor is it purely quantum mechanical. It is an unresolved issue whether or not this enhances or diminishes transfer; however, theoretical studies indicate that energy transfer in this regime enhances transfer rates beyond that of classical hopping and purely quantum transport (Yin et al. 2008; Rebentrost et al. 2009).
There is growing consensus that most observed quantum beat signals that are relevant to energy transport are of a mixed vibronic nature. Vibronic states result from mixing of vibrational states with electronic states in a non-separable manner. Vibronic coherences are likely to be important in energy transport within pigment/protein complexes when the vibrational energy is isoenergetic with the exciton energy gaps. Taken together, there is considerable evidence for non-trivial quantum effects within individual light harvesting and photosynthetic proteins. The presence of strongly coupled chromophores leads to exciton states, with broader spectral coverage and possibly coherent dynamics. Vibronic coherences may play a role in excitation transfer through the protein’s chromophore system, depositing the excitation in some final acceptor or trap state.
In contrast, there is very little evidence for quantum effects being involved in energy transfer between individual proteins. Thus, the pathway from the initially excited protein to the final destination in the reaction centre/photosystem is likely to proceed via a series of incoherent transfers (FRET). Coherent superpositions of chromophores within a protein effectively enhance the rate of transfer to other proteins by increasing the dipole moment leading to super-transfer. For soluble protein systems such as the cryptophytes or the dinoflagellates, little is known about the ultrastructural organisation of these soluble luminal proteins, hence, the pathway to the photosystem remains a mystery. The phycobilisome, however, appears to be a prime example of a very successful light harvesting system that does not utilise coherent phenomena. To date, there is no experimental evidence of any large-scale quantum effects in the assembly. There is, however, evidence for delocalised excitonic states within the individual phycobiliprotein trimers and hexamers, extending their spectral properties and potentially assisting in efficient energy funnelling through the PBS rod structures. The phycobilisome appears to have optimised light harvesting photon efficiency by constructing an antenna with coordinated energy and spatial gradients from the high-energy rod tips to the lowest energy chromophores near the junction of the PBS with the photosystem.
Finally, there is debatable evidence for process coherence in light harvesting systems, namely, coherences that occur between different pathways of a reaction. Specifically, these coherences are observed in reaction centres where several initiating states are coherent before a pathway is selected. Some evidence suggests this occurs during transport through the FMO complex. In purple bacteria, coherences between the exciton states of the B800 ring appear to have quantum control via a phase shift when transported down to the B850 ring. This phase shift would suggest a selection of pathway from an initial superposition based on the state of the environment. This potentially manifests as a means of increasing robustness in energy transfer.
This review only contains a brief overview of observations and analysis of quantum beats in biological light harvesting systems. Some systems have been omitted for the sake of brevity and many more studies exist for most of the systems discussed. While being worthy of discussion, we have omitted some analyses due to their complexity; however, these can be found within the references. New systems are being discovered and more precise methods are being developed to tease apart the nature and origin of quantum beats in light harvesting systems, and more generally, light-activated biological systems.
New horizons
The development of new techniques is needed to further dissect energy transfer pathways. Spectroscopic techniques that show promise in extracting the nature of coherences and the mixing of vibrational and excitonic coherences are higher-dimensional (3D and above) pump-probe techniques (Fidler et al. 2010; Zhang et al. 2013; Oliver et al. 2014; Loukianov et al. 2017; Goodknight and Aspuru-Guzik 2017; Harel 2018; Hutson et al. 2018). In these techniques, different parts of the spectrum (optical and infrared) are excited to generate a fifth-order correlation map of vibrational and excitonic coherences and can also be coupled to different optical phenomena (such as the Stark effect and circular dichroism).
Current experimental techniques require the use of femtosecond laser pulses to prepare the initial state of the system. An open question that needs to be addressed is the initial correlation between chromophore excitations as prepared by the laser. As all the frequencies of the laser pulse are coherent with one another (unlike sunlight), initial correlations can be prepared by the laser between energetically different exciton states. Furthermore, if an excitation is localised to a single chromophore in a coupled system, it is in a superposition of exciton states. Hence, beats would be present in a localised system when excited in the energy basis.
One further issue with most spectroscopic measurements is that all system measurements suffer averaging. That is to say that different members of the ensemble are performing different processes at once and upon averaging, much of the coherent nature is coarse grained away. Using single-molecule techniques, it is possible to forage for individual pathways, their probability and the distribution of time scales for coherence in these systems.
The wrong question: “does evolution select for non-trivial quantum effects?”
The existence of quantum coherent mechanisms takes different forms and plays different roles in different organisms. Namely, quantum coherence can be used for coupling of chromophores via excitons as a bridge between states; efficient funnelling of energy from a higher to a lower exciton state via vibronic coherence; and wavelike transport through clusters and superpositions of states that allow for robustness in choice of energy transfer pathways. The word used is possibly a bad turn of phrase as it implies that quantum mechanisms can be switched on and off, which really gets to the key point about quantum coherence in nature: quantum mechanics is ubiquitous. In many biological processes, the quantum mechanisms are obvious; however, the presence or role of more wavelike transport properties is still debated.
The question of whether or not quantum coherence is selected for may not be the right one to ask. Nature is inherently quantum mechanical and, as such, any interaction must have some obligate quantumness that cannot be switched off. Teasing apart whether efficiency in ordered chromophore assemblies, for example, is driven by coherence or by large interaction energies is an unanswerable question. Analysis of similar light harvesting systems with different coherence times due to temperature or chromophore separation (as in cryptophytes) may be of some benefit in understanding the nature of quantum processes in light harvesting. Regardless, with large interaction energies comes quantum effects which means one cannot discern whether or not coherence is a spandrel or a property that is selected for during evolution.
Acknowledgements
The authors wish to thank Dr.’s Roger Hiller and Ivan Kassal for their detailed critiques of the manuscript and many fruitful discussions.
Funding
HWR acknowledges the support of an Australian Government Research Training Program scholarship. This work was funded by grants from the Australia Research Council grant (DP180103964) and the US Air Force Office of Scientific Research through the Asian Office of Aerospace Research and Development (FA2386-17-1-4101) grants to PMGC.
Conflict of interest
Harry W. Rathbone declares that he has no conflict of interest. Jeffery A. Davis declares that he has no conflict of interest. Katharine A. Michie declares that she has no conflict of interest. Sophia C. Goodchild declares that she has no conflict of interest. Neil O. Robertson declares that he has no conflict of interest. Paul M.G. Curmi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Adolphs J, Renger T. How proteins trigger excitation energy transfer in the FMO complex of green sulfur bacteria. Biophys J. 2006;91:2778–2797. doi: 10.1529/biophysj.105.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin PC, Turner DB, McClure SD, et al. Spectroscopic studies of cryptophyte light harvesting proteins: vibrations and coherent oscillations. J Phys Chem B. 2015;119:10025–10034. doi: 10.1021/acs.jpcb.5b04704. [DOI] [PubMed] [Google Scholar]
- Baghbanzadeh S, Kassal I. Geometry, supertransfer, and optimality in the light harvesting of purple bacteria. J Phys Chem Lett. 2016;7:3804–3811. doi: 10.1021/acs.jpclett.6b01779. [DOI] [PubMed] [Google Scholar]
- Book LD, Ostafin AE, Ponomarenko N, et al. Exciton delocalization and initial dephasing dynamics of purple bacterial LH2. J Phys Chem B. 2000;104:8295–8307. [Google Scholar]
- Bricker WP, Lo CS. Efficient pathways of excitation energy transfer from delocalized S2 excitons in the peridinin-chlorophyll a-protein complex. J Phys Chem B. 2015;119:5755–5764. doi: 10.1021/jp511766j. [DOI] [PubMed] [Google Scholar]
- Cardona T. A fresh look at the evolution and diversification of photochemical reaction centres. Photosynth Res. 2014;126:111–134. doi: 10.1007/s11120-014-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachisvilis M, Kühn O, Pullerits T, Sundström V. Excitons in photosynthetic purple bacteria: wavelike motion or incoherent hopping? J Phys Chem B. 1997;101:7275–7283. [Google Scholar]
- Chachisvilis M, Pullerits T, Jones MR, et al. Vibrational dynamics in the light-harvesting complexes of the photosynthetic bacterium Rhodobacter sphaeroides. Chem Phys Lett. 1994;224:345–354. [Google Scholar]
- Chachisvilis M, Sundström V. Femtosecond vibrational dynamics and relaxation in the core light-harvesting complex of photosynthetic purple bacteria. Chem Phys Lett. 1996;261:165–174. [Google Scholar]
- Chmeliov J, Songaila E, Rancova O, et al. Excitons in the LH3 complexes from purple bacteria. J Phys Chem B. 2013;117:11058–11068. doi: 10.1021/jp400239z. [DOI] [PubMed] [Google Scholar]
- Cohen Stuart TA, Vengris M, Novoderezhkin VI, et al. Direct visualization of exciton reequilibration in the LH1 and LH2 complexes of Rhodobacter sphaeroides by multipulse spectroscopy. Biophys J. 2011;100:2226–2233. doi: 10.1016/j.bpj.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collini E (2012) Differences among coherent dynamics in evolutionary related light-harvesting complexes: evidence for subtle quantum-mechanical strategies for energy transfer optimization. In: Quantum Optics II
- Collini E, Wong CY, Wilk KE, et al. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature. 2010;463:644–647. doi: 10.1038/nature08811. [DOI] [PubMed] [Google Scholar]
- Curtis BA, Tanifuji G, Burki F, et al. Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature. 2012;492:59–65. doi: 10.1038/nature11681. [DOI] [PubMed] [Google Scholar]
- Dahlberg PD, Norris GJ, Wang C, et al. Communication: coherences observed in vivo in photosynthetic bacteria using two-dimensional electronic spectroscopy. J Chem Phys. 2015;143:101101. doi: 10.1063/1.4930539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JC, Mirkovic T, Toa ZSD, et al. Vibronic enhancement of algae light harvesting. Chem. 2016;1:858–872. [Google Scholar]
- Delaye L, Valadez-Cano C, Pérez-Zamorano B (2016) How really ancient is Paulinella chromatophora? PLoS Curr 8 10.1371/currents.tol.e68a099364bb1a1e129a17b4e06b0c6b [DOI] [PMC free article] [PubMed]
- Diffey WM, Homoelle BJ, Edington MD, Beck WF. Excited-state vibrational coherence and anisotropy decay in the bacteriochlorophyll a dimer protein B820. J Phys Chem B. 1998;102:2776–2786. [Google Scholar]
- Dostál J, Mančal T, Vácha F, et al. Unraveling the nature of coherent beatings in chlorosomes. J Chem Phys. 2014;140:115103. doi: 10.1063/1.4868557. [DOI] [PubMed] [Google Scholar]
- Dostál J, Pšenčík J, Zigmantas D. In situ mapping of the energy flow through the entire photosynthetic apparatus. Nat Chem. 2016;8:705–710. doi: 10.1038/nchem.2525. [DOI] [PubMed] [Google Scholar]
- Doust AB, Marai CNJ, Harrop SJ, et al. Developing a structure–function model for the cryptophyte phycoerythrin 545 using ultrahigh resolution crystallography and ultrafast laser spectroscopy. J Mol Biol. 2004;344:135–153. doi: 10.1016/j.jmb.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Duan H-G, Prokhorenko VI, Cogdell RJ, et al. Nature does not rely on long-lived electronic quantum coherence for photosynthetic energy transfer. Proc Natl Acad Sci. 2017;114:8493–8498. doi: 10.1073/pnas.1702261114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H-G, Stevens AL, Nalbach P, et al. Two-dimensional electronic spectroscopy of light-harvesting complex II at ambient temperature: a joint experimental and theoretical study. J Phys Chem B. 2015;119:12017–12027. doi: 10.1021/acs.jpcb.5b05592. [DOI] [PubMed] [Google Scholar]
- Edington MD, Riter RE, Beck WF. Evidence for coherent energy transfer in allophycocyanin trimers. J Phys Chem. 1995;99:15699–15704. [Google Scholar]
- Engel GS, Calhoun TR, Read EL, et al. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature. 2007;446:782–786. doi: 10.1038/nature05678. [DOI] [PubMed] [Google Scholar]
- Ferretti M, Novoderezhkin VI, Romero E, et al. The nature of coherences in the B820 bacteriochlorophyll dimer revealed by two-dimensional electronic spectroscopy. Phys Chem Chem Phys. 2014;16:9930–9939. doi: 10.1039/c3cp54634a. [DOI] [PubMed] [Google Scholar]
- Fidler AF, Harel E, Engel GS. Dissecting hidden couplings using fifth-order three-dimensional electronic spectroscopy. J Phys Chem Lett. 2010;1:2876–2880. [Google Scholar]
- Fidler AF, Singh VP, Long PD, et al. Time scales of coherent dynamics in the light-harvesting complex 2 (LH2) of Rhodobacter sphaeroides. J Phys Chem Lett. 2013;4:1404–1409. doi: 10.1021/jz400438m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller FD, Pan J, Gelzinis A, et al. Vibronic coherence in oxygenic photosynthesis. Nat Chem. 2014;6:706–711. doi: 10.1038/nchem.2005. [DOI] [PubMed] [Google Scholar]
- Goodknight J, Aspuru-Guzik A. Taking six-dimensional spectra in finite time. Science. 2017;356:1333. doi: 10.1126/science.aan2842. [DOI] [PubMed] [Google Scholar]
- Gould SB, Fan E, Hempel F, et al. Translocation of a phycoerythrin α subunit across five biological membranes. J Biol Chem. 2007;282:30295–30302. doi: 10.1074/jbc.M701869200. [DOI] [PubMed] [Google Scholar]
- Harel E. Zooming in on vibronic structure by lowest-value projection reconstructed 4D coherent spectroscopy. J Chem Phys. 2018;148:194201. doi: 10.1063/1.5030402. [DOI] [PubMed] [Google Scholar]
- Harel E, Engel GS. Quantum coherence spectroscopy reveals complex dynamics in bacterial light-harvesting complex 2 (LH2) Proc Natl Acad Sci U S A. 2012;109:706–711. doi: 10.1073/pnas.1110312109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop SJ, Wilk KE, Dinshaw R, et al. Single-residue insertion switches the quaternary structure and exciton states of cryptophyte light-harvesting proteins. Proc Natl Acad Sci U S A. 2014;111:E2666–E2675. doi: 10.1073/pnas.1402538111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D, Panitchayangkoon G, Fransted KA, et al. Dynamics of electronic dephasing in the Fenna–Matthews–Olson complex. New J Phys. 2010;12:065042. [Google Scholar]
- Hayes D, Wen J, Panitchayangkoon G, et al. Robustness of electronic coherence in the Fenna–Matthews–Olson complex to vibronic and structural modifications. Faraday Discuss. 2011;150:459. doi: 10.1039/c0fd00030b. [DOI] [PubMed] [Google Scholar]
- Hildner R, Brinks D, Nieder JB, et al. Quantum coherent energy transfer over varying pathways in single light-harvesting complexes. Science. 2013;340:1448–1451. doi: 10.1126/science.1235820. [DOI] [PubMed] [Google Scholar]
- Hofmann E, Wrench PM, Sharples FP, et al. Structural basis of light harvesting by carotenoids: peridinin-chlorophyll-protein from Amphidinium carterae. Science. 1996;272:1788–1791. doi: 10.1126/science.272.5269.1788. [DOI] [PubMed] [Google Scholar]
- Hutson WO, Spencer AP, Harel E. Ultrafast four-dimensional coherent spectroscopy by projection reconstruction. J Phys Chem Lett. 2018;9:1034–1040. doi: 10.1021/acs.jpclett.8b00122. [DOI] [PubMed] [Google Scholar]
- Jun S, Yang C, Isaji M, et al. Coherent oscillations in chlorosome elucidated by two-dimensional electronic spectroscopy. J Phys Chem Lett. 2014;5:1386–1392. doi: 10.1021/jz500328w. [DOI] [PubMed] [Google Scholar]
- Karpulevich AA, Maksimov EG, Sluchanko NN, et al. Highly efficient energy transfer from quantum dot to allophycocyanin in hybrid structures. J Photochem Photobiol B. 2016;160:96–101. doi: 10.1016/j.jphotobiol.2016.03.048. [DOI] [PubMed] [Google Scholar]
- Kassal I, Yuen-Zhou J, Rahimi-Keshari S. Does coherence enhance transport in photosynthesis? J Phys Chem Lett. 2013;4:362–367. doi: 10.1021/jz301872b. [DOI] [PubMed] [Google Scholar]
- Kraack JP, Buckup T, Hampp N, Motzkus M. Ground- and excited-state vibrational coherence dynamics in bacteriorhodopsin probed with degenerate four-wave-mixing experiments. Chemphyschem. 2011;12:1851–1859. doi: 10.1002/cphc.201100032. [DOI] [PubMed] [Google Scholar]
- Lee H, Cheng Y-C, Fleming GR. Coherence dynamics in photosynthesis: protein protection of excitonic coherence. Science. 2007;316:1462–1465. doi: 10.1126/science.1142188. [DOI] [PubMed] [Google Scholar]
- Lewis Nicholas H. C., Gruenke Natalie L., Oliver Thomas A. A., Ballottari Matteo, Bassi Roberto, Fleming Graham R. Observation of Electronic Excitation Transfer Through Light Harvesting Complex II Using Two-Dimensional Electronic–Vibrational Spectroscopy. The Journal of Physical Chemistry Letters. 2016;7(20):4197–4206. doi: 10.1021/acs.jpclett.6b02280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G, Berthold T, Bechtold M, et al. Structure of the P700(+ )A1(−) radical pair intermediate in photosystem I by high time resolution multifrequency electron paramagnetic resonance: analysis of quantum beat oscillations. J Am Chem Soc. 2001;123:4211–4222. doi: 10.1021/ja003382h. [DOI] [PubMed] [Google Scholar]
- Loukianov A, Niedringhaus A, Berg B, et al. Two-dimensional electronic stark spectroscopy. J Phys Chem Lett. 2017;8:679–683. doi: 10.1021/acs.jpclett.6b02695. [DOI] [PubMed] [Google Scholar]
- MacColl R, Eisele LE, Marrone J. Fluorescence polarization studies on four biliproteins and a bilin model for phycoerythrin 545. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999;1412:230–239. doi: 10.1016/s0005-2728(99)00063-8. [DOI] [PubMed] [Google Scholar]
- Ma F, Romero E, Jones MR, et al. Vibronic coherence in the charge separation process of the Rhodobacter sphaeroides reaction centre. J Phys Chem Lett. 2018;9:1827–1832. doi: 10.1021/acs.jpclett.8b00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Yu L-J, Hendrikx R, et al. Excitonic and vibrational coherence in the excitation relaxation process of two LH1 complexes as revealed by two-dimensional electronic spectroscopy. J Phys Chem Lett. 2017;8:2751–2756. doi: 10.1021/acs.jpclett.7b00824. [DOI] [PubMed] [Google Scholar]
- Maiuri M, Ostroumov EE, Saer RG, et al. Coherent wavepackets in the Fenna-Matthews-Olson complex are robust to excitonic-structure perturbations caused by mutagenesis. Nat Chem. 2018;10:177–183. doi: 10.1038/nchem.2910. [DOI] [PubMed] [Google Scholar]
- Marin A, Doust AB, Scholes GD, et al. Flow of excitation energy in the cryptophyte light-harvesting antenna phycocyanin 645. Biophys J. 2011;101:1004–1013. doi: 10.1016/j.bpj.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-Z, Aschenbrücker J, Miller M, Gillbro T. Ground-state vibrational coherence in chlorosomes of the green sulfur photosynthetic bacterium Chlorobium phaeobacteroides. Chem Phys Lett. 1999;300:465–472. [Google Scholar]
- McClure SD, Turner DB, Arpin PC, et al. Coherent oscillations in the PC577 cryptophyte antenna occur in the excited electronic state. J Phys Chem B. 2014;118:1296–1308. doi: 10.1021/jp411924c. [DOI] [PubMed] [Google Scholar]
- Müh F, Renger T. Refined structure-based simulation of plant light-harvesting complex II: linear optical spectra of trimers and aggregates. Biochim Biophys Acta. 2012;1817:1446–1460. doi: 10.1016/j.bbabio.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Nango E, Royant A, Kubo M, et al. A three-dimensional movie of structural changes in bacteriorhodopsin. Science. 2016;354:1552–1557. doi: 10.1126/science.aah3497. [DOI] [PubMed] [Google Scholar]
- Neilson JAD, Durnford DG. Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes. Photosynth Res. 2010;106:57–71. doi: 10.1007/s11120-010-9576-2. [DOI] [PubMed] [Google Scholar]
- Novelli F, Nazir A, Richards GH, et al. Vibronic resonances facilitate excited-state coherence in light-harvesting proteins at room temperature. J Phys Chem Lett. 2015;6:4573–4580. doi: 10.1021/acs.jpclett.5b02058. [DOI] [PubMed] [Google Scholar]
- Oliver TAA, Lewis NHC, Fleming GR. Correlating the motion of electrons and nuclei with two-dimensional electronic-vibrational spectroscopy. Proc Natl Acad Sci U S A. 2014;111:10061–10066. doi: 10.1073/pnas.1409207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleček D, Edlund P, Westenhoff S, Zigmantas D. Quantum coherence as a witness of vibronically hot energy transfer in bacterial reaction centre. Sci Adv. 2017;3:e1603141. doi: 10.1126/sciadv.1603141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitchayangkoon G, Hayes D, Fransted KA, et al. Long-lived quantum coherence in photosynthetic complexes at physiological temperature. Proc Natl Acad Sci U S A. 2010;107:12766–12770. doi: 10.1073/pnas.1005484107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitchayangkoon G, Voronine DV, Abramavicius D, et al. Direct evidence of quantum transport in photosynthetic light-harvesting complexes. Proc Natl Acad Sci U S A. 2011;108:20908–20912. doi: 10.1073/pnas.1105234108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhorenko VI, Nagy AM, Waschuk SA, et al. Coherent control of retinal isomerization in bacteriorhodopsin. Science. 2006;313:1257–1261. doi: 10.1126/science.1130747. [DOI] [PubMed] [Google Scholar]
- Prokhorenko VI, Steensgaard DB, Holzwarth AR. Exciton dynamics in the chlorosomal antennae of the green bacteria Chloroflexus aurantiacus and Chlorobium tepidum. Biophys J. 2000;79:2105–2120. doi: 10.1016/S0006-3495(00)76458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan C, Ferretti M, van Roon H, et al. Evidence for coherent mixing of excited and charge-transfer states in the major plant light-harvesting antenna, LHCII. Phys Chem Chem Phys. 2017;19:22877–22886. doi: 10.1039/c7cp03038j. [DOI] [PubMed] [Google Scholar]
- Rebentrost P, Mohseni M, Kassal I, et al. Environment-assisted quantum transport. New J Phys. 2009;11:033003. [Google Scholar]
- Richards GH, Wilk KE, Curmi PMG, Davis JA. Disentangling electronic and vibrational coherence in the phycocyanin-645 light-harvesting complex. J Phys Chem Lett. 2014;5:43–49. doi: 10.1021/jz402217j. [DOI] [PubMed] [Google Scholar]
- Rolczynski BS, Zheng H, Singh VP, et al. Correlated protein environments drive quantum coherence lifetimes in photosynthetic pigment-protein complexes. Chem. 2018;4:138–149. [Google Scholar]
- Romero E, Augulis R, Novoderezhkin VI, et al. Quantum coherence in photosynthesis for efficient solar-energy conversion. Nat Phys. 2014;10:676–682. doi: 10.1038/nphys3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero E, Prior J, Chin AW, et al (2017) Quantum – coherent dynamics in photosynthetic charge separation revealed by wavelet analysis. Sci Rep 7. 10.1038/s41598-017-02906-7 [DOI] [PMC free article] [PubMed]
- Roscioli JD, Ghosh S, LaFountain AM, et al. Quantum coherent excitation energy transfer by carotenoids in photosynthetic light harvesting. J Phys Chem Lett. 2017;8:5141–5147. doi: 10.1021/acs.jpclett.7b01791. [DOI] [PubMed] [Google Scholar]
- Sarkisov OM, Gostev FE, Shelaev IV, et al. Long-lived coherent oscillations of the femtosecond transients in cyanobacterial photosystem I. Phys Chem Chem Phys. 2006;8:5671–5678. doi: 10.1039/b605660a. [DOI] [PubMed] [Google Scholar]
- Savikhin S, van Noort PI, Blankenship RE, Struve WS. Femtosecond probe of structural analogies between chlorosomes and bacteriochlorophyll c aggregates. Biophys J. 1995;69:1100–1104. doi: 10.1016/S0006-3495(95)79983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savikhin S, van Noort PI, Zhu Y, et al. Ultrafast energy transfer in light-harvesting chlorosomes from the green sulfur bacterium Chlorobium tepidum. Chem Phys. 1995;194:245–258. doi: 10.1016/0301-0104(95)00019-k. [DOI] [PubMed] [Google Scholar]
- Savikhin S, Zhu Y, Lin S, et al. Femtosecond spectroscopy of chlorosome antennas from the green photosynthetic bacterium Chloroflexus aurantiacus. J Phys Chem. 1994;98:10322–10334. doi: 10.1021/jp953734k. [DOI] [PubMed] [Google Scholar]
- Schlau-Cohen GS, Ishizaki A, Calhoun TR, et al. Elucidation of the timescales and origins of quantum electronic coherence in LHCII. Nat Chem. 2012;4:389–395. doi: 10.1038/nchem.1303. [DOI] [PubMed] [Google Scholar]
- Singh VP, Westberg M, Wang C, et al. Towards quantification of vibronic coupling in photosynthetic antenna complexes. J Chem Phys. 2015;142:212446. doi: 10.1063/1.4921324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spörlein S, Zinth W, Wachtveitl J. Vibrational coherence in photosynthetic reaction centres observed in the bacteriochlorophyll anion band. J Phys Chem B. 1998;102:7492–7496. [Google Scholar]
- Streltsov AM, Vulto SIE, Ya Shkuropatov A, et al. BA and BB absorbance perturbations induced by coherent nuclear motions in reaction centres from Rhodobacter sphaeroidesupon 30-fs excitation of the primary donor. J Phys Chem B. 1998;102:7293–7298. [Google Scholar]
- Strümpfer J, Schulten K. Light harvesting complex II B850 excitation dynamics. J Chem Phys. 2009;131:225101. doi: 10.1063/1.3271348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strümpfer J, Şener M, Schulten K. How quantum coherence assists photosynthetic light-harvesting. J Phys Chem Lett. 2012;3:536–542. doi: 10.1021/jz201459c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyrhaug Erling, Tempelaar Roel, Alcocer Marcelo J. P., Žídek Karel, Bína David, Knoester Jasper, Jansen Thomas L. C., Zigmantas Donatas. Identification and characterization of diverse coherences in the Fenna–Matthews–Olson complex. Nature Chemistry. 2018;10(7):780–786. doi: 10.1038/s41557-018-0060-5. [DOI] [PubMed] [Google Scholar]
- Thyrhaug E, Žídek K, Dostál J, et al. Exciton structure and energy transfer in the Fenna–Matthews–Olson complex. J Phys Chem Lett. 2016;7:1653–1660. doi: 10.1021/acs.jpclett.6b00534. [DOI] [PubMed] [Google Scholar]
- Turner DB, Dinshaw R, Lee K-K, et al. Quantitative investigations of quantum coherence for a light-harvesting protein at conditions simulating photosynthesis. Phys Chem Chem Phys. 2012;14:4857–4874. doi: 10.1039/c2cp23670b. [DOI] [PubMed] [Google Scholar]
- Turner DB, Wilk KE, Curmi PMG, Scholes GD. Comparison of electronic and vibrational coherence measured by two-dimensional electronic spectroscopy. J Phys Chem Lett. 2011;2:1904–1911. [Google Scholar]
- Vos MH, Jones MR, Hunter CN, et al. Coherent dynamics during the primary electron-transfer reaction in membrane-bound reaction centres of Rhodobacter sphaeroides. Biochemistry. 1994;33:6750–6757. doi: 10.1021/bi00188a002. [DOI] [PubMed] [Google Scholar]
- Vos MH, Jones MR, Martin J-L. Vibrational coherence in bacterial reaction centres: spectroscopic characterisation of motions active during primary electron transfer. Chem Phys. 1998;233:179–190. [Google Scholar]
- Vos MH, Lambry JC, Robles SJ, et al. Direct observation of vibrational coherence in bacterial reaction centres using femtosecond absorption spectroscopy. Proc Natl Acad Sci U S A. 1991;88:8885–8889. doi: 10.1073/pnas.88.20.8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrmeyer W. Zur Feinstruktur der Chloroplasten einiger photoautotropher Cryptophyceen. Arch Mikrobiol. 1970;71:367–383. [Google Scholar]
- Wells KL, Lambrev PH, Zhang Z, et al. Pathways of energy transfer in LHCII revealed by room-temperature 2D electronic spectroscopy. Phys Chem Chem Phys. 2014;16:11640–11646. doi: 10.1039/c4cp00876f. [DOI] [PubMed] [Google Scholar]
- Westenhoff S, Palec̆ek D, Edlund P, et al. Coherent picosecond exciton dynamics in a photosynthetic reaction centre. J Am Chem Soc. 2012;134:16484–16487. doi: 10.1021/ja3065478. [DOI] [PubMed] [Google Scholar]
- Wilk KE, Harrop SJ, Jankova L, et al. Evolution of a light-harvesting protein by addition of new subunits and rearrangement of conserved elements: crystal structure of a cryptophyte phycoerythrin at 1.63-A resolution. Proc Natl Acad Sci U S A. 1999;96:8901–8906. doi: 10.1073/pnas.96.16.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womick JM, Liu H, Moran AM. Exciton delocalization and energy transport mechanisms in R-phycoerythrin. J Phys Chem A. 2011;115:2471–2482. doi: 10.1021/jp111720a. [DOI] [PubMed] [Google Scholar]
- Womick JM, Moran AM. Exciton coherence and energy transport in the light-harvesting dimers of allophycocyanin. J Phys Chem B. 2009;113:15747–15759. doi: 10.1021/jp907644h. [DOI] [PubMed] [Google Scholar]
- Womick JM, West BA, Scherer NF, Moran AM. Vibronic effects in the spectroscopy and dynamics of C-phycocyanin. J Phys B Atomic Mol Phys. 2012;45:154016. [Google Scholar]
- Wong CY, Alvey RM, Turner DB, et al. Electronic coherence lineshapes reveal hidden excitonic correlations in photosynthetic light harvesting. Nat Chem. 2012;4:396–404. doi: 10.1038/nchem.1302. [DOI] [PubMed] [Google Scholar]
- Yamazaki I, Mimuro M, Murao T, et al. Excitation energy transfer in the light harvesting antenna system of the red alga Porphyridium cruentum and the blue-green alga Anacystis nidulans: analysis of time-resolved fluorescence spectra. Photochem Photobiol. 1984;39:233–240. [Google Scholar]
- Yin Y, Katsanos DE, Evangelou SN (2008) Quantum walks on a random environment. Phys Rev A 77. 10.1103/physreva.77.022302
- Zhang J, Ma J, Liu D, et al. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature. 2017;551:57–63. doi: 10.1038/nature24278. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Shiu YJ, Hayashi M, et al. Investigations of ultrafast exciton dynamics in allophycocyanin trimer†. J Phys Chem A. 2001;105:8878–8891. [Google Scholar]
- Zhang Z, Wells KL, Seidel MT, Tan H-S. Fifth-order three-dimensional electronic spectroscopy using a pump-probe configuration. J Phys Chem B. 2013;117:15369–15385. doi: 10.1021/jp4046403. [DOI] [PubMed] [Google Scholar]