Abstract
Mechanical stimuli acting on the cellular membrane are linked to intracellular signaling events and downstream effectors via different mechanoreceptors. Mechanosensitive (MS) ion channels are the fastest known primary mechano-electrical transducers, which convert mechanical stimuli into meaningful intracellular signals on a submillisecond time scale. Much of our understanding of the biophysical principles that underlie and regulate conversion of mechanical force into conformational changes in MS channels comes from studies based on MS channel reconstitution into lipid bilayers. The bilayer reconstitution methods have enabled researchers to investigate the structure-function relationship in MS channels and probe their specific interactions with their membrane lipid environment. This brief review focuses on close interactions between MS channels and the lipid bilayer and emphasizes the central role that the transbilayer pressure profile plays in mechanosensitivity and gating of these fascinating membrane proteins.
Keywords: Mechanosensitive ion channels, MscL channels, MscS channels, Piezo channels, Lipid bilayer, Amphipaths, Bilayer curvature
Introduction
Among the mechanosensors found in organisms, from all niches of life, mechanosensitive (MS) ion channels are the fastest primary mechano-electrical transducers, which couple mechanical stimuli to intracellular signaling pathways on a submillisecond time scale. These force-gated (MS) channels have been implicated in numerous mechanosensory transduction processes ranging from cellular osmoregulation in bacteria to the senses of touch and hearing in animals and humans (Martinac and Cox 2016). More recently, our understanding of the common biophysical principles underlying their gating mechanism has been significantly enriched thanks to the progress made in identification and structural information of eukaryotic K2P-type TREK and TRAAK as well as Piezo1 MS channels (Brohawn et al. 2012; Coste et al. 2010; Saotome et al. 2018; Zhao et al. 2018; Guo and MacKinnon 2017). This information has been aided by a series of functional studies investigating inherent mechanosensitive properties of these eukaryotic MS channel proteins. These studies show that the eukaryotic MS channels although structurally very different, are functionally very similar to bacterial MscL and MscS channels (Brohawn et al. 2014a; Berrier et al. 2013; Cox et al. 2016; Syeda et al. 2016).
This short review focuses on close interactions between MS channels and the lipid bilayer. A few examples of protein-lipid interactions between MS channels and the lipid bilayer are given, highlighting the crucial role that the transbilayer pressure profile plays in channel mechanosensitivity and gating by mechanical force.
Mechanosensing at the membrane interface
Force transmission pathways in biological cells includes a variety of cellular structures, such as extracellular and cytoskeletal elements, cell-cell junctions, cell-surface interactions, and membrane receptor complexes (e.g., GPCRs, MS channels) (Cox et al. 2017; Mofrad 2006). Central to the function of all these structures is the lipid bilayer, which preceded evolution of different life forms on Earth by forming spontaneously in archaic prebiotic vesicles as a product of the “hydrophobic effect” and the amphipathic structure of the lipids of cellular membranes (Chandler 2005). Direct evidence for the existence of prebiotic liposomes as self-assembling collections of amphiphiles that could withstand membrane tensions of several mN/m comes from the detection of fatty acids in the interior of the Murchison meteorite from Australia (Chen and Walde 2010; Morris 2002), whose organic extracts were shown to form boundary membranes when rehydrated (Deamer and Pashley 1989). By minimizing the contact between the hydrophobic tails and water, the bilayer serves as the matrix that holds various membrane structures together. It thus provides a physical barrier for ions and water between the extracellular and intracellular compartments. Moreover, the lipid bilayer is fluid and dynamic, as originally proposed by Singer and Nicholson (Singer and Nicolson 1972), allowing for a dynamic rearrangement of proteins and lipids via Brownian motion. Although instrumental for understanding many membrane phenomena observed in biological cells, the original “fluid mosaic model” has not been sufficient to explain many others. More recent studies have indicated that membrane components are compartmentalized and organized into functional domains, which are important for selective and effective signal transduction. The new “dynamically structured mosaic model” emphasizes membrane mosaicism allowing proteins and lipids to organize themselves in molecular-scale clusters as well as larger sub-micron clusters often referred to as “lipid rafts” (Vereb et al. 2003; Simons and Ikonen 1997). In this new model, membrane fluidity enables and facilitates dynamic restructuring of the clusters as dictated by the environment in which biological cells function.
Cellular membranes are major targets of mechanical force, which exists in many forms depending on its interaction with cells. With the exception of gravity, which is force field acting at distance, mechanical force exerts its effect through direct contact by stretching, bending, curving, breaking, or deforming the cell membrane and its components by shear stress or osmotic pressure. Given the essential role that water plays for the existence of life, osmotic pressure has been the primordial mechanical force inherent to all life forms. Organisms from different evolutionary origins have dealt in various ways with the hyper- or hypo-osmotic challenges they face in their living environments. The cell membrane of cell-walled organisms such as bacteria, archaea, fungi, and plants is protected from sudden changes in environmental osmolarity by thick cell walls able to resist osmotic pressure corresponding to 20–30 atm pressure difference across the cell envelope (Martinac and Kloda 2003; Wilson et al. 2013; Hamilton et al. 2015; Peyronnet et al. 2014). Mammalian cells, which normally do not experience large changes in osmotic pressure, have developed different strategies based on meshed and relatively soft cell structures including the cytoskeleton and extracellular matrix. They protect the membrane lipid bilayer from being ruptured by excessive stress while preserving cell deformability (Hamill and Martinac 2001). In addition, animal cell membranes are characterized by significant membrane folding, which by unfolding reduces or releases excessive mechanical stimuli (Hamill 2006).
MscL and MscS: “workhorses” of mechanosignalling
Microbial cells, such as animal and human gut bacteria, are frequently forced to adapt to the fluctuations in osmolarity of the environments they exist in. They are particularly well equipped with osmoregulatory genes encoding specialized membrane proteins designed to meet the osmotic challenges (Stokes et al. 2003; Bialecka-Fornal et al. 2015). Two types of such membrane proteins are MscL- and MscS-like mechanosensitive channels (Fig. 1a). They form two families of ion channels functioning in mechanotransduction processes of a large number of bacteria and archaea (Booth and Blount 2012). However, they have also been found in eukaryotic single-celled microbes such as fungi and green algae as well as multicellular plant organisms (Fig. 1b) (Martinac et al. 2013; Pivetti et al. 2003). MscL and MscS channels are potential descendants of the primordial sensors of osmotic and other forms of mechanical force that evolved as primary signaling molecules supporting the mechanosensory physiology of biological cells (Cox et al. 2018). For many years, both MscL and MscS were the only MS channels of a known 3D structure (Chang et al. 1998; Bass et al. 2002). This helped enormously in studying the relationship between their structure and function, including lipid protein interactions essential for their mechanosensitivity (Perozo et al. 2001, 2002a, b; Moe and Blount 2005; Sukharev et al. 1999; Betanzos et al. 2002; Sukharev 2002; Iscla et al. 2008, 2011; Nomura et al. 2012; Bavi et al. 2016a; Ridone et al. 2018). These studies took many years, providing great insight into the basic physical principles of MS channel mechanosensitivity starting with the initial evidence for MS channel gating according to the “force-from-lipids” principle (Martinac et al. 1990; Teng et al. 2015).
Fig. 1.
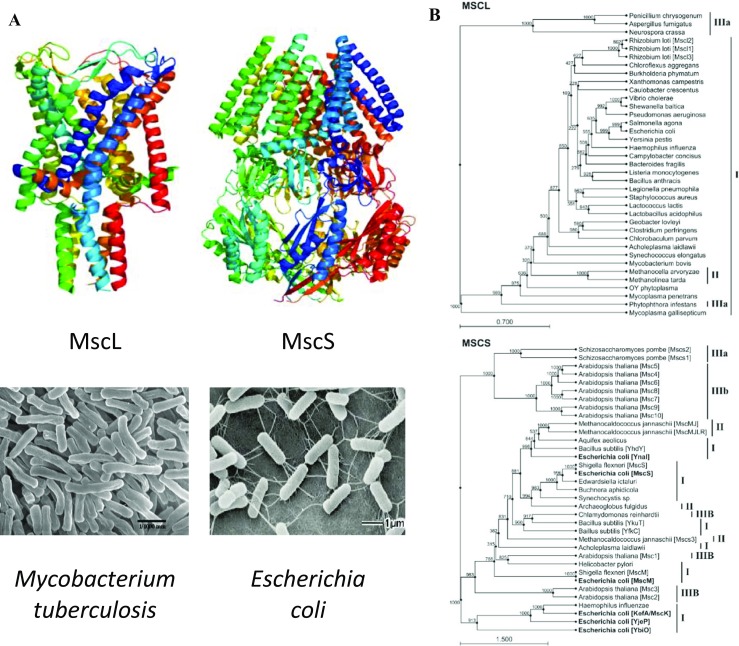
Structure and phylogeny of bacterial mechanosensitive channels. a 3D structure of the MscL pentamer of Mycobacterium tuberculosis (Chang et al. 1998) and MscS heptamer of E. coli (Bass et al. 2002) (top) and scanning electronmicrographs of the corresponding bacterial species (bottom). b A reduced phylogenetic trees showing the distribution of MscL and MscS homologs, which are found across all three domains of life. MscL homologs are found in cell-walled and wall-less bacteria (I), archaea (II), and fungi and oomycetes (belonging to the domain eukaryota, IIIa). MscS homologs have additionally been identified in plants and unicellular green algae (IIIb) with multiple MscS homologs often found in a single organism. The different MscS homologs in E. coli are in bold typeface to highlight the presence of at least three distinct clusters: (i) MscS-like, (ii) MscM-like, and (iii) MscK-like, which suggests that the acquisition of multiple MscS-like homologs might have occurred at a much earlier evolutionary time point than the rare cases in which this has occurred for MscL (e.g., Rhizobium loti). Phylogenetic trees were constructed using 1000 bootstrapped iterations of the UPGMA algorithm in CLC Sequence Viewer (CLC bio, Aarhus Denmark). Scale bar indicates substitutions per site. For simplicity, organisms that contain multiple, uncharacterized MscS-like proteins have had them sequentially designated MscsN, where N begins with the number of characterized homologs plus one and increments. M. jannaschii Mscs3 = Uniprot ID Q58111; Schizosaccharomyces pombe Mscs1 = O74839; S. pombe Mscs2 = O14050 (adapted from (Martinac et al. 2013) with permission)
“Force-from-lipids” principle
The bilayer reconstitution methods have for a number of years served as a “gold standard” for determining inherent mechanosensitivity of MS channels (Fig. 2a) (Martinac et al. 2010). They have allowed researchers to examine the effect of bilayer properties, including thickness and stiffness/fluidity as well as its specific lipid components, on the structural dynamics and function of these channels. Moreover, these methods helped to firmly establish the “force-from-lipids” (FFL) principle as an evolutionary conserved physico-chemical principle that can be used to understand and explain the basic mechanism of MS channel gating (Martinac et al. 1990; Teng et al. 2015; Kung 2005) ranging from prokaryotic (bacterial and archaeal) MS channels (MscL, MscS) (Perozo et al. 2002a, b; Nomura et al. 2012; Bavi et al. 2016a; Martinac et al. 1990) to eukaryotic MS channels of known structure and function (Piezo1, TREK-1, TRAAK) (Brohawn et al. 2014a; Berrier et al. 2013; Cox et al. 2016; Syeda et al. 2016; Cox et al. 2017; Brohawn et al. 2014b).
Fig. 2.
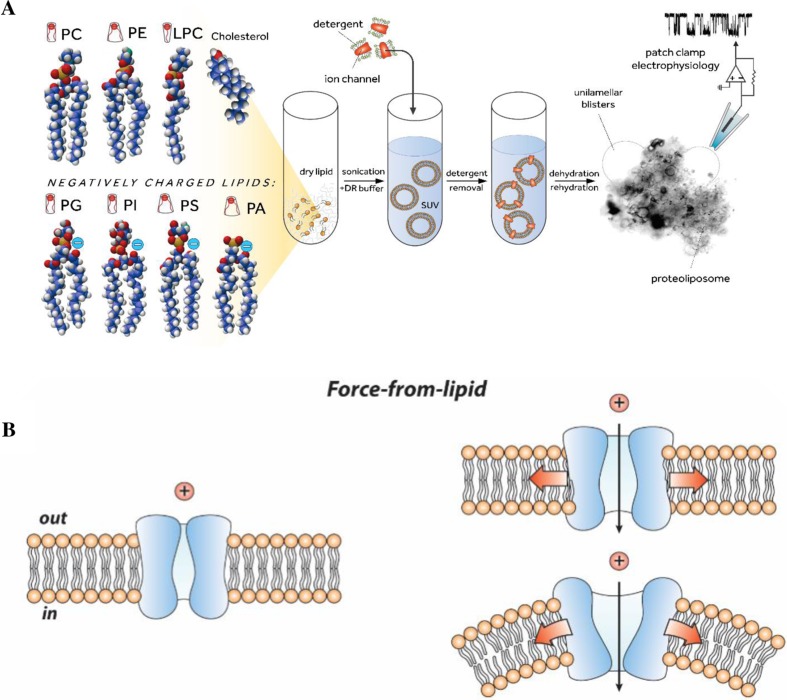
Liposomal reconstitution of MS channels and basic paradigms for the molecular activation of MS channels. a Reconstitution of ion channels into liposomes using dehydration/rehydration (D/R) method. Ball and stick representation of different types of phospholipids (left). The lipid head and tail group differences induce changes in the thickness and shape of the lipid bilayer membrane that is formed on contact of the lipid with water. Reconstitution of proteoliposomes containing ion channel proteins for patch-clamp recording (center): (i) pure lipids are dried and resuspended in the D/R buffer, (ii) after bath-sonication of the liposome suspension, detergent-solubilized ion channel proteins are added to form small unilamellar vesicles (SUVs). Detergent is removed using BioBeads, SUVs are spun down by centrifugation and the pellet is rehydrated in D/R buffer. An aliquot of rehydrated liposomes is then added to the experimental chamber for the patch clamp recording (right). A gigaohm seal between the pipette glass and liposome membrane is formed across a small opening in the patch pipette and suction is applied to activate MS channels (adapted from (Battle et al. 2015)). b MS channel embedded in the lipid bilayer (left) is activated by the FFL either by membrane tension (top right) or local membrane curvature (bottom right)
Computer modeling of mechanical stress experienced by liposome membrane patches, during patch clamp recording, provided new insights into mechanical force distribution corresponding to membrane tension and/or membrane curvature (“force-from-lipids”) that activates and gates MS ion channels (Fig. 2b). Finite element (FE) modeling, for example, has shown that unlike cell-attached patch mode, the stress in two lipid monolayers of a membrane patch differs significantly in the excised patch configuration, with the stress in the upper monolayer being ∼ 30% higher compared to the lower monolayer (Bavi et al. 2014). As it will become clearer towards the end of this review, this stress difference between the monolayers may have been instrumental for the discovery of mechanosensitive ion channels (Hamill and Martinac 2001; Brehm et al. 1984; Guharay and Sachs 1984; Martinac et al. 1987) facilitated by the advent of the patch clamp recording technique (Hamill et al. 1981). In addition, computational modeling, including FE and molecular dynamics (MD) simulations, of MS channel gating in bilayers has provided further insights about the self-assembly of lipid molecules into the membrane bilayers (Chandler 2005). One of the consequences of this self-assembly is severe stress heterogeneity across the bilayer thickness referred to in the literature as the “transbilayer pressure profile” (Fig. 3a). The strong anisotropic internal bilayer stress has been determined to be in the order of hundreds of atmospheres (Ridone et al. 2018; Cantor 1999; Gullingsrud and Schulten 2004).
Fig. 3.
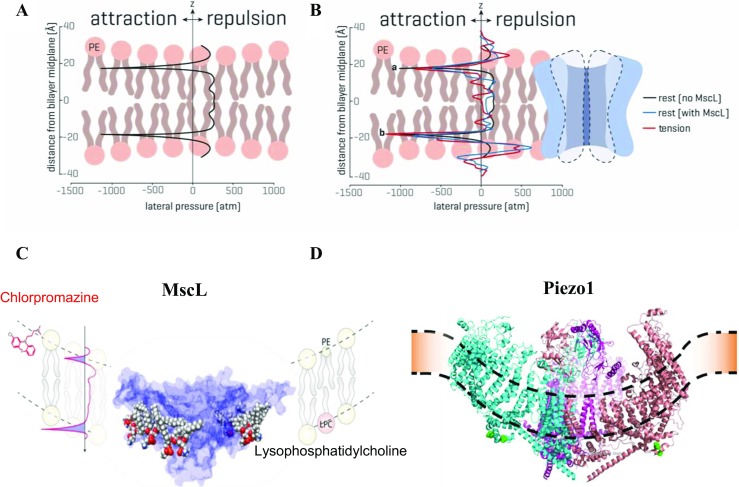
The transbilayer pressure profile. a The transbilayer pressure profile results from mainly three factors: (i) a region with positive lateral pressure caused by the repulsion among the hydrophilic headgroups, (ii) a negative pressure area (surface tension) around the glycerol backbone, which prevents water access to the hydrophobic lipid tails, and (iii) a central area around the middle of the bilayer characterized by positive pressure due to the entropic (steric) repulsion between the lipid tails (left). b Transbilayer pressure profiles from MD simulations of a POPE bilayer with E. coli MscL inserted in it is shown on the right. The lateral pressure profile is shown at rest without (black) and with (blue) MscL inserted. Note how in the presence of the protein the pressure profile in a symmetrical lipid bilayer has become distinctly asymmetric with asymmetry further increasing when tension is applied (red). Peak a and peak b represent the rise in the pressure profile at the lipid solvent interface (modified from (Cox et al. 2017)). c The pressure profile, including surface tension (the area highlighted in blue) representing the stress at the interface of two phases, can be modulated by a number of amphipathic molecules (e.g., chlorpromazine, lysophosphatidylcholine) inserted in one leaflet of the bilayer, which results in activation and opening of MscL. d Piezo1 insertion into a bilayer has been proposed to curve the membrane (Guo and MacKinnon 2017)
Transbilayer pressure profile
The anisotropy of the transbilayer pressure profile results from mainly three factors explained in Fig. 3a (Bavi et al. 2017a). There is repulsion between the head groups and steric repulsion in the tails, which is strongly dependent on the degree of lipid saturation (Ridone et al. 2018). Therefore, there is a sharp stretch on the solvent bilayer interface to disallow water to access the hydrophobic core of the bilayer. Thus, surface tension corresponding to the area highlighted blue in Fig. 3c is the stress at the interface of two phases (i.e., lipid and water). In an idealized lipid bilayer consisting of two identical monolayers, the symmetric transbilayer pressure profile shows characteristic negative peaks at the water-lipid interface and repulsive positive peaks in the headgroup and tail region (Fig. 3a). In the presence of a membrane protein such as an MS ion channel, the pressure profile becomes asymmetric (Fig. 3b). The asymmetry of the pressure profile increases further upon stretching the bilayer, which is a consequence of readjustment of the energy equilibrium between the protein and the bilayer. Stretching thins a bilayer, which results in hydrophobic mismatch between the hydrophobic length of the channel and the bilayer, which may influence MS channel gating (Martinac and Cox 2016). MscL was shown to be sensitive to bilayer thickness such that thinner bilayers drive its gating (Perozo et al. 2002a; Nomura et al. 2012). Another example illustrating that mechanosensitivity is not dictated solely by the channel structure, which can vary with the physical properties of the lipid bilayer, is gramicidin A (gA). This simple bacterial peptide that forms cation channels in lipid bilayers by the transmembrane association of one monomer from each monolayer was shown to switch between stretch activation and stretch inactivation depending on bilayer thickness (Martinac and Hamill 2002). Reverting the response polarity by subnanometer changes in the bilayer thickness of this model channel is also in qualitative agreement with a recent continuum elastic model showing that the lateral tension of the membrane may either activate or inactivate the channel activity of gA depending on the relation of the hydrophobic thickness of the bilayer and the length of the gA dimer (Kondrashov et al. 2018).
The transbilayer pressure profile, including surface tension, has also been shown to be modulated by a large number of amphipathic molecules, including conical lipids such as lysophosphatidylcholine (LPC), antipsychotic drug chlorpromazine (CPZ), or various local anesthetics. Their effect on bacterial MS channels has been reported numerous times (Fig. 3c) (Perozo et al. 2002a, b; Nomura et al. 2012; Martinac et al. 1990; Mukherjee et al. 2014; Dimitrova et al. 2016). As indicated by MD simulations, the asymmetric incorporation of amphipaths into lipid bilayers may ultimately distort the membrane and cause local curvatures (wrinkles) (Fig. 2b), which provides an alternative mechanism for activation of MS channels besides the membrane stretch (tension) (Yoo and Cui 2009). However, a global curvature corresponding to a radius of a membrane patch of ~ 1 μm, visible under light microscopy during patch clamp experiments, is itself of negligible energetic consequence for the activation of an MS channel. In this case, stretching the curved membrane patch activates the channels because the global patch curvature is practically flat from a single channel perspective. Only a curvature corresponding to a radius of < 50 nm, which is of comparable size to MS channel dimensions, can cause sufficient changes of several mN/m in the stress distribution along the thickness of the membrane necessary for activation of MS channels (Bavi et al. 2016b). Importantly, such local curvatures are induced by unilateral insertion of amphipaths, which thus cause an increase in asymmetry of the transbilayer pressure profile similar to stretching the bilayer (Fig. 3b). The relevance of membrane curvature in gating of MS channels by FFL is further indicated by membrane-curving properties of the Piezo1 channel based on its 3D structure determined by cryo-EM (Fig. 3d) (Guo and MacKinnon 2017).
A recent study investigating the effect of unilateral insertion of amphipaths into the lipid bilayer uncovered subtleties of the effect amphipaths exert on different types of MS channels (Bavi et al. 2017b). Crucially, the study showed that FFL activation of a particular MS channel was strongly dependent on its structure. Using the surface active agent 2,2,2-trifluoroethanol (TFE) as a pharmacological tool, TFE could facilitate the activation of MscL if added to either leaflet of the bilayer. In contrast, TREK-1 could be activated only if added to the cytoplasmic side, whereas Piezo1 and MscS could be activated only from the extracellular side. Activation curvature of MscL, upon addition of TFE from either side, seems consistent with its cylindrical shape. MscS and Piezo1, which are both conical, were only activated from the extracellular side, while the activation of TREK-1 by TFE added to the cytoplasmic side was equally consistent with the curved membrane conforming to its non-planar curved shape (Bavi et al. 2017a). In addition, MD simulations revealed that TFE increased the asymmetry of the transbilayer pressure profile markedly, indicating a close relationship between MS channel shapes and their activation mechanism by surface tension perturbations (Bavi et al. 2017b). These findings provide strong evidence that changes in asymmetry of the transbilayer pressure profile are the core mechanical force which gates MS ion channels. Consequently, the FFL principle is founded on tuning the asymmetry of the transbilayer pressure profile to the mechanosensitivity of MS channels according to their shape.
Effect of asymmetric insertion of drugs and amphipaths into lipid bilayers goes far beyond bacterial MS channels and arguably is more physiologically relevant to eukaryotic MS channels. Several studies have already shown the effect of LPC, chlorpromazine (CPZ), Arachidonic acid (AA), and LPA on activity of K2P channels (TREK and TRAAK) and Piezo channels (Syeda et al. 2016; Honoré et al. 2006; Brohawn et al. 2014b; Kloda et al. 2007). However, it is yet to be conclusively shown whether their effect is through their specific interactions with the protein or through perturbation of the transbilayer pressure profile (non-specific interactions).
Interestingly, plasma membranes of living cells are intrinsically asymmetric as the lipid composition of their outer leaflet is largely different from the composition of the inner leaflet. A recent study has clearly shown the importance of such asymmetry, specifically due to (phosphatidylserine) PS lipids being present only in the inner leaflet while the cells are alive. The study shows that existence of PS lipids in the inner leaflet is crucial for normal Piezo-1 function and subsequently for PIEZO1-mediated myotube formation (Tsuchiya et al. 2018). Moreover, Tucker and colleagues have computationally and experimentally shown that TREK-2 is more sensitive to the bilayer tension generated in the inner compared to the outer monolayer (Clausen et al. 2017).
Conclusions
The force-from-lipids principle is a fundamental physicochemical principle based on the large and anisotropic forces defined by the transbilayer pressure profile inherent to biological membranes. MS channels, embedded in the lipid bilayer, are subjected to push and pull forces resulting from the interactions between MS channels and the lipid bilayer. These interactions occur either through hydrophobic mismatch or local bilayer curvature. Collectively, all membrane deformations, including those affecting extracellular matrix and cytoskeleton, that can gate an MS ion channel and modulate its activity are inherently linked to the changes in the transbilayer pressure profile asymmetry. This asymmetry underlies the FFL principle as the unifying paradigm of all mechanotransduction processes originating at the cell membrane.
Acknowledgements
We thank Dr. Charles D Cox for critical reading of the manuscript. We acknowledge the Japanese Society for Promotion of Science (JSPS) for a fellowship to YN, the University of Chicago for a postdoctoral fellowship to NB, University of Newcastle for a PhD Scholarship to YAN, University of New South Wales for a PhD Scholarship to PR, Australian Government Research Training Program Scholarship and a UNSW Prince of Wales Clinical School Scholarship to ADM, and the National Health and Medical Research Council of Australia for a Principal Research Fellowship to BM.
Authors’ contributions
All authors contributed to writing of the manuscript.
Funding
This work was supported by DP160103993 grant from the Australian Research Council.
Conflict of interest
Boris Martinac declares that he has no conflict of interest. Navid Bavi declares that he has no conflict of interest. Pietro Ridone declares that he has no conflict of interest. Yury A. Nikolaev declares that he has no conflict of interest. Adam D. Martinac declares that he has no conflict of interest. Yoshitaka Nakayama declares that he has no conflict of interest. Paul R. Rohde declares that he has no conflict of interest. Omid Bavi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- Battle AR, Ridone P, Bavi N, Nakayama Y, Nikolaev YA, Martinac B. Lipid–protein interactions: lessons learned from stress. Biochim Biophys Acta. 2015;1848:1744–1756. doi: 10.1016/j.bbamem.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Bavi N, Nakayama Y, Bavi O, Cox CD, Qin QH, Martinac B. Biophysical implications of lipid bilayer rheometry for mechanosensitive channels. Proc Natl Acad Sci U S A. 2014;111:13864–13869. doi: 10.1073/pnas.1409011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavi N, Cortes DM, Cox CD, Rohde PR, Liu W, Deitmer JW, et al. The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels. Nature Commun. 2016;7:11984. doi: 10.1038/ncomms11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavi Omid, Cox Charles, Vossoughi Manouchehr, Naghdabadi Reza, Jamali Yousef, Martinac Boris. Influence of Global and Local Membrane Curvature on Mechanosensitive Ion Channels: A Finite Element Approach. Membranes. 2016;6(1):14. doi: 10.3390/membranes6010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavi N, Nikolaev YA, Bavi O, Ridone P, Martinac AD, Nakayama Y, Cox CD, Martinac B. Principles of Mechanosensing at the Membrane Interface. In: Epand RM, Ruysschaert J-M, editors. The Biophysics of Cell Membranes. Singapore: Springer Nature; 2017. pp. 85–119. [Google Scholar]
- Bavi N, Cox CD, Bavi O, Martinac B. Perturbation of bilayer surface tension differentially modulates mechanosensitive ion channels. Biophys J. 2017;112:416a. doi: 10.1016/j.bpj.2016.11.2229. [DOI] [Google Scholar]
- Berrier C, Pozza A, de Lacroix de Lavalette A, Chardonnet S, Mesneau A, Jaxel C, et al. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. J Biol Chem. 2013;288:27307–27314. doi: 10.1074/jbc.M113.478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos M, Chiang CS, Guy HR, Sukharev S. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat Struct Biol. 2002;9:704–710. doi: 10.1038/nsb828. [DOI] [PubMed] [Google Scholar]
- Bialecka-Fornal M, Lee HJ, Phillips R. The rate of osmotic downshock determines the survival probability of bacterial mechanosensitive channel mutants. J Bacteriol. 2015;197:231–237. doi: 10.1128/JB.02175-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR, Blount P. The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J Bacteriol. 2012;194:4802–4809. doi: 10.1128/JB.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P, Kullberg R, Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014;516:126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A. 2014;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor RS. Lipid composition and the lateral pressure profile in bilayers. Biophys J. 1999;76:2625–2639. doi: 10.1016/S0006-3495(99)77415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature. 2005;437:640–647. doi: 10.1038/nature04162. [DOI] [PubMed] [Google Scholar]
- Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- Chen IA, Walde P. From self-assembled vesicles to protocells. Cold Spring Harb Perspect Biol. 2010;2:a002170. doi: 10.1101/cshperspect.a002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen Michael V., Jarerattanachat Viwan, Carpenter Elisabeth P., Sansom Mark S. P., Tucker Stephen J. Asymmetric mechanosensitivity in a eukaryotic ion channel. Proceedings of the National Academy of Sciences. 2017;114(40):E8343–E8351. doi: 10.1073/pnas.1708990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nature Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bavi N, Martinac B. Origin of the force: the force-from-lipids principle applied to piezo channels. Curr Top Membr. 2017;79:59–96. doi: 10.1016/bs.ctm.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Cox CD, Bavi N, Martinac B. Bacterial Mechanosensors. Annu Rev Physiol. 2018;80:71–93. doi: 10.1146/annurev-physiol-021317-121351. [DOI] [PubMed] [Google Scholar]
- Deamer DW, Pashley RM. Amphiphilic components of the Murchison carbonaceous chondrite: surface properties and membrane formation. Orig Life Evol Biosph. 1989;19:21–38. doi: 10.1007/BF01808285. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Walko M, Hashemi Shabestari M, Kumar P, Huber M, Kocer A. In situ, reversible gating of a mechanosensitive Ion Channel through protein-lipid interactions. Front Physiol. 2016;7:409. doi: 10.3389/fphys.2016.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullingsrud J, Schulten K. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys J. 2004;86:3496–3509. doi: 10.1529/biophysj.103.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YR, MacKinnon R (2017) Structure-based membrane dome mechanism for piezo mechanosensitivity. eLife 6:e33660 [DOI] [PMC free article] [PubMed]
- Hamill OP. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006;453:333–351. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton ES, Jensen GS, Maksaev G, Katims A, Sherp AM, Haswell ES. Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science. 2015;350:438–441. doi: 10.1126/science.aac6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci U S A. 2006;103(18):6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscla I, Wray R, Blount P. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys J. 2008;95:2283–2291. doi: 10.1529/biophysj.107.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscla I, Wray R, Blount P. An in vivo screen reveals protein-lipid interactions crucial for gating a mechanosensitive channel. FASEB J. 2011;25:694–702. doi: 10.1096/fj.10-170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda A., Lua L., Hall R., Adams D. J., Martinac B. Liposome reconstitution and modulation of recombinant N-methyl-D-aspartate receptor channels by membrane stretch. Proceedings of the National Academy of Sciences. 2007;104(5):1540–1545. doi: 10.1073/pnas.0609649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov Oleg V., Galimzyanov Timur R., Pavlov Konstantin V., Kotova Elena A., Antonenko Yuri N., Akimov Sergey A. Membrane Elastic Deformations Modulate Gramicidin A Transbilayer Dimerization and Lateral Clustering. Biophysical Journal. 2018;115(3):478–493. doi: 10.1016/j.bpj.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- Martinac B, Cox CD. Mechanosensory transduction: focus on ion channels. Reference module in life sciences. New York: Elsevier; 2016. [Google Scholar]
- Martinac B., Hamill O. P. Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proceedings of the National Academy of Sciences. 2002;99(7):4308–4312. doi: 10.1073/pnas.072632899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B, Kloda A. Evolutionary origins of mechanosensitive ion channels. Prog Biophys Mol Biol. 2003;82:11–24. doi: 10.1016/S0079-6107(03)00002-6. [DOI] [PubMed] [Google Scholar]
- Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. Coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- Martinac B, Rohde PR, Battle AR, Petrov E, Pal P, Foo AF, et al. Studying mechanosensitive ion channels using liposomes. Methods Mol Biol. 2010;606:31–53. doi: 10.1007/978-1-60761-447-0_4. [DOI] [PubMed] [Google Scholar]
- Martinac Boris, Nomura Takeshi, Chi Gamma, Petrov Evgeny, Rohde Paul R., Battle Andrew R., Foo Alexander, Constantine Maryrose, Rothnagel Rosalba, Carne Sonia, Deplazes Evelyne, Cornell Bruce, Cranfield Charles G., Hankamer Ben, Landsberg Michael J. Bacterial Mechanosensitive Channels: Models for Studying Mechanosensory Transduction. Antioxidants & Redox Signaling. 2014;20(6):952–969. doi: 10.1089/ars.2013.5471. [DOI] [PubMed] [Google Scholar]
- Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemist. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- Mofrad MRK, Cytoskeletal Mechanics RD. Models and measurements. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Morris CE. How did cells get their size? Anat Rec. 2002;268:239–251. doi: 10.1002/ar.10158. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Jose MD, Birkner JP, Walko M, Ingolfsson HI, Dimitrova A, et al. The activation mode of the mechanosensitive ion channel, MscL, by lysophosphatidylcholine differs from tension-induced gating. FASEB J. 2014;28:4292–4302. doi: 10.1096/fj.14-251579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Cranfield CG, Deplazes E, Owen DM, Macmillan A, Battle AR, et al. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci U S A. 2012;109:8770–8775. doi: 10.1073/pnas.1200051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Site-directed spin-labeling analysis of reconstituted MscL in the closed state. J Gen Physiol. 2001;118:193–206. doi: 10.1085/jgp.118.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo Eduardo, Cortes D. Marien, Sompornpisut Pornthep, Kloda Anna, Martinac Boris. Open channel structure of MscL and the gating mechanism ofmechanosensitive channels. Nature. 2002;418(6901):942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- Perozo Eduardo, Kloda Anna, Cortes D. Marien, Martinac Boris. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature Structural Biology. 2002;9(9):696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Peyronnet R, Tran D, Girault T, Frachisse JM. Mechanosensitive channels: feeling tension in a world under pressure. Front Plant Sci. 2014;5:558. doi: 10.3389/fpls.2014.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, et al. Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridone P, Grage SL, Patkunarajah A, Battle AR, Ulrich AS, Martinac B. "Force-from-lipids" gating of mechanosensitive channels modulated by PUFAs. J Mech Behav Biomed Mater. 2018;79:158–167. doi: 10.1016/j.jmbbm.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554:481–486. doi: 10.1038/nature25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Stokes NR, Murray HD, Subramaniam C, Gourse RL, Louis P, Bartlett W, et al. A role for mechanosensitive channels in survival of stationary phase: regulation of channel expression by RpoS. Proc Natl Acad Sci U S A. 2003;100:15959–15964. doi: 10.1073/pnas.2536607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83:290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Sigurdson WJ, Kung C, Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J Gen Physiol. 1999;113:525–540. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, et al. Piezo1 channels are inherently mechanosensitive. Cell Rep. 2016;17:1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J, Loukin S, Anishkin A, Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch. 2015;467:27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya M, Hara Y, Okuda M, Itoh K, Nishioka R, Shiomi A, Nagao K, Mori M, Mori Y, Ikenouchi J, Suzuki R, Tanaka M, Ohwada T, Aoki J, Kanagawa M, Toda T, Nagata Y, Matsuda R, Takayama Y, Tominaga M, Umeda M. Cell surface flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. Nat Commun. 2018;9:2049. doi: 10.1038/s41467-018-04436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereb G, Szollosi J, Matko J, Nagy P, Farkas T, Vigh L, et al. Dynamic, yet structured: the cell membrane three decades after the singer-Nicolson model. Proc Natl Acad Sci U S A. 2003;100:8053–8058. doi: 10.1073/pnas.1332550100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Maksaev G, Haswell ES. MscS-like mechanosensitive channels in plants and microbes. Biochemist. 2013;52:5708–5722. doi: 10.1021/bi400804z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Cui Q. Curvature generation and pressure profile modulation in membrane by lysolipids: insights from coarse-grained simulations. Biophys J. 2009;97:2267–2276. doi: 10.1016/j.bpj.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554:487–492. doi: 10.1038/nature25743. [DOI] [PubMed] [Google Scholar]