Abstract
With the advent of improved experimental techniques and enhanced precision, laser-induced breakdown spectroscopy (LIBS) offers a robust tool for probing the chemical constituents of samples of interest in biological sciences. As the interest continues to grow rapidly, the domain of study encompasses a variety of applications vis-à-vis biological species and microbes. LIBS is basically an atomic emission spectroscopy of plasma produced by the high-power pulsed laser which is tightly focused on the surface of any kinds of target materials in any phase. Due to its experimental simplicity, and versatility, LIBS has achieved its high degree of interest particularly in the fields of agricultural science, environmental science, medical science, forensic sciences, and biology. It has become a strong and sensitive elemental analysis tool as compared to the traditional gold standard techniques. As such, it offers a handy, rapid, and flexible elemental measurement of the sample compositions, together with the added benefits of less cumbersome sample preparation requirements. This technique has extensively been used to detect various microorganisms, extending the horizon from bacteria, molds, to yeasts, and spores on surfaces, while also being successful in sensing disease-causing viruses. LIBS-based probe has also enabled successful detection of bacteria in agriculture as well. In order for good quality processing of food, LIBS is also being used to detect and identify bacteria such as Salmonella enteric serovar typhimurium that causes food contamination. Differences in soil bacteria isolated from different mining sites are a very good indicator of relative environmental soil quality. In this connection, LIBS has effectively been employed to discriminate both the inter- and intra-site differences of the soil quality across varying mining sites. Therefore, this article summarizes the basic theory and use of LIBS for identifying microbes causing serious agricultural and environmental infectious diseases.
Keywords: Laser-induced breakdown spectroscopy (LIBS), Microorganisms, Bacteria, Fungus, Viruses, Trace and toxic elements, Multivariate analysis
Introduction
Human health and well-being bear the direct impact of a hierarchy of microorganisms that are largely grouped together as pathogens like bacteria, viruses, molds, prions, amoebae, fungi, etc. Microbes are the omnipresent companions to human existence and prevalent in the surroundings (Musazzi and Perini, 2014). In fact, various microbiological cells exist in a healthy adult which has a great influence on human health and also these microbes can be very hazardous even at very low concentrations. Hence, different techniques have been explored to develop a reliable detection system for these microbes (Truant 2002). The detection and control of these biohazards are also desirable medical field and also to monitor hygiene in food-processing industries. Typically, biological identification methods so far have relied on certain biochemical, microbiological, and immunological processes which while being quite reliable and sensitive are also very time-consuming and limited to few specific compounds only. Moreover, these methods require extensive sampling and preparation steps, which in turn contaminate the target samples. Accordingly, a reliable, sensitive and rapid technique is demanded to be applied on line as early-warning systems to adopt suitable precautionary measures like the use of respirators, gloves, room isolation, etc. and subsequently use more polished tools for precise identification.
The early in-situ identification of the microbes would help for successful diagnosis of the disease for proper treatment without delay, and also the integration of new technology would play a pivotal role in the early detection of outbreaks of diseases such as tuberculosis (TB) (George et al. 1986). Biosensing technology is clinically important and provides an immediate identification of dangerous pathogens in food industries such as infections due to salmonella and Escherichia coli (E. coli) which may be dangerous. In contrast with the past, microbial-scientist still depends extensively on ancient techniques based on wide categorization of bacteria employing Gram-staining. The techniques to identify bacteria are mainly put in three different categories as: (i) phenotypic (morphology), (ii) immunological (serological), and (iii) genotypic methods (Madigan et al., 1996).
Morphological methods largely depend on Gram-staining and on the size and shape of cells. While it requires starting with a pure culture, such method of identification is also slow (Kaneko et al. 1990) and problems arise in cases when multiple bacteria types are present in the sample. In case of the latter, one requires streaking plate method to isolate pure strain of bacterial colonies from the single species of microorganism (containing multiple bacteria) for perfect identification subsequently. In all such situations, identification stand in need of microbiological expertise which is not only expensive but also a labor-extensive process and so is time-consuming as well. Serological blood test methods to diagnose various disease conditions through detection and measurement of levels of antibodies as a result of exposure to a particular pathogens involve the interaction of a microbial antigen (Pryor 2001). In enzyme-linked immunosorbent assay (ELISA) method, a single bacterium can be identified an antibody that binds to a bacterial antigen. The disbenefits of this ELISA method are the selectivity of antibodies, where any single test is only attuned to one specific bacterium.
Further, polymerase chain reaction (PCR) is one kind of genotypic technique employed to identify the pathogenic microbes quickly in blood, cerebrospinal fluid, sputum, and urine. PCR requires a peculiar sequence of deoxyribonucleic acid to be escalated, producing millions of copies (Santo Domingo and Sadowsky, 2007). PCR requires pure DNA that must be extracted. Furthermore, employing PCR to study clinical specimens has several difficulties like problem to detect specifically targeted bacterial pathogens in a blend of sample. In such cases, cell culture accompanied by isolation and diagnosis of the bacterial DNA may be needed. Fluorescence in situ hybridization (FISH) is another widely employed method to locate microbial particles which is based on the absence and presence of peculiar DNA sequences. After that, fluorescence microscope is used for visualizing and quantification. However, FISH has some disadvantages when it applies to applications for clinical purposes because of the difficulty involve in assembling probes and counting of the total number of probe-target base pairings (Hoshino et al. 2008).
For all the reasons stated above, a new diagnostic technology capable of rapidly identifying pathogens without any prior information of nucleic acid sequences as PCR requirement and antibodies against known bacterial antigens as required in fluorescence immuno-assay techniques. Nowadays, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) is employed for fast recognition of microorganisms (Lay 2001). This technology requires a high-power laser beam to ablate bacteria to produce ions (proteins) that are accelerated in an external electric field. Then, to detect the time of flight (TOF), an analyzer is generally used for each ions. The separated ion fractions are detected and mass-spectrum is referred as a fingerprint of the microorganism. Yet, this technique stands in need of minimal amount of bio-samples, simple step sampling, and good reproducibility; an expensive high-vacuum mass spectrometry system is required to perform the experiments by an experienced person. Raman spectroscopy is another molecular detection technology widely used for identification of bacteria (Petry et al., 2003; Pappas et al., 2000). Hence, it is quite possible to develop an advanced and real-time pathogen identification technology without imposing any constraints.
Here, we argue that LIBS technology is an attractive alternative compared to the alternatives. LIBS is emerging as a popular and promising technology detecting and identifying the bacteria quickly based on their unique atomic compositions of the laser-induced plasma produced on the sample surface. This technology has proven track record for carefully investigating microbiological diversity and pathogens identification in a wide range of systems. Significant research efforts are focused in the area of bio-LIBS particularly identifying pathogens. Before highlighting the use and importance of LIBS for pathogen detection, it is quite important to discuss some basic and fundamentals of LIBS technique, which would help the reader to understand the basic concepts of LIBS in microbiology. In the following sections, we will briefly highlight some fundamentals of LIBS and its biomedical applications and also, we discuss the applications of LIBS in microbial systems in detail. The LIBS-based elemental analysis of different bacterial systems is also included with their advantages, limitations, and future prospects.
Principles and fundamentals of LIBS
The technical details about LIBS technology can be found elsewhere by the group of LIBS pioneer workers Miziolek and Cremers (Miziolek et al. 2006; Cremers and Radziemski, 2006). In the LIBS process, the following sequence of events takes place, where (i) a short and high-power pulsed laser beam are focused onto a target material; (ii) the incident laser energy vaporizes a very little quantity of the sample and interaction of incident laser beam with the vapor plume to produce hot micro-plasma of very high temperature; (iii) an optical fiber bundle, equipped with collection optics, is used to collect the light and then a high-resolution grating is used to disseminate the light produced by the emission from the hot plasma; and (iv) the accompanying LIBS signal are then analyzed to predict and quantify the relative presence of the chemical constituents of the target materials.
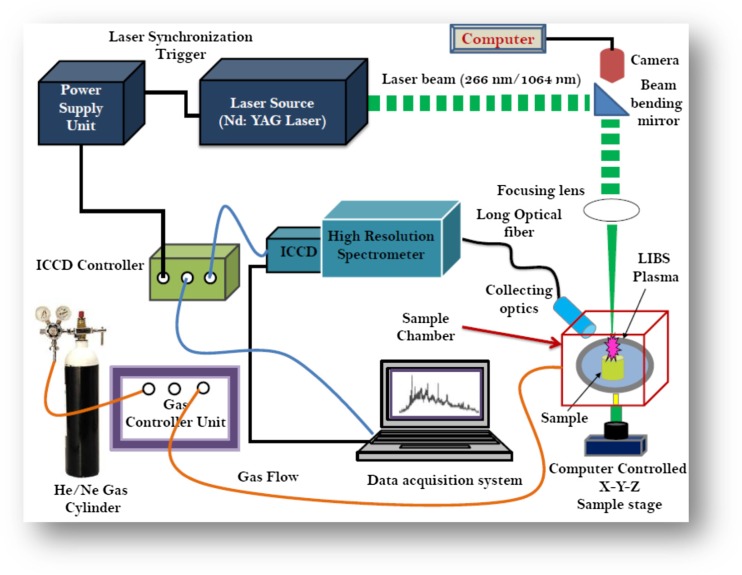
An illustrative experimental setup of LIBS technique is presented in Fig. 1. A pulsed Nd:YAG laser of typical wavelength 1064 nm with nanosecond pulse width is widely utilized excitation source for LIBS due to its reliability, ease of use, and its high peak pulse energies. The second and third harmonic wavelengths of 532, 355, and 266 nm from Nd:YAG laser using nonlinear process are also used as excitation sources (Miziolek et al. 2006; Gondal et al., 2014, 2016a, b, 2018). Femtosecond (fs) lasers have also been used in LIBS for many applications (Cremers and Radziemski, 2006; Chin 2009). In LIBS method, the spot size of the laser beam and the energy of the laser pulse are very important to impart the sufficient fluence on the sample surface (order of mJ/pulse) (Pasquini et al. 2007).
Fig.1.
A typical schematics diagram of laser-induced breakdown spectroscopy (LIBS) for the study of biological specimens
The mechanism of LIBS experiment typically can be divided into (i) primary laser-matter interaction which involves the process of heating, evaporation, and then bond breaking; (ii) creation of micro-plasma plume; and (iii) expansion of plasma produced and its cooling.
Initially, the pulsed laser beam of sufficient energy is concentrated on the surface of the bio-samples (i.e., bacteria) with a smallest possible spot size, with a laser of pulse widths of nanosecond, picoseconds, or femtosecond (Baudelet et al., 2006a, b, c; Singh and Thakur, 2007; Gondal et al. 2007 a,b). The energy absorbed by the surface of the samples is then converted into heat energy and a little amount from the sample material vaporizes and subsequently, vapor plume of high-pressure is formed at the surface of the material. The mass (M) vaporizes after the ablation by the laser pulse is determined using the following equation (Cremers and Radziemski, 2006):
| 1.1 |
where R represents the reflectivity of surface, Cp represents the specific heat, Tb and T0 denotes the boiling point and the room temperature respectively in K. The term E and Lv represent the laser pulse energy and the latent heat of vaporization respectively.
The time duration of this process is shorter than that from the pulse duration which is of nanosecond (ns) order, the laser beam continues to brighten the vapor plume that leads to further absorption of laser energy within the vapor plume and then eventually ionized plasma is formed. This vapor plasma shields the sample from further illumination and ablation that determines the mass ablated. The process that leads to plasma shielding is the absorption of the laser pulse energy by the electrons and multi-photon ionization. This is described by the absorption of the laser radiation under the condition that the laser frequency must be equal to the plasma frequency. This process results in a higher temperature of the laser produced plasma that increases the degree of ionization and further dissociates the ablated tiny particles (Cremers and Radziemski, 2006; Singh and Thakur, 2007).
Two prime steps are generally involved that lead to the breakdown of the produced plasma in the form of vapor plume. Firstly, some free electrons are present in the focal region of the laser pulse which is due to natural and local radioactivity (Singh and Thakur, 2007). Secondly, occurrence of electron cascade ionization (ECI) within the vapor plume in the focal volume of the laser. The laser irradiance is of up to the order of 108–1010 W/cm2 (Cremers and Radziemski, 2006). At such high laser irradiance, the densities of electron and ion are executed via cascade ionization rather than multi-photon production which can be explained by Eq. (1.2) given below:
| 1.2 |
where the number of photons is represented by n and X represents the target atom.
In these phenomena, an atom is ionized by absorption of photons from the laser field (Joachain et al. 2001). Voronov and Delone (1966) observed this process firstly while using ruby laser as an excitation source to ionize xenon via the absorption of photons. The above process takes place at high intensity of laser radiation with the condition that the photon energy should be less than the atomic ionization energy. It is possible that the atom absorbs various photons “simultaneously” and is excited to a bound-free transition and consequently a free electron is released. It is already mentioned above that most of the electron generation occurs via an electron cascade phenomenon rather than multi-photon absorption process. Classically, the generated electrons are accelerated by the field produced by the laser during collisions with neutral species via the inverse Bremsstrahlung process. Further, the electrons are quickly thermalized and gain enough energy to collisionally ionize an atom which can be understood by the equation given by (1.3):
| 1.3 |
As clear from Eq. (1.3), more free electrons are thus produced which gain energy from the electric field of the laser again and leads to more ionization and eventually increases the overall value of electron density (Cremers and Radziemski, 2006; Singh and Thakur, 2007).
In the end, sufficient free electrons and ions are produced to create plasma plume which consists of atoms, ions, molecules, and free electrons as well. After the exponential increase in the generation of free electrons and ions, the laser plasma expands and propagates from the sample surface toward the surrounding gas/atmosphere. The produced laser plasma compresses the surrounding atmosphere producing a shock wave. Finally, the plasma will become opaque at the plasma frequency equals to the laser frequency. The foregoing conditions are attained at a critical electron density nc ~ (1021/λ2)/cm3, where λ is measured in microns. Thereafter, at some region, electron density and plasma frequency increase continuously. In condition of higher plasma frequency as compared to the laser frequency, the laser radiations are reflected by the plasma and leads to plasma cooling (Pasquini et al. 2007). After significant expansion of the plasma, cooling has occurred and electromagnetic radiation form plasma is collected for LIBS analysis by introducing a time delay which is a time gap between the laser excitation and data collection.
The electromagnetic emission from the plasma gives the chemical compositional information of the target material and also gives a broadband non-specific component, i.e., continuum emission (due to the free-free and free-bound transitions), which appears as a background. The free-free transitions in the field of an atom/ion are represented by Eq. (1.4) and Eq. (1.5) gives the information of free-free transition in the field of an atom/ion.
| 1.4 |
| 1.5 |
In the recombination radiation, a free electron is trapped in an atomic energy level and the electron gives up its excess kinetic energy (KE) as a continuum radiation as per Eq. (1.6).
| 1.6 |
The characteristic elemental radiation is due to the bound-bound transitions. An atom or ion in its excited state undergoes a transition to a lower state emitting photon whose energy will depend on the energy differences of upper and lower states (Singh and Thakur, 2007).
| 1.7 |
| 1.8 |
In LIBS, the elemental compositions of a sample are determined using the intensities of the observed spectral lines which are the finger print of the species present in the target material. Intensity of observed atomic line in LIBS generally relies on oscillator strength value and also on the excitation conditions particularly on the density of emitters within the plasma. Oscillator strength illustrates the comparative intensity of any transitions that took place in emission and absorption processes. In this case of “optically thin” plasma, atomic lines of sufficient intensity are measured to achieve and better quantitative results in LIBS analysis (Miziolek et al. 2006; Cremers and Radziemski, 2006; Singh and Thakur, 2007).
The intensity of a spectral line observed in LIBS spectra is then computed by the plasma temperature by measuring the relative intensities of two lines. The relative intensities of any two lines from a given atomic species are estimated employing the Boltzmann and Saha Eqs. (1.9) which are related to each other and also the temperature.
| 1.9 |
Where A1 and A2 represents the transition probabilities of two transitions having wavelengths λ1 and λ2, g1 and g2 corresponds to the statistical weights of their upper energy levels having energies E1 and E2 of their upper levels respectively. T represents the plasma temperature which can be estimated directly using Eq. (1.9) by evaluating the relative intensities of the two observed lines.
To estimate the plasma temperature, Boltzmann equation is generally used generating a Boltzmann plot. The intensity of a spectral line corresponding to the transition from energy levels Ek to Ei of an atomic species is given by:
| 1.10 |
where Ns represents the number density (particle/cm3) for the respective atomic species, Aki represents the transition probability, gk is the degeneracy of the kth energy level, and Us(T) represents the partition function of the atomic species at temperature T. The Eq. (1.9) can be rearranged in a linear form by defining some scaled intensities (I/gkAki) and then after taking the natural logarithm of Eq. (1.10) of both sides, it takes the form:
| 1.11 |
If one plots the rescaled intensities of corresponding atomic species versus the upper state energy KE and fits it to a straight line, one can plot the slope of the line as (1/KBT) from which the plasma temperature can be estimated (Borgia et al. 2000).
In case one transition line is from a neutral atom and another is from an ionic species of the same atom, then the Saha-Boltzmann equation is given by:
| 1.12 |
where I stands for the intensity of the ion/atom, ne represents the electron density (cm−3), A represents the Einstein coefficient for emission of the upper level (s−1), V+ represents the ionization potential of the atom, Eion and Eatom are the upper energy level of the ionic line and atomic line, and KB is the Boltzmann constant (Samek et al. 2000). The Eq. (1.12) is written assuming thermodynamic equilibrium condition for the plasma, i.e., Tion = Tatom = T). However, single temperature parameter is generally used to describe the plasma emission. Moreover, the electron density ne is required to measure the temperature of the laser plasma. The electron density and spectral width of the emission line are related to each other and are given by the relation:
| 1.13 |
In Eq. (1.13), constant C is used to enumerate electron density (ne) that can be found in a book by Griem (1964).
Biomedical applications of LIBS
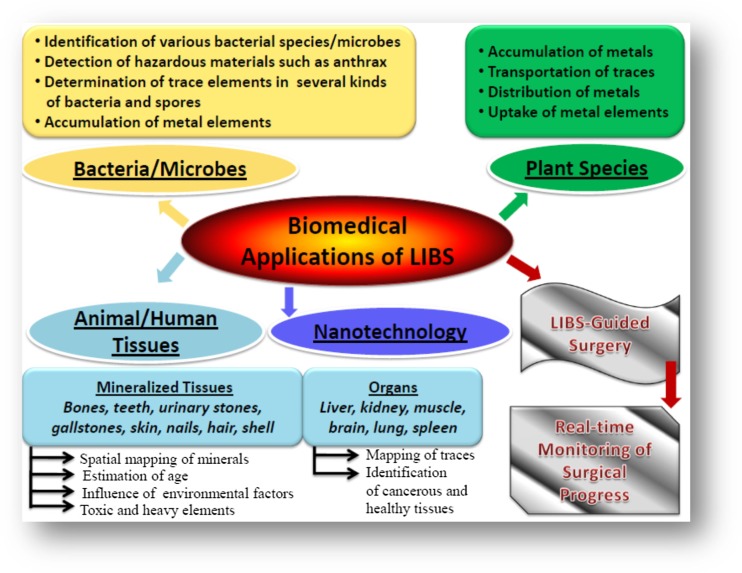
Biological samples cover a broad range of materials, which include very common materials such as wood, skin, or paper. In the last few decades, high-resolution LIBS technique has been extensively employed for different kinds of biological systems such as wood and tree samples, plant systems, and vegetables to detect their inherent variations in context of cellular biochemistry and in situ trace metal accumulations (Miziolek et al. 2006; Cremers and Radziemski, 2006; Pasquini et al. 2007; Singh and Thakur, 2007; Gondal et al. 2016c). In these applications, LIBS has proven its capability to analyze the organic and inorganic cellular compositions with high spatial resolution typically 1–100 μm. LIBS is one of the fast and furious techniques that is now emerging in the research area of bio-LIBS. The unique LIBS applications particularly in biomedical field can mainly be categorized in two principal categories: (i) investigation of clinical samples of humans such as bones, teeth, urinary stones or gallstones, nails, hair, blood, human tissues, bio-fluids, and (ii) investigation of microorganisms such as bacteria, fungi, molds, and yeasts that cause disease. LIBS is a superb technology to investigate biological specimens listed above and has also proven its capability in forensic science (Miziolek et al. 2006; Cremers and Radziemski, 2006; Pasquini et al. 2007; Singh and Thakur, 2007; Gondal et al., 2016d, Almessiere et al., 2018). The most recent biomedical LIBS applications have been condensed in Table 1 (Sinescu et al. 2008; Negrutiu et al. 2008; Hamzaoui et al. 2011; Singh et al. 2008, 2009a, b; Pathak et al. 2011; Jaswal et al., 2016a, b; Anzano and Lasheras 2009; Yueh et al. 2009; El-Hussein et al. 2010; Wu et al. 2008; Martin et al. 2003, 2005; Melikechi et al. 2008; Lee et al. 2017; Samek et al. 1999; Riberdy et al. 2017).
Table 1.
Recent progress for biomedical applications of LIBS
| Biomedical applications | Sample | Elements analyzed | Issue studied using LIBS | Authors et al. |
|---|---|---|---|---|
| In dentistry | Teeth | Ca, Mg, P, Zn, Cr, Co, and I | Microleakage in dentistry; microleakage between infrastructure and veneer materials in dentures | Sinescu et al. 2008; Negrutiu et al. 2008 |
| Analysis of nails | Nails | Ca, Na, and K | Quantitative analysis of normal and pathological nails | Hamzaoui et al. 2011 |
| In gastroenterology | Gallbladder stone | • C, Ca, Cu, Fe, K, Mg, Mn, N, Na, O, P, Sr, and Zn • C2 swan bands and CN violet bands • Na, Cr, Fe, Ti, K, Br, Si, C, K, Sn, Ca, Cl, Pb, F, N, F, Mg, Br, Ag, and Ni |
qualitative and quantitative analysis of different kinds of gallstones and variations in the elemental composition across their width Elements detected in cholesterol and pigment gallstones |
Singh et al. 2008; Pathak et al. 2011; Jaswal et al., 2016a, b |
| Laser ablation of stones and application in nephrology | Kidney stones, urinary stones | • Ca, Mg, Cu, Fe, Zn, Sr, Na, K, C, H, N, O, P, S, and Cl • C, H, N, O, Ca, Mg, Na, Sr, K, and Pb • Ca, Si, Sn, K, Mg, Fe, Cu, Ti, F, C, and N |
Qualitative and quantitative analysis of different kinds of kidney stones, urinary stones, and cross-sectional study Elements detected in calcium oxalate type kidney stones |
Singh et al. 2009b; Anzano and Lasheras 2009; Jaswal et al., 2016a, b |
| Tissue classification | Brain, lung, spleen, liver, kidney, and skeletal muscle | Ca, Al, Fe, Cu, Na, Zn, Cr, Mg, K, P, C, Li, Ni, Mo, Sn, Sc | Characterization of different normal animal tissues | Yueh et al. 2009 |
| In diagnosis | Malignant tissues | Ca and Mg | Spectrochemical analysis to identify and characterize some types of human malignancies | El-Hussein et al. 2010 |
| Analysis of body fluids | Sample solution composed of glucose and NaCl. | Analysis of organic matter as glucose and metal elements | Wu et al. 2008 | |
| LIBS for the detection of metals in dry membranes | Wet and dry membranes | Pd and Ag | Detection of metal dispersed in bacterial cellulose membranes using wet and dry metal-doped membranes. | Martin et al. 2003 |
| Analysis of blood | Blood samples | O, H, C, N, Fe, Mg, Ca, K, and Na | Qualitative analysis of blood and other liquid organic compounds | Melikechi et al. 2008 |
| Analysis of biological specimens | Salt and soil | • Ca, Mg, Na, K, Al, Si, Sr, Ti, Zn, Fe, S, C, H, N, O, C, and N • Cl, S, Mg, O |
Analysis of common edible salts, soils, and heterogeneous biological samples Accurate analysis of S in edible salts |
Singh 2009b; Martin et al. 2005; Lee et al. 2017 |
| Laser ablation for mineral analysis | Skin, finger nail and hair | • Mineral elements: B, Ca, Cr, Cu, Fe, Si, Zn, and Toxic elements: Al, Cd, Pb, and Hg • C, Ca, Mg, Na Zn, and CN in human fingernail clippings |
Combined application of LIBS and LIFS to the analysis of important minerals and toxic elements within the different parts of the body including specimens of skin tissue, finger nails and teeth. Absolute concentration of Zn in human fingernail using LIBS |
Samek et al. 1999; Riberdy et al. 2017 |
Figure 2 shows different LIBS applications to analyze different kinds of bacterial samples, plant samples, animal and human tissues, and skin samples to map of metal elements in tissues and to analyze bio minerals. Generally, high level interest in LIBS-based examination of biological materials is motivated by its native advantages including real-time analysis without any need for cumbersome sample preparation with chemicals, antibodies, or biomolecular markers. Coupled with chemometrics, and neural networking (NN), LIBS investigation provides promising, real-time, and highly sensitive point-of-care diagnosis for important applications in biomedical area of research.
Fig. 2.
Unique capabilities of LIBS towards biological applications
Analysis of microorganisms causing human disease
A bacterium is a living complex biological structure and generally composed of a genome, cytoplasm, and various membranes (Rehse et al. 2012; Kubitschek 1990; Chaleard et al., 1997; Dixon and Hahn 2005; Boyain-Goitia et al., 2003; Beddows and Telle 2005; Samuels et al., 2003; Morel et al. 2003). The size and mass of the bacterium (~ 1 μm, equivalent to a cell mass of 1 pg) make bacterial pathogens more suitable for LIBS measurements than the viral pathogens of mass a thousand times smaller (Kubitschek 1990). Elemental detection using LIBS is to investigate some typical atomic markers that are authentic to provide useful statistics about living microorganisms and their probable pathogenic capabilities.
Chaleard et al. (1997) measured the mass of Al ablated per laser pulse which was approximately 200–400 ng using laser ablation optical emission spectroscopy (LA-OES). For the first time, authors executed a series of experiments on Al alloys, steel, and brass and demonstrated the possibility of using laser-based method such as LIBS for matrix-independent measurement with a sufficient accuracy up to few percent. Dixon and Hahn (2005) reported the use and application of LIBS for single-particle measurements of bacterial spores suspended in water and aerosolized. The authors were able to measure Ca in single-spore single-shot spectra with an uncertainty of about 35%. The authors addressed the feasibility of using LIBS to detect individual bioaerosol particles in terms of detection limit and precision. In a similar manner, Boyain-Goitia et al., 2003 and Beddows and Telle 2005 reported the work done employing LIBS on different kinds of bioaerosols. In the beginning of 2003, and motivated by the Bacillus anthracis “anthrax” bioterrorism attacks, various breakthrough LIBS experiments were performed to indicate its potential usefulness for rapid detection of injurious Gram-negative and Gram-positive bacteria and spores (Samuels et al. 2003; Morel et al., 2003; Hybl et al. 2003; Kim et al. 2004; Kiel et al. 2005; Leone et al. 2004; Delucia et al. 2005).
Subsequent more in-depth investigations using LIBS were performed and documented for rapid bacterial identification by Baudelet et al. (2006a, b, c). These authors investigated Acinetobacterbaylyi, Bacillus subtilis, Erwiniachrysanthemi, Escherichia coli, and Shewanellaoneidensis using both nanosecond (ns) and femtosecond (fs) laser pulses as excitation sources. They achieved genus-level discrimination using the intensity of elements such as sodium, magnesium, phosphorous, potassium, calcium, and iron of the bacterial cell. The supremacy of femtosecond LIBS (fs-LIBS) apparatus intense molecular CN emission band relative to atomic carbon emission was found (Baudelet et al., 2006a, b, c). Baudelet et al. (2007) has also confirmed this result employing a nanosecond UV laser pulses. Because of the formation of intense fs “filaments,” fs-LIBS opens up a possibility to detect and to sense dangerous pathogens. These can transmit over large distances without losing light intensity and focusing capabilities, which allow the proper ablation of biological specimens at inconsistent distances. Adequate signal-to-noise (S/N) analysis were executed on biological samples at large distances of 32 m (Xu et al. 2007; Chin et al. 2009), yet the same has not been carried out on pathogens.
Merdes et al. (2007) studied and discriminated the bacterial target (B. subtilis spores) from non-pathogenic biological confusant targets including pollens, molds, starch, and egg albumin. Thereafter, in 2008, Snyder et al. (2008) mainly developed the chemometric algorithm models for the identification of residues of dangerous biological agents and pathogens. At the same time, Gottfried et al. (2007) applied standoff LIBS (dual pulse, DP) to monitor high S/N LIBS spectra from Bacillus globigii and a mold (Alternia alternate) at a distance of 20 m. Single-shot LIBS spectra were recorded from residues of B. globigii and the ricin surrogate ovalbumin which was fixed on double-sided adhesive tape (Gottfried et al. 2008). Employing PLS-DA, they explained the selection of variables and used to create the PLS-DA model which is extremely important mainly to avoid over-fitting and to maximize the predictive power of the model. Carefully constructing PLS-DA models, Gottfried et al. were able to discriminate Bacillus atrophaeus spores, E. coli, MS-2 bacteriophage, α-hemolysin, and Staphylococcus aureus employing portable LIBS system. Gottfried et al. (2011) deposited BW simulants and interferants on different substrates (aluminum, polycarbonate, and steel) and used PLS-DA to classify pure samples and mixtures of residues, both with a single-substrate approach and by combining different substrates. The conclusions drawn by the authors indicated the importance of building robust training datasets, including a larger number of residues and of substrates, in order to prevent models from being misled by the presence of interferants, contaminants, and spectral features from the substrates.
Recently, Cisewski et al. (2012) studied the use of a support vector machine chemometric algorithm in order to classify LIBS spectra obtained from a variety of powdered confusant materials including B. atrophaeus, Bacillus cereus, Bacillus thuringiensis, and Bacillus stearothermophilus. The authors were able to show good classification with 3.3% of error particularly for the spores.
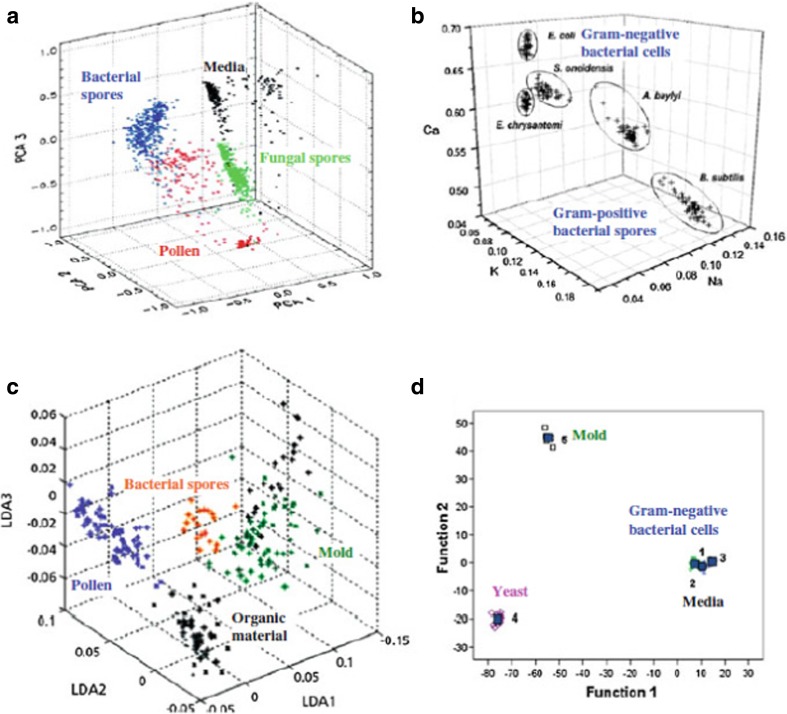
Studies reported by Hybl et al. (2003), Baudelet et al. (2006a, b, c), Merdes et al. (2007), and Diedrich et al. (2007a, b) indicated that investigation of employing LIBS allowed them to discriminate the multiple biotypes such as bacteria, fungi, mold, pollen, and yeast. In all situations, the discrimination was truly based on the intensity of observed atomic lines of elemental constituents present in microorganism and described in Fig. 3. The first three principal components of PCA were intelligent enough to discriminate bacteria, fungi, and pollen from the nutrient medium employed to culture the microorganisms (Fig. 3a: Hybl et al. 2003). Straightforward discrimination was noticed between Gram-positive spores and Gram-negative bacteria using the LIBS spectral fingerprinting from elemental contents of calcium, potassium, and sodium (Fig. 3b: Baudelet et al., 2006a, b, c). The first three scores in a linear discriminant analysis provided discrimination between Gram-positive bacterial spores, pollen, mold, and other organics like egg albumin and starch (Fig. 3c: Merdes et al. 2007). The first two discriminant function scores provided discrimination in a DFA between yeast, mold, and E. coli, as well as the nutrient medium (Fig. 3d: Diedrich 2007a).
Fig. 3.
A LIBS-based elemental analysis of microorganisms allows discrimination between multiple biotypes, including bacteria, pollen, mold, fungi, and yeast. a The first three principal components of a principal component analysis were able to differentiate bacteria, fungi, pollen, and a nutrient medium used to culture the microorganisms (Hybl 2003). b Discrimination was easily observed between Gram-negative bacteria and Gram-positive spores in a trace element hyperspace classification on the basis of their Ca, K, and Na content (Baudelet 2006). c The first three scores in a linear discriminant analysis were able to provide discrimination between Gram-positive bacterial spores, pollen, mold, and other organic materials such as starch and egg albumin (Merdes et al. 2007). d The first two discriminant function scores provided discrimination in a discriminant function analysis between specimens of yeast, mold, and Gram-negative bacteria (E. coli), as well as the nutrient medium on which they were cultured (Diedrich 2007a). Figure adapted from S.J. Rehse, Springer series in Optical Science 182, doi: 10.1007/978-3-642-45,085-3_17, pp. 457–488, 2014, with the permission from Springer Nature
About the same time, Diedrich et al. (2007a, b) reported microbiological aspects of using LIBS to identify pathogens in order to perform detailed analyses on bacterial specimens keeping in mind the interest of the medical science under clinically relevant situations including testing of live bacteria from a variety of Gram-positive and Gram-negative species. Initially, DFA yielded reproducible differences in LIBS spectra obtained from the strains of E. coli including the pathogen enterohemorraghic (E.colior O157:H7), an environmental mold, and the Candidaalbicans yeast (Diedrich et al., 2007a, b). The spectra were obtained using LIBS method using 1064 nm laser in open atmosphere. The authors performed their experiment on bacterial cultured using different nutrient media including TSA. The authors were able to show that differences in nutritional environment of the cells during cell reproduction do not affect LIBS identification.
Rehse et al. (2007) reported LIBS-based pathogens analysis with biological fluids as a growth media. In this case, the investigated pathogenic bacterium was Pseudomonas aeruginosa which are responsible for many antibiotic-resistant hospital-acquired infections (HAI). The three growths medium selected were TSA; TSA with the addition of blood, simulating samples from a patient with a blood infection; and MacConkey plus lactose agar plate (MAC) with the addition of bile salts. Addition of bile salts makes the LIBS detection a case study of great relevance in clinical diagnosis. P. aeruginosa grown on TSA and on TSA with blood resulted indistinguishable, as desirable in view of potential clinical applications. The bacterium grown in the presence of bile salts was clearly separated from the others, due to alterations in Ca and Mg content induced by the bile salts themselves. They were also able to observe the discrimination of P. aeruginosa and two non-pathogenic strains of E. coli grown on one of the substrates (TSA). Marcos-Martinez et al. (2011) also analyzed three bacteria strains P. aeruginosa, E. coli, and Salmonella typhimurium, responsible for HAI. They performed three sets of validation procedures, to investigate the capability of model constructed to identify samples in different situations (mixtures of different bacteria in the same culture medium; same bacterium cultivated in different days; unknown samples not included in the learning process of the network). The authors concluded that the method used was robust even when few single-shot spectra were included in the calculation.
After all these initial tests, Rehse et al. (2009) employed LIBS technique to ablate all the bacteria samples in different atmosphere of argon and helium and performed detailed investigations (Rehse et al. 2009). Repeated experiments of Gram-negative bacteria on a variety of media revealed that changes in the Ca and Mg content in bacterial cell in the outer membrane can be examined with the LIBS spectrum (Rehse et al. 2009). For the Gram-negative bacterium, the outer membrane was mainly a permeability barrier, primarily due to its polysaccharide content; it possessed many of the important characteristics of the Gram-negative bacterial cell (Nikaido 1973; Nikaido and Takae 1979). The authors explained that the outer layer of the outer membrane contained some phospholipids; however, it is formed mainly by a amphilic molecule composed of lipopolysaccharide (LPS) (Kamio and Nikaido 1976; Raetz 1990). Particularly, Ca2+ and Mg2+ play an important role in stabilizing the outer membrane by binding adjacent LPS molecules. The correct phenomenon of the stabilization of the cations is complexly understood, but remarkable changes in membrane permeability as a function of the concentrations of cations Ba, Ca, Mg, and Na have been observed which are straightforwardly associated with antibiotic efficacy against the bacteria (Leive, 1974; Pink et al. 2003; Ibrahim et al. 1997). LIBS is very sensitive to these peculiar cations due to the presence of highly intense atomic lines and their concentrations. Using LIBS, authors were able to observe changes when cells were exposed to known membrane detergents. Finally, authors reported a remarkable connection of LIBS assay and the membrane composition and suggest a possible serological interpretation of the LIBS-based identification.
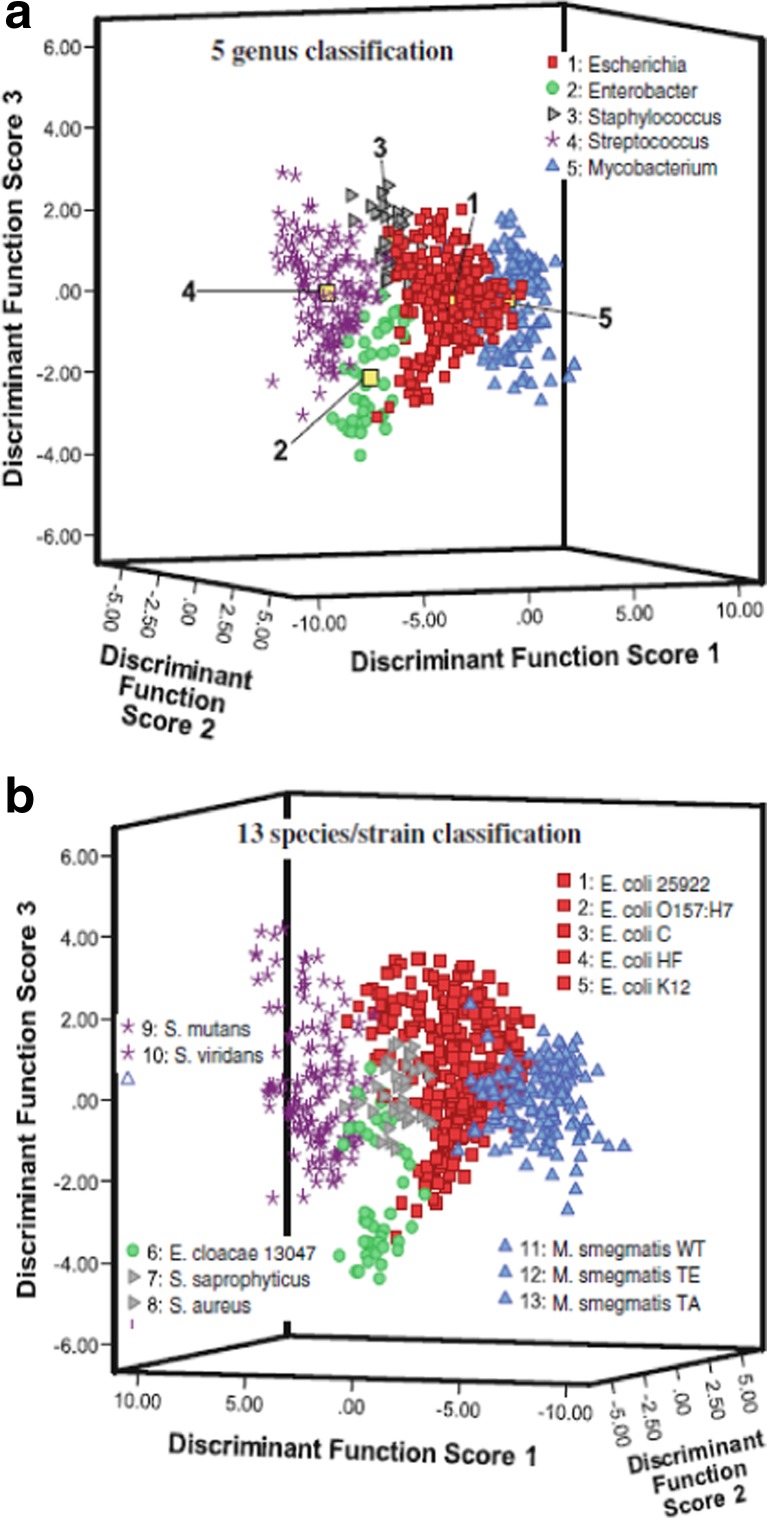
Rehse et al. (2010) also analyzed mixtures of different bacteria in many clinical samples. They used two different bacteria, the Gram-positive Mycobacterium smegmatis and the Gram-negative E. coli, and prepared samples with fractions of E. coli from 0 to 100%. The mixtures with mixing ratio up to 80:20 classified 100% of the times as M. smegmatis (the classification accuracy predictably declined with a further increase of the fraction of E. coli), suggesting that in case of contamination between different pathogens, LIBS would be able to accurately classify the main pathogen. Moreover, the authors evaluated the sensitivity of the discrimination accuracy in terms of the number of the bacterial cells interrogated by LIBS. Mohaidat et al. (2011), further, demonstrated that the accuracy of bacteria classification was not affected by a series of stressful circumstances, commonly occurring in actual biomedical samples. These include changes in the nutrition medium, inactivation or death by UV radiation, and deprivation of nutrients (starvation). This work showed that stressing conditions are not able to induce significant changes in the elemental composition of bacteria. The authors were able to prove that LIBS is an equally sensitive and accurate technique for its use on live bacteria or deactivated ones. In fact, an interesting implication of this observation could be the possibility to analyze deactivated pathogenic samples. Mohaidat et al. (2012) again came back to the issue of discriminating pathogenic bacteria in mixtures in clinical practices. One of them was the discrimination of one of the bacteria involved in urinary tract infections. The authors built the training models for their algorithms using only water as the matrix to reduce the operational complexity and to evaluate the response of the presence of the solutes in urine. The authors were able to discriminate Staphylococcus epidermidis from other staphylococci with 100% accuracy. Moreover, they demonstrated acceptable genus- and strain-discrimination (with sensitivity ranging from 62 to 90% and specificity higher than 95%) with a training model built using all the spectra acquired from bacteria of 5 genera and 13 strains which can be clearly seen in Fig. 4. Finally, they showed that the use of sequential classification testing could improve the discrimination without adding important complications in terms of time and computational expense.
Fig. 4.
The first three discriminant function scores of a DFA performed on 13 elemental emission lines in the LIBS spectra from live bacterial specimens. Sensitive and specific discrimination/classification of bacterial pathogens and non-pathogenic bacteria was observed in external validation tests performed with these two models. a The bacterial specimens were grouped by genus in the model showing the possibility of a rapid genus-level identification of an unknown sample utilizing the LIBS spectrum (Mohaidat 2012). b Thirteen bacterial classes of strain and species were ungrouped and no association between samples was provided to the model. The natural clustering of the classes by species and genus demonstrated that there were reproducible and consistent elemental differences in the bacterial cell which can be quantified by the LIBS assay and used as a basis for identification of unknown specimens (Mohaidat 2012). Figure adapted from S.J. Rehse, Springer series in Optical Science 182, doi: 10.1007/978-3-642-45,085-3_17, pp. 457–488, 2014, with the permission from Springer Nature
Multari et al. (2010) also demonstrated the use of LIBS on non-deactivated pathogenic bacteria. They demonstrated the ability of LIBS to identify unknown pathogens by comparing them to a defined sample set. They carried out a blind analysis, i.e., they analyzed two sets of samples (E. coli and four strains of Staphylococcus aureus) without knowing their contents. They used first set to build and validate a chemometric model based on PCA, which they then applied to the second set. Further, Multari et al. (2012) applied the same method to the differentiation of different live pathogens and killed viruses. In this work, pathogens were analyzed in different forms: lawns (i.e., uniform layers of bacterial growth) on agar, colonies on agar, dilutions (i.e., colony samples mixed into a liquid) on agar, and dilutions on glass slides, while viruses were deactivated by UV and interrogated as dilutions on slides. The authors found that the prediction results were better for those deposited on glass slides. Finally, the authors were able to show that the choice of an appropriate substrate is a critical parameter for pathogen discrimination and in general, for applications of chemometrics to LIBS.
LIBS analyses have also identified pathogens in context of food quality and safety such as E. coli and Salmonella enteric, a very common Gram-negative food-borne pathogen (Diedrich et al., 2007a, b; Rehse et al. 2007; Yao et al. 2010). Using Nd:YAG laser (266 nm, fourth harmonics), Barnett et al. (2011) employed LIBS system to detect pathogenic bacterium Salmonella enterica in one growth medium (Brain-Heart Infusion, BHI) and in milk, chicken broth, deposited on silicon substrates. They demonstrated discrimination of plain BIH from BIH containing S. enterica, and even different concentrations of the bacterium artificially inoculated in milk and chicken broth. The authors compared their LIBS results with the results from PCR and quantitative real-time PCR and concluded that LIBS was less sensitive than PCR in the BIH and chicken broth case, but outperformed it in the milk case. The authors also suggested LIBS as competitive with biomolecular assays with careful optimization of experimental parameters.
Lewis et al. (2011) employed femtosecond LIBS (fs-LIBS) system on the isolated bacteria which indicated the presence of sodium, magnesium, potassium, zinc, and calcium in the LIBS spectrum. The authors employed PCA and PLSR analysis on the LIBS spectra of bacterial species and demonstrated the potential capabilities of LIBS in order to discriminate and differentiate among bacteria in the unmined and reclaimed chronosequence of bauxite soils isolated on Luria-Bertani agar media.
Recently, Putnam et al. (2013) carried out a comparison of two different multivariate techniques mainly DFA and PLS-DA with the purpose to point out possible differences between the two classification models and choose the more appropriate for bacteria discrimination. In addition to the usual line selection approaches, two more methods for independent variable selection were investigated with DFA, and the one providing the highest sensitivity and specificity was then used with PLS-DA. The performance of the two chemometric techniques was similar which was based on the specific metrics obtained and hence the authors suggested DFA as the most appropriate choice to obtain a genus-level classification of completely unknown samples, and PLS-DA for finer species- or strain-level classification. Recently, Malenfant et al. (2016) made a further step ahead toward the introduction of LIBS in the clinical practice and adopted a sample preparation convention and a substrate of nitrocellulose filter paper more familiar to pathologists and clinicians. The author used DFA and PLS-DA model to discriminate the bacteria E. coli, S. epidermidis, M. smegmatis, and P. aeruginosa and evaluated the capacity of DFA model to distinguish live and autoclaved E. coli. The authors observed that a larger number of bacteria were required to obtain detectable signals in case of using filter paper. In their work, both classification methods provided higher classification sensitivity and specificity than with agar substrates.
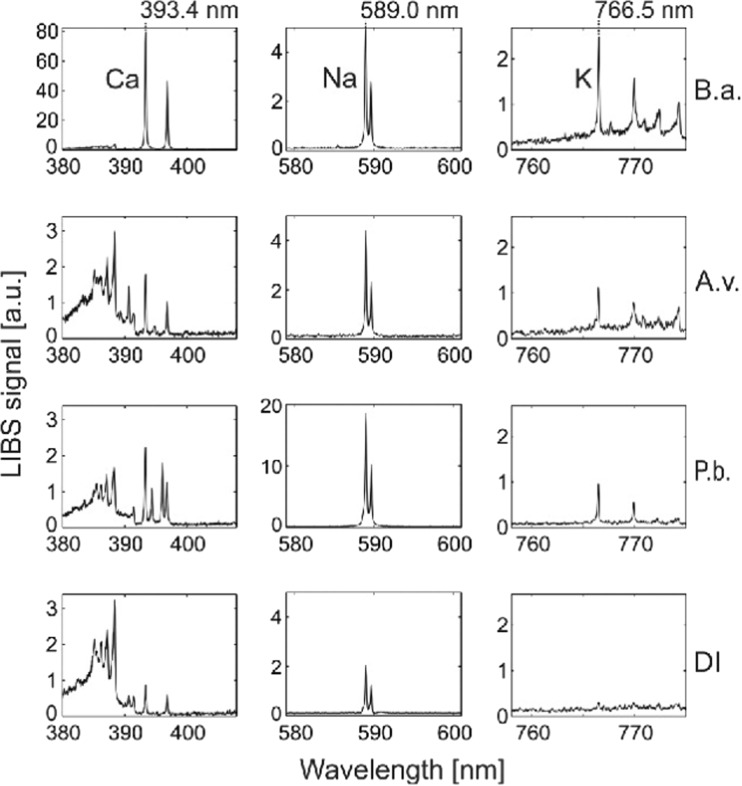
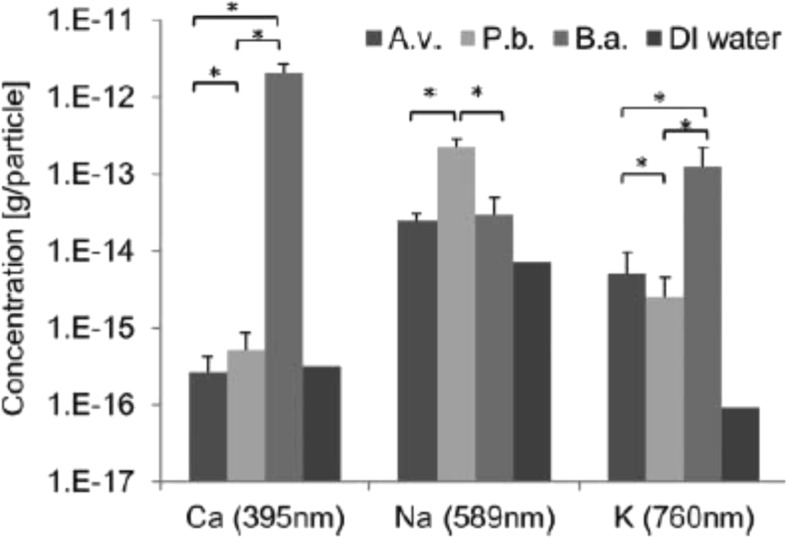
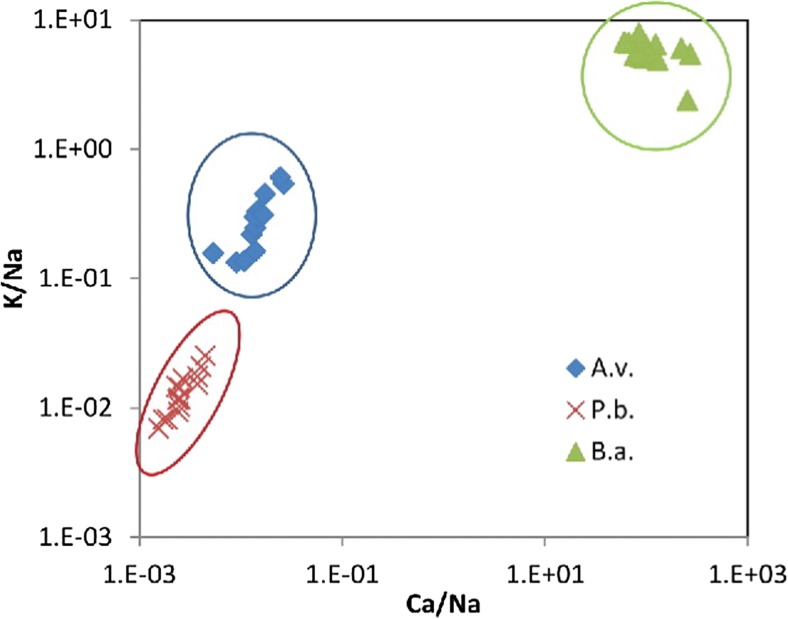
While discussing the issues related to health and climatic relevance, the online identification of fungal and bacterial spores is quite important and requires attention. Recently, Saari et al. (2016) employed electro-dynamic balance-assisted LIBS and LIF technique together to identify online the single fungal spores (Aspergillus versicolor and Penicillium brevicompactum) and bacteria (Bacillus aureus). LIBS technique enabled sensitive and repetitive detection of elemental constituent’s calcium, sodium, and potassium from fungal and bacterial spores. LIBS spectra using single laser shot (Fig. 5) were recorded from B. aureus (B.a.), A. versicolor (A.v.), and P. brevicompactum (P.b.), and DI water particles. Strong and characteristic line of Na at 589.0 and 589.6 nm were observed for microbial particles as well as aerosolized particles. Bacterial particles showed the presence of strong emission peaks of Ca and K than that from fungal spores, A.v. and P.b.. The geometric means of 20 trials and standard deviations of the elemental contents (g/particle) are given in Fig. 6. The authors reported that B.a. bacterial spores contained Ca three times greater (2 × 10−12 g/particle) than the fungal spores (3–5 × 10−16 g/particle). The highest Na content (2.3 × 10−13 g/particle) was observed in the P.b. fungal spores, whereas the Na content of A.v. fungal spores and B.a. bacterial spores were found to be 2.5 × 10−14 and 3 × 10−14 g/particle, respectively. The higher concentration of sodium in the P.b. particles was attributed due to the different surface morphology of the spores. The content of sodium and potassium were found above the background levels of DI water in all fungal spores and bacteria. The authors were able to find the good reproducibility in LIBS analysis of microbial suspensions with relatively small standard deviations. In order for precise monitoring, the laser beam in the LIBS system should be focused tightly to obtain sufficient laser fluence with 9 mJ laser pulse energy on the microbe surface to generate the plasma. Figure 7 presents the normalized Ca/Na and K/Na value derived from LIBS data of fungal and bacterial spores. Figure 7 clearly distinguishes fungal spores from bacterial spores and even readily distinguished A.v. and P.b. Finally, the authors successfully applied LIBS method for identification of fungal spores and bacteria on line and established the fact that LIBS could be a nice candidate for characterization of different varieties of aerosols and water microbes online.
Fig. 5.
Typical single-shot LIBS spectra of bacterial (Bacillus aureus, B.a.) and fungal spores (Aspergillus versicolor, A.v., and Penicillium brevicompactum, P.b.) as well as background signals from de-ionized water (DI). Characteristic peaks of Ca, Na, and K are 393.4, 589.0, and 766.5 nm, respectively. Figure adapted from S. Sari, Aerosol Science and Technology, 50, 126–132, 2016, with the permission from Taylor and Francis
Fig. 6.
Geometric mean concentrations (g/particle) of elemental components for fungal (Aspergillus versicolor, A.v., and Penicillium brevicompactum, P.b.) and bacterial (Bacillus aureus, B.a.) spores. De-ionized (DI) water represents the limit of detection based on the background from DI water. Error bars represent geometric standard deviations of 20 repeats. Asterisks represent statistically significant difference (p < 0.01) between species. Figure adapted from S. Sari, Aerosol Science and Technology, 50, 126–132, 2016, with the permission from Taylor and Francis
Fig. 7.
Normalized Ca/Na and K/Na signals from single-shot LIBS spectra for fungal (Aspergillus versicolor, A.v., and Penicillium brevicompactum, P.b.) and bacterial (Bacillus aureus, B.a.) spores. Figure adapted from S. Sari, Aerosol Science and Technology, 50, 126–132, 2016, with the permission from Taylor and Francis
Recently, Gamble et al. (2016) focused mainly on the experimental aspects of sample handling and its impact on classification accuracy. In their experiment, the authors used 266 nm laser beam to ablate four bacteria specimens and reported few good practices to improve the LIBS sensitivity to classify biological specimens. The authors kept pH stable by excluding atmospheric CO2 from the bacterial suspensions and also avoided contamination with non-native inorganic salts by preparing samples with DI water and selected organic buffers than that of inorganic ones which generally contains metallic cations.
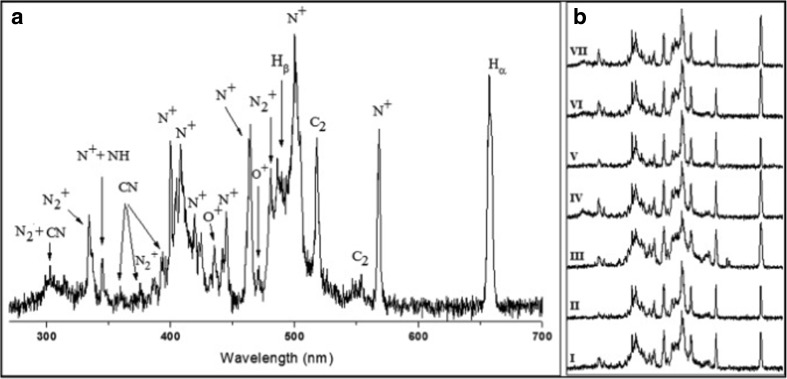
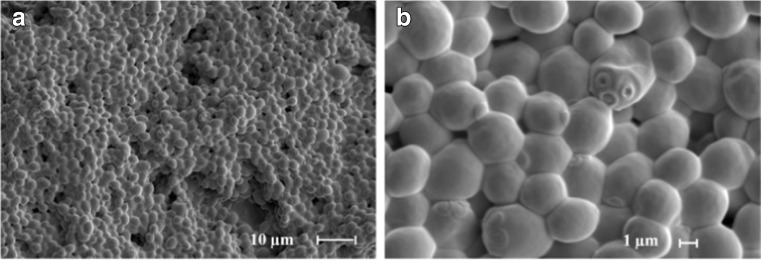
Recently, Manzoor et al. (2016) utilized LIBS technology and neural networking (NN) to discriminate different strains of various species of Candida. The authors (Manzoor et al. 2016) studied 21 strains related to 7 species of Candida. These are the predominant fungal pathogenic species causing infection to humans (Alam et al. 2014). It is also an opportunist pathogen which is responsible for mucocutaneous and disseminated infections in humans (Pulcrano et al. 2013). Some species of Candida can also be a part of the endogenous microbiota of digestive, urinary tract, and genital organs (Bedout and Gomez 2010; Sardi et al. 2013) and also have been frequently observed in cancer patients and also observed as a leading cause of nosocomial infections (Maksymiuk et al. 1984). The more frequent species of Candida includes Candidaalbicans, Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei than other species such as Candida guillermondii, Candidakefyr, and Candida dubliniensis (Bedout and Gomez 2010; Brown et al. 2012). In the present work, the authors studied Candida strains using LIBS and NN were C. parapsilosis, C. dubliniensis, C. krusei, C. glabrata, C. guillermondii, C. tropicalis, and C. albicans. The authors studied the cellular structure, 3D visualization, and chemical compositional analysis of Candida species employing SEM and EDS. The recorded LIBS data were normalized using the intensity of Hα (656 nm) line in order to stay away from data variations. Figure 8a represents the typical LIBS spectra of Candida guilliermondii. Molecular and ionic bands and atomic emission lines have also been observed (Fig. 8a) which revealed the presence of atomic signature of carbon, nitrogen, oxygen, and hydrogen which are the organic building blocks of fungal cells. The authors could not observe any atomic lines from inorganic constituents. These same kinds of elements were analyzed in the emission spectra of all the strains which can be seen in Fig. 8b. The comparison of LIBS atomic lines showed the similarities among the distinct LIBS spectral finger prints which is attributed to a very similar chemical constituents of analyzed fungal cells. Authors also performed SEM-EDS analyses to explain the absence of LIBS emission lines from inorganic elements and also to investigate the elemental composition of Candida cultures. The SEM measurement was shown in Fig. 9 which reveals the morphology of Candida culture. However, EDS provides the elemental composition these strains in weight % of each element. The major contribution was found due to the presence of elements carbon, nitrogen, and oxygen, whereas sodium, potassium, phosphorous, and sulfur were observed at very low level. The EDS results were supported by LIBS data. It was not possible to detect hydrogen using EDS technique; however, LIBS spectra showed the presence of atomic lines from Hɑ and Hβ (Fig. 8), the main constituents of organic samples. LIBS analysis indicated the presence of C2 Swan bands at 516 nm (C2: d3Πg → a3Πu) due to the organic compounds, whereas emissions from CN (B2Σ+ → X2Σ+), N, and O were also observed from the sample. The results obtained for C, N, H, O, CN and C/H, C/O, and C/N intensity ratios demonstrated highly usefulness of LIBS to characterize the compounds or organic samples. Thus, the cellular chemical structure of Candida revealed the spectral fingerprint with LIBS emissions from C, N, H, O, and CN (Lopez et al. 2006). LIBS spectral results were carried out using statistical methods by employing NN to develop the models for classification. The authors used NN model to inspect LIBS spectral data obtained from species of Candida. The NN models were selected to improve the identification capability and hence to get the classification of the Candida strains, the NN model was evaluated using training set of libraries from all the strains of 21 Candida and then validated using test set of libraries. All the LIBS spectra for Candida strains have been designated precisely to their species and achieved 100% correlation. The results also demonstrated the ability of NN in combination with LIBS data to deal with more number of samples in less computing timing. However, no misidentified test spectra of Candida strains were observed. The authors were able to classify all the strains successfully according to their species and strain. The authors gathered sufficient and useful information to discriminate all 21 Candida strains from the CN, C2 swan band system, and emission lines from H, N, and O appeared in the LIBS spectra. Hence, the authors were able to present the first LIBS-based analysis emphasized to identify fungal samples (Candida), with an outstanding clinical significance.
Fig. 8.
a Normalized LIBS spectrum of Candida guilliermondii with the assignation of emission lines. b Normalized spectra of (I) C. parapsilosis (ATCCCP22019), (II) C. dubliniensis (CBS7987), (III) C. krusei (ATCC6258), (IV) C. guilliermondii (CNM-CL9533), (V) C. glabrata (CBS138), (VI) C. tropicalis (CNM-CL9537), and (VII) C. albicans (CNM-CL9534) within the spectral range of 300–700 nm. Figure adapted from S. Manzoor, Talanta, 155, 101–106, 2016, with the permission from Elsevier
Fig. 9.
a Wide and b magnified view of Candida albicans (CNM-CL9535) by scanning electron microscopy (SEM). Figure adapted from S. Manzoor, Talanta, 155, 101–106, 2016, with the permission from Elsevier
High degrees of resolution are generally required to analyze cells, sub-cells, and hazardous microorganisms using optical techniques. The biomedical applications of LIBS particularly to analyze pathogenic microorganism affecting human being are not very common. Due to the unique advantages of LIBS technique, it is more commonly used for the analysis of bacterial samples than that for the animal tissues. To quantify various elements in bacteria, viruses, and other microorganisms, LIBS method is extensively employed. In Table 2, LIBS data for cells and microorganisms used in this study are summarized. Also, it is reported in the literature that LIBS technique has been employed to analyze hazardous materials and also showed his potential capabilities to find the presence of microorganism. LIBS studies have also been made on variety of biomaterials in the medical field with encouraging results. In Table 3, we have also summarized the advantages, limitations, and some possible future biomedical applications of LIBS (Jaswal et al., 2016a, b; Rehse et al. 2012; Singh and Rai 2011; Pathak et al. 2012; Jaswal and Singh 2015; Singh et al. 2014; Kaiser et al. 2012). Moreover, LIBS technique provides rapid data from samples containing multi-elements, including the light elements.
Table 2.
LIBS studies on microorganisms together with the investigated species, the elements studied, and the appropriate references are given
| S.No. | Investigated species | Elements studied | Author et al. |
|---|---|---|---|
| 1. | Alternia alternata | Al, Cr, Cu, Au, In, Pb, Ag | Gottfried et al. 2007 |
| 2. | Bacillus cereus | Ca, C, Mn, Ag | Samuels et al. 2003 |
| 3. | Bacillus megaterium | Ca, Fe, Mn, K, Na | Kim et al. 2004 |
| 4. | Bacillus subtilis | Al, Ca, C, Cr, Cu, H, Au, In, Fe, Pb, Mg, Mn, N, K, Ag, Na | Samuels et al. 2003; Morel et al. 2003; Kim et al. 2004; Baudelet et al. 2006a, b, c; Gottfried et al. 2007 |
| 5. | Bacillus thuringiensis | Ca, C, Fe, Mg, Mn, K, Ag, Na | Samuels et al. 2003; Morel et al. 2003; Kim et al. 2004 |
| 6. | Candida albicans | Ca, C, Mg, P, K, Na | Diedrich 2007a |
| 7. | Escherichia coli | Ca, C, Cl, H, Fe, Mg, Mn, N, O, P, K, Na | Baudelet et al. 2006a, b, c; Morel et al. 2003; Kim et al. 2004; Diedrich 2007a; Rehse and Mohaidat 2009; Rehse et al. 2010; Multari et al. 2010 |
| 8. | Mycobacterium smegmatis | Ca, C, Mg, P, Na | Rehse et al. 2010 |
| 9. | Proteus mirabilis | Ca, Mg, K | Morel et al. 2003 |
| 10. | Pseudomonas aeruginosa | Ca, C, Mg, P, Na | Rehse 2007; Rehse and Mohaidat, 2009 |
| 11. | Stachybotrys chartarum | Ca, C, Mg, P, K, Na | Diedrich 2007a |
| 12. | Staphylococcus aureus | Ca, C, Mg, P, K, Na | Morel et al. 2003; Rehse et al. 2010; Multari et al. 2010 |
| 13. | Staphylococcus saprophyticus | Ca, C, Mg, P, Na | Mohaidat et al. 2012 |
| 14. | Streptococcus mutans | Ca, C, Mg, P, Na | Mohaidat et al. 2012 |
| 15. | Streptococcus viridans | Ca, C, Mg, P, Na | Mohaidat et al. 2012 |
Table 3.
Advantages, limitations, and some possible future biomedical applications of LIBS
| LIBS | Analytical features |
|---|---|
| Advantages | i. Quick and in-situ analysis. ii. Capability of in-vivo analysis. iii. Capability to analyze solids, liquids, gases, as well as microorganism. iv. Simultaneous and multi-elemental analysis capability. v. Point detection capability. vi. Capability to analyze the samples in hostile environment. vii. Capability of stand-off detection. viii. Potential for underwater analysis. ix. Lack of tedious sample preparation steps. x. Potential of LIBS for direct real-time analysis of biomedical specimens without the addition of any biochemical precursors, antigenic, genetic amplification steps, acid digestion steps, etc. xi. Potential to deliver a LIBS-based medical probe for current medical practitioner in surgery and dentistry. |
| Limitations | i. One potential limitation is the destructive nature of the analysis of very small biological samples. ii. Limited utility for the analysis of proteins and biochemical disease markers generally composed of C, H, N, and O. iii. Difficulty in effective quantification of the lighter elements such as C, H, N, and O. iv. Limits of detection (typically in ppm range) are generally not as good as those of the conventional techniques. v. Difficult to get suitable matrix-matched standards in case of biological samples such as gallstones, kidney stones, tissues, etc. vi. Precision is poor as compared to conventional techniques. |
| Future prospects for biomedical applications | i. In vivo discriminate between malignant tissues from non-malignant tissues. ii. In vivo or in vitro study of kidney stones, gall bladder stones, urinary stones. iii. Real-time identification of caries dental tissue. iv. In-vivo measurement of heavy metal elements with high spatial resolution of in bones, in joints, or in other tissues. v. Identification of bacteria in human fluid specimens. vi. Rapid classification bacterial strain for epidemiology and infection control in hospitals and clinics. vii. Monitoring of surface contamination. viii. Real-time testing of urinary tract bacterial infection. |
Use of femtosecond LIBS to analyze bacteria
Here, in the present section, we presented some critical points of using femtosecond lasers (particularly fs-LIBS) in comparison with nanosecond lasers with the purpose to identify the most suitable source for LIBS of bacteria. Baudelet et al. (2006a, b, c) documented that ultra-short pulses could be beneficial for the discrimination of bacteria using LIBS. The authors came to this conclusion by performing a time-resolved kinetic study, which showed that the native and recombination CN bands (from a graphite sample) have a different time evolution. In a subsequent work, Baudelet et al. (2006a, b, c) also addressed the femtosecond-LIBS of bacteria from a different point of view. In this work, authors analyzed the relative contents of sodium, magnesium, phosphorous, potassium, calcium, and iron pressed pellets of lyophilized bacteria and reported these data in a six-dimension hyperspace. Interestingly, they obtained significant separation between clusters of the different samples, even using 3D plots. The same research group continued their study about CN bands, where they investigated the plasma produced by irradiating the organic polymer Nylon 6,6 with femtosecond and nanosecond laser pulses (UV and IR) (Baudelet et al. 2009). The authors analyzed the shockwave expansion and the kinetics of formation and evolution of the CN molecular band in the different ablation regimes, using Nylon as a model target. The time-resolved studies demonstrated that CN bands observed in the plasmas produced with IR nanosecond lasers were due to recombination of carbon atom of micro-plasma and atmospherics nitrogen. In contrast, UV nanosecond lasers and femtosecond lasers were able to ablate molecular fragments from the target. Therefore, in the latter ablation conditions, these signals could be used as discriminating spectral features in the analysis of biological samples. Two other groups used femtosecond lasers for the analysis of bacteria. Although these groups did not focus their work on strictly biomedical applications, they are relevant to the general issue of bacteria discrimination through LIBS and chemometrics. Lewis et al. (2011) used femtosecond-LIBS and PCA and PLS to discriminate bacteria contained in bauxite soils to monitor the level of rehabilitation of these particular ecosystems. The combination of fs-LIBS and chemometrics did yield capture of inter- and intra-site differences and differentiation of both bacterial species and strain. However, its usefulness and feasibility for in-situ measurements were limited because of a relatively complex sample preparation. Sivakumar et al. (2015) carried out a comparative investigation between ns-LIBS and fs-LIBS to discriminate between live and dead E. coli (sonicated and autoclaved). Their work was motivated by the need to develop technologies able to identify possible contaminations from terrestrial biological material in astrobiological applications aimed at ascertaining the presence of life in extraterrestrial planets. They found that some key elements such as magnesium, phosphorous, potassium, sodium, and calcium had statistically different emission intensities in live and dead bacteria due to damages induced in the bacterial membranes by the deactivation procedures. Due to sample splashing induced by nanosecond lasers, the authors suggested that femtosecond lasers were more suitable for the analysis of this kind of samples. Finally, an interesting application of femtosecond lasers was proposed by Xu et al. (2006, 2007), which the authors denominated FIBS and was based on the phenomenon of femtosecond laser filamentation. FIBS was used to acquire time-resolved elemental and molecular spectra of biological materials at standoff distances of 3.5 m (Xu et al. 2006) and 4.7 m (Xu et al. 2007), while a sample spectrum was also acquired at 50 m (Xu et al. 2007) to exhibit the capability of the method at longer distances. The samples employed in these studies were not bacteria or other hazardous biological agents yeast and egg white in (Xu et al. 2006), barley, corn, and wheat grain (Xu et al. 2007), in both cases, but the described methodology could find potential application in the standoff determination of biological and chemical warfare.
Conclusion and future prospects
LIBS is in a stage of great vitality as an analytical technology particularly for investigations of biomedical samples. We have reviewed the utility of LIBS to detect and identify pathogens and several other kinds of bacterial systems. LIBS system has the capability to detect lighter elements in the bacterial systems in few seconds. This detection system does not need cumbersome-specific sample preparation. It is also a non-invasive technique and provides spatial resolution of higher orders in micron. Among other positive features, the major advantages are minimal sample preparation requirements and good spatial resolution with instant detection of the emission from micro-plasma. We have also reviewed the utility of LIBS and its applications to broad spectrum of biological species and elements detected in these specimens along with bacterial species causing human disease. In addition to traditional applications to analyze calcified tissues and biological materials of various kinds, some new research trends have emerged in recent years. These unique capabilities are likely to play an important role in potential development of LIBS technique as well as in its penetration in the medical community. In the LIBS detection system, pathogens have been investigated broadly related to bacterial biosensing. An increasingly important role is played by chemometrics, in particular for sample classification, dimensionality reduction of the datasets and pattern recognition, and in view of the development of automatic procedures for routine analysis of large number of samples (e.g., screening tests for cancer or for bacterial pathogens). In the future, this area may attain a great deal of research scope in broad context using LIBS technology. The convergence of LIBS, machine learning, and nanotechnology can give the birth of new diagnosis technology for its applications in biomedical field precisely. In the future, this area may be a potential area of research interest in broad context using LIBS technology. Further efforts in this sense are crucial to promote biomedical applications LIBS in a new phase of its evolution and fill the gap between the spectroscopic community and the medical one.
Conflict of interest
Vivek K. Singh declares that he has no conflict of interest. Jitendra Sharma declares that he has no conflict of interest. Ashok K. Pathak declares that he has no conflict of interest. Charles T. Ghany declares that he has no conflict of interest. M.A. Gondal declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by the any of the authors.
References
- Anzano J, Lasheras RJ. Strategies for the identification of urinary calculus by laser induced breakdown spectroscopy. Talanta. 2009;79:352–360. doi: 10.1016/j.talanta.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Alam MZ, Alam Q, Jiman-Fatani A, Kamal MA, Abuzenadah AM, Chaudhary AG, et al. Candida identification: a journey from conventional to molecular methods in medical mycology. World J Microbiol Biotechnol. 2014;30:1437–1451. doi: 10.1007/s11274-013-1574-z. [DOI] [PubMed] [Google Scholar]
- Baudelet M, Boueri M, Yu J, Mao SS, Piscitelli V, Mao X, Russo RE. Time-resolved laser-induced breakdown spectroscopy for organic material analysis. Spectrochem Acta B. 2007;62(12):1329–1339. [Google Scholar]
- Bedout De C, Gómez BL. Candida y candidiasis invasora: unreto continuo para su diagnostic temprano. Infectio. 2010;14:S159–S171. [Google Scholar]
- Baudelet M, Guyon L, Yu J, Wolf JP, Amodeo T, Frejafon E, Laloi P. Femtosecond time-resolved laser-induced breakdown spectroscopy for detection and identification of bacteria: a comparison to the nanosecond regime. J Appl Phys. 2006;99(8):084701. [Google Scholar]
- Baudelet M, Yu J, Bossu M, Jovelet J, Wolf JP, Amodeo T, Frejafon E, Laloi P. Discrimination of microbiological samples using femtosecond laser-induced breakdown spectroscopy. Appl Phys Lett. 2006;89(16):163903. [Google Scholar]
- Baudelet M, Guyon L, Yu J, Wolf JP, Amodeo T, Frejafon T, Laloi P. Spectral signature of native CN bonds for bacterium detection and identification using femtosecond laser-induced breakdown spectroscopy. Appl Phys Lett. 2006;88(6):063901. [Google Scholar]
- Baudelet M, Boueri M, Yu J, Mao X, Mao SS, Russo R. Laser ablation of organic materials for discrimination of bacteria in an inorganic background. Proc SPIE. 2009;7214:72140J. [Google Scholar]
- Boyain-Goitia AR, Beddows DCS, Griffiths BC, Telle HH. Single-pollen analysis by laser-induced breakdown spectroscopy and Raman microscopy. Appl Opt. 2003;42(30):6119–6132. doi: 10.1364/ao.42.006119. [DOI] [PubMed] [Google Scholar]
- Borgia I, Burgio LMF, Corsi M, Fantoni R, Palleschi V, Salvetti A, Squarcialupi MC, Tognoni E. Self-calibrated quantitative elemental analysis by laser-induced plasma spectroscopy: application to pigment analysis. J Cult Herit. 2000;1(2):S281–S286. [Google Scholar]
- Beddows DCS, Telle HH. Laser-induced plasma spectroscopy and applications (LIBS 2004) third international conference LIBS Malaga. Spectrochem Acta B. 2005;60(7):1040–1242. [Google Scholar]
- Barnett C, Bell C, Vig K, Akpovo CA, Johnson L, Pillai S, Singh S. Development of LIBS assay for the detection of salmonella entericaserovar Typhimurium from food. Anal Bioanal Chem. 2011;400(10):3323–3330. doi: 10.1007/s00216-011-4844-3. [DOI] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science. 2012;336(6082):647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- Cremers D, Radziemski L (2006) Handbook of laser-induced breakdown spectroscopy. John Wiley & Sons ltd. ISBN 978–0–470-09300-9
- Chin S L (2009) Femtosecond laser filamentation. Springer series on atomic, optical and plasma physics. ISBN 978–1–4419-0688-5
- Cisewski J, Snyder E, Hannig J, Oudejans L. Support vector machine classification of suspect powders using laser-induced breakdown spectroscopy(LIBS) spectral data. J Chemom. 2012;26:143–149. [Google Scholar]
- Chin SL, Xu HL, Luo Q, Théberge F, Liu W, Daigle JF, Kamali Y, Simard PT, Bernhardt J, Hosseini SA, Sharifi M, Méjean G, Azarm A, Marceau C, Kosareva O, Kandidov VP, Aközbek N, Becker A, Roy G, Mathieu P, Simard JR, Châteauneuf M, Dubois J. Filamentation remote sensing of chemical and biological agents/pollutants using only one femtosecond laser source. Appl Phys. 2009;95(1):1–12. [Google Scholar]
- Chaleard C, Mauchien P, Andre N, Uebbing J, Lacour JL, Geertsen C. Correction matrix effects in quantitative elemental analysis with laser ablation optical emission spectrometry. J Anal At Spectrom. 1997;12(2):183–188. [Google Scholar]
- Dixon PB, Hahn DW. Feasibility of detection and identification of individual bioaerosols using laser-induced breakdown spectroscopy. Anal Chem. 2005;77(2):631–638. doi: 10.1021/ac048838i. [DOI] [PubMed] [Google Scholar]
- DeLucia FC, Samuels AC, Harmon RS, Walter RA, McNesby KL, LaPointe A, WinkelJr RJ, Miziolek AW. Laser-induced breakdown spectroscopy (LIBS): a promising versatile chemical sensor technology for hazardous material detection. IEEE Sensors J. 2005;50:681–689. [Google Scholar]
- Diedrich J, Rehse SJ, Palchaudhuri S. Escherichia coli identification and strain discrimination using nanosecond laser-induced breakdown spectroscopy. Appl Phys Lett. 2007;90(16):163901. [Google Scholar]
- Diedrich J, Rehse SJ, Palchaudhuri S. Pathogenic Escherichia coli strain discrimination using laser-induced breakdown spectroscopy. J Appl Phys. 2007;102(1):014702. [Google Scholar]
- El-Hussein A, Kassem AK, Ismail H, Harith MA. Exploiting LIBS as a spectrochemical analytical technique in diagnosis of some types of human malignancies. Talanta. 2010;82:495–501. doi: 10.1016/j.talanta.2010.04.064. [DOI] [PubMed] [Google Scholar]
- Almessiere MA, Altuwiriqi R, Gondal MA, Al Dakheel RK, Alotaibi HF. Qualitative and quantitative analysis of human nails to find correlation between nutrients and vitamin D deficiency using LIBS and ICP-AES. Talanta. 2018;185:61–70. doi: 10.1016/j.talanta.2018.03.057. [DOI] [PubMed] [Google Scholar]
- Gamble GR, Park B, Yoon S-C, Lawrence KC. Effect of sample preparation on the discrimination of bacterial isolates cultured in liquid nutrient media using laser-induced breakdown spectroscopy (LIBS) Appl Spectrosc. 2016;70:494–504. doi: 10.1177/0003702815626679. [DOI] [PubMed] [Google Scholar]
- Gondal MA, Habibullah YB, Baig U, Oloore LE. Direct spectral analysis of tea samples using 266 nm UV pulsed laser-induced breakdown spectroscopy and cross validation of LIBS results with ICP-MS. Talanta. 2016;152:341–352. doi: 10.1016/j.talanta.2016.02.030. [DOI] [PubMed] [Google Scholar]
- Gondal MA, Shemis MA, Khalil AAI, Nasr MM, Gondal B. Laser produced plasma diagnosis of carcinogenic heavy metals in gallstones. J Anal Spectrom. 2016;31:506–514. [Google Scholar]
- Gondal MA, Maganda YW, Dastageer MA, Al Adel FF, Naqvi AA, Qahatan TF. Detection of carcinogenic chromium in synthetic hair dyes using laser induced breakdown spectroscopy. Appl Opt. 2014;53:1636–1643. doi: 10.1364/AO.53.001636. [DOI] [PubMed] [Google Scholar]
- Gondal MA, Hussain T, Yamani ZH, Baig MA. The role of various binding materials for trace elemental analysis of powder samples using laser induced breakdown spectroscopy. Talanta. 2007;72:642–649. doi: 10.1016/j.talanta.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Gondal MA, Hussain T. Determination of poisonous metals in waste water collected from paint manufacturing plant using laser- induced breakdown spectroscopy. Talanta. 2007;71:73–80. doi: 10.1016/j.talanta.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Gondal B, Gondal MA, Siddiqui MF, Sarwar M, Bissonnette M (2016c) Colon cancer and heavy metal exposure: exploration of etiology and risk factors using advanced laser Spectro-chemical analysis. Am J Gastroenterol, Oct issue 2016
- Gondal MA, Shemis MA, Gondal B, Khalil AAI. Gallbladder stones analysis using pulsed UV laser induced breakdown spectroscopy. J Med Bioeng. 2016;5:85–88. [Google Scholar]
- George RH, Gully PR, Gill N, Innest JA, Bakhshig SS, Connolly M. An outbreak of tuberculosis in a children’s hospital. J Hosp Infect. 1986;8(2):129–142. doi: 10.1016/0195-6701(86)90039-3. [DOI] [PubMed] [Google Scholar]
- Griem HR. Plasma Spectroscopy. New York: McGraw-Hill; 1964. [Google Scholar]
- Gottfried JL, De Lucia FC, Jr, Munson CA, Miziolek AW. Double pulse standoff laser-induced breakdown spectroscopy for versatile hazardous materials detection. Spectrochem Acta B. 2007;62:1405–1411. [Google Scholar]
- Gottfried JL, De LJFC, Munson CA, Miziolek AW. Standoff detection of chemical and biological threats using laser-induced breakdown spectroscopy. Appl Spectrosc. 2008;62:353–363. doi: 10.1366/000370208784046759. [DOI] [PubMed] [Google Scholar]
- Gottfried JL. Discrimination of biological and chemical threat simulants in residue mixtures on multiple substrates. Anal Bioanal Chem. 2011;400:3289–3301. doi: 10.1007/s00216-011-4746-4. [DOI] [PubMed] [Google Scholar]
- Nikaido H. In: Bacterial membranes and walls. Leive L, editor. New York: Marcel Dekker; 1973. [Google Scholar]
- Hoshino Q, Yilmaz LS, Noguera DR, Daims H, Wagner M. Quantification of target molecules need to detect microorganisms by fluorescence in situ hybridization (FISH) and catalyzed reporter deposition-FISH. Appl Environ Microbiol. 2008;74(16):5068–5077. doi: 10.1128/AEM.00208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybl JD, Lithgow GA, Buckley SG. Laser-induced breakdown spectroscopy detection and classification of biological aerosols. Appl Spectrosc. 2003;57(7):1207–1215. doi: 10.1366/000370203769699054. [DOI] [PubMed] [Google Scholar]
- Hamzaoui S, Khleifia R, Jaidane N, Lakhdar ZB. Quantitative analysis of pathological nails using laser-induced breakdown spectroscopy (LIBS) technique. Lasers Med Sci. 2011;26(1):79–83. doi: 10.1007/s10103-010-0821-x. [DOI] [PubMed] [Google Scholar]
- Ibrahim HR, Higashiguck S, Sugimot Y, Aoki T. Role of divalent cations in the novel bactericidal activity of the partially unfolded lysozyme. J Agric Food Chem. 1997;45:89–94. [Google Scholar]
- Joachain CJ, Chester AN, Martellucci S, Batani D. Atoms, solids, and plasmas in super-intense laser. 1. New York: Springer, Kluwer Academic/Plenum Publishers; 2001. pp. 15–36. [Google Scholar]
- Jaswal BBS, Kumar V, Sharma J, Rai PK, Gondal MK, Gondal B, Singh VK. Analysis of heterogeneous gallstones using laser-induced breakdown spectroscopy (LIBS) and wavelength dispersive X-ray fluorescence (WD-XRF) Lasers Med Sci. 2016;31:573–579. doi: 10.1007/s10103-016-1905-z. [DOI] [PubMed] [Google Scholar]
- Jaswal BBS, Rai PK, Gondal MA, Singh VK. Spectroscopic investigations of calcium oxalate kidney stone using Fourier transform infra-red spectroscopy and laser-induced breakdown spectroscopy. Mater Focus. 2016;6:82–86. [Google Scholar]
- Jaswal BBS, Singh VK. Analytical assessments of gallstones and urinary stones: a comprehensive review of the development from laser to LIBS. Appl Spectrosc Rev. 2015;50:473–498. [Google Scholar]
- Kaiser J, Novotny K, Martin MZ, Hrdlicka A, Malinaa R, Hartl M, Adam V, Kizek R. Trace elemental analysis by laser-induced breakdown spectroscopy-biological applications. Surf Sci Rep. 2012;67:233–243. [Google Scholar]
- Kamio Y, Nikaido H. Outer membrane of salmonella typhimurium: accessibility of phospholipids head group to phospholipase C and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976;15(12):2561. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- Kim T, Specht ZG, Vary PS, Lin CT. Spectral fingerprints of bacterial strains by laser-induced breakdown spectroscopy. J Phys Chem B. 2004;108(17):5477–5482. [Google Scholar]
- Kiel JL, Holwitt EA, Parker JE, Vivekananda J, Franz V, Sloan MA, Miziolek AW, DeLucia FC, Munson CA, Mattley YD. Specific biological agent Taggants. Proc SPIE. 2005;5795:39–45. [Google Scholar]
- Kubitschek HE. Cell volume increase in Escherichia coli after shifts to richer media. J Bacteriol. 1990;172(1):94–101. doi: 10.1128/jb.172.1.94-101.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Qndera O, Miyatake T, Tsuji S. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction (PCR) Neurology. 1990;40(10):1617–1618. doi: 10.1212/wnl.40.10.1617. [DOI] [PubMed] [Google Scholar]
- Lee Yonghoon, Chirinos Jose, Gonzalez Jhanis, Oropeza Dayana, Zorba Vassilia, Mao Xianglei, Yoo Jonghyun, Russo Richard E. Laser-Ablation Sampling for Accurate Analysis of Sulfur in Edible Salts. Applied Spectroscopy. 2017;71(4):651–658. doi: 10.1177/0003702817691288. [DOI] [PubMed] [Google Scholar]
- Lewis DE, Martinez J, Akpovo CA, Johnson L, Chauhan A, Edington MD. Discrimination of bacteria from Jamaican bauxite soils using laser-induced breakdown spectroscopy. Anal Bioanal Chem. 2011;401:2225–2230. doi: 10.1007/s00216-011-5274-y. [DOI] [PubMed] [Google Scholar]
- Lay JO. MALDI-TOF mass spectrometry of bacteria. Mass Spectrom Rev. 2001;20(4):172–194. doi: 10.1002/mas.10003. [DOI] [PubMed] [Google Scholar]
- Leone N, Arthur GD, Adam P, Amouroux J. Detection of bacterial deposits and bio-aerosols by time-resolved laser-induced breakdown spectroscopy(TRELIBS) High Technol Plasma Process. 2004;8(1):1–22. [Google Scholar]
- Leive L. The barrier function of gram-negative envelope. Ann N Y Acad Sci. 1974;235:109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Malenfant DJ, Gillies DJ, Rehse SJ. Bacterial suspensions deposited on microbiological filter material for rapid laser-induced breakdown spectroscopy identification. Appl Spectrosc. 2016;70:485–493. doi: 10.1177/0003702815626673. [DOI] [PubMed] [Google Scholar]
- Madigan M, Martinko J, Parker J. Brock biology of microorganisms. 9. Upper Sadder, New Jersey: Prentice Hall; 1996. p. 992. [Google Scholar]
- Musazzi S, Perini U, editors. laser-induced breakdown spectroscopy. Berlin Heidelberg: Springer-Verlag; 2014. [Google Scholar]
- Martin M, Evans B, O’Neill H, Woodward J. Laser induced breakdown spectroscopy used to detect palladium and silver metal dispersed in bacterial cellulose membranes. Appl Opt. 2003;42(30):6174–6178. doi: 10.1364/ao.42.006174. [DOI] [PubMed] [Google Scholar]
- Melikechi N, Ding H, Rock S, Marcano OA, Connolly D. Laser-induced breakdown spectroscopy of whole blood and other liquid organic compounds. Proc SPIE. 2008;6863:68630O. [Google Scholar]
- Morel S, Leone M, Adam P, Amouroux J. Detection of Bacteria by time-resolved laser-induced breakdown spectroscopy. Appl Opt. 2003;42(30):6184–6193. doi: 10.1364/ao.42.006184. [DOI] [PubMed] [Google Scholar]
- Merdes DW, Suhan JM, Keay JM, Hadka DM, Bradley WR. The investigation of laser induced breakdown spectroscopy for the detection of biological contaminants on surface. Spectroscopy. 2007;22:28–32. [Google Scholar]
- Mohaidat QI, Sheikh K, Palchaudhuri S, Rehse SJ. Pathogen identification with laser-induced breakdown spectroscopy: the effect of bacterial and biofluid specimen contamination. Appl Opt. 2012;51:B99–B107. doi: 10.1364/AO.51.000B99. [DOI] [PubMed] [Google Scholar]
- Mohaidat QI, Palchaudhuri S, Rehse SJ. The effect of bacterial environmental and metabolic stresses on a laser-induced breakdown spectroscopy(LIBS) based identification of Escherichia coli and streptococcus viridans. Appl Spectrosc. 2011;65(4):386–392. doi: 10.1366/10-06178. [DOI] [PubMed] [Google Scholar]
- Multari R, Cremers DA, Dupre JM, Gustafson JE. The use of laser-induced breakdown spectroscopy for distinguishing bacterial pathogen species and strains. Appl Spectrosc. 2010;64(7):750–759. doi: 10.1366/000370210791666183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multari R, Cremers DA, Bostian ML. Use of laser-induced breakdown spectroscopy for the differentiation of pathogens and viruses on substrates. Appl Opt. 2012;51(7):B57–B64. doi: 10.1364/AO.51.000B57. [DOI] [PubMed] [Google Scholar]
- Marcos-Martinez D, Ayala JA, Izquierdo-Hornillos RC, Manuel de Villena FJ, Caceres JO. Identification and Dicrimination of bacterial strains by laser-induced breakdown spectroscopy and neural networks. Talanta. 2011;84(3):730–737. doi: 10.1016/j.talanta.2011.01.069. [DOI] [PubMed] [Google Scholar]
- Manzoor S, Ugena L, Tornero-Lopéz J, Martín H, Molina M, Camacho JJ, Cáceres JO. Laser induced breakdown spectroscopy for the discrimination of Candida strains. Talanta. 2016;155:101–106. doi: 10.1016/j.talanta.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Martin MZ, Wullschleger SD, Garten CT, Palumbo AV, Smith JG. Elemental analysis of environmental and biological samples using laser-induced breakdown spectroscopy and pulsed Raman spectroscopy. J Dispers Sci Technol. 2005;25(5):687–694. [Google Scholar]
- Miziolek AW, Palleschi V, Schechter I. Laser induced breakdown spectroscopy. 1. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Maksymiuk AW, Thongprasert S, Hopfer R, Luna M, Fainstein V, Bodey GP. Systemic candidiasis in cancer patients. Am J Med. 1984;77:20–27. [PubMed] [Google Scholar]
- Nikaido H, Takae T. The outer membrane of gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Negrutiu ML, Sinescu C, Draganescu G, Todea C, Dodenciu D, Rominu R. Micro spectral analysis with laser in microleakage evaluation between infrastructure and veneer materials in fixed partial dentures. Proc SPIE. 2008;6843:68430Q. [Google Scholar]
- Petry R, Schmitt M, Popp J. Raman spectroscopy—a prospective tool in the life sciences. Eur J Chem Phys Phys Chem. 2003;4(1):14–30. doi: 10.1002/cphc.200390004. [DOI] [PubMed] [Google Scholar]
- Pappas D, Smith BW, Winefordner JD. Raman spectroscopy in bioanalysis. Talanta. 2000;51:131–144. doi: 10.1016/s0039-9140(99)00254-4. [DOI] [PubMed] [Google Scholar]
- Pasquini C, Cortez J, Silva L, Gonzaga F. Laser induced breakdown spectroscopy. J Braz Chem Soc. 2007;18(3):463–512. [Google Scholar]
- Pryor WA. Bio-assays for oxidative stress status (BOSS) Amesterdam: Elsevier Science B.V; 2001. [Google Scholar]
- Pathak AK, Singh VK, Rai NK, Rai AK, Rai PK, Rai PK, Rai S, Baruah GD. Study of different concentric rings inside gallstones with LIBS. Lasers Med Sci. 2011;26:531–537. doi: 10.1007/s10103-011-0886-1. [DOI] [PubMed] [Google Scholar]
- Putnam RA, Mohaidat QI, Daabous A, Rehse SJ. A comparison of multivariate analysis techniques and variable selection strategies in a laser-induced breakdown spectroscopy bacterial classification. Spectrochem Acta B. 2013;87:161–167. [Google Scholar]
- Pink DA, Truelstrup Hansen L, Gill TA, Quinn BE, Sjericho BH, Beveridge TJ. Divalent calcium ions inhibit the penetration of protamine through the polysaccharide brush of the outer membrane of Gram-negative bacteria. Langmuir. 2003;19:8852–8858. [Google Scholar]
- Pulcrano G, Iula DV, Vollaro A, Tucci A, Cerullo M, Esposito M, et al. Rapid and reliable MALDI-TOF mass spectrometsry identification of Candida non-al-bicans isolates from blood stream infections. J Microbiol Methods. 2013;94:262–266. doi: 10.1016/j.mimet.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Pathak AK, Kumar K, Singh VK, Agrawal R, Rai S, Rai AK. Assessment of LIBS for spectrochemical analysis: a review. Appl Spectrosc. 2012;47:14–40. [Google Scholar]
- Rehse SJ, Diedrich J, Palchaudhuri S. Identification and discrimination of Pseudomonas aeruginosa bacteria grown in blood and bile by laser-induced breakdown spectroscopy. Spectrochem Acta B. 2007;62(10):1169–1176. [Google Scholar]
- Rehse SJ, Mohaidat QI. The effect of sequential dual-gas testing on laser-induced breakdown spectroscopy-based discrimination: application to brass samples and bacterial strains. Spectrochem Acta B. 2009;64(10):1020–1027. [Google Scholar]
- Riberdy AV, Christopher FJ, Rehse Steven J. Determination of the zinc concentration in human fingernails using laser-induced breakdown spectroscopy. Appl Spectrosc. 2017;71(4):567–582. doi: 10.1177/0003702816687568. [DOI] [PubMed] [Google Scholar]
- Rehse SJ, Salimnia H, Miziolek AW. Laser induced breakdown spectroscopy (LIBS): an overview of recent progress and future potential for biomedical applications. J Med Eng Technol. 2012;36(2):77–89. doi: 10.3109/03091902.2011.645946. [DOI] [PubMed] [Google Scholar]
- Rehse SJ, Jeyasingham N, Diedrich J, Palchaudhuri S. A membrane basis for bacterial identification and discrimination using laser-induced breakdown spectroscopy. J Appl Phys. 2009;105(10):102034. [Google Scholar]
- Raetz CRH. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Rehse SJ, Mohaidat QI, Palchaudhuri S. Towards the clinical applicationof laser-induced breakdown spectroscopy for rapid pathogen diagnosis: the effect of mixed cultures and sample dilution on bacterial identification. Appl Opt. 2010;49(13):C27–C35. [Google Scholar]
- Singh VK, Rai NK, Pandhija S, Rai AK, Rai PK. Investigation of common Indian edible salts suitable for kidney disease by laser induced breakdown spectroscopy. Lasers Med Sci. 2009;24(6):917–924. doi: 10.1007/s10103-009-0659-2. [DOI] [PubMed] [Google Scholar]
- Samek O, Liska M, Kaiser J, Krzyzanek V, Beddows DCS, Belenkevitch A, Morris GW, Telle HH. Laser ablation for mineral analysis in the human body: integration of LIFS with LIBS. Proc SPIE. 1999;3570:263. [Google Scholar]
- Samuels AC, DeLuciaJr FC, McNesby KL, Miziolek AW. Laser-induced breakdown spectroscopy of bacterial spores, molds, pollens and proteins: initial studies of discrimination potential. Appl Opt. 2003;42(30):6205–6209. doi: 10.1364/ao.42.006205. [DOI] [PubMed] [Google Scholar]
- Snyder EG, Munson CA, Gottfried JL, De Lucia FC, Jr, Gullett B, Miziolek AW. Laser-induced breakdown spectroscopy for the classification of unknown powders. Appl Opt. 2008;47:G80–G87. doi: 10.1364/ao.47.000g80. [DOI] [PubMed] [Google Scholar]
- Saari S, Järvinen S, Reponen T, Mensah-Attipoe J, Pasanen P, Toivonen J, Keskinen J. Identification of single microbial particles using electro-dynamic balance assisted laser-induced breakdown and fluorescence spectroscopy. Aerosol Sci Technol. 2016;50(2):126–132. [Google Scholar]
- Sardi J De CO, Pitangui N De S, Gullo FP, Giannini A M F A e M J S M A mini review of Candida species in hospital infection: epidemiology, virulence factor and drugs resistance and prophylaxis. Trop Med Surg. 2013;1:1–7. [Google Scholar]
- Singh VK, Rai AK. Prospects for laser-induced breakdown spectroscopy for biomedical applications: a review. Lasers Med Sci. 2011;26:673–687. doi: 10.1007/s10103-011-0921-2. [DOI] [PubMed] [Google Scholar]
- Singh VK, Kumar V, Sharma J. Importance of laser-induced breakdown spectroscopy for hard tissues (bone, teeth) and other calcified tissue materials. Lasers Med Sci. 2014;30:1763–1778. doi: 10.1007/s10103-014-1549-9. [DOI] [PubMed] [Google Scholar]
- Santo Domingo JS, Sadowsky MJ, editors. Microbial Source Tracking. Washington DC: ASM press; 2007. pp. 235–277. [Google Scholar]
- Singh JP, Thakur SN, editors. Laser-induced breakdown spectroscopy. Amsterdam: Elsevier; 2007. [Google Scholar]
- Samek O, Beddows DCS, Kukhlevsky SV, Liska M, Telle HH, Young J. Application of laser-induced breakdown spectroscopy to in situ analysis of liquid samples. Opt Eng. 2000;39(8):2248–2262. [Google Scholar]
- Sinescu C, Negrutiu M, Draganescu G, Todea C, Dodenciu D, Florita Z, Pop D. Microleakage in dentistry: new methods for investigating the gaps in biomaterials. Lasers in dentistry XIV. Proc SPIE. 2008;6843:0277–0786. [Google Scholar]
- Singh VK, Singh V, Rai AK, Thakur SN, Rai PK, Singh JP. Quantitative analysis of gallstones using laser-induced breakdown spectroscopy. Appl Opt. 2008;47:G38–G47. doi: 10.1364/ao.47.000g38. [DOI] [PubMed] [Google Scholar]
- Singh VK, Rai AK, Rai PK, Jindal PK. Cross-sectional study of kidney stones by laser-induced breakdown spectroscopy. Lasers Med Sci. 2009;24(5):749–759. doi: 10.1007/s10103-008-0635-2. [DOI] [PubMed] [Google Scholar]
- Sivakumar P, Fernández-Bravo A, Taleh L, Biddle JF, Melikechi N. Detection and classification of live and dead Escherichia coli by laser-induced breakdown spectroscopy. Astrobio. 2015;15:144. doi: 10.1089/ast.2014.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truant AL. Manual of commercial methods in clinical microbiology. Washington DC: ASM Press; 2002. pp. 12–21. [Google Scholar]
- Voronov GS, Delone NB. Many-photon ionization of the xenon atom by ruby laser radiation. Sov Phys - JETP. 1966;23(1):78–84. [Google Scholar]
- Wu J, Zhang W, Shao X, Lin Z, Liu X. Simulated body fluid by laser-induced breakdown spectroscopy. Chin J Laser B. 2008;35:445–447. [Google Scholar]
- Xu HL, Liu W, Chin SL. Remote time-resolved filament-induced breakdown spectroscopy of biological materials. Opt Lett. 2006;31:1540. doi: 10.1364/ol.31.001540. [DOI] [PubMed] [Google Scholar]
- Xu HL, Mejean G, Liu W, Kamali Y, Daigle JF, Azarm A, Simard PT, Mathieu P, Roy G, Simard JR, Chin SL. Remote detection of similar biological material using femtosecond-filament induced breakdown spectroscopy. Appl Phys B Lasers Opt. 2007;87(1):151–156. [Google Scholar]
- Yueh FY, Zheng H, Singh JP, Burgess S. Preliminary evaluation of laser-induced breakdown spectroscopy for tissue classification. Spectrochim Acta B. 2009;64(1):1059–1067. [Google Scholar]
- Yao M, Lin J, Li Q, Lei Z, Huang L (2010) Proc. of BMEI 2010. IEEE:302–305