Abstract
α-Tocopherol is known to show different activity depending on the concentration and food matrix. Effects of α-tocopherol at the concentrations of 0, 0.1, 0.5, and 1.0 mM were determined in oil-in-water (O/W) emulsions containing anionic, neutral, and cationic emulsifiers under different types of oxidative stress including riboflavin photosensitization, photooxidation, and autoxidation. Headspace oxygen depletion, lipid hydroperoxides, and conjugated dienes were analyzed to determine the oxidative stability of O/W emulsions. α-Tocopherol served as an antioxidant in O/W emulsion with a cationic emulsifier irrespective of oxidative stress. α-Tocopherol acted as an antioxidant in O/W emulsion with a neutral emulsifier at riboflavin photosensitization while a prooxidant at photooxidation. However, in samples with an anionic emulsifier, α-tocopherol activity differed from the concentration and types of oxidative stress. Therefore, cationic transition metals or reactive oxygen species generated from RF photosensitization could play key roles of α-tocopherol in O/W emulsion.
Keywords: α-Tocopherol, O/W emulsion, Riboflavin photosensitization, Photooxidation, Autoxidation, Emulsifier charge
Introduction
Lipid oxidation, which is a chemical reaction between unsaturated lipids and oxygen molecules, greatly influences the sensory attributes of foods and decreases their nutritional value. Many factors such as unsaturation of lipids, types of oxygen, the presence of antioxidants or prooxidants, and the food matrices have significant effects on the lipid oxidation. A major site of lipid oxidation is known to be an oil–water interface; therefore, the rate of lipid oxidation is much faster in oil-in-water (O/W) emulsion than bulk oil (Frankel, 1996).
O/W emulsions, which consist of oil, water, and emulsifiers, are characterized by charges, hydrophilic–lipophilic balance, and concentration of the emulsifiers, polarity and concentration of antioxidative or prooxidative compounds, types and concentrations of salts, pH of the continuous phase, and the presence of transition metals (Chaiyasit et al., 2007; McClements and Decker, 2000; Mei et al., 1998; Lee and Decker 2011). Many studies have shown that lipid oxidation in O/W emulsion is influenced by the emulsifier charge. Mei et al. (1998) reported that oxidative stability is higher in O/W emulsion with an anionic emulsifier than a neutral or cationic emulsifier owing to the attraction of transition metals. Nevertheless, according to Kancheva and Kasaikina (2012), a cationic emulsifier promoted the decomposition of hydroperoxides and increased the rate of lipid oxidation. Besides, Yi et al. (2016) demonstrated that the O/W emulsion containing cetyltrimethylammonium bromide (CTAB), a cationic emulsifier, had the lowest oxidative stability, also the addition of a transition metal or metal chelator into the emulsion with a cationic emulsifier had a different influence during lipid oxidation as compared to an anionic or neutral charged emulsifier.
α-Tocopherol is a lipophilic antioxidant with a 16–carbon non-polar side chain. α-Tocopherol is known as a free–radical scavenger as well as a singlet oxygen quencher. Rate constants of α-tocopherol toward peroxyl radicals for scavenging the free radical is 106–108 M−1 s−1 (Simic, 1981), whereas that toward singlet oxygen is reported to be 2.5 × 108 M−1 s−1 (King and Min, 1998). α-Tocopherol is known to exert different activities depending on the concentration and food matrix (Evans et al., 2002; Frankel 1996; Huang et al., 1994; Jung and Min, 1990). In bulk oil, α-tocopherol at the low concentration decreased the rate of lipid oxidation, whereas at the high concentration accelerated (Evans et al., 2002; Jung and Min 1990). Nevertheless, a higher concentration of α-tocopherol in O/W emulsion showed high antioxidant activity based on the formation of hexanal (Huang et al., 1994).
Photosensitization, which is oxidation occurred when foods containing photosensitizer are stored under light irradiation, plays a significant role in the oxidation stability of lipid-based foods. Photosensitizers transfer the light energy to other compounds and promote lipid oxidation (Evans et al., 2002). Riboflavin (RF), one of well-known photosensitizers, promotes oxidation via type I and type II pathways of photosensitization (Choe et al., 2005). The type I pathway produces radicals from the substrates, whereas the type II pathway generates a singlet oxygen from a triplet oxygen (Choe et al., 2005). The rate ratio of oleate, linoleate, and linolenate were 1:27:77 in triplet oxygen oxidation, whereas those were 30,000:40,000:70,000 during singlet oxygen oxidation (Min, 1998).
Usually, foods are stored under light irradiation for display purposes or in the dark for storage purposes. Therefore, it is necessary to compare the oxidative stability of foods depending on oxidative stresses to extend the shelf–life of food products. Yi et al. (2016) compared the effects of RF photosensitization in O/W emulsion with different emulsifier charge. Nonetheless, there are no reports on the effects of α-tocopherol concentration and different oxidative stresses including RF photosensitization, photooxidation, and autoxidation on the oxidative stability of O/W emulsion containing differently charged emulsifiers.
The objective of this study was to determine the effect of α-tocopherol on the oxidative stability in O/W emulsion depending on the emulsifier charges and different oxidative stress.
Materials and methods
Materials
Sodium dodecyl sulfate (SDS), polyoxyethylene sorbitan monolaurate (Tween 20), CTAB, and RF were purchased from Sigma-Aldrich (St. Louis, MO, USA). Corn oil was acquired at a local grocery market (Suwon, Korea). Other reagent grade chemicals were purchased from Daejung Chemical Co. (Seoul, Korea).
Sample preparation for the emulsion and photosensitized oxidation
O/W emulsions were prepared according to previous reports (Yi et al., 2015). α-Tocopherol was dissolved in n-hexane and mixed with corn oil, and then we removed n-hexane using nitrogen flushing. The anionic, neutral, and cationic emulsifiers were SDS, Tween 20, and CTAB, respectively. An emulsifier was added to deionized water at the concentration of 0.25% (w/w), and then we combined the mixture with 2.5% (w/w) in corn oil containing α-tocopherol. A coarse emulsion was prepared by homogenizing the mixture for 3 min using an HB501 (Tefal, Haute-Savoie, France) and the coarse emulsion was then passed through a nano disperser (ISA-LM100, Ilshinautoclave Co., Ltd., Daejeon, Korea) at 34.47 MPa with three-time passes. The final concentration of α-tocopherol was 0, 0.1, 0.5, or 1.0 mM.
For the evaluation of RF photosensitization, RF was added to the O/W emulsion at the concentration of 0.13 mM. Two milliliters of each sample was placed in a 10 mL vial with an air-tight seal. Sample vials were stored under fluorescent light at 1333-Lux light intensity for 24 h and were analyzed at 0, 12, and 24 h. In the dark group, air-tight vials were wrapped with foil and stored under the same conditions as above; such samples were regarded as autoxidation samples. For the photooxidation analysis, O/W emulsions without RF were described as above. Samples were prepared in triplicate at each sampling time point.
Analysis of headspace oxygen content
The headspace oxygen in air-tight sample bottles was analyzed according to the method of Yi et al. (2015). Namely, 30 μL of headspace gas was removed from a sample vial with an air-tight syringe and oxygen content was determined on a gas chromatograph (GC) equipped with a thermal conductivity detector (TCD). A Hewlett-Packard 7890 GC system (Agilent Technologies, Inc., CA, USA) equipped with a 60/80 packed column (3.0 m × 2 mm ID, Restek Ltd., PA, USA) and a TCD from Agilent Technologies (CA, USA) was used. The flow rate of helium gas was 200 mL/min. Temperatures of an oven, an injector, and a TCD were 60, 180, and 180 °C, respectively.
Analysis of lipid hydroperoxides
Lipid hydroperoxides were quantified by a modified method from other reports (Kim et al., 2012; Yi et al., 2015). A sample (0.2 mL) was mixed with 1.5 mL of isooctane: 2-propanol (3:2, v/v), vortex-mixed three times for 10 s each and centrifuged for 3 min at 2000 × g. The upper layer (0.1 mL) was collected and mixed with 1.4 mL of methanol: 1-butanol (2:1, v/v) and 30 μL of a thiocyanate/Fe2+ solution by vortexing for 10 s. The thiocyanate/Fe2+ solution was prepared by mixing equal volumes of a 3.94 M thiocyanate solution with 0.072 M Fe2+ solution (obtained from the supernatant of the mixture of one part of 0.144 M FeSO4 and one part of 0.132 M BaCl2 in 0.4 M HCl). The samples were incubated for 20 min at room temperature and the absorbance at 510 nm was measured on a UV/VIS-spectrometer (Model UV-1650PC, Shimadzu, Kyoto, Japan). The concentration of a lipid hydroperoxide was calculated by means of a cumene hydroperoxide standard curve.
Analysis of conjugated dienes
Conjugated dienes in samples were determined by a method of Yi et al. (2015). A 120-μL sample was mixed with 2.7 mL of methanol: 1-butanol (2:1, v/v) and vortexed three times for 10 s each. Absorbance at 233 nm was measured on a UV/VIS-spectrometer (Model UV-1650PC). If the range of absorbance was not within 0.2–0.8, the sample was diluted with the mixture of methanol and 1-butanol.
The relative percentage of oxidation products
Data on oxidation products, which are lipid hydroperoxides and conjugated dienes, are shown as relative percentage. Values measured in O/W emulsion with 0.1 or 1.0 mM α-tocopherol were divided by those without α-tocopherol. The relative percentage of oxidation products below 100% indicates that O/W emulsion with α-tocopherol forms fewer oxidation products than controls, which means that α-tocopherol added into samples acted as an antioxidant.
Statistical analysis
Data on headspace oxygen content, lipid hydroperoxides, and conjugated dienes were assessed statistically by ANOVA and Duncan’s multiple-range test in the SPSS, program version 22 (SPSS Inc., IL, USA). A difference of p value < 0.05 was considered significant. The statistical significance of the relative percentage of oxidation products was analyzed relative to 0 mM α-tocopherol samples by Student’s t test (GraphPad Software, Inc., CA, USA) (*p < 0.05; **p < 0.01).
Results and discussion
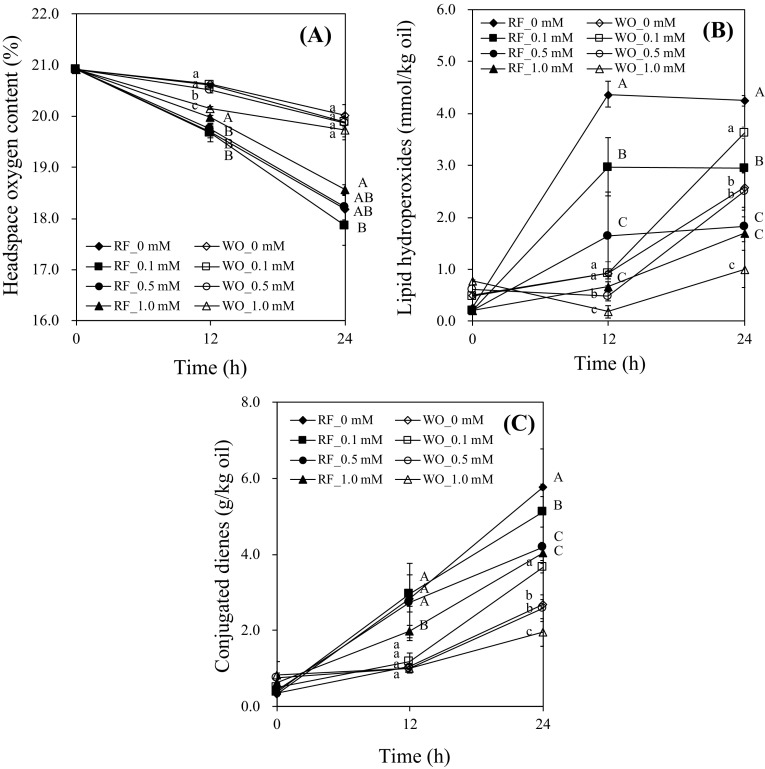
Effects of α-tocopherol on changes in headspace oxygen (a), lipid hydroperoxides (b), and conjugated dienes (c) in SDS-stabilized O/W emulsions during RF photosensitization or photooxidation for 24 h are shown in Fig. 1. Samples subjected to RF photosensitization had lower headspace oxygen content than those under photooxidation [Fig. 1(A)]. This result implies that RF photosensitization accelerated the consumption of headspace oxygen. This consumption in samples containing 1.0 mM α-tocopherol was significantly lower in comparison with samples with 0.1 mM α-tocopherol during RF photosensitization (p < 0.05), whereas headspace oxygen content in samples was not significantly different in SDS-stabilized O/W emulsion during photooxidation [p > 0.05; Fig. 1(A)]. In the results on lipid hydroperoxides and conjugated dienes, α-tocopherol showed antioxidant activity during RF photosensitization in a concentration–dependent manner, whereas reaction with 0.5 and 1.0 mM α-tocopherol were not significantly different [p > 0.05; Fig. 1(B, C)]. In a case of photooxidation experiment, 0.1 mM α-tocopherol acted as a prooxidant while 1.0 mM α-tocopherols had significant antioxidant properties in O/W emulsion [p < 0.05; Fig. 1(B, C)]. This result indicates that during RF photosensitization, α-tocopherol was an antioxidant in O/W emulsion with SDS whereas, during photooxidation, α-tocopherol showed different patterns of antioxidant or prooxidant actions depending on the concentration of α-tocopherol.
Fig. 1.
Effects of α-tocopherol on the headspace oxygen content (A), lipid hydroperoxides (B), and conjugated dienes (C) in oil-in-water emulsions with SDS with or without riboflavin under light for 24 h. Different capital and small letters are significantly different among the same treatment time with or without riboflavin under light at 0.05. ‘RF_0 mM’, ‘RF_0.1 mM’, ‘RF_0.5 mM’, and ‘RF_1.0 mM’ are O/W emulsion containing 0, 0.1, 0.5, and 1.0 mM α-tocopherol under riboflavin photosensitization. ‘WO_0 mM’, ‘WO_0.1 mM’, ‘WO _0.5 mM’, and ‘WO _1.0 mM’ are O/W emulsion containing 0, 0.1, 0.5, and 1.0 mM α-tocopherol under light without riboflavin
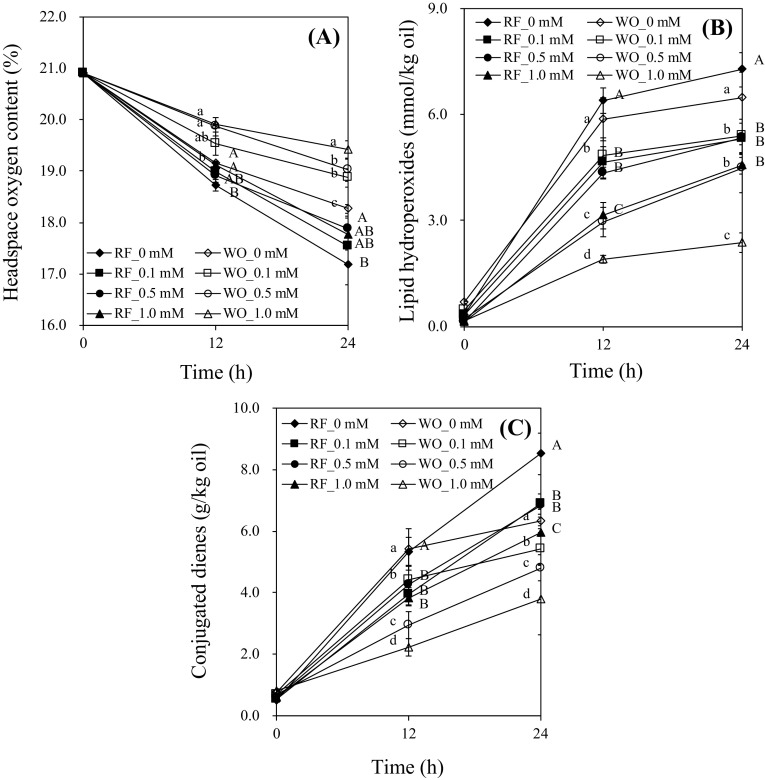
Effects of α-tocopherol on headspace oxygen content (a), lipid hydroperoxides (b), and conjugated dienes (c) in Tween 20-stabilized O/W emulsions during RF photosensitization or photooxidation for 24 h are shown in Fig. 2. Samples subjected to RF photosensitization had lower headspace oxygen content, higher lipid hydroperoxides, and higher concentration of conjugated dienes as compared to those undergoing photooxidation (Fig. 2); this result implies that RF photosensitization accelerated the rates of lipid oxidation in O/W emulsion containing Tween 20. Headspace oxygen content at 1.0 mM α-tocopherol was significantly higher than that in control samples during RF photosensitization (p < 0.05). As α-tocopherol concentration increased from 0 to 1.0 mM, amounts of lipid hydroperoxides and conjugated dienes decreased in O/W emulsion undergoing RF photosensitization [Fig. 2(B, C)]. On the other hand, the higher concentration of α-tocopherol, e.g. 1.0 mM significantly upregulated lipid hydroperoxides and conjugated dienes in Tween 20-stabilized O/W emulsion during photooxidation [p < 0.05; Fig. 2(B, C)]. α-Tocopherol acted as an antioxidant in Tween 20-stabilized O/W emulsion during RF photosensitization and served as a prooxidant at the 0.1 mM concentration during photooxidation.
Fig. 2.
Effects of α-tocopherol on the headspace oxygen content (A), lipid hydroperoxides (B), and conjugated dienes (C) in oil-in-water emulsions with Tween 20 with or without riboflavin under light for 24 h. Different capital and small letters are significantly different among the same treatment time with or without riboflavin under light at 0.05. Abbreviation are shown in the captions of Fig. 1
Effects of α-tocopherol on headspace oxygen content (a), lipid hydroperoxides (b), and conjugated dienes (c) in CTAB-stabilized O/W emulsions during RF photosensitization or photooxidation for 24 h are shown in Fig. 3. Samples with CTAB during photooxidation had relatively lower headspace oxygen content, higher concentration of lipid hydroperoxides, and higher concentration of conjugated dienes as compared to the results of O/W emulsion with SDS or Tween 20 during photooxidation. Headspace oxygen content in SDS-, Tween 20-, or CTAB-stabilized O/W emulsion without α-tocopherol was 20.02, 20.84, and 18.27%, respectively, after 24 h; this founding indicates that oxidative stability in CTAB-stabilized O/W emulsion during photooxidation was significantly lower than that in O/W emulsion containing SDS or Tween 20 [Figs. 1(A), 2(A), 3(A)]. As the concentration of α-tocopherol in CTAB-stabilized O/W emulsion increased from 0 to 1.0 mM, concentration of lipid hydroperoxides and conjugated dienes decreased; this result implies that oxidative stability increased in a concentration–dependent manner under photooxidation stress [Fig. 3(B, C)]. As for RF photosensitization, added α-tocopherol acted as an antioxidant according to the results on lipid hydroperoxides and conjugated dienes in O/W emulsion with CTAB as compared to the results of photooxidation (Fig. 3).
Fig. 3.
Effects of α-tocopherol on the headspace oxygen content (A), lipid hydroperoxides (B), and conjugated dienes (C) in oil-in-water emulsions with CTAB with or without riboflavin under light for 24 h. Different capital and small letters are significantly different among the same treatment time with or without riboflavin under light at 0.05. Abbreviation are shown in the captions of Fig. 1
Changes of headspace oxygen content, lipid hydroperoxides, and conjugated dienes in O/W emulsion containing 0–1.0 mM α-tocopherol with or without RF in the dark after 24 h are shown in Table 1. In SDS-stabilized O/W emulsion, 1.0 mM α-tocopherol served as an antioxidant in samples with RF, in agreement with results under light (Fig. 1), whereas α-tocopherol acted as a prooxidant in samples without RF. The addition of α-tocopherol accelerated the lipid oxidation in Tween 20-stabilized O/W emulsion but significantly decreased the rate of lipid oxidation in CTAB-stabilized O/W emulsion (p < 0.05), regardless of the addition of RF.
Table 1.
Changes of headspace oxygen content, lipid hydroperoxides, and conjugated dienes in O/W emulsion containing 0–1.0 mM α-tocopherol with or without riboflavin in the dark after 24 h
| Headspace oxygen content (%) | Lipid hydroperoxides (mmol/kg oil) | Conjugated dienes (g/kg oil) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 mMa | 0.1 mM | 0.5 mM | 1.0 mM | 0 mM | 0.1 mM | 0.5 mM | 1.0 mM | 0 mM | 0.1 mM | 0.5 mM | 1.0 mM | ||
| w/RFb | SDSc | 18.64 ± 0.70dbe | 18.09 ± 0.71b | 19.98 ± 0.75a | 20.21 ± 0.63a | 3.84 ± 0.14a | 3.76 ± 0.97a | 3.45 ± 0.40a | 1.14 ± 0.10b | 2.34 ± 0.35ab | 2.83 ± 0.19a | 1.68 ± 0.63bc | 1.10 ± 0.62c |
| Tween 20 | 20.58 ± 0.45a | 20.78 ± 0.05a | 20.55 ± 0.15a | 20.38 ± 0.05a | 0.75 ± 0.02d | 0.91 ± 0.05c | 1.83 ± 0.03b | 2.37 ± 0.16a | 1.48 ± 1.03b | 1.29 ± 0.10b | 2.10 ± 0.01ab | 2.77 ± 0.07a | |
| CTAB | 18.57 ± 0.21b | 18.77 ± 0.16b | 18.85 ± 0.16b | 19.79 ± 0.09a | 7.14 ± 0.12a | 5.62 ± 1.28b | 4.97 ± 0.32b | 2.75 ± 0.15c | 7.99 ± 0.19a | 5.77 ± 0.94b | 5.58 ± 0.44b | 3.94 ± 0.28c | |
| w/o RF | SDS | 20.52 ± 0.01a | 20.46 ± 0.07a | 20.32 ± 0.11a | 19.74 ± 0.22b | 1.27 ± 0.14a | 1.30 ± 0.20a | 1.14 ± 0.31a | 1.66 ± 0.96a | 1.49 ± 0.04b | 1.57 ± 0.20b | 1.52 ± 0.12b | 2.27 ± 0.68a |
| Tween 20 | 20.86 ± 0.03a | 20.71 ± 0.01b | 20.43 ± 0.03c | 20.16 ± 0.05d | 0.12 ± 0.05d | 0.70 ± 0.04c | 1.62 ± 0.03b | 2.50 ± 0.41a | 0.67 ± 0.06d | 1.20 ± 0.02c | 2.09 ± 0.22b | 2.75 ± 0.10a | |
| CTAB | 18.31 ± 0.13c | 18.94 ± 0.10b | 19.52 ± 0.07a | 19.53 ± 0.06a | 6.58 ± 0.64a | 6.40 ± 1.09a | 3.17 ± 0.16b | 2.48 ± 0.35b | 6.60 ± 0.40a | 4.79 ± 0.77a | 3.37 ± 0.26b | 3.29 ± 0.16b | |
aSamples with alpha-tocopherol concentration
b‘w/RF’ and ‘w/o RF’ mean O/W emulsions with or without riboflavin addition, respectively
cAnionic emulsifier, Sodium dodecyl sulfate (SDS); Neutral emulsifier; Cationic emulsifier, Cetyltrimethylammonium bromide (CTAB)
dMean ± standard deviation (n = 3)
eDifference letters are significantly different in the same row of the same methods
Generally, addition of α-tocopherol showed antioxidant properties in O/W emulsion under strong oxidative stress such as RF photosensitization and in the presence of CTAB. In contrast, under weak oxidative stress such as photooxidation, autoxidation, or Tween 20 supplementation, α-tocopherol accelerated the rates of oxidation (Table 2). In SDS-stabilized O/W emulsion, α-tocopherol showed different activities depending on the concentration under light but antioxidant properties in the dark.
Table 2.
Summarization of α-tocopherol activity in O/W emulsions with different charged emulsifier depending on the oxidative stress
| SDSa | Tween 20 | CTAB | |||||
|---|---|---|---|---|---|---|---|
| Light | Dark | Light | Dark | Light | Dark | ||
| w/RFb | Antic | Anti | Anti | Pro | Anti | Anti | |
| 0.1 mM Pro | |||||||
| w/o RF | 0.5 mM No | Pro | Pro | Pro | Anti | Anti | |
| 1.0 mM Anti | |||||||
aAnionic emulsifier, Sodium dodecyl sulfate (SDS); Neutral emulsifier; Cationic emulsifier, Cetyltrimethylammonium bromide (CTAB)
bSamples with or without riboflavin
cAnti, Antioxidant properties; No, No effects; Pro, Prooxidant properties
When α-tocopherol was not added, the rate of lipid oxidation in O/W emulsion was high in the following order: a cationic, nonionic, and anionic emulsifier at RF photosensitization. In contrast, the ranking of the rates of lipid oxidation changed during autoxidation and photooxidation, e.g. in the presence of CTAB, SDS, or Tween 20.
Yoshida et al. (1994) reported consumption of α-tocopherol induced by CuCl2 was faster in anionic emulsifier (SDS)-stabilized micelles than that in micelles with cationic emulsifier. When expose to the weak stress, such as photooxidation and autoxidation in SDS- and Tween 20-stabilized O/W emulsion, cationic metal ions might accelerate the consumption of α-tocopherol at oil–water interface and/or generate more α-tocopheryl radicals, which speeds up the rates of lipid oxidation. However, α- tocopherol might be used as antioxidants against reactive oxygen species like singlet oxygen generated by RF photosensitization (Min, 1998). Therefore, α-tocopherol showed different activity depending on the mechanisms of oxidation.
The prooxidative properties of CTAB could be due to the effects of decomposition of hydroperoxides, neutral pH, and extended stabilit of RF. Cationic emulsifiers had ability to promote decomposition of hydroperoxides and accelerate lipid oxidation (Kancheva and Kasaikina, 2012). Kim et al. (2012) reported that pH in a solution affects the oxidative stability in a bulk oil–water model, and the oxidative stability in oil decreases in the order of pH level: 1 > 10 > 7 > 4 > 13. Furthermore, Mancuso et al. (1999) found that a high-pH solution accelerates lipid oxidation in salmon O/W emulsion containing Tween 20 and CTAB, although oxidative stability in SDS-stabilized O/W emulsions is not affected by the changes of pH. A preliminary study revealed that pH in a 0.25% (w/v) solution with SDS, Tween 20, or CTAB was 6.08, 5.45, and 7.21, respectively. Relatively high pH in presence of CTAB could decrease the oxidative stability in O/W emulsion.
At RF photosensitization, the fast oxidation rates in samples containing CTAB and Tween 20 could be due to the higher stability of RF. Fluorescence intensity of RF depends on the emulsifier charge (Ghann, 2008). The increased concentration of SDS does not change the fluorescence intensity of RF, whereas Triton X-100, a neutral emulsifier, and CTAB enhance the fluorescence of RF (Ghann, 2008). Therefore, the remaining RF could accelerate the rates of lipid oxidation in samples with CTAB and Tween 20 as compared to samples with SDS.
RF photosensitization can produce singlet oxygen via the type II pathway, and lipid radicals can also be formed via the type I pathway. α-Tocopherol can work as an antioxidant for both singlet oxygen and lipid radicals. The singlet oxygen-quenching rate of α-tocopherol was reported to be 2.7 × 107 M−1 s−1 (Min and Lee, 1999). Fahrenholtz et al. (1974) stated that D-α-tocopherol can physically quench singlet oxygen, and 120 molecules of 1O2 were successfully deactivated by α-tocopherol. Regarding the hydrogen-donating ability, α-tocopherol inhibited the methyl linoleate oxidation at the rate of 5.1 × 105 M−1 s−1 (Niki et al., 1984). In addition, according to Fukuzawa et al. (1982), up to 220 polyunsaturated fatty acid molecules could be protected by one molecule of α-tocopherol.
Whether α-tocopherol manifests antioxidant or prooxidant properties depends on the concentration of α-tocopherol in the matrix. Huang et al. (1994) reported that antioxidant or prooxidant properties of α-tocopherol differ among food matrices such as bulk oil and O/W emulsion and depend on the concentration, oxidation time, and the measurement method. In O/W emulsion here, the antioxidant activity of α-tocopherol did not show a clear-cut pattern depending on the concentration (Huang et al., 1994). As the concentration of α-tocopherol increased from 0 to 0.16%, antioxidative effect increased in O/W emulsion with 5% soybean oil and 1% lecithin (soybean phosphatidylcholine) (Ruben and Larsson, 1985). Kim et al. (2012) reported that α-tocopherol in Tween 20-stabilized O/W emulsion during RF photosensitization manifests a different activity depending on the concentration. The addition of 0.01 or 0.1 μM α-tocopherol increased the rate of lipid oxidation whereas 1.0 mM α-tocopherol acted as an antioxidant judging by the headspace oxygen content and headspace volatile compounds.
In conclusion, antioxidant or prooxidant properties of α-tocopherol in O/W emulsion are influenced by the concentration of α-tocopherol, emulsifier charges, and types of oxidative stress. Generally, antioxidant properties of α-tocopherol are clearly observed in O/W emulsion under strong oxidative stress including RF photosensitization and cationic emulsifier. However, α-tocopherol did not show such effects under photooxidation and autoxidation. Cationic transition metals or reactive oxygen species generated from RF photosensitization could affect the roles of α-tocopherol in O/W emulsion, which needs further studies. O/W emulsified foods, which may be exposed to strong oxidative stress like RF photosensitization, may need supplementation with α-tocopherol to extend the shelf-life through retardation of the rates of lipid oxidation.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2013R1A2A2A0106729) and (NRF-2017R1A2B4002613).
References
- Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. Nutr. 2007;47:299–317. doi: 10.1080/10408390600754248. [DOI] [PubMed] [Google Scholar]
- Choe E, Huang R, Min DB. Chemical reacitions and stability of riboflavin in foods. J. Food Sci. 2005;70:R28–R36. doi: 10.1111/j.1365-2621.2005.tb09055.x. [DOI] [Google Scholar]
- Evans JC, Kodali DR, Addis PB. Optimal tocopherol concentrations to inhibit soybean oil oxidation. J. Am. Oil Chem. Soc. 2002;79:47–51. doi: 10.1007/s11746-002-0433-6. [DOI] [Google Scholar]
- Fahrenholtz SR, Doleiden FH, Trozzolo AM, Lamola AA. On the quenching of singlet oxygen by α-tocopherol. Photochem. Photobiol. 1974;20:505–509. doi: 10.1111/j.1751-1097.1974.tb06610.x. [DOI] [PubMed] [Google Scholar]
- Frannkel EN. Antioxidants in lipid foods and their impact on food quality. Food Chem. 1996;57:51–55. doi: 10.1016/0308-8146(96)00067-2. [DOI] [Google Scholar]
- Fukuzawa K, Tokumura A, Ouchi S, Tsukatani H. Antioxidant activities of tocopherols on Fe2+-ascorbate-induced lipid peroxidation in lecithin liposomes. Lipids. 1982;17:511–513. doi: 10.1007/BF02535334. [DOI] [PubMed] [Google Scholar]
- Ghann WE. Studies of surfactants effect on riboflavin fluorescence and its determination in commercial food products and vitamin tablets. Electronic Theses and Dissertations. Paper 2000 (2008)
- Huang SW, Frankel EN, German JB. Antioxidant activity of α- and γ-tocopherols in bulk oils and in oil-in-water emulsions. J. Agric. Food Chem. 1994;42:2108–2221. doi: 10.1021/jf00046a007. [DOI] [Google Scholar]
- Jung MY, Min DB. Effects of α -, γ -, and δ-tocopherols on the oxidative stability of purified soybean oil. J. Food Sci. 1990;55:1464–1465. doi: 10.1111/j.1365-2621.1990.tb03960.x. [DOI] [Google Scholar]
- Kancheva VD, Kasaikina, OT. Lipid oxidation in homogeneous and microheterogeneous media in presence of prooxidants, antioxidants, and surfactants. pp. 31–62. In: Lipid Peroxidation. Catala A (ed). In Tech Open Access Publ., Rijeka, Croatia (2012)
- Kim TS, Decker EA, Lee JH. Antioxidant capacities of α-tocopherol, trolox, ascorbic acid, and ascorbyl palmitate in riboflavin photosensitized O/W emulsion systems. Food Chem. 2012;133:68–75. doi: 10.1016/j.foodchem.2011.12.069. [DOI] [Google Scholar]
- King JM, Min DB. Riboflavin photosensitized singlet oxygen oxidation of vitamin D. J. Food Sci. 1998;63:31–34. doi: 10.1111/j.1365-2621.1998.tb15669.x. [DOI] [Google Scholar]
- Lee JH, Decker EA. Effects of metal chelator, sodium azide, and superoxide dismutase (SOD) on the oxidative stability in riboflavin photosensitized O/W emulsion systems. J. Agr. Food Chem. 2011;59:6271–6276. doi: 10.1021/jf2001537. [DOI] [PubMed] [Google Scholar]
- Mancuso JR, McClements DJ, Decker EA. Ability of iron to promote surfactant peroxide decomposition and oxidize α-tocopherol. J. Agric. Food Chem. 1999;47:4146–4149. doi: 10.1021/jf990453r. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65:1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Mei L, McClements DJ, Wu J, Decker EA. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl. Food Chem. 1998;61:307–312. doi: 10.1016/S0308-8146(97)00058-7. [DOI] [Google Scholar]
- Min DB. Lipid oxidation of edible oil. pp. 283–296. In: Food Lipids: Chemistry, Nutrition, and Biotechnology. Akoh CC and Min DB (ed). Marcel Dekker, New York. (1998)
- Min David B., Lee Hyung-Ok. Flavor Chemistry. Boston, MA: Springer US; 1999. Chemistry of Lipid Oxidation; pp. 175–187. [Google Scholar]
- Niki E, Saito T, Kawakami A, Kamiya Y. Inhibition of oxidation of methyl linoleate in solution by Vitamin E and Vitamin C. J. Biol. Chem. 1984;259:4177–4182. [PubMed] [Google Scholar]
- Ruben C, Larsson K. Relations between antioxidant effect of α-tocopherol and emulsion structure. J. Dispers. Sci. Technol. 1985;6:213–221. doi: 10.1080/01932698508943945. [DOI] [Google Scholar]
- Simic MG. Vitamin E radicals. pp. 109–115. In: Oyxgen and Oxy-radicals in Chemistry and Biology. Rodgers MAJ, Powers EL (ed). In Academic Press Inc., New York, USA (1981)
- Yi BR, Ka HJ, Kim MJ, Lee JH. Effects of curcumin on the oxidative stability of oils depending on type of matrix, photosensitizers, and temperature. J. Am. Oil Chem. Soc. 2015;92:685–691. doi: 10.1007/s11746-015-2639-y. [DOI] [Google Scholar]
- Yi BR, Kim MJ, Lee JH. Effects of emulsifier charges on the oxidative stability in oil-in-water emulsions under riboflavin photosensitization. Food Sci. Biotechnol. 2016;25:1003–1009. doi: 10.1007/s10068-016-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Tsuchiya J, Niki E. Interaction of α-tocopherol with copper and its effect on lipid peroxidation. Biochim. Biophys. Acta. 1994;1200:85–92. doi: 10.1016/0304-4165(94)90121-X. [DOI] [PubMed] [Google Scholar]