Abstract
This study investigated the effects of aging methods (wet aging and dry aging) and aging times (7 and 14 days) on the physicochemical and sensory characteristics of meat quality using pork loin. Dry-aged loin (DA) had significantly lower moisture content and higher crude fat protein content than wet-aged loin (WA). The pH of DA was significantly higher than that of WA and it increased with the aging time. DA showed lower cooking loss and higher aging loss than WA (p < 0.001). Lipid oxidation and metmyoglobin content of DA were higher than those of WA (p < 0.001). Shear force in DA was lower than that in WA (p < 0.001) and myofibril protein index (MFI) increased in DA. In addition, DA recorded higher scores of roast color, flavor and overall acceptability compared to WA. These results suggested that the application of dry-aging on pork improved physicochemical, textural and sensory characteristics.
Keywords: Dry-aging, Pork, Meat quality, Flavor, Aging time
Introduction
Wet-aged meat is stored the meat in a sealed vacuum package at refrigerated temperatures, whereas in dry-aged meat is unpackaged and controlled relative humidity and temperature to exposed meat. The aging process in animals (lamb and beef) is widely recognized with an increase in tenderness and flavor in the meat industry (Choe et al., 2016; Obuz et al., 2014; Vitale et al., 2014). Myofibrillar and cytoskeletal proteins are degraded through proteolytic enzyme activities in accordance with improving tenderness and water-holding capacity (Nowak, 2011). A comparison between dry-aging and wet-aging of beef has reported that consumers who preferred to consume dry-aged beef has better overall flavor than wet-aged beef provided/gave a relatively higher value (Sitz et al., 2006). In addition, the dry aging process contributes to enhance the dry-aged flavor, tenderness, and juiciness compared to non-aged and/or dry-aged beef at 7 days aging time (Campbell et al., 2001).
Information on dry-aged pork loin is limited, and recently, dry aging versus wet aging of pork loins has been gradually researched with respect to meat quality and sensory characteristics of aged pork loins (Juarez et al., 2001; Kim et al., 2016; Lee et al., 2016). Min et al. (2008) suggested that oxidative stability of pork loin was higher than that of beef loin although unsaturated fatty acids in pork loin were higher than those in beef loin since the non-heme iron content and lipoxygenase-like activities were increased in beef loins compared to pork loins. This finding is in accordance with that described by Rhee et al. (1996) who reported that beef was more vulnerable to lipid oxidation compared to pork.
Although several researches have been performed to prove the difference in beef quality according to the aging methods and aging times, limited researches have been performed to compare dry-aged pork loin and wet-aged pork loin at different aging times. Therefore, the aim of this study was to evaluate the effects of dry aging versus wet aging and two aging times (7 and 14 days) on the physicochemical and sensory characteristics of pork loins.
Materials and methods
Animals and samples
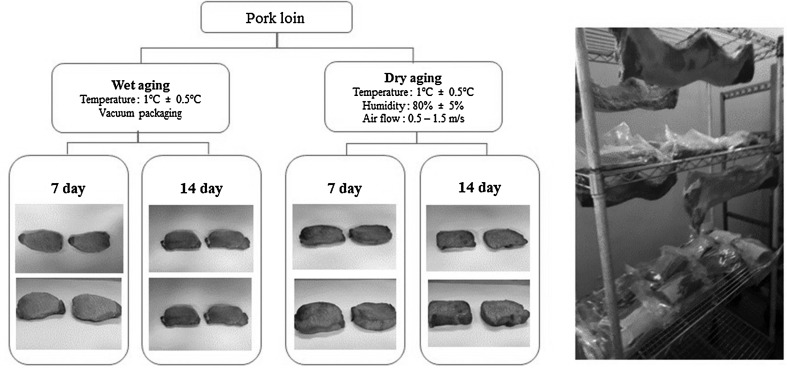
Twenty pork loins (Longissimus lumborum muscles, n = 20) from twenty carcasses of three-way crossbred (Landrace × Yorkshire × Duroc) pigs were collected from a commercial slaughter house after 24 h of slaughter, and they were immediately transported to Konkuk University (Seoul, Republic of Korea). Pork loins were divided into two equal groups and were randomly allocated to the postmortem aging methods (dry-aging and wet-aging) and postmortem times (7 and 14 days). For the purpose of wet-aging, the pork loin was individually packaged under vacuum using polyethylene/nylon vacuum bags (Cryovac, Duncan, SC) and stored in a refrigerated room at 1°C ± 0.5 for 14 days. For the purpose of dry-aging, the pork loin was placed on a stainless-steel tray in a refrigerated room at 1°C ± 0.5, a relative humidity of 80 ± 5% and an air flow rate of 0.5–1.5 m/s.
Proximate composition analysis
Moisture, crude fat, crude protein, and ash of samples were determined according to the method of Association of Official Analytical Chemists (AOAC, 1995).
pH measurement
Each sample (2 g) was homogenized with 18 mL of distilled water. The pH value of homogenized samples was measured by using a pH meter (pH 900, Precisa, Co, UK).
Aging loss and cooking loss
Aging loss of each sample (wet aging and dry aging at 7 and 14 days) was calculated as the difference in weight of samples with aged before and after
Each sample with a weight of approximately 100 g of steaks with 3 cm was heated in an oven. The cooking process was conducted by AMSA (1995). The samples were heated for 10 min to the core temperature of 71.1°C, and afterwards, they were cooled down to room temperature. Cooking loss of each sample was calculated as a percentage of weight loss before and after cooking.
2-Thiobarbituric acid reactive substances (TBARS)
TBARS measurement was performed according to the method of Buege and Aust (1978). Five grams of the sample was homogenized with 15 mL distilled water and 100 μL 6% butylated hydroxytoluene (BHT) in ethanol. One aliquot of the homogenate was reacted with 2 mL of TBA reagent (20 mM 2-thiobarbituric acid in 15% tricholoroacetic acid solution). The mixture was heated for 15 min in a water bath at 80°C. After cooling, the sample was centrifuged at 2000g for 10 min. The supernatant was filtered through a Whatman paper No. 1 and the absorbance of samples was evaluated at 531 nm by using a spectrophotometer (Optizen 2120UV, Mecasys, Seoul, Korea). The amount of TBARS was expressed as malondialdehyde (MDA) per kg of meat by using the molar extinction coefficient of 1.56 × 105 M−1 cm−1.
Protein solubility
Assessment of protein solubility in samples was conducted according to the method of Bowker and Zhuang (2016). Samples (1 g) were homogenized with 10 volumes of cold buffer for solubility (0.025 M potassium phosphate buffer (pH 7.2) for Sarcoplasmic protein solubility and 0.55 M potassium iodide in 0.05 M potassium phosphate buffer (pH 7.2) for Total protein solubility). Homogenized samples were stored in a refrigerator at 4°C for 20 h. After refrigeration, the samples were centrifuged at 2600g and at 4°C for 30 min. The protein concentration of the supernatant was determined using the biuret test. Myofibrillar protein solubility was calculated as the difference in solubility between total and sarcoplasmic proteins (= Total protein − Sarcoplasmic protein). Protein solubility was expressed as mg of soluble protein per g of meat.
Myofibril fragmentation index (MFI)
Assessment of the myofibril fragmentation index (MFI) of samples was conducted according to the method of Culler et al. (1978) with little modifications. Samples (4 g) were homogenized for 30 s with MFI buffer solution (20 mM potassium phosphate buffer at pH 7.0 with 100 mM KCl, 1 mM EDTA, 1 mM NaN3, and 1 mM MgCl2). Homogenized samples were centrifuged at 1000g and at 2°C for 15 min. The supernatant was removed and the samples were centrifuged again with addition of MFI buffer solution using the same parameters. The supernatant was removed and added to homogenize, and then filtered through a strainer. MFI buffer solution was added to the filtrate to make a protein concentration at 0.5 mg/mL. Absorbance value was evaluated at 540 nm by using a spectrophotometer (Optizen 2120UV, Mecasys, Seoul, Korea). The value of MFI was calculated using the following formula: MFI = 200 × Absorbance.
Metmyoglobin measurement
Assessment of metmyoglobin in samples was conducted according to the method of Chu et al. (1987) with little modifications. Samples (4 g) were homogenized for 10 s with 0.04 M phosphate buffer solution (pH 6.8). Homogenized samples were stored in a refrigerator at 4°C for 1 h. After refrigeration, samples were centrifuged at 5000g and at 5°C for 30 min. The supernatant was filtered through a Whatman filter paper No. 1. The absorbance of samples was evaluated at 525, 572, and 730 nm by using a spectrophotometer (Optizen 2120UV, Mecasys, Seoul, Korea). The metmyoglobin measurement values were calculated using the following formula:
Color measurement
Color measurement of samples was conducted before and after cooking and blooming for 30 min at room temperature. Each sample were performed inside measurements. The color of samples was expressed as CIE L*(lightness), CIE a*(redness), and CIE b*(yellowness). The colorimeter was calibrated using a white plate (CIE L* = + 94.48, a* = − 0.67, b* = + 3.31). Color measurement values of samples were determined using a colorimeter (NR-300, Nippon Denshoku, Tokyo, Japan).
Shear force
After cooking loss measurement, samples were cut from each steak into cubes of 100 mm2 at 30 mm in length. Shear force was recorded as peak force (kg) on a minimum of 10 strips from each sample using TX-XT2 (Stable Micro System Ltd, England) equipped with a triangular blade. The condition of this machine was designed as 50 kg load cell and 200 mm/min cross-head speed.
Sensory evaluation
Each steak was cut into approximately 2.54 cm of thickness and 100 g of weight. The steak was cooked at a core temperature of 72°C using the air conventional oven and cooled at room temperature for 30 min. Each cooked steak was cut into 1.5 × 1.5 × 2.54 cm pieces and randomly served using three-digit codes. Sensory evaluation was conducted by trained panelists and the scoring of each sample was obtained in terms of off-flavor intensity, flavor, juiciness, tenderness, chewiness, roast color, and overall palatability using the 7-hedonic scale test (1 = extremely intense, bland, dry, tough, not chewy, gray, non-acceptable and 7 = none, intense, juicy, tender, chewy, red cherry color, very acceptable).
Statistical analysis
The experiment was designed in a 2 × 2 factorial arrangement with the aging methods (wet and dry) and aging time (7 and 14 days). Data were analyzed using the two-way ANOVA of SPSS version 24.0. A significant difference between the aging method (dry and wet), aging time (20 and 40 days) and the aging method × time interaction effects were tested by the Student t test (p < 0.05).
Results and discussion
Proximate composition
Results of proximate composition of dry-aged and wet-aged pork loins during the aging time are shown in Table 1. Dry-aged pork loins had significantly lower moisture content than wet-aged pork loins (66.69 and 70.03%, respectively), while dry-aged pork loins had significantly higher crude protein, fat, and ash contents (24.50, 3.57, and 1.58%) than wet-aged pork loins (23.70, 2.65, and 1.18%). With respect to the aging time, moisture and protein contents of aged pork loins were slightly decreased, whereas crude fat and ash contents increased from 7 to 14 days (p < 0.001). The aging method and time interaction effects on crude fat content were detected (p < 0.01), because fat content of dry-aged pork loins was more affected by the aging time. The interaction result showed that the moisture content of dry-aged pork loins was more easily evaporated since the surface area of meat was directly exposed to the air. A similar result was observed by Lee et al. (2016) who reported that dry-aged pork loins at 40 days had lower moisture content than non-aged pork loins.
Table 1.
Proximate compositions of aging method and aging time on loin from pork
| Moisture (%) | Crude protein (%) | Crude fat (%) | Ash (%) | |
|---|---|---|---|---|
| Aging method | ||||
| Wet aging | 70.03 | 23.70 | 2.65 | 1.18 |
| Dry aging | 66.69 | 24.50 | 3.57 | 1.58 |
| p value | < 0.001 | 0.006 | < 0.001 | < 0.001 |
| SEMa | 0.215 | 0.169 | 0.056 | 0.022 |
| Aging time | ||||
| 7 days | 68.62 | 25.01 | 2.31 | 1.28 |
| 14 days | 68.09 | 23.09 | 3.91 | 1.48 |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| SEM | 0.215 | 0.169 | 0.056 | 0.001 |
| Aging method × aging time | ||||
| Wet aging | ||||
| ×7 days | 70.66 | 24.80 | 1.98 | 1.14 |
| ×14 days | 70.62 | 22.60 | 3.33 | 1.23 |
| Dry aging | ||||
| ×7 days | 67.81 | 25.22 | 2.64 | 1.43 |
| ×14 days | 66.79 | 23.79 | 4.49 | 1.73 |
| p value | 0.088 | 0.132 | 0.005 | 0.024 |
| SEM | 0.035 | 0.239 | 0.080 | 0.031 |
aSEM, standard error of mean
pH, aging loss, and cooking loss
The pH, aging loss, and cooking loss of dry-aged and wet-aged pork loins at 7 and 14 days are expressed in Table 2. The pH increased significantly owing to the dry-aging process and 14 days aging time (p < 0.001). Cooking loss of dry-aged pork loins was significantly lower than that of wet-aged pork loins (20.30 and 27.75%, respectively). Lower cooking loss and higher pH of dry-aged meat than vacuum-packed meat was in agreement with the result presented by Li et al. (2013) and Obuz et al. (2014). Recently, enhancement of cooking yield and water-holding capacity of dry-aged beef was reported by Choe and Kim (2017). Several studies have indicated that water-holding capacity increases with disintegration of protein during the aging time since there is an increase in ionic net charge through absorption and release of K+ and Ca2+ (Lawrie, 1998; Naveena et al., 2015). Aging loss of wet-aged pork loins was significantly lower than that of dry-aged pork loins (4.94 and 21.20%, respectively), which was in agreement with previous studies (Li et al., 2013; Warren and Kastner, 1992).
Table 2.
Effect of aging method and aging time on pH, aging loss, cooking loss, TBARS, protein solubility (sarcoplasmic, myofibrillar and total protein) of loin form pork
| pH | Aging loss (%) | Cooking loss (%) | TBARSa | Sarcoplasmic protein (mg/g) | Myofibrillar protein (mg/g) | Total protein (mg/g) | |
|---|---|---|---|---|---|---|---|
| Aging method | |||||||
| Wet aging | 5.41 | 4.94 | 27.75 | 0.17 | 79.23 | 43.11 | 112.34 |
| Dry aging | 5.51 | 21.20 | 20.30 | 1.66 | 76.80 | 42.07 | 118.86 |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.153 | 0.594 | 0.008 |
| SEMb | 0.011 | 1.460 | 0.545 | 0.041 | 1.092 | 1.331 | 0.698 |
| Aging time | |||||||
| 7 days | 5.43 | 10.57 | 25.10 | 0.77 | 81.93 | 39.37 | 118.78 |
| 14 days | 5.9 | 15.57 | 22.95 | 1.07 | 79.14 | 45.81 | 122.43 |
| p value | < 0.001 | 0.022 | 0.545 | < 0.001 | 0.109 | 0.009 | 0.006 |
| SEM | 0.001 | 1.460 | 0.008 | 0.041 | 1.092 | 1.331 | 0.698 |
| Aging method × aging time | |||||||
| Wet aging | |||||||
| ×7 days | 5.4 | 3.69 | 29.44 | 0.11 | 84.76 | 38.34 | 119.54 |
| ×14 days | 5.48 | 6.18 | 26.05 | 0.23 | 80.83 | 47.88 | 125.14 |
| Dry aging | |||||||
| ×7 days | 5.50 | 17.44 | 20.75 | 1.42 | 81.18 | 40.40 | 118.01 |
| ×14 days | 5.57 | 24.96 | 19.85 | 1.90 | 79.54 | 43.74 | 119.72 |
| p value | 0.845 | 0.234 | 0.116 | 0.205 | 0.478 | 0.139 | 0.084 |
| SEM | 0.017 | 2.064 | 0.770 | 0.006 | 1.545 | 1.882 | 0.987 |
aUnit, MDA mg/kg meat
bSEM, standard error of mean
TBARS
As shown in Table 2, TBARS level in dry-aged pork loin from 7 to 14 days aging time (1.42–1.90 MDA mg/kg of meat) was significantly increased compared to that in wet-aged pork loin (0.11–0.23 MDA mg/kg of meat). The aging method and the aging times interaction effect on TBARS was not observed (p = 0.205). DeGeer et al. (2009) found that increasing TBARS values in dry-aged beef between 21 and 28 days did not have a negative effect on flavor, whereas the lipid oxidation positively contributed to aged-flavor of dry-aged beef. A similar result was observed by Naveena et al. (2015) Exposure to the air could lead to sequential acceleration of lipid oxidation in meat under a dry-aging condition.
Protein solubility and myofibril fragmentation index (MFI)
The range of sarcoplasmic protein solubility from 76.80 to 79.23 mg/g and that of myofibrillar protein solubility from 42.07 to 43.11 mg/g was observed in dry-aged and wet-aged pork loins (Table 2), whereas no significant differences in sarcoplasmic and myofibrillar protein solubility were detected (p > 0.05). Moreover, sarcoplasmic protein solubility was not affected by the aging time, while total protein and myofibrillar protein solubility increased from 7 to 14 days (p < 0.01). Moreover, dry-aged pork loin showed higher total protein solubility than wet-aged pork loin (p < 0.01). The interaction effect of protein solubility was not observed in this study (p > 0.05). Wang and Xiong (2005) proved that the increase in protein solubility is related to the degree of protein hydrolysis. An increase in protein solubility of bovine muscle during the aging time has been reported in previous studies (Hopkins and Thompson, 2001; Naveena et al., 2015).
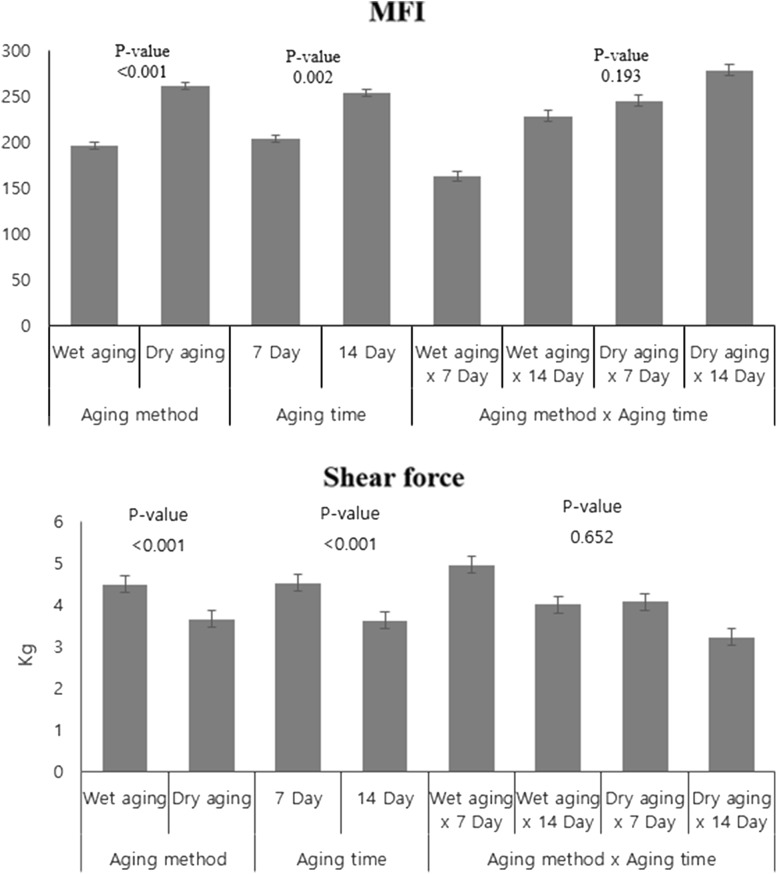
Figure 1 shows the changes in MFI of pork loin with the aging method and aging time. MFI of pork loins was significantly affected by the aging method (p < 0.001) and the aging time (p = 0.002). The aging method and the time interaction effect on MFI was not detected (p > 0.05). Marino et al. (2013) observed that MFI of beef under the vacuum packaging condition at 21 days aging time increased from 152.25 to 158.00 compared to that at 0 days aging time (MFI = 38.14–5.85). A similar result was reported by Silva et al. (1999) who found an increasing MFI of wet-aged beef during postmortem (13 days). The increase in MFI during the aging time can be explained by the fact that proteolysis is responsible for the degradation of myofibrillar proteins (Gil et al., 1999). With respect to the aging method, a high protein degradation rate was also reported in dry-aged beef compared with wet-aged beef in accordance with the result of protein solubility (Kim et al., 2016).
Fig. 1.
Effect of aging time and method on experiment design of loin muscle from pork
Metmyoglobin measurement
A significant interaction effect between the aging method and the aging time on metmyoglobin (%) was observed (p < 0.001; Table 3). Metmyoglobin content of dry-aged pork loins (7 days, 53.40% and 14 days, 64.29%) was more significantly affected by the aging time compared to that of wet-aged pork loins (7 days, 28.26% and 14 days, 39.15%). Naveena et al. (2015) have reported that metmyoglobin content of aerobically packaged emu meat was higher than that of vacuum packaged emu meat at 9d aging time (55.93 and 6.97%, respectively). Metmyoglobin was formed from myoglobin oxidation (Faustman et al., 2010). Metmyoglobin accumulation in meat was influenced by exogenous factors such as postmortem aging, display time, and muscle type (Madhavi and Carpenter, 1993). Lindahl (2011) observed a similar result of high metmyoglobin accumulation in aged meat in high oxygen modified atmosphere packaging compared to aged meat in vacuum packaging. Therefore, this result might be caused due to the fact that oxygen penetration in dry-aged pork loins was enhanced since the surface of dry-aged pork loins was entirely exposed to the air compared to that of wet-aged pork loins.
Table 3.
Effect of aging method and aging time Metmyoglobin measurement, Uncooked color (L*, a*,b*), Cooked color (L*, a*, b*) of loin muscle form pork
| Metmyoglobin (%) | Uncooked meat color | Cooked meat color | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||
| Aging method | |||||||
| Wet aging | 33.71 | 54.85 | 7.66 | 6.10 | 67.88 | 4.18 | 12.58 |
| Dry aging | 58.84 | 54.45 | 5.89 | 6.52 | 68.57 | 4.05 | 17.44 |
| p value | < 0.001 | 0.586 | < 0.001 | 0.101 | 0.366 | 0.508 | < 0.001 |
| SEMa | 1.1591 | 0.524 | 0.262 | 0.178 | 0.531 | 0.139 | 0.337 |
| Aging time | |||||||
| 7 days | 40.83 | 54.78 | 7.32 | 6.48 | 66.98 | 4.91 | 15.52 |
| 14 days | 51.72 | 54.52 | 6.23 | 6.14 | 69.47 | 3.31 | 14.50 |
| p value | < 0.001 | 0.731 | < 0.001 | 0.181 | 0.003 | < 0.001 | 0.043 |
| SEM | 1.591 | 0.524 | 0.262 | 0.178 | 0.531 | 0.139 | 0.337 |
| Aging method × aging time | |||||||
| Wet aging | |||||||
| ×7 days | 28.26 | 53.55 | 8.42 | 5.99 | 66.61 | 4.96 | 11.95 |
| ×14 days | 39.15 | 56.15 | 6.90 | 6.20 | 69.15 | 3.40 | 13.22 |
| Dry aging | |||||||
| ×7 days | 53.40 | 56.00 | 6.22 | 6.22 | 67.35 | 4.86 | 19.10 |
| ×14 days | 64.29 | 52.89 | 5.56 | 5.56 | 69.79 | 3.23 | 15.78 |
| p value | < 0.001 | < 0.001 | 0.252 | 0.034 | 0.941 | 0.888 | 0.001 |
| SEM | 1.984 | 0.741 | 0.371 | 0.251 | 0.751 | 0.196 | 0.477 |
aSEM, standard error of mean
Color of raw and cooked pork loins based on the aging methods and aging times
The effect of aging methods and aging times on color (L*, lightness; a*, redness and b*, yellowness) of raw meat and cooked meat was evaluated (Table 3). Interaction effects on lightness and yellowness of uncooked meat were observed (p < 0.05). Interaction effects could be explained by the fact that the lightness and yellowness of wet-aged pork loins increased from 7 to 14 days, whereas the lightness and yellowness of dry-aged pork loins decreased. Increased lightness of wet-aged pork loins can be explained by the fact that the high moisture content contributed to increasing the reflectance spectra of wet-aged pork loins (Bertram et al., 2004). Similarly, Hwa et al. (2016) observed that the aging time under vacuum packaging resulted in an increase in lightness. In addition, the redness of dry-aged pork loins was lower (p < 0.001) than that of wet-aged pork loins, and the reduction in redness occurred in both dry-aged and wet-aged pork loins from 7 to 14 days (p < 0.001). This finding is in agreement with the result described by Kim et al. (2016) who reported that dry-aged beef had a relatively declining tendency of colors (lightness, redness, and yellowness) compared to wet-aged beef. In cooked pork loins, dry and wet aging methods did not influence the lightness and redness of aged pork loins, whereas an increase in lightness with a decrease in redness occurred from 7 to 14 days (P < 0.001). The interaction effect on yellowness of cooked pork was observed (p < 0.001). Yellowness of dry-aged pork loins was significantly higher than that of wet-aged pork loins regardless of the aging time. As reported by Seyfert et al. (2004), premature browning of cooked meat at 71.1°C was accelerated in the presence of high oxygen levels. Therefore, high oxygen penetration to the interior of meat during aging may contribute to the transformation of myoglobin status and the color of meat (Vitale et al., 2014).
Shear force
Shear force of aged pork loin was significantly (p < 0.001) affected by the aging methods and aging times (Fig. 2). The shear force value of wet-aged dry-aged pork loins was expressed as 4.65 and 3.61 kg, respectively. Furthermore, shear force of aged pork loins decreased from 4.68 to 3.59 kg during aging. The interaction effect on shear force was not observed (p > 0.05). Campbell et al. (2001) noted that shear force was reduced in beef longissimus muscle between 14 and 21 days dry aging time. Improvement in shear force (instrumental tenderness) during aging occurred from the degradation of proteins such as myofibrillar and cytoskeletal proteins (Nowak, 2011). Destefanis et al. (2008) demonstrated that the perception of tenderness was defined as the range of shear force (4.37–5.2 kg, intermediate tender and 3.36–4.36 kg, tender). Lee et al. (2016) concluded that dry-aged pork at 40 days aging time had a lower shear force than non-aged pork (2.50 and 4.64 kg, respectively).
Fig. 2.
Effect of aging time and method on MFI (A) and shear force (B) of loin muscle from pork
Sensory evaluation
The effect of aging methods and aging times on sensory characteristics of pork loins (four treatments, dry-aged pork loins and wet-aged pork loins at 7 and 14 days aging times) is shown in Table 4. There was no difference in off-odor between wet-aged pork loins at 7 and 14 days, whereas dry-aged pork loins showed a significantly lower amount of off-odor than wet-aged pork loins (p < 0.01). In addition, flavor score of wet-aged pork loins increased, while flavor score increased in dry-aged pork loins during the aging time (p < 0.01). Juiciness of dry-aged pork loins was slightly lower than that of wet-aged pork loins and tenderness of dry-aged pork loins at 14 days aging time showed the highest score. In agreement with previous studies (Campbell et al., 2001; Juarez et al., 2001), tenderness, juiciness, and flavor of dry-aged meat increased with aging. Roast color and overall acceptability of dry-aged pork loins were significantly higher than those of wet-aged pork loins (p < 0.01), which is in agreement with the result presented by Warren and Kastner (1992) who reported that brown-roasted flavor of dry-aged beef was increased compared to that of vacuum-aged beef.
Table 4.
Effect of aging method and aging time on sensory characteristics of loin muscle form pork
| Off flavor | Aroma | Juiciness | Tenderness | Chewiness | Roast color | Overall | |
|---|---|---|---|---|---|---|---|
| Aging method | |||||||
| Wet aging | 3.813 | 3.438 | 3.500 | 3.625 | 4.313 | 3.188 | 3.375 |
| Dry aging | 2.438 | 2.438 | 4.813 | 4.250 | 3.563 | 3.563 | 5.188 |
| p value | 0.001 | 0.030 | 0.010 | 0.122 | 0.091 | 0.477 | < 0.001 |
| SEMa | 0.262 | 0.309 | 0.334 | 0.278 | 0.303 | 0.368 | 0.311 |
| Aging time | |||||||
| 7 days | 3.313 | 2.938 | 4.063 | 3.500 | 4.188 | 3.250 | 4.125 |
| 14 days | 2.938 | 2.938 | 4.250 | 4.375 | 3.688 | 3.500 | 4.438 |
| p value | 0.320 | 1.000 | 0.695 | 0.034 | 0.254 | 0.635 | 0.483 |
| SEM | 0.262 | 0.309 | 0.334 | 0.278 | 0.303 | 0.368 | 0.311 |
| Aging method × aging time | |||||||
| Wet aging × 7 days | 3.875 | 3.125 | 3.375 | 3.125 | 4.625 | 2.750 | 3.250 |
| Dry aging × 7 days | 2.750 | 2.750 | 4.750 | 3.875 | 3.750 | 3.750 | 5.000 |
| Wet aging × 14 days | 3.750 | 3.750 | 3.625 | 4.125 | 4.000 | 3.625 | 3.500 |
| Dry aging × 14 days | 2.125 | 2.125 | 4.875 | 4.625 | 3.375 | 3.375 | 5.375 |
| p value | 0.505 | 0.164 | 0.896 | 0.752 | 0.773 | 0.240 | 0.888 |
| SEM | 0.371 | 0.437 | 0.473 | 0.392 | 0.429 | 0.521 | 0.440 |
aSEM, standard error of mean
In terms of the aging method, dry aging process induced an improvement in instrumental tenderness and sensory characteristics of pork loins although adverse effects on total loss (the sum of aging loss and cooking loss) and pigment oxidation compared with those of wet-aged pork loins in this study. In addition, meat aged for 14 days by aging time improved meat quality more than aged meat for 7 days regardless of the aging method. The increase in crude fat and crude protein of dry-aged pork is due to the loss of meat moisture and, a decrease in shear force during aging. Therefore, this result concluded that the dry-aged pork with 14 days of the aging time improved physicochemical and sensory characteristics. For better improvement in dry-aging on pork quality, further studies are required for determining different conditions (temperature and relative humidity) and packaging (high/low oxygen penetration) response to the total loss, brown color of cooked pork loins, and lipid/protein oxidation and nutrient contents (fatty acid, free amino acid and mineral contents).
Acknowledgements
This paper was supported by Konkuk University in 2017.
References
- AMSA. American Meat Science Association. Research guidelines for cookery, sensory evaluation and instrumental tenderness measurement of fresh meat. Chicago, IL, USA (1995)
- AOAC. Official Methods of Analysis. 16th ed. Method 39.1-39.23. Association of Official Analytical Chemists, Arlington, VA, USA (1995)
- Bertram H, Engelsen S, Busk H, Karlsson A, Andersen S. Water properties during cooking of pork studied by low-field NMR relaxation: effects of curing and the RN-gene. Meat Sci. 2004;66(2):437–446. doi: 10.1016/S0309-1740(03)00132-3. [DOI] [PubMed] [Google Scholar]
- Bowker B, Zhuang H. Impact of white striping on functionality attributes of broiler breast meat. Poult Sci. 2016;95(8):1957–1965. doi: 10.3382/ps/pew115. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Method Enzymol. 1978;30:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Hunt MC, Levis P, Chambers E. Dry-aging effects on palatability of beef longissimus muscle. J. Food Sci. 2001;66:196–199. doi: 10.1111/j.1365-2621.2001.tb11315.x. [DOI] [Google Scholar]
- Choe JH, Stuart A, Kim YBH. Effect of different aging temperatures prior to freezing on meat quality attributes of frozen/thawed lamb loins. Meat Sci. 2016;116:158–164. doi: 10.1016/j.meatsci.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Choe JH, Kim HY. Effects of dry aging on physicochemical properties of beef cattle loins. Korean J. Food Sci. Technol. 2017;49(2):158–161. doi: 10.9721/KJFST.2017.49.2.158. [DOI] [Google Scholar]
- Chu YH, Huffman DL, Trout GR, Egbert WR. Color and color stability of frozen restructured beef steaks: effect of sodium chloride, tripolyphosphate, nitrogen atmosphere, and processing procedures. J. Food Sci. 1987;52:869–875. doi: 10.1111/j.1365-2621.1987.tb14230.x. [DOI] [Google Scholar]
- Culler RD, Parrish FCJR, Smith GC, Cross HR. Relationship of myofibril fragmentation index to certain chemical, physical, and sensory characteristics of bovine longissimus muscle. J. Food Sci. 1978;43:1177. doi: 10.1111/j.1365-2621.1978.tb15263.x. [DOI] [Google Scholar]
- DeGeer SL, Hunt MC, Bratcher CL, Crozier-Dodson BA, Johnson DE, Stika JF. Effects of dry aging of bone-in and boneless strip loins using two aging processes for two aging times. Meat Sci. 2009;83:768–774. doi: 10.1016/j.meatsci.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Destefanis G, Brugiapaglia A, Barge MT, Dal Molin E. Relationship between beef consumer tenderness perception and Warner-Bratzler shear force. Meat Sci. 2008;78:153–156. doi: 10.1016/j.meatsci.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Faustman C, Sun Q, Mancini R, Suman SP. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010;86:86–94. doi: 10.1016/j.meatsci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Gil M, Guerrero L, Sarraga C. The effect of meat quality, salt and ageing time on biochemical parameters of dry-cured Longissimus dorsi muscle. Meat Sci. 1999;51:329–337. doi: 10.1016/S0309-1740(98)00129-6. [DOI] [PubMed] [Google Scholar]
- Hopkins DL, Thompson JM. Inhibition of protease activity. Part 1. The effect on tenderness and indicators of proteolysis in ovine muscle. Meat Sci. 2001;59:175–185. doi: 10.1016/S0309-1740(01)00068-7. [DOI] [PubMed] [Google Scholar]
- Hwa SH, Kim JH, Kim JH, Jang HJ, Ju MG, Cho WY, Lee CH. Effect of dietary processed sulfur on the meat quality in pork under aging. Korean. J. Food. Sci. An. 2016;36(6):760–768. doi: 10.5851/kosfa.2016.36.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez M, Cainea WR, Dugana MER, Hidirogloub N, Larsena IL, Uttaroa B, Aalus JL. Effect of dry-ageing on pork quality characteristics in different genotypes. Meat Sci. 2001;88:117–121. doi: 10.1016/j.meatsci.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Kim YHB, Kemp R, Samuelsson LM. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016;111:168–176. doi: 10.1016/j.meatsci.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Lawrie RA. Meat Science. 6. New York: Pergamon Press; 1998. [Google Scholar]
- Lee CW, Lee JR, Kim MK, Jo C, Lee KH, You I, Jung S. Quality improvement of pork loin by dry aging. Korean J. Food Sci. An. 2016;36(3):369–376. doi: 10.5851/kosfa.2016.36.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G. Colour stability of steaks from large beef cuts aged under vacuum or high oxygen modified atmosphere. Meat Sci. 2011;87:428–443. doi: 10.1016/j.meatsci.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Li X, Babol J, Wallby A, Lundsotrom K. Meat quality, microbiological status and consumer preference of beef gluteus medius aged in a dry ageing bag or vacuum. Meat Sci. 2013;95:229–234. doi: 10.1016/j.meatsci.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Madhavi DL, Carpenter CE. Aging and processing affect color, metmyoglobin reductase and oxygen consumption of beef muscles. J. Food Sci. 1993;58(5):939–947. doi: 10.1111/j.1365-2621.1993.tb06083.x. [DOI] [Google Scholar]
- Marino R, Albenzio M, Malva Ad, Santillo A, Loizzo P, Sevi A. Proteolytic pattern of myofibrillar protein and meat tenderness as affected by breed and aging time. Meat Sci. 2013;95:281–287. doi: 10.1016/j.meatsci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Min B, Nam KC, Cordray J, Ahn DU. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J. Food Sci. 2008;73(6):C439–C446. doi: 10.1111/j.1750-3841.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Naveena BM, Muthukumar M, Kulkarni VV, Kumar YP, Rani KU, Kiran M. Effect of aging on the physicochemical, textural, microbial and proteome changes in emu (Dromaius Novaehollandiae) meat under different packaging conditions. J. Food Process. Preserv. 2015;30:2497–2506. doi: 10.1111/jfpp.12499. [DOI] [Google Scholar]
- Nowak D. Enzymes in tenderization of meat—the system of calpains and other systems—a review. Pol. J. Food Nutr. Sci. 2011;61:231–237. [Google Scholar]
- Obuz E, Akkaya L, Gok V, Dikeman ME. Effects of blade tenderization, aging method and aging time on meat quality characteristics of longissimus lumborum steaks from cull Holstein cows. Meat Sci. 2014;96:1227–1232. doi: 10.1016/j.meatsci.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Rhee KS, Anderson LM, Sams AR. Lipid oxidation potential of beef, chicken, and pork. J. Food Sci. 1996;61:8–12. doi: 10.1111/j.1365-2621.1996.tb14714.x. [DOI] [Google Scholar]
- Seyfert M, Hunt MC, Mancini RA, Kropf DH, Stroda SL. Internal premature browning in cooked steaks from enhanced beef round muscles packaged in high-oxygen and ultra-low oxygen modified atmospheres. J. Food Sci. 2004;69(2):142–146. [Google Scholar]
- Silva JA, Patarata L, Martins C. Influence of ultimate pH on bovine meat tenderness during ageing. Meat Sci. 1999;52:453–459. doi: 10.1016/S0309-1740(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Sitz BM, Calkins CR, Feuz DM, Umberger WJ, Eskridge KM. Consumer sensory acceptance and value of wet-aged and dry-aged beef steaks. J. Anim. Sci. 2006;84:1221–1226. doi: 10.2527/2006.8451221x. [DOI] [PubMed] [Google Scholar]
- Vitale M, Perez-Juan Lloret E, Arnau J, Realini CE. Effect of aging time in vacuum on tenderness, and color and lipid stability of beef from mature cows during display in high oxygen atmosphere package. Meat Sci. 2014;96:270–277. doi: 10.1016/j.meatsci.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Wang LL, Xiong YL. Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability. J. Agric. Food Chem. 2005;53:9186–9192. doi: 10.1021/jf051213g. [DOI] [PubMed] [Google Scholar]
- Warren KE, Kastner CL. A comparison of dry-aged and vacuum-aged beef strip loins. J Muscle Foods. 1992;3:151–157. doi: 10.1111/j.1745-4573.1992.tb00471.x. [DOI] [Google Scholar]